Abstract
The tongue plays a key role in various volitional and automatic functions such as swallowing, maintenance of airway patency, and speech. Precisely how hypoglossal motor neurons, which control the tongue, receive and process their often concurrent input drives is a subject of ongoing research. We investigated common synaptic input to the hypoglossal motor nucleus by measuring the coordination of spike timing, firing rate, and oscillatory activity across motor units recorded from unilateral (i.e., within a belly) or bilateral (i.e., across both bellies) locations within the genioglossus (GG), the primary protruder muscle of the tongue. Simultaneously recorded pairs of motor units were obtained from 14 healthy adult volunteers using tungsten microelectrodes inserted percutaneously into the GG while the subjects were engaged in volitional tongue protrusion or rest breathing. Bilateral motor unit pairs showed concurrent low frequency alterations in firing rate (common drive) with no significant difference between tasks. Unilateral motor unit pairs showed significantly stronger common drive in the protrusion task compared with rest breathing, as well as higher indices of synchronous spiking (short-term synchrony). Common oscillatory input was assessed using coherence analysis and was observed in all conditions for frequencies up to ∼5 Hz. Coherence at frequencies up to ∼10 Hz was strongest in motor unit pairs recorded from the same GG belly in tongue protrusion. Taken together, our results suggest that cortical drive increases motor unit coordination within but not across GG bellies, while input drive during rest breathing is distributed uniformly to both bellies of the muscle.
INTRODUCTION
The tongue is a unique structure both with respect to its composition and its function. Its muscles are innervated by the hypoglossal nerve (cranial nerve XII) the cell bodies of which are located in the hypoglossal motor nucleus (HMN) of the caudal medulla. The HMN receives input from multiple sources, including the primary motor cortex and several brain stem central pattern generators related to critical functions such as chewing, swallowing and respiration (reviewed in Sawczuk and Mosier 2001).
Neurophysiological studies of the tongue have typically focused on its primary protruder muscle, the genioglossus (GG). The GG is composed of a right and left belly, each controlled by the ipsilateral HMN. Within each HMN, GG motor neurons receive projections from the contralateral primary motor cortex (Snell 1980) along with bilaterally distributed drive from respiration-related premotor neurons (Ezure and Tanaka 2006; Peever and Duffin 2001; Tarras-Wahlberg and Rekling 2009).
The effect that synaptic input has on the output of a motor neuron pool (i.e., firing rates, burst patterns) varies as a function of the source of the input and its distribution onto the motor nucleus. Of particular interest is how different inputs form and organize groups of motor neurons in the pool to subserve a particular behavior. One means of assessing this in vivo is to examine the correlated activities of simultaneously active single motor units under a range of conditions (e.g., Datta and Stephens 1990; De Luca and Erim 1994; De Luca et al. 1982; Farmer et al. 1993; Rosenberg et al. 1989; Sears and Stagg 1976). Thus common fluctuations in firing rates and synchronous spiking among a population of motor neurons can be used to characterize the organization of different sources of synaptic input.
In the present study, we sought to characterize the strength and temporal patterns of correlated motor unit activity within and across bellies of the GG in rest breathing and voluntary tongue protrusion. This allowed us to compare automatic, brain-stem mediated control with voluntary control from the cortex. We predicted that in the rest breathing condition, we would measure a high degree of correlated activity in motor units recorded both from within and across bellies of the GG because respiratory drive to the HMN is bilaterally distributed. Conversely, because cortical premotor neurons that control the GG project to the contralateral HMN, we predicted that tongue protrusion would increase correlated activity between motor units within but not across bellies of the GG.
METHODS
Recordings were obtained from 14 (8 male, 6 female) healthy adult volunteers [mean body mass index (BMI) = 21.9 ± 2.3 (SD), mean age = 24.4 ± 3.5 yr]. All procedures were approved by the Human Subjects Committee at the University of Arizona, and all subjects gave informed consent prior to participation.
General procedures
For each subject, the depth to the inferior border of the GG muscle was determined using ultrasonography (Pro Sound 3500, Aloka, Tokyo, Japan) (Eastwood et al. 2003). Paired single motor unit recordings were obtained in rest breathing or volitional tongue protrusion. Each motor unit pair was recorded under one experimental condition. For rest breathing, subjects lay supine in a dental chair and were given no instructions other than to rest quietly with their eyes open. No audio or visual feedback was provided in this task. For volitional tongue protrusion, subjects sat upright in the dental chair and were instructed to slowly move the tongue forward toward the teeth until motor units were recruited. In this task, subjects were provided audio and/or visual feedback from one of the recorded units and asked to maintain a steady firing rate. In both tasks recordings were sustained for ∼2 min (126 ± 55 s).
Electromyographic recordings
Single motor unit action potentials were recorded using tungsten needle electrodes (100 kΩ at 1 kHz, 1–5 μM tip diam., 250 μM shaft diam., Frederick Haer, Bowdoinham, ME) inserted percutaneously into the GG and referenced to surface electrodes on the mastoid process. A ground electrode was positioned on the right clavicle. Pairs of simultaneously recorded single motor units were obtained from one side of the GG (unilateral placement) or opposite sides of the GG (bilateral placement). Electrodes were positioned ∼1.0 cm from the midline and ∼2.0 cm from the other electrode. Electromyographic (EMG) signals were sampled at 33 kHz, amplified (20,000 times), and band-pass filtered from 0.3 to 3 kHz (Model 15 Grass Instruments, West Warwick, RI). The signals were acquired and stored using a Cambridge Electronic Design 1401 interface and Spike2 software (Cambridge Electronic Design, Cambridge UK).
