Abstract
The brain stem provides most of the noradrenaline (NA) present in the spinal cord, which functions to both increase spinal motoneuron excitability and inhibit sensory afferent transmission to motoneurons (excitatory postsynaptic potentials; EPSPs). NA increases motoneuron excitability by facilitating calcium-mediated persistent inward currents (Ca PICs) that are crucial for sustained motoneuron firing. Spinal cord transection eliminates most NA and accordingly causes an immediate loss of PICs and emergence of exaggerated EPSPs. However, with time PICs recover, and thus the exaggerated EPSPs can then readily trigger these PICs, which in turn produce muscle spasms. Here we examined the contribution of adrenergic receptors to spasms in chronic spinal rats. Selective activation of the α1A adrenergic receptor with the agonists methoxamine or A61603 facilitated Ca PIC and spasm activity, recorded both in vivo and in vitro. In contrast, the α2 receptor agonists clonidine and UK14303 did not facilitate Ca PICs, but did decrease the EPSPs that trigger spasms. Moreover, in the absence of agonists, spasms recorded in vivo were inhibited by the α1 receptor antagonists WB4010, prazosin, and REC15/2739, and increased by the α2 receptor antagonist RX821001, suggesting that both adrenergic receptors were endogenously active. In contrast, spasm activity recorded in the isolated in vitro cord was inhibited only by the α1 antagonists that block constitutive receptor activity (activity in the absence of NA; inverse agonists, WB4010 and prazosin) and not by the neutral antagonist REC15/2739, which only blocks conventional NA-mediated receptor activity. RX821001 had no effect in vitro even though it is an α2 receptor inverse agonist. Our results suggest that after chronic spinal cord injury Ca PICs and spasms are facilitated, in part, by constitutive activity in α1 adrenergic receptors. Additionally, peripherally derived NA (or similar ligand) activates both α1 and α2 adrenergic receptors, controlling PICs and EPSPs, respectively.
INTRODUCTION
In the months following a spinal cord injury (SCI), individuals often develop a debilitating spastic syndrome, consisting of increased muscle tone, clonus, exaggerated reflexes, and associated widespread muscle spasms (Ashby 1987; Bennett et al. 2004; Gorassini et al. 2004; Kuhn and Macht 1949; Maynard et al. 1990; Young 1994). These involuntary muscle spasms can be triggered by very brief innocuous stimulation, including cutaneous stimulation, and can last for many seconds, disrupting residual motor function and compromising rehabilitation efforts. As shown in a rat model of SCI (Bennett et al. 2004; Li et al. 2004a,b) and verified in humans with SCI (Gorassini et al. 2004; Norton et al. 2008), spasms result in large part from two factors: a permanently heightened motoneuron excitability, which paradoxically develops in the months following spinal cord transection (Bennett et al. 2004; Button et al. 2008; Hultborn et al. 2004; Li et al. 2004a), and a lack of inhibitory control over sensory afferent transmission, leading to exaggerated synaptic inputs to motoneurons (Li et al. 2004a; Norton et al. 2008). The goal of this paper was to understand the role of adrenergic receptors in these two processes.
Normally the control of both motoneuron excitability and sensory transmission depends on descending monoaminergic drive, including noradrenaline (NA) and serotonin (5-HT), originating primarily from the brain stem and providing the spinal cord with a state-dependent control of excitability (Fung et al. 1991; Hochman et al. 2003; Jacobs et al. 2002; Jankowska et al. 1993; Jordan et al. 2008; Lundberg 1982; Millan 2002; Perrier and Delgado-Lezama 2005; Rekling et al. 2000; Schmidt and Jordan 2000). NA and other monoamines increase motoneuron excitability (Adachi et al. 2005; Elliott and Wallis 1992; Funk et al. 1994; Li et al. 2004b; Rekling et al. 2000) by facilitating persistent inward currents (PICs), consisting of low-voltage activated calcium (Ca PIC) and sodium (Na PIC) currents (Harvey et al. 2006b,c; Lee and Heckman 1999). These monoamine-dependent PICs are essential for motoneuron function, amplifying synaptic inputs to motoneurons and providing the basic capacity for sustained depolarization and firing (Harvey et al. 2006b; Hounsgaard et al. 1988; Lee and Heckman 2000). Importantly, adrenergic facilitation of PICs occurs in animals like cats and rats (Harvey et al. 2006b; Lee and Heckman 1999), but also in humans (Udina et al. 2010). The elimination of necessary brain-stem-derived monoamines following SCI leads immediately to a dramatic loss of PICs and motoneuron excitability with some motoneurons incapable of even basic repetitive firing (Harvey et al. 2006c; Hounsgaard et al. 1988).
Paradoxically, in the weeks following the spinal transection, there is a spontaneous recovery of large PICs in motoneurons across species, including rats, cats, and humans (Button et al. 2008; Gorassini et al. 2004; Hultborn et al. 2004; Li and Bennett 2003; Li et al. 2004a), despite the continued lack of brain-stem-derived monoaminergic input to the spinal cord. However, unlike before injury, these PICs are permanently elevated, without brain stem control, leaving motoneurons in a permanently excitable state. Furthermore, as we discuss later, excitatory sensory-evoked synaptic inputs to motoneurons are augmented after injury, and thus the low-threshold PICs are readily activated by sensory stimulation (Li et al. 2004a). Finally, the powerfully depolarizing actions of these PICs, especially Ca PICs, are difficult to terminate voluntarily because after SCI the motoneurons have weaker inhibitory inputs (e.g., postsynaptic glycine currents) (Boulenguez et al. 2010; Crone et al. 2003; Li et al. 2004a; Norton et al. 2008), especially from interneurons that are normally regulated by descending systems (Baldissera et al. 1981; Hammar and Jankowska 2003; Jankowska et al. 2000; Lundberg 1982; Shefchyk and Jordan 1985). The ultimate outcome is unchecked motoneuron firing and associated muscle spasms, produced by Ca PICs, which are readily triggered by normally innocuous stimulation and are not easily terminated (lasting many seconds, to minutes) (Bennett et al. 2001, 2004).
The spontaneous recovery of motoneuron excitability and the re-emergence of PICs, despite the absence of essential brain stem monoaminergic input, has been somewhat difficult to reconcile. However, by using receptor antagonists to inhibit the PICs, Harvey et al. (2006b) recently demonstrated that this recovery involves the spontaneous activation of both 5-HT2 and α1 adrenergic receptors, though the origin of this spontaneous activity was undetermined. Subsequently, Murray et al. (2010) demonstrated that spontaneous activity of the 5-HT2 receptors occurs in the absence of any residual 5-HT, due to constitutive receptor activity (defined as receptor activity in the absence of any neurotransmitter). Similar constitutive activity may account for spontaneous adrenergic receptor activity in SCI, because adrenergic receptors are known to exhibit constitutive activity in reduced single cells systems (Rossier et al. 1999; Seifert and Wenzel-Seifert 2002). Additionally, it is likely that residual NA in the spinal cord may also contribute to adrenergic receptor activity because increasing endogenous NA release via amphetamine increases reflexes, spasms, and Ca PICs after complete spinal cord transection (Nozaki et al. 1980; Rank et al. 2007). We tested these ideas in the present paper, employing both a novel antagonist that blocks only conventional NA-activated receptor activity (neutral antagonist, REC15/2739) (Rossier et al. 1999) and antagonists that block constitutive receptor activity (inverse agonists, WB4101 and prazosin) (Rossier et al. 1999; Seifert and Wenzel-Seifert 2002).
While Harvey et al. (2006b) suggest that α1 adrenergic receptors contribute to spasms after SCI, the specific receptor subtype is unknown (α1A, α1B,α1D). Furthermore, even in the normal motoneurons it remains uncertain which adrenergic receptors modulate motoneuron PICs because previous studies of adrenergic modulation of PICs (Lee and Heckman 1999) employed nonselective agonists (e.g., methoxamine) that likely activated both α1 and α2 adrenergic receptors (U'Prichard et al. 1977). Thus, prior to examining the origin of the spontaneous adrenergic receptor activity, we first identified the specific receptors that modulate PICs, excitatory postsynaptic potentials (EPSPs) and spasms, using selective activation of receptor subtypes with agonists.
In addition to the facilitatory actions of descending NA on spinal motoneurons, NA also inhibits sensory afferent transmission to motoneurons and ascending sensory systems (Jankowska et al. 1993; Lundberg 1982; Millan 2002; Yoshimura and Furue 2006). For example, NA inhibits afferent transmission from low threshold group I and II muscle and cutaneous afferents, thereby inhibiting polysynaptic flexor reflexes (Clarke et al. 2002; Li et al. 2004b; Lundberg 1982). Thus with SCI there is an immediate loss of this inhibition (disinhibition), leading to the emergence of unusually long polysynaptic EPSPs evoked by stimulation of group I and II afferents in both rats (Baker and Chandler 1987; Li et al. 2004a) and humans (Norton et al. 2008). Because Ca PICs require depolarizations of about half a second to be fully activated (Li and Bennett 2007; Li et al. 2004a), these long EPSPs are critical for activating the Ca PICs, which in turn produce sustained motoneuron firing and uncontrolled muscle contractions.
It remains uncertain though which subclass of adrenergic receptors regulate these unusually long EPSPs responsible for triggering spasms or whether these receptors are constitutively active. The α2 adrenergic receptor is an ideal candidate for the regulation of the long EPSPs because α2 receptors are known to play a role in the control of afferent transmission (Clarke et al. 2002; Jankowska and Hammar 2002; Rekling et al. 2000). Furthermore, after SCI, inhibition over sensory transmission and reflexes can be restored by application of α2 receptor agonists (Chau et al. 1998; Clarke et al. 2002; Jankowska and Hammar 2002; Sakitama 1993; Tremblay and Bedard 1995). Thus another goal of this paper was to determine whether the α2 receptor inhibits the long EPSP that mediates spasms and whether loss of ligand-activated receptor activity after SCI is partly compensated for by spontaneous activity in α2 adrenergic receptors.
