Abstract
Proprioception is considered important for maintaining spinal stability and for controlling posture and movement in the low back. Previous studies demonstrate the presence of thixotropic properties in lumbar muscle spindles, wherein a vertebra's positional history alters spindle responsiveness to position and movement. This study investigated whether a vertebra's movement history affects the velocity sensitivity of paraspinal muscle spindles in the low back. Afferent activity from multifidus and longissimus muscle spindles was recorded in the L6 dorsal root in 30 anesthetized cats. To alter movement history, a feedback-controlled motor attached to the L6 spinous process held (conditioned for 4 s) the L6 vertebra at an intermediate position or at positions that either lengthened or shortened the muscles. With the vertebra returned to the intermediate position, resting spindle discharge was measured over the next 0.5 s (static test) and then during a dynamic test consisting of ramp vertebral movement at four velocities (0.2, 0.5, 1.0, 2.0 mm/s). Spindle activity during the tests was measured relative to hold-intermediate. For both tests, hold-long decreased and hold-short increased muscle spindle responsiveness. For the static test position responsiveness was not different among the velocity protocols for either hold-long or hold-short (P = 0.42 and 0.24, respectively). During the dynamic test, hold-long conditioning significantly decreased [F(3,119) = 7.99, P < 0.001] spindle responsiveness to increasing velocity. Mean velocity sensitivity was 4.44, 3.39, and 1.41 (impulses/s)/(mm/s) for the hold-short, hold-intermediate, and hold-long protocols, respectively. The nearly 2.5-fold decrease in velocity sensitivity following hold-long was significantly less than that for either hold-intermediate (P = 0.005) or hold-short conditioning (P < 0.001). Hold-short conditioning had little effect on velocity responses during the dynamic test [F(3,119) = 0.23, P = 0.87]. In conclusion, only movement histories that stretch but not shorten muscle spindles alter their velocity sensitivity. In the low back, forward flexion and lateral bending postures would likely be the most provocative.
INTRODUCTION
The neuromuscular system plays a crucial role in posture and movement of the lumbar vertebral column. The lumbar spine is a complicated structure biomechanically, being multisegmented with contiguous vertebral segments being coupled by three articulations (two gliding diarthroses and one amphiarthrosis) that permit 6 degrees of freedom of motion. Passive properties alone of the paraspinal tissues are insufficient to support spinal loading, with the spine buckling as either a long or short column under compressive loads less than the torso's weight (Crisco et al. 1992; Wilder et al. 1988). Consequently, the neuromuscular control system must simultaneously control regional activity of the trunk (e.g., bending and twisting) and the accompanying intervertebral translations and rotations that underlie the regional activity (Cholewicki and McGill 1996; Macdonald et al. 2006; Panjabi 1992). In the lumbar spine, over 45 paired fascicles of the multifidus and erector spinae muscles (not including the paired intersegmental intertransversarii and interspinales muscles) contribute to this motor control through their dorsal attachments to the lumbar vertebra (Bogduk et al. 1992; Macintosh and Bogduk 1987). Unsuccessful neuromuscular control resulting in excessive rotation at a single lumbar vertebra during trunk flexion has been captured by videofluoroscopy and was painful (Cholewicki and McGill 1992).
Recent evidence indicates that proprioceptive feedback plays a particularly important role in motor control of the trunk (Granata et al. 2001). Although feedforward antagonist cocontraction contributes to precision control of limb movement and to stabilization of the trunk during anticipated postural perturbations, precision control of trunk movement uses sensory feedback more than the cocontraction strategy (Willigenburg et al. 2010). The use of feedback appears more pronounced during forward and lateral flexion than during trunk extension (Willigenburg et al. 2010), movements likely controlled to a large degree by eccentric contraction of the paraspinal muscles. Reflex mechanisms alone may account for ≥40% of the trunk stiffness to maintain a stable posture during sudden loading (Moorhouse and Granata 2007). The apparent importance of feedback for precision control of the trunk may have a functional counterpart in the greater position and velocity sensitivity of lumbar paraspinal muscle spindles, compared with appendicular muscle spindles (Cao et al. 2009a,b).
Muscle spindles are well known for signaling at least three kinematic variables: muscle length, changes in muscle length, and the velocity at which the change occurs (Matthews 1972). In general, they can contribute not only to consciously perceived sensations, but to unconscious, automatic postural adjustments as well (Gandevia 1996; Prochazka 1996; Proske 2005). In the human lumbar spine, they have been shown to contribute to conscious awareness of low back position and the velocity of movement (Brumagne et al. 1999; Gade and Wilson 2007; Soltys and Wilson 2008). Vibratory activation of paraspinal muscle spindles causes undershoot errors in lumbopelvic repositioning by evoking the illusion that the paraspinal muscles are stretched and therefore that the spinal joints are erroneously extended (Brumagne et al. 1999, 2000). Similarly, lumbar vibration causes individuals to slow their trunk movement relative to a target velocity due to the illusion that the paraspinal muscles are being stretched at a rate faster than actuality (Soltys and Wilson 2008).
