Abstract
Electrically excitable cells have voltage-dependent ion channels on the plasma membrane that regulate membrane permeability to specific ions. Voltage-gated Ca2+ channels (VGCCs) are especially important as Ca2+ serves as both a charge carrier and second messenger. Zebrafish (Danio rerio) are an important model vertebrate for studies of neuronal excitability, circuits, and behavior. However, electrophysiological properties of zebrafish VGCCs remain largely unexplored because a suitable preparation for whole cell voltage-clamp studies is lacking. Rohon-Beard (R-B) sensory neurons represent an attractive candidate for this purpose because of their relatively large somata and functional homology to mammalian dorsal root ganglia (DRG) neurons. Transgenic zebrafish expressing green fluorescent protein in R-B neurons, (Isl2b:EGFP)ZC7, were used to identify dissociated neurons suitable for whole cell patch-clamp experiments. Based on biophysical and pharmacological properties, zebrafish R-B neurons express both high- and low-voltage-gated Ca2+ current (HVA- and LVA-ICa, respectively). Ni+-sensitive LVA-ICa occur in the minority of R-B neurons (30%) and ω-conotoxin GVIA-sensitive CaV2.2 (N-type) Ca2+ channels underlie the vast majority (90%) of HVA-ICa. To identify G protein coupled receptors (GPCRs) that modulate HVA-ICa, a panel of neurotransmitters was screened. Application of GABA/baclofen or serotonin produced a voltage-dependent inhibition while application of the mu-opioid agonist DAMGO resulted in a voltage-independent inhibition. Unlike in mammalian neurons, GPCR-mediated voltage-dependent modulation of ICa appears to be transduced primarily via a cholera toxin-sensitive Gα subunit. These results provide the basis for using the zebrafish model system to understanding Ca2+ channel function, and in turn, how Ca2+ channels contribute to mechanosensory function.
INTRODUCTION
In excitable cells, such as neurons, Ca2+ influx through voltage-gated Ca2+ channels (VGCCs) contribute to electrical excitability, synaptic transmission, and signaling pathways involved with gene expression. Therefore identification of VGCC subtypes, cellular distribution, and sensitivity to neuromodulators is fundamental for understanding neuronal physiology. VGCCs have been well characterized in neurons of mammalian and avian primary sensory ganglia (Scroggs and Fox 1992a). VGCCs on the readily accessible soma provide a model system for exploring the molecular underpinnings of G protein coupled receptors (GPCRs) modulation and, by inference, presynaptic mechanisms underlying alteration of synaptic transmission at the less accessible presynaptic nerve terminal.
VGCCs are composed of an α1 subunit and different auxiliary β and α2δ subunits. Ten distinct genes encoding different α1 subunit have been identified and classified based on pharmacological and electrophysiological properties (Catterall et al. 2005). The CaV1 (α1C, α1D, α1F, α1s) subfamily encodes high-voltage-activated (HVA) L-type channels that are sensitive to dihydropyridine antagonists (Bean 1984). The CaV2 subfamily, comprised of the α1A, α1B, and α1E genes products, correspond to HVA P/Q-, N-, and R-type channels, respectively, are selectively inhibited by ω-agatoxin IVA (Mintz and Bean 1993), ω-conotoxins GVIA or MVIIA (Mintz et al. 1991; Olivera et al. 1994), and SNX-482 (Newcomb et al. 1998). The CaV2 class of VGCCs provide the main source of Ca2+ to support neurotransmitter release and are commonly modulated by heterotrimeric G proteins acting downstream from GPCR activation (Dolphin 2003; Tedford and Zamponi 2006a). Lastly, members of the CaV3 Ca2+ channel family, α1G, α1H, α1I, correspond to low voltage-activated (LVA) T-type Ca2+ channels (Catterall et al. 2005).
Despite the wealth of information accumulated for mammalian and avian sensory neuron VGCCs, little is known about zebrafish (Danio rerio) VGCCs and modulation by GPCRs. Zebrafish represent an important model vertebrate for studies of neuronal excitability, circuits, and behavior as the cost of housing, ease of genetic manipulation, optical transparency, and ex vivo development are advantageous when compared with mammalian systems. However, VGCCs in zebrafish neurons remain largely unexplored due to the difficulty in isolating single identified neurons suitable for whole cell voltage-clamp studies. Here we utilized a transgenic zebrafish line expressing green fluorescent protein to develop a technique for isolating Rohon-Beard (R-B) neurons at 24 h postfertilization (hpf). R-B neurons are primary sensory neurons that exist transiently during the early embryonic development of anamniotes (Clarke et al. 1984; Lamborghini 1987; Soffe 1991; Williams et al. 2000) and are functionally replaced by dorsal root ganglion (DRG) neurons at later stages of development. Zebrafish embryos acquire a tactile response to mechanical stimulation during the initial phase of development that relies solely on R-B neuron function (Roberts 2000). Therefore R-B neurons provide a simple, but functionally mature, sensory system at a time point when transient genetic manipulations such a morpholino antisense oligonucleotide-mediated suppression of protein translation are efficacious.
In this study, we show that enzymatically isolated R-B neurons are readily identified from GFP fluorescence and suitable for whole cell voltage-clamp studies. Using this technique, we characterized LVA- and HVA-Ca2+ currents (ICa). HVA-ICa arose almost exclusively from CaV2.2 (N-type) channels as inferred from toxin-occlusion studies. Finally, we provide evidence that 5-HT, GABA, and DAMGO modulate HVA-Ca2+ channels via different G protein dependent mechanisms.
METHODS
Animals
Zebrafish (D. rerio) were housed at 28°C in the fish facility on a 14/10 h light/dark cycle. Embryos were maintained at 28°C in embryo media consisting of 1 mM NaCl and 10-4 % methylene blue. Staging was based on external morphology (Kimmel et al. 1995) and defined as hpf or days postfertilization (dpf). Wild-type embryos were obtained from AB, TL, or TAB type fish. The transgenic fish line, Tg(Islet2b:EGFP)ZC7, was kindly provided by Dr. Chi-Bin Chien (University of Utah Medical Center). The transgenic fish line Tg(HuC:mCherry) expressed the mCherry fluorescent protein driven by the HuC promoter. Adult fish were bred according to guidelines outlined in the Zebrafish Book (Westerfield 1995). After crossing, heterozygote embryos were selected using a fluorescence stereomicroscope. Maintenance of adult fish and experiments using embryos were performed following Institutional Animal Care and Use Committee guidelines of the National Institutes of Health/NIAAA.
Preparation of R-B neurons
Larvae (<30 hpf) expressing enhanced green fluoresent protein (EGFP) were anesthetized in embryo media containing 0.02% tricaine (Sigma-Aldrich, St. Louis, MO). Dechorionated embryos were killed by transection at a level caudal to the yolk sac (see Fig. 2A). The trunk of the embryo was transferred to dissociation buffer containing (in mM) 0.6 EDTA, 5.5 Glucose, 5.4 KCl, 136.8 NaCl, 5.5 NaHCO3, and 2 mg/ml trypsin TRL (Worthington Biochemical, Lakewood, NJ) and incubated for 30 min at room temperature. After incubation, partially dissociated tissues were triturated with a Pasteur pipette in culture medium consisting of 60% L-15 (Invitrogen, Carlsbad, CA), 1 mM Na-HEPES (Sigma-Aldrich), 1% penicillin-streptomycin (Invitrogen), and 0.5% horse serum (Invitrogen). Dissociated R-B neurons were plated on poly-l-lysine (Sigma-Aldrich) coated tissue culture dishes and maintained overnight at room temperature prior to recording. In some experiments, neurons were incubated with either Bordetella pertussis (PTX; 500 ng/ml) or Vibrio cholerae (CTX; 500 ng/ml) toxin.
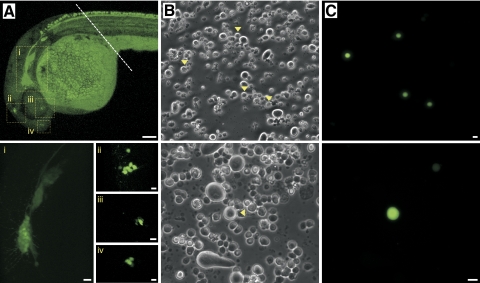
Fig. 2.
