Abstract
Neurons of the nucleus locus coeruleus (LC) discharge with phasic bursts of activity superimposed on highly regular tonic discharge rates. Phasic bursts are elicited by bottom-up input mechanisms involving novel/salient sensory stimuli and top-down decision making processes; whereas tonic rates largely fluctuate according to arousal levels and behavioral states. Although it is generally believed that these two modes of activity differentially modulate information processing in LC targets, the unique role of phasic versus tonic LC output on signal processing in cells, circuits, and neural networks of waking animals is not well understood. In the current study, simultaneous recordings of individual neurons within ventral posterior medial thalamus and barrel field cortex of conscious rats provided evidence that each mode of LC output produces a unique modulatory impact on single neuron responsiveness to sensory-driven synaptic input and representations of sensory information across ensembles of simultaneously recorded cells. Each mode of LC activation specifically modulated the relationship between sensory-stimulus intensity and the subsequent responses of individual neurons and neural ensembles. Overall these results indicate that phasic versus tonic modes of LC discharge exert fundamentally different modulatory effects on target neuronal circuits within the rodent trigeminal somatosensory system. As such, each mode of LC output may differentially influence signal processing as a means of optimizing behaviorally relevant neural computations within this sensory network. Likely the ability of the LC system to differentially regulate neural responses and local circuit operations according to behavioral demands extends to other brain regions including those involved in higher cognitive functions.
INTRODUCTION
The ability to regulate information processing under diverse behavioral conditions and ever changing motivational contingencies is an essential property of the CNS. Within this context, considerable evidence indicates that the locus coeruleus-norepinephrine (LC-NE) projection system regulates behavioral state and state dependent processing of sensory information (for reviews, see Aston-Jones and Cohen 2005b; Berridge and Waterhouse 2003). LC neurons exhibit distinct phasic and tonic modes of output, which differ in both the pattern of spike discharge and NE release properties (Aston-Jones et al. 1980; Florin-Lechner et al. 1996). Phasic discharge is characterized by brief 10–20 Hz bursts of two to three action potentials that is often, but not always, followed by a sustained suppression of spontaneous activity (200–500 ms) (Akaike 1982; Aston-Jones and Bloom 1981b; Clayton et al. 2004). Phasic bursts are elicited by novel or salient polymodal sensory stimuli as well as top-down decision- and response-related signals from prefrontal cortical regions (Aston-Jones and Bloom 1981b, 2005a,b; Berridge and Waterhouse 2003). Tonic activity is exemplified by stochastic discharge across a range of relatively slow rates (0.1–5.0 Hz). Increasing tonic rates are coarsely related to arousal levels within the sleep/waking continuum (Aston-Jones and Bloom 1981a; Berridge and Waterhouse 2003; Foote et al. 1980; Hobson et al. 1975), but during waking, these intermediate rates are more subtly related to goal-directed task flexibility (Aston-Jones et al. 1997, 2005b).
The rat trigeminal somatosensory (vibrissa) system has been used extensively as a model to better understand the representation and transfer of sensory information within the brain as well as a model system to investigate the impact of LC-NE output on target neuron function. Individual neurons within specialized cytoarchitectonic units of the ventral posteriomedial (VPM) thalamus (i.e., “barreloids”) and primary somatosensory cortex (i.e., “barrels”) (Van der Loos 1976) provide dynamic information about the status of single preferred whiskers on the contralateral muzzle by encoding sensory feature primitives (e.g., magnitude, velocity, and direction of vibrissa movement) (Ahissar et al. 2000; Armstrong-James et al. 1992; Ito 1988; Shoykhet et al. 2000; Simons 1978; Waite 1973). Whisker-related information is gathered in awake animals, both through passive deflection of vibrissae (as the rodent moves past objects) as well as by actively whisking an object. Although both strategies have behavioral significance, passive sensory encoding lacks complex top-down influences invoked during active whisking (Krupa et al. 2001; Lottem and Azouz 2009; Mehta et al. 2007; Ritt et al. 2008). Nevertheless, the reduced complexity of passive sensory encoding has proved essential to begin to understand the intricate modulatory impact of the LC-NE system on sensory signal processing.
Discharge rate of individual somatosensory neurons accounts for the majority of amplitude and velocity information and is likely important for discrimination of objects and course textures (Maravall et al. 2007; Shoykhet et al. 2000; von Heimendahl et al. 2007). However, neural population coding schemes and temporal codes have also been shown to be important for representing these primary sensory features (Ahissar et al. 1997, 2000; Chapin and Nicolelis 1999; Curtis and Kleinfeld 2009; Harris 2005; Lottem and Azouz 2009; Maravall et al. 2007; Pinto et al. 2000). For example, neural ensembles or functionally related assemblies of cells accomplish complex computations within this system (Churchland 1989; Erickson 1968; Katchalsky et al. 1974) by representing movement of stimuli across multiple whiskers as distributed patterns of activity across these neural ensembles at thalamic and cortical levels of the trigeminal somatosensory pathway (Chapin and Nicolelis 1999; Devilbiss et al. 2006; Nicolelis et al. 1994; Nicolelis 1996). Using principal component (PC) analysis to quantify distributed patterns of neuronal activity, these prior studies provide evidence that overall amplitude of displacement of vibrissae is represented in the correlated activity of VPM neuronal ensembles (PC1). More specific sensory features such as directional vectors of vibrissa movement are represented in higher dimensional relationships between neural activity patterns (e.g., PC2).
Within sensory circuits, previous reports have demonstrated that LC-NE output can dynamically regulate the excitability of target neurons and information processing capabilities of neurons and neural assemblies (Devilbiss et al. 2006; Hurley et al. 2004). These studies have demonstrated that endogenous tonic-like release of NE from LC terminals or continuous iontophoretic application of NE produces a dose dependent inverted-U facilitation of target neuronal responses to excitatory synaptic inputs. Although the sensitivity of individual neurons to the modulatory effects of LC output is dependent on brain region (i.e., thalamus vs. cortex), tonic LC discharge rates of ∼1.0 Hz produce the optimal change in individual neuron and neural ensemble coding strategies (Devilbiss and Waterhouse 2004; Devilbiss et al. 2006). Characterization of the effects of phasic-like LC output on sensory signal processing has been limited to a relatively small number of studies where neuronal responses to sensory-driven inputs were facilitated by high-frequency stimulation of the LC efferent path (see review: Berridge and Waterhouse 2003; Devilbiss and Waterhouse 2004). However to date, it remains unclear as to whether phasic and tonic modes of LC discharge differentially impact individual neuron and neural ensemble responses to sensory inputs at multiple sites along forebrain pathways in the intact, conscious animal. To address these questions and advance our understanding of the role of the LC-NE system in sensory signal processing, we examined the impact of phasic and tonic LC activation on individual neuron and neural ensemble response properties of VPM thalamus and barrel field somatosensory (BF) cortical neurons of waking animals. Additionally, we determined the extent to which the modulatory effects of phasic versus tonic LC output altered neural representations of increasing sensory input intensities.
METHODS
Ethics statement
All experiments were conducted in accordance with National Institutes of Health guidelines on research animal care and under approval and guidance of the Institutional Animal Care and Use Committee of Drexel University.
Subjects and surgery
Five adult male Long-Evans hooded rats (Charles River Laboratories, Wilmington, MA) weighing 250–450 g were included in this study for extracellular recordings of multiple single units from the BF cortex and the ipsilateral VPM thalamus.
Animals were unilaterally implanted with microwire recording electrodes into the somatosensory BF cortex, ipsilateral VPM thalamus, and ipsilateral LC. A stimulation electrode was additionally inserted subcutaneously within the whisker pad contralateral to the BF cortex and VPM implants as previously described (Devilbiss and Waterhouse 2002). Briefly, anesthesia was induced with 390 mg/kg chloral hydrate (Sigma, St. Louis, MO) and 25 mg/kg pentobarbital sodium (IP; Abbott Laboratories, North Chicago, IL) and supplemented with a 100 mg/kg chloral hydrate-10 mg/kg pentobarbital solution to maintain a surgical plane of anesthesia. A microwire recording bundle (n = 8, 50 μm diam wire; SB103, NB Labs, Dennison, TX) was stereotaxically placed within the LC (∼1.2 lateral ∼3.6 caudal to the intersection of the midline and lambda; head placed at a 15° angle; nose down). Positions of microwire electrode tips were confirmed electrophysiologically by monitoring neuronal electrical activity throughout the descent of the recording probe (∼50 μm/min; ∼6.0 dorsoventral). Characteristic spontaneous discharge rate (∼0.1–5 Hz) and biphasic response to tail pinch (Akaike 1982) were two criteria used to identify putative LC neurons. The wires of a second electrode bundle were cut on a diagonal to correspond to the anatomical structure of VPM barreloids, bound with a silk suture, and implanted into the ipsilateral VPM thalamus (flat skull; −3.3 caudal, −2.8 lateral, ∼5.5 dorsoventral). Electrophysiological recordings were made to evaluate microwire position as they approached the C3 whisker representation of the VPM thalamus. A microwire array (S103, NB Labs) was implanted into the somatosensory BF cortex (−2.5 caudal and −5.8 lateral) at an approximate depth of 1.2 mm. Neuronal activity was again monitored during probe insertion to evaluate the position of the microwire bundle as it approached a final target in layer V of the C3 whisker representation in the BF cortex. Dura from each implant site was covered with Gelfoam and each probe was secured to the skull with dental cement. Last, a flexible bipolar stimulating electrode (a twisted pair of 7-stranded stainless steel wires) was implanted around the base of a single (C3) vibrissae of the rat's whisker pad. Electrode implantation was facilitated by inserting the bipolar electrode inside a 20 gauge needle, cut blunt and polished, and tunneling under the skin from the initial site of the skull incision to the rat's whisker pad. Before the skull screws were encapsulated with dental acrylic, the grounding wires from each of the electrode assemblies were wrapped around the exposed screws for electrical grounding. Electrode connectors were attached to the skull with screws and dental acrylic. The skin was loosely sealed around the dental cement and the animal was allowed to recover for 5–10 days.
Experimental procedure
Animals used in this study were habituated to the experimenter, the testing chamber, and the experimental procedure (whisker pad stimulation) for 2 h before testing began (see: Devilbiss and Waterhouse 2002, 2004; Devilbiss et al. 2006; Rutter et al. 2005). Only one recording session was permitted for a given day with at least a 3-day inter-session interval between testing. During animal habituation to its environment, putative single units of the VPM thalamus and BF cortex were initially discriminated using template matching algorithms (RASPUTIN, Plexon, Dallas, TX). After each experimental session, preestablished off-line criteria were used to verify that waveforms assigned to each discriminated “unit” originated from a single neuron. These criteria were based on unit waveform properties and spike train discharge patterns (Devilbiss and Waterhouse 2002), including: peak waveform voltage, slope(s) of waveform from peak to peak, clustering of scattergram points from the waveform's first two principal components, and spike train autocorrelegram. Although neural action potential waveforms were not explicitly analyzed to classify neuronal subtype, it is likely that the vast majority of our discriminated cortical units were regular spiking neurons. Small amplitude waveforms that emanate from fast spiking neurons at rapid discharge rates were not routinely discriminated from the underlying field potential activity and irregular burst discharges were not readily observed in our sample of neurons. Moreover, our recording locations in layer V bias sampling of neurons in favor of pyramidal cells rather than fast spiking interneurons. Spike trains from all verified neurons were examined for “unreasonable levels of correlation” among spike trains to determine whether a neuron was recorded from more than one electrode. As such, if a pair of neurons exhibited a correlation >0.6 across the duration of the experiment, one of the neurons was excluded from the analysis. Determination that neurons were not recorded across multiple recording sessions was made by examining waveform shape, discharge pattern (interspike interval), and response properties for each recorded neuron. Our unpublished data indicate that recording from the same neuron across multiple recording sessions (separated by several days) occurs infrequently. However, if a single neuron was identified across multiple recording sessions, the analysis for that neuron was limited to the first session in which it was isolated.