METHODS
Adult female Sprague-Dawley rats with chronic SCI resulting in fully developed spasticity, and normal, previously unlesioned adult rats were utilized in this study. Rats received a complete sacral spinal (S2) transection at 45–55 days old as previously detailed (Bennett et al. 1999, 2004). All experiments on chronic spinal rats were conducted after full spasticity had developed in the axial muscles of the tail (2–3 mo after transection). Experiments on normal rats were conducted at a similar age (3–6 mo old). Recordings were made from sacrocaudal motoneurons and ventral roots of normal and chronic spinal rats in vitro (Li et al. 2004a,b). Muscle spasms were also recorded in vivo via percutaneous EMG placed in the axial tail muscles of spastic chronic spinal rats (Murray et al. 2010). All procedures were approved by the University of Alberta Animal Care and Use Committee: Health Sciences.
In vitro preparation
Details of the in vitro preparation have been previously described in detail (Li et al. 2004a,b), and are only briefly summarized here. Rats were deeply anesthetized with urethane (0.18 g/100 g; with a maximum dose of 0.45 g), and the whole sacrocaudal spinal cord was removed and transferred to a dissection chamber filled with modified artificial cerebrospinal fluid (mACSF) maintained at a constant temperature of 20°C. To remove the cord in chronic spinal rats, a transection was made just above the chronic injury (at upper S2 level). In normal adult rats, the cord was cut at the same location (upper S2) for removal, and they are therefore termed acute spinal rats. All dorsal and ventral spinal roots were removed with the exception of the sacral S4 and caudal Ca1 ventral roots and the Ca1 dorsal roots. The cord was then allowed to rest in the dissection chamber for 1.5 h. Following this rest period, the cord was transferred to a recording chamber containing continuously flowing normal artificial cerebrospinal fluid (nACSF) maintained near 24°C and with a flow rate >5 ml/min. Following a 60 min nACSF wash out period to clear any residual anesthetic and mACSF, the nACSF was recycled in a closed system with a peristaltic pump.
Intracellular recordings and analysis
Intracellular recordings were made from motoneurons in the sacrocaudal spinal cord of chronic spinal rats as detailed elsewhere (Li et al. 2004a) and are only briefly summarized here. Sharp intracellular electrodes were made from thick-walled glass capillary tubes (1.5 mm OD; Warner GC 150F-10) using a Sutter P-87 micropipette puller. Electrodes were back-filled with a combination of 1 M potassium acetate and 1 M KCl. A stepper-motor micromanipulator (660 Kopf) was used to advance into motoneurons. After penetration, motoneuron identification was made with antidromic stimulation of the S4 and Ca1 ventral roots noting ventral horn location, input resistance and time constant (>6 ms for motoneurons) (Li et al. 2007). Data were collected with an Axoclamp 2b intracellular amplifier (Axon Instruments, Burlingame, CA) running in discontinuous current clamp (DCC, switching rate 4–6 kHz, output bandwidth 3.0 kHz, sample rate of 6.7 kHz) or discontinuous single-electrode voltage clamp (SEVC; gain 0.8 to 2.5 nA/mV) modes. To measure the basic electrical properties of motoneurons, slow triangular current ramps (0.4 nA/s) and voltage ramps (ramp speed, 3.5 mV/s) were applied. Resting potential (Vm) was recorded 15 min after cell penetration, allowing time for the cell to stabilize, with a bias current of 0 nA. The input resistance (Rm) was measured during the voltage ramps over a 5 mV range near resting membrane potential and subthreshold to PIC onset. PIC measurements were made during the slow triangular voltage ramps. First the passive leak current was estimated during the upward portion of the ramp where the current response initially increases linearly with voltage in response to the passive leak conductance. A linear relation was fit to the subthreshold current response 5–10 mV below the negative-slope region of the PIC onset and then extrapolated to more positive voltages. The PIC amplitude was then estimated by subtracting this leak current from the total recorded current (leak-subtracted current). The onset voltage for the PIC (Von) was measured at the beginning of the first negative slope region in the current (where first 0 slope in current response occurred). The peak current of the PIC was measured from the leak subtracted current, where the downward deviation below the leak line reached peak amplitude. EPSPs and associated reflexes were also measured in motoneurons after stimulating the Ca1 dorsal roots (at 3× threshold, T) while applying a hyperpolarizing bias current to block the PICs and peak value quantified at ∼200 ms after the stimulation (long polysynaptic EPSP). Data were analyzed in Clampfit 8.0 (Axon Instruments).
Ventral root reflex recording and averaging
A detailed description of these procedures can be found in Li et al. (2004b). Briefly, two dorsal roots (left and right Ca1) and two to four ventral roots (left and right S4 and/or Ca1) were mounted on chlorided silver wires suspended above the ACSF of the recording chamber for monopolar stimulation and recording, respectively. The roots were wrapped around the wire above the ACSF and covered with a 1:1 mixture by weight of petroleum jelly:mineral oil. Ventral root reflexes were recorded in response to a single low threshold stimulation pulse (0.1 ms, 0.02 mA; Isoflex stimulator, AMPI) to the dorsal root (3 × reflex threshold, T ≈0.007 mA), consistent with stimulation of group I and II afferents, including mainly cutaneous afferents (Bennett et al. 2004; Li et al. 2004a,b). Dorsal root stimulation was repeated five times consecutively with an interstimulus interval of 10 s to provide multiple ventral root reflexes for averaging. Ventral root reflexes were recorded via a custom built differential preamplifier with one lead connected to the root and the second to the reference wire in the ACSF [high-pass, 100 Hz; low-pass, 3 kHz; amplified by 2,000 times; sampling rate 6.7 kHz (Axoscope 8, Axon Instruments)]. Ventral root reflexes were recorded every 12 min. When drugs were used, they were added to the bath immediately after a recording so as to ensure the actions of the drug could be recorded at the subsequent 12 min recording session. Dose response curves were constructed by administering increasing doses of the drug every 12 min. Ventral root reflexes were quantified using custom written software (MatLab 7.0.4, MathWorks, Natick, MA). That is, ventral root recordings were high-pass filtered (at 800 Hz, using a 1st order Butterworth filter), rectified, and then averaged over a time window 500–4,000 ms post stimulation, which we term the long-lasting reflex (LLR). This reflex period has previously been shown to result mainly from a sustained depolarization of the motoneurons by the Ca PIC (Bennett et al. 2004; Li et al. 2004a), although the activation of the Ca PIC itself depends on a long polysynaptic EPSP evoked by the stimulation (Li et al. 2004a).
Drugs and solutions
Two kinds of artificial cerebrospinal fluid were used in these experiments; a modified ACSF (mACSF) used during dissection and recovery to minimize neural and metabolic activity and a normal ACSF (nACSF) in the recording chamber. The mACSF was composed of (in mM) 118 NaCl, 24 NaHCO3, 1.5 CaCl2, 3 KCl, 5 MgCl2, 1.4 NaH2PO4, 1.3 MgSO4, 25 d-glucose, and 1 kynurenic acid. nACSF was composed of (in mM) 122 NaCl, 24 NaHCO3, 2.5 CaCl2, 3 KCl, 1 MgCl2, and 12 d-glucose. Both types of ACSF were saturated with 95% O2-5% CO2 and maintained at pH 7.4. During intracellular recordings, transient and persistent sodium currents were sometimes blocked with tetrodotoxin (TTX Alamone Labs, Israel) to isolate the Ca PIC. Other drugs used include methoxamine, strychnine (Sigma-Aldrich, Oakville, ON, Canada), A61603, prazosin, WB4101, clonidine, RX821002, UK14304 (Tocris Biosciences, Ellisville, MO) and Recordati15/2739 (abbreviated REC15/2739; generously donated by Recordati S.p.A., Milano, Italy).
Percutaneous electromyogram (EMG) reflex recording
Awake chronic spinal rats were housed inside a clear Plexiglass tube with the tail protruding and held horizontally by taping it to a bar. The tail was kept warm with a radiant heat lamp. Multi-stranded stainless steel wires (AS631; Cooner Wire, Chatsworth, CA) were bared 1 cm at each end and inserted percutaneously into the axial muscles of the mid-tail. EMG electrode placement into the tail muscles was standardized using the 12th coccygeal vertebra as a reference point. Recording electrodes were placed 1 and then 2 cm rostral to this point with the ground electrode placed 1 cm caudal to this point. Long-lasting cutaneous reflexes (termed LLR or spasms) were elicited with two stimulating electrodes inserted percutaneously on the distal tip of the tail, separated from each other by 1.5 cm. As the tip of the tail contains very little muscle, stimulation via the electrodes placed here provides relatively pure cutaneous stimulation (Bennett et al. 1999, 2004). To prevent movement of the wires, each wire was fixed to the skin using a small amount of cyanoacrylate glue. Spasms were evoked by single pulse stimulation (width, 0.2 ms) at 10 mA (Isoflex Stimulator, AMPI; ∼3–5 × T) every 10 s, and repeated six times. LLRs (spasms) were recorded with the EMG wires using a custom-built amplifier and Axoscope hardware and software (Digidata 1322A, Axoscope; Axon Instruments; amplified 2,000 times, low-pass filtered at 1,000 Hz, high-pass filtered at 100 Hz and sampled at 5 kHz). LLRs were quantified in a similar manner to that used for ventral root reflexes (see preceding text). To summarize, data were rectified and the LLR was computed by averaging the rectified EMG over a time-window 500–4,000 ms post stimulation.
In vivo drug injection
Unless otherwise listed, all drugs were administered in vivo via transcutaneous intrathecal (IT) injection under light isofluorane anesthetic. A 1-in, 25 - gauge needle connected to a 100 μl glass Hamilton syringe was inserted into the tissues between the L5 and L6 vertebrae on the dorsal side, perpendicular to the spinal column as per Mestre et al. (1994). This injection site was selected because of easy intervertebral accessibility to the spinal cord as well as a reduced possibility of spinal cord damage because the injection site is restricted to the area near the cauda equina. As the needle entered the spinal canal, the tail would produce an abrupt lateral twitch caused by the needle entering the proximity of the ventral roots, and this sign was used to positively confirm the injection site. The drug solution was slowly injected over ∼5 s. Drugs injected IT included A616103, prazosin, WB4101, and REC15/2739. All drugs were dissolved in sterile saline at a constant volume of 30 μl for each IT injection. Rats woke up within minutes of removal of light anesthetic at which point reflex testing resumed. Neither the anesthetic nor the saline vehicle influenced the reflexes as tested by control saline IT injections. Chronic spinal animals received multiple IT injections per experimental session, up to a maximum of four injections with ≥90 min separating each injection.