Data analysis
Single motor unit action potentials were discriminated off-line in Spike2, using a template-matching algorithm based on waveform shape and amplitude. The results were checked by visual inspection and, where necessary, corrected manually. Indices of short-term synchrony and common drive were obtained for motor unit pairs using custom Spike2 scripts, and coherence analysis was carried out in Matlab 7.04 (The MathWorks, Natick, MA). For the purpose of comparison, the recordings were split into four groups according to each combination of electrode location (unilateral or bilateral) and task condition (protrusion or rest breathing). All statistical analyses were carried out in Matlab.
Details regarding the derivation and analysis of each measure of motor unit correlation are provided in the following text.
Common drive index
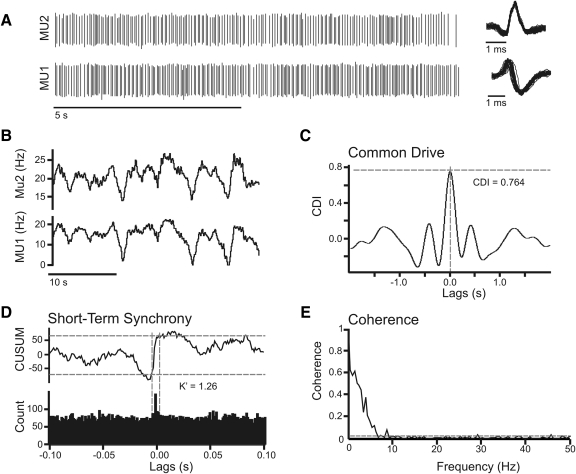
Concurrent fluctuations in the firing rates of motor units across a pool reflects a widespread common input drive that can be quantified using the method described by De Luca et al. (1982), herein referred to as the common drive index (CDI). Briefly, motor unit spike times were used to construct binary impulse trains (1 ms resolution), which were then convolved with a 400 ms Hann window and high-pass filtered (0.75 Hz) using a third order Butterworth filter. The resulting smoothed firing rate traces were used to construct a cross-correlogram from each motor unit pair with the largest correlation coefficient within ±100 ms of zero lag taken as the CDI. The CDI is therefore a number between −1 and 1 with 1 indicating perfect correlation and 0 representing no correlation between the spike firing rates. The key aspects of this calculation are presented in Fig. 1 (A–C). A depicts a short time span (10 s) of discriminated action potentials for a motor unit pair. Following conversion of spike trains to smoothed firing rate traces (Fig. 1B), it is evident that the two motor units show common fluctuations in mean firing rate. The extent to which motor unit firing rates are similarly modulated by synaptic input is reflected in the cross-correlation of their smoothed firing rate traces, calculated over the entire recording duration (Fig. 1C). The cross-correlogram shows a peak of 0.76, which is the CDI. CDI values were compared across experimental groups using a Kruskal-Wallis ANOVA with Tukey's HSD post hoc tests used for pairwise comparisons.
Fig. 1.
Evaluation of common drive, short-term synchrony, and coherence using a pair of motor units recorded during tongue protrusion. A: a 10 s epoch of discriminated motor unit activity for 2 simultaneously active genioglossus (GG) motor units. To the right of each spike train is an expanded time view showing the superimposition of 50 consecutive action potential waveforms. Smoothed firing rate traces (B) were constructed for the same motor unit pair. Smoothed firing rate traces were filtered (0.75 Hz) and used to construct a cross-correlogram (C). The common drive index (CDI) was calculated as the peak correlation coefficient within 100 ms of the 0 lag. To evaluate short-term synchrony, the spike timings were used to construct a cross-correlation histogram (D, bottom) the peak region of which is defined using the cumulative sum derivative (CUSUM) of the histogram (D, top). The ratio of spikes within the peak region (vertical lines) relative to that expected by chance (off-peak region), yields the K′ index of short-term synchrony. In the coherence analysis (E), the strength of linear correlation between the 2 signals at each frequency was calculated. The horizontal line shows the 95% confidence level for significant coherence.
Short-term synchrony
Short-term synchrony refers to the tendency of single motor units to fire simultaneously more often than expected by chance. The increased probability of synchronous firing occurs when motor neurons share a physical connection to a common presynaptic source (Datta and Stephens 1990; Sears and Stagg 1976). We quantified the magnitude of short-term synchrony by calculating the K′ index, which is a ratio expressing the number of synchronous spikes relative to the number expected by chance (Ellaway and Murthy 1985). Figure 1D demonstrates several aspects of the calculation. The spike times were used to construct a cross-correlation histogram (Fig. 1D, bottom, bin size = 1 ms, lags = ±100 ms). A peak-region (vertical dashed lines) was identified using the cumulative sum derivative (Ellaway 1978) of the cross-correlation histogram (Fig. 1D, top). The borders of the peak-region were identified as the points at which the cumulative sum derivative trace exceeded 10 and 90% of the difference between its maximum and minimum values (Schmied et al. 1993). Histogram bins falling within the peak region are taken to represent synchronous spiking. The mean and SD of the off-peak bin counts (i.e., the region outside the ±40 ms range) were used to estimate the level of synchronous firing attributable to chance (Keen and Fuglevand 2004). The K′ index was then calculated by dividing the average count in the peak region by that of the off-peak region. The average spike count in the peak region was then compared with chance using the mean and SD of off-peak bin counts (z-score). If the level of synchronous activity did not exceed chance (z-score <1.64, translating to a one-tailed P value <0.05), K′ was re-calculated using a fixed peak-region of 11 ms centered at zero lag (Semmler and Nordstrom 1995). As with the common drive analysis, K′ values were compared across experimental groups using a Kruskal-Wallis ANOVA with Tukey's HSD post hoc tests used for pairwise comparisons.
