Abstract
In this study we examined the electrophysiological and morphological properties of inhibitory neurons located just ventrolateral to the hypoglossal motor (XII) nucleus in the Nucleus of Roller (NR). In vitro experiments were performed on medullary slices derived from postnatal day 5 (P5) to P15 GAD67-GFP knock-in mouse pups. on cell recordings from GFP+ cells in NR in rhythmic slices revealed that these neurons are spontaneously active, although their spiking activity does not exhibit inspiratory phase modulation. Morphologically, GFP+ cells were bi- or multipolar cells with small- to medium-sized cell bodies and small dendritic trees that were often oriented parallel to the border of the XII nucleus. GFP+ cells were classified as either tonic or phasic based on their firing responses to depolarizing step current stimulation in whole cell current clamp. Tonic GFP+ cells fired a regular train of action potentials (APs) throughout the duration of the pulse and often showed rebound spikes after a hyperpolarizing step. In contrast, phasic GFP+ neurons did not fire throughout the depolarizing current step but instead fired fewer than four APs at the onset of the pulse or fired multiple APs, but only after a marked delay. Phasic cells had a significantly smaller input resistance and shorter membrane time constant than tonic GFP+ cells. In addition, phasic GFP+ cells differed from tonic cells in the shape and time course of their spike afterpotentials, the minimum firing frequency at threshold current amplitude, and the slope of their current–frequency relationship. These results suggest that GABAergic neurons in the NR are morphologically and electrophysiologically heterogeneous cells that could provide tonic inhibitory synaptic input to HMs.
INTRODUCTION
Hypoglossal motoneurons (HMs) are a diverse group of brain stem neurons that innervate the tongue muscles. The tongue is involved in various basic tasks such as sucking, mastication, swallowing, and vocalization. The tongue is also active in respiration and controls upper airway patency. HMs are thought to play a role in the pathogenesis of obstructive sleep apnea (Horner 2007). Since HMs are involved in a variety of oropharyngeal behaviors, it is not surprising that they are controlled by a host of brain stem neural networks, including inhibitory networks. The role of synaptic inhibition in generating HM output is not well understood, however. Functionally, synaptic inhibition has been shown to contribute to inspiratory but not expiratory HM membrane potential trajectories (Saywell and Feldman 2004; Withington-Wray et al. 1988; Woch and Kubin 1995). Exogenously applied γ-aminobutyric acid (GABA) or glycine depresses spike firing of HMs stimulated with intracellular current pulses (Marchetti et al. 2002). There is also a significant tonic component to the synaptic inhibition received by HMs (Paton and Richter 1995) and blockade of this tonic inhibition results in an increase in input resistance and membrane time constant in HMs (Nunez-Abades et al. 2000).
Both glycinergic and GABAergic synaptic terminals are found on the somata and dendrites of HMs (Aldes et al. 1988). The immunostaining for glutamic acid decarboxylase (GAD) is most dense in the ventromedial part of the nucleus (Aldes et al. 1988), a region that contains predominantly motoneurons that innervate the genioglossus muscle of the tongue (Krammer et al. 1979). By combining patch-clamp recordings and immunocytochemistry it was shown that HMs from the ventrolateral part of the XII nucleus have spontaneous miniature inhibitory synaptic currents with a large GABAA receptor component and HMs in this part of the nucleus show more dense labeling for GABAA receptors than HMs in other parts of the nucleus (O'Brien and Berger 2001). These findings suggest that GABA innervation of the XII nucleus is not uniform, but is strongest in the ventral part of the nucleus. The source of these inhibitory synaptic terminals is not known, but they are believed to originate from the reticular formation adjacent to the XII nucleus (Donato and Nistri 2000; Umemiya and Berger 1995) or from interneurons located within the XII nucleus itself (Takasu and Hashimoto 1988). Immunocytochemistry for GABA and its synthesizing enzyme GAD has revealed that a population of inhibitory neurons is clustered just ventral to the XII nucleus (Aldes et al. 1988) in and around the Nucleus of Roller (NR). Combined retrograde labeling and GAD immunocytochemstry indicates that inhibitory premotor neurons that innervate HMs are located in this part of the reticular formation (Li et al. 1997). Local stimulation of the NR elicits glycinergic inhibitory postsynaptic currents (IPSCs) in HMs (Hulsmann et al. 2000), but it is not known whether GABAergic IPSCs can be evoked by stimulation of the area. It has been suggested that interneurons in the NR inhibit HMs as part of the trigemino-hypoglossal reflex (Sumino and Nakamura 1974). Most HMs receive synaptic inhibition during inspiration and this inhibition is presumed to be mediated by GABAergic premotor neurons that receive excitatory inspiratory drive (Sanchez et al. 2009; Saywell and Feldman 2004). Disruption of GABA synthesis in neonatal mice leads to an irregular respiratory rhythm characterized by a short inspiratory period and a paucity of firing during the inspiratory phase in brain stem respiratory neurons (Kuwana et al. 2003). Blocking GABAA receptors in the rhythmically active rat brain stem preparation leads to an increase in inspiratory burst frequency (Bou-Flores and Berger 2001).
HM axons do not possess collaterals (Mosfeldt Laursen and Rekling 1989; Withington-Wray et al. 1988), suggesting that there is little or no feedback inhibition. The nature of the feedforward inhibitory network that controls the output of HMs to the tongue muscles is not well understood. A major drawback in studying these inhibitory networks has been the difficulty encountered in identifying these neurons in living brain tissue. The recent development of mice expressing green fluorescence protein (GFP) under the control of the endogenous GAD67 promotor has made it possible to easily identify GABAergic neurons in brain tissue (Tamamaki et al. 2003), including the respiratory brain stem (Kuwana et al. 2006).
In this study we examined the morphological and electrophysiological properties of GAD67-GFP (GFP+) interneurons located in the NR in both rhythmic and quiescent slices of the juvenile mouse brain stem. We show that GFP+ cells are tonically active in the rhythmic slice, but this activity is not modulated by ongoing inspiratory activity. Focal electrical stimulation of the NR evokes GABAergic postsynaptic currents in HMs. Furthermore, our results show that GFP+ interneurons are morphologically and electrophysiologically heterogeneous, suggesting that they play different roles in modulating the motor output of HMs.
METHODS
Experimental procedures
In vitro slice experiments were performed on medullary slices derived from heterozygous neonatal (P5 to P15) GAD67-GFP knock-in mouse pups (Tamamaki et al. 2003). Since both the GFP and the GAD67 genes in the knock-in mice are identically controlled, the parallel GFP protein expression accurately reflects the expression of GAD67 in GABA neurons (Acuna-Goycolea et al. 2005; Brown et al. 2008; Ono et al. 2005; Tamamaki et al. 2003). Newborn pups were phenotyped on postnatal days P1–P3 by examining the head of the mouse using light from a “miner's lamp” apparatus (Model GFsP-5; BLS, Budapest). With this device the brains and olfactory bulbs (as visualized through the thin skull of the newborn) of GAD67-GFP knock-in mice glowed green (Brown et al. 2008). Those whose brains and olfactory bulbs did not glow green were considered to be wild-type and were immediately killed in accordance with the regulations of the University of Washington Institutional Animal Care and Use Committee. On the experimental day GAD67-GFP knock-in mouse pups were anesthetized by exposing them to air containing isoflurane and then were rapidly killed by decapitation in accordance with the regulations of the University of Washington Institutional Animal Care and Use Committee.
Nonrhythmic medullary slice preparations
We have previously described the methods for obtaining nonrhythmic medullary slices (van Brederode and Berger 2008). Briefly, following decapitation a tissue block containing the caudal brain stem and upper cervical spinal cord were removed from the anesthetized mouse pup and submerged in ice-cold carbogen-gassed (95% O2-5% CO2) artificial cerebrospinal fluid (ACSF). The ACSF contained (in mM): 118 NaCl, 3 KCl, 1 MgCl2, 1 NaH2PO4, 25 NaHCO3, 30 d-glucose, and 1.5 CaCl2. The tissue block was placed on a cutting platform of a Vibratome (TPI, St. Louis, MO), while this block was submerged in ice-cold carbogen-gassed ACSF transverse slices (250–300 μm thick) were cut. Three to four slices were then transferred for 1 h at 37°C to an incubation chamber containing carbogen-gassed ACSF. Subsequently, slices were held at room temperature until being transferred to the perfusion-recording chamber. The perfusion-recording chamber was mounted on an upright Zeiss microscope (Axioscope), equipped with both infrared differential interference contrast (IR-DIC) optics and epifluoresence illumination. The fluorescence filter set was chosen to reveal, in the absence of infrared illumination, GFP-containing neurons (QuantaMax-Green filter set, excitation/emission 450–490/510–560 nm; Omega Optical, Brattleboro, VT).
The slices in the heated perfusion-recording chamber were bathed at 26 ± 1°C with carbogen-gassed ACSF. For whole cell patch recordings electrodes were pulled from thin-walled borosilicate glass to a resistance of 3–7 MΩ for HMs and 5–10 MΩ for interneurons. For current-clamp recordings electrodes were filled with an intracellular solution composed of (in mM): 115 K-gluconate, 25 KCl, 1 MgCl2, 9 NaCl, 10 HEPES, 3 K2-ATP, 1 Na-ATP, and 0.2 K-EGTA. The pH was adjusted to 7.3 with KOH. In some recordings biocytin (0.2 wt %) was added to this intracellular solution to allow for post hoc morphological identification of the cells. A calculated junction potential of −12 mV was subtracted from the voltage values recorded in current clamp.