Following on-line discrimination of VPM thalamic and BF cortical units and confirmation that units were responsive to whisker pad stimulation, electrophysiological experiments were initiated. Biphasic pulses (range: 1–3 mA; 1 ms duration) were delivered through the bipolar stimulating electrode implanted around the base of the vibrissae the rat's whisker pad. For each animal, a maximal stimulus intensity was determined that was threshold for producing a rostral twitch of that single vibrissae. This intensity was the upper limit of a range of stimulus pulses (Sham Stim, 0.25*Max, 0.5*Max, Max) presented randomly to generate stimulus-response functions. This approach normalized differences between electrode impedances and anatomical placement within the whisker pad. Exact timing of sensory-stimulus presentation was randomly generated within a Gaussian distribution (mean interstimulus interval. 3 s; range, 2.5–3.5 s) to prevent stimulus habituation of the somatosensory system. A control condition was always presented first; 400 whisker pad stimuli were delivered to animals that randomly varied in time. Following the initial control period, animals received 30 min blocks of both phasic and 1.0 Hz tonic electrical stimulation of the LC presented in a random order. Irrespective of the pattern of impulse activity (phasic or tonic), this paradigm resulted in identical numbers of stimuli being delivered to the LC during stimulus periods. Additionally, during periods of LC activation, whisker pad stimuli were presented in the same manner as control conditions; however, during tonic LC stimulation, the timing of whisker-pad stimulus pulses were adjusted such that LC and whisker-pad pulses were never allowed to occur simultaneously. Whisker-pad pulses were delayed or advanced in time with respect to tonic stimulus presentation, thus avoiding consistent time-locked interactions between single tonic LC pulses and whisker-pad stimulation as occurs under phasic LC stimulation conditions.
Putative changes in LC discharge or target neuron response properties that relate to changes in behavioral state were controlled by confirming via videotape analysis that the animal remained in a state of quiet rest throughout the experiment. The quiet resting state was defined behaviorally as a sternal recumbent position with head elevated above the chamber floor. Neither phasic nor tonic stimulation of the LC altered the overt resting behavioral state. Such periods of quiet resting exhibit a desynchronous cortical activation state (as determined by EEG; see results, Fig. 3). Furthermore, unpublished studies indicate that manipulations of the LC output via presentation of auditory stressors do not alter EEG desynchronous activity or the frequency-power spectra of EEG when analysis is confined to intervals of quiet resting. Videotape recordings were made of the entire experimental session with a video counter timer providing time stamps (resolution = 0.1 s) synchronized to the multi-unit recording and stimulus control systems.
Fig. 3.
Neuronal recording, electrocorticogram trace, and power spectrum during periods of quiet resting. A: representative waveform discrimination of 2 neurons recorded from layer V of the BF cortex. This putative single neuron (unit A) was isolated from other neuronal activity and met strict off-line criteria including separation of clustered data in principal component space (inset) and visually distinct waveform patterns. B: representative waveform discrimination of ≥3 neurons recorded from the VPM thalamus. Only unit B met the strict off-line verification criteria and was permitted to be included for further analyses. C: the example electrocorticogram (ECoG) represents gross electrical activity monitored from the BF cortex recording sites and plotted over time. This trace illustrates the cortical activation state that corresponds to quiet resting as determined from video tape recordings of animal behavior. All electrophysiological analyses were based on these periods of quiet resting conditions. Whisker stimulation artifact in the ECoG trace is indicated by the above raster plotting occurrence of whisker pad stimulation (whisker stim). D: the power spectrum calculated from this epoch confirms a mixture of dominant frequencies within the 3–15 Hz range. x axis represents the frequencies extracted from the ECoG; y axis represents the percent of total power for each frequency.
Activation of the LC
Under normal, physiologic conditions, LC cells exhibit phasic bursts of activity (2–3 spikes; 10–20 Hz) in response to salient sensory stimuli and processes of decision making that are superimposed on tonic discharge patterns (0.1–5.0 Hz) (Aston-Jones and Cohen 2005a; Aston-Jones and Gold 2009; Clayton et al. 2004; Foote et al. 1983; Ivanova et al. 1997; Nieuwenhuis et al. 2005). In the present study, phasic and tonic electrical LC stimulation parameters were carefully chosen to mimic physiological discharge of LC neurons, minimize current spread to other reticular formation structures, and replicate studies demonstrating NE release in forebrain circuits. Stimulus pulses delivered to the LC were monopolar current pulses delivered across two of the eight wires of the microwire bundle implant. Currents used for LC stimulation were determined independently for each animal (range, 3–300 μA) by selecting the level of LC stimulation that led to the maximal facilitation of BF cortical and VPM thalamic neuron responsiveness during 1.0 Hz tonic stimulation. Each LC phasic stimulation train was delivered 300 ms before presentation of the whisker pad stimulus as trains of three pulses at 10 Hz (train duration, 300 ms) delivered every 3 s. Under these conditions, phasic LC stimulation (10 Hz train of 3 spike/train every 3 s) evoked bursts of LC discharge like those observed under physiologic conditions (Fig. 2). Tonic activation of LC was achieved by delivering LC stimulation pulses at 1.0 Hz. Previous studies confirm that tonic LC discharge rates are increased in an additive manner using this tonic stimulation protocol (Devilbiss et al. 2006). Stimulus currents within 3–300 μA activate cells within a maximal radius of 150 μm (smaller distance for smaller cells) (Snow et al. 1999; Stoney et al. 1967; Yeomans 1990). Thus the stimulus parameters used here were selected so as to minimize current spread beyond the borders of the LC nucleus. Moreover the interval between trains of phasic LC stimulation and whisker pad stimulus was chosen to produce the maximal modulatory effect for both excitatory and inhibitory target neuron responses (see discussion) (see also Holdefer and Jacobs 1994; Waterhouse et al. 1998).
Fig. 2.
Selective response of an LC neuron to electrical stimulation adjacent to the LC and thalamic neuron to whisker stimulation. A: action potential waveforms from an exemplar LC neuron (black) and background neuronal activity. These waveforms were also separated from background activity when plotted in principal component space (inset). B: peristimulus time histogram (PSTH) illustrates a typical LC multi-neuron response to phasic electrical stimulation (n = 300 trains). The y axis represents the probability of discharge, and the x axis represents time (seconds) before and after presentation of the 1st pulse of the phasic stimulus train (diamond = occurrence of each LC stimulus pulse; n = 3; internal frequency = 10 Hz; train duration = 300 ms; train-train frequency = 0.33 Hz). Horizontal line indicates 99% Poisson confidence interval of spontaneous discharge rate. Note: LC neuron is not significantly responsive to low intensity whisker stim (arrowhead). C: a raster timeline illustrating the temporal relationship between the 3 LC stimulation pulses (LC stim) comprising a phasic stimulation train and the whisker-pad stimulation (whisker stim). 0 s = onset of whisker-pad stimulation; LC stimulation occurs within a window 300–500 ms before each whisker stim. Additionally, the occurrence of spiking activity from an exemplar LC Neuron in relation to LC stim and whisker stim was plotted. D: PSTH of a VPM thalamic neuron illustrating a significant response to whisker stim (n = 300). No response to LC stimulation was observed for this VPM neuron (brief periods of reduced spontaneous activity following each LC stimulus pulse were occasionally observed as a result of amplifier saturation from LC simulation; amplifier saturation did not effect the ability to determine the degree to which LC stimulation could evoke whisker responses). PSTH conventions are the same as B.
Analysis of electrophysiological data
VPM thalamic and BF cortical neuron discharge patterns were quantified from computer-stored time stamps of discriminated waveforms as previously described (see Devilbiss and Waterhouse 2002, 2004). Briefly, sensory-evoked patterns of discharge for individual neurons were analyzed with trial-by-trial bin counts collected during periods of quiet resting for either control or LC stimulation conditions (NeuroExplorer, Nex Technologies, NC, and custom Matlab functions: Mathworks, Natick, MA).
Initially trial bin counts were summarized as peristimulus time histograms (PSTHs) and quantified. From each PSTH the mean probability of discharge during the whisker stimulus-evoked response was calculated; the response window was defined by the duration of evoked activity exceeding Poisson 99% confidence intervals of spontaneous discharge rates. Functional changes in the ensembles of simultaneously recorded VPM thalamic and BF cortical neurons during periods of phasic or tonic LC stimulation were determined by analyzing differences in the distributed patterns of stimulus related discharge with principal components analysis (PCA) and with all-pairwise cross-correlational analyses. These methods have previously been described in detail (Chapin and Nicolelis 1999; Devilbiss et al. 2006). Briefly, eigenvalue decomposition by PCA was determined from correlation matrices generated from neuronal spike trains of simultaneously recorded neurons within an anatomical region (mean frequency within 3 ms bins) (Chapin and Nicolelis 1999; Devilbiss et al. 2006; Wilent and Contreras 2004). Binned spike train data for each neuron from the entire experimental session served as independent variables for the PCA analysis. Eigenfunctions for each PC were then calculated as the sum of eigenvalue-weighted neuronal spike trains. PSTHs were generated from these eigenfunctions to access the effects of phasic and tonic LC activation on responses of the VPM and BF cortical ensembles.
To determine the relationship between increasing afferent stimulus levels and properties of evoked responses, individual neuronal response properties and neuronal population eigenfunctions were expressed as a percentage of the maximal stimulation presented in an experimental session. The maximal stimulation used in a session was unique to each animal (range, 1–3 mA; 1 ms duration) and was titrated to be the minimum current necessary to elicit an overt twitch of a single whisker. The effects of phasic and tonic LC stimulation were then compared with these normalized input-output curves across animals.
Functional relationships between neurons were further examined by generating cross-correlegrams from individual neural spike train data 3 ms before and 90 ms after stimulation of vibrissa afferents (Aertsen et al. 1985, 1989; Devilbiss et al. 2006; Eggermont 1992, 1994). Again spike trains were discretized with 3 ms time bins and used to determine the magnitude of correlation between neuronal pairs. A peristimulus trial-by-trial shift predictor was used to control stimulus-induced correlation when generating each neuron pair cross correlegram (Aertsen et al. 1989; Aertsen and Gerstein 1985; Devilbiss et al. 2006; Eggermont 1992, 1994). The shift predictor was also used to generate confidence intervals to determine the level of statistical significance of correlated activity (Supplemental Fig. S31). Across all pairs of simultaneously recorded neurons, the mean significant correlation was calculated for 30 ms before and after each action potential of the reference neuron as a scattergram. These methods permitted assessment of functional connectivity during the processing of whisker pad stimulation, while minimizing the impact of correlations related to simultaneous activation of VPM or BF cortical neurons by excitatory afferent input.
Phasic or tonic LC stimulation effects on the magnitude of stimulus-evoked discharge, response latency measures, PCA eigenfunction measures, and cross-correlation measures were accessed for statistical differences between control conditions and between phasic and tonic LC output conditions. Individual cells demonstrating either increases (>10%), decreases (<10%), or no change in response parameters were categorized and effects on stimulus-evoked response properties were subjected to appropriate χ2 analysis. One way ANOVA analysis (modulation) was performed on normalized data for data of Figs. 4 and 5. Two way repeated measures ANOVA was used for data of Figs. 6– 8 with whisker stimulation intensity and anatomical location as factors of Figs. 6A and 8 and whisker stimulation intensity and LC activation mode as factors for the remaining comparisons. LSD post hoc tests were used to make comparisons between individual groups.
Fig. 4.
Phasic LC activation elicits a spectrum of modulatory effects on sensory-evoked responses of VPM thalamus and BF cortical neurons. Peristimulus time histograms (PSTHs) illustrate spontaneous and vibrissa pad stimulus-evoked discharge from neurons (1 each row) of the BF cortex (A, i and ii) and VPM thalamus (B, i and ii), during control (left) and phasic LC stimulation (right) conditions. Phasic stimulation of the LC either facilitated (Ai and Bi) or suppressed (Aii and Bii) neural responses to somatosensory input. The y axis of each histogram represents the probability that the cell discharged in a given time bin represented by the x axis with 0 depicting the time at which the whisker pad stimulation occurred. The inset numbers represent the summed probability of discharge for the stimulus-evoked excitatory response. Statistically significant responses exceed Poisson 99% confidence intervals of prestimulus activity (· · ·). C: the average magnitude of whisker response discharge probability was calculated before and during phasic LC stimulation conditions for each neuron and plotted. Neuronal responses from either the VPM thalamus or BFC that were facilitated (left – n = 20/41, VPM; 33/69 BFC) or suppressed (right – n = 15/41 VPM; 25/69 BFC) from control levels were grouped and plotted as a percent change from control. (* = P < 0.05).
Fig. 5.