Isolation of single R-B neurons from Isl2b:EGFP transgenic zebrafish embryos. A: at 24 hpf, several clusters of neurons (labeled i–iv) in the cranial region displayed intense EGFP expression. - - -, where embryos were transected prior to enzymatic and mechanical dissociation of the trunk region. The fluorescence in the yolk sac was auto-fluorescence. B and C: phase-contrast (B) and fluorescent (C) photomicrographs of acutely dissociated R-B neurons (▶) from 24 hpf Isl2b:EGFP embryos. Scale bar in A (top) represents 1 mm. The remainders of the scale represent 10 μm.
Electrophysiological recording
ICa was recorded using the conventional whole cell patch-clamp configuration (Hamill et al. 1981). Patch electrodes were fabricated from borosilicate glass capillaries (1.5 mm OD, 0.84 mm ID; WPI, Sarasota, FL) using a model P-97 micropipette puller (Sutter Instrument, Novato, CA). The patch electrodes were coated with silicone elastomer (Sylgard 184, Dow Corning, Midland, MI) and fire-polished to a final resistance of ∼8–9 MΩ when filled with the pipette solution described in the following text. After rupturing the cell membrane, the mean access resistance was 18.5 ± 0.5 (SE) MΩ. The cell membrane capacitance was cancelled and series resistance was routinely compensated (>85% for both prediction and compensation; lag set to 10 μs) with an Axopatch 200B patch-clamp amplifier (Molecular Devices, Sunnyvale, CA). The bath was grounded by an Ag/AgCl pellet connected via a 0.15 M NaCl/agar bridge. The liquid junction potential between the pipette and external solutions used for ICa isolation was approximately −1 mV and not corrected for. Voltage protocol generation and data acquisition were performed using custom-designed software (S5) on a Macintosh G4 computer (Apple Computer, Cupertino, CA) equipped with an ITC-18 data acquisition interface (HEKA Instruments, Bellmore, NY). Currents were filtered at 2 kHz (−3 dB) using a four-pole low-pass Bessel filter, digitized at 10 kHz with a 16-bit analog-to digital converter in the ITC-18 data acquisition interface, and stored on the computer. All drug and bath solutions were applied to single neurons via a gravity-fed fused silica capillary tube connected to an array of seven polyethylene tubes. Drug application was started by switching the bath solution to a drug solution to avoid flow-induced artifacts. All recordings were performed at room temperature (21–24°C).
Solutions and chemicals
To isolate ICa, patch electrodes were filled with a solution containing (in mM) 120 N-methyl-d-glucamine, 20 tetraethylammonium hydroxide (TEA-OH), 11 EGTA, 10 HEPES, 1 CaCl2, 20 HCl, 4 MgATP, 0.1 Na2GTP, and 14 Tris creatine phosphate (pH 7.2, 295 mmo/kg H2O). The bath solution contained (in mM) 140 methanesulfonic acid, 145 TEA-OH, 10 HEPES, 10 glucose, 10 CaCl2, and 0.0003 tetrodotoxin (pH 7.4, 320 mmo/kg H2O). Stock solutions were made for the following drugs: γ-aminobutyric acid (GABA), (rs)-baclofen, l-glutamic acid hydrochloride, oxotremorine M (all from TOCRIS Cookson, Ellisville, MO), nifedipine (EMD Chemicals, Gibbstown, NJ), tetrodotoxin (TTX), ω-agatoxin IVA, ω-conotoxin GVIA, ω-conotoxin MVIIA, SNX-482 (all from Alomone Labs, Jerusalem, Israel), guanylyl 5′-imidodiphosphate [Gpp(NH)p], (±)-norepinephrine, serotonin hydrochloride (5-hydroxytryptamine, 5-HT), NiCl2, and CdCl2 (all from Sigma-Aldrich). Drugs were prepared in distilled water, except for nifedipine, which was dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO was 0.1%, which did not affect ICa. For experiments with peptide toxins, bovine serum albumin (BSA) was added to all external solution at 0.5 mg/ml to minimize nonspecific binding. PTX and CTX holotoxins were purchased from List Biological Laboratories (Campbell, CA). All drugs were diluted to the final concentrations from stock solutions on the day of the experiment.
Imaging
Fluorescence images of zebrafish embryos were acquired with a confocal microscope (Zeiss LSM510 META, Chester, VA) using a water immersion 25× 0.8 NA objective. For EGFP fluorescence, excitation was 488 nm and emission was band-pass-filtered between 500 and 550 nm. The mCherry fluorescence was imaged using 565 nm excitation and emission was band-pass-filtered between 576 and 704 nm. Dissociated cells were imaged on an inverted fluorescence microscope (Eclipse TE2000-U, Nikon, Melville, NY). Screening for heterozygote transgenic embryos was performed on a dissecting microscope with fluorescence attachment (MVX10, Olympus, Center Valley, PA).
Data analysis
Current traces were analyzed using Igor Pro version 6 (WaveMetrics, Portland, OR), and statistical tests were performed with GraphPad Prism 5 for Mac OS X (GraphPad Software, La Jolla, CA). All data were expressed as means ± SE. The percentage inhibition of ICa (%) was determined using the equation (Icon − Idrug)/Icon × 100, where Icon and Idrug are the ICa amplitudes before and after drug application, respectively. Statistical significance between two groups was determined using the paired or unpaired Student's t-test as appropriate. The modified Wald method (http://www.graphpad.com/quickcalcs/ConfInterval1.cfm) was used to compute confidence intervals of a proportion. Multiple comparisons were performed with a one-way ANOVA followed by Dunnett's post hoc test as indicated. P < 0.05 was considered significant.
RESULTS
Preparation of R-B neurons using Tg(Isl2b:EGFP)ZC7 zebrafish
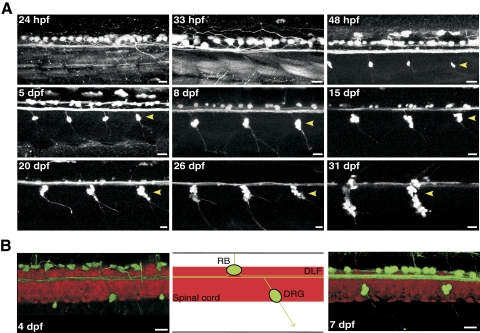
To obtain identified R-B neurons, a transgenic zebrafish line, Tg(Isl2b:EGFP)ZC7 was utilized. Tg(Isl2b:EGFP)ZC7, hereafter termed Isl2b:EGFP, is an Isl2b-promoter driven EGFP transgenic zebrafish line originally designed to label retinal ganglion cells that also facilitates identification of sensory neurons (Pittman et al. 2008). Previous studies revealed that the number of zebrafish R-B neurons peaks by 34 hpf and then gradually decreases as a result of programmed cell death (PCD), an event coincident with DRG development (Reyes et al. 2004; Svoboda et al. 2001; Williams et al. 2000). Therefore we followed the development of sensory neurons in Isl2b:EGFP embryos to determine the best stage for isolation of a pure R-B population. Figure 1A shows EGFP-labeled sensory neurons in the trunk of living Isl2b:EGFP embryos imaged using confocal microscopy over a 24–31 hpf period. Prior to 33 hpf, R-B neurons were the sole EGFP-expressing cell type in the embryo trunk. However, beginning at 48 hpf, DRG neurons (▶) became apparent distributed segmentally along the entire trunk ventral to the R-B neurons (Fig. 1). Contrary to previous studies (Patten et al. 2007; Svoboda et al. 2001; Williams et al. 2000), R-B neurons in Isl2b:EGFP embryos were observed for ≤2 wk post fertilization. During the latter part of this observation period, R-B neurons decreased in both size and number. To confirm the identity of the EGFP-labeled cells, we generated double-labeled zebrafish by crossing the Isl2b:EGFP and HuC:mCherry transgenic lines. The Tg(HuC:mCherry) line expresses the fluorescent protein mCherry driven by the HuC RNA-binding protein promoter (Park et al. 2000a,b), which serves a pan-neuronal marker (Kim et al. 1996; Park et al. 2000a). As illustrated in Fig. 1B, R-B neurons could be recognized by several morphological features such as large soma size, dorsal position in the spinal cord, axonal trajectories, and double labeling with both EGFP and mCherry. The boundaries of the spinal cord in these images (Fig. 1B) are delineated by the mCherry fluorescence. Based on these results, we utilized embryos prior to 30 hpf for preparing R-B cells to avoid contamination with DRG neurons. In addition to DRG neurons, additional neurons expressing EGFP under the control of Isl2b promoter were observed in the head of Isl2b:EGFP embryos at 24 hpf (Fig. 2A). These possibly included trigeminal, olfactory, retinal, and posterior lateral line ganglion neurons (Dambly-Chaudière et al. 2003). Therefore the cranial portion of the embryo was removed prior to dissociation to assure a pure population of R-B neurons. Figure 2B shows fields of cells obtained following enzymatic and mechanical dissociation of the entire zebrafish trunk prior to 30 hpf. Although the phase contrast image shows a wide variety of cell types all displaying a similar appearance (Fig. 2B), individual dissociated single R-B neurons could be easily identified based on EGFP fluorescence (Fig. 2C). The acutely dissociated R-B neurons were spherical with a mean diameter of 10.4 ± 1.1 μm (n = 34) and devoid of neuronal processes; an ideal geometry for voltage-clamp studies. We confirmed the adequacy of space clamp in the isolated neurons by recording voltage-gated Na+ currents from the dissociated R-B neuron. Comparison of the Na+ current biophysical properties (Supplementary Fig. S1)1 with previous published results acquired using the nucleated patch-clamp technique (Pineda et al. 2005) were in good agreement.