The location of the hypoglossal motor nucleus (nucleus XII) was determined as described in previous publications from this laboratory (Viana et al. 1993a). The densely packed multipolar cells that together form the XII nucleus were identified under high-power optics using IR-DIC illumination and we selected for recordings large HMs that were located in the ventrolateral area of the nucleus, an area in which motoneurons that innervate the genioglossus tongue muscle are located (Krammer et al. 1979). We targeted GFP+ interneurons under epifluorescent illumination. We selected from a large group of GFP+ cells only those labeled cells that were located adjacent and just ventral and lateral to the XII nucleus, in an anatomical area called the Nucleus of Roller (NR) (Franklin and Paxinos 1997; see Fig. 1). After verifying that the targeted interneuron was GFP positive we turned off the fluorescent illumination to prevent cell damage and patched onto the cell using IR-DIC illumination. Electrodes were advanced onto visually identified neurons, using positive pressure and, after formation of a gigaohm seal, the cell membrane was ruptured by applying brief suction to the patch. Whole cell intracellular recordings were made using an Axoclamp 2B amplifier, a Digidata A/D board and Clampex software (Molecular Devices, Sunnyvale, CA). Current and voltage traces were amplified, low-pass filtered at 10 kHz, digitized at 20 kHz, and stored on hard disc. Off-line analysis was done with the help of Clampfit software (Molecular Devices) and customized procedures in conjunction with the Neuromatics software package (Jason Rothman) written for IGOR Pro (WaveMetrics). For recording of intrinsic membrane properties the ACSF contained blockers to minimize background synaptic noise. These included blockers of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)–mediated glutamatergic [6,7-dinitroquinoxaline-2,3-dione (DNQX), 10 μM], GABAergic [2-(3-carboxypropyl)-3-amino-6-methoxyphenyl-pyridazinium bromide (SR95531), 0.5 μM], and glycinergic [strychnine, 1 μM] synaptic transmission.
Fig. 1.
Nucleus of Roller (NR), located just ventrolateral to the hypoglossal motor nucleus (XII), contains a high-density of GAD67-GFP+ neurons. A1: confocal microscope image of a nonrhythmic transverse brain stem slice shows the presence in the NR of a high density of GAD67-GFP+ neurons. The arrow points to the NR, which is the large overall cluster of GAD67-GFP+ neurons just ventrolateral to XII (see also Fig. 91 in Franklin and Paxinos 1997). The XII is almost devoid of green fluorescence protein (GFP)–containing neurons. A2: higher magnification confocal microscope image of the NR from the same brain stem slice as in A1. Data in A from a postnatal day 7 (P7) mouse. B1: infrared differential interference contrast (IR-DIC) image of a nonrhythmic living transverse brain stem slice (250 μm thick) shows the tip of a patch recording electrode and the recorded GAD67-GFP+ neuron in the NR. The downward arrow points to the recorded GAD67-GFP+ neuron. B2: same image as in B1, but in fluorescence, shows that the recorded neuron in B1 is GFP positive. Data from a P5 mouse. Scale is the same as that in B1 and B2, orientation indicators shown (D, dorsal; L, lateral; M, medial; V, ventral).
In some recordings we placed a borosilicate glass capillary stimulating electrode (5- to 10-μm tip diameter) into the NR to evoke inhibitory postsynaptic currents (eIPSCs) in the recorded HMs. Stimuli (40–80 V, 0.3-ms pulse duration, 0.5 Hz) were delivered through a stimulus isolation unit (Grass Instruments), driven by Clampex software in the presence of 10 μM DNQX and 1 μM strychnine. Postsynaptic responses were recorded in voltage clamp using an Axopatch 200B amplifier (Molecular Devices) at a holding potential of −70 mV. The intracellular solution for these recordings was composed of (in mM): 140 CsCl, 1 CaCl2, 4 Cs-BAPTA, 10 HEPES, 5 Mg-ATP, and 10 QX-314. The pH was adjusted to 7.3 with CsOH.
Rhythmic medullary slice preparations
on-cell recording of unitary spike activity was made from HMs and NR GFP+ neurons in the rhythmic mouse pup (P5–P9) medullary slice preparation (Smith et al. 1991). We previously described the methods our laboratory uses for obtaining these rhythmic medullary slices (Sebe and Berger 2008; Sebe et al. 2006). In brief, we cut a single rhythmic slice (600 to 700 μm thick) from the medullary tissue block; the latter was prepared as described earlier in the case of the nonrhythmic slice preparation. Once cut the rhythmic slice was immediately placed in the recording-perfusion chamber described earlier (perfusion rate 4–6 ml/min). Over the course of 30 min extracellular K+ was raised to 8 mM in the carbogen-gassed ACSF (no synaptic blockers added) perfusing the slice. Chamber temperature was also gradually raised to 27–28°C before recording commenced. As an index of global rhythmic inspiratory-phase–related activity in the slice we recorded this activity using a perfusion fluid–filled glass electrode that had a tip diameter of 50–100 μm. This field-recording electrode was positioned on the caudal surface of the slice in a region overlying the ventral respiratory center, just ventral to the compact zone of the nucleus ambiguus, a region that is known to contain the pre-Bötzinger complex (preBötC). The preBötC generates rhythmic inspiratory activity in this slice preparation (Smith et al. 1991). In several experiments we recorded simultaneously using field recording electrodes from both the preBötC and the XII. We found that in these cases the rhythmic inspiratory activity was linked and occurred simultaneously in both regions. Rhythmic preBötC field activity was sampled at 20 kHz, amplified, and band-pass filtered (10 Hz to 2.4 kHz) using a CyberAmp 320 amplifier and pCLAMP 10 software (Molecular Devices). Both HMs and GFP+ interneurons whose on-cell spike activity was recorded were identified as described earlier. on-cell recording was made using patch recording electrodes described earlier for GFP+ interneurons, except in this case the electrode was filled with the chamber perfusion fluid. on-cell spike activity was achieved by first approaching the visually identified cell with the recording pipette under positive pressure and then when the pipette tip was in contact with the somal membrane the pipette's pressure was made slightly negative to develop a high-resistance seal. With this seal the electrode was then able to record well-isolated spikes that were sampled at 20 kHz, amplified, and band-pass filtered (10 Hz to 1.6 kHz) using a CyberAmp 320 amplifier and pCLAMP 10 software (Molecular Devices). Spikes from these recordings were detected off-line with a threshold method. Peristimulus time histograms (PSTHs) were constructed by collecting spikes in 50-ms bins for a 2-s time period coinciding with inspiratory activity recorded extracellularly in the preBötC. PSTHs were constructed from spikes collected during 15–30 cycles of inspiratory activity.
Subthreshold electrophysiological properties
Long (1-s duration) subthreshold membrane voltage responses to injected current (I) pulses were used to investigate the passive electrical properties of the neurons. Input resistance (Rn) was calculated from the steady-state voltage (Vss) response to small (maximum voltage deflection of between −5 and −10 mV) hyperpolarizing DC current pulses at rest (Vrest): Rn = Vss/I. The membrane time constant (tau) was calculated from exponential curve fits to the initial 50–100 ms of the same membrane voltage traces. Membrane potential “sag” was calculated from the ratio of the initial peak voltage (Vpeak) response and steady-state voltage response (Vss) recorded during the last 100 ms of the current pulse: sag ratio = Vpeak/Vss.
Analysis of firing properties
The neurons were classified based on their spiking pattern in response to DC current pulses. After electrical access to the cell interior was achieved and membrane potential stabilized we recorded the membrane voltage responses to a series of depolarizing and hyperpolarizing DC current steps of 1-s duration at a 10-s interval. Individual spikes in the voltage trace were detected off-line with a threshold set at a voltage 20 to 30 mV below the peak amplitude of the spike. Interspike interval (ISI) and its reciprocal instantaneous firing frequency (f) were calculated from the measured spike threshold crossings. Stimulus–response curves were created by plotting instantaneous firing frequency against current pulse amplitude (f–I curves). For each cell we measured the minimum current necessary to fire at least one action potential (AP) and this current was defined as the threshold current (1T). Subsequent current pulses were adjusted such that their amplitudes were multiples of this threshold current (2T, 3T, etc.).
The shape of single APs was determined from spikes evoked by just-suprathreshold current pulses or from rebound APs at the end of a hyperpolarizing current pulse. Action potential threshold (APth) was measured from the inflection point at the beginning of the AP (dVm/dt > 5 mV/s). Action potential height (APh) was calculated from the difference between the peak spike voltage and APth. Action potential half-width (APhw) is reported as the width of the spike at half-maximal amplitude. The spike afterhyperpolarization (AHP) was measured as the peak hyperpolarization during the initial 100 ms following a single spike relative to APth.
Morphological classification
After completing the intracellular recording protocol, and in those instances where the intracellular electrodes contained biocytin, the electrodes were carefully withdrawn from the cell. Slices were fixed overnight in 4% paraformaldehyde sandwiched between two pieces of filter paper. Slices were reacted for biocytin with the ABC Elite kit (Vectastain). The reaction product was visualized with the help of tablets of diaminobenzidine and H2O2 (Sigma fast kit). Stained tissue sections were cleared and dehydrated in a graded series of ethanol and xylene and coverslipped. About 50% of biocytin-filled GFP+ cells and all filled HMs were recovered by this procedure. All filled cells were photographed with a camera mounted on a microscope. Selected cells were reconstructed under high power (×40 or ×100 oil objective) by drawing the outline of the cells on paper with the aid of a camera lucida. We measured the long- and short-axis diameters of each cell somata and calculated the soma area from these measurements, assuming an elliptical shape of the soma. We also counted the number of primary processes coming off the soma.
Statistics
Statistical comparisons were performed using the t-test (paired comparisons) or ANOVA, with significance set at P < 0.05. If a significant difference between group means was found with an ANOVA, a post hoc Tukey test was performed to determine which groups differed from each other. Statistical correlations were performed using either linear regression techniques or using Spearman's rank correlation. Results are expressed as mean ± SE unless otherwise noted.