The proprioceptive role of muscle spindles and the apparent importance of feedback mechanisms for motor control of the trunk led us to investigate whether movement history of a lumbar vertebra affects the signaling characteristics of that segment's muscle spindles (Cao and Pickar 2009a; Ge and Pickar 2008; Ge et al. 2005; Pickar et al. 2008, in preliminary form). In the limbs, the history-dependent property of muscle spindles changes their responsiveness to a new joint position in a direction that depends on the parent muscle's previous length history (Proske et al. 1993). Such movement history causes limb repositioning errors, creates changes in the timing and magnitude of tendon reflexes, and alters the level of bias for spinal reflex excitability (Gregory et al. 1987, 1988; Wise et al. 1999; Wood et al. 1996). Although experiments in both the spine and limbs reveal that directionally opposite movement histories (i.e., whether they lengthen or shorten a muscle) have an opposite effect on the spindle's subsequent responsiveness to a new joint position (Ge et al. 2005; Gregory et al. 1986), the effect of movement history on a spindle's velocity sensitivity is not known. We hypothesized that, similar to its effect on spindle responsiveness to position, movement history that decreases position responsiveness will also decrease the spindle's velocity sensitivity and, conversely, that movement history that increases position responsiveness will also increase velocity sensitivity.
METHODS
Preparation
Experiments were performed on 30 deeply anesthetized adult cats (weighting 3.0–5.7 kg). All cats were treated in accordance with the Guiding Principles in the Care and Use of Animals approved by the American Physiological Society. All procedures have been described previously (Cao and Pickar 2009b; Ge et al. 2005). Briefly, deep anesthesia was initiated with pentobarbital sodium (35 mg/kg, administered intravenously [iv]) and maintained with additional dosages (∼5 mg/kg, iv). Cats were mechanically ventilated (model 681; Harvard Apparatus, Holliston, MA). Arterial pH, Pco2, and Po2 were measured every 90 min using the i-STAT System (i-STAT, East Windsor, NJ) and were maintained within normal range (pH 7.32–7.43; Pco2, 32–37 mmHg; Po2, >85 mmHg).
Paraspinal tissue dissection and a bilateral laminectomy limited to the caudal half of L4 and the entire L5 vertebra provided access to the L6 dorsal roots. The low back from L6 caudalward remained intact. The L6 spinal nerve innervates fascicles of the multifidus and longissimus muscles that likely attach to the L6 vertebra (Bogduk 1976, 1980). To record from muscle spindle afferents from these muscles, we made an incision through the dura mater, identified the L6 dorsal roots, and cut them close to their entrance into the spinal cord. Rootlets were successively placed on a small platform and thin filaments teased from them using sharpened forceps under a dissecting microscope and successively placed on a stainless steel wire electrode until impulse activity from a single unit with a receptive field in the paraspinal muscles could be identified. Recordings were made differentially (P511K; Grass Instrument, Quincy, MA) with the second electrode inserted into the exposed multifidus muscle at the cranial edge of the laminectomy. Action potentials were identified using a PC-based data acquisition system (Spike2, Cambridge Electronic Design, Cambridge, UK). Activity from a putative muscle spindle in the lumbar spine was first identified when gentle, manual compression of the lumbar paraspinal tissues evoked a high frequency discharge. Only afferents were used whose discharge was highest in response to probing the back muscles compared with the gluteal, hip, or leg regions, and which responded to manual movement of the L6 vertebra in the dorsal–ventral directions. Following the experimental protocols (described in the following text), the back muscles were mechanically isolated by removing the lumbococcygeus muscle. That a receptive ending in the lumbar longissimus or multifidus muscles was the source of neural activity was determined using von Frey hairs (Stoelting, Wood Dale, IL), to confirm that the most sensitive area for mechanically activating the afferent was in the low back. Several methods were used to confirm that neural activity was from a muscle spindle: 1) the afferent's ability to follow vibration (90 Hz, 0.06 mm; Mini-Vibrator, model NC70209; North Coast Medical, Morgan Hill, CA) applied to the muscle (Bianconi and van der Meulen 1963; Brown et al. 1967; Durbaba et al. 2006); 2) a discharge pattern during a ramp muscle stretch, now recognized as classic for primary and secondary spindle afferent endings (Matthews 1972; Scott 1990), where primaries display an abrupt increase in discharge during the constant velocity stretch (exclusive of the initial burst at the ramp's acceleration) followed either by a plateau or slower increase during the remainder of the ramp stretch, and secondaries display a graded increase during the ramp stretch; 3) decreased discharge to a direct muscle twitch; and 4) increased discharge to succinylcholine injection (100–300 μg/kg, intraarterial).