Phasic and tonic LC stimulation do not produce equivalent effects on evoked-activity for a given neuron. A: scattergrams of data collected from the VPM thalamus (Ai) and BF cortex (Aii) illustrate the unique and cell-specific modulatory effects of phasic vs. tonic LC stimulation on the magnitude of sensory-evoked responses for a given neuron. Equivalent modulatory actions of phasic and tonic LC activation would result in data plotted on or near the equivalency line (45° diagonal magenta dotted line). A linear regression of these data (red dotted line) and 95% confidence intervals (green dotted lines) was calculated and plotted revealing the level of dependency between the effects of phasic and tonic LC output. y axis represents the modulatory action of phasic LC activation as a percent of control; x axis represents tonic LC mediated changes as a percent of control. Several neurons that demonstrated a modulatory effect >450% of control were not plotted for clarity (but included in the linear regression). B: contour plots were generated by fitting a spline model to the absolute difference between the effects of phasic vs. tonic LC stimulation for each VPM and BF cortical neuron. Green to red shading indicates least to greatest difference between the effect of phasic and tonic LC output, respectively. Phasic LC activation produces the greatest difference in modulatory action for VPM neurons, whereas tonic LC output produces the greatest modulatory difference in the cortex. x axis represents tonic LC mediated changes as a percent of control, y axis indicates phasic LC modulation as a percent of control, z axis is the absolute difference between the modulatory effects of tonic vs. phasic output. Note that the x and y axis in Bi and Bii are rotated in space and are of different scales to provide an unobstructed view of the 3D data and include data not shown in A.
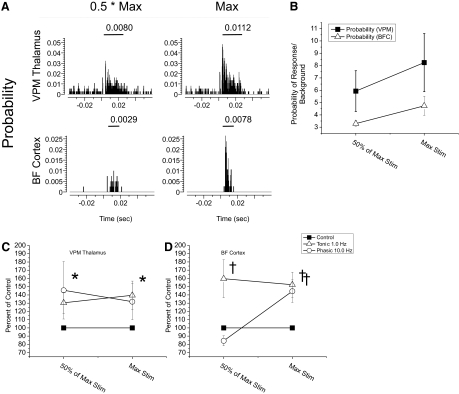
Fig. 6.
A comparison of the effects of phasic vs. tonic LC activation on responses of VPM thalamic and BF cortical neurons to increasing sensory stimulus intensities. A: PSTHs illustrate evoked responses of a VPM thalamic (top) and BF cortical (bottom) neuron to increasing levels of vibrissa pad electrical stimulation (left = 0.5*Max; right = maximal stimulation delivered). Stimulus currents were normalized for each animal so that maximal stimulus current (Max) was just threshold for eliciting overt movement of a single vibrissa (0.5*Max = ½ of the maximal stimulus current). y axis = probability of discharge in a time bin; x axis = time before and after stimulus presentation; numerical inset = mean probability of response (—). B: the 2-point stimulus response function illustrates the relationship between the average responses of VPM thalamic and BF cortical neurons to 0.5 Max and Max stimulus currents under control conditions. y axis = mean signal/noise of the probability of response across all animals. C: the effects of each mode of LC activation on VPM neuron stimulus-response functions illustrate that both phasic and tonic LC output facilitated responses of VPM neurons similarly across stimulus input levels. Plotting the effects of LC activation at each stimulus intensity as a percentage change from discharge under control conditions further illustrates the probability of discharge is globally facilitated without changing the slope of the stimulus-response function. D: plot of the modulatory actions within BF cortex during phasic vs. tonic LC activation. Phasic and tonic LC stimulation produced modulatory actions that were dependent on stimulus input strength. This unique action of phasic LC output altered the slope of the stimulus-response function for cortical neurons. (*, P < 0.05; †, P < 0.01). Error bars indicate SE.
Fig. 7.
The effects of phasic vs. tonic LC stimulation on representations of sensory stimulus properties by ensembles of VPM thalamic and BF cortical neurons. A: PSTHs illustrate the response of the 1st (top) and 2nd (bottom) PC eigenfunctions calculated from a VPM thalamic (n = 16) ensemble and BF cortical ensemble (n = 20) of simultaneously recorded neurons to increasing whisker stimulus intensities. Generation of PC eigenvectors are described in depth in Chapin and Nicolelis (1999), Nicolelis et al. (1994, 1996) and Devilbiss et al. (2006). Numerical insets indicate magnitude of PC response (vertical bar overlay indicates peak calculation of PC1 and PC2). B: plots of input-output functions for the 1st 2 PC eigenfunctions (1 each row; PC1, PC2) generated for VPM thalamic (left) and BF cortical (right) neural ensembles. As in Fig. 6, the effects of each mode of LC activation were plotted for each stimulus intensity as the average percent change from control across all animals tested. These plots demonstrate that VPM thalamus and BF cortical ensembles respond differently to LC output. Both modes of LC output facilitated sensory information represented across the distributed activity of VPM thalamic ensembles (PC1, PC2). However, phasic LC output significantly altered the slope of the stimulus-response function of PC2 derived from cortical neural activity. (*, P < 0.05; †, P < 0.01; ‡, P < 0.001). Error bars indicate SE.
Fig. 8.
Effects of phasic vs. tonic LC stimulation on functional connectivity across thalamic and cortical networks. A: representative cross-correlegrams between 2 neurons recorded from the VPM thalamus (Ai), the VPM thalamus and BF cortex (Aii), and BF cortex. A VPM thalamic neuron of Ai and a BF cortical neuron of Aiii were used to generate the cross-correlegram for the thalamic and cortical pair of neurons of Aii. During control conditions, significant correlation between spike trains of these neuron pairs indicates that they are functionally connected. Probability of correlated activity between neuron pairs were subtracted from a trial-by-trial shift predictor and plotted (black line) along with 99% confidence intervals for the correlegram (blue …). For each cross-correlegram the y axis represents the probability of discharge; x axis represents time before or after the reference neuron action-potential (0 ms indicated by green line). Correlated activity that was greater or <99% confidence intervals and was between ±30 ms (indicated by outer green vertical lines) was further analyzed. B: functional connectivity maps, defined by temporal correlation of spike discharges between neuron pairs, were generated during control periods (B, i and iii) and during periods of phasic LC stimulation (Bii) or tonic LC activation Biv. Each column of the x axis represents the cross-correlegram calculated for a neuron pair from the VPM thalamus (red fiduciary), BF cortex (purple fiduciary), and VPM-BF cortical pair (green fiduciary). The y axis represents time before and after discharge of the reference neuron (0 ms; red line). For each neuron pair, the absolute strength of only significant correlations was indicated by tick color (see color bar). C: plots of the average connectivity across neuron pairs generated from data of B, i–iv. Changes in overall functional connectivity within the BF cortex (Ci), between the VPM and BF cortex (Cii), and within the VPM (Ciii) between control conditions and modes of LC activation indicate that functional connectivity within the VPM thalamus and between the VPM thalamus and BF cortex was preferentially enhanced by phasic LC output. D: bar graphs summarize changes in functional connectivity across all animals tested for both the number of significantly correlated neuron pairs observed (xCorr Count) and the mean strength of correlated activity (xCorr Mean). Phasic LC output induces the greatest change in connectivity in either region and significantly increases the functional connectivity between the thalamus and cortex. Additionally, these data indicate that LC output has opposite effects on neuronal connectivity within the VPM thalamus vs. BF cortex. *, P < 0.05 from control; †, P < 0.05 between tonic vs. phasic. Error bars indicate SE.
Histology
At the conclusion of the study, the placement of the recording electrodes within the VPM thalamus, BF cortex, and LC were verified. Following induction of anesthesia with a terminal dosage of pentobarbital (100 mg/kg), 60 μA of current was passed across two of the eight microwires in a recording bundle for 45 s. Animals were then perfused transcardially with 0.9% saline followed by a 10% formalin solution containing 5% potassium ferrocyanide to produce a Prussian blue reaction product. The brains were then removed and stored in phosphate buffer containing 15% sucrose. Coronal 80 μm sections were cut on a freezing microtome and mounted and cover-slipped. Illustrations of typical electrode placement in the VPM thalamus, SI cortex, and LC can be seen in Fig. 1.
Fig. 1.
Schematics and coronal sections of the rat brain illustrating the microwire electrode placements within the ventral posteriomedial (VPM), thalamus barrel field somatosensory (BF) cortex, and locus coeruleus (LC). A: schematic of the ascending trigeminal system illustrates the flow of somatosensory information from the vibrissa pad to the thalamus and cortex. BFC, barrel field cortex; PO, posterior thalamus; V trigeminal nucleus. B: a low power photomicrograph of a forebrain coronal section illustrating the placement of recording surfaces of an electrode bundle of 8 microwires (→) in the VPM thalamus and in layer V of the BF cortex. C: low power photomicrograph of a brain stem coronal section depicts the recording/stimulation site at the lateral edge of the LC. This placement was the most lateral recording/stimulation site (arrow head) and demonstrates the most tissue damage but was near enough to the LC to elicit neuromodulatory effects on sensory signal processing. These sections were mounted on slides and photographed immediately following tissue sectioning and before dehydration and coverslipping. D: schematic representation of electrode placement within and adjacent to the LC. These coronal section drawings at the level of the LC are arranged from most caudal (left) to most rostral (right). *, location of the central electrode of the recording/stimulation bundle as determined by histological examination of pontine tissue sections for each animal. 4V, fourth ventricle; Bar, Barrington's nucleus; DTN, dorsal tegmental nucleus; g7, genu of the facial nerve; LDT, lateral dorsal tegmental nucleus; Me5, mesencephalic trigeminal nucleus; mlf, medial longitudinal fasciculus; LC, locus coeruleus.
RESULTS
Effects of phasic electrical stimulation on LC neuron discharge properties
For each animal in the current set of studies, the final location of microelectrodes was histologically verified to confirm accurate placement within the VPM subdivision of the thalamus, layer V of the of the BF cortex (Fig. 1, A and B), and within or adjacent to the LC (C). Only animals with stimulation electrodes placed within or immediately adjacent to the LC (<250 μm from the LC core; Fig. 1D) were considered for analysis. No differences were observed in the LC modulatory profiles across animals when these criteria for electrode placement were applied. Similar electrode placements and stimulus parameters have been shown previously to elicit LC neuron discharge (Aston-Jones et al. 1980; Florin-Lechner et al. 1996) and to increase NE efflux in frontal circuits, including the VPM thalamus (Devilbiss et al. 2006; Florin-Lechner et al. 1996).
In a limited number of animals (n = 2), LC multi-neuron unit activity was recorded simultaneously with LC stimulation (Fig. 2). On one occasion, the activity of a single LC neuron was discriminated from multi-neuron waveforms (Fig. 2A). LC tonic discharge rates for these units averaged 0.88 ± 0.23 (SE) Hz under control, quiet resting conditions. During similar quiet resting conditions, 1.0 Hz tonic LC stimulation increased LC discharge rate to 1.40 ± 0.41 Hz. Phasic stimulation of the LC nucleus elicited multi-neuron excitatory discharge with no overt change in overall tonic discharge rates (Fig. 2B). Examination of the precise timing between LC neuron activation and LC stimulation pulses for the sample neuron shown in Fig. 2A demonstrates a response to each stimulus pulse with an action potential nearly coincident with approximately each of the three stimulus pulses (as confirmed by the raster in Fig. 2C). Importantly, LC stimulation did not activate somatosensory neurons through indirect activation of the neighboring mesencephalic trigeminal nucleus (Fig. 2D). In combination with previous LC stimulation and microdialysis studies from our laboratory and others, these findings suggest that phasic and tonic stimulation evoke LC neuron discharge and elicit increased NE efflux in terminal fields such as the VPM thalamus and BF cortex with different temporal patterns (Devilbiss et al. 2006; Florin-Lechner et al. 1996; Waterhouse et al. 1998).