RESULTS
Distribution of GAD67-GFP+ neurons in the dorsal medulla
Previous studies have shown that between 88 and 97% of GAD67-GFP+ neurons in the knock-in mouse stain for GABA immunoreactivity (Ono et al. 2005; Tamamaki et al. 2003) and thus we will consider them to be GABAergic interneurons for the purpose of this study. GAD67-GFP+ neurons were distributed throughout the brain stem, but showed a dense cluster near the ventrolateral part of the XII nucleus in an area that is also known as the NR (Fig. 1). Labeled GFP+ neurons were only rarely found inside the XII nucleus. For whole cell patch-clamp recording we targeted for recording GFP+ cells in the NR close to the border with the XII nucleus under high-power IR-DIC illumination (Fig. 1). GFP fluorescence was again confirmed on establishing the whole cell recording configuration.
The quality of the biocytin filling of GFP+ cells varied considerably from cell to cell, with some cells showing only a relatively vague labeling of the soma and proximal dendrites, whereas in other cells a more complete filling of the dendritic tree and, in some cases, the axonal arborizations were achieved. Biocytin-labeling of intracellularly recorded GFP+ cells revealed two major cell types based on the shape and number of primary processes. The large majority of biocytin-labeled neurons (18 of 21 recovered GFP+ cells) had more than two primary processes emanating from their cell bodies and were classified as multipolar interneurons (Fig. 2). The remaining three GFP+ neurons had two primary processes emanating from opposite poles of their cell body and were classified as fusiform in shape. Multipolar cells had small- to medium-sized cell bodies (mean soma area = 111.2 ± 8.7 μm2, n = 18) from which originated an average of 4.8 ± 0.4 processes. All three fusiform neurons had cell bodies that were <75 μm2 in area (mean soma area = 63.7 ± 5.8 μm2). Because many of the primary processes were relatively thin it was often difficult to tell which process was a dendrite and which was an axon. However, in some well-filled cells we were able to identify axons with confidence and follow them for some distance in the slice. The terminal branches of these processes showed the characteristic small swellings on axon terminals indicative of putative synaptic contacts (Fig. 2B). In other cells the axon appeared to be cut off by the slicing procedure and the terminal end showed a characteristic “bulb,” where it left the slice (not shown). Although most of the GFP+ cells we filled were located with their cell bodies immediately adjacent to the ventrolateral border of the XII nucleus, their processes tended to avoid entering the XII nucleus itself. In about half of the GFP+ cells their processes were oriented with their axes parallel to the capsule that surrounds the XII nucleus (Fig. 2, B and D). In one such cell we were able to trace its axon to the contralateral side where it terminated in the capsule surrounding the XII nucleus on that side (Fig. 2D). Other GFP+ cells were not oriented in a particular orientation relative to the XII nucleus (Fig. 2C). In two GFP+ cells the filled axon ran toward the ventrolateral side of the brain stem slice away from the XII nucleus (Fig. 2C). In this study we also filled HMs and these cells had medium- to large-sized multipolar cell bodies (mean soma area = 292.9 ± 30.7 μm2, n = 6, P < 0.05 vs. GFP+ cells). All HMs were located in the ventrolateral part of the nucleus, in the part that innervates the genioglossus muscle (Fig. 2A). The morphology of the filled HMs in this study was similar to what has been described in previous studies for this type of cell (Nunez-Abades et al. 1994). These cells have extensive dendritic trees that branch repeatedly within the nucleus and often extend well beyond the borders of the XII nucleus into the reticular areas adjacent to the nucleus (Fig. 2A).
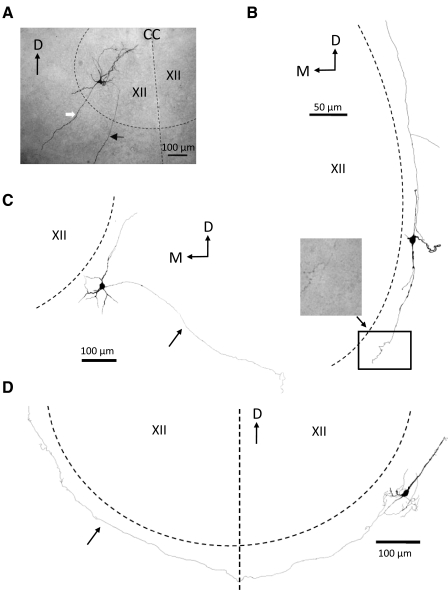
Fig. 2.
Examples of several morphological types of biocytin-filled mouse brain stem neurons examined in this study. A: photomicrograph of a HM filled with biocytin through a whole cell intracellular recording electrode (see methods). Slice (250 μm thick, nonrhythmic) was processed for biocytin with ABC-peroxidase as a whole mount. The cell body was located in the ventrolateral part of the XII nucleus (therefore likely a genioglossus motoneuron). The approximate boundary of the nucleus and the midline of the brain stem are indicated by the dashed black lines. The black arrow points toward the axon that, after leaving the nucleus, ran ventrolaterally in the slice toward the ventromedial surface of the slice. Note the absence of axon collaterals. The white arrow marks a large dendritic process that extends well beyond the boundary of the XII nucleus. B: camera lucida reconstruction of a multipolar biocytin-filled GFP+ cell located in the NR. The processes of this cell ran parallel to the boundary of the XII nucleus (dashed line marks approximate boundary location). Note the many axonal swellings—presumed synaptic contacts—in the photomicrograph of the terminal axonal arborization of this cell corresponding to the rectangular area in the drawing. C: camera lucida reconstruction of a multi-polar GFP+ cell located in the NR. Arrow points towards the axon which ran in a ventro-lateral direction in the slice away from the XII nucleus. D: camera lucida reconstruction of a multipolar GFP+ cell located in the NR. Arrow points to the cell axon which ran along the border of the XII nucleus (dashed line), crossed the midline (vertical dashed line), and ran along the border of the contralateral XII nucleus. D, dorsal; M, medial; CC, central canal; XII, hypoglossal nucleus.
Stimulation of the NR evokes inhibitory postsynaptic currents in HM neurons
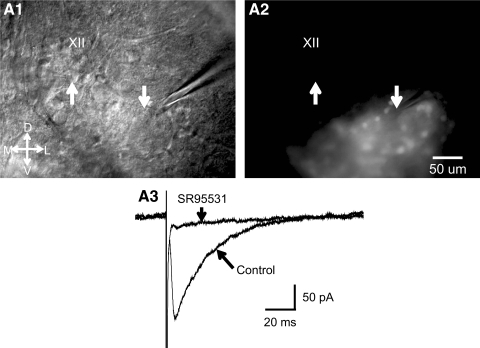
It has been known from anatomical studies that the NR contains neurons that stain for GABA (Aldes et al. 1988). Electrical stimulation of NR in neonatal mice evokes glycinergic IPSCs in HM neurons (Hulsmann et al. 2000; Umemiya and Berger 1995). When we placed a glass stimulating microelectrode into the NR in brain stem slices (two mice, P7 and P9) and applied brief current pulses we obtained reliable evoked inhibitory postsynaptic currents (eIPSCs) in HMs (n = 4 cells) in the presence of 10 μM DNQX and 1 μM strychnine (Fig. 3). These eIPSCs were abolished or severely reduced by bath application of SR95531 (0.5 μM), indicating that they were mediated chiefly by GABAA receptors. GABAergic IPSCs were also evoked in HMs by brief puffs of a high potassium solution (140 mM K+) from a glass pipette (1- to 2-μm tip diameter) placed near GFP+ cells in the NR; these responses disappeared when we moved the puffing pipette away from the stimulated cells (data not shown). These results, together with the anatomical data presented earlier, suggest that GFP+ cells inhibit HMs, perhaps through synaptic contacts onto their distal dendrites. This result is consistent with the observation in the cat that the NR contains inhibitory neurons that are premotor to HMs (Ono et al. 1998).
Fig. 3.
Electrical stimulation of the perihypoglossal region of a nonrhythmic slice containing GAD67-GFP+ neurons results in γ-aminobutyric acid type A (GABAA) receptor-mediated synaptic currents in a hypoglossal motoneuron (HM). A1: IR-DIC image of slice shows electrical stimulation electrode in the perihypoglossal area just ventrolateral to the hypoglossal motor nucleus and the simultaneously recorded HM. Tip of the stimulating electrode indicated by the downward arrow, tip of patch recording electrode, and the recorded HM indicated by the upward arrow. A2: same image as that in A1, but in fluorescence showing the cluster of GAD67-GFP+ neurons in the NR. Upward and downward arrows in same position as in A1 and A2. Scale bar same in A1 and A2, orientation indicator shown (D, dorsal; L, lateral; M, medial; V, ventral; XII, hypoglossal motor nucleus). A3: average traces of GABAA receptor-mediated evoked IPSCs before and during bath application of SR95531 (0.5 μM). Experiments were performed in the presence of DNQX (10 μM) and strychnine (1 μM). Additional blockade of GABAA receptors with SR95531 almost completely abolished the evoked response. Data shown were obtained from the stimulating and recording electrodes shown in A and the responses shown are average responses to 10 consecutive stimuli in control and during SR95531 application; data taken from a P9 mouse. DNQX, 6,7-dinitroquinoxaline-2,3-dione; IPSC, inhibitory postsynaptic current; SR95531, 2-(3-carboxypropyl)-3-amino-6-methoxyphenyl-pyridazinium bromide.