Statistics
All data are shown as means ± SE throughout the text and figures. Statistical differences were computed at a significance level of P < 0.05 with a paired Student's t-test where data were before and after drug applications in the same animals, and otherwise with an unpaired Student's t-test or ANOVA as needed. A Kolmogorov-Smirnov test for normality was applied to each data set with the level set for significance set to P = 0.05, to verify normality, as is required for a t-test. Where dose-response curves are presented, a standard sigmoidal curve (with a Hill slope of unity) was fit to LLR responses with increasing drug doses (in log units). Drug potency, as indicated by the dose at which 50% of the maximal effect was observed (EC50), was measured from the curve. All calculations of EC50 values and accompanying statistics comparing EC50 values were carried out using the logarithm of dose.
RESULTS
Ca PICs are increased by α1 receptors
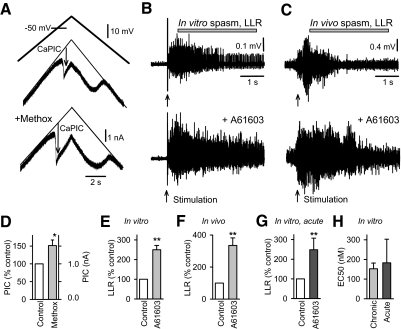
Considering that Ca PICs in motoneurons are a major underlying cause of spasms after SCI, we began our study by examining the effect of α1 receptor activation on Ca PICs. The Ca PICs were quantified in vitro during intracellular recordings from motoneurons in the sacral spinal cord of chronic spinal rats (Fig. 1). TTX (2 μM) was applied to synaptically isolate the motoneuron (blocking spike-mediated transmission) and to block the Na PIC that otherwise can obscure the Ca PIC, as previously described (Li and Bennett 2003). We quantified the Ca PIC using slow voltage ramps (under voltage-clamp conditions) to inactivate transient currents and enable the full voltage dependence of the Ca PIC to be evaluated. During these slow voltage ramps, the Ca PIC was activated at –57.94 ± 7.63 mV (Von, n = 9 motoneurons) and produced a downward deflection in the recorded current of 1.05 ± 0.26 nA, which we considered an estimate of the Ca PIC amplitude (Fig. 1A, arrow; previously verified to be mediated by L-type calcium channels; nimodipine-sensitive) (Li and Bennett 2003; Li et al. 2004a). Application of the moderately selective α1A adrenergic receptor agonist methoxamine significantly increased this Ca PIC amplitude (151.32%; Fig. 1, A and D). In contrast, methoxamine had no effect on the Ca PIC threshold Von, the motoneuron resistance or resting membrane potential (changes with drug: –0.14 ± 1.42 mV, 0.84 ± 0.69 MΩ, –0.13 ± 2.53 mV, respectively; not significant, P > 0.05, n = 9).
Fig. 1.
Activation of the α1 adrenergic receptor increases calcium-mediated persistent inward currents (Ca PICs) and spasms. A: intracellular recording of Ca PIC in motoneuron, recorded in whole sacrocaudal spinal cord below a chronic transection, in vitro. Ca PIC measured in isolation by slowly increasing the membrane potential (top plot) in presence of 2 μM TTX, and quantified at its initial peak, where it produced a downward deflection in the recorded current (thick black plot, at arrow, Ca PIC) relative to the leak current (thin line in middle plot). Bottom plot: increase in Ca PIC with addition of the α1 adrenergic receptor agonist methoxamine to the bath (10 μM). B: long-lasting reflex (LLR) triggered by dorsal root stimulation (single pulse, 3 × T) and recorded from the ventral roots (LLR, quantified during horizontal bar; counterpart of spasms) before and after application of the α1 receptor agonist A61603 (0.1 μM). C: LLR spasm in awake chronic spinal rat evoked by electrical/cutaneous stimulation of the tail (0.2 ms pulse, 10 mA) and recorded with tail muscle electromyogram (EMG) before and after local intrathecal (IT) injection of A61603 (0.03 mM in 30 μl). Spasm quantified during horizontal bar (LLR). D: group mean of increase in Ca PIC with methoxamine (abbreviated methox; 10–40 μM) in chronic spinal rats (n = 8), normalized (left axis) and in absolute current values (right axis). E and F: normalized group mean of increase in LLR with application of A61603 to the isolated in vitro spinal cord of chronic spinal rats (0.03–1 μM; n = 42) and to the in vivo spinal cord in the awake chronic spinal rat (0.03 mM in 30 μl; IT injection, n = 5). G: normalized group mean of increase in LLR with application of A61603 (0.03 – 1 μM; n = 18) to the isolated in vitro spinal cord removed from normal rats (termed acute spinal). Control values were taken in strychnine (3 μM) to produce a similar LLR to that under control untreated condition in chronic spinal rats. H: normalized group mean of the dose to produce a 50% increase in the LLR (EC50) with increasing doses of A61603, recorded both in chronic spinal (n = 30) and acute spinal (n = 11) conditions in vitro. *, P < 0.05; **, P < 0.01. Error bars, SE. All recordings in B–H were made in the presence of RX821002 (in vitro: 0.5 μM; in vivo: intraperitoneal injection, 1 mg/kg) to prevent involvement the α2 adrenergic receptor.
LLRs are increased by α1A receptors
To examine the functional consequences of α1 adrenergic receptors, we measured the effects of adrenergic receptor agonists on LLRs (quantified 500–4,000 ms post stimulation) recorded on the ventral roots in response to a brief dorsal root stimuli in vitro (single pulse, 3 × T), which have previously been shown to depend on Ca PICs (Li et al. 2004a). Neither the moderately selective α1A adrenergic receptor agonist methoxamine (0.1–30 μM) (Minneman et al. 1994; Shibata et al. 1995) nor the more selective and potent α1A receptor agonist A61603 (0.03–10 μM) (Craig et al. 1997; Knepper et al. 1995; Mehrotra et al. 2007) consistently changed the LLR (nonsignificant increase of 24.3 ± 31.0 and 10.7 ± 15.5% for methoxamine and A61603, respectively, P > 0.05, n = 14 each). This was initially unexpected, considering that following the transient dorsal root evoked EPSP (<500 ms) known to trigger the Ca PICs, the remaining many-second-long portion of the LLR that we quantified is almost entirely mediated by the Ca PICs on motoneurons (see introduction) (Li et al. 2004a) (Murray et al. 2011). However, in retrospect, we realized that this was due to a potent inhibition of the EPSP by these agonists, mediated by α2 receptors, as we describe later, and this counterbalanced the increase in the Ca PICs mediated by α1 receptors. This occurred because methoxamine and A61603, as well as other available α1 agonists, have substantial binding affinity for α2 as well as α1 receptors (Craig et al. 1997; Mehrotra et al. 2007; Minneman et al. 1994; Shibata et al. 1995; U'Prichard et al. 1977), and the negative effects of α2 receptors in our preparation were unexpectedly large (see later section). This poor α1 verses α2 selectivity had not been anticipated, especially for A61603, because A61603 has otherwise negligible binding at all other receptors previously tested, including α1B and β adrenergic, 5-HT, and dopamine receptors (Craig et al. 1997).
To study the effects of the α1 receptor in isolation, we next blocked the α2 receptors with the selective α2 receptor antagonist RX821002 (0.3–0.5 μM) (Jasper et al. 1998; Sanders et al. 2006) prior to applying the agonist A61603. This effectively made A61603 highly selective for the α1A adrenergic receptor (Craig et al. 1997). Under these conditions, A61603 significantly increased the LLR (Figs. 1 and 2), more than doubling the LLR amplitude when given at doses >30 nM. This is consistent with an α1A receptor mediated increase in the PIC. The facilitation of the LLR increased with increasing doses of A61603 (≤1,000 nM), and this dose-response relation was well approximated by a sigmoidal function with half-maximal effects at about 150 nM (EC50, Figs. 1H and 2C, sigmoid had Hill slope of 1.0), consistent with the known high affinity of A61603 to the α1A receptor (Ki = 80 nM) (Craig et al. 1997; Mehrotra et al. 2007).
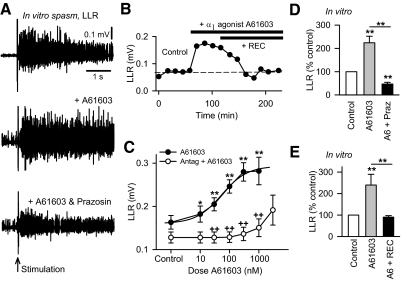
Fig. 2.
The α1A adrenergic receptor agonist A61603 is antagonized by selective α1 receptor antagonists. A: LLR evoked in the isolated in vitro spinal cord of a chronic spinal rat, as described in Fig. 1 (top plot), with bath application of the α1A receptor agonist A61603 (0.1 μM, middle plot), and with subsequent application of α1 receptor antagonist prazosin (1 μM, bottom plot). B: amplitude of LLR (quantified 0.5–4 s post stimulus, as in Fig. 1) of a chronic spinal rat measured repeatedly over time under control conditions (left), with application of A61603 (upper horizontal black bar; 0.03 μM) and subsequent application of the highly specific α1A receptor neutral antagonist REC15/2739 (abbreviated REC; lower horizontal black bar; 10 μM). C: mean LLR amplitude in response to increasing doses of A61603 (dose response) recorded in chronic spinal rat in vitro (●, upper line; n > 18 for each dose) and for A61603 applied after the α1 receptor antagonists prazosin (1 μM) or WB4101 (3 μM) (○, lower line; n > 8 for each dose, ++P < 0.01). D: normalized group mean of LLR with application of A61603 alone (0.03–0.3 μM;  , n = 42) and A61603 with subsequent treatment with prazosin (abbreviated A6 + Praz; ■, n = 16), recorded in chronic spinal rat in vitro. E: same as D except treatment with A61603 alone (0.03 μM; n = 15), and A61603 with subsequent application of REC15/2739 (abbreviated A6 + REC; 10 μM; n = 15). *P < 0.05, **P < 0.01. Error bars, SE. All recordings were made in the presence of RX821002 (0.5 μM).