While recording afferent activity from lumbar paraspinal muscle spindles, actuation of the L6 vertebra was induced using an electronic feedback control system (Lever System Model 310; Aurora Scientific, Aurora, Ontario, Canada). A horizontally aligned lever arm attached to the motor's rotary drive shaft was coupled to the L6 spinous process via a pair of adjustable tissue forceps vertically (152.4 mm long, 1 × 2 teeth). The forceps were clamped tightly onto the lateral surfaces of the L6 spinous process through a thin slit along either side (Ge and Pickar 2008; Ge et al. 2005). Controlled displacements of the lever arm were applied along the cat's dorsal–ventral axis.
Vertebral history
The conditioning magnitude used to create history was established for each cat by the displacement of the motor's lever arm that loaded the L6 vertebra between 35% and 45% of the cat's body weight (BW) and also increased afferent discharge by 5 impulses/s. A ramp-and-hold displacement (ramp: 1 mm/s; hold: 2 s) was applied in the direction that increased each afferent's discharge. The afferent's discharge pattern during the ramp was used to help confirm that the afferent recording was from a muscle spindle (see earlier text). Past experience had shown that displacements >2 mm would likely tear the nerve filament, so the maximum conditioning magnitude was 2 mm except in two cats, where 2.1 and 2.2 mm were used. Thus conditioning magnitude in four cats was <35% BW: 34%, 33%, 30%, and 24%. Conditioning magnitudes ranged between 1.1 and 2.2 mm.
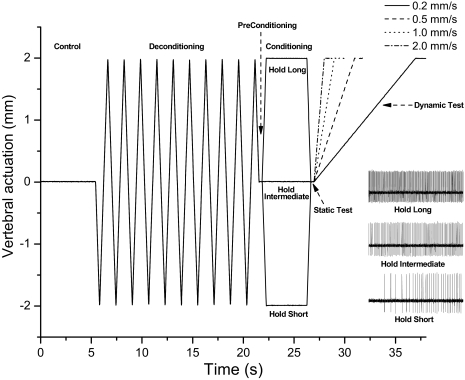
Spinal history or “conditioning” was created by moving and holding the L6 vertebra in a static position that either shortened (hold-short), lengthened (hold-long), or maintained the attached the muscles at an intermediate (hold-intermediate) position for 4.0 s (Fig. 1). At the intermediate position, the spinal tissues exerted no net vertical force on the motor. The direction of hold-short conditioning was identified by a reduction in spindle discharge and hold-long by an increase in spindle discharge. Because the previous lengthening history of a muscle can influence subsequent muscle spindle discharge (Proske et al. 1993), the system was initially placed in a predefined state by the application of identical histories prior to conditioning: the L6 vertebra was moved back and forth 10 times, rapidly (10 mm/s) to the same magnitude as the conditioning amplitude, stretching, and shortening the attached muscles. All protocols were conducted in the ventralward or dorsalward direction.
Fig. 1.
Schematic of the experimental protocol and representative response (inset) of one spindle to 3 conditioning protocols in the dorsal–ventral direction. Loading protocol shows the change in vertebral position relative to the intermediate position. Note that at the beginning of the static test, the vertebra was positioned identically for each of 3 protocols. Four velocities of vertebral actuation were used during the dynamic test.
Similar to previous studies (Cao and Pickar 2009b; Ge and Pickar 2008; Ge et al. 2005) the effects of spinal history on muscle spindle discharge were identified using a static test and dynamic test. The static test occurred immediately following conditioning and consisted of a 0.5-s time interval with the vertebra returned to the intermediate position. The dynamic test followed the static test and the vertebra was moved at different velocities (0.2, 0.5, 1.0, 2.0 mm/s) in a direction that loaded the muscle spindle to the same amplitude as that during conditioning. Muscle spindle discharge was compared between hold-intermediate and hold-long or hold-short during each of these tests.
Each cat received 12 protocols, receiving all combinations of the three hold conditionings and the four velocities. Each protocol was separated by ≥5 min. The 12 protocols were divided into four groupings characterized by the velocity of the dynamic test. The presentation order of velocity groupings was randomized across cats. The presentation order of the three hold conditionings was randomized within a grouping. For all 12 protocols in an individual cat, deconditioning and conditioning magnitudes were identical and the intermediate position for the static test and the magnitude of the dynamic test were also identical.