GAD67-GFP+ neurons in the NR are tonically active in the rhythmic slice
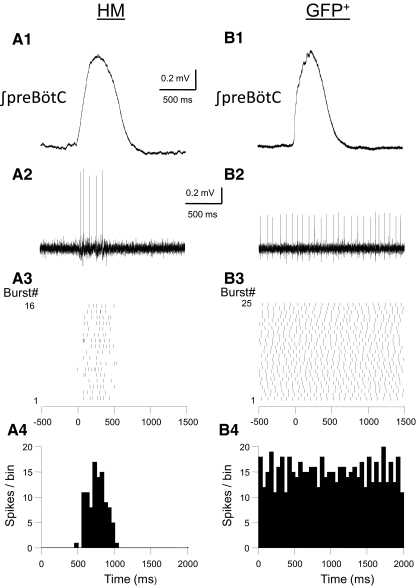
To establish the activity pattern of GFP+ cells under “in vivo-like” conditions we recorded spiking activity of GFP+ cells in the rhythmic mouse pup (P5–P9) medullary slice preparation (Sebe and Berger 2008; Smith et al. 1991; van Brederode and Berger 2008). Rhythmic activity in this slice preparation was recorded using field electrodes located in the preBötC. When extracellular K+ is raised to 8 mM these slices generate a regular respiratory-related rhythm recorded as inspiratory burst activity in the preBötC. In these experiments we did not add synaptic blockers to the bath. In a representative sample of 13 GFP+ cells in the NR we found that all recorded cells (100% of cells tested) fired spontaneously in rhythmic slices (Fig. 4). GFP+ cells fired spontaneous spikes with a mean firing frequency of 5.5 ± 1.3 Hz (range: 1.4–17.5 Hz). Spontaneous firing was highly regular with a mean coefficient of variation (CV, calculated from the ratio of the SD and the mean of the spontaneous firing rate of individual neurons) of 0.28 ± 0.05 and was maintained with little variation in mean spontaneous rate for recording periods ≤30 min (data not shown). Importantly, this ongoing tonic activity was not modulated by coincident inspiratory burst activity in the preBötC (Fig. 4B). The spontaneous firing rate of GFP+ cells in the NR was unaffected by adding a cocktail of blockers of glutamatergic (10 μM DNQX in combination with 10 μM 2-amino-5-phosphonopentanoic acid [AP-5]), GABAergic (0.5 μM SR95531), and glycinergic (1 μM strychnine) neurotransmission to the bath (n = 5 cells, P < 0.05), suggesting that autorhythmicity is an intrinsic property in these cells, independent of synaptic drive. HMs, in contrast, were generally not tonically active in this preparation, but instead showed spiking activity only during and coincident with inspiratory burst activity in the preBötC (Fig. 4A).
Fig. 4.
Examples of the spiking patterns of an HM (A) and NR GFP+ neuron (B) recorded in rhythmically active mouse brain stem slices. A1 and B1, top traces: integrated electrical activity recorded with a field electrode placed in the ventral medulla in the vicinity of the pre-Bötzinger complex (preBötC). Raw field recordings were rectified, integrated, and aligned at the start of each inspiratory burst and averaged. A2 and B2: examples of the spikes recorded simultaneously with a cell-attached whole cell recording electrode during a single inspiratory burst. GFP+ cell was tonically active at a mean rate of 12.7 Hz (with a coefficient of variation [CV] of 0.16), whereas the HM fired only during the inspiratory burst. A3 and B3: raster plots for the spiking activity during 16 (left) and 25 (right) inspiratory cycles in the 2 neurons. The start of each inspiratory burst was aligned at 0 ms. Spike time occurrences in individual bursts were measured over a time interval starting 500 ms before the start of the burst for a total duration of 2 s. A4 and B4: peristimulus time histograms calculated for the raster plots shown above. Bin width: 50 ms. Note the prominent modulation of firing rate of the HM during the burst and the absence of inspiratory-burst modulated activity in the NR GFP+ cell.
Comparison of the electrophysiological properties of GAD67-GFP+ and HM neurons
We obtained stable whole cell intracellular recordings from a total of 43 GAD67-GFP+ neurons in the NR. All whole cell intracellular recordings of intrinsic membrane properties were obtained with blockers of glutamatergic, GABAergic, and glycinergic neurotransmission present in the bath (nonrhythmic slice; see methods). We only report on GFP+ neurons that had overshooting (>0 mV) action potentials and input resistances >200 MΩ. In the nonrhythmic slice (P5–P15 mouse pups), slightly more than half of the GFP+ neurons (24 of 43) fired spontaneous spikes at rest at a rate of 5.9 ± 1.3 Hz (mean ± SE, n = 12). For the analysis of firing properties (see following text) it was necessary to inject a small amount of DC hyperpolarizing current into these cells to prevent spontaneous spiking. The amount of negative current was adjusted in this group of cells such that the membrane voltage was just below the threshold for spontaneous spiking (average hyperpolarizing injected current was −17.0 ± 2.26 pA). Mean resting membrane potential of quiescent and spontaneously active GAD67-GFP+ cells, after injection of hyperpolarizing holding current, was not significantly different between these two groups (−69.2 vs. −64.5 mV, respectively, P > 0.05). Mean input resistance (Rn) was higher in spontaneously active cells than that in quiescent GFP+ cells (973 vs. 523 MΩ, respectively, P < 0.05). In addition, action potential height and membrane time constant were not significantly different between these two groups, suggesting that spontaneous firing at rest was not due to increased leakage current resulting from cell damage or other factors leading to unhealthy or unstable cells. We believe that the spontaneous activity in GFP+ cells in the NR in the nonrhythmic slice is a reflection of the spontaneous firing that we found with on-cell recordings in the rhythmic slice as described earlier. For the purpose of analyzing the firing properties of GFP+ cells we combined the data from spontaneously active and quiescent cells. To compare GFP+ cell firing patterns in this study to those of HMs (van Brederode and Berger 2008; Viana et al. 1995) we also obtained recordings from seven HMs located in the genioglossus division of the XII nucleus. None of the recorded HMs were spontaneously active at rest in the nonrhythmic slice.
Firing patterns in response to depolarizing current steps
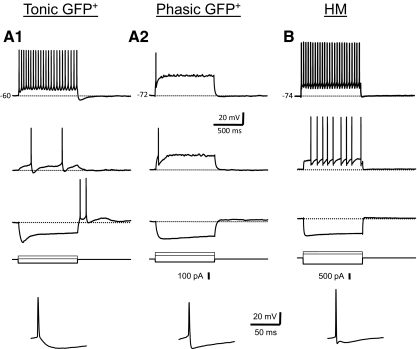
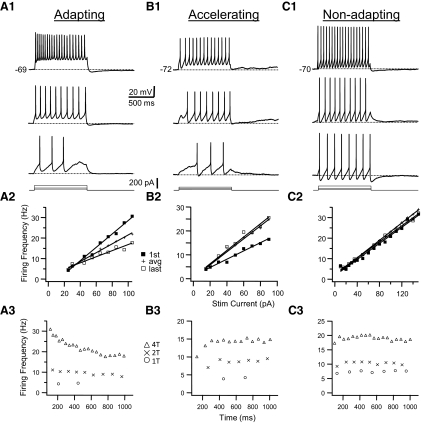
We found that GFP+ neurons in the brain stem are electrophysiologically heterogeneous. We were able to distinguish two different basic firing patterns—tonic and phasic spiking—based on the responses to long (1-s) step depolarizing current stimulation. These different firing patterns were most obvious when cells were stimulated with just-suprathreshold current pulses at resting membrane potential. Examples of these two basic firing patterns are shown in Fig. 5A. Tonic cells (n = 36) fired a regular train of action potentials in response to positive current pulses (Fig. 5A1). At just-suprathreshold current amplitude (threshold current or 1T amplitude; see methods) these cells fired multiple spikes at low frequency throughout the duration of the pulse. Tonic cells could be further subdivided into three subcategories based on their firing patterns (Fig. 6). Tonic, adapting cells (n = 17) showed a gradual lengthening of ISIs with successive spikes in the train (i.e., spike-frequency adaptation, Fig. 6A). Tonic, nonadapting cells (n = 10) showed little or no such spike-frequency adaptation; spike intervals did not vary systematically from the beginning to the end of the train (Fig. 6C). Tonic, accelerating cells (n = 9) showed a decrease in ISI (or an increase in instantaneous spike frequency) with successive spikes in the train (Fig. 6B). A minority of cells in this latter group (4 of 9) had a long first ISI and relatively long delay to the first action potential in the train at just-suprathreshold stimulus current (Fig. 6B). To quantify differences in firing types among tonic GFP+ interneurons we made frequency–time (f–t) plots based on the response of neurons to DC current stimulation. The amount of spike-frequency adaptation was dependent on the amplitude of the current pulse and membrane depolarization. To permit a classification of neurons based on f–t relationships we calculated the adaptation ratio (first spike interval frequency/last spike interval frequency) for a stimulus of twofold the threshold amplitude (2T) to elicit repetitive firing at resting membrane potential. This adaptation ratio was used to classify tonic GFP+ interneurons (Table 1). Tonic interneurons that had adaptation ratios <0.90 were classified as tonic, accelerating cells, neurons with ratios between 0.90 and 1.10 as nonadapting cells, and neurons with ratios >1.1 as adapting cells.
Fig. 5.
Examples of the electrophysiological properties of the 2 main classes of GFP+ neurons (tonic, A1; phasic, A2) and HMs (B). Shown are membrane voltage responses (resting membrane potential indicated in the top traces) to 2 depolarizing steps of different amplitude (just-suprathreshold voltage response is shown in the middle) and a hyperpolarizing current step recorded at resting membrane potential (current pulses shown below voltage traces). Tonic cells fired a regular train of action potentials (APs) in response to depolarizing current pulses (A1). These cells often showed rebound APs at the end of a hyperpolarizing current pulse (bottom voltage trace). Note the large depolarizing “sag” in the membrane potential trace in response to the hyperpolarizing current step. Single APs (bottom trace; note expanded timescale) were followed by a relatively slow single hyperpolarizing afterpotential (AHP). Phasic cells (A2) fired a single AP or only a few APs (<4) at the start of the pulse. Single APs (bottom) were followed by a fast AHP, which slowly decayed back to threshold. HMs (B) fired a regular train of APs in response to depolarizing current pulses. Single APs were usually followed by 3 afterpotentials, a fast AHP, an afterdepolarization (ADP), and a medium AHP. All the data in this and the remaining figures were obtained from nonrhythmic slices in the presence of glutamatergic, GABAergic, and glycinergic antagonists (see methods).