Effects of phasic LC stimulation on responses of VPM thalamic and BF cortex neurons to sensory input
The effect of phasic electrical activation of the LC on neuronal responsiveness to afferent sensory input was examined in 139 units recorded from the VPM thalamus and 158 units recorded from the BF cortex of five awake, unrestrained animals. Detailed analysis of spike train activity was limited to units that met strict off-line verification criteria and were subsequently classified with high confidence as single neurons (Fig. 3, A and B) (Devilbiss and Waterhouse 2002). On average, 8 ± 7 VPM and 13 ± 6 BF cortical verified single neurons were recorded per animal yielding 1–1.5 neurons per electrode. Analyses were further restricted to periods when the animals were quietly resting to control for the relationship between behavioral state and LC discharge. Periods of quiet resting were determined by manually scoring time-stamped video records of the experiment. Our results from other studies (unpublished findings) indicate that animals in this behavioral state exhibit a predominance of desynchronous electroencephalographic (EEG) activity (Fig. 3B) with occasional bouts of lower frequency activity (C). Accordingly, in this behavioral state 41 VPM and 76 BF cortical units were verified as single neurons having a baseline mean spontaneous discharge rate of 1.84 ± 0.30 and 1.12 ± 0.16 Hz, respectively during quiet resting behaviors. Examination of individual VPM and BF cortical neuron spontaneous firing rates revealed little appreciable effect of either tonic or phasic LC stimulation on a cell-to-cell basis (Supplemental Fig. S1). Sensory-evoked responses were observed in all VPM neurons; seven BF cortical neurons did not exhibit a sensory-evoked response that was significantly above spontaneous activity levels.
Initially, the impact of phasic LC stimulation on sensory-evoked responses of VPM and BF cortical neurons were cataloged to determine whether heterogeneous modulatory effects could be observed across target neurons. Phasic LC activation produced an array of modulatory influences on sensory-evoked responses of VPM and BF cortical neurons (Fig. 4). These actions were cell specific and, in general, could be classified as either facilitation (Fig. 4, Ai and Bi) or suppression (Aii and Bii) of stimulus-evoked activity. Facilitation of sensory-evoked responses during phasic LC activation was the foremost observation made across VPM and BF cortical neurons (n = 20/41, 49% VPM cells; n = 33/69, 48% BF cortical cells). Suppression of sensory-evoked discharge was also observed in each brain region (n = 15/41, 37% VPM cells; n = 25/69, 36% BF cortical cells) as well as cases where LC stimulation yielded little or no change in sensory evoked activity (less than ±10% of control conditions) and therefore was deemed to have no effect (n = 6/41, 14% VPM cells; n = 11/69, 16% BF cortical cells). Phasic LC stimulation did produce “gating” in a number of VPM and BF cortical neurons, as defined by a stimulus-induced response that was not statistically different from spontaneous discharge during control conditions but became prominent and achieved significance during periods of LC activation (n = 7/41, 17% VPM cells; n = 15/69, 21% BF cortical cells). In the current study, we classified neurons that displayed gating effects as a subset of those whose responses were facilitated (e.g., Fig. 4Bi) and included them in the preceding counts.
Within each anatomical region, the preference for sensory neurons to respond to phasic LC output with facilitation of sensory-evoked responses was statistically significant (χ2, P < 0.01). However, this modulatory pattern was essentially identical between the VPM thalamus and BF cortex (χ2, P = 0.979). This distribution of effects was further investigated by determining the degree to which neuronal responses in the VPM and BF cortex were modulated for groups of neurons demonstrating each neuromodulatory classification (i.e., facilitation or suppression; Fig. 4C). The average LC-mediated enhancement of sensory-evoked discharge in VPM thalamic neurons was a 2.02-fold increase over control responses. This degree of facilitation was nearly double the magnitude of suppression (1.64-fold) observed across VPM neurons [ANOVA; F(1,40) = 12.271, P = 0.001]. To a similar degree, evoked-responses of BF cortical neurons were significantly facilitated (2.22-fold) or suppressed (1.32-fold) from control values during periods of phasic LC activation [ANOVA; F(1,40) = 4.010, P = 0.049]. These data indicate that at the circuit level, the responsiveness of neurons to somatosensory input was generally facilitated by phasic LC output and the degree to which these modulatory effects were observed was nearly equivalent across VPM and BF cortical target regions.
Direct comparison of phasic versus tonic LC stimulation on thalamic and cortical neuron stimulus-response properties
Previous evidence suggests that phasic and tonic modes of LC activity may differentially regulate signal processing within target circuitry (Aston-Jones et al. 1980; Florin-Lechner et al. 1996). To test this hypothesis, direct comparisons of the effects of phasic versus tonic LC stimulation on sensory-response properties were made for each VPM thalamic and BF cortical neuron. Phasic and tonic activation of the LC produced effects on sensory-evoked discharges that were unique to individual thalamic and cortical target neurons (Supplemental Fig. S2). These effects were directly compared by constructing scattergrams of the percentage change in sensory-evoked response for tonic versus phasic LC stimulation conditions (Fig. 5A). Equivalent modulatory actions of phasic and tonic LC stimulation would result in data points plotted on or close to the 45° equivalency line (dotted magenta diagonal line). However, as indicated by the scattergram, phasic and tonic modes of LC output differentially modulated sensory-evoked responses of individual cells in both the VPM thalamus (Fig. 5Ai) and BF cortex (Aii). This relationship was further studied with linear regression analysis. The linear regression (red solid line) of the modulatory effects observed for VPM thalamic sensory-evoked responses demonstrated a slightly positive slope, {f(x) = 0.11x +195 [r2 = 0.001; ANOVA F(1,40) = 0.037]}, whereas the regression of data from the BF cortex resulted in a negative slope {f(x) = −0.05x +124 [r2 = 0.014, ANOVA F(1,55) = 0.762]}. However, as indicated by the r2 values, the fit of these data to a linear model was equally poor in both anatomical regions. To further understand differences in the magnitude of neuromodulatory effects generated by phasic versus tonic LC activation and the impact of gating, absolute differences between the modulatory effects produced by phasic and tonic LC activation were calculated and fitted with a spline model (Fig. 5B). The contour graph of this model illustrates that VPM thalamic neurons exhibit a larger dynamic range of neuromodulation (yellow-red) under conditions of phasic LC activation. In contrast, modeling BF cortical cells in this manner predicts that these neurons exhibit the largest range of modulation under conditions of tonic LC output. Overall, these scattergrams and contour graphs show that neural circuit responsiveness to sensory input is regulated on a cell-by-cell basis by LC output, VPM thalamic and BF cortical regions respond differently to LC output, and phasic and tonic modes of LC discharge exert different modulatory influences on neuron responsiveness to sensory driven synaptic inputs.
Functionally, the neuromodulatory effects of phasic versus tonic LC activation may differentially regulate the dynamics of neuronal response properties. To initially test this hypothesis, the impact of phasic versus tonic LC output on neural representations of increasing levels of sensory stimulation was examined (Fig. 6). Under control conditions, VPM and BF cortical neurons responded to increasing whisker pad stimulus intensities with increased discharge rates (Fig. 6A). The series of normalized peripheral stimulation intensities presented to each animal to generate stimulus-response (S-R) functions was adjusted to be threshold for producing an overt twitch of a single vibrissa at the maximal intensity stimulus pulse (250–500 μm) (Devilbiss and Waterhouse 2002; Rutter et al. 2005). Although ¼-maximum stimulus intensity and a sham stimulus were presented, these stimulus intensities did not yield consistently quantifiable responses and could not be used to generate a three-point curve. Neuronal two-point S-R functions were generally similar across populations of recorded VPM and BF cortical neurons (Fig. 6B). S-R slopes for VPM neurons [f(x) = 2.32x + 3.61] on average were similar to BF cortical neurons [f(x) = 1.44x + 1.85]; however, responses of BF cortical neurons to each stimulus intensity level were generally smaller than evoked-responses of VPM neurons [ANOVA(anatomical location); F(1,162) = 5.094, P = 0.0253]. A comparison of effects elicited by phasic versus tonic LC activation across all neurons regardless of cell-specific modulatory effects revealed region specific actions that were dependent on the specific mode of LC output. Within the VPM thalamus, phasic and tonic LC activation facilitated target neuron responses to a similar extent (range = 1.3- to 1.45-fold) regardless of sensory-stimulus intensity [Fig. 6C; ANOVA(LC activation mode); F(2,116) = 4.6229, P = 0.0117]. In contrast, modulation of sensory-evoked responses in the BF cortex was dependent on both stimulus intensity and mode of LC output [Fig. 6D; ANOVA(sensory stim. intensity × LC mode); F(2,138) = 5.5047, P = 0.0050]. For example, under phasic LC stimulation conditions, responses to maximal stimulus input levels were facilitated (1.45-fold), whereas responses to half-maximal stimulus intensities demonstrated little change from control conditions (0.84-fold suppression that did not reach statistical significance). Tonic LC activation produced a similar degree of facilitation across both sensory-stimulus levels in BF cortex (1.52- to 1.60-fold from control). The extent to which VPM and BF cortical sensory-evoked responses were facilitated by tonic LC activation were consistent with previous reports (Devilbiss et al. 2004, 2006; Snow et al. 1999).
In summary, phasic and tonic LC output produced cell-specific modulatory actions across groups of sampled neurons from the VPM and BF cortex. The net effect of tonic LC activation was to facilitate sensory responses to a similar degree across all stimulus intensities regardless of neuromodulatory profiles of individual neurons. In contrast, phasic LC output produced a net, selective facilitation of responses of BF cortical neurons to strong stimulus input. These interactions between different modes of LC activation and regional and stimulus intensity-dependent effects indicate potential biologically relevant changes in the ability of the subject to encode stimulus intensities during conditions that elicit phasic patterns of LC discharge.
Phasic versus tonic LC stimulation on thalamic and cortical ensemble stimulus-response curves
Representations of sensory features distributed across discharge patterns of ensembles of neurons within the VPM thalamus and BF cortex were further studied with principal component (PC) analysis. Previous studies have demonstrated that tonic LC output can alter distributed neural representations of sensory features within the VPM thalamus (Devilbiss et al. 2006). To initially address whether phasic versus tonic LC activation differentially altered ensemble representations of sensory input, PC eigenfunctions were generated from spike train data recorded from the VPM thalamus or BF cortex of each experimental animal. Spike train activity was averaged across each simultaneously recorded neuron of an ensemble weighted by one of the principal component eigenvectors (Chapin and Nicolelis 1999; Devilbiss et al. 2002, 2006). On average, 16 VPM (range, 10–21 cell/animal) or 20 BF cortical (range, 14–23 cell/animal) neurons were used to generate PC eigenfunctions for analysis.
Analysis of PC eigenfunction activity revealed a monotonic relationship between PC eigenfunction responses and increasing intensities of sensory-stimuli (Fig. 7A). The S-R relationship of PC1 calculated from VPM or BF cortical neurons was generally not affected by phasic or tonic LC activation (Fig. 7B, top). However, one notable exception was that tonic LC stimulation facilitated PC1 representations of VPM ensemble activity at all stimulus intensities examined [ANOVA(LC activation mode); F(2,20) = 12.635, P < 0.0003]. The magnitude of facilitation demonstrated by PC1 during tonic LC output conditions (1.21- to 1.27-fold) was similar to that previously reported for 1.0 Hz tonic LC stimulation (Devilbiss et al. 2006). Responses of PC2 to increasing sensory-stimulus intensities were markedly more sensitive to the modulatory actions of phasic versus tonic LC output (Fig. 7B, bottom). Across VPM thalamic neuronal ensembles, phasic and tonic LC activation facilitated PC2 responses to each sensory-stimulus intensity tested [ANOVA(LC activation mode); F(2,20) = 13.670, P < 0.0002]. Phasic LC activation produced roughly equivalent facilitation of VPM ensemble responses to all stimulus intensity levels (range, 1.34- to 1.4-fold change from control). Likewise, tonic LC activation produced similar facilitation across stimulus intensity levels (range, 1.55- to 1.81-fold from control). Although a trend for tonic LC activation to elicit greater facilitation of PC2 responses to maximal sensory-stimulus intensities was apparent, this effect was not statistically significant. In contrast, PC2 eigenfunctions calculated from BF cortical ensembles demonstrated fundamentally different LC-mediated modulatory patterns compared with those observed from the VPM thalamus.
Phasic LC activation produced significantly different modulatory effects for cortical PC2 S-R relationships than those observed under tonic LC output conditions [ANOVA(LC activation mode); F(2,20) = 19.622, P < 0.00002]. Specifically, phasic LC output facilitated sensory-evoked responses of PC2 to maximal stimulus intensities (1.19-fold) and suppressed PC2 responses to low stimulus intensities (0.8-fold). In contrast, under tonic LC output conditions PC2 responses were facilitated to a similar degree for all sensory-stimulus intensities. Although low sensory-stimulus intensities tended to be facilitated to the largest degree (1.51-fold), these actions were again not statistically different from facilitation of maximal stimulus intensities (1.27-fold). Importantly, the interaction between increasing sensory-stimulus intensities and the modulatory actions of phasic LC output was statistically significant (ANOVA(sensory stim. intensity × LC mode); F(2,20) = 9.816, P < 0.0011]. These data indicate that cell ensembles are differentially regulated by phasic versus tonic LC output modes at each level of the trigeminal somatosensory network in a manner that is consistent across animals.