Fig. 1.
Time course of Rohon-Beard (R-B) neuron degeneration in Isl2b:EGFP transgenic zebrafish. A: enhanced green fluoresent protein (EGFP) expression driven by Isl2b promoter facilitates observation of R-B neurons degeneration concurrent with development of dorsal root ganglia (DRG, arrow heads). All images of the spinal cord are maximum intensity projections of stacks acquired in the lateral plane using confocal microscopy over a period from 24 h postfertilization (hpf) to 31 days postfertilization (dpf). B: merged confocal images (left and right) and schematic representation (middle) of the double-labeled transgenic line (Isl2b:EGFP/HuC:mCherry) imaged in the lateral plane. The contrast and transparency of the images were adjusted to emphasize EGFP-labeled sensory neurons. The boundaries of the spinal cord are delineated by the mCherry expression. Note the anterior and posterior axonal projections and location of EGFP-labeled sensory neurons over the dorsal longitudinal fasciculus (DLF) in the double-labeled embryo spinal cord. All scale bars represent 20 μm and all images are oriented with anterior to the left and dorsal to the top.
Voltage-gated ICa in R-B neurons
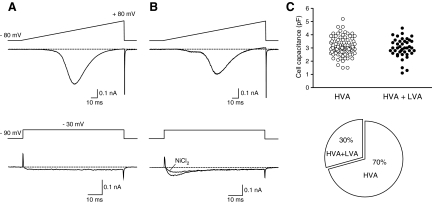
ICa from single dissociated R-B neurons were recorded using the whole cell variant of the patch-clamp technique in solutions designed to isolate ICa (see methods). Recordings from R-B neurons of voltage-activated inward ICa evoked with a 160 ms ramp command pulse from holding potential of −80 to +80 mV revealed two distinct subpopulations based on current trajectory. One group displayed only a high-threshold inward ICa component based on the ramp current-voltage (I-V) curve, which initiated near −40 mV and displayed a monotonic activation component (Fig. 3A). In contrast, the other group displayed a small but prominent hump at low-voltage potentials (−60 to −30 mV) in addition to a high-threshold current component (Fig. 3B). To further explore whether the LVA ICa component originated from T-type Ca2+ channels, ICa was evoked with a depolarizing step pulse to −30 mV from holding potential of −90 mV. As depicted in Fig. 3B (bottom traces), a TTX-insensitive (300 nM) and Ni+-sensitive (100 μM) rapidly inactivating ICa was recorded from R-B neurons with a hump in the ramp I-V; characteristics consistent with ICa arising from T-type Ca2+ channels. In contrast, only a minimally inactivating (over the 70 ms step pulse) ICa was evoked from the population lacking an overt hump at low voltages (Fig. 3A, bottom). The mean membrane capacitance (Cm) of R-B neurons with and without T-type Ca2+ channels was similar, 3.0 ± 0.1 (n = 42) and 3.1 ± 0.1 (n = 98) pF, respectively, and not significantly different (P = 0.51, unpaired t-test). R-B neurons possessing both LVA- and HVA-ICa comprised 30% (0.23–0.38, 95% CI, modified Wald method) of the total recorded R-B neuron population (Fig. 3C, right).
Fig. 3.
Distinct subpopulations of R-B neurons based on Ca2+ current components. A and B: representative traces of inward Ca2+ currents (ICa) from dissociated R-B neurons acquired using whole cell patch clamp. ICa were evoked by a 160 ms ramp from −80 to +80 mV (top traces) and test pulses to −30 mV from a holding potential of −90 mV (bottom traces, same neurons as above). R-B neuron with a “hump” in the ramp current displayed a transient Ni2+-sensitive (100 μM) low-voltage-cativated (LVA) T-type ICa component. C: scatter plot representing membrane capacitances of R-B neurons with (●) and without (○) T-type Ca2+ channels. The pie graph (bottom) displays the proportion of neurons for each subpopulation.
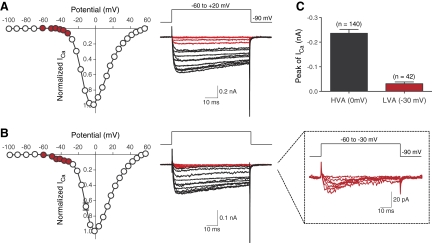
Figure 4 depicts normalized (to maximal currents) ICa I-V relationships and superimposed current traces evoked by test pulses over the range −100 to +60 mV from a holding potential of −90 mV. In neurons lacking an LVA-ICa component (Fig. 4A), ICa began to activate around −40 mV, reached a peak near 0 mV, and declined thereafter approaching a zero current asymptote near +60 mV. The ICa trajectory was minimally inactivating at low-voltage potentials (Fig. 4A, red traces) although greater inactivation (20–30% over 70 ms) was evident near voltages where the current was maximal. In neurons displaying an LVA-component, ICa first appeared between −60 and −50 mV, again peaked near 0 mV, and declined thereafter (Fig. 4B). The rapidly inactivating LVA component was apparent at more hyperpolarized potentials than HVA-ICa components (Fig. 4B, middle and right traces). The mean ICa amplitudes for the LVA (−30 mV) and HVA (0 mV) components were −0.02 ± 0.002 (n = 42) and −0.24 ± 0.02 nA (n = 102), respectively (Fig. 4C). Mean current densities, determined with 10 mM Ca2+ as the charge carrier, were 10.2 ± 2.3 and 78.7 ± 5.3 pA/pF for LVA- and HVA-current components, respectively.
Fig. 4.
Whole cell ICa from R-B neurons with and without a LVA current component. A and B: mean normalized (to maximal amplitude) Ca2+ current-voltage (I-V) relationships (left). Each symbol represents the mean ± SE. of 9 (A) or 10 (B) neurons, respectively. Current traces (right) were evoked by a series of voltages steps to potentials ranging between −100 and +60 mV from a holding potential of −90 mV. For illustrative purposes, only traces from −60 to −20 mV are shown. Red colored traces in each group represent ICa components at low-voltage potentials (approximately −60 to −30 mV) in I-V relationships (red circles). Enlarged LVA-ICa traces are shown to emphasize transient current trajectory (B, right inset). C: bar graph represents the mean amplitudes of HVA- and LVA-ICa. The number of neurons tested is shown in parentheses.
