Abstract
GABAA receptors are found on the somatodendritic compartment and on the axon initial segment of many principal neurons. The function of axonal receptors remains obscure, although it is widely assumed that axonal receptors must have a strong effect on excitability. We found that activation of GABAA receptors on the dentate granule neuron axon initial segment altered excitability by depolarizing the voltage threshold for action potential initiation under conditions that minimally affected overall cell input resistance. In contrast, activation of somatic GABAA receptors strongly depressed the input resistance of granule neurons without affecting the voltage threshold of action potential initiation. Although these effects were observed over a range of intracellular chloride concentrations, average voltage threshold was unaffected when ECl rendered GABAA axon initial segment responses explicitly excitatory. A compartment model of a granule neuron confirmed these experimental observations. Low ambient agonist concentrations designed to activate granule neuron tonic currents did not stimulate axonal receptors sufficiently to raise voltage threshold. Using excitatory postsynaptic current (EPSC)-like depolarizations, we show physiological consequences of axonal versus somatic GABAA receptor activation. With axonal inhibition, individual excitatory postsynaptic potentials (EPSPs) largely retained their amplitude and time course, but EPSPs that were suprathreshold under basal conditions failed to reach threshold with GABAA activation. By contrast, somatic inhibition depressed individual EPSPs because of strong shunting. Our results suggest that axonal GABAA receptors have a privileged effect on voltage threshold and that two major measures of neuronal excitability, voltage threshold and rheobase, are differentially affected by axonal and somatic GABAA receptor activation.
INTRODUCTION
GABAA receptors have a compartmentalized distribution within many neurons, but the functional implications of this compartmentalization are still incompletely defined. Axon initial segment (AIS) GABAA receptors, the postsynaptic target of axoaxonic synapses formed by chandelier interneurons (axoaxonic cells), have fascinated neurophysiologists and neuroanatomists (Freund and Buzsaki 1996; Howard et al. 2005; Woodruff and Yuste 2008). Although some recent evidence favors the idea of different physiological effects of AIS versus somatic GABAA receptors (Khirug et al. 2008; Szabadics et al. 2006; but see Glickfeld et al. 2009), AIS GABAA receptors are often viewed as misplaced somatodendritic receptors, with similar consequences on excitability (Kullmann et al. 2005). Here we study whether AIS receptors might influence excitability differently than somatic receptors because of the proximity of axonal GABAA receptors to the voltage-gated sodium channels responsible for action potential initiation (Colbert and Johnston 1996; Kole et al. 2008; Kress et al. 2008; Schmidt-Hieber et al. 2008).
Granule neurons of the dentate gyrus are good subjects for studying the influence of compartmentalized subcellular GABAA receptor activation on excitability. These cells receive AIS GABAergic input like other principal cells of the hippocampus and cortex (Soriano et al. 1990). However, these cells are more electrotonically compact than other cortical principal cells (Schmidt-Hieber et al. 2007). Therefore interpretation of subcellular agonist applications to the axon and somatodendritic compartments is less clouded by artifacts of electrotonic remoteness of these compartments from the site of recording, typically the soma. For instance, in pyramidal neurons, the electrotonic distance of the action potential initiation site from the somatic recording site can lead to errors in the measurement of the voltage threshold for action potential initiation (Kole and Stuart 2008; Kress et al. 2010). These errors are minimized in granule neurons because of a proximal initiation site and electrotonically compact structure (Kress et al. 2010). In addition, granule neurons have no basal dendrites, which in pyramidal neurons are problematic for attempts to localize drug applications to the AIS.
Cellular excitability can be defined in several ways. Two primary definitions include the voltage threshold for action potential initiation and the minimal amount of current required to evoke an action potential (current threshold; rheobase). Because of the small excitatory postsynaptic potential (EPSP) size in granule neurons (Bekkers and Clements 1999; McNaughton et al. 1981), voltage threshold increases of even a millivolt or less will be physiologically significant, requiring recruitment of substantial numbers of additional presynaptic inputs. Rheobase changes are usually associated with a change in input resistance of the cell, such that additional current is needed to reach voltage threshold. Although these two measures of cellular excitability are both used in the literature to define excitability, the impact of compartmentalized subcellular conductances on the two measures are unclear. Here we show that GABA receptor activation on the AIS primarily alters voltage threshold. By contrast, somatic GABAA receptor activation strongly alters neuronal input resistance and rheobase for action potential initiation, without altering voltage threshold.
METHODS
Slice preparation
Animal use protocols were approved by the Washington University Animal Studies committee. Hippocampal slices were prepared from postnatal day 18 (P18) to P22 Sprague-Dawley rats. Rats were anesthetized with isofluorane and decapitated. Brains were removed and dissected in an oxygenated ice-cold slicing buffer containing (in mM) 87 NaCl, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCal2, 3 MgCl2, 25 glucose, 75 sucrose, and 0.75 kynurenic acid. Transverse slices of 300 μm were made with a VT 1200 vibrating slicer (Leica, Bannockburn, IL) and were immediately transferred to modified artificial cerebrospinal fluid (ACSF) containing (in mM) 125 NaCl, 25 glucose, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, and 2 kynurenic acid at 32°C for 30 min. Slices were subsequently stored at room temperature. All drugs were obtained from Sigma-Aldrich (St. Louis, MO). Alexa Fluor reagents were from Invitrogen (Carlsbad, CA).
Electrophysiology
Hippocampal slices were perfused at 2 ml/min with oxygenated ACSF at 25 ± 1°C. Patch electrodes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL) with a P-97 Flaming/Brown micropipette puller (Sutter Instruments, Novato, CA). Intracellular solutions contained (in mM) 130 potassium gluconate, 4 NaCl, 0.5 CaCl2, 5 EGTA, 10 HEPES, 4 Mg-ATP, 0.3 Na-ATP, 10 phosphocreatine, and 100 μM Alexa Fluor 488 hydrazide. The latter four components were omitted from cell-attached patch recordings described below. For experiments with moderately depolarized chloride equilibirium potential (ECl), KCl was increased by 12.7 mM and potassium gluconate was decreased to 117.7 mM, for a final [Cl−]i concentration of 17.7 mM. Uncorrected ECl was therefore −55 mV for this solution. For high pipette [Cl−], we used 33.4 mM for an uncorrected ECl of −35 mV. Puffer application pipettes were filled with the above ACSF solution buffered with 10 mM HEPES, pH 7.4, and 160 μM Alexa Fluor 568. Pipette resistance was 2.5–4 MΩ for patch pipettes and 3–5 MΩ for puffer pipettes.
Whole cell current-clamp recordings were obtained from somas of visually identified granule cells of the upper blade of the dentate gyrus. Cells were identified under infrared differential interference contrast microscopy on a Nikon FN-1 microscope (Nikon, Melville, NY) and with a cooled digital camera (Roper Scientific, Tucson, AZ). Somatic recordings were obtained with a Multiclamp 700B patch-clamp amplifier and Clampex 9.2 software (Molecular Devices, Sunnyvale, CA). Bridge balance and whole cell capacitance were adjusted by Multiclamp Commander software (Molecular Devices). Data were acquired at 100 kHz using a DigiData 1322A converter (Molecular Devices) and filtered at 10 kHz using an 8-pole Bessel filter. Somatic access resistance was monitored continuously, and cells with initial access resistance larger than 25 MΩ or access resistance that increased >20% were rejected. When necessary, a small bias current was injected to maintain the resting potential near −70 mV. After seal rupture, cells were allowed to stabilize for 2–3 min while fluorescent dye filled the cell. All cells studied had electrophysiological and morphological characteristics of mature DG cells (Liu et al. 2000). Only cells with axons longer than 100 μm were used in this study.
To assay voltage threshold in the presence and absence of muscimol, 20 ms pulse depolarizing current injections with the amplitude necessary to evoke one action potential near the middle of the pulse were delivered at 30 s intervals. In all cases, the depolarizing current amplitude for interleaved control and agonist application trials was held constant for a cell to avoid confounds of altered current amplitude on action potential threshold (see results). To locally apply muscimol, the puffer pipette was positioned 5 μm from the target region of the fluorescently filled axon. We used 25–50 ms digital pulses to a PV800 Picospritzer (World Precision Instruments) at 5–8 psi. The puffer pipette was mounted on the second headstage of the Multiclamp 700B amplifier, allowing acquisition of junction currents as an indicator of solution ejection. At the low pressures used in this study (5–8 psi), a 20–30 ms lag was observed between the TTL pulse and the change in junction current. Muscimol application alternated with control sweeps every other current pulse. For every condition (control and muscimol), 2–5 pulses were delivered, and threshold was taken as the condition's average value. For comparison of effects of tonic GABAA receptor activation in Fig. 8, we used values obtained from the last minute of recording in each condition. To generate synaptic-like stimulation for Fig. 9, EPSPs were generated by a current waveform that was the sum of rising and decay exponential functions. Time constants were 1 and 7 ms for rise and decay, respectively, corresponding with previously reported values (Clements and Bekkers 1997; Schmidt-Hieber et al. 2007). These artificial postsynaptic currents (aPSCs) were delivered at 50 Hz. As with depolarizing pulses, the amplitude of aPSCs was held constant for control and interleaved agonist application trials. All voltages throughout are presented uncorrected for liquid junction potentials. These were estimated at −14 mV for the low chloride pipette solution, at −13 mV for our moderately elevated pipette solution, and −12 mV for high pipette [Cl−] as calculated by Clampex 9.2.
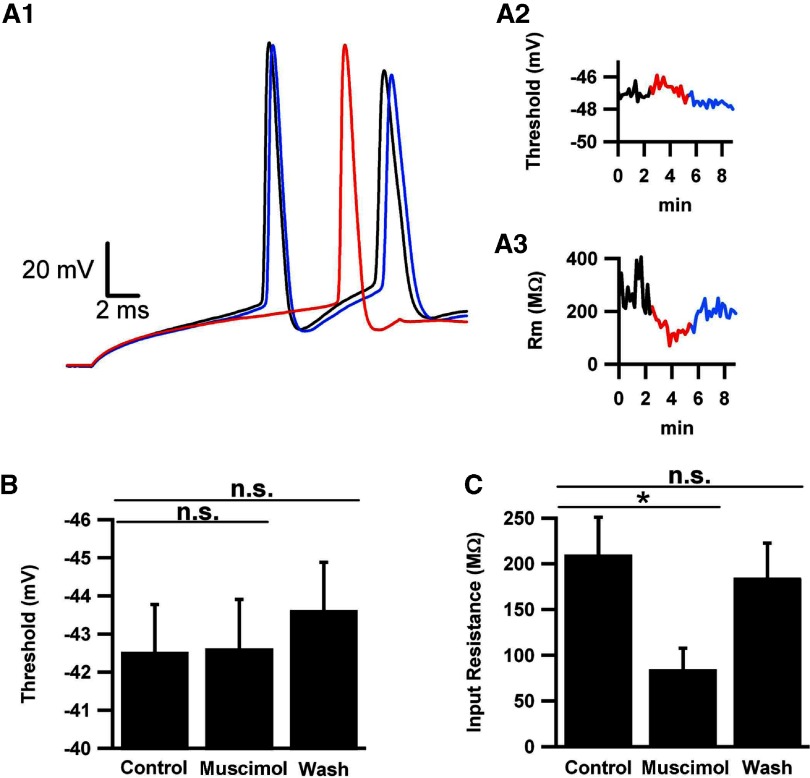
Fig. 8.