Coherence
Different synaptic inputs impinging onto a MN pool vary in regard to their frequency composition. Coherence analysis is a frequency domain technique that can reveal distinct oscillatory drives to MN pools using pairs of simultaneously recorded motor units (e.g., Farmer et al. 1993; Rosenberg et al. 1989). This method produces a coefficient between 0 and 1 that describes the correlation between the spike trains at each frequency. Here we converted raw spike times into impulse trains (sampling frequency = 1,000 Hz), and coherence was calculated with Matlab's mscohere function using un-weighted, nonoverlapping data segments 2,048 ms in length. This yielded coherence estimates at a frequency resolution of 0.49 Hz. The significance of coherence was assessed by determining the 95% confidence level for each motor unit pair according to the equation 1-0.05[1/(N-1)], where N is the number of disjoint data segments used in the analysis (Carter 1987; Rosenberg et al. 1989). Figure 1E shows the coherence analysis of the same motor unit pair as shown in A. The horizontal line marks the 95% confidence limit. This motor unit pair shows significantly correlated activity at frequencies up to ∼10 Hz.
Two methods were used to assess coherence across experimental conditions. First, the number of motor unit pairs exhibiting significant coherence at a given frequency was determined for each experimental group. Fisher's exact test was then used to compare these numbers across groups. Second, we compared coherence magnitudes. To facilitate this type of comparison, coherence values (C) were normalized using Fisher's transform [z = atanh(√C)] and weighted according to the number of segments used in determining the initial coherence estimates. A two-tailed unequal variance t-test was then used to compare the transformed coherence values across groups at every frequency ≤500 Hz. The average within-group coherence for each frequency was calculated by re-transforming the weighted mean of Fisher's z-scores.
RESULTS
A total of 91 motor unit pairs were recorded. The average single motor unit firing rate was 18.8 ± 4.3 Hz. Table 1 shows the mean ± SD of CDI and K′ values grouped according to experimental condition. Also shown in Table 1 are the numbers of motor unit pairs recorded in each location and task.
Table 1.
Common drive index (CDI) and short-term synchrony K′ measures across experimental conditions
| Task | Location | CDI | K′ | No. of pairs |
|---|---|---|---|---|
| Protrusion | Unilateral | 0.63 ± 0.22 | 1.18 ± 0.28 | 25 |
| Bilateral | 0.38 ± 0.17 | 1.06 ± 0.06 | 23 | |
| Rest breathing | Unilateral | 0.46 ± 0.19 | 1.03 ± 0.05 | 20 |
| Bilateral | 0.43 ± 0.23 | 1.01 ± 0.05 | 23 |
Unilateral recordings taken during volitional tongue protrusion have the largest common drive and short-term synchrony measures.
Common drive
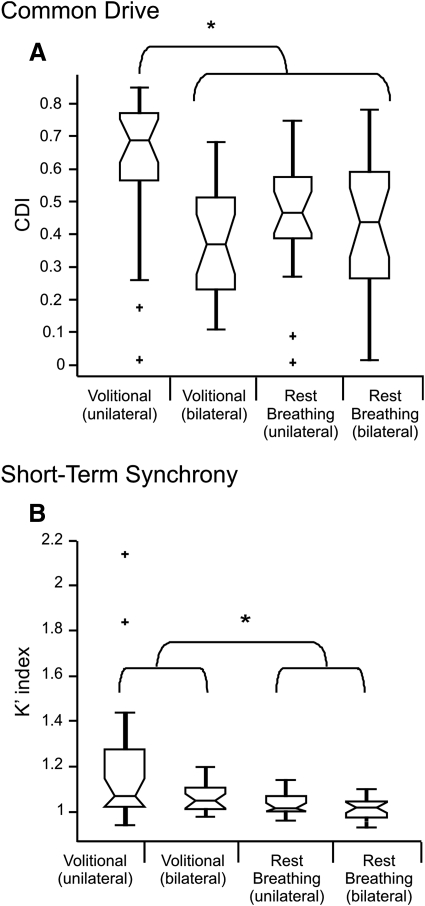
The results of the common drive analysis are summarized in Fig. 2A. The box- and- whisker plots show the median (midline), inter-quartile range (box and whiskers), and outliers (plus signs) of the CDI values calculated for motor unit pairs within each experimental condition. Common drive values did vary significantly across experimental groups (P < 0.001, Kruskal-Wallis ANOVA). More specifically, motor unit pairs recorded unilaterally in the tongue protrusion task had substantially higher levels of common drive than the other experimental conditions, which were not significantly different from each other. The influence of volitional input on common drive seems to be reflected only in motor unit pairs located within the same belly of the GG.
Fig. 2.
Differences in short-term synchrony and common drive across task conditions. A: how common drive, quantified as the CDI, varied across recording conditions. The distribution of CDI values are displayed as a box-and-whisker plots, with each plot showing the median CDI value (midline), inter-quartile range (box and whiskers), and outliers (+). Statistical comparison across groups was accomplished using a Kruskal-Wallis ANOVA with Tukey's HSD post hoc tests for pairwise comparisons. Significant differences between groups are marked with an asterisk (*). B: the results of the short-term synchrony analysis, quantified by the K′ index. The highest CDI and K′ values were measured from unilaterally located units recorded during tongue protrusion.