, n = 42) and A61603 with subsequent treatment with prazosin (abbreviated A6 + Praz; ■, n = 16), recorded in chronic spinal rat in vitro. E: same as D except treatment with A61603 alone (0.03 μM; n = 15), and A61603 with subsequent application of REC15/2739 (abbreviated A6 + REC; 10 μM; n = 15). *P < 0.05, **P < 0.01. Error bars, SE. All recordings were made in the presence of RX821002 (0.5 μM).
Spasms in awake rats are increased by α1A receptors
We also examined the effects of α1 receptor activation on spasms triggered by brief cutaneous stimulation at the tip of the tail (3 × T) and recorded from the axial tail muscles of awake chronic spinal rats with implanted EMG wires. These spasms are the equivalent of the LLR recorded in vitro (Bennett et al. 2004; Li et al. 2004a), lasting many seconds, and were quantified over the same time window (500–4,000 ms, LLR and spasm used interchangeably; Fig. 1C). The adrenergic agonists A61603, methoxamine, and phenylephrine were applied locally to the spinal cord by intrathecal injection (IT, 0.1–1 mM in 30 μl saline) to avoid systemic effects. Again we found that, by themselves, none of these agonists increased LLRs (spasms) in all rats tested (n = 7/7 rats tested; data not shown), although in two of these animals, A61603 induced a regular rhythmic movement of the tail in the absence of spasm-triggering stimulation. In contrast, after a prior application of RX821002 (1–3 mg/kg intraperitoneal (ip) to block possible nonselective actions on α2 receptors, LLRs (tail spasms) were significantly increased by an IT injection of A61603 (Fig. 1, C and F). Control saline injections had no significant effect (P > 0.05, n = 5; not shown). These results further demonstrate that activation of the α1A adrenergic receptor increases spasticity and underlying Ca PICs in chronic spinal rats.
Chronic spinal rats are not supersensitive to α1 receptor activation
The increases in LLRs resulting from α1 adrenergic receptor activation were not limited to chronic spinal animals. Application of A61603 also led to increases in LLRs recorded in normal control rats studied in vitro (considered acute spinal because of cord removal for in vitro recording; Fig. 1G; in RX821002). For these acutely spinalized rats, LLRs were initially absent (i.e., animals were not spastic). To ensure similar preliminary conditions, a low dose of strychnine was administered in vitro that resulted in LLRs that were similar in magnitude to those in chronic spinal rats (only slightly smaller; Fig. 1, E vs. G). The increase in LLRs produced by A61603 in these acutely spinalized control rats, with strychnine, was comparable to that seen in chronic spinal animals (Fig. 1, E vs. G). Moreover the dose at which A61603 exerted half of its maximal effect on in vitro LLRs (EC50) was similar in both chronic and acutely lesioned rats, indicating a lack of supersensitivity to α1 receptor activation with A61603.
Blocking the α1 adrenergic receptor reverses agonist-mediated increase in spasms
As mentioned, agonists of α1 adrenergic receptors generally demonstrate only limited selectivity over other adrenergic receptor subtypes (e.g., α2 receptors). In contrast, antagonists of α1 adrenergic receptors are more selective (including REC15/2739, prazosin, and WB4101) (Doxey et al. 1983; Ford et al. 1997; Sanders et al. 2006; Schwinn et al. 1995; Shibata et al. 1995), and for that reason, we used these drugs to confirm the involvement of the α1 receptors in facilitating LLRs in chronic spinal rats. We found that the facilitation of the LLR by A61603 (in presence of RX821002, as in the preceding text) was significantly inhibited by a subsequent application of prazosin or REC15/2739 (Fig. 2, A, B, D, and E), in vitro. The typical time course of the facilitation of the LLR by the α1 agonist and subsequent inhibition by and the α1 antagonist is shown in Fig. 2B with the antagonist acting relatively slowly, taking >30 min to reach peak effect. Part of this antagonist-mediated inhibition might have resulted from a block of endogenously active α1 receptors (see following text). Thus we also evaluated the action of increasing doses of the agonist A61603 on the LLR after first applying the antagonist and giving time for the intrinsic effects of this antagonist, if any, to reach steady state (agonist given >30 min after antagonist). In this situation, increasing doses of the agonist A61603 had no effect until very high doses were reached (1,000 nM), whereas without the antagonist, A61603 increased the LLR at doses as low as 10 nM, demonstrating that this agonist indeed increased the LLR and associated PICs via α1 receptors. These experiments were performed in the presence of RX821002 to rule out any nonselective action of A61603 on the α2 receptor.
Endogenous activity in α1 receptors from peripherally derived neurotransmitter
The drug REC15/2739 is special because it has previously been shown to act as a neutral antagonist at α1A adrenergic receptors, meaning that it blocks only the action of a ligand (such as NA) at the α1A receptor and not constitutive receptor activity (Rossier et al. 1999). REC15/2739 is therefore useful in determining whether the α1A receptors are activated by endogenous NA, or another natural ligand, that somehow persists below a chronic spinal injury. We found that administration of REC15/2739 alone had no effect on the ventral root LLRs in the isolated in vitro spinal cord (Fig. 3, A and C) even though it readily antagonized the α1A agonist A61603 (Fig. 2E). This suggests that, at least in vitro, the α1A receptor is not endogenously activated by residual NA in the spinal cord. In contrast, when we administered REC15/2739 in awake spastic rats in vivo, with a localized IT injection, there was a significant decrease in LLRs (spasms Fig. 3, B and D). This demonstrates that the α1A adrenergic receptor is activated by some endogenous ligand, likely NA, in vivo, but not in vitro, indicating that any residual endogenous NA that affects the spinal cord may originate from the periphery (see discussion).
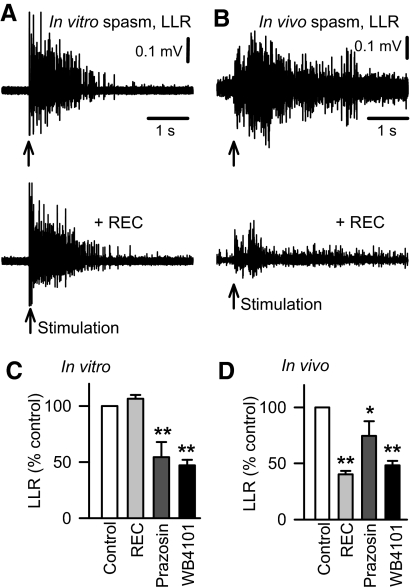
Fig. 3.
Endogenous activation of the α1 adrenergic receptor is the result of constitutive activity in vitro, but a combination of constitutive and ligand activity in vivo. A: LLR in chronic spinal rat, evoked in the isolated in vitro spinal cord (as described in Fig. 1, top plot) and after blocking the action of endogenous NA (or similar ligand) with application of the α1A neutral antagonist REC15/2739 (abbreviated REC; 10 μM, bottom plot). B: long-lasting reflex spasm in awake chronic spinal rat evoked by electrical/cutaneous stimulation of the tail and recorded with tail muscle EMG (LLR computed 0.5–4 s post stimulus, as in Fig. 1) before (top plot) and after blocking endogenous action of NA at the α1A receptor with local intrathecal (IT) injection of REC15/2739 (5 mM in 30 μl). Normalized group mean of chronic spinal rat LLRs recorded in vitro (C) and in vivo (D) after application of the α1 receptor neutral antagonist REC15/2739 (gray bars, in vitro: 5–10 μM, n = 24; in vivo: IT injection of 3–10 mM in 30 μl; n = 5), and after application of inverse α1 receptor agonists prazosin (dark gray bars, in vitro: 1 μM, n = 24; in vivo: IT injection, 1 mM in 30 μl, n = 9) and WB4101 (black bars, in vitro: 3–5 μM, n = 16; in vivo: IT injection, 1–3 mM in 30 μl; n = 5). *, P < 0.05, **, P < 0.01. Error bars, SE. All recordings were made in the presence of RX821002 (in vitro: 0.5–1 μM; in vivo: intraperitoneal injection, 1 mg/kg).
Constitutive activity in α1 receptors
Interestingly, application of the α1 antagonists WB4101 or prazosin significantly decreased LLRs recorded in vitro (without prior agonist application, Fig. 3, C and D), even though REC15/2739 did not. WB4101 and prazosin have been previously shown to act as potent inverse agonists at α1 receptors (Noguera et al. 1996; Rossier et al. 1999; Seifert and Wenzel-Seifert 2002), which means that they block constitutive activity in α1 receptors in addition to blocking traditional ligand-mediated activation of the receptor. Thus the inhibitory action of WB4101 and prazosin on the LLR, together with the lack of action of the neutral antagonist (REC15/2739; no ligand activated receptors), suggests that the α1 receptors exhibit substantial constitutive activity, at least in vitro. When applied in vivo, both WB4101 and prazosin (IT) likewise inhibited the LLRs (spasms Fig. 3D), which is likely due to both a block of ligand-activated receptors (residual NA) and constitutively activated receptors.
α2 adrenergic receptor modulates the EPSP but not the Ca PIC
Considering that we suspected an inhibitory effect of α2 receptors on the EPSPs that trigger LLRs (spasms), we next measured how the moderately selective α2 adrenergic (and imidazoline I1) receptor agonist clonidine, and the highly selective α2A adrenergic receptor agonist UK14304, affected ventral root LLRs in vitro. Treatment with both these α2 agonists significantly decreased LLRs (Fig. 4, A and D), and this decrease was reversed by subsequent treatment with the selective α2 adrenergic antagonist RX821002 (Fig. 4, A and D). Furthermore the decease in the LLR with clonidine was dose-dependent with a very low EC50 of 25 ± 7 nM, consistent with the high binding affinity of clonidine to the α2A receptor (Ki = 31 nM) (Millan et al. 2000), and inconsistent with the 10 times lower affinity of clonidine to α1 receptors (e.g., Ki = 300 nM at α1A receptor) (Millan et al. 2000). These results suggest that α2A adrenergic receptors inhibit LLRs and resulting spasms after chronic SCI.
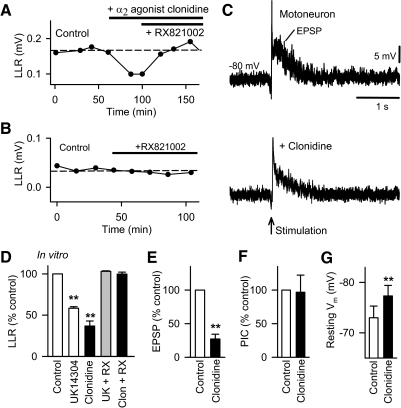
Fig. 4.