Tonic activation of GABAA receptors does not change voltage threshold. Action potentials were evoked every 10 s for 2 min, then 1 μM muscimol was applied for 2 min, and finally washed out for 1 min. Pipette chloride was standard low chloride concentration to facilitate detection of effects on threshold. A1: representative action potentials in the absence (black), presence (red), and washout (blue) of 1 μM muscimol. Inset: rising part of the phase plot, with voltage threshold indicated by the horizontal line at 10 mV/ms. A2: time course of voltage threshold change with time under the various experimental conditions. Same color codes as in A1. A3: time course of input resistance (Rm). The example shows 1 of the larger changes observed. B and C: average values for thresholds (B) and input resistance (C) for each condition (n = 10 cells).
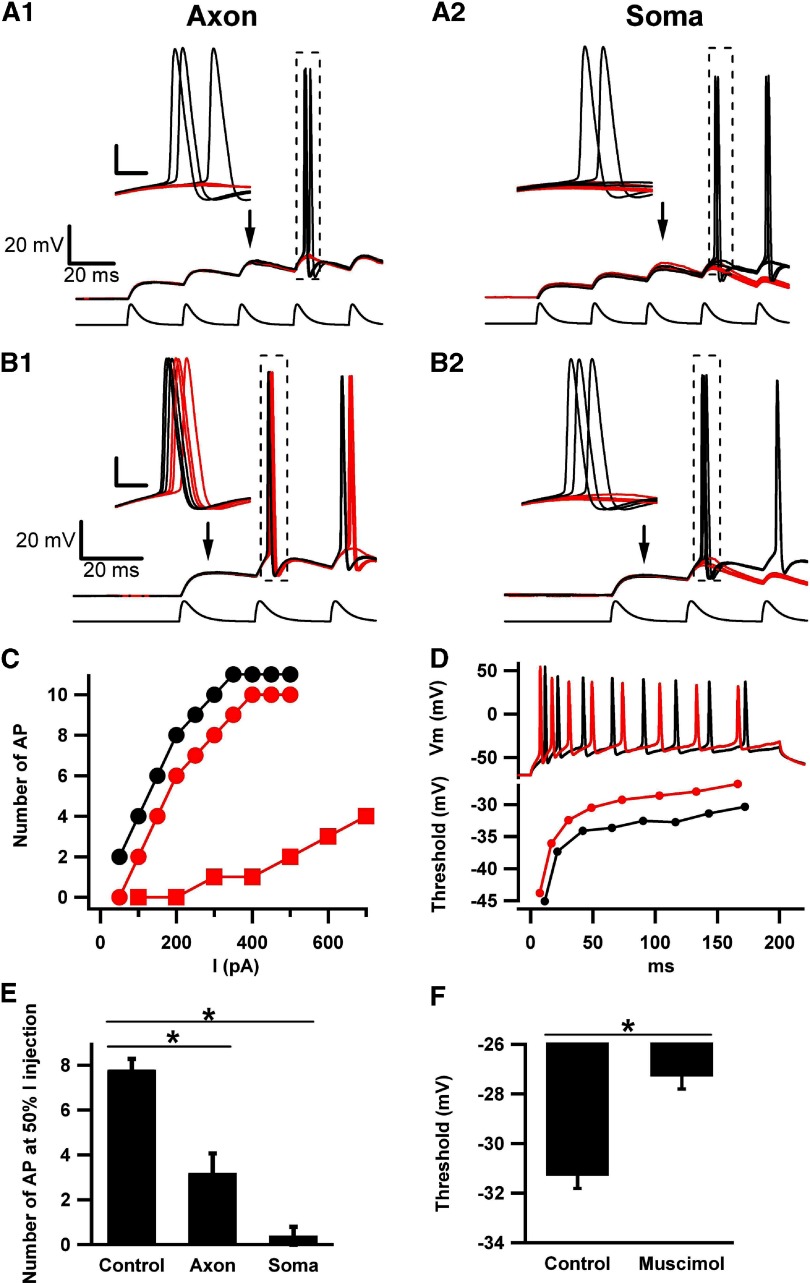
Fig. 9.
Somatic and AIS muscimol differentially affect temporal summation of excitatory postsynaptic potential (EPSP)-like events (aPSPs) and input–output relationships. A1: muscimol application to the AIS failed to evoke an action potential during the 4th aPSP, which routinely reached threshold in interleaved control sweeps. Notice that aPSP size and shape are similar in the absence (red traces) and presence (black traces) of muscimol. Bottom: current waveform used to stimulate the cell. The peak amplitude was 175 pA. All insets are magnification of the boxed area, with calibration bars 20 mV and 2 ms. A2: somatic muscimol failed to evoke an action potential at the 4th aPSP. Notice the difference in aPSP peak and time course in the absence (black) and presence (red) of muscimol. B1: with larger current amplitude (250 pA peak amplitude), muscimol application to AIS just before the 2nd aPSP depolarized the voltage threshold and delayed firing. B2: somatic muscimol under the same current-injection conditions as B1 caused an action potential failure at the 2nd aPSP. All traces are from the same cell, which is representative of the 4 cells tested. C: input/output curves for a representative granule cell subjected to 200 ms current pulses of varied amplitudes, with muscimol applied for 50 ms to either the AIS or to the soma immediately before current onset. The y-axis gives the number of action potentials (APs) elicited by the given current amplitude. Muscimol concentration was 5 μM, applied either to the AIS (red circles) or to the soma (red squares). Baseline input/output curve is given as black circles. D: raw traces (top) and voltage thresholds (bottom) in the absence and presence of AIS muscimol application for the cell represented in C. Traces were matched for number of action potentials (8), which corresponded to a current amplitude of 200 pA for control (black trace) and 250 pA for muscimol (red trace). Bottom: voltage thresholds calculated from phase-plot analysis for each action potential in the train. The effect of muscimol on voltage threshold may be reduced compared with the effect in other figures as a result of the larger amplitude of current injection used. Increased current amplitudes tended to hyperpolarize action potential threshold. E: summary of effect represented by the example in C (n = 5). The number of action potentials at a fixed current amplitude is plotted. The current amplitude was that needed to elicit 50% of the maximum firing rate under baseline conditions (175 pA for the example in C). F: summary of effect represented by the example in D (n = 5). Average action potential threshold for the final 4 action potentials in each condition is plotted.
To examine the influence of AIS GABAA receptor activation in cells with a chloride gradient that was not perturbed by whole cell recording, we electroporated neurons with Alexa Fluor hydrazide dye (Khirug et al. 2008) using an Axoporator 800A (−6 V, 1 ms pulses at 100 Hz for 2 s). After allowing time for dye diffusion into the proximal axon (10−30 min), we performed cell attached recordings on the visualized neurons. Cells were typically quiescent, so we enhanced granule cell excitability in these experiments with decreased extracellular divalent concentration (0.25 mM CaCl2 and 0.25 mm MgCl2) and increased [K+]o to 4 mM K+ to achieve a target spontaneous firing rate of 2–10 Hz. Cells without detectable spontaneous firing were excluded from analysis. Compared with each cell's baseline firing rate, we evaluated the effect of a 50 ms local AIS muscimol application every 20 s. Firing frequency in the 0.8–1.5 s immediately preceding muscimol application was compared with the same time period immediately following the puff.
Data analysis
Data were acquired using pClamp 9.2 (Molecular Devices) and analyzed using custom routines written in Igor Pro (Wavemetrics, Lake Oswego, OR). Phase plots were constructed for purposes of calculating voltage threshold by plotting the first derivative of membrane voltage versus the membrane voltage (Bean 2007). Before calculation of threshold, we subtracted the passive change in membrane potential resulting from pipette current injection (before the action potential) from phase plots. The passive dV/dt value subtracted was typically 1–3 mV/ms, and all phase plots are shown after this subtraction. Supplemental Fig. S1 details the subtraction process.1 Action potential threshold was denoted as the voltage at which the first derivative (dV/dt), after subtracting the passive dV/dt, reached 10 mV/ms (Kress et al. 2008; Naundorf et al. 2006; Shu et al. 2007a). To calculate input resistance, voltage pulses of −10 mV were applied from a holding voltage of −90 mV for 100 ms. To calculate the reversal potential of muscimol-elicited currents, 200 ms ramps from −90 to −40 mV were applied every 30 s, with interleaved muscimol puffs. Control and muscimol ramps were averaged, and the muscimol-evoked current component was obtained by digital subtraction. Reversal potential was obtained by linear regression fit to the current surrounding the region of zero current. All values in the text are represented as means ± SE; error bars in figures represent SE.
Simulations
For simulation of the effects of tonic GABAA receptor–mediated current on action potential initiation, we constructed a simplified model based on our prior simulations of dentate gyrus granule cells (Kress et al. 2010). Sodium and potassium channel properties were as described (Kress et al. 2010). The model consisted of a single somatic compartment (length 10 μm, diameter 10 μm), a single dendritic compartment (length 40 μm, diameter 3 μm), and a five-compartment axon (lengths 20, 20, 40, 40, and 120 μm; diameters 1.5, 1.5, 1.25, 1.0, and 0.75 μm). Membrane resistivity (Rm) was 10,000 Ω cm2 with a reversal potential of −70 mV, membrane capacitance (Cm) was 1 μF/cm2, and axial resistivity (Ra) was 200 Ω cm for all compartments. Sodium channel density was 0.025 S/cm2 in all compartments except for the first and second axonal compartments, where it was 0.125 S/cm2. This average fivefold higher sodium conductance in the initial segment is consistent with recent observations (Kress et al. 2010; Schmidt-Hieber and Bischofberger 2010). However, we also tried increasing the sodium conductance to 50-fold above the rest of the axon (10-fold above the AIS density in the standard simulation) while retaining a constant GABA conductance in the initial segment. Although this caused the peak of phase plots to exceed anything ever observed experimentally (Supplemental Fig. S2), introducing the AIS GABA conductance produced qualitatively similar effects on threshold as the standard simulation (Supplemental Fig. S2), suggesting the observations are robust. Delayed rectifier potassium channel density was 0.002 S/cm2 for all compartments. Action potential threshold in granule cells has been shown under our conditions of brief current injection to be independent of voltage-gated potassium conductances (Kress et al. 2010). Each compartment had an additional passive conductance to simulate tonic GABAA receptor–mediated current. Conductances and reversal potentials were set for each compartment as described in Fig. 5; otherwise, the conductances were set to zero. The AIS GABA conductance was titrated to minimize effects on rheobase from the baseline condition (with no added GABA conductance). The soma conductance was reduced threefold relative to the AIS conductance as described in Fig. 5 to simulate greater spread and dilution of muscimol from the pipette tip in actual experiments and to reproduce the degree of input resistance change observed. All simulations were performed in NEURON versions 6.2 and 7.0 (Hines and Carnevale 2001).