Short-term synchrony
Figure 2B shows box-and-whisker plots summarizing the distribution of K′ values calculated from motor unit pairs in each experimental condition. No motor unit pairs showed significant short-term synchrony in the rest breathing task, whereas seven motor unit pairs showed significant synchronization in the tongue protrusion task. All seven pairs showing significant synchronization were recorded from unilateral sites and had peak widths <10 ms. K′ values generally were small but varied significantly across groups (P = 0.007, Kruskal-Wallis ANOVA). The effect of task on short-term synchrony became most apparent when recording location was ignored and motor unit pairs recorded in protrusion were compared with those recorded in rest breathing. Tongue protrusion was associated with larger K′ values, and although the magnitude of this difference was small, it was strongly significant (P < 0.001, Kruskal-Wallis ANOVA). In fact, the K′ values in the protrusion task remained significantly larger than those of rest breathing even after all large K′ values (z > 1.64) were excluded from the data set (P = 0.017, Kruskal-Wallis ANOVA). In contrast, there was no significant difference in K′ values across recording locations (uni- vs. bilateral) when task was ignored (P = 0.205, Kruskal-Wallis ANOVA). To rule out any effects that firing rate may have had on these results, we calculated the geometric mean firing rate for each MU pair and found no difference across tasks (P = 0.06, t-test), and no correlation between firing rate and K′ values in general (Pearson's rho = −0.17, P = 0.115). Importantly, recording durations were no different for each task (P = 0.096, t-test).
Comparing common drive and short-term synchrony across all recorded motor unit pairs, we found a significant correlation between K′ and CDI values (Pearson's rho = 0.38, P < 0.001). In contrast to the short-term synchrony analysis, when CDI values were grouped by task (ignoring recording location), no significant effect of task was found (P = 0.14, Kruskal-Wallis ANOVA).
Coherence
Coherence was analyzed in two ways, first we assessed the proportion of motor unit pairs within each group that showed significant coherence, and second, we assessed coherence magnitudes directly.
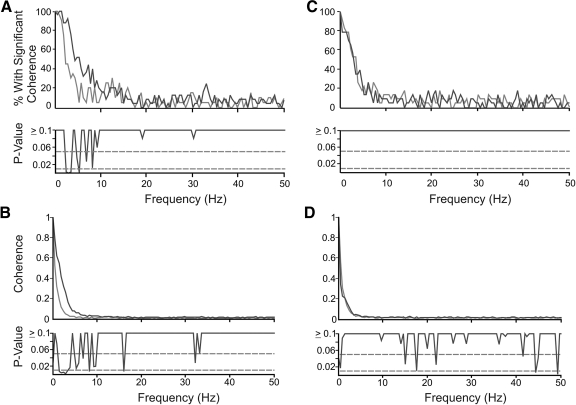
The results of the first analysis are depicted in the top two panels of Fig. 3, which show the proportion of motor unit pairs having significant coherence at each frequency. A compares unilateral motor unit pairs recorded in tongue protrusion (black) and rest breathing (gray), and C compares bilateral motor unit pairs recorded in tongue protrusion (black) and rest breathing (gray). The histograms in A and C were tested for statistical differences at each frequency using Fisher's exact test. The resulting P values are plotted for each frequency below the coherence histograms. Because frequencies above ∼50 Hz showed no relevant effects (in either type of analysis), we have displayed frequencies ≤50 Hz in Fig. 3. There was evidence of significant coherence for frequencies <2.0 Hz in the majority of recordings. At higher frequencies (up to ∼10 Hz), motor unit pairs recorded unilaterally during tongue protrusion showed significant coherence more often than any other group, all of which had similar coherence profiles to each other.
Fig. 3.
Incidence and strength of coherence. A and B: comparison of unilateral motor unit pairs recorded during tongue protrusion (black, n = 25) and rest breathing (gray, n = 20). C and D: comparison of bilateral motor unit pairs recorded during tongue protrusion (black, n = 23) and rest breathing (gray, n = 23). A and C, top: plots are coherence histograms depicting the number of motor unit pairs that showed above-chance levels of coherence (P < 0.05) at each frequency. The coherence histograms compared in A and C were tested for statistical differences at each frequency using Fisher's exact test. The resulting P values are plotted below the histograms. Horizontal dashed lines mark the P < 0.05 and P < 0.01 significance levels. The coherence profiles in B and D compare the average coherence values for motor unit pairs within each group. To compare coherence magnitudes across groups, individual coherences were normalized using Fisher's z- transform and weighted in proportion to recording duration. The resulting values were either averaged and re-transformed back to coherence (shown in B and D), or directly compared across groups using a 2-sample t-test. All conditions showed high levels of coherence at frequencies under 5 Hz in large proportions of the recordings. Both the incidence and strength of coherence at frequencies under ∼10 Hz was strongest for unilateral motor units recorded during tongue protrusion. In unilateral recordings, the most extreme difference between tongue protrusion and rest breathing was at 3 Hz for both types of analyses.
The outcome of the second analysis is shown in B and D, which compare average coherence magnitudes as a function of recording location and condition. Again results of the statistical comparison between the groups (t-test) at each frequency are plotted at the bottom of B and D. Similar to the proportion analysis, coherence at frequencies up to ∼10 Hz was strongest on average in motor unit pairs recorded unilaterally in the tongue protrusion condition. The average coherence profiles of all other groups were very similar to each other.
DISCUSSION
We explored synaptic drives associated with voluntary and involuntary control of the human tongue. The influence of synaptic drive onto a motor neuron pool can be evaluated in terms of concurrent fluctuations in motor unit firing rates, simultaneous spiking, and correlated oscillatory activity. Accordingly, we measured common drive, short-term synchrony, and coherence between simultaneously recorded GG motor units in rest breathing and tongue protrusion.