Data analysis
Spindle activity was quantified as mean instantaneous frequency (MIF) over the course of the static test and mean frequency (MF) over the course of the dynamic test as in our previous studies (Cao and Pickar 2009b). MIF was calculated by averaging the reciprocal of each time interval between consecutive action potentials. MF was calculated by dividing the total number of action potentials by the dynamic test's duration. Static responsiveness was defined as the change in MIF between the hold-intermediate and the hold-short (ΔMIFshort) or hold-long (ΔMIFlong) protocols.
How conditioning affected the spindle's dynamic responsiveness was assessed using two metrics: velocity responsiveness and velocity sensitivity. Similar to static responsiveness, velocity responsiveness was defined as the change in MF between hold-intermediate and hold-short (ΔMFshort) or hold-long (ΔMFlong) protocols. Velocity responsiveness was therefore measured in impulses/s. A positive value indicated an increase in muscle spindle responsiveness and, conversely, a negative value indicated a reduction in muscle spindle responsiveness. Values close to zero indicated that conditioning had little or no effect. Spindle responses are reported as means (lower 95% confidence limit, upper 95% confidence limit) unless otherwise indicated. The second metric, velocity sensitivity, was determined separately for each of the three hold conditions. For each of the 12 protocols (3 holds × 4 velocities), the average baseline discharge frequency during the 0.5 s at the end of deconditioning just prior to conditioning was subtracted from MF during the dynamic test (ΔMFbaseline). Velocity sensitivity was defined as the slope of the relationship between ΔMFbaseline and the velocity of the dynamic test. Slopes were determined by regressing ΔMFbaseline on velocity using the least-squares method for each muscle spindle. Velocity sensitivities in response to hold-long, hold-intermediate, and hold-short conditioning were measured in (impulses/s)/(mm/s).
A priori planned analyses compared the effect of conditioning history on static and velocity responsiveness and on velocity sensitivity. ΔMIFs and ΔMFs for each velocity were compared with a one-way ANOVA (ANOVA for a balanced, randomized complete block design [RBD]). Comparisons were performed separately for ΔMIFlong and ΔMIFshort and ΔMFlong and ΔMFshort. The effect of conditioning on velocity sensitivity was also analyzed using a one-way ANOVA for RBD to compare the mean slopes between hold-long, hold-intermediate, and hold-short. We also determined whether the effects on ΔMF during the dynamic test were consistent across time by first dividing the dynamic test into 20 bins, each bin representing 5% of the dynamic test's total movement. MFs were calculated for each bin. To examine the effects of conditioning on the time course of velocity responsiveness, mixed linear effects models were fit for ΔMFshort and ΔMFlong with terms for velocity, time, and velocity × time interaction using a first-order autoregressive variance–covariance structure.
Statistical significance was set at the P < 0.05 level for the entire study. Post hoc pairwise comparisons were performed when significance reached P < 0.05 and were adjusted for multiple comparisons using the Bonferroni method. Statistical analyses were conducted using SAS (version 9.1; SAS Institute, Cary, NC).
RESULTS
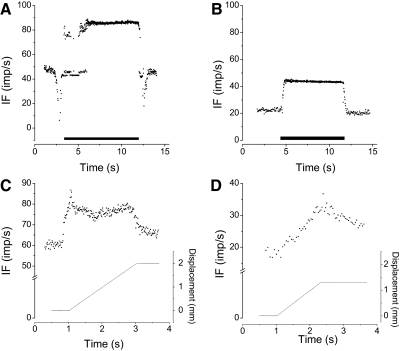
Vibration activated all 30 units when applied indirectly through the thoracolumbar fascia or directly to the muscle's exposed surface. During vibration through the fascia, 24 units were driven 1:1 (85–105 impulses/s) (Fig. 2A) and 6 were activated at 2:1 (43–48 impulses/s) (Fig. 2B), i.e., their discharge was half the driving frequency. With direct muscle stimulation, one of the activated units became driven (90 impulses/s) and the discharge of one decreased from 48 to 32 impulses/s. The latter unit was silenced by twitch and activated by succinylcholine. Three driven units and one activated unit could not be tested with direct muscle vibration because they died before the protocol could be completed. Twenty-seven afferents were considered as supplying the muscle with primary endings to the muscle spindle based on its discharge pattern (Fig. 2C). Twenty-one of these 27 primaries were driven by vibration. The remaining 6 primaries were activated at the first harmonic of the vibration frequency, i.e., at 2:1. Three afferents were classified as terminating in secondary endings (Fig. 2D); one was driven at a subharmonic of the vibration frequency (Fig. 2C) and two were driven at the vibration frequency.
Fig. 2.
Representative responses from 2 afferents to paraspinal muscle vibration and ramp-and-hold movement of a vertebra. A and C: from the same afferent. B and D: from the same afferent. A: driven by the vibratory stimulus. B: stimulated but not driven 1:1 by vibration. C: response of a primary afferent ending. D: response of a secondary afferent ending. See methods for description of the criteria. Solid horizontal bar in A and B indicate where vibrator was on. In A, the decrease in spindle discharge at the beginning occurred as the vibrator was positioned and at the end as the vibrator was removed.