Activation of the α2 adrenergic receptor does not directly affect the Ca PIC but instead inhibits EPSPs. A: amplitude of LLR recorded in the isolated in vitro spinal cord of chronic spinal rat and measured repeatedly over time (LLR quantified 0.5–4 s post stimulus, as in Fig. 2). Control values are shown on left, followed by activation of α2 adrenergic receptors with the agonist clonidine (top bar 0.1 μM) and subsequent application of the α2 receptor antagonist RX821002 (0.3 μM; bottom bar). B: same format as A with application of RX821002 alone (0.5 μM). C: intracellular motoneuron recording of long-latency polysynaptic EPSP (abbreviated EPSP) evoked by dorsal root stimulation (0.1 ms at 3 × T) of chronic spinal rat (quantified at 200 ms post stimulus) during hyperpolarizing bias current before (top plot) and after blocking the α2 receptor with bath application of clonidine (0.3 μM, bottom plot). D: normalized group mean for LLR in chronic spinal rats in vitro with application of the α2 receptor agonists UK14304 (0.03 μM; n = 8), and clonidine (0.1 μM; n = 5), and application α2 antagonist RX821002 (0.3 μM) after UK14304 (abbreviated UK14 + RX; n = 8) and after clonidine (abbreviated Clon + RX; n = 8). Normalized group mean of intracellulary recorded polysynaptic EPSP (E) evoked by 3 × T dorsal root stimulation, PIC (F), and resting membrane potential (Vm; G) before and after application of clonidine in chronic spinal rats (0.1–1 μM; n = 5). *P < 0.05, **P < 0.01. Error bars, SE.
We next investigated whether this inhibitory effect of α2 receptors was mediated by a reduction in the dorsal root evoked long polysynaptic EPSP that triggers the PICs or the PICs themselves that ultimately cause the many seconds of firing during the LLRs (spasms). We recorded EPSPs in motoneurons of chronic spinal rats in response to our standard brief dorsal root stimulation (0.1 ms, 3 × T; Fig. 4C). The motoneurons were held with a hyperpolarizing bias current to prevent PIC activation and spiking (holding cell at −80 mV) and thus allow us to investigate the EPSP in isolation (Li et al. 2004a). Under these conditions, a long EPSP was evoked with a 5–10 ms latency, lasting about 500–1,000 ms, and with a mean amplitude of 5.2 ± 2.1 mV measured at 250 ms poststimulation (at main peak after transient peak at 5–10 ms). The α2 receptor agonist clonidine decreased this long polysynaptic EPSP significantly (Fig. 4, C and E). In contrast, clonidine had no effect on the PIC (Fig. 4F; recorded under voltage clamp, as described in Fig. 1A). Interestingly, clonidine significantly hyperpolarized the resting membrane potential by about –4 mV (Fig. 4). These data suggest that the inhibitory effect of the α2 receptor on spasms is mediated by a reduction of the long polysynaptic EPSP that trigger the PICs (and associated spasms), rather than by a reduction in the PICs themselves. Additionally this receptor may act by hyperpolarizing the motoneurons.
Lack of constitutive activity in α2 receptors
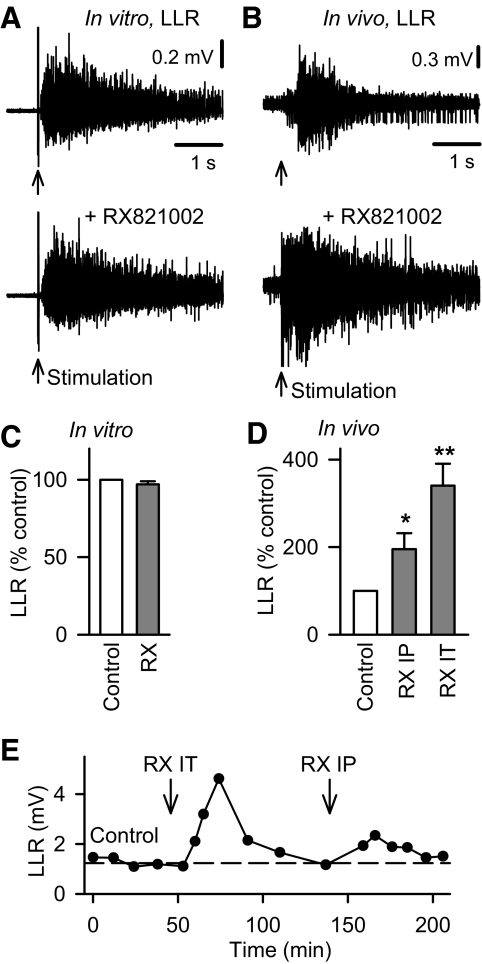
Application of the α2 adrenergic receptor antagonist RX821002 alone, without agonists, had no effect on the LLRs (Figs. 4B and 5, A and C) measured in the isolated spinal cord in vitro, even though it is a potent α2 receptor inverse agonist that is capable of blocking constitutively active α2 receptors (Pauwels et al. 2000). In contrast, RX821002 significantly increased the spasms recorded in the awake spastic rat in vivo, both with systemic intraperitoneal (IP) or local spinal intrathecal injection of RX821002 (Fig. 5, D and E). This suggests that although the α2 adrenergic receptor is not constitutively active in the isolated in vitro spinal cord, it is activated by some endogenous ligand (NA) present below the lesion in the awake rat after chronic SCI, similar to the activation of the α1 receptor.
Fig. 5.
The α2 adrenergic receptor is endogenously active in vivo, but not in vitro. A: LLR evoked in the isolated in vitro spinal cord (as described in Fig. 1) of a chronic spinal rat before (top plot) and after blocking possible endogenously activated α2 adrenergic receptors with the selective α2 antagonist RX821002 (0.5 μM, bottom plot). B: LLR spasm in chronic spinal rat in vivo evoked by electrical/cutaneous stimulation of the tail and recorded with tail muscle EMG (LLR computed 0.5–4 s post stimulus, as described in Fig. 1) before (top plot) and after blocking endogenously activated α2 receptors with a systemic intraperitoneal (ip) injection of RX821002 (1 mg/kg). C: normalized group mean for chronic spinal rat LLRs before and after bath application of RX821002 (abbreviated RX) in vitro (0.3–0.5 μM; n = 42) and for systemic ip injection of RX2821001 (D; abbreviated RX IP, 1–3 mg/kg; n = 11) and local intrathecal injection (abbreviated RX IT, 0.3–1 mM in 30 μl; n = 5) in vivo. E: amplitude of in vivo tail spasms of awake chronic spinal rat recorded with EMG (LLR) and measured repeatedly over time under control conditions (left), and after blocking the endogenous activation of the α2 adrenergic receptor with either local IT injection of RX821002 (abbreviated IT RX, left ↓; 0.3 mM in 30 μl) or systemic IP injection of RX821002 (abbreviated RX IP, right ↓; 1 mg/kg). *P < 0.05, **P < 0.01. Error bars, SE.
DISCUSSION
The results of our study characterize for the first time the roles of two adrenergic receptor subtypes (α1 and α2) in the recovery of motoneuron excitability and spasms after chronic SCI. We find that α1 receptors increase motoneuron excitability and the α2 receptors decrease synaptic transmission of sensory inputs to motoneurons and thus have opposing effects on motor output and spasms after injury, broadly consistent with our understanding of the function of these receptors in normal uninjured animals (Jankowska and Hammar 2002; Jankowska et al. 1993, 2000; Lundberg 1982; Millan 2002; Rekling et al. 2000). Notably we demonstrate a previously undescribed mechanism for compensating for loss of adrenergic innervation with SCI: α1 receptors become constitutively active (active in absence of NA), and this ultimately contributes to both the recovery of motoneuron excitability (PICs) and emergence of spasms (uncontrolled PICs). Interestingly, we find that a peripheral source of NA, or potentially another ligand, additionally activates the α1 and α2 receptors. In contrast, the α2 receptors do not seem to exhibit constitutive activity, suggesting that these receptors respond differently to injury.
α1A receptor subtype on motoneurons facilitates the Ca PICs and spasms
Our results specifically establish that activation of the α1A adrenergic receptor facilitates the Ca PIC in motoneurons, thereby increasing its excitability and ultimately increasing the many-second-long spasms (LLRs) known to be mediated by the Ca PIC. These conclusions are based on α1A receptor agonist-induced increases in the Ca PICs, measured both directly with intracellular recordings and indirectly by assessing the many seconds long ventral root LLRs produced by the Ca PICs, the latter allowing more detailed pharmacological testing not possible during intracellular recordings. We specifically used the highly selective α1A agonist A61603 that has negligible binding affinity for most other receptors, including other α1 adrenergic receptor subtypes (α1B, α1D), β adrenergic receptors, dopamine receptors and 5-HT receptors (Craig et al. 1997; Mehrotra et al. 2007). The only nonselective action of A61603 is to bind with high affinity to α2 receptors (Craig et al. 1997), which initially thwarted our efforts to demonstrate α1A receptor-mediated increases in the LLR, and thus we subsequently applied A61603 in the presence of the α2 antagonist RX821002 to make it highly selective to α1A receptors. Under these conditions, we found that A61603 consistently increases the LLR, demonstrating the presence of an α1A adrenergic receptor that facilitates the Ca PIC on motoneurons.
Consistent with the involvement of the α1 receptor in facilitating motoneuron excitability, we found that A61603 increases the LLR at a dose (EC50 of 150 nM) that is remarkably consistent with the binding affinity of A61603 to the α1A receptor measured in isolated cells (Ki = 80 nM) and not other receptors (Craig et al. 1997). We do not know why the EC50 is so close to this Ki value obtained from binding to α1 receptors in isolated cells, whereas with 5-HT2 receptor agonists we find that the EC50 for increasing the LLR is consistently ∼10 times the agonist binding affinity at the 5-HT2 receptors (Murray et al. 2011). One possibility is that the α1A receptors may be located near the surface of the spinal cord on the distal dendrites of motoneurons, where the drug can easy diffuse to, when applied in vitro. In contrast, the 5-HT2 receptors are located deep in the spinal cord, including on the motoneuron soma (Murray et al. 2010), where drugs reach less easily, although this needs to be further investigated. We know that the α1A receptors that facilitate Ca PICs and spasms must be located somewhere on motoneurons because the facilitation of the Ca PIC by α1 agonists occurs in the presence of a sodium channel block with TTX, which renders the motoneurons synaptically silent, essentially isolated from inputs (Li and Bennett 2003). This is consistent with previous reports of widespread expression of the α1 receptors in the spinal cord, including high levels on motoneurons (Giroux et al. 1999; Rekling et al. 2000; Roudet et al. 1993).