Common drive
Common drive has primarily been studied in hand muscles during isometric contractions (De Luca and Mambrito 1987; De Luca et al. 1982, 2009; Erim et al. 1999; Garland and Miles 1997; Kamen et al. 1992; Marsden et al. 1999; Negro et al. 2009; Sauvage et al. 2006; Semmler and Nordstrom 1995, 1998; Semmler et al. 1997), where typical CDI values fall between 0.4 and 0.6. Although the absolute level of force does not appear to affect common drive (De Luca et al. 1982; Erim et al. 1999), common fluctuations in mean firing rate are thought to underlie fluctuations in force output (De Luca et al. 1982; reviewed in De Luca 1985). In general, the strength of common drive declines in tasks which require fine motor control, whereas gross motor activities are associated with stronger common drive values. For example, common drive values for motor units of the first dorsal interosseus muscle are larger after cerebellar stroke (Sauvage et al. 2006) and reduced in skilled musicians (Semmler and Nordstrom 1998). Consistent with this general idea are findings that common drive is typically greater in muscles with lower spindle density, i.e., with less proprioceptive feedback (De Luca et al. 2009; Kamen et al. 1992), in fatigued muscles (Contessa et al. 2009), in trunk muscles when used for postural control (Mochizuki et al. 2006), and across synergist muscle/ motor unit pools (De Luca and Mambrito 1987; Marsden et al. 1999).
In the present study, we were able to investigate levels of common drive in a muscle which fulfills a respiratory postural role (i.e., related to airway patency) and yet is subject to a high degree of volitional control by the motor cortex. Conveniently, we were able to probe the distribution of common drive to unilateral or bilateral hypoglossal nuclei because right and left sides of the GG are controlled from the ipsilateral HMN (Snell 1980). Our findings confirmed our initial prediction that in rest breathing, the magnitude of common drive would be of equal strength for motor unit pairs recorded within and across bellies of the GG. The actual CDI values were fairly strong, comparable with what the above-cited studies recorded for finger muscles under volitional control.
Because cortical projections to GG motor units are primarily unilateral, we predicted that levels of common drive would be greater for uni- versus bilateral recording locations. Our findings confirmed this prediction. Interestingly, during tongue protrusion, CDI values differed from those recorded during rest breathing only in unilaterally recorded motor units. The lack of any detectable effect of tongue protrusion on CDI values measured from bilaterally recorded motor unit pairs could indicate that cortical premotor activity is not temporally correlated across hemispheres (at least within the frequency range measured by the CDI). An additional consideration is that cortical input to the two HMN may still be subject to slightly different local processing within each individual HMN and that such processing might de-correlate the final motor outputs.
Short-term synchrony
The extent of short-term synchronization among motor neurons is affected by the density of their premotor inputs (Lemon 1993) as well as factors that modify the overall influence of those projections on the final output of the motor neuron pool. For example, factors such as task (Adams et al. 1989; Bremner et al. 1991) or attention (Schmied et al. 2000) can affect short-term synchrony. Like common drive, short-term synchrony has been found in synergist muscle/ motor unit pairs (Powers et al. 1989); however, in studies where proprioceptive feedback or fatigue altered common drive, synchrony remained unchanged (Contessa et al. 2009; Garland and Miles 1997). Thus while common drive and short-term synchrony can be correlated (Semmler et al. 1997), they do not appear to arise from the same source (Negro et al. 2009; Semmler et al. 1997). Our observations are in agreement with these previous findings, as CDI and K′ values were moderately correlated but not necessarily predictive of one another.
We expected that in rest breathing, when the GG acts to maintain airway tone, we would observe similar levels of short-term synchrony in uni- and bilateral motor unit pairs. Our data confirmed this prediction; however, it is worth noting the complete absence of short-term synchrony in either uni- or bilateral motor unit pairs. This indicates that, of the motor neurons that are active during rest breathing, few share premotor axon branches the input of which is strong enough to evoke simultaneous spiking. The implication that individual motor neurons may respond weakly to involuntary drive is supported by previous findings that whole muscle EMG recorded from the GG reaches only ∼20–25% of its maximum inducible level when respiratory drive is high, as in exercise (Williams et al. 2000), airway obstruction (McGinley et al. 2008), and hypercapnia (Richardson and Bailey 2010). Further, a relative weakness of respiratory drive compared with direct cortical input could explain why common modulation of firing rates, as measured by the common drive index and coherence, was larger in the protrusion condition compared with rest breathing.
All motor unit pairs that showed significant levels of short-term synchrony were recorded unilaterally during the protrusion task. The fact that significant levels of short-term synchrony were only observed in unilateral motor unit pairs appears to reflect the unilateral nature of cortical projections to the HMN (Haerer 1992; Snell 1980). Ignoring recording location, motor unit pairs had slightly larger K′ values in the tongue protrusion condition than the rest breathing condition, even when all significantly large K′ values were excluded from the data set. This implies a weak bilateral effect of cortical drive on short-term synchrony in addition to the stronger unilateral effect. Aside from shared axonal input, synchronization in the activity of premotor neurons can increase the probability of simultaneous spiking (i.e., higher K′ values) between motor neurons (Kirkwood et al. 1982; McAuley et al. 1997; Murthey and Fetz 1996). We did not observe the characteristic broad peaks or fast oscillations that are associated with premotor synchronization (Kirkwood et al. 1982). Another way in which cortical drive may have slightly increased synchronous firing in bilateral motor unit pairs arises from the anatomy of HMN motor neurons themselves. Motor neurons may extend their dendrites from one HMN to the other (Altschuler et al. 1994) and therefore receive cortical input projected onto the opposite HMN. If such connections were rare or weak, their influence on short-term synchrony could remain undetectable at the level of individual motor unit pairs while still influencing group comparisons.