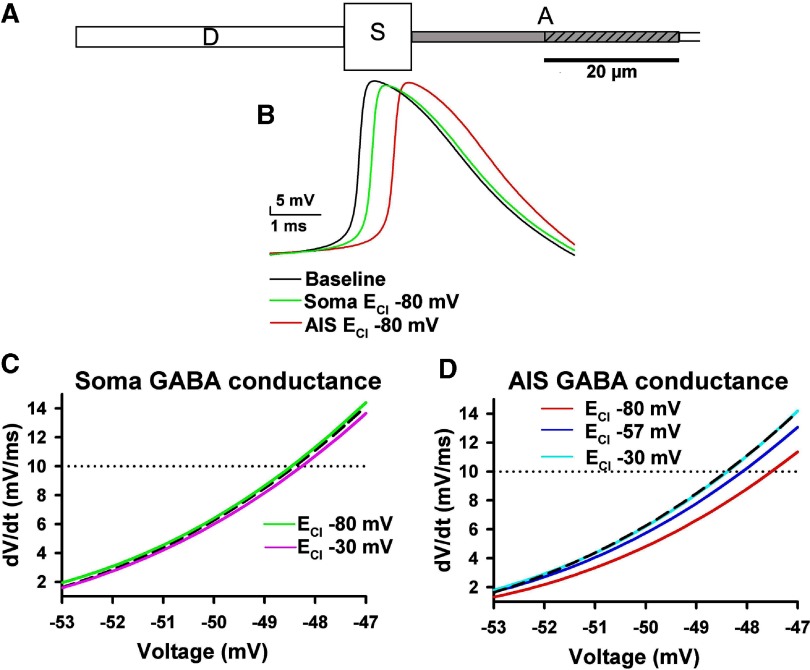
Fig. 5.
Simulations of the effects of somatic and AIS GABAA conductances. A: schematic of the cell created in the NEURON simulation environment to test the effect of AIS and somatic Cl− conductance on action potential threshold. Labels indicate dendrite (D), soma (S), and axon (A). The 1st 2 axon compartments, representing the 40 μm long AIS, are shown, although the entire simulated axon extended 240 μm, tapering from 1.5 μm in diameter in the 1st 2 compartments to 0.75 μm diam. The gray shading indicates the region of high sodium channel density (5-fold higher than somatodendritic compartment). The diagonal hatches represent the compartment of the AIS (20–40 μm distal) in which the GABA conductance was introduced. B: action potentials obtained from simulated soma recordings of the model cell. The black trace is a baseline trace (no Cl− conductance). The red trace shows the simulated action potential elicited in the presence of a sustained conductance introduced into the proximal AIS (ECl = −80 mV), 20–40 μm from the soma (0.003 S/cm2). The green trace represents the conductance introduced into the soma compartment. For the soma conductance, we decreased the conductance density to 0.001 S/cm2 to keep the rheobase for the conditions within a similar range (90 pA for soma vs. 70 pA for AIS conductance). C: initial portions of phase plots obtained from the simulation with a Cl− conductance introduced at the soma and with ECl at −80 and −30 mV for the green and pink traces, respectively. The dashed black trace is the baseline trace. The black and green phase plots were derived from the action potentials in B. The dotted horizontal line indicates the threshold of 10 mV/ms. D: phase plot rises show the effect of AIS Cl− conductance on action potential threshold at 3 different ECl values, as indicated. The vertical dashed lines give the membrane potential at threshold. The light blue line (ECl = −30 mV) and the black dashed baseline line are superimposed and nearly indistinguishable. Both ECl = −57 mV (dark blue trace) and ECl = −80 mV (red trace) conditions yielded depolarization of action potential threshold. The red trace is derived from the red action potential in B.
RESULTS
Differential effects of localized GABAA agonist
We reasoned that, if inhibition occurs near the site of action potential initiation (the proximal AIS in granule cells) (Kress et al. 2008; Schmidt-Hieber et al. 2008), voltage-gated sodium currents responsible for initiation will be shunted by the local chloride conductance, possibly resulting in different effects on excitability than inhibition occurring distal from the site of initiation. We evoked action potentials with just-suprathreshold current pulses (20 ms) in whole cell recordings from dentate granule neurons. A fluorescent dye fill identified the axon for targeted local AIS GABAA receptor activation. Axonal drug applications were aimed the AIS 20–40 μm distal to the axon hillock (Fig. 1A). We used the nontransportable selective GABAA agonist muscimol to activate GABAA receptors. This helped ensure that the full concentration of agonist was delivered (uninfluenced by GABA transporters) and that the effect did not result from GABAB receptor activation. Muscimol has a similar concentration–response relationship to GABA at all tested GABAA receptor subunit combinations (Ebert et al. 1994, 1997; Storustovu and Ebert 2006). To help limit effects of agonist spread, we timed the local application to occur 25–50 ms prior to current injection, and we kept current injections brief. Figure 1A shows an example of the experimental setup, including the placement of the muscimol application pipettes (arrows in Fig. 1A) and the whole cell recording pipette (asterisk in Fig. 1A).
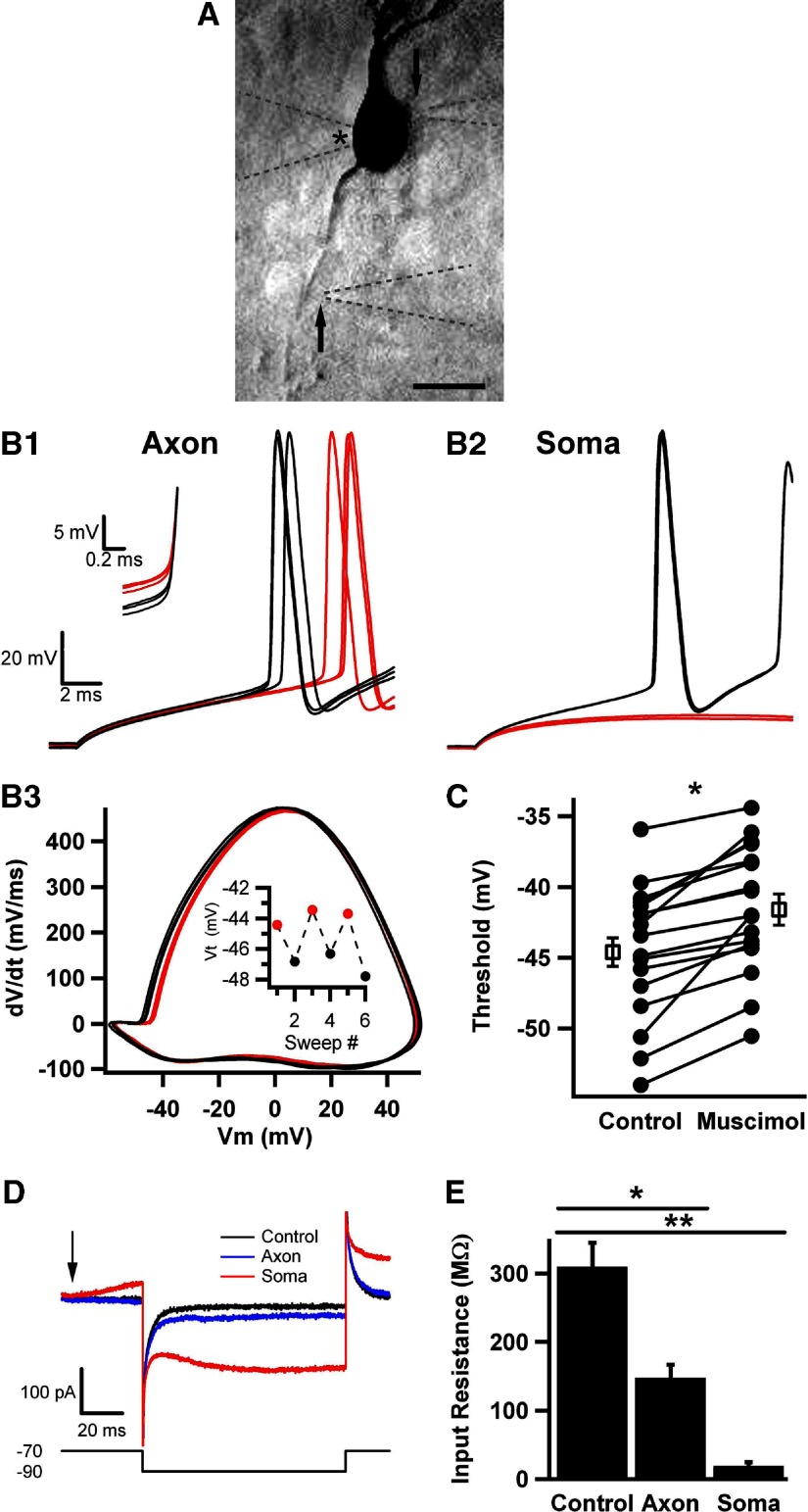
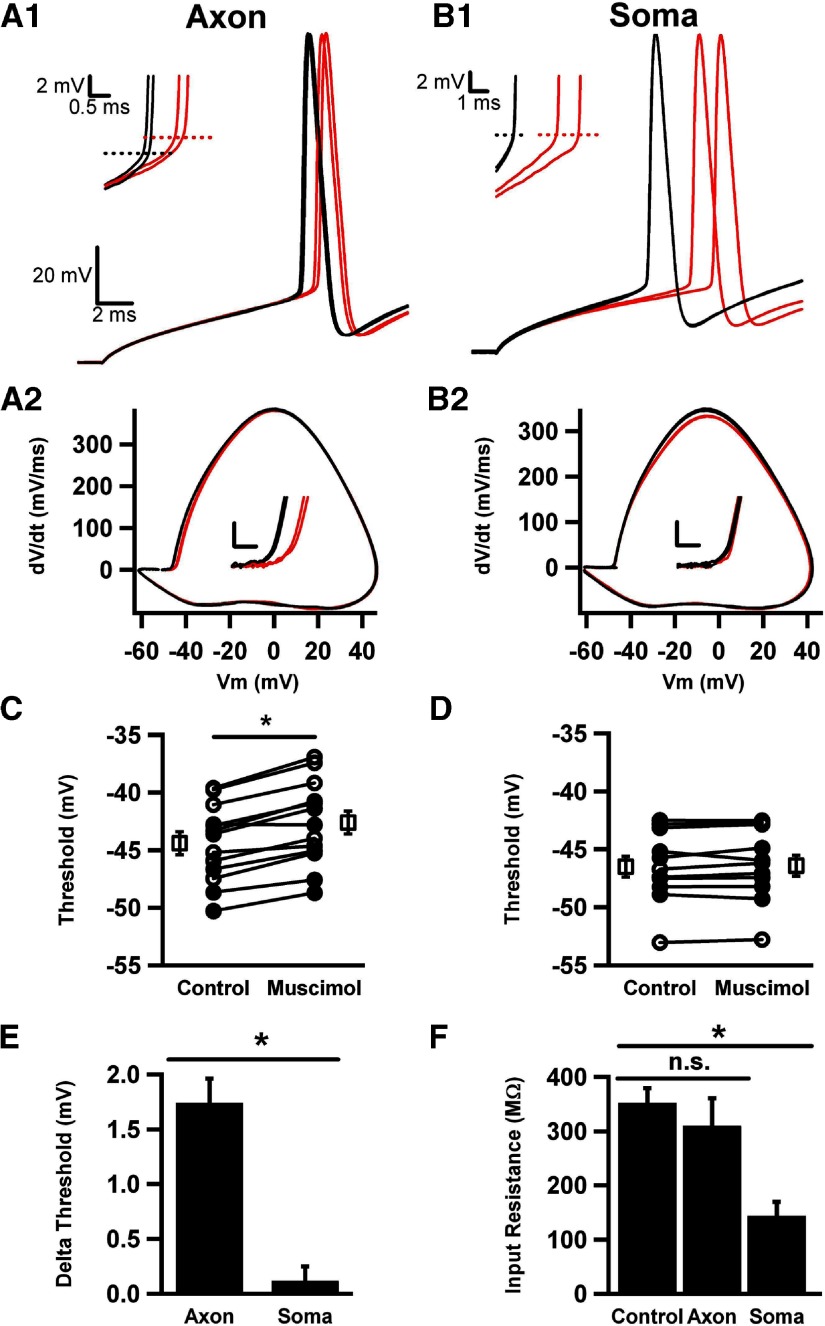
Fig. 1.