Bipolar muscle stimulation silenced 24 of the 30 afferents. The potential for silencing could not be assessed in 6 afferents because the recordings were lost before the protocol could be completed. Succinylcholine increased the discharge of 28 afferents. Discharge began to increase within 3 to 35 s and increased between 6 and 132 impulses/s. The increased discharge was maintained for ≥1 min, similar to that previously reported for muscle spindles from the lumbar spine (Cao et al. 2009b; Durbaba et al. 2006; Pickar and Ge 2006). Of the two remaining afferents, one died before succinylcholine was injected and one did not respond to succinylcholine, perhaps because it was vascularly inaccessible. However, both of these units were driven by vibration. The 28 afferents responding to succinylcholine had an average resting discharge of 32.5 (SD 12.1, range: 13 and 60 impulses/s) impulses/s; the remaining 2 afferents had similar resting discharges of 35.6 (6.0) and 37.0 (1.0) impulses/s, respectively.
The receptive fields for 29 spindle afferents were located in the lumbar longissimus muscle and only one afferent's receptive field was in the multifidus muscle. The most sensitive portion of each receptive field was most often located near the L6–7 facet joint; 3 in the longissimus muscle were close to the L7–S1 facet joint. Mechanical threshold of lumbar paraspinal muscle spindles ranged between 3.9 and 744.3 mN [104.0 (152.0) mN; mean (SD)]. Among the 30 spindle afferents, ventralward translation of the L6 vertebra loaded 26 muscle spindles and dorsalward translation loaded the remaining 4 spindles.
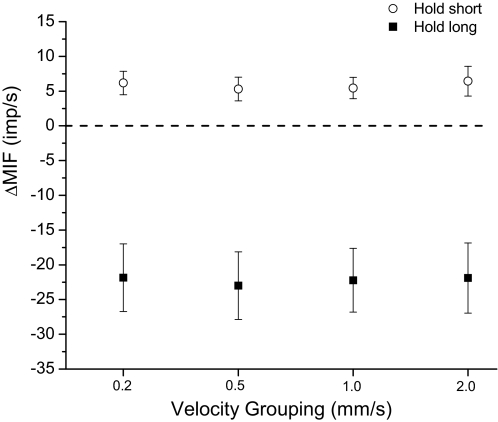
For the static test, hold-long compared with hold-intermediate conditioning decreased resting muscle spindle discharge −22.2 (SD 0.5) impulses/s on average, whereas hold-short increased it by 5.8 (0.5) impulses/s. This result is consistent with findings from previous studies (Cao and Pickar 2009b; Ge and Pickar 2008; Ge et al. 2005). Regardless of the velocity grouping, none of the 95% confidence intervals for either ΔMIFshort or ΔMIFlong crossed zero (Fig. 3). The absolute magnitudes for ΔMIFlong were consistently and substantially larger compared with ΔMIFshort. Among the four velocity groupings, ΔMIFs were not significantly different following either hold-long or hold-short conditioning (Fig. 3, P = 0.42 and 0.24, respectively), indicating each preparation was stable across protocols and enabling comparison of the four velocity groupings during the dynamic test.
Fig. 3.
Effect of paraspinal muscle history on resting muscle spindle discharge during the static test. The y-axis represents the change in muscle spindle discharge following the hold-long or hold-short compared with the hold-intermediate conditionings. Each symbol represents the mean ± 95% confidence interval (CI) of 30 spindles. ΔMIF, change in mean instantaneous frequency.
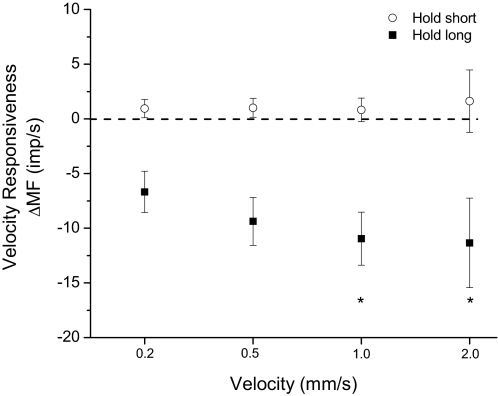
For the dynamic test, hold-long conditioning had a significant effect on velocity responsiveness [F(3,119) = 7.99, P < 0.001]. ΔMFlong decreased −6.7 (−8.6, −4.8), −9.4 (−11.6, −7.2), −11.0 (−13.4, −8.5), and −11.4 (−15.5, −7.3) impulses/s from 0.2 to 2.0 mm/s (Fig. 4). Velocity responsiveness was significantly smaller during the fastest movements (1.0 and 2.0 mm/s) compared with the slowest movements (0.2 mm/s) (P < 0.001). The decrease in velocity responsiveness between 0.2 and 0.5 mm/s approached the significance criterion (P < 0.08). In response to hold-short conditioning ΔMFshort increased 0.9 (0.1, 1.7), 1.0 (0.1, 1.8), 0.8 (−0.3, 2.1), and 1.6 (−1.2, 5.7) impulses/s from 0.2 to 2.0 mm/s. Velocity responsiveness was not significantly affected by the hold-short history [F(3,119) = 0.23, P < 0.87].