Pharmacology of ICa
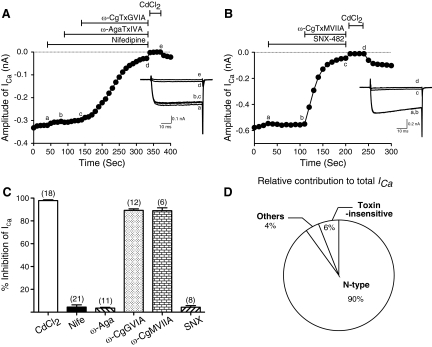
We next sought to identify the types of HVA-Ca2+ channels functionally expressed in R-B neurons by sequentially applying pharmacological antagonists and toxins that occlude specific Ca2+ channel types (Adams et al. 1993; Randall and Tsien 1995). ICa was evoked every 10 s by a test pulse to 0 mV from a holding potential of −80 mV. Figure 5, A and B, illustrates the time course of ICa amplitude reduction following sequential cumulative application of different subtype-specific Ca2+ channel blockers. Application of 10 μM nifedipine, a dihydropyridine L-type antagonist (Bean 1984), produced a slight block (5.9%) of ICa. In the continued presence of nifedipine, addition of 0.5 μM ω-agatoxin IVA, a concentration sufficient to block both P-and Q-types of ICa (Bernhardt et al. 1992; Mintz and Bean 1993), produced no additional overt reduction of ICa. Addition of ω-conotoxin GVIA to this mixture at a saturating concentration (3 μM) (Mintz et al. 1991; Olivera et al. 1994) resulted in a 90% block of total ICa, indicating that the large majority of ICa arose from N-type (Cav2.2) channels. The small residual current remaining following application of the three antagonists was abolished by application of the nonselective Ca2+ channel blocker Cd2+(100 μM). To further characterize the toxin-resistant ICa component, SNX-482 (300 nM), a selective antagonist of recombinant α1E channels, (Newcomb et al. 1998), was applied to R-B neurons but failed to produce overt block of ICa (Fig. 5B). Subsequent addition of ω-conotoxin MVIIA (3 μM), another N-type channel blocker (Olivera et al. 1994) resulted in an ∼90% of reduction of total ICa further substantiating the interpretation that ICa, under these recording conditions, was carried almost exclusively by N-type Ca2+ channels. As summarized in Fig. 5C, 98 ± 1% of total inward current was the Cd2+-sensitive HVA-ICa, and 89 ± 1 and 87 ± 2% of inward currents were blocked by ω-conotoxin GVIA or ω-conotoxin MVIIA, respectively. There was no apparent difference in the spectrum of expressed HVA-Ca2+ channel isoforms in R-B neurons displaying a LVA-ICa component (89 ± 1 vs. 92 ± 2% for N-type; 3 ± 1 vs. 2 ± 1% for L-type; 4 ± 1 vs. 3 ± 1% for P/Q-type; 6 ± 1 vs. 2 ± 3% for R-type in R-B neurons with and without LVA-ICa component, respectively). We conclude from these data that N-type Ca2+ channels underlie the vast majority of VGCCs in R-B neurons and that other Ca2+ channel isoforms (L-, P/Q-, and SNX-482 sensitive R-type) contributed <5% of the total ICa (Fig. 5D).
Fig. 5.
Pharmacological dissection of high-voltage-activated (HVA)-ICa in R-B neurons. A and B: time courses of ICa amplitude during serial application of 10 μM nifedipine, 0.5 μM ω-agatoxin IVA, 3 μM ω-conotoxin GVIA, and 100 μM CdCl2 (A) or 300 nM SNX-482, 3 μM ω-conotoxin MVIIA, and 100 μM CdCl2 (B). ICa was evoked every 10 s by 70 ms test pulses to 0 mV from a holding potential of −80 mV. The horizontal bars indicate the duration of drug application. Inset: superimposed current traces obtained at different time points during drug application (labeled as a-e or a-d). C: bar graph representing the mean ICa inhibition (%) produced by application of the indicated antagonists or toxins. The number of neurons tested is indicated in parentheses. D: summary of relative contribution of N- and other types of currents to total ICa in R-B neurons.
Endogenous G protein modulation of N-type Ca2+ channels in R-B neurons
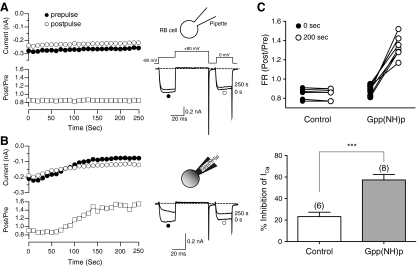
A number of neurotransmitters modulate N-type Ca2+ channels and the major modulation mechanism occurs via activation of heterotrimeric G-proteins (Herlitze et al. 1996; Hille 1994; Ikeda 1996). We thus examined the possible involvement of heterotrimeric G-proteins in modulating zebrafish N-type Ca2+ channels. ICa was evoked by a modified double-pulse voltage protocol (Elmslie et al. 1990) consisting of a test pulse to 0 mV (the “prepulse”), a strong depolarizing conditioning pulse to +80 mV, and second identical test pulse to 0 mV (the “postpulse”; Fig. 6A). From the resulting ICa, we determined the ratio of the postpulse to prepulse current amplitude measured isochronally at 10 ms after the start of the test pulse; a parameter known as the facilitation ratio (FR). The FR is an often-used metric for voltage-dependent Ca2+ channel inhibition mediated by Gβγ subunits (Herlitze et al. 1996; Ikeda 1996). When determined in the absence of GPCR agonists, the basal FR is an indicator of tonic G protein activation (Ikeda 1991). The mean basal FR of dissociated R-B neurons was 0.87 ± 0.01 (n = 66); a result indicating little tonic G protein activation. Previous studies have shown that Gpp(NH)p, a hydrolysis-resistant GTP analogue, produces tonic inhibition of the ICa when introduced into mammalian sympathetic neurons, presumably by irreversibly binding to and activating Gα during basal nucleotide exchange (Ikeda 1996). To examine whether endogenous G proteins modulate N-type Ca2+ channels in R-B neurons, 500 μM Gpp(NH)p was dialyzed into R-B neurons via the patch pipette solution. Prepulse amplitude (●), postpulse amplitude (○), and the FR (☐) were plotted as illustrated in Fig. 6, A and B, together with superimposed current traces (right). Dialysis of normal pipette solution (which contain 0.1 mM GTP) into R-B neurons produced no significant change of FR during the 250 s recording period (Fig. 6A). In contrast, inclusion of 500 μM Gpp(NH)p in the pipette solution resulted, after a short delay, in a slowly increasing FR and ICa traces with kinetic slowing during the prepulse, a hallmark of voltage-dependent modulation (Fig. 6B). A comparison of FRs determined just following patch rupture (●) and after 200 s of whole cell recording (○) are summarized in Fig. 6C. The mean FR increased from 0.89 ± 0.02 to 1.35 ± 0.04 (n = 8; P < 0.001, paired t-test) during dialysis with Gpp(NH)p compared with the control group which did not change (0.85 ± 0.02 to 0.85 ± 0.06, n = 6; P = 0.74) during an equivalent time period. Figure 6B, bottom, also shows that dialysis with Gpp(NH)p produced apparent inhibition of prepulse ICa amplitude when compared with control group (57.3 ± 5 vs. 23.2 ± 4%, respectively). These results indicate that N-type Ca2+ channels in zebrafish R-B neurons, isolated at an early stage of development, display all the properties of heterotrimeric G protein modulation observed in adult mammalian peripheral and central neurons.
Fig. 6.
Dialysis of 500 μM Gpp(NH)p into R-B neurons modulates HVA-ICa. A and B: Time courses of ICa (left) and superimposed current traces (right) evoked with the voltage protocol illustrated (inset on the traces in A) from control and Gpp(NH)p dialyzed R-B neurons, respectively. The ICa amplitude generated by the prepulse (●) and postpulse (○) are plotted. Facilitation ratio (FR) was calculated as the ratio of the postpulse to prepulse ICa amplitude (☐). Cartoons above each figure depict the experimental paradigm. C, top: summary graph of the FR just after patch rupture (0 s, ●) or following 200 s of whole cell recording (○) under the conditions indicated. Bottom: summary for the mean ICa inhibition (%) produced by control (☐) or Gpp(NH)p (■) containing pipette solution after 200 s of whole cell recording. The numbers in parentheses indicate the tested number of neurons. Error bars represent SE. *** P < 0.001 by unpaired t-test.