Strong axonal GABAA channel activation increases voltage threshold. A: reverse fluorescence image of an Alexa Fluor–filled dentate granule neuron, with pipettes positioned for axon initial segment (AIS) and soma application of muscimol. Soma and AIS puffer pipettes are barely visible to the right of the cell and indicated by arrows. The whole cell recording pipette is indicated with an asterisk in the lumen, to the left of the soma. All 3 pipettes are outlined by dashed lines for clarity. Scale bar is 10 μm. B1: action potentials in response to current injections in the presence of muscimol (30 μM, red traces) interleaved with trials in the absence of muscimol (black traces). Muscimol applications started 50 ms before depolarizing current injection. Action potentials were elicited by 20 ms current injection from a resting potential of −70 mV. B2: from the same cell as in A1, responses to current injections in the presence (red traces) and absence (black traces) of interleaved somatic muscimol applications. B3: phase plot of control (black) and muscimol conditioned (red) action potentials shown in A1. Vm is membrane potential. Inset: voltage thresholds (Vt) as a function of sweep number. C: summary of voltage thresholds for control and muscimol conditions. Every symbol pair represents a single cell's average values for interleaved control and muscimol sweeps (n = 16). In this and subsequent figures, the open squares in the paired scatter plot represent the grand average of all the cells in the baseline and treatment conditions. Here and subsequent figures: *significantly different at the P < 0.05 level. D: representative traces used to obtain input resistance values. The arrow indicates the onset of a muscimol puff to either the AIS (red trace) or soma (blue trace), and the shift in baseline in the red trace before the voltage pulse results from the onset of GABAA receptor activation. The lower waveform gives the voltage protocol (mV values are given to the left). E: average input resistance for control, axonal, and somatic muscimol application (n = 4), shown in D.
Figure 1B shows the response of a single dentate granule neuron to local application of 30 μM muscimol to either the AIS (Fig. 1B1) or to the soma (Fig. 1B2). Muscimol applications were interleaved with control responses in the absence of drug application. Current injection amplitude ranged from 150 to 300 pA and was set to yield an action potential under baseline conditions midway through the 20 ms current pulse. For a given cell, the amplitude of depolarizing current was fixed and was not altered during the local muscimol applications. At this concentration of muscimol applied to the AIS, action potentials were still elicited with the fixed-amplitude current injection. We found that this same current amplitude never effectively elicited action potentials when we applied the muscimol to the soma (Fig. 1B2, red traces). In fact, with 20 ms pulses of ≤500 pA, we were no longer able to elicit action potentials in any of the cells examined with 30 μM muscimol application. Therefore rheobase (minimum current required to elicit an action potential) clearly increased with somatic muscimol application. Increased current injection amplitude alone, in the absence of muscimol, hyperpolarized voltage threshold. For instance, in five cells tested in the absence of muscimol, increasing depolarizing current intensity from 200 to 700 pA hyperpolarized voltage threshold by 1.7 ± 0.3 mV (P < 0.05). By depolarizing cells faster (with a larger current stimulus), there may be less opportunity for sodium channel inactivation and potassium channel activation to affect threshold. Furthermore, strong current injection may recruit somatodendritic sodium channels that do not ordinarily contribute to threshold, resulting in a hyperpolarized action potential threshold. Thus to avoid confounding current amplitude effects with muscimol effects, we did not analyze the effects of stronger current injection during somatic muscimol application. The strong effects of somatic GABAA receptor activation likely result primarily from a larger membrane surface area activated by application, resulting in opening of more channels. A higher somatic receptor density, including extrasynaptic and synaptic receptors, could also participate in the strong effects.
With axonal muscimol applications, we examined the threshold for action potentials elicited in the presence and absence of muscimol. To quantify the muscimol effects on voltage threshold, we created action potential phase plots, graphs of the first time derivative of membrane potential versus the membrane potential (Fig. 1B3). These plots also made other changes to the action potential waveform more evident, such as the maximum slope and the peak amplitude of action potentials recorded at the soma. As a standardized criterion for threshold, we used the voltage at which the first time derivative reached 10 mV/ms (Kress et al. 2008; Naundorf et al. 2006; Shu et al. 2007a). Threshold depolarized for all cells to which muscimol was applied by an average of 2.7 ± 0.5 mV (n = 16; Fig. 1C), and the effect was completely reversible, as shown by the interleaved design (Fig. 1B3, inset). In control tests of local application of HEPES-buffered extracellular saline alone to the AIS, we observed no change in threshold (−0.1 ± 0.2 mV; n = 9 cells). Action potential threshold exhibited considerable variability in different cells at baseline (note scatter in Fig. 1C, Control). It seems likely that this may be caused by cell-to-cell differences in density, functional properties, or relative position of the voltage-gated sodium channels underlying initiation (Kress et al. 2010). Regardless of the reason for the variability, the muscimol-induced change was robust in virtually all cells.
To explore the basis for the excitability changes with axonal versus somatic muscimol application, we examined cell input resistance in the presence and absence of muscimol. We found that input resistance was very strongly reduced by somatic muscimol application, accounting for the difficulty in eliciting excitable responses (Fig. 1, D and E). Axonal application caused a significantly smaller decrease in overall input resistance than somatic application (Fig. 1E).
To determine whether the overall input resistance decrease was necessary for the inhibitory effects of axonal GABAA receptor activation and to decrease somatic inhibition to levels allowing spike analysis, we assessed the effect of lower muscimol concentration (5–10 μM). In this case, AIS muscimol application still depolarized action potential threshold (1.7 ± 0.2 mV, n = 13; Fig. 2, A1, A2, C, and E). This AIS muscimol effect persisted in four cells tested at elevated temperature (1.4 ± 0.4 mV depolarization at 25°C vs. 1.5 ± 0.6 mV depolarization at 33°C).
Fig. 2.
Effects of moderate axonal and somatic activation of GABAA channels. A1: AIS applications of 5 μM muscimol (red traces) depolarized the voltage threshold compared with control (black traces). Inset: magnification of the action potential early upswing, with dotted horizontal lines indicating the average threshold voltage. A2: phase plots of control (black) and muscimol-conditioned (red) action potentials shown in A1. Inset: magnification of the rising part of phase plot, calibration bars are 10 mV/ms and 2 mV. B1: somatic muscimol application produced a delay in action potentials without affecting voltage threshold (inset, same calibration as in A1). B2: phase plots of traces shown in B1. Notice the change in the peak dV/dt, without an effect on voltage threshold (inset, same calibration as A2). C and D: summary of AIS (C) and somatic (D) muscimol applications, with every dot pair representing a single cell (n = 13). Closed symbols represent 5 μM muscimol; open symbols represent 10 μM muscimol. E: change in voltage threshold in response to axonal and somatic muscimol, from C and D. F: input resistance under control (no application), axonal, or somatic muscimol application (n = 5), n.s., not significantly different.
Axonal muscimol also delayed action potential initiation by 1.1 ± 0.3 ms compared with interleaved control responses (n = 13, P < 0.05), as seen in the cell of Fig. 2A1, but produced no significant change in overall cell input resistance (Fig. 2F). Soma application of 5–10 μM muscimol reduced input resistance (Fig. 2F), with effects similar in magnitude to those of 30 μM muscimol applied to the axon (compare with Fig. 1C). Fixed current injections were now capable of eliciting single spikes during somatic puffs (Fig. 2B1). However, in marked contrast to the effect of axonal application, somatic muscimol caused no effect on action potential voltage threshold (Fig. 2, B1, B2, D, and E).