Phasic versus tonic LC stimulation and functional connectivity of VPM, BF cortical, and thalamic-cortical ensembles
To further study the differential impact of phasic and tonic LC output on sensory signal coding across neural ensembles of the thalamic and cortical circuitry, we examined the effects of phasic and tonic LC activation on correlated or near-coincident discharge between simultaneously recorded cells of the VPM thalamus and BF cortex. Cross-correlegrams were generated for each neuron pair within the VPM thalamus and BF cortex as well as between neurons between each region correcting for stimulus-dependent changes in discharge rates (Fig. 8A and Supplemental Fig. S3) (Aertsen et al. 1985, 1989). Functional connectivity mappings were then generated by plotting incidences of significant correlation [exceeding 99% confidence intervals (CIs) between each neuron recorded from a given animal Fig. 8B]. Within each LC stimulation condition, significant correlated discharge between two neurons was observed typically within 30 ms of reference neuron discharge. Moreover, correlated activity was observed for neurons recorded from the same recording microelectrode as well as on separate electrodes (after confirming that a single neuron was not recorded on multiple electrodes; see methods). A statistically significant correlation observed for a neuron pair indicates a functional relationship between these cells, although not necessarily a direct synaptic connection between the neural pair (Aertsen et al. 1985, 1989; Devilbiss et al. 2002, 2006).
Phasic and tonic LC stimulation produced several distinct changes in functional connectivity mappings between neurons of the VPM thalamus and the BF cortex as well as between these anatomical regions within a given experimental subject. First, patterns of correlated activity across neuron pairs were altered between baseline and either tonic or phasic LC output conditions, such that a given neuronal pair either initially demonstrated no significant relationship during control conditions but correlated activity during either tonic or phasic LC activation conditions or vice versa. Second, when significant correlated activity was averaged across cell pairs in a given anatomical region, these data revealed that functional connectivity among VPM thalamic neurons was the most sensitive to LC output.
Functional connectivity plots generated from a representative animal (Fig. 8B) illustrate that significant correlated discharge was observed for 48% of VPM neuron pairs during the 30 ms window following presentation of sensory stimuli (mean, 1,777 of 3,673 neural pairs) under control conditions. Phasic activation of the LC increased the number of significant correlations 2.13-fold accompanied by a 4.0-fold increase in the mean correlational strength (Fig. 8Ciii, right). In comparison, tonic LC output increased the number of functionally connected pairs 1.87-fold resulting in a 1.49-fold increase in the strength of correlation between VPM neuron pairs (Fig. 8Ciii, left). Interestingly, phasic LC stimulation increased correlated activity prior to and after the reference neuron discharge, whereas during tonic LC stimulation conditions the increases in correlated activity principally occurred following the reference neuron discharge.
Within the BF cortex, correlated activity between pairs neurons was initially 0.87 fold of that observed for thalamic neurons under control conditions (Fig. 8B). Among these BF cortical neuron pairs, the modulatory effects of phasic and tonic LC stimulation were much smaller relative to what was observed in the VPM thalamus (Fig. 8Ci). For example, phasic LC output had little effect on either the number of incidences of correlation (1.18-fold increase) or the mean correlational strength (1.19-fold increase) between cortical neuron pairs. Likewise, tonic LC stimulation only produced minor effects on the number of significant correlations between cortical neurons (1.25-fold increase) in addition to a relatively small 1.39-fold increase in mean correlational strength.
Between the VPM thalamus and BF cortex, functional connectivity between pairs of neurons was preferentially affected by phasic LC activation. Under control conditions, significant correlated activity was observed for a number of thalamic and cortical neuron pairs. This correlated activity was evenly distributed across the ±30 ms analysis window (2,154/4,970; Fig. 8B). Phasic LC activation modestly increased both the number of significant correlations (1.57-fold) and mean correlational strength (2.38-fold) between neurons in these regions (Fig. 8Cii). This increase occurred principally within 10 ms of the reference neuron discharge, but was also evident as a late correlation (10–20 ms after the reference neuron discharge). In contrast, tonic LC stimulation produced much smaller increases in both of these measures (1.3-fold, number of significant correlations; 1.16-fold correlational strength) and the overall distribution of these relationships within the 30 ms window remained flat.
These correlational analyses were performed on datasets from all subjects yielding similar results (Fig. 8D). Across all animals tested, LC stimulation altered the number of significant correlations and correlational strength [ANOVA(LC Activation Mode); F(2,60) = 4.659, P = 0.0132]. Again, these effects were specific to the anatomical location of neuron pairs [ANOVA(anatomical location × LC mode); F(4,60) = 2.871, P = 0.0304]. Accordingly, these data indicate that LC output exerts a qualitatively different and larger effect on functional connectivity within thalamic ensembles as opposed to cortical networks. This action is reflected, in part, by the additional increase of functional connectivity between thalamic and cortical neuron pairs. Overall both modes of LC output increase thalamic neuron functional connectivity as well as connectivity between thalamic and cortical neurons while coincidently increasing the independence of cortical neurons. Importantly, phasic LC output produced a qualitatively different and larger effect on functional connectivity compared with tonic LC activation.
DISCUSSION
The present study demonstrates that phasic and tonic modes of LC output differentially regulate single unit, local circuit, and neural network representations of sensory information within the trigeminal thalamocortical circuitry of awake, quietly resting animals. Phasic activation of the LC facilitated the majority of VPM thalamic and BF cortical neuronal responses to peripheral somatosensory input. The modulatory actions of phasic LC activation were cell-specific and differed from the effects of tonic LC stimulation when these two modes of LC output were directly compared within a given sensory neuron. Within the BF cortex, modulation of sensory-evoked responses by phasic LC activation was dependent on the intensity of sensory stimuli. This unique interaction was apparent for sensory-evoked responses of single neurons and distributed representations of sensory stimuli by neural ensembles as measured by PCA. Coincident with these actions, phasic LC activation increased functional connectivity to noise sources among VPM neurons to a greater extent than tonic LC output. Additionally, this increase in functional connectivity was not observed among BF cortical neurons. Together these observations provide insight regarding the physiologically relevant differences between phasic versus tonic modes of LC output and their impact on neural circuit operations and sensory signal processing.
Neuromodulatory mechanisms
SPECIFICITY OF NE ACTIONS.
Direct comparisons of the effects of phasic versus tonic activation of the LC can only be made using electrical stimulation paradigms; although disadvantages to this technique have been discussed and include activation of adjacent tissue in the pons, fibers of passage, and non-LC neurons (Berridge and Abercrombie 1999; Foote et al. 1991). Importantly, the combination of low stimulus currents shown to have minimal current spread (Snow et al. 1999; Stoney et al. 1967; Yeomans 1990), electrophysiological confirmation that stimulation electrodes were within or immediately adjacent to the LC during surgery (Akaike 1982), and postmortem with histological methods, suggests that in the current study activation was likely confined to the LC and peri-LC regions. Moreover, the lack of overt whisker movement and lack of somatosensory neuron response to LC stimulation as a result of activating the neighboring mesencephalic trigeminal nucleus further supports the likelihood that our LC electrical stimulation had a minimal sphere of influence. Thus in our view, it is unlikely that the stimulus protocol employed here was sufficient to activate other reticular modulatory systems that project to thalamus and cortex as is observed following strong LC activation or associated cholinergic pathway activation (Berridge and Foote 1991; Buzsaki et al. 1988; McLin et al. 2002).
Previous studies suggest that in addition to release of NE, phasic patterns of discharge may be sufficient for releasing neuroactive peptides from axon terminals (Dutton and Dyball 1979; Karhunen et al. 2001; Verhage et al. 1991). Galanin and neuropeptide Y are colocalized with NE in LC neurons and suppress excitatory neurotransmission (Bijak 2000; Colmers et al. 1987; Pieribone et al. 1998; Schlifke et al. 2006; Simpson et al. 1999, 2006). Although the present experiments did not address the degree to which LC peptide co-transmitters contribute to observed effects, increases in thalamic and cortical neural responsiveness to sensory input are contrary to the previously reported actions of galanin and neuropeptide Y. Instead the observed effects are generally similar to those reported for locally administered NE (see review, Berridge and Waterhouse 2003). Nonetheless differences observed between tonic and phasic modes of LC activation could, in part, result from differential release of NE versus peptide.
HETEROGENEITY OF EFFECTS ON TARGET NEURONS.
Previous studies have demonstrated that NE modulates responses of VPM and BF cortical neurons in a manner that is specific to individual target neurons. This heterogeneity in target cell action has been observed under a number of experimental conditions including: phasic LC stimulation in anesthetized rat (Lecas 2001, 2004; Snow et al. 1999; Waterhouse et al. 1988, 1998), local iontophoretic administration of NE (Armstrong-James and Fox 1983; Devilbiss and Waterhouse 2000, 2004; Foote et al. 1975; George 1992; Holdefer and Jacobs 1994; Kossl and Vater 1989; McCormick et al. 1991; Rogawski and Aghajanian 1980; Sato and Kayama 1983; Snow et al. 1999; Waterhouse et al. 1990; Waterhouse and Woodward 1980), and tonic LC stimulation in the awake freely moving rat (Devilbiss and Waterhouse 2004; Devilbiss et al. 2006). Most likely, this heterogeneity is a result of the neuronal expression of adrenergic receptor subtypes, receptor sensitivity for NE binding, and local concentrations of NE (Berridge and Waterhouse 2003). For example, in sensory circuits, facilitation of excitatory responses has been shown to be principally mediated by the alpha-1 receptor, whereas suppression of evoked-activity is likely a result of NE acting on postsynaptic alpha-2 or beta receptors (Devilbiss and Waterhouse 2000). Increasing frequencies of tonic LC stimulation elevates tissue levels of NE within LC terminal fields (Abercrombie and Finlay 1991; Berridge and Abercrombie 1999; Devilbiss et al. 2006; Florin-Lechner et al. 1996). Increasing tonic LC output or local iontophoretic delivery of NE produces a net facilitation of somatosensory neuron responses to afferent synaptic inputs across an inverted-U dose-response profile even though individual neurons demonstrate cell-specific modulatory profiles (Devilbiss and Waterhouse 2004; Waterhouse et al. 1980, 1998). Phasic LC stimulation yields fourfold higher NE efflux compared with tonic stimulation at 1.0 Hz (Florin-Lechner et al. 1996). However, neither differences in phasic versus tonic NE efflux levels nor the inverted-U modulatory profile of target neuron evoked responses across increasing tonic LC output simply predicted the net modulatory effects of phasic LC output on sensory-evoked responses observed in the current studies. Phasic activation of the LC produced a unique array of modulatory profiles of individual neurons across VPM and BF cortical ensembles distinct from that observed during tonic LC output. This diversity of individual neuronal responses likely provides for a richer representation of information within a population of neurons (Chelaru and Dragoi 2008; Shamir and Sompolinsky 2006). Together, this evidence and that from the present study suggests that phasic LC output provide for more fine-grained coding of sensory information.
Anatomical studies have demonstrated LC collateral innervation of multiple sites along the ascending somatosensory path (Simpson et al. 1997). Such organizational features of the LC efferent system indicate that activation of the LC is capable of NE release and modulation of stimulus-driven signals at multiple sites along the ascending somatosensory pathway. Current findings and previous reports (Devilbiss and Waterhouse 2004) indicate that the effects of LC stimulation and NE application are region specific. However, as shown here, the impact of either phasic or tonic LC-NE output on sensory signals was not simply additive as signals progressed through sequentially higher-order brain regions. Accordingly, cell type, local circuit mechanisms of inhibition and excitation that define receptive field properties, and differences in terminal field NE concentration could be responsible for these regional neuromodulatory differences. Given the prominence of these regional effects, it will be important for future studies to consider these differences in addition to patterns of LC-NE innervation in theories of LC function.
TEMPORAL RELATIONSHIP BETWEEN LC ACTIVITY AND TARGET NEURON ACTIONS.