Coupling of GPCRs to N-type Ca2+ channels in R-B neurons
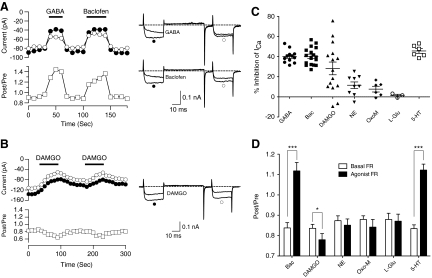
To identify GPCRs that modulate Ca2+ channels in R-B neurons, we screened agonists that activate GABAb (GABA or baclofen, 100 μM), μ-opioid (DAMGO, 1 μM), adrenergic (norepinephrine, 10 μM), muscarinic ACh (oxotremorine-M, 10 μM), metabotropic glutamate (l-glutamate, 100 μM), or serotonin (5-HT, 10 μM) receptors. Of these agonists, robust ICa modulation was observed following application of GABA/baclofen, 5-HT, or DAMGO. Figure 7A illustrates representative time courses of prepulse amplitude (●), postpulse amplitude (○), and FR (☐) prior to, during (—), and after agonist washout. Extracellular perfusion of either GABA (100 μM) or the specific GABAbR agonist baclofen (100 μM) resulted in ICa inhibition via a voltage-dependent mechanism. The onset and washout of agonist effect was rapid occurring with the 10 s interval between test pulses. The ICa modulation displayed all of the hallmarks of voltage-dependent Gβγ-mediated inhibition, namely slowing of activation during the prepulse, relief of inhibition during the postpulse, reversal of kinetic slowing in the postpulse, and increased FR (Ikeda 1991, 1996). Similar phenomenology was seen following application of 5-HT. In contrast, application of DAMGO produced a predominately voltage-independent inhibition of ICa based on the absence of overt kinetic slowing in the prepulse trace or increase in the FR during agonist-mediated inhibition (Fig. 7B). The effects of neurotransmitter application on R-B neuron ICa are summarized in Fig. 7, C and D. GABA/baclofen and 5-HT produced a mean ICa inhibition of between 40 and 45% that was relatively consistent; inhibition was >20% in all neurons tested and the coefficient of variation (CV) was 15–22%. DAMGO produced a more variable inhibition with a mean inhibition of 28% and a CV of 87%. Given the sample size (n = 15), it was unclear whether this variation arose from a multimodal distribution. NE and Oxo-M produced small and variable inhibitions, 11.5 ± 3.3 (n = 9) and 7.7 ± 2.8% (n = 6), respectively, while a small sampling of l-glutamate treated neurons failed to display an overt inhibition (1.3 ± 1.2%, n = 3).
Fig. 7.
Modulation of HVA-ICa by neurotransmitters. A and B: time courses of ICa (left) and superimposed current traces (right) evoked with the double-pulses voltage protocol during application of GABA(100 μM)/baclofen (100 μM) and DAMGO (1 μM), respectively. The horizontal bars indicate the duration of drugs application. C: scatter plots represent inhibition of prepulse ICa (%) produced by application of GABA, baclofen (Bac), DAMGO, norepinephrine (NE, 10 μM), oxotremorine M (OxoM, 10 μM), l-glutamic acid hydrochloride (l-Glu, 100 μM) and 5-HT (10 μM). Means and SE are shown. D: summary of the mean FR (Post/Pre) before (basal, ☐) or after (■) application of agonists. Data are presented as means ± SE. * P < 0.05, *** P < 0.001 by paired t-test.
Mean FRs during agonist application are shown in Fig. 7D. Activation of either GABAbR (via baclofen) or 5-HT receptors resulted in mean FRs of 1.12 ± 0.04 (n = 17) and 1.12 ± 0.03 (n = 6), respectively. The basal FR (prior to drug application) for these conditions, 0.84 ± 0.03 and 0.83 ± 0.02, respectively, were comparable to the basal FR reported in Fig. 6. Although the increases in FR during agonist application were not as large as those reported for comparable conditions in mammalian neurons (e.g., often ≥2), the results support the idea that activation of GABAbR or metabotropic 5-HTRs result in ICa modulation with a voltage-dependent component. Conversely, application of DAMGO produced a small decrease in mean FR (mean difference of −0.06, paired t-test, P = 0.014, n = 15) when controlled with predrug values. Hence, DAMGO-mediated ICa inhibition was voltage-independent, suggesting a discrete signaling mechanism when compared with the GABAbR or 5-HTR responses.
Effects of Pertussis and Cholera toxin pretreatment
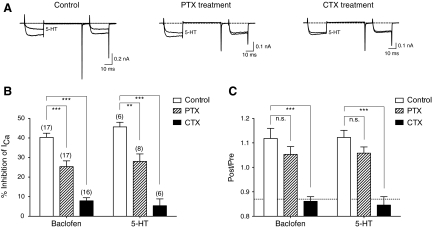
The class of heterotrimeric G protein involved in GABAbR and 5-HTR modulation of ICa was examined by preincubating dissociated R-B neurons in either PTX (500 ng/ml) or CTX (500 ng/ml) for 15–17 h prior to recording. In general, PTX pretreatment uncouples Gi/o G proteins from GPCRs by ADP-ribosylating a cysteine residue near the carboxyl-terminus of susceptible Gα subunits. The actions of CTX are more complex. Under in vivo conditions, CTX ADP-ribosylates an arginine residue in Gs and Golf (GNAL) containing G proteins. Initially this produces an activation of Gα-subunit leading to stimulation of adenylate cyclase and elevations in cAMP levels. However, the modified Gα subunit is rapidly degraded leading to functional uncoupling from GPCR (Zhu and Ikeda 1994). We examined the GABAbR and 5-HTR responses because the consistent response (Fig. 7C) facilitated comparisons of toxin-treated and control neuron populations.
Examples of ICa traces in the presence or absence of 5-HT for control, PTX-treated, and CTX-treated R-B neuron are shown in Fig. 8A. PTX-treatment appeared to attenuate inhibition although a clear voltage-dependent component remained. However, CTX-treatment abolished the majority of the inhibition. Pretreatment with either toxin did not alter the mean ICa amplitude when compared with untreated neurons (1-way ANOVA, Dunnett's test, P > 0.05). Similar effects were seen with the baclofen-mediated response (traces not shown).
Fig. 8.
Effects of Pertussis and Cholera toxin pretreatment on ICa modulation in R-B neurons. A: superimposed ICa traces evoked with the double-pulses voltage protocol from control (left), Bordetella pertussis holotoxin (PTX, 500 ng/ml, middle), and Vibrio cholerae holotoxin (CTX, 500 ng/ml, right) preincubating R-B neurons. B and C: bar graphs summarizing the effects of control, PTX and CTX pretreatment on the mean ICa inhibition and facilitation ratio (FR) produced by baclofen or serotonin (5-HT) in R-B neurons, respectively. - - - in bar graph of C indicates the mean FR determined in absence of agonists. Data are presented as means ± SE. The number of neurons tested is indicated in parentheses on the graph. * P < 0.05, *** P < 0.001 1-way ANOVA followed by Dunnett's post hoc tests with control.
A summary of the toxin pretreatment experiments are shown in Fig. 8, B and C. In PTX preincubated neurons, ICa inhibition by baclofen was reduced from 40.3 ± 2.1 (n = 17) to 25.4 ± 3.0% (n = 17). Treatment with CTX, however, reduced ICa inhibition further to 7.9 ± 1.6% (n = 16). A very similar profile was seen with 5-HT-mediated inhibition which was reduced from 45.7 ± 2.3 (n = 6) to 28.0 ± 3.8 (n = 8) and 5.4 ± 3.5 (n = 6)%, respectively, for PTX- and CTX-treated neurons. PTX treatment did not affect the agonist-induced increase in FR for either baclofen or 5-HT (Fig. 8C). However, CTX treatment reduced the FR to nearly basal levels for both agonists consistent with the near ablation of modulation. These results suggest that both metabotropic 5-HT and GABAb receptors inhibit N-type Ca2+ channels of zebrafish R-B neurons via a process dominated by CTX-sensitive Gα-subunits.