Fig. 6.
Examples of the firing patterns in response to step-current pulses in a tonic adapting (column A), tonic accelerating (column B), and a tonic nonadapting (column C) GFP+ interneuron. Top panel (A1–C1): for each cell membrane voltage responses to 1,000-ms-long depolarizing current pulses at 3 different amplitudes are shown. Current amplitude increases from bottom to top. The smallest current pulse that resulted in repetitive firing is the threshold current (1T). Resting membrane potential is indicated in the top trace of each panel. Middle panel (A2–C2): relationship between instantaneous firing frequency and current amplitude (f–I plot) for the cells shown in the top panel. Plotted are the firing frequency for the first spike interval in the train (first; filled squares), last interval (last; open squares), and average frequency (avg; pluses). Graphs for first, last, and average frequencies and current amplitudes were fitted with straight lines and the slope of these lines was used to quantify f–I relationships with current amplitude expressed relative to the threshold current (1T). Bottom panel (A3–C3): relationship between the instantaneous firing frequency and the time from the start of the pulse (f–t plot) for the cells shown in the top panel. For each cell the f–t plots at 3 different current amplitudes (expressed as multiples of the threshold current) are shown corresponding to the 3 traces in the top panel. Note the gradual lengthening of interspike intervals (ISIs) in the adapting cell in A. The cell in B increases its firing rate rapidly for the first few intervals and more gradually later in the train. Spike intervals remain virtually constant in the nonadapting cell in C.
Table 1.
Intrinsic membrane properties of GAD67-GFP+ interneurons and hypoglossal motoneurons
| Cell Type | n | Rn, mΩ | Tau, ms | C, pF | Sag-ratio | Vrest |
|---|---|---|---|---|---|---|
| A. Passive properties | ||||||
| Interneurons | ||||||
| Tonic, adapting | 17 | 888 ± 130 | 47 ± 5 | 62 ± 6 | 1.17 ± 0.05 | −69.0 ± 2.1 |
| Tonic, nonadapting | 10 | 788 ± 146 | 57 ± 12 | 50 ± 7 | 1.30 ± 0.09 | −69.3 ± 2.0 |
| Tonic, accelerating | 9 | 808 ± 81 | 47 ± 5 | 62 ± 7 | 1.13 ± 0.03 | −72.1 ± 2.7 |
| Phasic | 7 | 375 ± 68† | 15 ± 3† | 42 ± 6 | 1.10 ± 0.02 | −68.3 ± 2.2 |
| HMs | 7 | 57 ± 130* | 6 ± 1* | 117 ± 28* | 1.31 ± 0.08 | −75.4 ± 1.7 |
| ANOVA, P value | <0.05 | <0.05 | <0.05 | >0.05 | >0.05 | |
| Cell Type | n | APth, mV | APh, ms | APhw, mV | AHP, mV | tAHP, ms |
|---|---|---|---|---|---|---|
| B. Action potential properties | ||||||
| Interneurons | ||||||
| Tonic, adapting | 17 | −51.6 ± 2.1 | 56.0 ± 4.0 | 1.76 ± 0.14 | −20.6 ± 0.8 | 44.5 ± 5.93 |
| Tonic, nonadapting | 10 | −54.0 ± 1.4 | 58.7 ± 3.3 | 1.28 ± 0.10 | −22.3 ± 1.1 | 33.6 ± 2.7 |
| Tonic, accelerating | 9 | −50.3 ± 2.5 | 59.3 ± 5.1 | 1.15 ± 0.08 | −23.1 ± 2.1 | 28.0 ± 3.6 |
| Phasic | 7 | −52.2 ± 1.3 | 50.7 ± 2.6 | 1.39 ± 0.17 | −17.1 ± 2.0† | 11.0 ± 1.8† |
| HMs | 7 | −54.3 ± 2.2 | 62.3 ± 4.9 | 0.81 ± 0.05* | −16.2 ± 1.4* | 9.5 ± 3.1* |
| ANOVA, P value | >0.05 | >0.05 | <0.05 | <0.05 | <0.05 | |
| Cell Type | n | Adap-ratio | Ith, pA | fth, Hz | fI slope, Hz | fL slope, Hz |
|---|---|---|---|---|---|---|
| C. Firing properties | ||||||
| Interneurons | ||||||
| Tonic, adapting | 17 | 1.31 ± 0.04 | 22.5 ± 4.2 | 4.4 ± 0.5 | 9.5 ± 1.7 | 4.2 ± 0.8 |
| Tonic, nonadapting | 10 | 1.01 ± 0.02 | 11.3 ± 1.7 | 5.0 ± 0.5 | 4.1 ± 0.6 | 2.8 ± 0.5 |
| Tonic, accelerating | 9 | 0.83 ± 0.03 | 16.3 ± 3.4 | 4.9 ± 0.7 | 4.1 ± 0.3 | 3.6 ± 0.4 |
| Phasic | 7 | NA | 42.9 ± 9.2† | 7.8 ± 2.3† | 12.8 ± 3.4 | 8.0 ± 1.9 |
| HMs | 7 | 0.86 ± 0.10* | 367.9 ± 77.8* | 8.8 ± 1.2* | 15.3 ± 3.7* | 17.4 ± 3.3* |
| ANOVA, P value | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | |
Values are means ± SE. Rn, input resistance; tau, membrane time constant; C, total cell capacitance; Sag, sag-ratio calculated from: Vpeak/Vss (see methods); Vrest, resting membrane potential; APth, action potential threshold; APh, action potential height; APhw, action potential half-width; AHP, magnitude of the maximum spike afterhyperpolarization; tAHP, time to the peak spike afterhyperpolarization; Adap-ratio, frequency adaptation ratio calculated from the firing response to square current pulses (see methods); Ith, minimum or threshold current amplitude needed to elicit repetitive firing; fth, threshold frequency for repetitive firing; f1slope, slope of the relationship between the instantaneous frequency of the first spike interval in the train and the amplitude of the injected current pulse normalized by expressing current as a multiple of the threshold current (threshold current = 1); fL slope, slope of the normalized f–I relationship for the last spike interval in the train. ANOVA was performed between all five groups of cells. Significant differences between groups are discussed in the results.
Significant difference between interneurons and HMs (t-test, P < 0.05).
Significant difference between phasic and tonic interneurons (Tukey test, P < 0.05).
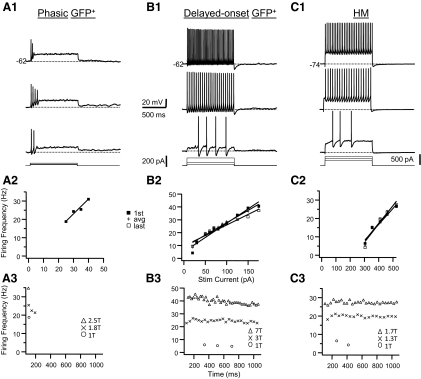
Phasic GFP+ cells (n = 7) did not fire throughout the pulse when they were stimulated with 1-s depolarizing current steps at just-suprathreshold current strength. Phasic GFP+ cells could be subdivided into cells that fired single spikes only (single spike cells; n = 2, Fig. 5A2), fired a brief burst of spikes (<4) at the beginning of the pulse (single burst cells; n = 2, Fig. 7A), or fired multiple spikes after a long delay to the first spike in the train (delayed onset cells; n = 3, Fig. 7B). Spontaneous oscillations of the membrane potential near spike threshold were common in these cells (Fig. 7, A and B). The firing characteristics of phasic GFP+ cells were dependent on stimulus strength and membrane depolarization. For instance, delayed onset were able to fire throughout the pulse after only a short delay when the stimulus amplitude was increased beyond threshold current (Fig. 7B) and single spike cells could fire more than one spike when they were depolarized to just below spike threshold (data not shown). Delayed onset cells often showed irregular firing (data not shown). Other studies have found that these subtypes of interneurons (single spike, single burst, or delayed onset) constitute separate cell classes (Prescott and De Koninck 2002), although we found too few of these subtypes to classify them into separate categories and, for the purpose of this study, they were lumped together in one group that we term phasic cells based on the distinction that they do not fire a regular train of spikes throughout the pulse. Furthermore, classification of these particular cell types was complicated by the observation that these firing patterns were also the most sensitive to changes in membrane potential and stimulus current.
Fig. 7.
Examples of the firing patterns in response to step current pulses for a phasic interneuron (A), a delayed onset interneuron (B), and an HM (C). Top panel (A1–C1): for each cell membrane voltage responses to 1,000-ms-long depolarizing current pulses at 3 different amplitudes are shown. Current amplitude increases from bottom to top. The smallest current pulse that resulted in repetitive firing is the threshold current (1T). Resting membrane potential is indicated in the top trace of each panel. Middle panel (A2–C2): relationship between instantaneous firing frequency and current amplitude (f–I plot) for the cells shown in the top panel. Plotted are the firing frequency for the first spike interval in the train (first; filled squares), last interval (last; open squares), and average frequency (avg; pluses). For the phasic cell only the first spike interval frequency is shown. Graphs for first, last, and average frequencies and current amplitudes were fitted with straight lines. Bottom panel (A3–C3): relationship between the instantaneous firing frequency and the time from the start of the pulse (f–t plot) for the cells shown in the top panel. For each cell the f–t plots at 3 different current amplitudes (expressed as multiples of the threshold current) are shown corresponding to the 3 traces in the top panel. Note the long delay to the first spike in the train at 1T current amplitude for the delayed onset cell and the HM cell. These 2 cell types also showed relatively little spike frequency adaptation.