Evidence of the compartmentalized effects of the local applications was observed in the phase plots of axonal versus somatic application of muscimol. For axonal application, the major effect of muscimol application was at the inflection point of the phase plot (Figs. 1B3 and 2A2), consistent with the idea that the early part of phase plots reflects action potential initiation in the AIS (Kress et al. 2008; Yu et al. 2008). By contrast, somatic muscimol application most influenced the later peak amplitude of the phase plot (Fig. 2B2; peak dV/dt was reduced by 9.5 ± 3.2 mV/ms by soma application, n = 13), consistent with the peak rate of rise reflecting recruitment of somatic channels involved in the action potential. As expected from the input resistance changes, axonal application had very little effect on the voltage trajectory preceding the action potential (Fig. 2A), whereas somatic application altered the passive membrane response and more strongly delayed action potential initiation (by 2.5 ± 0.4 ms compared with interleaved control responses; n = 13, P < 0.01; Fig. 2B1). Because both somatic and AIS muscimol application delayed action potential initiation, but only AIS application altered threshold, muscimol-induced delays in spiking cannot account for the effects of AIS GABAA receptor activation. Somatic muscimol application modestly decreased the action potential peak amplitude by 0.9 ± 0.5 mV (P = 0.07, n = 13). The trend might result from direct shunting by the somatic GABA conductance or by slightly stronger steady-state sodium channel inactivation in the soma because of the delay preceding initiation (Fig. 2B1).
As noted above, somatic muscimol applications in the same cell yielded larger input resistance changes than AIS muscimol applications (Fig. 2F). This is likely in large part because of the different membrane surface areas activated by muscimol. To better compare effects matched for membrane surface area, in a separate set of experiments, we also compared AIS, soma, and dendritic applications of GABA. In this case, dendrite applications better reflected the input resistance changes induced by AIS application, yet, like soma applications, failed to induce voltage threshold alteration (n = 4; Supplemental Fig. S3). This result supports the idea that AIS GABAA receptor activation has a privileged effect on voltage threshold for action potential initiation.
Intracellular [Cl−] manipulations
The effects of muscimol in Figs. 1 and 2 were achieved with a low Cl− concentration in the whole cell pipette. Recent evidence has suggested that a range of intracellular chloride concentrations is relevant to the AIS, probably depending on cell type, developmental stage, specifics of slice preparation, and other variables that affect expression of chloride transporters (Holmgren et al. 2010). In recent results, activation of AIS GABAA receptors can be hyperpolarizing (Glickfeld et al. 2009), depolarizing but still inhibitory (Khirug et al. 2008; Woodruff et al. 2009), or overtly excitatory (Szabadics et al. 2006). We sought to explore the effect of this range of intracellular chloride concentrations on the effect of AIS GABAA receptor activation.
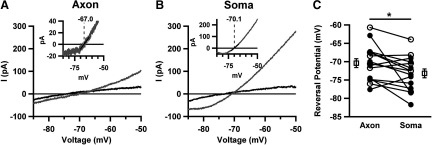
We first probed the reversal potential of muscimol currents when applied to the AIS versus the soma under conditions of low pipette chloride used for Figs. 1 and 2. Voltage ramps (200 ms) were applied in the presence or absence of 25 ms puffs to both axon and soma of the same cells (Fig. 3, A and B). We digitally averaged two to three interleaved traces and subtracted the muscimol-evoked response (Fig. 3, A and B, insets). To obtain the reversal potential, a linear regression around the zero current point of the current-voltage curve was performed (Fig. 3, A and B, insets). Because of the small response amplitude to 10 μM muscimol, we also examined the responses to 30 μM muscimol (open symbols in Fig. 3C). In both data sets, we found that the AIS had a more depolarized Cl− reversal potential than the soma (Fig. 3C), although neither was far from the expected reversal potential of −72 mV (see methods). In pooled data from 10 and 30 μM muscimol, axonal reversal potential was 2.8 ± 1.1 mV more depolarized than the soma (n = 15). This difference is smaller than observed in previous studies of this cell type and other cell types measured with gramicidin perforated patch (Khirug et al. 2008; Szabadics et al. 2006) and with whole cell pipettes (Khirug et al. 2008). The smaller soma-axon ECl differential in our experiments was likely caused by our use of low-resistance pipettes to minimize and stabilize access resistance errors (2.5–4 vs. 6.5–7.5 MΩ in Khirug et al. 2008). Use of such low-resistance pipettes will more readily outstrip the actions of transporters in the soma and AIS (Pusch and Neher 1988). Nevertheless, even with low-resistance pipettes, the different ECl between soma and AIS suggests that the AIS maintains a more depolarized ECl than that predicted by pipette [Cl−] (Khirug et al. 2008; Szabadics et al. 2006). Our results suggest that we have reasonably good control over ECl in both compartments.
Fig. 3.
Evidence for compartamentalized axonal Cl−. A: averaged current traces elicited by voltage (250 mV/s) ramps in presence (gray) or absence (black) of 10 μM axonal AIS muscimol application. Inset: digitally subtracted muscimol-dependent component. The reversal potential was obtained from linear fit of current near the voltage axis crossing (black line). B: response to somatic muscimol application in the same cell as in A. Muscimol-evoked currents were larger than axonal-evoked currents and exhibited a more hyperpolarized reversal potential. C: summary of reversal potentials obtained in paired AIS and somatic applications of 10 (●) and 30 μM (○) muscimol.
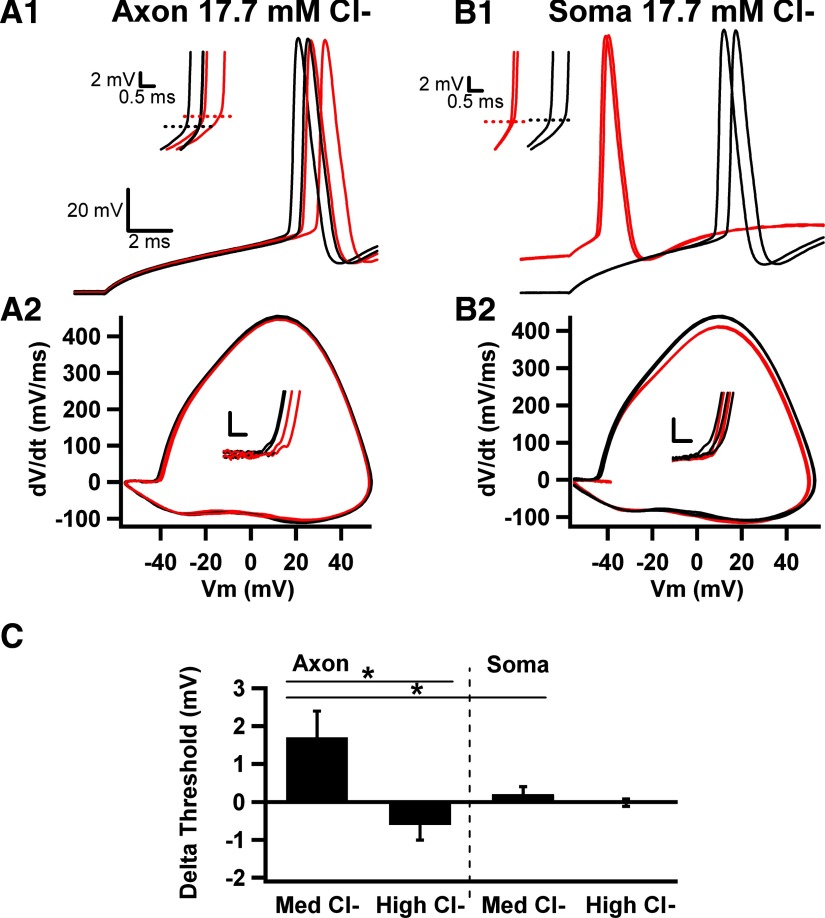
We examined moderately elevated and strongly elevated pipette chloride concentrations (17.7 and 33.4 mM, respectively; see methods), which represent depolarizing but still inhibitory GABA actions (Khirug et al. 2008; Woodruff et al. 2009) and overtly excitatory GABA actions (Szabadics et al. 2006), respectively. With moderately elevated pipette [Cl−] (17.7 mM), local AIS muscimol (10 μM) still depolarized action potential voltage threshold (Fig. 4, A1, A2, and C). As with recordings using low pipette [Cl−], AIS muscimol application did not reliably elicit overt depolarization or hyperpolarization of the cell, and it did not elicit spiking in the absence of current injection, probably because ECl was negative to action potential threshold. Unfortunately, we were unable to accurately estimate the AIS ECl with voltage ramps because active conductances at the voltages near ECl made reliable subtractions impractical. However, it was clear that ECl was more depolarized than with low pipette [Cl−], and apparently ECl did not exceed voltage threshold for action potential initiation at the AIS.
Fig. 4.
Altered AIS voltage threshold effects with varied intracellular [Cl−]. A1: local muscimol applications with pipette [Cl−]i elevated from 5 (Figs. 1–3) to 17.7 mM still depolarized voltage threshold. Red traces are muscimol; black traces are interleaved control sweeps. Inset: magnification of action potential upswing shows the depolarized threshold. A2: phase plots from traces in A1 in presence (red) and absence (black) of muscimol applications to the AIS. Inset: change in voltage threshold, calibration bars are 10 mV/ms and 2 mV. B1: in the same cell as A1, somatic muscimol application (red traces) depolarized membrane voltage before current injection but did not alter threshold (inset). B2: phase plots from traces in B1 in presence (red) or absence (black) of somatic application. Notice the decrease in peak dV/dt with muscimol, without changes in threshold (inset). C: summary of action potential threshold changes for control and muscimol application during axonal and somatic applications with a pipette Cl− concentration of 17.7 mM (Med Cl−; n = 8). The bar graph also shows the lack of significant overall change in threshold for 18 cells filled with 33.4 (High Cl−; n = 18 for axons and 6 for somas), so that GABAA receptor activation was explicitly excitatory.
Somatic applications with 17.7 mM (moderate) pipette [Cl−] again failed to alter voltage threshold (Fig. 4, B1, B2, and C). Nevertheless, somatic muscimol (10 μM) exhibited marked differences from responses obtained with low pipette [Cl−]. Although overt membrane potential changes were not apparent with soma applications to cells filled with low [Cl−] (Vm before puff: −71.1 ± 0.3 mV; after puff −71.5 ± 0.4 mV; Fig. 2B1), somatic application to Cl−-filled cells elicited depolarization (−69 ± 0.9 to −61 ± 2 mV, n = 7; Fig. 4B1) before current injection. Furthermore, with moderate pipette [Cl−], somatic muscimol puffs decreased the latency to firing on current injection (by 2.7 ± 0.9 ms, n = 7, P < 0.02; Fig. 4B1), because the muscimol depolarization brought membrane voltage closer to threshold. This is in contrast to the increased latency observed with low pipette [Cl−].
Although the delay to initiation was briefer with somatic muscimol applications, phase plots showed that both rate of rise and action potential peak were smaller compared with control traces (Fig. 4B2). This suggests some degree of shunting of the action potential waveform at the level of the soma, which could be important for the backpropagation of action potentials toward the dendrites of neurons. Average absolute peak amplitude of action potentials was decreased by 1.1 ± 0.3 mV (P < 0.05, n = 7). Average maximum dV/dt was decreased by 7.4 ± 2.8 mV/ms (P < 0.05, n = 7). Therefore somatic shunting effects on the action potential waveform were observed at both low and moderate pipette [Cl−]. These observations suggest that AIS GABAA agonist application has an inhibitory influence on voltage threshold under multiple conditions, whereas somatic application has a mixture of actions: latency changes according to pipette [Cl−] and shunting effects on the action potential under multiple conditions. These observations also give additional validity to the conclusion that muscimol applications were largely restricted to one compartment of the cell.