DISCUSSION
Zebrafish R-B neurons as model system for investigating voltage-gated Ca2+ channels
At present, the transient nature of morpholino antisense knockdown of gene translation and heterologous expression of proteins from cRNA microinjection limits the usefulness of these techniques to the first few days following fertilization. Accordingly, we chose R-B neurons as a platform to investigate VGCC properties as these neurons appear rapidly following fertilization and form functional circuits within hours (Rossi et al. 2009; Saint-Amant 2006). Unlike in R-B neurons of Xenopus larvae and lamprey (el Manira and Bussières 1997; el Manira et al. 1996), voltage-clamp studies of zebrafish R-B neurons are few (Ribera and Nüsslein-Volhard 1998) due to the difficulty in isolating identified neurons for electrophysiology. Here we demonstrate the suitability of using enzymatically dissociated single R-B neurons from zebrafish expressing Isl2b-promoter driven EGFP as marker for primary sensory neurons (Pittman et al. 2008; Tokumoto et al. 1995). Dissociated single R-B neurons were amenable to conventional whole cell patch-clamp studies, and the spherical geometry and lack of neurites facilitated spatial voltage control. Although R-B neurons are smaller than rodent peripheral neurons (e.g., 10 vs. 20–30 μm), the HVA-ICa density (78 pA/pF) was approximately two– to threefold greater than what we measure in adult rat sympathetic neurons, thus providing a favorable signal-to-noise ratio.
Ca2+ channel currents in zebrafish R-B neurons
Based on the presence or absence of LVA-ICa component, zebrafish R-B neurons were divided into two subpopulations. Thirty percent of the R-B neurons recorded from displayed an LVA-ICa component. However, no overt characteristic examined differentiated the two R-B neuron populations. Previous studies have reported that ∼40% of R-B neurons at 24 hpf express PKC isoforms (Patten et al. 2007; Slatter et al. 2005) and thus the presence of LVA-ICa and expression of PKC isoforms may be correlated although this relationship was not examined. The predominant ICa component was HVA and present in all R-B neurons tested. Pharmacological dissection of the HVA-ICa revealed that ω-conotoxin GVIA-sensitive N-type Ca2+ channels accounted for 90% of the current amplitude. A residual ICa component (ca. 6%) resistant to the subtype-specific Ca2+ channel antagonists but blocked by Cd2+ was observed. The toxins used in these experiments, especially ω-conotoxin GVIA, have the advantage of identifying functional channels as well as specific subtypes of VGCCs (Adams et al. 1993; Feng et al. 2001; Kerr and Yoshikami 1984; Randall and Tsien 1995). A tacit assumption underlying our interpretations is that pharmacological specificity in zebrafish was comparable to that documented for mammalian/avian preparations. The lack of heterogeneity in HVA-Ca2+ channel subtype composition within and between cells is an advantage for studying GPCR-mediated modulation, and N-type channels are more highly modulated than other CaV2.x subtypes. Mammalian DRG neurons are known to express a wide variety of different HVA-Ca2+ channel subtypes which varies greatly from cell to cell (Scroggs and Fox 1992a,b). Among R-B neurons, Xenopus larvae have an HVA- ICa composition of N (70%)-, P/Q (25.5%)-, and L-type (<5.5%) channels (Sun and Dale 1997) while mechanosensory dorsal cells in lamprey HVA-ICa is comprised of N (70%)-, P/Q (6%)-, and L-type (12%) Ca2+ channels (el Manira and Bussières 1997). Conversely, acutely dissociated embryonic (11–12 days) avian DRG, like zebrafish R-B neurons, exhibit HVA-ICa composed of nearly pure population of N-type channels (Cox and Dunlap 1992).
The precise composition of R-B neuron N-type Ca2+ channel is unclear at this point. Each of the major subunits families, i.e., α1, β, α2-δ, has undergone gene duplication in Danio rerio leading to a somewhat complex picture. In terms of α1 subunits (CACNA1), 16 genes are listed in the Ensembl database with multiple members from CaV1.x, 2.x, and 3.x represented. Two genes corresponding to CaV2.2 (CACNA1B) α1 subunits are annotated; however, it is unclear whether both are functional or correctly annotated at this point. It seems likely the ω-conotoxin GVIA-sensitive ICa component arises from one or both of these genes. Likewise, the zebrafish Ca2+ channel α2-δ family (CACNA2) is complex with eight members likely representing duplication of each of the four α2-δ genes found in mammals (Davies et al. 2007). The zebrafish Ca2+ channel β-subunit (CACNB) family (β1–4) has also been duplicated with the exception of β1 (CACNB1)—hence there are seven members. Expression patterns of Ca2+ channel β-subunit in zebrafish embryos determined using in situ hybridization (Zhou et al. 2008) demonstrate that orthologs of mammalian CACNB3, cacnb3a, and cacnb3b, are expressed in R-B neurons. Based on these data, cacnb3a/b likely contribute to the channel phenotype observed in our study.
G protein modulation of Ca2+ channels in R-B neurons
Modulation of VGCCs, especially N- and P/Q-type Ca2+ channels, by GPCRs has been intensively studied for many years (Dolphin 2003; Elmslie 2003; Hille 1994; Ikeda 1991,1996; Ikeda and Dunlap 1999; Jeong and Ikeda 2000; Tedford and Zamponi 2006; Zamponi and Snutch 1998; Zhu and Ikeda 1994). Activation of heterotrimeric G proteins produces two classes of ICa inhibition, voltage dependent and independent, which are not mutually exclusive. All of the signature properties of voltage-dependent pathway, usually attributed to Gβγ were observed with both direct G protein activation and GPCR-mediated (GABAbR and metabotropic 5-HTR) inhibition in R-B neurons. In contrast, DAMGO, presumably acting via μ-opioid receptors, produced a pure voltage-independent block of ICa as indicated by the absence of these characteristics during agonist application. These interpretations are predicated on the assumption of similarity between zebrafish and mammalian/avian N-type Ca2+ channels with respect to the biophysical characteristics of modulation.
A number of inhibitory neurotransmitters modulate glutamate release from primary sensory neurons onto interneurons in spinal circuit models of lampreys and tadpoles (Grillner 2003; Hale et al. 2001; Sillar and Simmers 1994; Sun and Dale 1997). An analogous circuit might exist in the zebrafish embryo with dorsal spinal 5-HT innervations (Grillner 2003; Hale et al. 2001; Sillar et al. 2002) and four types of GABAergic spinal interneurons; KA, DoLA, CoSA, and VeLD (Bernhardt et al. 1992; Higashijima et al. 2004). Therefore our results predict that GABAb or 5-HT receptors may participate in presynaptic modulation of sensory transmission in zebrafish R-B neurons by inhibiting Ca2+ channel involved in synaptic release.
Further examination of the GABAbR and 5-HTR signaling pathway revealed some interesting properties. Both responses were partially blocked by pretreatment with PTX but nearly completely blocked by CTX pretreatment. As 5-HT is a nonselective agonist and there are numerous subtypes of metabotropic 5-HT that coupled with different G-proteins families, a complex signaling pathway might be anticipated. However, the GABAbR response was unexpected as analogous modulation in mammalian neurons is predominately mediated by PTX-sensitive G proteins (Menon-Johansson et al. 1993; Scholz and Miller 1991). The nearly complete block of the modulation by CTX pretreatment has several possible explanations. First, GABAb receptors in zebrafish may, unlike their mammalian counterparts, primarily couple to CTX-sensitive Gs family G proteins including Gs and Golf. Previous studies of VIP-type receptors in mammalian neurons show voltage-dependent N-type ICa inhibition mediated by a CTX-sensitive pathway (Zhu and Ikeda 1994). Second, Gα subunits in zebrafish may not respond to bacterial toxins identically to their mammalian orthologs. Although PTX requires a cysteine residue near the C-terminus to be effective, the arginine residue modified by CTX is found in nearly all Gα subunits. What controls the specificity of CTX in terms of ADP-ribosylating particular Gα subunits is unclear and somewhat dependent on experimental conditions (e.g., in vivo vs. in vitro). Finally, the diversity of Gα subunits in zebrafish is actually greater than that of mammals. Recently a fifth class of Gα protein, termed Gv, was identified in zebrafish (Oka et al. 2009). Although related to the Gi family, this class of G proteins appears to be under stringent negative selection and is found in only a few organisms. The Gv proteins lack the cysteine required to be a PTX substrate but possess an arginine that might be a substrate for CTX. Thus a novel Gα containing G protein may couple with GABAbR in zebrafish to produce voltage-dependent modulation (which we assume occurs via the Gβγ subunit); a scenario that could provide insight in to the specificity of GPCR coupling.