Fig. 4.
Effect of paraspinal muscle history on velocity responsiveness of lumbar paraspinal muscle spindles during the dynamic test. The y-axis represents the change in muscle spindle discharge following the hold-long or hold-short compared with the hold-intermediate conditionings averaged over the entire movement of the dynamic test. Each symbol represents the mean ± 95% CI of 30 spindles. ΔMF, change in mean frequency. *P < 0.05 compared with 0.2 mm/s.
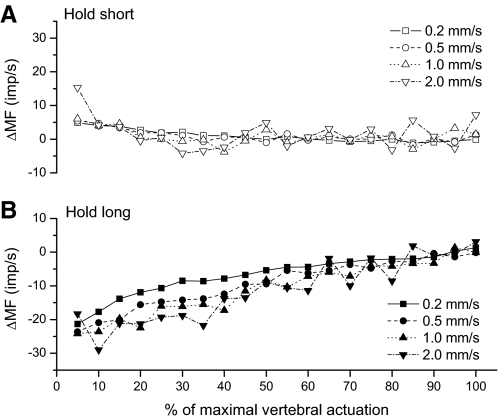
Figure 5 illustrates the time course of ΔMF during the dynamic test for each movement velocity. Significant interaction occurred between movement velocity and ΔMFlong's time course [F(3,2,363) = 3.64, P = 0.01]. Visual inspection of Fig. 5 indicates the interaction took a form where ΔMFlong was most affected by velocity toward the first half of the movement and converged for the four velocities during the last 40–50% of the dynamic test. ΔMFlong approached zero as the movement magnitude approached the conditioning magnitude. The main effect of hold-long conditioning on velocity responsiveness and time course therefore was not directly interpretable. On the other hand, there was no interaction effect in response to hold-short conditioning. Although hold-short conditioning did not affect the spindle's velocity responsiveness [F(3,2,363) = 0.41, P = 0.74], the main effect for time course was significant [F(1,2,363) = 21.14, P < 0.001]. Hold-short's effect was abolished as movement magnitude approached the conditioning magnitude, similar to the pattern seen for hold-long conditioning.
Fig. 5.
Time course of changes in muscle spindle discharge during the dynamic test for (A) hold-short compared with hold-intermediate and (B) hold-long compared with hold-intermediate. The x-axis is normalized to the maximal actuation used for each spindle based on the displacement that loaded the spine to 35 to 45% of body weight (see methods). 100% ranged between 1.1 and 2.2 mm for the 30 cats. MF, mean frequency.
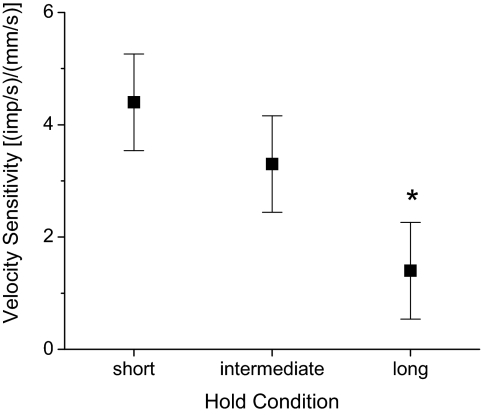
Figure 6 shows the effect of conditioning direction on velocity sensitivity of the lumbar paraspinal muscle spindles. Mean velocity sensitivity was 4.44, 3.39, and 1.41 (impulses/s)/(mm/s) for the hold-short, hold-intermediate, and hold-long protocols, respectively. Conditioning direction significantly affected velocity sensitivity [F(2,89) = 12.98, P < 0.001]. Table 1 shows mean differences between the conditioning positions from the post hoc analysis. The nearly 2.5-fold decrease in velocity sensitivity following hold-long conditioning was significantly less than the velocity sensitivity following hold-intermediate (P = 0.005) and hold-short conditioning (P < 0.001). Hold-short's effect on velocity sensitivity was not significantly different from hold-intermediate's effect.
Fig. 6.