The relative timing of phasic LC bursts to temporally locked information processing events in target brain regions is dependent on the timing of LC activation, conduction velocity of LC axons, and dynamics of NE release. Under physiological conditions, nonnoxious sensory stimuli evoke excitatory LC discharge with an onset latency of 20–70 ms that terminates after 100–300 ms (Aston-Jones and Bloom 1981b; Berridge and Waterhouse 2003; Chiang and Aston-Jones 1993; Sara et al. 1994). Differences in LC excitatory response durations have been shown to be specific to sensory modality and dependent on the level of arousal (Aston-Jones and Bloom 1981b). Slow conduction velocities of LC neurons induce a further 60–70 ms delay between action-potential generation and arrival in terminal cortical regions (Aston-Jones et al. 1980, 1985). In total, the latency between a strong and salient sensory event (unlike marginal-intensity sensory stimuli used in the current study) and putative NE release in the somatosensory cortex is estimated to occur within the range of 100–350 ms. Moreover, top-down activation of the LC, generated by internal decision-making processes occurs ∼200 ms before behavioral motor-responses (Rajkowski et al. 2004). These estimates are further supported by evidence by Waterhouse et al. (1998) suggesting that a 200–300 ms gap between phasic LC stimulation pulses and afferent input maximally facilitates target cell excitatory activity. The time frame of ∼300 ms was also demonstrated to maximally modulate spontaneous activity by Holdfeller and Jacobs (1994). Thus regardless of the circuitry activating the LC, optimization of target circuit functions occurs with a latency of 200–300 ms after phasic LC discharge. Such temporal organization of LC actions are likely important for optimizing the feature extraction properties of target circuitry and enabling these signal processing operations to appropriately guide decisions and motor responses.
ORIGIN OF VPM CORRELATED ACTIVITY.
Current findings and previous reports show that increased LC output enhances correlated discharge of VPM neurons within a recorded ensemble. The timing of correlated discharge between VPM neurons was nearly coincident and occurred within a narrow time window. This pattern of correlated discharge can be generated by a number of mechanisms including reciprocal connectivity among cells or a common input (Bartho et al. 2004; Csicsvari et al. 1998; Lampl et al. 1999; Marshall et al. 2002). Reciprocal connectivity is not supported as a potential mechanism in this case because neurons of the rodent VPM are not anatomically interconnected (Barbaresi et al. 1986). Common inputs capable of generating correlated discharge between neural pairs include sensory stimuli eliciting simultaneous discharge or a common excitatory source of neural noise that may not necessarily represent a direct monosynaptic connection between a neural pair (Aertsen and Gerstein 1985; Aertsen et al. 1989; Devilbiss and Waterhouse 2002; Devilbiss et al. 2006). Stimulus-induced correlations were minimized by application of a trial-by-trial shift predictor (Gerstein et al. 1989), thus suggesting a common excitatory neural noise source driving VPM correlated activity. The most likely source of these noise-induced VPM correlations is spontaneous excitatory input from principal (PrV) trigeminal nucleus projections or spinal trigeminal nucleus (SpV) projections to VPM thalamus (Chiaia et al. 1991; Friedberg et al. 2004; Timofeeva et al. 2004; Wang and Ohara 1993). Other well described circuit mechanisms could also produce noise correlations among VPM neurons including descending projections from the BF cortex (Bal et al. 1995; Bal and McCormick 1996; Friedberg et al. 2004; Harris 2005; Lampl et al. 1999; Nicolelis et al. 1995; Ritz and Sejnowski 1997; Singer 1999). In any case, origins of VPM correlated activity are poorly understood. Thus future studies will need to address the mechanisms through which LC output exerts these varied influences on connectivity within local circuits and across neural networks as well as consider the net impact of these effects on bottom-up signal processing versus top-down corticothalamic influences.
Estimates of noise correlations between VPM neurons were made by applying a trial-by-trial shift predictor to correlegrams. The shuffle-corrected cross-correlegram is generally a straightforward approach and minimizes the effects of stimulus-induced signal correlations when Brody's rules-of-thumb (Brody 1999) are observed and relative changes in correlation are of interest over absolute determination of correlation. Although this technique has been shown to be limited in its interpretation in some cases (Brody 1999; Schneidman et al. 2003), analysis of our cross-correlational data was not impeded by corresponding increases in spontaneous discharge rates (see supplementary material). Nevertheless, future approaches may benefit by using Bayesian approaches in place of correlational measures to measure dependence between neural spike trains.
Functional relevance to sensory signal processing
MODULATION OF SINGLE NEURON ACTIVITY.
A number of studies have demonstrated that sensory signal encoding is dynamical by nature. For example, it is well known that sensory signal processing is regulated by behavioral state, depth of anesthesia, adaptation during sequential deflections of a single or multiple whiskers, and active whisking versus passive stimulus presentation (Fanselow and Nicolelis 1999; Friedberg et al. 1999; Krupa et al. 2001; Kleinfeld et al. 2006; Lottem and Azouz 2009; Maravall et al. 2007; Ritt et al. 2008; Simons et al. 1992). However, output from the LC-NE system likely provides another mode of control over the intrinsic dynamics of the rodent vibrissa circuitry that tunes this system to favor either detectability or discriminability of stimulus. For instance, our previous work demonstrated that tonic stimulation of the LC at 0.5–1.0 Hz produced changes in a number of sensory stimulus encoding strategies including discharge rate, spike timing, correlated discharge, and neuronal population codes (Devilbiss and Waterhouse 2004, 2006). From this body of work, we posited that under conditions of optimal tonic firing rate LC output primes the somatosensory system to facilitate transmission of sensory signaling through thalamic and cortical circuitry. The results of the current study show that tonic LC output facilitates low-level sensory input to the same degree as high stimulus-intensities and therefore extends this hypothesis to suggest that optimal tonic LC output increases the overall gain of ascending excitatory signals through the thalamic and cortical networks. Moreover this facilitation of neuronal responses to a broad range of stimulus intensities likely enhances detection of not only threshold but also marginal and subliminal sensory inputs.
Phasic bursts of LC activity demonstrated a number of modulatory effects that were similar to those elicited by tonic LC output. However, a number of important differences were observed with respect to the intensity of the sensory stimulus and the slope of the stimulus-response function. Current and previous evidence suggests that phasic LC output likely increases sensory signal transmission through thalamic and cortical circuitry. For example, current results are consistent with previous one cell-at-a-time recording studies in sensory cortex of anesthetized rats demonstrating a net facilitating action of phasic LC output on responses to strong and salient sensory input (Lecas 2001, 2004; Snow et al. 1999; Waterhouse et al. 1988, 1998) as well as instances of “gating” following local application of NE (Hurley et al. 2004; Manunta and Edeline 1998; Rogawski and Aghajanian 1982). Importantly, when comparisons were made between the impact of phasic versus tonic LC output for the same neural ensembles, it was evident that modulation of sensory-evoked discharge of BF cortical neurons was dependent on the intensity of the input stimulus. Other lines of research have documented the relationship between intensity of stimulus input and sensory feature primitives such as amplitude, velocity, and direction of whisker deflection and discharge rates of VPM and BF cortical neurons (Ahissar et al. 2000; Armstrong-James et al. 1992; Ito 1988; Shoykhet et al. 2000; Simons 1978; Waite 1973). Moreover it has been shown by others that rate encoding of these sensory properties is important for discriminating objects and textures (Maravall et al. 2007; Shoykhet et al. 2000; von Heimendahl et al. 2007). Although the behavioral impact of LC-mediated changes to neuronal and neural network input/output functions remains open to speculation, we propose that selective enhancement of responses to large amplitude stimulus inputs over marginal inputs permits better discrimination between whisker deflection magnitudes.
MODULATION OF FUNCTIONAL CONNECTIVITY.
Correlated neural discharge patterns during peripheral stimulus response epochs were used as a measure of effective synaptic weight between pairs of neurons and to define patterns of functional connectivity across ensembles of neurons (Gerstein et al. 1989). In general, phasic LC stimulation increased both the number and strength of functional connections among neurons in the VPM circuitry, nearly doubling the effective size of the VPM cellular ensemble across animals studied. As such, highly correlated discharge across VPM neurons can provide a strong afferent drive for cortical neurons (Salinas and Sejnowski 2000; Sejnowski and Paulsen 2006) through coincidence of closely timed thalamic inputs and subsequent temporal summation of excitatory post synaptic potentials (Wilent and Contreras 2005). Noise correlations, as increased by LC output across VPM neurons, likely has an important role in information encoding by neural ensembles (Latham and Nirenberg 2005; Schneidman et al. 2003). For example, enhanced functional connectivity to noise sources observed during phasic LC activation theoretically could boost signal detection by VPM neurons through stochastic resonance mechanisms (Moss et al. 2004). Furthermore within sensory systems, downstream regions of the VPM thalamus (i.e., BF cortex) may more easily remove correlated noise by taking advantage of the redundancies present across ensembles of neurons and as a result permit compression of the sensory information by reducing its dimensionality (Averbeck et al. 2006). Within the BF cortex noise correlations are reduced, albeit to a small degree, with phasic LC activation (see Fig. 8Dii). It is likely that these reductions in correlated noise within the cortex during signal processing permits a more efficient and sparse neural coding scheme that is generally more sensitive to noise dependent errors. Mechanistically, we posit that the opposing effects of phasic LC output on noise correlations in the BF cortex and VPM thalamus are likely a result of LC effects on inhibitory and excitatory cortical neurons across cortical layers; however, the precise mechanism underlying these brain region differences will need to be investigated in future studies. Thus differences in connectivity to noise sources across ensembles during phasic versus tonic LC output conditions intuitively suggests that this reorganization of functional connectivity is likely beneficial for the neural population to represent small fluctuations in stimulus intensities and enhance the signal processing capabilities of sensory circuitry.
MODULATION OF POPULATION CODES.
Behaviorally relevant information about whisker movements including amplitude and direction is represented in the coordinated discharge patterns of sensory system neuronal ensembles (Chapin and Nicolelis 1999; Devilbiss et al. 2002, 2006; Laurent et al. 1996; Nicolelis et al. 1995). PCA was used to quantify these distributed discharge patterns, as each statistically orthogonal component reflects independent sensory-stimulus properties encoded in the variance of discharge patterns across neural populations. Unlike single neuron analyses, unweighted averages across large numbers of neurons, or field potential analysis, principal component representations above PC1 are unrelated to the firing rates of individual neurons. Functionally, the eigenfunction of PC1 is a measure of the generalized magnitude of discharge across a population of neurons and reflects information pertaining to the overall magnitude of whisker deflection, whereas PC2 specifically relates to directional movement of the vibrissae along the rostrocaudal axis (Chapin and Nicolelis 1999). Information pertaining to the overall magnitude of whisker deflection as well as directional information represented by PC2 have previously been shown to be represented in neural responses to whisker-pad stimuli used in the current study (Devilbiss et al. 2006). Directional information may result from a number of putative mechanisms including activation of a subset of afferent nerve terminals including Merkel cells or as a result of very small stimulus-induced contractions of the musculature below threshold for producing visible movement of the whiskers.
Phasic and tonic LC output conditions produced significant changes in the average response of PC2 to sensory stimuli. Within the BF cortex, the increase in slope of the stimulus-response function suggests that under conditions of phasic LC output the representation of directional-specific information by these neurons is altered. We hypothesize that selective facilitation of PC2 responses to intense stimulus input permits the disambiguation of directional vectors and increases the ability of the animal to discriminate directionality of whisker movement. In contrast, a general facilitation of PC2 regardless of stimulus intensity during tonic LC output conditions was observed in current and previous studies (Devilbiss et al. 2006). Likely, the general facilitation of PC2 under tonic LC activation conditions reflects the enhancement of detectability of marginal and subliminal sensory inputs.
Relevance to current theories of LC function
Modern theories of LC function account for its role in controlling mechanisms of adaptive gain (both tonic and phasic modes of LC output) (Aston-Jones and Cohen 2005b), mediating unexpected uncertainty (phasic and tonic output) (Dayan and Yu 2006; Yu and Dayan 2005), and network resetting (phasic output only Bouret and Sara 2005; Dayan and Yu 2006). Moreover these theories have argued either for a top-down cortical regulation of LC activity versus bottom-up sensory driven activation of LC output. In general, these ideas derive from LC unit recording studies in behaving animals or computational models that translate LC activity states to behavioral outcomes. Such predictions lack detail regarding the impact of LC output on target neurons and target neuronal networks. The adaptive gain model proposed by Aston-Jones and Cohen (2005b) predicts that tonic LC activity increases the “gain” or global responsivity of cells in noradrenergic terminal fields to any excitatory input, whereas phasic LC output increases the “gain” of all units in a manner that produces an “all or nothing” response profile to excitatory input. A further understanding of the impact of adaptive gain modulation on discharge properties of target neurons is provided by recent studies in primary visual cortex suggesting that under high gain conditions (as would occur following phasic bursts of LC discharge), weakly active neurons are suppressed concordant with a competitive enhancement of strongly active neurons (Schafer et al. 2009). Although the LC adaptive gain theory was originally proposed for associational cortices and decision making networks, our current data support this hypothesis by demonstrating that phasic LC output generally increases the responsiveness of neurons in sensory thalamus and cortical regions to excitatory afferent stimuli and alters stimulus input-response output function of cortical neuron responses to increasing afferent input strength, whereas tonic LC output facilitates cortical responses of individual neurons to all levels of afferent input.