Conclusions and summary
In terms of investigating modulation of Ca2+ channels, what are the advantages using zebrafish R-B neurons? First, single identified R-B neurons can be isolated from transgenic fish lines, here Isl2b:EGFP, and are suitable for patch-clamp recording of Ca2+ and other voltage-gated channels. Second, the R-B neurons appear and are functional (i.e., participate in a macroscopic phenotype) during the brief period when transient gene manipulation is convenient and feasible in this model organism. Hence linking Ca2+ channel modulation to integrative function might be possible. Third, R-B neurons are present during an early stage of development when zebrafish embryos are transparent and easily imaged. Because we have demonstrated intact G protein coupling at this early stage, R-B neurons might represent a platform for the testing genetically based optical sensors for G protein activation in a living organism. Fourth, the neurons are relatively homogenous, when compared with mammalian DRG neurons, in terms of both HVA Ca2+ channel composition, consisting primarily of N-type channels, and some GPCR responses. Finally, the genetic divergence in orthologous genes provides insight into conserved domains underlying the numerous similarities between mammalian and zebrafish Ca2+ channel modulation. On the other hand, there are challenges to using zebrafish R-B neurons not present in conventional mammalian preparations. First, molecular complexity in terms of signaling component numbers is, paradoxically, greater in the zebrafish system. This arises not only from gene duplication but also, unexpectedly, from divergent evolutionary paths. For example, as mentioned earlier, zebrafish have an additional Gα subunit family not found in the majority of vertebrate species. Second, attempts to ablate specific components during early development can have unexpected, albeit interesting, consequences. For example, knockdown of zebrafish CACNB4 (Ca2+ channel β4 subunit) blocks initiation of epiboly in a manner independent of Ca2+ channel function resulting in an embryonic lethal phenotype (Ebert et al. 2008). Therefore novel developmental properties for proteins with seemingly well-defined functions (in the adult) may be uncovered at the expense of diversion from the original intent of the experiment.
In summary, we have used the zebrafish Isl2b:EGFP transgenic line to isolate single identified R-B neurons and obtain whole cell voltage-clamp recordings of VGCC. R-B neurons contain both LVA- and HVA-ICa components, the latter comprised almost entirely of ω-conotoxin GVIA-sensitive N-type channels. The N-type channels are modulated by endogenous GPCRs in both a voltage-dependent and -independent manner. Unlike in mammalian systems, GPCR-mediated voltage-dependent modulation of ICa appears to be transduced via a CTX-sensitive Gα subunit. These results provide the basis for using the zebrafish model system to understanding Ca2+ channel function and in turn, how Ca2+ channels contribute to zebrafish mechanosensory function.
GRANTS
This research was supported by the Intramural Research Program of the National Institutes of Health—National Institute on Alcohol Abuse and Alcoholism.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Chi-Bin Chien (University of Utah Medical Center) for generously providing the Tg(Isl2b:EGFP)ZC7 fish line and are grateful to Dr. Takanori Ikenaga (University of Hyogo) for many helpful discussions.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Adams ME, Mintz IM, Reily MD, Thanabal V, Bean BP. Structure and properties of omega-agatoxin IVB, a new antagonist of P-type calcium channels. Mol Pharmacol 44: 681–688, 1993 [PubMed] [Google Scholar]
- Bean BP. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci USA 81: 6388–6392, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt RR, Patel CK, Wilson SW, Kuwada JY. Axonal trajectories and distribution of GABAergic spinal neurons in wildtype and mutant zebrafish lacking floor plate cells. J Comp Neurol 326: 263–272, 1992 [DOI] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 57: 411–425, 2005 [DOI] [PubMed] [Google Scholar]
- Clarke JD, Hayes BP, Hunt SP, Roberts A. Sensory physiology, anatomy and immunohistochemistry of Rohon-Beard neurones in embryos of Xenopus laevis. J Physiol 348: 511–525, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DH, Dunlap K. Pharmacological discrimination of N-type from L-type calcium current and its selective modulation by transmitters. J Neurosci 12: 906–914, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambly-Chaudière C, Sapède D, Soubiran F, Decorde K, Gompel N, Ghysen A. The lateral line of zebrafish: a model system for the analysis of morphogenesis and neural development in vertebrates. Biol Cell 95: 579–587, 2003 [DOI] [PubMed] [Google Scholar]
- Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol Sci 28: 220–228, 2007 [DOI] [PubMed] [Google Scholar]
- Dolphin AC. G protein modulation of voltage-gated calcium channels. Pharmacol Rev 55: 607–627, 2003 [DOI] [PubMed] [Google Scholar]
- Ebert AM, McAnelly CA, Srinivasan A, Linker JL, Horne WA, Garrity DM. Ca2+ channel-independent requirement for MAGUK family CACNB4 genes in initiation of zebrafish epiboly. Proc Natl Acad Sci USA 105: 198–203, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Manira A, Bussières N. Calcium channel subtypes in lamprey sensory and motor neurons. J Neurophysiol 78: 1334–1340, 1997 [DOI] [PubMed] [Google Scholar]
- el Manira A, Shupliakov O, Fagerstedt P, Grillner S. Monosynaptic input from cutaneous sensory afferents to fin motoneurons in lamprey. J Comp Neurol 369: 533–542, 1996 [DOI] [PubMed] [Google Scholar]
- Elmslie KS. Neurotransmitter modulation of neuronal calcium channels. J Bioenerg Biomembr 35: 477–489, 2003 [DOI] [PubMed] [Google Scholar]
- Elmslie KS, Zhou W, Jones SW. LHRH and GTP-gamma-S modify calcium current activation in bullfrog sympathetic neurons. Neuron 5: 75–80, 1990 [DOI] [PubMed] [Google Scholar]
- Feng ZP, Hamid J, Doering C, Bosey GM, Snutch TP, Zamponi GW. Residue Gly1326 of the N-type calcium channel alpha 1B subunit controls reversibility of omega-conotoxin GVIA and MVIIA block. J Biol Chem 276: 15728–15735, 2001 [DOI] [PubMed] [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci 4: 573–586, 2003 [DOI] [PubMed] [Google Scholar]
- Hale ME, Ritter DA, Fetcho JR. A confocal study of spinal interneurons in living larval zebrafish. J Comp Neurol 437: 1–16, 2001 [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pfluegers 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature 380: 258–262, 1996 [DOI] [PubMed] [Google Scholar]
- Higashijima S, Mandel G, Fetcho JR. Distribution of prospective glutamatergic, glycinergic, and GABAergic neurons in embryonic and larval zebrafish. J Comp Neurol 480: 1–18, 2004 [DOI] [PubMed] [Google Scholar]
- Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci 17: 531–536, 1994 [DOI] [PubMed] [Google Scholar]
- Ikeda SR. Double-pulse calcium channel current facilitation in adult rat sympathetic neurones. J Physiol 439: 181–214, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature 380: 255–258, 1996 [DOI] [PubMed] [Google Scholar]
- Ikeda SR, Dunlap K. Voltage-dependent modulation of N-type calcium channels: role of G protein subunits. Adv Second Messenger Phosphoprotein Res 33: 131–151, 1999 [DOI] [PubMed] [Google Scholar]