Comparison of paraspinal muscle history's effect on the velocity sensitivity of lumbar paraspinal muscle spindle. The y-axis represents the slope of the relationship between the average mean frequency during movement and the velocity of movement (see methods). Error bars represent the 95% CI based on the statistical model. *P ≤ 0.005 compared with hold-intermediate and hold-short (see Table 1).
Table 1.
Comparison of positional history on velocity sensitivity
| Positional History | Change in Sensitivity, (impulses/s)/(mm/s) | 95% Confidence Interval, (impulses/s)/(mm/s) | Significance Level |
|---|---|---|---|
| Hold-short vs. -intermediate | 1.05 | −0.44, 2.54 | NS |
| Hold-long vs. -intermediate | 1.98 | 0.49, 3.47 | P = 0.005 |
| Hold-long vs. -short | 3.03 | 1.54, 4.52 | P < 0.001 |
DISCUSSION
This study confirms previous findings regarding the effect of a lumbar vertebra's movement history on the position and movement responsiveness of muscle spindles in paraspinal muscles stretched or shortened by the movement (Ge and Pickar 2008; Ge et al. 2005). Whereas a lengthening history decreases their responsiveness to both position (Fig. 3) and movement (Fig. 5), a shortening history increases it. Following the lengthening history, the change in responsiveness is substantially larger in magnitude for position and more prolonged in time during movement compared with the shortening history. The new information and novel finding from this study is that lengthening and shortening histories do not have opposite effects on the spindles' velocity sensitivity: lengthening history decreases velocity sensitivity, whereas shortening history has no significant effect when compared with an intermediate movement history (Figs. 4 and 6). Decreased sensitivity is most pronounced during the early part of movement (Fig. 5) The decreased sensitivity may be consistent with history-dependent kinesthetic effects previously shown in a forearm extensor muscle, the triceps surae muscle where, following a lengthening history, as movement velocity decreases, movement detection thresholds for the elbow joint increase (i.e., movement sensitivity decreases) more compared with a shortening history (Wise et al. 1996).
From a material properties perspective, velocity sensitivity is attributed to viscosity. Although components of the passive muscle spindle that underlie this property are not yet known, they are thought to lie in the polar regions, where the intrafusal fibers predominate (Crowe and Matthews 1964; McMahon 1984). Intrafusal fibers are also considered responsible for the effects of movement history wherein the time-dependent formation of stable, nonrecycling cross-bridges do not develop active force, yet stiffen the spindle at the new muscle length (Hill 1968; Proske and Morgan 1999; Proske et al. 1993). Subsequent passive shortening is thought to slacken and partially unload the intrafusal fibers, whereas passive lengthening is thought to increase their tension (Proske et al. 1993). Based on this mechanism, it could be expected that slackened intrafusal fibers following a lengthening history disengage a mechanical, viscous component, perhaps by increasing interfilamentary distances. As shown in Fig. 4, some velocity sensitivity remained following the lengthening history, suggesting that either the intrafusal fibers alone are not responsible for the viscous component or that the induced slack was insufficient to fully disengage them. However, why would the increased intrafusal tautness following the shortening history not augment their engagement and increase velocity sensitivity? This was initially expected because increased intrafusal fiber tension caused by fusimotor stimulation increases a spindle's velocity sensitivity (Crowe and Matthews 1964). Although the issue cannot be resolved with the current experiment, increasing tension in the nonrecycling cross-bridges may contribute little to changing the spindle's viscosity sensitivity compared with the rate of tension formation created by the recycling cross-bridges activated during intrafusal excitation. Long ago it was shown in whole skeletal muscle that the velocity sensitivity of active extrafusal fibers arises from the rate of chemical work (Fenn and Marsh 1935), now known to represent active cross-bridge formation.
Velocity sensitivity appears important for spinal function. In the lumbar spine, velocity information can be appreciated at a conscious level. Inaccurate muscle spindle input, having been experimentally increased using a vibratory stimulus, impairs velocity detection (Soltys and Wilson 2008). Lumbar paraspinal muscle spindles have greater velocity sensitivity when compared with appendicular muscle spindles (Cao et al. 2009a). In both the spine and appendicular skeleton, the ability to detect movement typically improves as movement velocity increases (Hall and McCloskey 1983; Taylor and McCloskey 1990). Detection of limb movement is less dependent on the movement's velocity for proximal versus distal joints unless velocity is normalized to fascicle length wherein detection thresholds become similar, at least for the finger and elbow (Hall and McCloskey 1983). For finger movement, detection thresholds increase as velocity becomes slower than 10°/s. For elbow and shoulder movement, thresholds increase as velocity becomes slower than about 2°/s (Hall and McCloskey 1983). In the lumbar spine, subjects can learn to move their lumbar spines at three different speeds: 9.5, 13.5, and 17.5°/s (Soltys and Wilson 2008). If this regional movement were distributed equally among the five lumbar joints, intersegmental rotations would be 1.9, 2.7, 3.5°/s, respectively. These same velocities would also detectable by the more proximal elbow and shoulder joints but not by the fingers.