HMs (n = 7) all fired a regular train of action potentials in response to depolarizing current pulses (Figs. 5B and 7C), similar to what has been described in HMs in the rodent brain stem in previous studies (Nunez-Abades et al. 1993; van Brederode and Berger 2008; Viana et al. 1995). HMs in this study showed either little or no frequency adaptation or frequency acceleration due to a long first ISI. Previous studies from our laboratory have shown that the majority of HMs in rats show frequency adaptation during the first postnatal week, whereas an accelerating firing pattern is more common during the second postnatal week (Viana et al. 1995). Consistent with this observation in rats we found that HMs that showed acceleration were recorded in slightly older mice (n = 3 neurons, mean age = 11.3 days) than HMs that showed adaptation (n = 4 neurons, mean age = 8.7 days). Three HMs showed a long delay to the first spike at just-suprathreshold current (Fig. 7C1). f–I curves for the first and last intervals in the spike trains of HMs were linear (Fig. 7C2), as previously described for genioglossus HMs in earlier studies (Nunez-Abades et al. 1993; Viana et al. 1995).
Passive properties of the four groups of interneurons and the group of HMs are given in Table 1. There was no significant animal age difference between different types of tonic GFP+ cells (ANOVA, P > 0.05) or between tonic and phasic GFP+ cells (t-test, P > 0.05), but HM recordings were obtained from slightly older animals than recordings from GFP+ cells (mean postnatal ages 10.1 vs. 7.2 days, respectively).
Neurons in all cell groups showed a variable amount of depolarizing sag in response to hyperpolarizing current pulses (Fig. 5, Table 1). Sag ratios, calculated from the membrane voltage response to 20 to 30 mV hyperpolarizing current pulses (see methods), were not significantly different among neuron groups. Sag ratio in GFP+ interneurons was not correlated with animal age (correlation coefficient r = 0.06, P > 0.05). Steady-state input resistance (Rn) was significantly larger in interneurons than that in HMs (t-test, P < 0.05, see Table 1). Phasic cells had a lower Rn than that in the other types of interneurons (Tukey test, P < 0.05). Whole cell membrane time constant (tau) was significantly longer in interneurons than that in HMs (t-test, P < 0.05, see Table 1). Phasic cells had a shorter tau than that of the other types of interneurons (P < 0.05) and their tau was not significantly different from the tau of HMs (Tukey test, P > 0.05). Whole cell capacitance (C) was substantially larger in HMs than that in interneurons (t-test, P < 0.05), which is a reflection of their much larger cell size. Whole cell capacitance was not different among different types of GFP+ interneurons (Table 1; ANOVA, P > 0.05).
In addition to differences in firing patterns and passive membrane properties we found that the subclasses of GFP+ cells and HMs differed based on the shape and time course of single action potentials (Table 1). Action potential threshold and peak height were not different between HMs and interneurons or among interneurons, but spike half-width was shorter in HMs than that in interneurons (Table 1; t-test and ANOVA, P < 0.05). Tonic, adapting neurons had significantly wider spikes than those of the other groups of GFP+ interneurons (Table 1; Tukey test, P < 0.05). In addition to differences in spike shape we found that neurons in each class could be distinguished based on the shape and time course of their spike afterpotentials (Fig. 5, Table 1). In all phasic GFP+ cells (n = 7) spikes were followed by a single fast afterhyperpolarization (AHP) that decayed gradually back to the threshold potential (Fig. 5A2). In the majority of tonic GFP+ cells spikes were followed by a single gradual medium duration AHP (Fig. 5A1; seen in 21 of 36 cells). Spikes in the other tonic GFP+ cells were characterized by afterpotentials composed of an afterdepolarization (ADP; sometimes visible only as a slight “hump” in the membrane trajectory following a spike) followed by an AHP (n = 14, data not shown), whereas in one tonic cell spike afterpotentials were triphasic consisting of fAHP, ADP, and mAHP (data not shown; see Schwindt et al. 1988). The peak value of the AHP was larger and the time to the peak of the AHP (tAHP) was slower in tonic interneurons than that in phasic interneurons or HMs (Table 1; Tukey test, P < 0.05). Triphasic afterpotentials (fAHP, ADP, and mAHP) were common (5 of 7 cells) in HMs (Fig. 5B; see also Viana et al. 1993b).
When we measured the lowest firing frequency in response to the smallest long depolarizing current pulse that resulted in repetitive firing (threshold frequency or fth) we found lower values for fth in tonic cells than in phasic cells or HMs (Table 1, Tukey test, P < 0.05). We plotted the value of the instantaneous firing frequency for the first interval (f1) and the last interval (fL) in the train against current amplitude (expressed as multiple of the threshold current or 1T; see methods) to construct f–I relationships. For small to moderate current amplitudes these f–I relationships were linear or nearly so (Figs. 6 and 7). The slope of the f1–I relationships (which presumably reflects the nonadapted state of the cell) was steeper in tonic adapting neurons than in tonic nonadapting or accelerating cells (Table 1; Tukey test, P < 0.05). In the adapted or steady state there was no difference between the slopes of the fL–I relationship between the three classes of tonic cells (Tukey test, P > 0.05). The slope of the f1–I relationship in phasic cells was steeper than that in tonic cells, but this difference was significant only versus tonic nonadapting and accelerating cells (Table 1, Tukey test, P < 0.05). The slopes of the f1–I and fL–I relationships were the steepest in HMs and significantly larger in HMs than in tonic interneurons (but not different from phasic interneurons). Beyond current strengths of 2T most tonic nonadapting cells began to show mild spike-frequency adaptation, especially in the first few intervals, and this is reflected in the smaller slope of the fL–I slope compared with the f1–I slope (Table 1, t-test, P < 0.05). In HMs the f1–I and fL–I relationships did not differ (t-test, P > 0.05), reflecting the lack of adaptation even at relatively high stimulus strengths in these cells. In tonic cells that had a spike ADP the f1–I relationships deviated from linear at high current strengths when these cells started to fire initial spike “doublets” (data not shown).
DISCUSSION
Summary of results
In this study we describe the morphological and electrophysiological properties of a population of GABAergic interneurons in the NR. We show that stimulation of this area in the brain stem evokes GABAergic postsynaptic responses in HMs in the adjacent XII nucleus. A large proportion of GFP+ interneurons in the NR were spontaneously active both in cell-attached and whole cell recordings, suggesting that these cells possess intrinsic spontaneous activity. Whole cell intracellular recording and filling of GFP+ interneurons indicate that they are electrophysiologically and morphologically heterogeneous.
Morphology of GFP+ cells
We found a dense cluster of GAD67-positive, small- to medium-sized cells located in the mouse NR, similar to what has been described in the rat NR (Tanaka et al. 2003). The somadendritic morphology of GFP+ interneurons in the NR was quite variable, but we found that the axonal and dendritic trees of these cells were often restricted to a narrow band surrounding the ventrolateral side of the XII nucleus. In addition, the main orientation of the cell processes often ran parallel to the border of the XII nucleus. The functional consequences of this particular anatomical arrangement remain to be established. The axonal anatomy of filled GFP+ cells in this study suggests that synaptic contacts are made with other neuronal structures in a narrow band surrounding the ventrolateral part of the XII nucleus on both the ipsilateral side and the contralateral side. Axonal branching patterns suggest that GFP+ cells in the NR make contacts with structures close to their own cell bodies and with remote structures. Our study did not identify those targets, nor did we establish whether the axonal boutons that we observed were actual synaptic contacts. However, if at least some of these boutons are synaptic connections between GFP+ cells and HMs, then this implies that these synapses are located on HM dendrites that extend beyond the boundaries of the XII nucleus. Dendrites that extend well beyond the border of the XII nucleus and into the lateral reticular formation have been described in the rodent brain stem (Altschuler et al. 1994; Nunez-Abades et al. 1994; Tarras-Wahlberg and Rekling 2009) and we confirm this observation. These distal dendrites are thus a possible location for synaptic contacts between the axon terminals of GFP+ cells in the NR and HMs.
We saw no filled interneuron axons enter the XII nucleus, consistent with a study in the monkey that found that no GABA-positive axonal pathways from outside the XII nucleus entered the nucleus itself; instead, the inhibitory synaptic terminals on HMs originated from GABAergic interneurons located inside the XII nucleus (Takasu et al. 1987). Rarely did we observe GAD67-GFP+ neurons with cell bodies located inside the XII nucleus, suggesting that there might be two inhibitory circuits impinging on HMs, one originating from inside the nucleus and the other from perihypoglossal regions in the reticular formation, including the NR.
Firing types of GFP+ cells and functional consequences for inhibitory circuitry
GAD67-GFP+ neurons in the NR in this study share electrophysiological and morphological characteristics with GAD67-GFP+ neurons located in the preBötC (Kuwana et al. 2006) and the inferior colliculus (Ono et al. 2005). A subpopulation of GAD67-GFP+ cells in the preBötC shows spontaneous tonic spiking (Kuwana et al. 2006), similar to what we find in the NR. GAD67-GFP+ cells in the inferior colliculus and preBötC include regular spiking cells with mild frequency adaptation, similar to our tonic adapting cells. In addition, Ono et al. (2005) also found GAD67-GFP+ cells with a long delay to the first spike (i.e., delayed onset cells), GFP+ cells with a long first spike interval combined with frequency acceleration, and phasic cells similar to these cell types described in our study. Similar firing types are also found in spinal cord interneurons (Prescott and De Koninck 2002), suggesting that certain circuit dynamics are similar across different brain stem and spinal cord regions and that they require similar types of interneurons. In the spinal cord these different firing types are associated with distinct morphological properties (Prescott and De Koninck 2002), but in our study we did not find such a correlation. This was perhaps attributable to the low percentage of phasic cells that we encountered in our study. Tonic inhibitory cells in the spinal cord express a persistent sodium current (INaP) and a persistent calcium current (ICaP). The intrinsic properties make these cells optimally suited to encode stimulus amplitude and function as integrators of synaptic input (Prescott and De Koninck 2002, 2005). The intrinsic properties of phasic cells in contrast were such that they functioned optimally as coincidence detectors (Prescott and De Koninck 2002). In the companion paper we show that a similar distinction can be applied to tonic and phasic GABAergic interneurons in the NR based on their responses to time-varying stimuli (van Brederode and Berger 2011).