Coherence
We used coherence to identify oscillatory drives descending on the HMN pool (Farmer et al. 1993; Rosenberg et al. 1989). The physiological interpretation of coherence varies depending on frequency (for reviews, see Brown 2000; Grosse et al. 2002). For example, coherence in the range of 1–5 Hz describes the same phenomenon that is measured by the common drive index (Myers et al. 2004). Coherent activity at frequencies between 6 and 12 Hz may reflect a distinct type of drive (Erimaki and Christakos 1999, 2008) associated with movement (Kakuda et al. 1999; Vallbo and Wessberg 1993), feedback from muscle spindles (Erimaki and Christakos 2008), or force-tremor (Marsden et al. 2001). Finally, coherence in beta (15–30 Hz) and gamma (30–60 Hz) frequency bands is associated with short-term synchrony (Farmer et al. 1993, 1997; Halliday et al. 1999; Kilner et al. 2002; Lowery et al. 2007; Moritz et al. 2005; Semmler et al. 2002, 2004).
We found significant coherence at frequencies under ∼2 Hz in nearly all GG motor unit pairs recorded. The fact that coherence in this range did not require cortical drive is in agreement with previous findings that motor unit firing rates remain coherent at these (low) frequencies even following capsular stroke (Farmer et al. 1993). At higher frequencies covered by time-domain common drive measures (typically up to ∼5 Hz), unilateral motor unit pairs recorded during tongue protrusion showed the strongest coherence, similar to the results obtained when using CDI as the measure of common firing rate modulation. The agreement of time domain (CDI) and frequency domain (coherence) measures of common firing rate modulation suggest that this is a robust feature of GG motor unit activity and one that is particularly responsive to cortical input. Also in the tongue protrusion task, there was increased coherence in the 6–10 Hz range. In our study, subjects did not perform dynamic tongue movements, but we cannot rule out a possible association with tremor or feedback. Because activity in the 6–10 Hz range is well below the average firing rate of GG motor units (∼20 Hz), coherence in this range may simply represent an extension of lower frequency common drive rather than a distinct type of input. Coherence >10 Hz was rare, but there may have been some small task-related differences in both uni- and bilateral motor unit pairs at higher frequencies. Although simulation studies (Lowery et al. 2007; Moritz et al. 2005) and experimental findings (Farmer et al. 1993, 1997; Halliday et al. 1999; Kilner et al. 2002; Semmler et al. 2002, 2004) indicate a close connection between short-term synchronization and coherence in the beta and gamma frequency ranges, we found the K′ index to be far more sensitive as a measure of short-term synchrony than high-frequency coherence.
In addition to characterizing descending drives onto the HMN, our results provide a starting point for more detailed investigations, particularly within a volitional framework (e.g., breath control, speech vs. nonspeech movement), and in clinical populations (e.g., tremor, stuttering). Further, motor unit coordination within the right and left bellies of the GG during speech or speech-like movement may reflect the lateralization of cortical activity associated with control of speech-related muscles (e.g., Simonyan et al. 2009; Sowman et al. 2009). Respiration-related control of the HMN may be further probed by experimentally manipulating inspired gas composition or airway resistance. Finally, it would be informative to compare common modulation of GG motor units to other muscles of the tongue, which do not play a central role in airway maintenance, and the motor units of which receive bilateral input from the motor cortex.
Overall, we found that in motor unit pairs recorded from the GG, coordination of spike timing and firing rates characterize a unilateral cortical input to the HMN in tongue protrusion, whereas a bilaterally distributed common drive characterizes synaptic input to the HMN in rest breathing. These results provide a basis for further investigation of synaptic drive onto the HMN through measures of motor unit coordination and suggest that the HMN may be a particularly useful target of such study given the diversity and experimental manipulability of its inputs.
GRANTS
The project described was supported by National Institute of Neurological Disorders and Stroke Grant 009587 to E. F. Bailey.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCD or the National Institutes of Health.
ACKNOWLEDGMENTS
The authors thank Dr. Andrew Fuglevand for providing us the Spike2 script used in the short-term synchrony analysis. We also acknowledge Drs. Andrew Fuglevand, Ralph Fregosi, and Douglas Stuart for helpful input and editing of the manuscript. We are grateful to C. Walls, I. Kidder, and J. Brittain for assistance in acquisition and analysis of the data and in the preparation of the manuscript.