We cannot entirely rule out the possibility that the α1 adrenergic receptor also increases the LLR and spasms by increasing other motoneuron properties or even the EPSPs that trigger the Ca PICs. The α1 receptor has been shown to depolarize other motoneurons (Rekling et al. 2000), bringing them closer to threshold and making them more likely to be involved in spastic reflexes (spasms). While we found that the α1 agonist methoxamine did not depolarize the resting potential of motoneurons, it is still possible that the α1 receptor depolarizes the sacral motoneurons we studied, but this is masked by the nonselective action of methoxamine on the α2 receptor, which hyperpolarizes motoneurons (see following text). We do not know whether the α1 receptors facilitate sensory afferent transmission (EPSPs), although if anything, they may do the opposite, by facilitation of inhibitory interneurons (Yoshimura and Furue 2006).
α1A receptors act similarly in normal and chronic spinal rats
In spinal cords from normal rats, we also found evidence for the presence of α1A receptor activation on motoneurons that likely act to increase the Ca PICs because A61603 application (with RX821002) increases sustained motoneuron output in spinal cords of normal rats. Interestingly, when we bring motoneurons of normal and chronic spinal rats to a similar initial level of excitability prior to testing with A61603, by applying a low dose of strychnine in normal rats, the estimated potency of this receptor agonist (EC50) is similar in normal and chronic spinal rats, suggesting that the α1A receptor-mediated responses may not become supersensitive with injury. This is contrary to previous suggestions (Li et al. 2004b) and unlike the supersensitivity of motoneuron PICs to 5-HT receptor activation after chronic injury (Harvey et al. 2006a). However, a lack of supersensitivity in chronic spinal rats (60–90 days post injury) is consistent with previous findings that, while the α1 receptor expression is upregulated transiently after SCI (Giroux et al. 1999; Roudet et al. 1993), it reverts back to normal expression at >30 days post injury. Caution must be taken in comparing receptor expression to agonist potency in facilitating reflexes though, because increasing receptor number does not necessarily increase the potency of agonists. Furthermore, in normal animals, agonists are more likely to be sequestered by the potent NA reuptake transporter (NET) than after SCI, where NET must be reduced with loss of NA innervation, considering its predominant localization on catecholamine neurons and not glial cells (Blakely et al. 1994). Currently, it is only clear that the α1A receptors appear to act similarly in spinal cords of normal and chronic spinal rats to increase motoneuron excitability.
Constitutive activity in α1A receptors contribute to recovery of motoneurons excitability
Our data demonstrate that when the spinal cord is isolated from peripheral influences (in vitro), the Ca PICs are facilitated by endogenous α1A receptor activity that is entirely mediated by constitutive receptor activity. Constitutive activity of wild-type α1A adrenergic receptors have recently been demonstrated in a variety of single cells systems with transfected cloned receptors, and across several species, including rats and humans (Seifert and Wenzel-Seifert 2002). Our findings represent the first time, however, that constitutive activity at the α1A receptor has been shown to play a functional role in the spinal cord. Our conclusions are based on the finding that the LLR (and associated Ca PIC) is reduced by blocking constitutive activity with inverse agonists (WB4101 or prazosin), whereas it is not affected by blocking possible residual NA with the neutral antagonist REC15/2739. In light of this new data (with REC15/2739), we can now re-interpret our previous finding that WB4101 also decreases sodium currents in motoneurons in chronic spinal rats (Harvey et al. 2006b). This now indicates that constitutive α1 adrenergic receptor activity also facilitates sodium currents, including Na PIC and the fast sodium currents underlying the spike.
Even though the antagonists WB4101, prazosin, and REC15/2739 are fairly selective to α1 receptors compared with other receptors, including α2 receptors, they are not very selective among the α1 receptor subtypes and bind potently to α1A, α1B, and α1D receptors (Doxey et al. 1983; Ford et al. 1997; Sanders et al. 2006; Schwinn et al. 1995; Shibata et al. 1995). Therefore, from our WB4101 and prazosin data alone, we only know that one of the α1 receptor types is constitutively active. However, while REC15/2739 is a neutral antagonist at α1A receptors, it is an inverse agonist at other α1 receptor subtypes, whereas WB4101 and prazosin are inverse agonists at all α1 receptor subtypes (Rossier et al. 1999). Thus, the inhibition of the LLR by WB4101 and prazosin, and not REC15/2739, indicates that the constitutive activity is mediated by the α1A receptor, further supporting the conclusion that the α1A receptor increases the Ca PIC.
Interestingly, previous reports have shown that the nonselective 5-HT2 receptor inverse agonists cyproheptadine and ketanserin inhibit the LLR substantially more than can be predicted from blocking constitutively active 5-HT2 receptors alone (Murray et al. 2011). In light of the present results and considering that these drugs bind to both adrenergic and serotonergic receptors (Yoshio et al. 2001), it now seems likely that these serotonergic drugs also block constitutive activity at α1 receptors. Constitutively active 5-HT2 receptors and α1 adrenergic receptors likely play an equally important role in facilitating spasms, because they contribute equally to PICs and blocking both these receptors essentially eliminates the PICs (Harvey et al. 2006b). This helps explain the particular effectiveness of cyproheptadine as an antispastic drug (Barbeau et al. 1982; Murray et al. 2010; Nance 1994) by its action in blocking both 5-HT2 receptors and α1 adrenergic receptors. However, broad spectrum drugs like cyproheptadine may also nonselectively block the α2 receptor, which may have a paradoxically pro-spastic action, increasing sensory afferent transmission and pain, as we discuss in the following text.
Possible peripheral source of NA after spinal cord injury
The lack of action of REC15/2739 on the LLR in the isolated spinal cord in vitro, despite its ability to antagonize exogenously applied α1 agonists, suggests that in the isolated spinal cord of chronic spinal rats there is no functional source of NA that accounts for activation of the α1 adrenergic receptors. Interestingly, a forced release of NA with application of amphetamine (Rothman et al. 2001) increases reflexes and motoneuron PICs after chronic SCI (Nozaki et al. 1980; Rank et al. 2007), even in the isolated spinal cord, and so there is a central store of NA, but this store does not appear to be actively released, at least under our experimental conditions in vitro. In contrast, the endogenous α1 receptor activity seen in the awake rats (in vivo) appears to additionally involve α1 receptors activated by an endogenous ligand (presumably NA) because both the inverse agonists and the neutral antagonist reduce spasms in this case. We do not know where this source of NA arises, but do know that it acts at the spinal level because we applied our antagonists locally to the spinal cord (IT injection).
There are consistent reports of small amounts of residual NA that persists in the spinal cord after SCI (Magnusson 1973; Roudet et al. 1993, 1994), although the origin of this NA remains a matter of dispute. Based on biochemical methods to visualize catecholamines in the spinal cord, McNicholas et al. (1980) suggested that this residual NA after SCI arises from small sympathetic efferents branching off of blood vessels in the spinal cord. This has more recently been given further support by reports of some residual dopamine β-hydroxylase, the enzyme essential for NA production, after chronic transection (McNicholas et al. 1980; Takeoka et al. 2010). This NA may partly account for the amphetamine-induced increases in the PICs and spasms that we observe in vitro (Rank et al. 2007), but we reiterate that this intrinsic source appears to be functionally inactive in the isolated spinal cord (in vitro; lack of effect of REC15/2739). Considering the very sparse distribution of these few residual NA fibers after SCI, and the lack of supersensitivity of α1 receptors to NA agonists, this sympathetic source of NA seems unlikely to account for the large PICs and spasms we see. Alternatively, because the blood brain barrier (BBB) is chronically compromised after SCI (Popovich et al. 1996), peripheral circulating NA originating in the autonomic system may cross into the spinal cord and activate the α1 receptors. While unconventional, this peripherally derived NA seems like a much larger source of NA, and we are currently investigating this possibility.
α2 receptors inhibit EPSPs but not PICs
Contrary to the α1 receptor function, our data demonstrate that the activation of the α2 adrenergic receptor has no direct effect on the Ca PIC in motoneurons (clonidine-resistant). Rather, activation of the α2 receptor with clonidine inhibits sensory synaptic transmission to the motoneuron, decreasing the polysynaptic EPSP and thereby preventing activation of the Ca PIC and ultimately reducing the activation of LLRs. We do not know where these α2 receptors are located, although they are likely on the terminals of the low threshold group I and II sensory afferents that we used to evoke LLRs and EPSPs or on the interneurons involved in the polysynaptic pathway that produces the EPSPs, consistent with previously reported locations of α2 receptors (Jankowska and Hammar 2002; Jankowska et al. 2000; Millan 2002; Rekling et al. 2000). Additionally, α2 receptors may be on motoneurons themselves, because we found that their direct activation with clonidine hyperpolarizes the motoneurons. Indeed, previous studies have shown that α2 receptors on motoneurons induce a hyperpolarization by blocking Ih currents (Adachi et al. 2005; Parkis and Berger 1997; Rekling et al. 2000), and such Ih currents contribute +10 mV to the resting potential in our chronic spinal rats (Li et al. 2007). Furthermore, α2 receptor expression can be detected throughout the spinal cord with the highest densities in the superficial dorsal horn and moderate densities in the portion of be ventral horn containing motoneurons (Giroux et al. 1999; Roudet et al. 1994). All together, α2 receptor agonists like clonidine or tizanidine are likely to produce their antispastic action primarily by inhibiting afferent transmission to motoneurons and secondarily by hyperpolarizing motoneurons, making motoneurons less likely to be activated during a muscle spasm.
Residual NA after spinal cord injury activates α2 receptors
Unlike the α1 receptor, the α2 receptor does not appear to be constitutively active after SCI because blocking possible constitutive activity with the α2 antagonist RX821002, an inverse agonist, does not affect the reflexes and associated EPSP recorded in vitro, even though this same drug readily antagonizes the action of exogenously applied α2 receptor agonists on the reflexes. However, the α2 receptor does appear to be spontaneously active in vivo, providing a tonic inhibition of reflex transmission, because the reflexes and spasms are facilitated by the α2 antagonists RX821002. We suggest that this spontaneous α2 receptor activity is due to a peripheral source of NA, which would also activate α1 receptors as discussed in the preceding text.