With 33.3 mM pipette [Cl−], we set ECl to be explicitly excitatory (Szabadics et al. 2006) (ECl more depolarized than action potential threshold). We found no significant effect of AIS muscimol on voltage threshold (Fig. 4C). We did observe, however, individual cells that exhibited either hyperpolarized or depolarized thresholds and a trend toward overall hyperpolarization of threshold (Fig. 4C). These results showing no significant overall effect on voltage threshold are in contrast to results from low and medium pipette Cl− conditions. The lack of significant effect is consistent with the idea that, when ECl is set to be depolarized with respect to action potential threshold, the current generated through GABAA receptors carries the membrane potential toward threshold, but voltage threshold itself will be set by the activation of sodium channels. As with previous experimental conditions, we found no effect of soma GABAA receptor activation on threshold (Fig. 4C).
Issues of drug spillover among compartments are difficult to exclude completely in localized application experiments. To obviate spillover issues and to test the general robustness of our observations, including those made at different chloride concentrations, we asked whether our experimentally observed excitability effects could be replicated in a simple neuron simulation, where we could unambiguously introduce a GABA conductance to the AIS or to the soma compartment. To minimize the number of free parameters in the model, we used a simple geometry that approximated granule cell morphology and passive membrane responses (see methods and Fig. 5A). We introduced a conductance either into the AIS or into the soma compartment, and we explored the effect on action potential threshold, as recorded from a somatic pipette. Simulated action potentials are shown in Fig. 5B. We observed qualtitatively similar effects of the AIS conductance and soma conductance as in actual experiments. At no ECl value did we observe an effect of the soma Cl− conductance on action potential threshold (Fig. 5C). By contrast, the simulated AIS Cl− conductance increased voltage threshold as long as ECl was hyperpolarized relative to threshold (Fig. 5D). The effect of the AIS Cl− conductance on voltage threshold was largest when ECl was strongly hyperpolarized (Fig. 5D, red line). However, the effect remained inhibitory at an ECl value that was depolarizing from rest but still hyperpolarized relative to action potential threshold (Fig. 5D, dark blue line). As ECl became overtly excitatory, there was no change in action potential threshold (Fig. 5D, light blue line, superimposed on and indistinguishable from baseline black line), as in actual experiments. These simulations confirm our experimental findings that compartmentalized effects of GABA receptor activation have distinguishable effects on excitability that extend over a range of intracellular [Cl−]. The simulation in Fig. 6 shows how modifications of the dynamics of action potential development in the AIS (Fig. 6, B and D) and in the somatodendritic compartment (Fig. 6, C and D) may alter the somatically recorded voltage threshold during AIS muscimol application, with the somatic waveform rising earlier relative to the shunted AIS compartment.
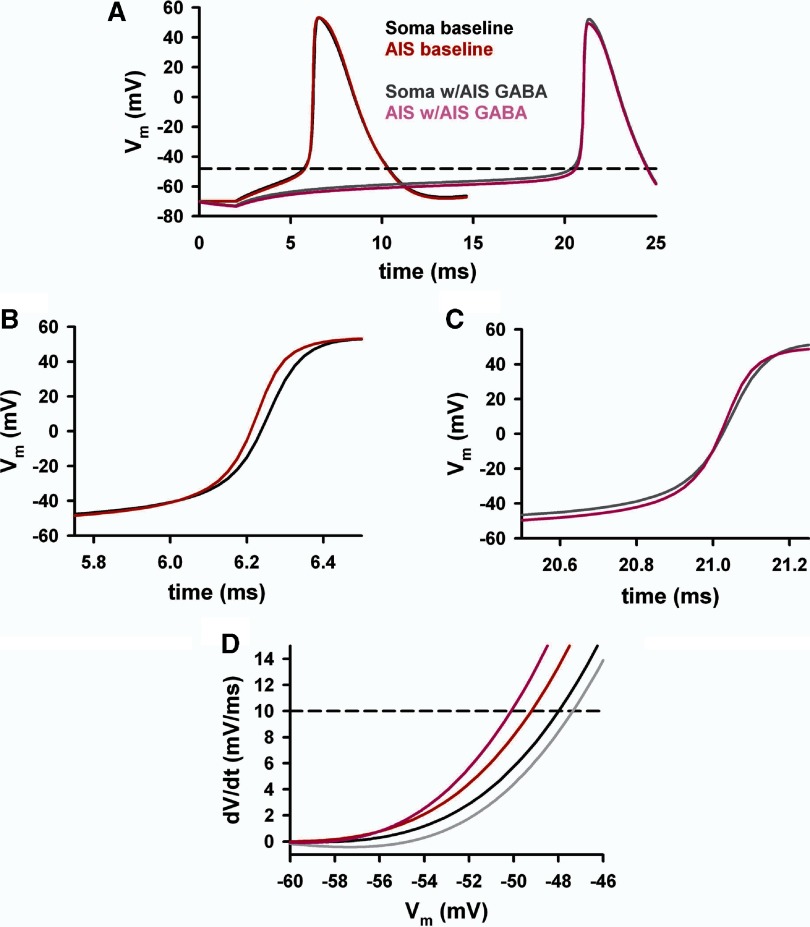
Fig. 6.
Simulated action potential waveforms in the soma and AIS. We used the simulations depicted in Fig. 5 to probe additional details of the likely mechanism of the depolarized threshold measured in somatic recordings. We used simulations with ECl set at −80 mV and with the GABA conductance introduced in the AIS. A: the action potential waveforms from the distal initial segment (AIS; gray crosshatched compartment in Fig. 5A) and from the soma compartment. B and C: the rises of the action potential waveforms are highlighted. Under baseline conditions, the AIS waveform rises 1st (B), consistent with the high density of sodium channels. The simulations show that the situation partially reverses with the presence of the AIS GABA conductance (C). Now the somatic waveform (gray trace) rises 1st at the very earliest stages of initiation. The AIS remains shunted by the GABA conductance (pink), but soon catches up and eventually crosses the somatic waveform as the dense AIS sodium channels are recruited. The resulting dynamics yield the surprising result that the AIS voltage threshold in the simulation actually hyperpolarizes in the presence of the GABA conductance. D: magnification of the rising part of phase plots constructed from the 4 simulations shown in A illustrating the voltage thresholds (dashed horizontal line).
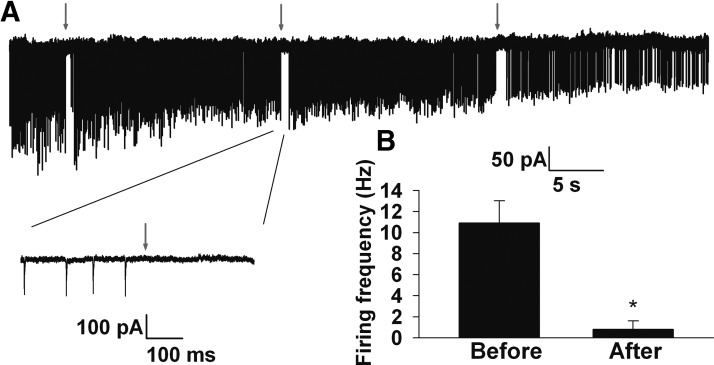
The experimental results in Fig. 4 and simulation results in Fig. 5 suggest that changes in voltage threshold will occur only when AIS GABAA receptor activation results in inhibition (i.e., when ECl is hyperpolarized relative to threshold). As previously noted, under some experimental conditions, and in some cell types, AIS GABAA receptor activation is excitatory (Szabadics et al. 2006). To test whether AIS GABAA receptor activation is inhibitory or excitatory when cytoplasmic [Cl−] is left unperturbed, we monitored spontaneous spiking in intact cells. We electroporated neurons with Alexa Fluor dye and allowed the dye to diffuse into the proximal axon for visualization and to target muscimol pipette placement. We performed cell-attached recordings from somas of the labeled granule neurons and monitored spontaneous firing in this recording configuration before and after local, brief AIS muscimol application (Fig. 7A). Because granule neurons are normally quite quiescent, we artificially increased excitability in these experiments, as described in methods, to have a strong baseline firing rate on which to evaluate muscimol effects. AIS muscimol pulses inhibited action potential firing in all four neurons tested. Average firing was reduced from 5.6 ± 2 Hz in the period immediately preceding local application to 0.6 ± 0.3 Hz immediately following muscimol (Fig. 7B; P < 0.05). The results strongly suggest that AIS GABAA receptor activation is inhibitory (ECl hyperpolarized relative to action potential threshold) in unperturbed granule neurons under our conditions and suggest that observations of voltage threshold increases in Figs. 1–5 are relevant to intact granule neurons.
Fig. 7.
AIS GABAA receptor activation is inhibitory in unperturbed granule neurons. A: slow sweep showing spontaneous spiking from a cell-attached recording of a granule neuron. The cell had been electroporated 15 min beforehand with Alexa Fluor dye for axon visualization. Downward deflections represent spontaneous action currents arising from spontaneous action potentials, shown in greater detail in the inset below. At the times indicated by red arrows, a 50 ms pulse application of muscimol was made to the initial segment 20–30 μm from the soma. B: summary of the firing frequency changes from the 3 epochs shown in A. For quantification, an 800 ms window before and after muscimol application was evaluated. Results are representative of 4 cells in which spiking was detected in the cell attached configuration.
AIS receptors likely respond only to synaptic GABA and not to ambient GABA
The results thus far indicate that stimulation of AIS GABAA receptors has an inhibitory effect on action potential initiation primarily by raising the voltage threshold for action potential initiation. By contrast, somatodendritic receptors lower the cell input resistance without a detectable effect on voltage threshold. Dentate granule neurons possess synaptic and extrasynaptic GABAA receptors. The latter receptors respond primarily to ambient GABA (<1 μM) thought to be present in vivo as a consequence of synaptic release (Glykys et al. 2008; Stell and Mody 2002). Although tonic, extrasynaptic GABAA receptors are believed to be primarily somatodendritic (Wei et al. 2003), our observations allow us to functionally verify that ambient agonist levels (≤1 μM) do not activate AIS GABA receptors.