REFERENCES
- Adams L, Datta AK, Guz A. Synchronization of motor unit firing during different respiratory and postural tasks in human sternocleidomastoid muscle. J Physiol 413: 213–231, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler SM, Bao X, Miselis RR. Dendritic architecture of hypoglossal motoneurons projecting to extrinsic tongue musculature in the rat. J Comp Neurol 342: 538–550, 1994 [DOI] [PubMed] [Google Scholar]
- Bremner FD, Baker JR, Stephens JA. Effect of task on the degree of synchronization of intrinsic hand muscle motor units in man. J Neurophysiol 66: 2072–2083, 1991 [DOI] [PubMed] [Google Scholar]
- Brown P. Cortical drives to human muscle: the Piper and related rhythms. Prog Neurobiol 60: 97–108, 2000 [DOI] [PubMed] [Google Scholar]
- Carter G. Coherence and time delay estimation. Proc IEEE 75: 236–255, 1987 [Google Scholar]
- Contessa P, Adam A, De Luca CJ. Motor unit control and force fluctuation during fatigue. J Appl Physiol 107: 235–243, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta AK, Stephens JA. Synchronization of motor unit activity during voluntary contraction in man. J Physiol 422: 397–419, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ. Control properties of motor units. J Exp Biol 115: 125–136, 1985 [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Erim Z. Common drive of motor units in regulation of muscle force. Trends Neurosci 17: 299–305, 1994 [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Gonzalez-Cueto JA, Bonato P, Adam A. Motor unit recruitment and proprioceptive feedback decrease the common drive. J Neurophysiol 101: 1620–1628, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Control scheme governing concurrently active human motor units during voluntary contractions. J Physiol 329: 129–142, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, Mambrito B. Voluntary control of motor units in human antagonist muscles: coactivation and reciprocal activation. J Neurophysiol 58: 525–542, 1987 [DOI] [PubMed] [Google Scholar]
- Eastwood PR, Allison GT, Shepherd KL, Szollosi I, Hillman DR. Heterogeneous activity of the human genioglossus muscle assessed by multiple bipolar fine-wire electrodes. J Appl Physiol 94: 1849–1858, 2003 [DOI] [PubMed] [Google Scholar]
- Ellaway PH. Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol 45: 302–304, 1978 [DOI] [PubMed] [Google Scholar]
- Ellaway PH, Murthy KS. The source and distribution of short-term synchrony between gamma-motoneurons in the cat. Q J Exp Physiol 70: 233–247, 1985 [DOI] [PubMed] [Google Scholar]
- Erim Z, Beg MF, Burke DT, de Luca CJ. Effects of aging on motor-unit control properties. J Neurophysiol 82: 2081–2091, 1999 [DOI] [PubMed] [Google Scholar]
- Erimaki S, Christakos CN. Occurrence of widespread motor-unit firing correlations in muscle contractions: their role in the generation of tremor and time-varying voluntary force. J Neurophysiol 82: 2839–2846, 1999 [DOI] [PubMed] [Google Scholar]
- Erimaki S, Christakos CN. Coherent motor unit rhythms in the 6–10 Hz range during time-varying voluntary muscle contractions: neural mechanism and relation to rhythmical motor control. J Neurophysiol 99: 473–483, 2008 [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. Distribution and medullary projection of respiratory neurons in the dorsolateral pons of the rat. Neuroscience 141: 1011–1023, 2006 [DOI] [PubMed] [Google Scholar]
- Farmer SF, Bremner FD, Halliday DM, Rosenberg JR, Stephens JA. The frequency content of common synaptic inputs to motoneurones studied during voluntary contraction in man. J Physiol 470: 127–155, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Halliday DM, Conway BA, Stephens JA, Rosenberg JR. A review of recent applications of cross-correlation methodologies to human motor unit recording. J Neurosci Methods 74: 175–187, 1997 [DOI] [PubMed] [Google Scholar]
- Garland SJ, Miles TS. Control of motor units in human flexor digitorum profundus under different proprioceptive conditions. J Physiol 502: 693–701, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse P, Cassidy MJ, Brown P. EEG-EMG, MEG-EMG and EMG-EMG frequency analysis: physiological principles and clinical applications. Clin Neurophysiol 113: 1523–1531, 2002 [DOI] [PubMed] [Google Scholar]
- Haerer AF. The hypoglossal nerve. In: DeJong's The Neurological Examination. Philadelphia: Lippincott, 1992, p. 251–257 [Google Scholar]
- Halliday DM, Conway BA, Farmer SF, Rosenberg JR. Load-independent contributions from motor-unit synchronization to human physiological tremor. J Neurophysiol 82: 664–675, 1999 [DOI] [PubMed] [Google Scholar]
- Kakuda N, Nagaoka M, Wessberg J. Common modulation of motor unit pairs during slow wrist movement in man. J Physiol 520: 929–940, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen G, Greenstein SS, De Luca CJ. Lateral dominance and motor unit firing behavior. Brain Res 576: 165–167, 1992 [DOI] [PubMed] [Google Scholar]
- Keen DA, Fuglevand AJ. Common input to motor neurons innervating the same and different compartments of the human extensor digitorum muscle. J Neurophysiol 91: 57–62, 2004 [DOI] [PubMed] [Google Scholar]
- Kilner JM, Alonso-Alonso M, Fisher R, Lemon RN. Modulation of synchrony between single motor units during precision grip tasks in humans. J Physiol 541: 937–948, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA, Tuck DL, Westgaard RH. Variations in the time course of the synchronization of intercostal motoneurons in the cat. J Physiol 327: 105–135, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN. The G. L. Brown Prize Lecture. Cortical control of the primate hand. Exp Physiol 78: 263–301, 1993 [DOI] [PubMed] [Google Scholar]
- Lowery MM, Myers LJ, Erim Z. Coherence between motor unit discharges in response to shared neural inputs. J Neurosci Methods 163: 384–391, 2007 [DOI] [PubMed] [Google Scholar]