The firing properties of GAD67-GFP+ interneurons in the NR will influence how they encode synaptic input and what type of stimulus is optimally suited to excite them. For instance, single spike cells and cells that fire only a few spikes at the beginning of the stimulus stopped firing well before the end of the current pulse and thus prefer short, nonsummating synaptic inputs. Single spike cells are also unable to encode stimulus intensity by changing their spike frequency. Tonic cells can encode stimulus intensity by linearly varying their firing frequency, depending on input amplitude and their ability to fire trains of action potentials allows them to integrate synaptic inputs over long time periods. If one combines these different firing patterns with the observed differences in threshold frequency and membrane time constant among GFP+ interneurons in the NR, it is not difficult to imagine that one can selectively activate a subgroup of interneurons in the NR based on the temporal pattern and intensity of the stimulus. It remains to be determined whether the different NR inhibitory interneuron cell types are involved in encoding different types of oropharyngeal behaviors and whether their input–output properties are matched to the specific task that each plays in these behaviors.
A subclass of GFP+ cells and a majority of HMs in this study showed spike-frequency acceleration in response to DC current step. This property has been described previously in HMs (Viana et al. 1995) and in neocortical pyramidal cells (Miller et al. 2008; Spain et al. 1991). In neocortical pyramidal cells spike-frequency acceleration is mediated by an ID-like potassium current that is sensitive to blockade by dendrotoxin-I and is associated with Kv1 subunit expression (Miller et al. 2008). Spike-frequency acceleration in neocortical pyramidal cells was accompanied by a relatively long delay to the first spike in the train and we found a similar association between the two properties in the group of tonic GFP+ cells that showed spike-frequency acceleration. The function of spike-frequency acceleration is not known, but it might enhance the gain and/or synchronization of neurons that express this property (Miller et al. 2008).
Tonic activity and its consequences
We showed that GFP+ neurons in the NR display spontaneous tonic firing that is not modulated by inspiratory synaptic drive in the rhythmically active in vitro slice preparation. This tonic spiking activity of GFP+ neurons was not dependent on synaptic drive and was confirmed in intracellular recordings in the nonrhythmic slice where fast synaptic transmission was blocked, suggesting that it is due to genuine autorhythmicity. We have not investigated the ionic mechanisms behind autorhythmicity in GFP+ NR neurons in this study. Autorhythmicity in other types of neurons is mediated by a combination of pacemaker currents responsible for interspike membrane depolarization and a “reset” mechanism composed of spike afterhyperpolarization mediated by K+ currents that are activated during the spike (Bevan and Wilson 1999; Forti et al. 2006). The tonic firing of GFP+ NR cells could provide a tonic inhibitory synaptic input to HMs, resulting in a sustained increase in their input conductance. This will lower the excitability of HMs and could alter their frequency tuning properties (van Brederode and Berger 2008). It has been shown that, in contrast to glycinergic currents, the frequency of GABAergic spontaneous synaptic currents in HMs is dramatically reduced by bath application of tetrodotoxin, suggesting that GABAergic interneurons that innervate HMs are spontaneously active and provide a network-driven source of inhibitory synaptic input to these cells (Donato and Nistri 2000). Spontaneously firing GAD67-GFP+ cells in the NR could be the source of this tonic inhibitory synaptic drive to HMs.
There is evidence that tonic inhibition plays a role in shaping the response properties of HMs. In vitro experiments in the rhythmically active mouse medullary slice preparation have demonstrated that blockade of GABAA-mediated synaptic inhibition with bicuculline increases the amplitude and duration of synaptic drive potentials recorded intracellularly in HMs (Paton and Richter 1995). Furthermore, bicuculline application leads to the appearance of slow (0.8–1.7 Hz) rhythmic oscillations of the membrane potential superimposed on a sustained respiratory-related depolarization of the membrane potential (Paton and Richter 1995). Also, blockade of GABAA receptors reduces the power of fast synchronous oscillations recorded in the XII nerve in this preparation (Sebe et al. 2006). It has been suggested that the effect of GABAA-mediated synaptic inhibition on HM firing is mediated through a tonic GABA conductance (Paton and Richter 1995), but others have seen mainly a phasic type of inhibition during inspiratory activity in the rhythmic slice (Saywell and Feldman 2004). Electrical stimulation of the lateral reticular formation adjacent to the XII nucleus decreases firing probability through a shunting type of inhibition mediated by GABA and glycine (Marchetti et al. 2002). This type of inhibition functions to reduce the excitability of the postsynaptic cells either by increasing the threshold for excitation and/or decreasing the gain of the postsynaptic neuron (reviewed by Semyanov et al. 2004). In support of this theory recent in vivo studies have shown that locally applied bicuculline increases the gain of the HM input–output relationship (Sanchez et al. 2009).
Functional significance
One of the roles of the genioglossus muscle is to maintain airway patency. There is increasing evidence that a disruption of reflex pathways involving the genioglossus muscle contributes significantly to obstructive sleep apnea (Wheatley et al. 1993). In vivo studies have shown that hypoglossal motoneurons are tonically inhibited by GABA and that antagonists of this inhibitory neurotransmitter increase the level of spiking activity in HMs (Liu et al. 2003; Morrison et al. 2003), but this was not observed for glycine receptor antagonists (Morrison et al. 2002). However, despite this powerful tonic synaptic inhibition no role of GABAergic inhibition was found in the suppression of genioglossal activity during rapid eye movement (REM) sleep (Morrison et al. 2003), but others have found that during cholinergically induced REM sleep a powerful glycinergic inhibitory premotor system suppresses hypoglossal motoneurons (Yamuy et al. 1999). Negative pressure in the upper airway causes a reflex increase in genioglossus activity, which is likely caused by a release from tonic inhibition of HMs mediated by inhibitory premotoneurons in the reticular formation (Chamberlin et al. 2007). Thus the regulation of the tonic activity of inhibitory premotoneurons could have important consequences for the reflex regulation of airway patency. Inhibitory premotoneurons are located in the NR (Sumino and Nakamura 1974). Modulation of the ionic currents underlying autorhythmicity in GFP+ interneurons could influence the amount of tonic inhibition that HMs receive and put their integrative properties under the control of a host of neuromodulator substances found in the brain stem that can affect ionic conductances.
In conclusion, our study suggests that GABAergic interneurons in the NR are a source of tonic inhibitory synaptic input to HMs. The heterogeneity of firing and morphological properties of these cells would suggest that they perform different tasks. What those tasks are and whether they are active during different behaviors remain to be established.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-49657 to A. J. Berger and H. van Brederode.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank J. Numata for expert technical assistance, help with data analysis, and drawing biocytin-filled cells and L.-S. Burton for drawing biocytin-filled cells.