Our previous work showed that, under our baseline conditions in the absence of added GABA agonists, there is no detectable influence of ambient endogenous GABA on action potential threshold (Kress et al. 2010), likely because of constant perfusion of the slice and limited synaptic release of GABA. Therefore we added exogenous muscimol (1 μM) to the slice bathing solution to test the effects of high-affinity receptor activation. During baseline, muscimol application (2 min periods each), and washout, we applied 20 ms depolarizing current pulses to elicit action potentials, and we delivered a hyperpolarizing 20 pA pulse (200 ms duration) to measure input resistance for verification of a muscimol effect. A representative example of effects is shown in Fig. 8A. Although this cell exhibited a slight change in threshold with muscimol (Fig. 8A2), overall we found no significant change in threshold (Fig. 8B) despite a large, partially reversible muscimol-induced decrease in input resistance (Fig. 8, A3 and C). These results suggest that tonic ambient GABA agonist levels do not activate axonal receptors and that the changes in voltage threshold evoked by AIS GABA receptor activation likely occur only under conditions when the receptors are synaptically activated.
Differential effects on temporal summation
GABA inhibition is known to have important effects on temporal summation of EPSPs (Johnston and Wu 1995). To highlight the differential effects of somatic versus AIS GABA receptor activation in the context of transient excitatory events, we evaluated local applications of muscimol during a barrage of EPSP-like depolarizations (aPSPs) elicited with transient, excitatory postsynaptic current (EPSC)-like current inputs (aPSCs) delivered through the patch pipette (Fig. 9). Both AIS and somatic application of muscimol (10 μM) decreased the reliable spiking that occurred with temporally summated aPSPs (Fig. 9, A1 and A2). However, with AIS application, the lack of spiking was not associated with a change in aPSP amplitude or time course (Fig. 9A1). In the same cells, action potential voltage threshold depolarized by 2.2 mV with muscimol application prior to 20 ms rectangular depolarizing current pulses. By contrast, somatic inhibition caused spike failures associated with smaller PSPs that decayed more quickly as a result of the lowered input resistance (Fig. 9A2). Therefore AIS inhibition did not change the impact of individual EPSPs, but more EPSPs were required to reach threshold. By contrast, somatic inhibition decreased the impact of individual EPSPs, making voltage threshold more difficult to achieve. Figure 9B1 shows that, even when strong EPSPs exceeded voltage threshold during AIS muscimol stimulation, the average latency of action potential initiation was increased by the increased threshold. The example is representative of effects observed in four of four cells challenged in this way. In the same neuron, strong EPSP activation during somatic muscimol application again reduced the impact of the EPSPs, causing failures of spike initiation (Fig. 9B2).
To highlight the functional impact of AIS GABAA receptor activation during trains of spike firing, we also examined localized muscimol (5–10 μM) applications during sustained (200 ms) depolarizing current pulses of varied amplitudes (Fig. 9, C and D). As expected, the input/output relationship of granule neurons was shifted to larger current injection amplitudes with the application of either AIS or somatic muscimol (5 μM). Therefore rheobase was shifted for both AIS and soma applications (Fig. 9C). However, in the case of AIS applications, the rheobase change was presumably caused by the primary change in voltage threshold rather than by the strong change in input resistance elicited by somatic muscimol application. To quantify this effect, the number of action potentials at the current injection that produced 50% of maximum effect was calculated for control, axonal, and somatic puffs (Fig. 9E). Axonal application decreased the number of action potentials by more than one half, whereas somatic puffs nearly abolished spiking. Figure 9D shows the effect of AIS muscimol application at a current injection level (250 pA) that produced eight action potentials, matched to the effect of 200 pA in baseline, which produced the same number of action potentials. It is clear from the voltage thresholds plotted as a function of time during the action potential trains (Fig. 9D, bottom) that spike history depolarized voltage threshold, but the effect of muscimol on voltage threshold was sustained over the duration of the spike train. To quantify this phenomenon we averaged the last five action potentials in each train, representing the quasi-steady state of combined muscimol and spike-history effects, for control and axonal application (Fig. 9F). Muscimol application produced a significant increase in the average voltage threshold.
DISCUSSION
Our main finding is that GABAA receptor activation in the AIS and somatodendritic compartment produces different effects on two major measures of cellular excitability, voltage threshold, and current threshold. This observation is significant in the general context of cellular excitability in part because rheobase and voltage threshold are both commonly used measures of excitability. How compartmentalized channels, especially ligand-gated channels, might interact with the two measures is unclear. We found that AIS GABAA receptor activation primarily influences voltage threshold by shunting the local sodium current responsible for normal initiation of the action potential. As a secondary consequence, current threshold increases. Somatodendritic receptor activation primarily raises current threshold, and despite strong input resistance changes, voltage threshold for action potential initiation is unaffected. We show that this differential influence applies under a number of different physiological conditions, including different intracellular chloride concentrations and during temporally summated EPSP-like depolarizations. AIS GABA receptor activation also shifts the input/output profile of granule neurons. There is abundant previous anatomical (Soriano et al. 1990) and physiological (Christie and Jahr 2009; Khirug et al. 2008; Szabadics et al. 2006) evidence for AIS synaptic GABAA receptor presence in principal neurons, but understanding of the impact of these receptors on measures of cellular excitability has been incomplete.
Physiological relevance
Granule cells are the entry point of the hippocampal tri-synaptic circuit. They are the weakly excitable gatekeepers of this circuit (Hsu 2007), and GABA inhibition is thought to play a strong role in this gatekeeper function (Coulter and Carlson 2007). Axonal GABA receptors are strategically poised to mediate strong inhibition in granule neurons (Soriano et al. 1990). Effects of AIS GABAA receptor stimulation in our experiments were modest in absolute terms (shifts of 1–4 mV depending on conditions). However, these shifts have important physiological consequences for granule neurons, which experience atypically small single-input EPSCs and EPSPs (Bekkers and Clements 1999; McNaughton et al. 1981). Single-input EPSPs are 0.1–0.5 mV in these cells (McNaughton et al. 1981). Therefore the shifts in voltage threshold observed here would require recruitment of significant additional numbers of presynaptic inputs to reach firing threshold in the postsynaptic granule neuron.
For selective activation of GABAA receptors, we used controlled agonist application to cellular compartments. We kept agonist concentration well below synaptic concentrations (estimated to approach 1 mM GABA; Jones and Westbrook 1995) to keep cross-compartmental spillover to a minimum. It is therefore possible that synaptic activation of these same receptors could have larger effects than those observed here, although the presynaptic release probability and spatial extent of synaptic receptor activation are also factors that warrant consideration. Ideally, true synaptic activation of AIS receptors would be desirable for exploration of effects on excitability. However, selective stimulation of the small population of axoaxonic (chandelier) neurons that give rise to the AIS inputs onto principal neurons remains technically prohibitive except in a few specialized cases (Szabadics et al. 2006; Woodruff et al. 2009).
Our major conclusion, drawn from results using subcellular drug application, superficially contradicts a recent dynamic clamp experiment (Fernandez and White 2010). Introduction of a somatic leak conductance in CA1 neurons depolarized voltage threshold. Although the leak conductance was introduced with a soma pipette, the conductance was probably not limited to the soma and likely interacted directly with AIS sodium channels, similar to our AIS muscimol application.
Our work does not directly address the physiological chloride concentration of the granule cell AIS, a question that was recently addressed by others (Khirug et al. 2008). This previous work showed that ECl of the AIS in dentate granule cells is depolarizing relative to rest, but effects of AIS GABAA receptor are still inhibitory because ECl was still quite negative to action potential threshold. Similar results were observed in superficial layers of cortex (Woodruff et al. 2009). Under our conditions, GABAA receptor activation on the initial segment was clearly inhibitory in granule neurons with unperturbed ECl (Fig. 7), although our experiments did not address whether this inhibition was associated with depolarization from rest (Khirug et al. 2008). Explicitly excitatory effects of AIS GABAA receptor activation have been observed in layer 2/3 pyramidal cells (Szabadics et al. 2006). Our systematic study of varied intracellular chloride concentrations suggests that, with explicitly excitatory GABA effects, voltage threshold is no longer affected (Fig. 4). In the case of high intracellular chloride, the chloride current presumably serves as a source of depolarization even at potentials near threshold. At these potentials, the Cl− current does not resist the voltage-gated sodium currents, but it is the voltage-gated channels that are ultimately responsible for initiation. Therefore no change from baseline voltage threshold is observed in either experimental or simulation results when ECl is explicitly excitatory (Figs. 4 and 5).
We also acknowledge that the precision of our control over the AIS [Cl−]i is unclear, because endogenous pumps likely worked against the supply of Cl− from our patch pipette. Nevertheless, the changes in excitability that we observed under varied pipette [Cl−] suggest reasonable control of AIS [Cl−]i. This likely resulted from our use of low-resistance patch pipettes, which facilitate rapid perfusion (Pusch and Neher 1988) and likely outstripped the actions of transporters acting to maintain the endogenous chloride gradient (Khirug et al. 2008).
Our results suggest that the main effect of tonic GABAA receptor activation is to decrease input resistance through somatodendritic receptor activation (Stell and Mody 2002). We failed to find evidence for a detectable effect of tonic receptor activation on voltage threshold (Fig. 8), but 1 μM bath muscimol induced a strong change in input resistance, presumably by activating somatodendritic receptors. These results are consistent with the somatodendric expression of GABAA receptor δ subunits in dentate granule neurons (Wei et al. 2003), which are thought to participate in the high affinity receptors that mediate tonic GABA effects on granule neurons (Glykys et al. 2008; Wei et al. 2003). By contrast, AIS GABAA receptors are believed to contain α2 and possibly α1 subunits, likely with a γ2 subunit (Essrich et al. 1998; Nyiri et al. 2001) and seem to be mainly synaptically localized (Soriano et al. 1990). By exercising a local shunting effect near the sodium channels responsible for initiation, these synaptic receptors, but not the more numerous somatodendritic receptors, are capable of raising the threshold voltage for action potential initiation.
AIS as a separate subcellular compartment
It has been known for many years that the constellation of voltage-dependent ion channels located in the soma participates in setting unique firing properties of different classes of neurons (Bean 2007). More recently, the role of channel compartmentalization has been explored. Early work showed that the initiation site of action potential is the AIS (Colbert and Johnston 1996; Stuart and Hausser 1994; Stuart et al. 1997), which spans from the axon hillock to a length of 40–80 μm depending on cell type. Axonal initiation has been verified in several cell types by direct intra-axonal recordings of cut axon blebs (Kole et al. 2008; Shu et al. 2007a). The high-density and favorable activation kinetics of voltage-gated sodium channels participate in setting the threshold for action potential initiation in the AIS (Colbert and Pan 2002; Kole et al. 2008; Royeck et al. 2008). Specific axonal K+ channels alter axonal spike waveform without necessarily altering voltage threshold (Kole et al. 2007; Shu et al. 2007b), although in some cases, low-threshold potassium conductances can influence voltage threshold in principal neurons (Goldberg et al. 2008; Shah et al. 2008). Other recent work suggests that low threshold voltage-gated axonal Ca2+ channels can influence voltage threshold (Bender and Trussell 2009). Most recently, it has been shown that the position and length of the AIS spike initiation zone is homeostatically regulated by activity (Grubb and Burrone 2010; Kuba et al. 2010). In this study, we showed that a ligand-gated channel can also affect action potential initiation by raising voltage threshold.