- Jeong SW, Ikeda SR. Effect of G protein heterotrimer composition on coupling of neurotransmitter receptors to N-type Ca(2+) channel modulation in sympathetic neurons. Proc Natl Acad Sci USA 97: 907–912, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr LM, Yoshikami D. A venom peptide with a novel presynaptic blocking action. Nature 308: 282–284, 1984 [DOI] [PubMed] [Google Scholar]
- Kim CH, Ueshima E, Muraoka O, Tanaka H, Yeo SY, Huh TL, Miki N. Zebrafish elav/HuC homologue as a very early neuronal marker. Neurosci Lett 216: 109–112, 1996 [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn 203: 253–310, 1995 [DOI] [PubMed] [Google Scholar]
- Lamborghini JE. Disappearance of Rohon-Beard neurons from the spinal cord of larval Xenopus laevis. J Comp Neurol 264: 47–55, 1987 [DOI] [PubMed] [Google Scholar]
- Menon-Johansson AS, Berrow N, Dolphin AC. G(o) transduces GABAB-receptor modulation of N-type calcium channels in cultured dorsal root ganglion neurons. Pfluegers 425: 335–343, 1993 [DOI] [PubMed] [Google Scholar]
- Mintz IM, Bean BP. Block of calcium channels in rat neurons by synthetic omega-Aga-IVA. Neuropharmacology 32: 1161–1169, 1993 [DOI] [PubMed] [Google Scholar]
- Mintz IM, Venema VJ, Adams ME, Bean BP. Inhibition of N- and L-type Ca2+ channels by the spider venom toxin omega-Aga-IIIA. Proc Natl Acad Sci USA 88: 6628–6631, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb R, Szoke B, Palma A, Wang G, Chen Xh, Hopkins W, Cong R, Miller J, Urge L, Tarczy-Hornoch K, Loo JA, Dooley DJ, Nadasdi L, Tsien RW, Lemos J, Miljanich G. Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry 37: 15353–15362, 1998 [DOI] [PubMed] [Google Scholar]
- Oka Y, Saraiva LR, Kwan YY, Korsching SI. The fifth class of Galpha proteins. Proc Natl Acad Sci USA 106: 1484–1489, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera BM, Miljanich GP, Ramachandran J, Adams ME. Calcium channel diversity and neurotransmitter release: the omega-conotoxins and omega-agatoxins. Annu Rev Biochem 63: 823–867, 1994 [DOI] [PubMed] [Google Scholar]
- Park HC, Hong SK, Kim HS, Kim SH, Yoon EJ, Kim CH, Miki N, Huh TL. Structural comparison of zebrafish Elav/Hu and their differential expressions during neurogenesis. Neurosci Lett 279: 81–84, 2000a [DOI] [PubMed] [Google Scholar]
- Park HC, Kim CH, Bae YK, Yeo SY, Kim SH, Hong SK, Shin J, Yoo KW, Hibi M, Hirano T, Miki N, Chitnis AB, Huh TL. Analysis of upstream elements in the HuC promoter leads to the establishment of transgenic zebrafish with fluorescent neurons. Dev Biol 227: 279–293, 2000b [DOI] [PubMed] [Google Scholar]
- Patten SA, Sihra RK, Dhami KS, Coutts CA, Ali DW. Differential expression of PKC isoforms in developing zebrafish. Int J Dev Neurosci 25: 155–164, 2007 [DOI] [PubMed] [Google Scholar]
- Pineda RH, Heiser RA, Ribera AB. Developmental, molecular, and genetic dissection of INa in vivo in embryonic zebrafish sensory neurons. J Neurophysiol 93: 3582–3593, 2005 [DOI] [PubMed] [Google Scholar]
- Pittman AJ, Law M-Y, Chien C-B. Pathfinding in a large vertebrate axon tract: isotypic interactions guide retinotectal axons at multiple choice points. Development 135: 2865–2871, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall A, Tsien RW. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci 15: 2995–3012, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes R, Haendel M, Grant D, Melancon E, Eisen JS. Slow degeneration of zebrafish Rohon-Beard neurons during programmed cell death. Dev Dyn 229: 30–41, 2004 [DOI] [PubMed] [Google Scholar]
- Ribera AB, Nüsslein-Volhard C. Zebrafish touch-insensitive mutants reveal an essential role for the developmental regulation of sodium current. J Neurosci 18: 9181–9191, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. Early functional organization of spinal neurons in developing lower vertebrates. Brain Res Bull 53: 585–593, 2000 [DOI] [PubMed] [Google Scholar]
- Rossi CC, Kaji T, Artinger KB. Transcriptional control of Rohon-Beard sensory neuron development at the neural plate border. Dev Dyn 238: 931–943, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Amant L. Development of motor networks in zebrafish embryos. Zebrafish 3: 173–190, 2006 [DOI] [PubMed] [Google Scholar]
- Scholz KP, Miller RJ. GABAB receptor-mediated inhibition of Ca2+ currents and synaptic transmission in cultured rat hippocampal neurons. J Physio 444: 669–686, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scroggs RS, Fox AP. Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons of different size. J Physiol 445: 639–658, 1992a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scroggs RS, Fox AP. Multiple Ca2+ currents elicited by action potential waveforms in acutely isolated adult rat dorsal root ganglion neurons. J Neurosci 12: 1789–1801, 1992b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillar KT, McLean DL, Fischer H, Merrywest SD. Fast inhibitory synapses: targets for neuromodulation and development of vertebrate motor behavior. Brain Res Brain Res Rev 40: 130–140, 2002 [DOI] [PubMed] [Google Scholar]
- Sillar KT, Simmers AJ. Presynaptic inhibition of primary afferent transmitter release by 5-hydroxytryptamine at a mechanosensory synapse in the vertebrate spinal cord. J Neurosci 14: 2636–2647, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatter CA, Kanji H, Coutts CA, Ali DW. Expression of PKC in the developing zebrafish, Danio rerio. J Neurobiol 62: 425–438, 2005 [DOI] [PubMed] [Google Scholar]
- Soffe SR. Centrally generated rhythmic and non-rhythmic behavioral responses in Rana temporaria embryos. J Exp Biol 156: 81–99, 1991 [DOI] [PubMed] [Google Scholar]
- Sun QQ, Dale N. Serotonergic inhibition of the T-type and high voltage-activated Ca2+ currents in the primary sensory neurons of Xenopus larvae. J Neurosci 17: 6839–6849, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda KR, Linares AE, Ribera AB. Activity regulates programmed cell death of zebrafish Rohon-Beard neurons. Development 128: 3511–3520, 2001 [DOI] [PubMed] [Google Scholar]
- Tedford HW, Zamponi GW. Direct G protein modulation of Cav2 calcium channels. Pharmacol Rev 58: 837–862, 2006 [DOI] [PubMed] [Google Scholar]
- Tokumoto M, Gong Z, Tsubokawa T, Hew CL, Uyemura K, Hotta Y, Okamoto H. Molecular heterogeneity among primary motoneurons and within myotomes revealed by the differential mRNA expression of novel islet-1 homologs in embryonic zebrafish. Dev Biol 171: 578–589, 1995 [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) (3rd ed.). Eugene, OR: University of Oregon Press, 1995 [Google Scholar]
- Williams JA, Barrios A, Gatchalian C, Rubin L, Wilson SW, Holder N. Programmed cell death in zebrafish rohon beard neurons is influenced by TrkC1/NT-3 signaling. Dev Biol 226: 220–230, 2000 [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Snutch TP. Modulation of voltage-dependent calcium channels by G proteins. Curr Opin Neurobiol 8: 351–356, 1998 [DOI] [PubMed] [Google Scholar]
- Zhou W, Horstick EJ, Hirata H, Kuwada JY. Identification and expression of voltage-gated calcium channel beta subunits in Zebrafish. Dev Dyn 237: 3842–3852, 2008 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Ikeda SR. VIP inhibits N-type Ca2+ channels of sympathetic neurons via a pertussis toxin-insensitive but cholera toxin-sensitive pathway. Neuron 13: 657–669, 1994 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.