Interestingly, our findings are in general agreement with computational models examining the putative neural adaptive gain mechanisms underlying LC mediated changes in task performance (Aston-Jones and Cohen 2005a; Eckhoff et al. 2009; Usher et al. 1999). Furthermore, our data are partially supportive of the hypothesis set forth by Bouret and Sara (2005). Their network reset model predicts that phasic LC discharge resets the network by dissolving existing functional connectivity structures. Subsequent to this reset, the network becomes permissive to form alternative network organizational structures during this step-wise process. However, we did not observe an overall degradation in connectivity structure among cortical neurons following phasic LC activation (i.e., global reduction in the number and strength of functional connections) but rather a nearly instantaneous reconfiguration of connectivity structure (i.e., increases and decreases in the number and strength of functional connections). Last, the model proposed by Dayan and colleagues. (Dayan and Yu 2006; Yu and Dayan 2005) predicts that LC output should suppress top-down information relative to ascending sensory-induced signals. However, in the current study, we did not distinguish between the top-down versus bottom up information and cannot provide support for or against this model.
Conclusions
Regardless of whether LC activity is generated by bottom-up sensory processes or top-down signals from associational cortical regions, our results are consistent with the notion that each mode of LC output subserves a different role in optimizing the neurocomputational ability of the circuits within the ascending somatosensory system. We hypothesize that increased functional connectivity within the VPM coupled with the direct actions of NE in the cortex during phasic LC output could rescale stimulus-response relationships. The adaptive rescaling of cortical neurons and ensemble stimulus-response relationships by phasic LC output is likely important for increasing the ability of the somatosensory system to discriminate differences in whisker movement amplitude, velocity, and direction. Likely these principles extend to other brain regions including those involved in higher cognitive functions to differentially regulate neural responses and local circuit operations according to behavioral demands.
GRANTS
This study was funded by National Institute of Drug Abuse Grant DA-017960 to B. D. Waterhouse.
DISCLOSURES
D. M. Devilbiss is the founder of NexStep Biomarkers, LLC. NexStep Biomarkers had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. NexStep Biomarkers, does not employ anyone who worked on this project, hold patents related to this project, sell products related to this project, or provided consultation on this project. This manuscript provides no financial gain for NexStep Biomarkers.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Drs. Craig Berridge, John Chapin, and Rita Valentino for timely discussions and helpful comments throughout various stages of the work and R. Markowitz for help with electrophysiological data collection.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Abercrombie ED, Finlay JM. Monitoring extracellular norepinephrine in brain using in vivo microdialysis and HPLC-EC. In: Microdialysis in the Neurosciences, edited by Robinson T, Justice J. Amsterdam: Elsevier, 1991, p. 253–274 [Google Scholar]
- Aertsen AM, Gerstein GL. Evaluation of neuronal connectivity: sensitivity of cross-correlation. Brain Res 340: 341–54, 1985 [DOI] [PubMed] [Google Scholar]
- Aertsen AM, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity.” J Neurophysiol 61: 900–917, 1989 [DOI] [PubMed] [Google Scholar]
- Ahissar E, Sosnik R, Haidarliu S. Transformation from temporal to rate coding in a somatosensory thalamocortical pathway. Nature 406: 302–306, 2000 [DOI] [PubMed] [Google Scholar]
- Ahissar E, Haidarliu S, Zacksenhouse M. Decoding temporally encoded sensory input by cortical oscillations and thalamic phase comparators. Proc Natl Acad Sci USA 94: 11633–11638, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike T. Periodic bursting activities of locus coerulleus neurons in the rat. Brain Res 239: 629–633, 1982 [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K. Effects of ionophoresed noradrenaline on the spontaneous activity of neurons in rat primary somatosensory cortex. J Physiol 335: 427–447, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K, Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurophysiol 68: 1345–1358, 1992 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci 1: 876–886, 1981a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Nonrepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci 1: 887–900, 1981b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol 493: 99–110, 2005a [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28: 403–450, 2005b [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Foote SL, Segal M. Impulse conduction properties of noradrenergic locus coeruleus axons projecting to monkey cerebrocortex. Neuroscience 15: 765–777, 1985 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Gold JI. How we say no: norepinephrine, inferior frontal gyrus, and response inhibition. Biol Psychiatry 65: 548–549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P. Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience 80: 697–715, 1997 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Segal M, Bloom FE. Brain aminergic axons exhibit marked variability in conduction velocity. Brain Res 195: 215–222, 1980 [DOI] [PubMed] [Google Scholar]
- Averbeck BB, Latham PE, Pouget A. Neural correlations, population coding and computation. Nat Rev Neurosci 7: 358–366, 2006 [DOI] [PubMed] [Google Scholar]
- Bal T, McCormick DA. What stops synchronized thalamocortical oscillations? Neuron 17: 297–308, 1996 [DOI] [PubMed] [Google Scholar]
- Bal T, von Krosigk M, McCormick DA. Synaptic and membrane mechanisms underlying synchronized oscillations in the ferret lateral geniculate nucleus in vitro. J Physiol 483: 641–663, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaresi P, Spreafico R, Frassoni C, Rustioni A. GABAergic neurons are present in the dorsal column nuclei but not in the ventroposterior complex of rats. Brain Res 382: 305–326, 1986 [DOI] [PubMed] [Google Scholar]
- Bartho P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsaki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol 92: 600–608, 2004 [DOI] [PubMed] [Google Scholar]
- Berridge CW, Abercrombie ED. Relationship between locus coeruleus discharge rates and rates of norepinephrine release within neocortex as assessed by in vivo microdialysis. Neuroscience 93: 1263–1270, 1999 [DOI] [PubMed] [Google Scholar]
- Berridge CW, Foote SL. Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J Neurosci 11: 3135–3145, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42: 33–84, 2003 [DOI] [PubMed] [Google Scholar]
- Bijak M. Neuropeptide Y reduces epileptiform discharges and excitatory synaptic transmission in rat frontal cortex in vitro. Neuroscience 96: 487–494, 2000 [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci 28: 574–582, 2005 [DOI] [PubMed] [Google Scholar]
- Brody CD. Correlations without synchrony. Neural Comput 11: 1537–1551, 1999 [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci 8: 4007–4026, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin JK, Nicolelis MA. Principal component analysis of neuronal ensemble activity reveals multidimensional somatosensory representations. J Neurosci Methods 94: 121–140, 1999 [DOI] [PubMed] [Google Scholar]
- Chelaru MI, Dragoi V. Efficient coding in heterogeneous neuronal populations. Proc Natl Acad Sci USA 105: 16344–16349, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaia NL, Rhoades RW, Bennett-Clarke CA, Fish SE, Killackey HP. Thalamic processing of vibrissal information in the rat. I. Afferent input to the medial ventral posterior and posterior nuclei. J Comp Neurol 314: 201–216, 1991 [DOI] [PubMed] [Google Scholar]
- Chiang C, Aston-Jones G. Response of locus coeruleus neurons to footshock stimulation is mediated by neurons in the rostral ventral medulla. Neuroscience 53: 705–715, 1993 [DOI] [PubMed] [Google Scholar]
- Churchland PM. A Neurocomputational Perspective. Cambridge, MA: MIT Press, 1989 [Google Scholar]
- Clayton EC, Rajkowski J, Cohen JD, Aston-Jones G. Phasic activation of monkey locus ceruleus neurons by simple decisions in a forced-choice task. J Neurosci 24: 9914–9920, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers WF, Lukowiak K, Pittman QJ. Presynaptic action of neuropeptide Y in area CA1 of the rat hippocampal slice. J Physiol 383: 285–299, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Buzsaki G. Reliability and state dependence of pyramidal cell-interneuron synapses in the hippocampus: an ensemble approach in the behaving rat. Neuron 21: 179–189, 1998 [DOI] [PubMed] [Google Scholar]
- Curtis JC, Kleinfeld D. Phase-to-rate transformations encode touch in cortical neurons of a scanning sensorimotor system. Nat Neurosci 12: 492–501, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, Yu AJ. Phasic norepinephrine: a neural interrupt signal for unexpected events. Network 17: 335–350, 2006 [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Page ME, Waterhouse BD. Locus ceruleus regulates sensory encoding by neurons and networks in waking animals. J Neurosci 26: 9860–9872, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. Norepinephrine exhibits two distinct profiles of action on sensory cortical neuron responses to excitatory synaptic stimuli. Synapse 37: 273–282, 2000 [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. Determination and quantification of pharmacological, physiological, or behavioral manipulations on ensembles of simultaneously recorded neurons in functionally related neural circuits. J Neurosci Methods 121: 181–198, 2002 [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. The effects of tonic locus ceruleus output on sensory-evoked responses of ventral posterior medial thalamic and barrel field cortical neurons in the awake rat. J Neurosci 24: 10773–10785, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton A, Dyball RE. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol 290: 433–440, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhoff P, Wong-Lin KF, Holmes P. Optimality and robustness of a biophysical decision-making model under norepinephrine modulation. J Neurosci 29: 4301–4311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ. Neural interaction in cat primary auditory cortex. Dependence on recording depth, electrode separation, and age. J Neurophysiol 68: 1216–1228, 1992 [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Neural interaction in cat primary auditory cortex. II. Effects of sound stimulation. J Neurophysiol 71: 246–270, 1994 [DOI] [PubMed] [Google Scholar]
- Erickson RP. Stimulus coding in topographic and nontopographic afferent modalities: on the significance of the activity of individual sensory neurons. Psychol Rev 75: 447–465, 1968 [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Nicolelis MA. Behavioral modulation of tactile responses in the rat somatosensory system. J Neurosci 19: 7603–7616, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin-Lechner SM, Druhan JP, Aston-Jones G, Valentino RJ. Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res 742: 89–97, 1996 [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci USA 77: 3033–3037, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Berridge CW, Adams LM, Pineda JA. Electrophysiological evidence for the involvement of the locus coeruleus in alerting, orienting, and attending. Prog Brain Res 88: 521–532, 1991 [DOI] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev 63: 844–914, 1983 [DOI] [PubMed] [Google Scholar]
- Foote SL, Freedman R, Oliver AP. Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. Brain Res 86: 229–242, 1975 [DOI] [PubMed] [Google Scholar]
- Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. J Neurophysiol 81: 2243–2252, 1999 [DOI] [PubMed] [Google Scholar]
- Friedberg MH, Lee SM, Ebner FF. The contribution of the principal and spinal trigeminal nuclei to the receptive field properties of thalamic VPM neurons in the rat. J Neurocytol 33: 75–85, 2004 [DOI] [PubMed] [Google Scholar]
- George MJ. Modification of receptive fields of posteriomedial barrel subfield neocortical single units by known concentrations of iontophoresed noradrenaline in the rat. Int J Neurosci 65: 69–81, 1992 [DOI] [PubMed] [Google Scholar]
- Gerstein GL, Bedenbaugh P, Aertsen MH. Neuronal assemblies. IEEE Trans Biomed Eng 36: 4–14, 1989 [DOI] [PubMed] [Google Scholar]
- Harris KD. Neural signatures of cell assembly organization. Nat Rev Neurosci 6: 399–407, 2005 [DOI] [PubMed] [Google Scholar]
- Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brain stem neuronal groups. Science 189: 55–58, 1975 [DOI] [PubMed] [Google Scholar]
- Holdefer RN, Jacobs BL. Phasic stimulation of the locus coeruleus: effects on activity in the lateral geniculate nucleus. Exp Brain Res 100: 444–452, 1994 [DOI] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol 14: 488–495, 2004 [DOI] [PubMed] [Google Scholar]
- Ito M. Response properties and topography of vibrissa-sensitive VPM neurons in the rat. J Neurophysiol 60: 1181–1197, 1988 [DOI] [PubMed] [Google Scholar]
- Ivanova S, Rajkowski J, Silakov V, Watanabe T, Aston-Jones G. Local chemomanipulations of locus coeruleus (LC) activity in monkeys alter cortical event-related potentials (ERPs) and task performance. Soc Neurosci Abstr 23: 1587, 1997 [Google Scholar]
- Karhunen T, Vilim FS, Alexeeva V, Weiss KR, Church PJ. Targeting of peptidergic vesicles in cotransmitting terminals. J Neurosci 21: RC127, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katchalsky AK, Rowland V, Blumenthal R. Dynamic Patterns of Brain Cell Assemblies : A Report Based on an NRP Work Session Held May 14–16, 1972, and Updated by Participants. Cambridge, MA: MIT Press, 1974 [Google Scholar]
- Kleinfeld D, Ahissar E, Diamond ME. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr Opin Neurobiol 16: 435–444, 2006 [DOI] [PubMed] [Google Scholar]
- Kossl M, Vater M. Noradrenaline enhances temporal auditory contrast and neuronal timing precision in the cochlear nucleus of the mustached bat. J Neurosci 9: 4169–4178, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa DJ, Matell MS, Brisben AJ, Oliveira LM, Nicolelis MA. Behavioral properties of the trigeminal somatosensory system in rats performing whisker-dependent tactile discriminations. J Neurosci 21: 5752–5763, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampl I, Reichova I, Ferster D. Synchronous membrane potential fluctuations in neurons of the cat visual cortex. Neuron 22: 361–374, 1999 [DOI] [PubMed] [Google Scholar]
- Latham PE, Nirenberg S. Synergy, redundancy, and independence in population codes, revisited. J Neurosci 25: 5195–5206, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G, Wehr M, Davidowitz H. Temporal representations of odors in an olfactory network. J Neurosci 16: 3837–3847, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecas JC. Noradrenergic modulation of tactile responses in rat cortex. Current source-density and unit analyses. C R Acad Sci III 324: 33–44, 2001 [DOI] [PubMed] [Google Scholar]
- Lecas JC. Locus coeruleus activation shortens synaptic drive while decreasing spike latency and jitter in sensorimotor cortex. Implications for neuronal integration. Eur J Neurosci 19: 2519–2530, 2004 [DOI] [PubMed] [Google Scholar]
- Lottem E, Azouz R. Mechanisms of tactile information transmission through whisker vibrations. J Neurosci 29: 11686–11697, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manunta Y, Edeline JM. Effects of noradrenaline on rate-level function of auditory cortex neurons: is there a “gating” effect of noradrenaline? Exp Brain Res 118: 361–372, 1998 [DOI] [PubMed] [Google Scholar]
- Maravall M, Petersen RS, Fairhall AL, Arabzadeh E, Diamond ME. Shifts in coding properties and maintenance of information transmission during adaptation in barrel cortex. PLoS Biol 5: e19, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Henze DA, Hirase H, Leinekugel X, Dragoi G, Buzsaki G. Hippocampal pyramidal cell-interneuron spike transmission is frequency dependent and responsible for place modulation of interneuron discharge. J Neurosci 22: RC197, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC, Williamson A. Actions of norepinephrine in the cerebral cortex and thalamus: implications for function of the central noradrenergic system. Prog Brain Res 88: 293–305, 1991 [DOI] [PubMed] [Google Scholar]
- McLin DE, 3rd, Miasnikov AA, Weinberger NM. The effects of electrical stimulation of the nucleus basalis on the electroencephalogram, heart rate, and respiration. Behav Neurosci 116: 795–806, 2002 [PubMed] [Google Scholar]
- Mehta SB, Whitmer D, Figueroa R, Williams BA, Kleinfeld D. Active spatial perception in the vibrissa scanning sensorimotor system. PLoS Biol 5: e15, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss F, Ward LM, Sannita WG. Stochastic resonance and sensory information processing: a tutorial and review of application. Clin Neurophysiol 115: 267–281, 2004 [DOI] [PubMed] [Google Scholar]
- Nicolelis MA. Beyond maps: a dynamic view of the somatosensory system. Braz J Med Biol Res 29: 401–12, 1996 [PubMed] [Google Scholar]
- Nicolelis MA, Baccala LA, Lin RC, Chapin JK. Sensorimotor encoding by synchronous neural ensemble activity at multiple levels of the somatosensory system. Science 268: 1353–1358, 1995 [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL, Chapin JK. Spatiotemporal structure of somatosensory responses of many-neuron ensembles in the rat ventral posterior medial nucleus of the thalamus. J Neurosci 14: 3511–3532, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychol Bull 131: 510–532, 2005 [DOI] [PubMed] [Google Scholar]
- Pieribone VA, Xu ZQ, Zhang X, Hokfelt T. Electrophysiologic effects of galanin on neurons of the central nervous system. Ann NY Acad Sci 863: 264–273, 1998 [DOI] [PubMed] [Google Scholar]
- Pinto DJ, Brumberg JC, Simons DJ. Circuit dynamics and coding strategies in rodent somatosensory cortex. J Neurophysiol 83: 1158–1166, 2000 [DOI] [PubMed] [Google Scholar]
- Rajkowski J, Majczynski H, Clayton E, Aston-Jones G. Activation of monkey locus coeruleus neurons varies with difficulty and performance in a target detection task. J Neurophysiol 92: 361–371, 2004 [DOI] [PubMed] [Google Scholar]
- Ritt JT, Andermann ML, Moore CI. Embodied information processing: vibrissa mechanics and texture features shape micromotions in actively sensing rats. Neuron 57: 599–613, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz R, Sejnowski TJ. Synchronous oscillatory activity in sensory systems: new vistas on mechanisms. Curr Opin Neurobiol 7: 536–546, 1997 [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Aghajanian GK. Norepinephrine and serotonin: opposite effects on the activity of lateral geniculate neurons evoked by optic pathway stimulation. Exp Neurol 69: 678–694, 1980 [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Aghajanian GK. Activation of lateral geniculate neurons by locus coeruleus or dorsal noradrenergic bundle stimulation: selective blockade by the alpha 1-adrenoceptor antagonist prazosin. Brain Res 250: 31–39, 1982 [DOI] [PubMed] [Google Scholar]
- Rutter JJ, Devilbiss DM, Waterhouse BD. Effects of systemically administered cocaine on sensory responses to peri-threshold vibrissae stimulation: individual cells, ensemble activity, and animal behavior. Eur J Neurosci 22: 3205–3216, 2005 [DOI] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Impact of correlated synaptic input on output firing rate and variability in simple neuronal models. J Neurosci 20: 6193–6209, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ, Vankov A, Herve A. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res Bull 35: 457–465, 1994 [DOI] [PubMed] [Google Scholar]
- Sato H, Kayama Y. Effects of noradrenaline applied iontophoretically on rat superior collicular neurons. Brain Res Bull 10: 453–457, 1983 [DOI] [PubMed] [Google Scholar]
- Schafer R, Vasilaki E, Senn W. Adaptive gain modulation in V1 explains contextual modifications during bisection learning. PLoS Comput Biol 5: e1000617, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlifke I, Kuteeva E, Hokfelt T, Kokaia M. Galanin expressed in the excitatory fibers attenuates synaptic strength and generalized seizures in the piriform cortex of mice. Exp Neurol 200: 398–406, 2006 [DOI] [PubMed] [Google Scholar]
- Schneidman E, Bialek W, Berry MJ. Synergy, redundancy, and independence in population codes. J Neurosci 23: 11539–11553, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejnowski TJ, Paulsen O. Network oscillations: emerging computational principles. J Neurosci 26: 1673–1676, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamir M, Sompolinsky H. Implications of neuronal diversity on population coding. Neural Comput 18: 1951–1986, 2006 [DOI] [PubMed] [Google Scholar]
- Shoykhet M, Doherty D, Simons DJ. Coding of deflection velocity and amplitude by whisker primary afferent neurons: implications for higher level processing. Somatosens Mot Res 17: 171–180, 2000 [DOI] [PubMed] [Google Scholar]
- Simons DJ. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol 41: 798–820, 1978 [DOI] [PubMed] [Google Scholar]
- Simons DJ, Carvell GE, Hershey AE, Bryant DP. Responses of barrel cortex neurons in awake rats and effects of urethan anesthesia. Exp Brain Res 91: 259–272, 1992 [DOI] [PubMed] [Google Scholar]
- Simpson KL, Altman DW, Wang L, Kirifides ML, Lin RCS, Waterhouse BD. Lateralization and functional organization of the locus coeruleus projection to the trigeminal somatosensory pathway in rat. J Comp Neurol 385: 135–148, 1997 [PubMed] [Google Scholar]
- Simpson KL, Waterhouse BD, Lin RC. Origin, distribution, and morphology of galaninergic fibers in the rodent trigeminal system. J Comp Neurol 411: 524–534, 1999 [DOI] [PubMed] [Google Scholar]
- Simpson KL, Waterhouse BD, Lin RC. Characterization of neurochemically specific projections from the locus coeruleus with respect to somatosensory-related barrels. Anat Rec A Discov Mol Cell Evol Biol 288: 166–173, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron 24: 49–65, 1999 [DOI] [PubMed] [Google Scholar]
- Snow PJ, Andre P, Pompeiano O. Effects of locus coeruleus stimulation on the responses of SI neurons of the rat to controlled natural and electrical stimulation of the skin. Arch Ital Biol 137: 1–28, 1999 [PubMed] [Google Scholar]
- Stoney SD, Jr., Thompson WD, Asanuma H. Excitation of pyramidal tract cells by intracortical microstimulation: effective extent of stimulating current. J Neurophysiol 13: 659–669, 1967 [DOI] [PubMed] [Google Scholar]
- Timofeeva E, Lavallee P, Arsenault D, Deschenes M. Synthesis of multiwhisker-receptive fields in subcortical stations of the vibrissa system. J Neurophysiol 91: 1510–1515, 2004 [DOI] [PubMed] [Google Scholar]
- Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science 283: 549–554, 1999 [DOI] [PubMed] [Google Scholar]
- Van der Loos H. Barreloids in mouse somatosensory thalamus. Neurosci Lett 2: 1–6, 1976 [DOI] [PubMed] [Google Scholar]
- Verhage M, McMahon HT, Ghijsen WE, Boomsma F, Scholten G, Wiegant VM, Nicholls DG. Differential release of amino acids, neuropeptides, and catecholamines from isolated nerve terminals. Neuron 6: 517–524, 1991 [DOI] [PubMed] [Google Scholar]
- von Heimendahl M, Itskov PM, Arabzadeh E, Diamond ME. Neuronal activity in rat barrel cortex underlying texture discrimination. PLoS Biol 5: e305, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite PM. The responses of cells in the rat thalamus to mechanical movements of the whiskers. J Physiol 228: 541–561, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BR, Ohara PT. Convergent projections of trigeminal afferents from the principal nucleus and subnucleus interpolaris upon rat ventral posteromedial thalamic neurons. Brain Res 629: 253–259, 1993 [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Azizi SA, Burne RA, Woodward DJ. Modulation of rat cortical area 17 neuronal responses to moving visual stimuli during norepinephrine and serotonin microiontophoresis. Brain Res 514: 276–292, 1990 [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ. Phasic activation of the locus coeruleus enhances responses of primary sensory cortical neurons to peripheral receptive field stimulation. Brain Res 790: 33–44, 1998 [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Sessler FM, Cheng JT, Woodward DJ, Azizi SA, Moises HC. New evidence for a gating action of norepinephrine in central neuronal circuits of mammalian brain. Brain Res Bull 21: 425–432, 1988 [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Woodward DJ. Interaction of norepinephrine with cerebrocortical activity evoked by stimulation of somatosensory afferent pathways in the rat. Exp Neurol 67: 11–34, 1980 [DOI] [PubMed] [Google Scholar]
- Wilent WB, Contreras D. Stimulus-dependent changes in spike threshold enhance feature selectivity in rat barrel cortex neurons. J Neurosci 25: 2983–2991, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilent WB, Contreras D. Synaptic responses to whisker deflections in rat barrel cortex as a function of cortical layer and stimulus intensity. J Neurosci 24: 3985–3998, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans JS. Principals of Brain Stimulation. New York, Oxford: Oxford University Press, 1990 [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron 46: 681–692, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.