Interestingly, our conclusions might also explain the recent surprising finding that the α2 receptor antagonist yohimbine markedly facilitates locomotion in transected mice (Lapointe et al. 2008). That is, a peripheral source of NA may tonically inhibit locomotor activity, perhaps by inhibiting reflex transmission, as we have seen, and antagonists may remove this inhibition. In contrast, α1 receptor activation facilitates rhythmic locomotor activity (Gabbay and Lev-Tov 2004), in addition to its facilitation of motoneuron excitability.
Implications for recovery of motor function
Considering the pronounced opposing effects of α1 and α2 receptors that we have uncovered after chronic SCI, the combined functional outcomes of activity in these two receptors remains to be considered. Because the α1 receptor is both constitutively active and activated by an endogenous source of NA (or other ligand; peripherally derived), whereas α2 receptors are only activated by endogenous NA, the former α1 receptor activity is likely to dominate when there is not much endogenous NA, ultimately increasing spasms. However, the levels of endogenous NA are likely to vary, especially as it appears to be peripherally derived (perhaps of autonomic origin) and thus it is interesting to consider what the net effect of this variable peripheral NA should be. Previously we have shown that very low concentrations of exogenously applied NA can facilitate spasms (LLRs) as well as increase motoneuron firing, whereas higher concentrations tend to decrease spasms (Li et al. 2004b), suggesting that there should be a similar biphasic action of endogenous NA. Interestingly, increasing release of endogenous NA with amphetamine increases spasms even at high doses (Rank et al. 2007), but this may be because amphetamine also binds directly to α2 adrenergic receptors (with similar affinity to NA itself) (Boyajian and Leslie 1987) and thus may competitively block the action of released NA on the α2 receptors, making the α1 receptor action of NA dominate. With more natural release of peripheral NA, the net effect of high levels of NA is likely to be inhibitory, or antispastic in action, as we have discussed. This fits with our understanding of the dual action of NA receptors because regardless of how large the α1 receptor-mediated PICs, if there are no EPSPs to trigger them, because of α2 receptor activity, there will not be spasms. Thus interventions that increase endogenous NA after SCI may well have antispastic benefits, while also increasing overall motor output (PICs) and motor functions (locomotion; via α1) (Gabbay and Lev-Tov 2004). This is consistent with the positive effects of transplanting brain stem-derived cells that produce NA and 5-HT at a SCI site (Gimenez y Ribotta and Privat 1998).
GRANTS
Funding was provided by National Institute of Neurological Disorders and Stroke Grants RO1 NS-47567-01 to D. J. Bennett and RO1 NS-48170-01 to M. A. Gorassini, Canadian Foundation for Innovation, the Canadian Institutes of Health Research, and the Alberta Heritage Foundation for Medical Research. The authors acknowledge Recordati S.p.A (Milano, Italy) for the generous donation of REC15/2739.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Special thanks to L. Sanelli and J. Nevett-Duchcherer for expert technical assistance.
REFERENCES
- Adachi T, Robinson DM, Miles GB, Funk GD. Noradrenergic modulation of XII motoneuron inspiratory activity does not involve alpha2-receptor inhibition of the Ih current or presynaptic glutamate release. J Appl Physiol 98: 1297–1308, 2005 [DOI] [PubMed] [Google Scholar]
- Ashby P. Neurophysiology of spinal spasticity. In: Handbook of the Spinal Cord, edited by Davidoff RA. New York: Dekker, 1987, p. 120–143 [Google Scholar]
- Baker LL, Chandler SH. Characterization of postsynaptic potentials evoked by sural nerve stimulation in hindlimb motoneurons from acute and chronic spinal cats. Brain Res 420: 340–350, 1987 [DOI] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Handbook of Physiology. The Nervous System. Motor Control, edited by Brooks VB. Bethesda: Am. Physiol. Soc., 1981, sect. 1, vol. II, p. 509–595 [Google Scholar]
- Barbeau H, Richards CL, Bedard PJ. Action of cyproheptadine in spastic paraparetic patients. J Neurol Neurosurg Psychiatry 45: 923–926, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, Gorassini M, Fouad K, Sanelli L, Han Y, Cheng J. Spasticity in rats with sacral spinal cord injury. J Neurotrauma 16: 69–84, 1999 [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol 86: 1972–1982, 2001 [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Sanelli L, Cooke CL, Harvey PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J Neurophysiol 91: 2247–2258, 2004 [DOI] [PubMed] [Google Scholar]
- Blakely RD, De Felice LJ, Hartzell HC. Molecular physiology of norepinephrine and serotonin transporters. J Exp Biol 196: 263–281, 1994 [DOI] [PubMed] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med 16: 302–307, 2010 [DOI] [PubMed] [Google Scholar]
- Boyajian CL, Leslie FM. Pharmacological evidence for alpha-2 adrenoceptor heterogeneity: differential binding properties of [3H]rauwolscine and [3H]idazoxan in rat brain. J Pharmacol Exp Ther 241: 1092–1098, 1987 [PubMed] [Google Scholar]
- Button DC, Kalmar JM, Gardiner K, Marqueste T, Zhong H, Roy RR, Edgerton VR, Gardiner PF. Does elimination of afferent input modify the changes in rat motoneurone properties that occur following chronic spinal cord transection? J Physiol 586: 529–544, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau C, Barbeau H, Rossignol S. Effects of intrathecal alpha1- and alpha2-noradrenergic agonists and norepinephrine on locomotion in chronic spinal cats. J Neurophysiol 79: 2941–2963, 1998 [DOI] [PubMed] [Google Scholar]
- Clarke RW, Eves S, Harris J, Peachey JE, Stuart E. Interactions between cutaneous afferent inputs to a withdrawal reflex in the decerebrated rabbit and their control by descending and segmental systems. Neuroscience 112: 555–571, 2002 [DOI] [PubMed] [Google Scholar]
- Craig DA, Forray CC, Gluchowski C, Branchek TA. Use of α1A-selective adrenoreceptor agonists for the treatment of urinary incontinence; US Patent, edited by Office USPaT United States of America: Synaptic Pharmaceutical, 1997, p. 23 [Google Scholar]
- Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain 126: 495–507, 2003 [DOI] [PubMed] [Google Scholar]
- Doxey JC, Roach AG, Smith CF. Studies on RX 781094: a selective, potent and specific antagonist of alpha 2-adrenoceptors. Br J Pharmacol 78: 489–505, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott P, Wallis DI. Serotonin and L-norepinephrine as mediators of altered excitability in neonatal rat motoneurons studied in vitro. Neuroscience 47: 533–544, 1992 [DOI] [PubMed] [Google Scholar]
- Ford AP, Daniels DV, Chang DJ, Gever JR, Jasper JR, Lesnick JD, Clarke DE. Pharmacological pleiotropism of the human recombinant alpha1A-adrenoceptor: implications for alpha1-adrenoceptor classification. Br J Pharmacol 121: 1127–1135, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Manzoni D, Chan JY, Pompeiano O, Barnes CD. Locus coeruleus control of spinal motor output. Prog Brain Res 88: 395–409, 1991 [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Development of thyrotropin-releasing hormone and norepinephrine potentiation of inspiratory-related hypoglossal motoneuron discharge in neonatal and juvenile mice in vitro. J Neurophysiol 72: 2538–2541, 1994 [DOI] [PubMed] [Google Scholar]
- Gabbay H, Lev-Tov A. Alpha-1 adrenoceptor agonists generate a “fast” NMDA receptor-independent motor rhythm in the neonatal rat spinal cord. J Neurophysiol 92: 997–1010, 2004 [DOI] [PubMed] [Google Scholar]
- Gimenez y Ribotta M, Privat A. Biological interventions for spinal cord injury. Curr Opin Neurol 11: 647–654, 1998 [DOI] [PubMed] [Google Scholar]
- Giroux N, Rossignol S, Reader TA. Autoradiographic study of alpha1- and alpha2-noradrenergic and serotonin1A receptors in the spinal cord of normal and chronically transected cats. J Comp Neurol 406: 402–414, 1999 [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain 127: 2247–2258, 2004 [DOI] [PubMed] [Google Scholar]
- Hammar I, Jankowska E. Modulatory effects of alpha1-, alpha2-, and beta -receptor agonists on feline spinal interneurons with monosynaptic input from group I muscle afferents. J Neurosci 23: 332–338, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 96: 1158–1170, 2006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol 96: 1171–1186, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol 96: 1141–1157, 2006c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman S, Garraway SM, Machacek DW, Shay BL. 5-HT receptors and the neuromodulatory control of spinal cord function. In: Neurobiology of the Spinal Cord, edited by Cope T. Washington, DC: CRC, 2003, p. 47–87 [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol 405: 345–367, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Brownstone RB, Toth TI, Gossard JP. Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res 143: 77–95, 2004 [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martin-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev 40: 45–52, 2002 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I. Spinal interneurones; how can studies in animals contribute to the understanding of spinal interneuronal systems in man? Brain Res Brain Res Rev 40: 19–28, 2002 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Chojnicka B, Heden CH. Effects of monoamines on interneurons in four spinal reflex pathways from group I and/or group II muscle afferents. Eur J Neurosci 12: 701–714, 2000 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Riddell JS, Skoog B, Noga BR. Gating of transmission to motoneurones by stimuli applied in the locus coeruleus and raphe nuclei of the cat. J Physiol 461: 705–722, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper JR, Lesnick JD, Chang LK, Yamanishi SS, Chang TK, Hsu SA, Daunt DA, Bonhaus DW, Eglen RM. Ligand efficacy and potency at recombinant alpha2 adrenergic receptors: agonist-mediated [35S]GTPgammaS binding. Biochem Pharmacol 55: 1035–1043, 1998 [DOI] [PubMed] [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev 57: 183–191, 2008 [DOI] [PubMed] [Google Scholar]