- Marsden JF, Brown P, Salenius S. Involvement of the sensorimotor cortex in physiological force and action tremor. Neuroreport 12: 1937–1941, 2001 [DOI] [PubMed] [Google Scholar]
- Marsden JF, Farmer SF, Halliday DM, Rosenberg JR, Brown P. The unilateral and bilateral control of motor unit pairs in the first dorsal interosseous and paraspinal muscles in man. J Physiol 521: 553–564, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley JH, Rothwell JC, Marsden CD. Frequency peaks of tremor, muscle vibration and electromyographic activity at 10 Hz, 20 Hz and 40 Hz during human finger muscle contraction may reflect rhythmicities of central neural firing. Exp Brain Res 114: 525–541, 1997 [DOI] [PubMed] [Google Scholar]
- McGinley BM, Schwartz AR, Schneider H, Kirkness JP, Smith PL, Patil SP. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol 105: 197–205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki G, Semmler JG, Ivanova TD, Garland SJ. Low-frequency common modulation of soleus motor unit discharge is enhanced during postural control in humans. Exp Brain Res 175: 584–595, 2006 [DOI] [PubMed] [Google Scholar]
- Moritz CT, Christou EA, Meyer FG, Enoka RM. Coherence at 16–32 Hz can be caused by short-term synchrony of motor units. J Neurophysiol 94: 105–118, 2005 [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Synchronization of neurons during local field potential oscillations in sensorimotor cortex of awake monkeys. J Neurophysiol 76: 3968–3982, 1996 [DOI] [PubMed] [Google Scholar]
- Myers LJ, Erim Z, Lowery MM. Time and frequency domain methods for quantifying common modulation of motor unit firing patterns. J Neuroeng Rehabil 1: 2, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negro F, Holobar A, Farina D. Fluctuations in isometric muscle force can be described by one linear projection of low-frequency components of motor unit discharge rates. J Physiol 587: 5925–5938, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peever JH, Duffin J. Bilateral synchronisation of respiratory motor output in rats: adult versus neonatal in vitro preparations. Pfluegers 442: 943–951, 2001 [DOI] [PubMed] [Google Scholar]
- Powers RK, Vanden Noven S, Rymer WZ. Evidence of shared, direct input to motoneurons supplying synergist muscles in humans. Neurosci Lett 102: 76–81, 1989 [DOI] [PubMed] [Google Scholar]
- Richardson PA, Bailey EF. Tonically discharging genioglossus motor units show no evidence of rate coding with hypercapnia. J Neurophysiol 103: 1315–1321, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs JE. Distinguishing between extrinsic and intrinsic tongue muscle weakness in unilateral hypoglossal palsy. Neurology 34: 1367–1368, 1984 [DOI] [PubMed] [Google Scholar]
- Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol 53: 1–31, 1989 [DOI] [PubMed] [Google Scholar]
- Sauvage C, Manto M, Adam A, Roark R, Jissendi P, De Luca CJ. Ordered motor-unit firing behavior in acute cerebellar stroke. J Neurophysiol 96: 2769–2774, 2006 [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Mosier KM. Neural control of tongue movement with respect to respiration and swallowing. Crit Rev Oral Biol Med 12: 18–37, 2001 [DOI] [PubMed] [Google Scholar]
- Schmied A, Ivarsson C, Fetz EE. Short-term synchronization of motor units in human extensor digitorum communis muscle: relation to contractile properties and voluntary control. Exp Brain Res 97: 159–172, 1993 [DOI] [PubMed] [Google Scholar]
- Schmied A, Pagni S, Sturm H, Vedel JP. Selective enhancement of motoneuron short-term synchrony during an attention-demanding task. Exp Brain Res 133: 377–390, 2000 [DOI] [PubMed] [Google Scholar]
- Sears TA, Stagg D. Short-term synchronization of intercostal motoneurone activity. J Physiol 263: 357–381, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmler JG, Kornatz KW, Dinenno DV, Zhou S, Enoka RM. Motor unit synchronization is enhanced during slow lengthening contractions of a hand muscle. J Physiol 545: 681–695, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmler JG, Nordstrom MA. Influence of handedness on motor unit discharge properties and force tremor. Exp Brain Res 104: 115–125, 1995 [DOI] [PubMed] [Google Scholar]
- Semmler JG, Nordstrom MA. Motor unit discharge and force tremor in skill- and strength-trained individuals. Exp Brain Res 119: 27–38, 1998 [DOI] [PubMed] [Google Scholar]
- Semmler JG, Nordstrom MA, Wallace CJ. Relationship between motor unit short-term synchronization and common drive in human first dorsal interosseous muscle. Brain Res 767: 314–320, 1997 [DOI] [PubMed] [Google Scholar]
- Semmler JG, Sale MV, Meyer FG, Nordstrom MA. Motor-unit coherence and its relation with synchrony are influenced by training. J Neurophysiol 92: 3320–3331, 2004 [DOI] [PubMed] [Google Scholar]
- Simonyan K, Ostuni J, Ludlow CL, Horwitz B. Functional but not structural networks of the human laryngeal motor cortex show left hemispheric lateralization during syllable but not breathing production. J Neurosci 29: 14912–14923, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell RS. Clinical Neuroanatomy for Medical Students. Boston: Little, Brown, 1980, p. 353–389 [Google Scholar]
- Sowman PF, Flavel SC, McShane CL, Sakuma S, Miles TS, Nordstrom MA. Asymmetric activation of motor cortex controlling human anterior digastric muscles during speech and target-directed jaw movements. J Neurophysiol 102: 159–166, 2009 [DOI] [PubMed] [Google Scholar]
- Tarras-Wahlberg S, Rekling JC. Hypoglossal motoneurons in newborn mice receive respiratory drive from both sides of the medulla. Neuroscience 161: 259–268, 2009 [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Wessberg J. Organization of motor output in slow finger movements in man. J Physiol 469: 673–691, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JS, Janssen PL, Fuller DD, Fregosi RF. Influence of posture and breathing route on neural drive to upper airway dilator muscles during exercise. J Appl Physiol 89: 590–598, 2000 [DOI] [PubMed] [Google Scholar]