REFERENCES
- Acuna-Goycolea C, Tamamaki N, Yanagawa Y, Obata K, van den Pol AN. Mechanisms of neuropeptide Y, peptide YY, and pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus. J Neurosci 25: 7406–7419, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldes LD, Chronister RB, Marco LA. Distribution of glutamic acid decarboxylase and gamma-aminobutyric acid in the hypoglossal nucleus in the rat. J Neurosci Res 19: 343–348, 1988 [DOI] [PubMed] [Google Scholar]
- Altschuler SM, Bao X, Miselis RR. Dendritic architecture of hypoglossal motoneurons projecting to extrinsic tongue musculature in the rat. J Comp Neurol 342: 538–550, 1994 [DOI] [PubMed] [Google Scholar]
- Bevan MD, Wilson CJ. Mechanisms underlying spontaneous oscillation and rhythmic firing in rat subthalamic neurons. J Neurosci 19: 7617–7628, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou-Flores C, Berger AJ. Gap junctions and inhibitory synapses modulate inspiratory motoneuron synchronization. J Neurophysiol 85: 1543–1551, 2001 [DOI] [PubMed] [Google Scholar]
- Brown RE, McKenna JT, Winston S, Basheer R, Yanagawa Y, Thakkar MM, McCarley RW. Characterization of GABAergic neurons in rapid-eye-movement sleep controlling regions of the brainstem reticular formation in GAD67-green fluorescent protein knock-in mice. Eur J Neurosci 27: 352–363, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol 579: 515–526, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato R, Nistri A. Relative contribution by GABA or glycine to Cl−-mediated synaptic transmission on rat hypoglossal motoneurons in vitro. J Neurophysiol 84: 2715–2724, 2000 [DOI] [PubMed] [Google Scholar]
- Forti L, Cesana E, Mapelli J, D'Angelo E. Ionic mechanisms of autorhythmic firing in rat cerebellar Golgi cells. J Physiol 574: 711–729, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press, 1997 [Google Scholar]
- Horner RL. Respiratory motor activity: influence of neuromodulators and implications for sleep disordered breathing. Can J Physiol Pharmacol 85: 155–165, 2007 [DOI] [PubMed] [Google Scholar]
- Hulsmann S, Oku Y, Zhang W, Richter DW. Metabotropic glutamate receptors and blockade of glial Krebs cycle depress glycinergic synaptic currents of mouse hypoglossal motoneurons. Eur J Neurosci 12: 239–246, 2000 [DOI] [PubMed] [Google Scholar]
- Krammer EB, Rath T, Lischka MF. Somatotopic organization of the hypoglossal nucleus: a HRP study in the rat. Brain Res 170: 533–537, 1979 [DOI] [PubMed] [Google Scholar]
- Kuwana S, Okada Y, Sugawara Y, Tsunekawa N, Obata K. Disturbance of neural respiratory control in neonatal mice lacking GABA synthesizing enzyme 67-kDa isoform of glutamic acid decarboxylase. Neuroscience 120: 861–870, 2003 [DOI] [PubMed] [Google Scholar]
- Kuwana S, Tsunekawa N, Yanagawa Y, Okada Y, Kuribayashi J, Obata K. Electrophysiological and morphological characteristics of GABAergic respiratory neurons in the mouse pre-Bötzinger complex. Eur J Neurosci 23: 667–674, 2006 [DOI] [PubMed] [Google Scholar]
- Li YQ, Takada M, Kaneko T, Mizuno N. Distribution of GABAergic and glycinergic premotor neurons projecting to the facial and hypoglossal nuclei in the rat. J Comp Neurol 378: 283–294, 1997 [DOI] [PubMed] [Google Scholar]
- Liu X, Sood S, Liu H, Nolan P, Morrison JL, Horner RL. Suppression of genioglossus muscle tone and activity during reflex hypercapnic stimulation by GABA(A) mechanisms at the hypoglossal motor nucleus in vivo. Neuroscience 116: 249–259, 2003 [DOI] [PubMed] [Google Scholar]
- Marchetti C, Pagnotta S, Donato R, Nistri A. Inhibition of spinal or hypoglossal motoneurons of the newborn rat by glycine or GABA. Eur J Neurosci 15: 975–983, 2002 [DOI] [PubMed] [Google Scholar]
- Miller MN, Okaty BW, Nelson SB. Region-specific spike-frequency acceleration in layer 5 pyramidal neurons mediated by Kv1 subunits. J Neurosci 28: 13716–13726, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu H, Park E, Nolan P, Horner RL. GABAA receptor antagonism at the hypoglossal motor nucleus increases genioglossus muscle activity in NREM but not REM sleep. J Physiol 548: 569–583, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu X, Liu H, Park E, Nolan P, Horner RL. Glycine at hypoglossal motor nucleus: genioglossus activity, CO(2) responses, and the additive effects of GABA. J Appl Physiol 93: 1786–1796, 2002 [DOI] [PubMed] [Google Scholar]
- Mosfeldt Laursen A, Rekling JC. Electrophysiological properties of hypoglossal motoneurons of guinea-pigs studied in vitro. Neuroscience 30: 619–637, 1989 [DOI] [PubMed] [Google Scholar]
- Nunez-Abades PA, He F, Barrionuevo G, Cameron WE. Morphology of developing rat genioglossal motoneurons studied in vitro: changes in length, branching pattern, and spatial distribution of dendrites. J Comp Neurol 339: 401–420, 1994 [DOI] [PubMed] [Google Scholar]
- Nunez-Abades PA, Pattillo JM, Hodgson TM, Cameron WE. Role of synaptic inputs in determining input resistance of developing brain stem motoneurons. J Neurophysiol 84: 2317–2329, 2000 [DOI] [PubMed] [Google Scholar]
- Nunez-Abades PA, Spielmann JM, Barrionuevo G, Cameron WE. In vitro electrophysiology of developing genioglossal motoneurons in the rat. J Neurophysiol 70: 1401–1411, 1993 [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Berger AJ. The nonuniform distribution of the GABA(A) receptor alpha 1 subunit influences inhibitory synaptic transmission to motoneurons within a motor nucleus. J Neurosci 21: 8482–8494, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Yanagawa Y, Koyano K. GABAergic neurons in inferior colliculus of the GAD67-GFP knock-in mouse: electrophysiological and morphological properties. Neurosci Res 51: 475–492, 2005 [DOI] [PubMed] [Google Scholar]
- Ono T, Ishiwata Y, Kuroda T, Nakamura Y. Swallowing-related perihypoglossal neurons projecting to hypoglossal motoneurons in the cat. J Dent Res 77: 351–360, 1998 [DOI] [PubMed] [Google Scholar]
- Paton JF, Richter DW. Role of fast inhibitory synaptic mechanisms in respiratory rhythm generation in the maturing mouse. J Physiol 484: 505–521, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y. Four cell types with distinctive membrane properties and morphologies in lamina I of the spinal dorsal horn of the adult rat. J Physiol 539: 817–836, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y. Integration time in a subset of spinal lamina I neurons is lengthened by sodium and calcium currents acting synergistically to prolong subthreshold depolarization. J Neurosci 25: 4743–4754, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, Mustapic S, Zuperku EJ, Stucke AG, Hopp FA, Stuth EA. Role of inhibitory neurotransmission in the control of canine hypoglossal motoneuron activity in vivo. J Neurophysiol 101: 1211–1221, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saywell SA, Feldman JL. Dynamic interactions of excitatory and inhibitory inputs in hypoglossal motoneurones: respiratory phasing and modulation by PKA. J Physiol 554: 879–889, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt PC, Spain WJ, Foehring RC, Stafstrom CE, Chubb MC, Crill WE. Multiple potassium conductances and their functions in neurons from cat sensorimotor cortex in vitro. J Neurophysiol 59: 424–449, 1988 [DOI] [PubMed] [Google Scholar]
- Sebe JY, Berger AJ. Inspiratory-phase short timescale synchrony in the brainstem slice is generated downstream of the pre-Bötzinger complex. Neuroscience 153: 1390–1401, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebe JY, van Brederode JF, Berger AJ. Inhibitory synaptic transmission governs inspiratory motoneuron synchronization. J Neurophysiol 96: 391–403, 2006 [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci 27: 262–269, 2004 [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain WJ, Schwindt PC, Crill WE. Post-inhibitory excitation and inhibition in layer V pyramidal neurones from cat sensorimotor cortex. J Physiol 434: 609–626, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumino R, Nakamura Y. Synaptic potentials of hypoglossal motoneurons and a common inhibitory interneuron in the trigemino-hypoglossal reflex. Brain Res 73: 439–454, 1974 [DOI] [PubMed] [Google Scholar]
- Takasu N, Hashimoto PH. Morphological identification of an interneuron in the hypoglossal nucleus of the rat: a combined Golgi-electron microscopic study. J Comp Neurol 271: 461–471, 1988 [DOI] [PubMed] [Google Scholar]
- Takasu N, Nakatani T, Arikuni T, Kimura H. Immunocytochemical localization of gamma-aminobutyric acid in the hypoglossal nucleus of the macaque monkey, Macaca fuscata: a light and electron microscopic study. J Comp Neurol 263: 42–53, 1987 [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol 467: 60–79, 2003 [DOI] [PubMed] [Google Scholar]
- Tanaka I, Ezure K, Kondo M. Distribution of glycine transporter 2 mRNA-containing neurons in relation to glutamic acid decarboxylase mRNA-containing neurons in rat medulla. Neurosci Res 47: 139–151, 2003 [DOI] [PubMed] [Google Scholar]
- Tarras-Wahlberg S, Rekling JC. Hypoglossal motoneurons in newborn mice receive respiratory drive from both sides of the medulla. Neuroscience 161: 259–268, 2009 [DOI] [PubMed] [Google Scholar]
- Umemiya M, Berger AJ. Presynaptic inhibition by serotonin of glycinergic inhibitory synaptic currents in the rat brain stem. J Neurophysiol 73: 1192–1201, 1995 [DOI] [PubMed] [Google Scholar]
- van Brederode JF, Berger AJ. Spike-firing resonance in hypoglossal motoneurons. J Neurophysiol 99: 2916–2928, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Brederode JF, Berger AJ. GAD67-GFP+ neurons in the Nucleus of Roller. II. Subthreshold and firing resonance properties. J Neurophysiol 105: 249–278, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana F, Bayliss DA, Berger AJ. Calcium conductances and their role in the firing behavior of neonatal rat hypoglossal motoneurons. J Neurophysiol 69: 2137–2149, 1993a [DOI] [PubMed] [Google Scholar]
- Viana F, Bayliss DA, Berger AJ. Multiple potassium conductances and their role in action potential repolarization and repetitive firing behavior of neonatal rat hypoglossal motoneurons. J Neurophysiol 69: 2150–2163, 1993b [DOI] [PubMed] [Google Scholar]
- Viana F, Bayliss DA, Berger AJ. Repetitive firing properties of developing rat brainstem motoneurones. J Physiol 486: 745–761, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley JR, Mezzanotte WS, Tangel DJ, White DP. Influence of sleep on genioglossus muscle activation by negative pressure in normal men. Am Rev Respir Dis 148: 597–605, 1993 [DOI] [PubMed] [Google Scholar]
- Withington-Wray DJ, Mifflin SW, Spyer KM. Intracellular analysis of respiratory-modulated hypoglossal motoneurons in the cat. Neuroscience 25: 1041–1051, 1988 [DOI] [PubMed] [Google Scholar]
- Woch G, Kubin L. Non-reciprocal control of rhythmic activity in respiratory-modulated XII motoneurons. Neuroreport 6: 2085–2088, 1995 [DOI] [PubMed] [Google Scholar]
- Yamuy J, Fung SJ, Xi M, Morales FR, Chase MH. Hypoglossal motoneurons are postsynaptically inhibited during carbachol-induced rapid eye movement sleep. Neuroscience 94: 11–15, 1999 [DOI] [PubMed] [Google Scholar]