The AIS is apparently also partly defined by other proteins and by additional functional differences from other cellular compartments. The AIS is defined in part by the scaffolding proteins ankyrin G, spectrin β IV, and gephyrin (Ogawa and Rasband 2008). Localization of sodium and potassium channels is associated with ankyrin G and spectrin β IV (Ogawa and Rasband 2008), and α2 GABA receptors bind to gephyrin (Tretter et al. 2008). The differential Cl− gradient between the AIS and soma noted above is explained by the subcellular distribution of the brain chloride transporters NKCC1 and KCC2 (Khirug et al. 2008). NKCC1 sequesters chloride, sodium, and potassium intracellularly and is located in the AIS. KCC2 extrudes chloride and potassium and is located in the soma (Khirug et al. 2008; Szabadics et al. 2006). Our results show an additional dimension of functional differences between soma and AIS GABAA receptor activation, based on local interactions between ligand-gated GABAA receptor channels and voltage-gated channels.
Our experimental and simulation results thus emphasize two fundamentally different effects on cellular excitability based on receptor compartmentalization relative to action potential–initiating voltage-gated sodium channels, which are located in the proximal AIS of granule neurons (Soriano et al. 1990). First, for AIS stimulation, voltage threshold is primarily affected. Second, somatic receptor activation can strongly alter overall input resistance of granule neurons in the absence of a voltage threshold change. Synaptic, but not ambient, AIS GABA alters voltage threshold in dentate granule neurons, thereby altering the likelihood and timing of firing in these cells. These differential effects can influence the impact of EPSPs and synaptic integration in granule neurons.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-54174 to S. Mennerick and Biotecza INNOVA-CORFO (Corporacion de Fomento de la Produccion) 06FC01IFC-71 to P. Rojas.
DISCLOSURES
The authors declare that they have no potential conflict of interest in the studies herein.
Supplementary Material
ACKNOWLEDGMENTS
We thank all laboratory members for helpful discussions and especially G. Kress for advice on experiments and data analysis.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci 8: 451–465, 2007. [DOI] [PubMed] [Google Scholar]
- Bekkers JM, Clements JD. Quantal amplitude and quantal variance of strontium-induced asynchronous EPSCs in rat dentate granule neurons. J Physiol 516: 227–248, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender KJ, Trussell LO. Axon initial segment Ca2+ channels influence action potential generation and timing. Neuron 61: 259–271, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Jahr CE. Selective expression of ligand-gated ion channels in L5 pyramidal cell axons. J Neurosci 29: 11441–11450, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Bekkers JM. Detection of spontaneous synaptic events with an optimally scaled template. Biophys J 73: 220–229, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert CM, Johnston D. Axonal action-potential initiation and Na+ channel densities in the soma and axon initial segment of subicular pyramidal neurons. J Neurosci 16: 6676–6686, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert CM, Pan E. Ion channel properties underlying axonal action potential initiation in pyramidal neurons. Nat Neurosci 5: 533–538, 2002. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Carlson GC. Functional regulation of the dentate gyrus by GABA-mediated inhibition. Prog Brain Res 163: 235–243, 2007. [DOI] [PubMed] [Google Scholar]
- Ebert B, Thompson SA, Saounatsou K, McKernan R, Krogsgaard-Larsen P, Wafford KA. Differences in agonist/antagonist binding affinity and receptor transduction using recombinant human gamma-aminobutyric acid type A receptors. Mol Pharmacol 52: 1150–1156, 1997. [PubMed] [Google Scholar]
- Ebert B, Wafford KA, Whiting PJ, Krogsgaard-Larsen P, Kemp JA. Molecular pharmacology of γ-aminobutyric acid type A receptor agonists and partial agonists in oocytes injected with different α, β, and γ receptor subunit combinations. Mol Pharmacol 46: 957–963, 1994. [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat Neurosci 1: 563–571, 1998. [DOI] [PubMed] [Google Scholar]
- Fernandez FR, White JA. Gain control in CA1 pyramidal cells using changes in somatic conductance. J Neurosci 30: 230–241, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus 6: 347–470, 1996. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Roberts JD, Somogyi P, Scanziani M. Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nat Neurosci 12: 21–23, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci 28: 1421–1426, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron 58: 387–400, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature 465: 1070–1074, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines ML, Carnevale NT. NEURON: a tool for neuroscientists. Neuroscientist 7: 123–135, 2001. [DOI] [PubMed] [Google Scholar]
- Holmgren CD, Mukhtarov M, Malkov AE, Popova IY, Bregestovski P, Zilberter Y. Energy substrate availability as a determinant of neuronal resting potential, GABA signaling and spontaneous network activity in the neonatal cortex in vitro. J Neurochem 112: 900–912, 2010. [DOI] [PubMed] [Google Scholar]
- Howard A, Tamas G, Soltesz I. Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci 28: 310–316, 2005. [DOI] [PubMed] [Google Scholar]
- Hsu D. The dentate gyrus as a filter or gate: a look back and a look ahead. Prog Brain Res 163: 601–613, 2007. [DOI] [PubMed] [Google Scholar]
- Johnston D, Wu SMS. Principles of Cellular Neurophysiology. Cambridge, MA: MIT Press, 1995. [Google Scholar]
- Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron 15: 181–191, 1995. [DOI] [PubMed] [Google Scholar]
- Khirug S, Yamada J, Afzalov R, Voipio J, Khiroug L, Kaila K. GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl cotransporter NKCC1. J Neurosci 28: 4635–4639, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ. Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci 11: 178–186, 2008. [DOI] [PubMed] [Google Scholar]
- Kole MH, Letzkus JJ, Stuart GJ. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron 55: 633–647, 2007. [DOI] [PubMed] [Google Scholar]
- Kole MH, Stuart GJ. Is action potential threshold lowest in the axon? Nat Neurosci 11: 1253–1255, 2008. [DOI] [PubMed] [Google Scholar]
- Kress GJ, Dowling MJ, Eisenman LN, Mennerick S. Axonal sodium channel distribution shapes the depolarized action potential threshold of dentate granule neurons. Hippocampus 20: 558–571, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress GJ, Dowling MJ, Meeks JP, Mennerick S. High threshold, proximal initiation, and slow conduction velocity of action potentials in dentate granule neuron mossy fibers. J Neurophysiol 100: 281–291, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba H, Oichi Y, Ohmori H. Presynaptic activity regulates Na(+) channel distribution at the axon initial segment. Nature 465: 1075–1078, 2010. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol 87: 33–46, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Tilwalli S, Ye G, Lio PA, Pasternak JF, Trommer BL. Morphologic and electrophysiologic maturation in developing dentate gyrus granule cells. Brain Res 856: 202–212, 2000. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Andersen P. Synaptic efficacy and EPSP summation in granule cells of rat fascia dentata studied in vitro. J Neurophysiol 46: 952–966, 1981. [DOI] [PubMed] [Google Scholar]
- Naundorf B, Wolf F, Volgushev M. Unique features of action potential initiation in cortical neurons. Nature 440: 1060–1063, 2006. [DOI] [PubMed] [Google Scholar]
- Nyiri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of α2-subunit-containing GABAA receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci 13: 428–442, 2001. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Rasband MN. The functional organization and assembly of the axon initial segment. Curr Opin Neurobiol 18: 307–313, 2008. [DOI] [PubMed] [Google Scholar]
- Pusch M, Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pfluegers 411: 204–211, 1988. [DOI] [PubMed] [Google Scholar]
- Royeck M, Horstmann MT, Remy S, Reitze M, Yaari Y, Beck H. Role of axonal NaV1.6 sodium channels in action potential initiation of CA1 pyramidal neurons. J Neurophysiol 100: 2361–2380, 2008. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Bischofberger J. Fast sodium channel gating supports localized and efficient axonal action potential initiation. J Neurosci 30: 10233–10242, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Action potential initiation and propagation in hippocampal mossy fibre axons. J Physiol 586: 1849–1857, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Subthreshold dendritic signal processing and coincidence detection in dentate gyrus granule cells. J Neurosci 27: 8430–8441, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Migliore M, Valencia I, Cooper EC, Brown DA. Functional significance of axonal Kv7 channels in hippocampal pyramidal neurons. Proc Natl Acad Sci USA 105: 7869–7874, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Duque A, Yu Y, Haider B, McCormick DA. Properties of action potential initiation in neocortical pyramidal cells: evidence from whole cell axon recordings. J Neurophysiol 97: 746–760, 2007a. [DOI] [PubMed] [Google Scholar]
- Shu Y, Yu Y, Yang J, McCormick DA. Selective control of cortical axonal spikes by a slowly inactivating K+ current. Proc Natl Acad Sci USA 104: 11453–11458, 2007b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano E, Nitsch R, Frotscher M. Axo-axonic chandelier cells in the rat fascia dentata: Golgi-electron microscopy and immunocytochemical studies. J Comp Neurol 293: 1–25, 1990. [DOI] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J Neurosci 22: RC223, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storustovu SI, Ebert B. Pharmacological characterization of agonists at δ-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J Pharmacol Exp Ther 316: 1351–1359, 2006. [DOI] [PubMed] [Google Scholar]
- Stuart G, Hausser M. Initiation and spread of sodium action potentials in cerebellar Purkinje cells. Neuron 13: 703–712, 1994. [DOI] [PubMed] [Google Scholar]
- Stuart G, Schiller J, Sakmann B. Action potential initiation and propagation in rat neocortical pyramidal neurons. J Physiol 505: 617–632, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 311: 233–235, 2006. [DOI] [PubMed] [Google Scholar]
- Tretter V, Jacob TC, Mukherjee J, Fritschy JM, Pangalos MN, Moss SJ. The clustering of GABAA receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor α2 subunits to gephyrin. J Neurosci 28: 1356–1365, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci 23: 10650–10661, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff A, Xu Q, Anderson SA, Yuste R. Depolarizing effect of neocortical chandelier neurons. Front Neural Circuits 3: 15, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff A, Yuste R. Of mice and men, and chandeliers. PLoS Biol 6: , 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Shu Y, McCormick DA. Cortical action potential backpropagation explains spike threshold variability and rapid-onset kinetics. J Neurosci 28: 7260–7272, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.