- Knepper SM, Buckner SA, Brune ME, DeBernardis JF, Meyer MD, Hancock AA. A-61603, a potent alpha 1-adrenergic receptor agonist, selective for the alpha 1A receptor subtype. J Pharmacol Exp Ther 274: 97–103, 1995 [PubMed] [Google Scholar]
- Kuhn RA, Macht MB. Some manifestations of reflex activity in spinal man with particular reference to the occurrence of extensor spasm. Bull Johns Hopkins Hosp 84: 43–75, 1949 [PubMed] [Google Scholar]
- Lapointe NP, Ung RV, Rouleau P, Guertin PA. Effects of spinal alpha(2)-adrenoceptor and I(1)-imidazoline receptor activation on hindlimb movement induction in spinal cord-injured mice. J Pharmacol Exp Ther 325: 994–1006, 2008 [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic alpha1 agonist methoxamine. J Neurophysiol 81: 2164–2174, 1999 [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci 20: 6734–6740, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Bennett DJ. Apamin-sensitive calcium-activated potassium currents (SK) are activated by persistent calcium currents in rat motoneurons. J Neurophysiol 97: 3314–3330, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Murray K, Harvey PJ, Ballou EW, Bennett DJ. Serotonin facilitates a persistent calcium current in motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 97: 1236–1246, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol 90: 857–869, 2003 [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91: 767–783, 2004a [DOI] [PubMed] [Google Scholar]
- Li Y, Harvey PJ, Li X, Bennett DJ. Spastic long-lasting reflexes of the chronic spinal rat studied in vitro. J Neurophysiol 91: 2236–2246, 2004b [DOI] [PubMed] [Google Scholar]
- Lundberg A. Inhibitory control from the brainstem of transmission from primary afferents to motoneurons, primary afferent terminals and ascending pathways. In: Brain Stem Control of Spinal Mechanisms, edited by Sjolund, Bjorklund A. New York: Elsevier, 1982, p. 179–224 [Google Scholar]
- Magnusson T. Effect of chronic transection on dopamine, noradrenaline and 5-hydroxytryptamine in the rat spinal cord. Naunyn Schmiedebergs Arch Pharmacol 278: 13–22, 1973 [DOI] [PubMed] [Google Scholar]
- Maynard FM, Karunas RS, Waring WP., 3rd Epidemiology of spasticity following traumatic spinal cord injury. Arch Phys Med Rehabil 71: 566–569, 1990 [PubMed] [Google Scholar]
- McNicholas LF, Martin WR, Sloan JW, Nozaki M. Innervation of the spinal cord by sympathetic fibers. Exp Neurol 69: 383–394, 1980 [DOI] [PubMed] [Google Scholar]
- Mehrotra S, Gupta S, Centurion D, Villalon CM, Saxena PR, VandenBrink AM. A61603-induced contractions of the porcine meningeal artery are mediated by alpha1- and alpha2-adrenoceptors. Basic Clin Pharmacol Toxicol 100: 279–285, 2007 [DOI] [PubMed] [Google Scholar]
- Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods 32: 197–200, 1994 [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol 66: 355–474, 2002 [DOI] [PubMed] [Google Scholar]
- Millan MJ, Dekeyne A, Newman-Tancredi A, Cussac D, Audinot V, Milligan G, Duqueyroix D, Girardon S, Mullot J, Boutin JA, Nicolas JP, Renouard-Try A, Lacoste JM, Cordi A. S18616, a highly potent, spiroimidazoline agonist at alpha(2)-adrenoceptors: I. Receptor profile, antinociceptive and hypothermic actions in comparison with dexmedetomidine and clonidine. J Pharmacol Exp Ther 295: 1192–1205, 2000 [PubMed] [Google Scholar]
- Minneman KP, Theroux TL, Hollinger S, Han C, Esbenshade TA. Selectivity of agonists for cloned alpha 1-adrenergic receptor subtypes. Mol Pharmacol 46: 929–936, 1994 [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, Rank M, D'Amico J, Harvey PJ, Li X, Harris RL, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT(2C) receptors. Nat Med 16: 694–700, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K, Stephens MJ, Ballou EW, Anelli R, Heckman CJ, Bennett DJ. Motoneuron excitability and muscle spasms are regulated by 5-HT2B and 5-HT2C receptor activity. J Neurophysiol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance PW. A comparison of clonidine, cyproheptadine and baclofen in spastic spinal cord injured patients. J Am Paraplegia Soc 17: 150–156, 1994 [DOI] [PubMed] [Google Scholar]
- Noguera MA, Ivorra MD, D'Ocon P. Functional evidence of inverse agonism in vascular smooth muscle. Br J Pharmacol 119: 158–164, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton JA, Bennett DJ, Knash ME, Murray KC, Gorassini MA. Changes in sensory-evoked synaptic activation of motoneurons after spinal cord injury in man. Brain 131: 1478–1491, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki M, Bell JA, Martin WR. Noradrenergic action of amphetamine following degeneration of descending monoaminergic fibers in the spinal cord. Psychopharmacology 67: 25–29, 1980 [DOI] [PubMed] [Google Scholar]
- Parkis MA, Berger AJ. Clonidine reduces hyperpolarization-activated inward current (Ih) in rat hypoglossal motoneurons. Brain Res 769: 108–118, 1997 [DOI] [PubMed] [Google Scholar]
- Pauwels PJ, Tardif S, Wurch T, Colpaert FC. Facilitation of constitutive alpha(2A)-adrenoceptor activity by both single amino acid mutation (Thr(373)Lys) and g(alphao) protein coexpression: evidence for inverse agonism. J Pharmacol Exp Ther 292: 654–663, 2000 [PubMed] [Google Scholar]
- Perrier JF, Delgado-Lezama R. Synaptic release of serotonin induced by stimulation of the raphe nucleus promotes plateau potentials in spinal motoneurons of the adult turtle. J Neurosci 25: 7993–7999, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG, Horner PJ, Mullin BB, Stokes BT. A quantitative spatial analysis of the blood-spinal cord barrier. I. Permeability changes after experimental spinal contusion injury. Exp Neurol 142: 258–275, 1996 [DOI] [PubMed] [Google Scholar]
- Rank MM, Li X, Bennett DJ, Gorassini MA. Role of endogenous release of norepinephrine in muscle spasms after chronic spinal cord injury. J Neurophysiol 97: 3166–3180, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev 80: 767–852, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier O, Abuin L, Fanelli F, Leonardi A, Cotecchia S. Inverse agonism and neutral antagonism at alpha(1a)- and alpha(1b)-adrenergic receptor subtypes. Mol Pharmacol 56: 858–866, 1999 [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39: 32–41, 2001 [DOI] [PubMed] [Google Scholar]
- Roudet C, Mouchet P, Feuerstein C, Savasta M. Normal distribution of alpha 2-adrenoceptors in the rat spinal cord and its modification after noradrenergic denervation: a quantitative autoradiographic study. J Neurosci Res 39: 319–329, 1994 [DOI] [PubMed] [Google Scholar]
- Roudet C, Savasta M, Feuerstein C. Normal distribution of alpha-1-adrenoceptors in the rat spinal cord and its modification after noradrenergic denervation: a quantitative autoradiographic study. J Neurosci Res 34: 44–53, 1993 [DOI] [PubMed] [Google Scholar]
- Sakitama K. Intrathecal noradrenaline facilitates and inhibits the flexor reflex mediated by group II afferent fibers via alpha 1- and alpha 2-receptors, respectively. Jpn J Pharmacol 62: 131–136, 1993 [DOI] [PubMed] [Google Scholar]
- Sanders JD, Szot P, Weinshenker D, Happe HK, Bylund DB, Murrin LC. Analysis of brain adrenergic receptors in dopamine-beta-hydroxylase knockout mice. Brain Res 1109: 45–53, 2006 [DOI] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull 53: 689–710, 2000 [DOI] [PubMed] [Google Scholar]
- Schwinn DA, Johnston GI, Page SO, Mosley MJ, Wilson KH, Worman NP, Campbell S, Fidock MD, Furness LM, Parry-Smith DJ, Peters B, Bailey DS. Cloning and pharmacological characterization of human alpha-1 adrenergic receptors: sequence corrections and direct comparison with other species homologues. J Pharmacol Exp Ther 272: 134–142, 1995 [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol 366: 381–416, 2002 [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ, Jordan LM. Excitatory and inhibitory postsynaptic potentials in alpha-motoneurons produced during fictive locomotion by stimulation of the mesencephalic locomotor region. J Neurophysiol 53: 1345–1355, 1985 [DOI] [PubMed] [Google Scholar]
- Shibata K, Foglar R, Horie K, Obika K, Sakamoto A, Ogawa S, Tsujimoto G. KMD-3213, a novel, potent, alpha 1a-adrenoceptor-selective antagonist: characterization using recombinant human alpha 1-adrenoceptors and native tissues. Mol Pharmacol 48: 250–258, 1995 [PubMed] [Google Scholar]
- Takeoka A, Kubasak MD, Zhong H, Kaplan J, Roy RR, Phelps PE. Noradrenergic innervation of the rat spinal cord caudal to a complete spinal cord transection: effects of olfactory ensheathing glia. Exp Neurol 222: 59–69, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay LE, Bedard PJ. Action of 5-hydroxytryptamine, substance P, thyrotropin releasing hormone and clonidine on spinal neuron excitability. J Spinal Cord Med 18: 42–46, 1995 [DOI] [PubMed] [Google Scholar]
- U'Prichard DC, Greenberg DA, Snyder SH. Binding characteristics of a radiolabeled agonist and antagonist at central nervous system alpha noradrenergic receptors. Mol Pharmacol 13: 454–473, 1977 [PubMed] [Google Scholar]
- Udina E, D'Amico J, Bergquist AJ, Gorassini MA. Amphetamine increases persistent inward currents in human motoneurons estimated from paired motor unit activity. J Neurophysiol 103: 1295–1303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Furue H. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J Pharmacol Sci 101: 107–117, 2006 [DOI] [PubMed] [Google Scholar]
- Yoshio R, Taniguchi T, Itoh H, Muramatsu I. Affinity of serotonin receptor antagonists and agonists to recombinant and native alpha1-adrenoceptor subtypes. Jpn J Pharmacol 86: 189–195, 2001 [DOI] [PubMed] [Google Scholar]
- Young RR. Spasticity: a review. Neurology 44: S12–20, 1994 [PubMed] [Google Scholar]