A further consideration suggests the joints of the lumbar spine may be considered the most proximal from both kinesthetic and anatomic perspectives. To our knowledge, changes in fascicle length for lumbar paraspinal muscles during vertebral movement have not been measured. Based on a qualitative consideration that the physical distance between vertebra appears shorter than that between sites of muscle attachment for elbow movement, fascicle lengths of intersegmental paraspinal muscles are likely shorter than those in muscles crossing the elbow joint. Consequently, it may be anticipated that normalizing velocity sensitivity in the spine to changes in fascicle length would make velocity sensitivity greater in the spine than in appendicular skeleton.
Our interest in proprioceptive errors produced by movement history is to understand whether such histories in the spinal column could affect feedback signals used for neuromuscular control of the low back. It might be argued that during natural movements, once paraspinal extensor muscles contract to produce spinal movement, the effects of movement history would be abolished presumably through gamma-motoneurons coactivated with the alphas (Gregory et al. 1986; Wise et al. 1999). However, the effect of muscle activation on removing movement history does not appear to be “all-or-none” (Allen et al. 2008). In the limbs, history-induced repositioning errors are reduced but not abolished by voluntary contractions at 10% of maximal voluntary contraction (MVC). Further reductions occur with contractions at 25% MVC and appear to be maximized at strengths <40% MVC (Allen et al. 2008). In the lumbar spine following a 10-s hold of the trunk in an upright position, positioning errors remained during active lengthening of the dorsal paraspinal muscles (i.e., during flexion repositioning) (Wilson and Granata 2003). Augmenting background extensor muscle activation during the repositioning, by creating a flexor moment with an anterior load (paraspinal extensor's %MVC was not determined), did not significantly reduce the lumbar repositioning error that followed the 10-s conditioning. Although this evidence suggests that the abolition of lengthening history is graded with the magnitude of muscle contraction, we do not yet have clear data from the spine.
Lengthening history and its ability to decrease the muscle spindle's velocity, movement, and position sensitivity may be particularly relevant in the lumbar spine. Maintained spinal postures that lengthen the dorsal paraspinal muscles (forward and lateral bending) comprise a substantial portion of daily activities. For example, forward flexion maintained by either isometric or eccentric contractions of dorsal paraspinal muscles while sitting in an armchair would create a lengthening history. If individuals were to return to an erect position using their arms, muscle spindles of the passively shortened paraspinal muscles would slacken. Because only low levels of paraspinal muscle contraction (1–3% MVC) are required to maintain lumbar spinal stability in the erect, neutral position (Cholewicki et al. 1996), paraspinal muscle activation that is low and below the threshold for abolishing the effect of movement history would receive diminished feedback support. The effect would be greatest at the onset of movement, where the consequences of lengthening history are the most pronounced (see Fig. 5). Although speculative movement history might contribute a component to the causal mechanism for low back injury, where low back pain or “throwing one's back out” is anecdotally reported to occur during light activities such as flexing to pick up a pencil. The diminished feedback support may cause a loss of segmental stability and unexpected vertebral displacements.
In conclusion, appropriate signaling from muscle spindles in the lumbar spine is subject to the previous positional history of a lumbar vertebra. Despite movements from an identical intermediate position, previous vertebral positions that lengthen the dorsal paraspinal muscles decrease the velocity sensitivity of their spindles. Thus similar vertebral kinematics could lead to different input from lumbar muscle spindles, depending on postural history, providing ambiguous sensory feedback to the CNS. In addition, the history-dependent spindle input would be discordant with proprioceptive input from skin and joint receptors not affected by movement history.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-46818. The work was conducted in a facility constructed with support from the National Center for Research Resources/Research Facilities Improvement Grant C06 RR-15433.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank R. Sozio for technical work and Dr. Cynthia Long and Y. Cao for statistical assistance.
REFERENCES
- Allen TJ, Ansems GE, Proske U. Evidence from proprioception of fusimotor coactivation during voluntary contractions in humans. Exp Physiol 93: 391–398, 2008 [DOI] [PubMed] [Google Scholar]
- Bianconi R, van der Meulen J. The response to vibration of the end organs of mammalian muscle spindles. J Neurophysiol 26: 177–190, 1963 [DOI] [PubMed] [Google Scholar]
- Bogduk N. The lumbosacral dorsal rami of the cat. J Anat 122: 653–662, 1976 [PMC free article] [PubMed] [Google Scholar]
- Bogduk N. The dorsal lumbar muscles of the cat. Acta Anz Jena 148: 55–67, 1980 [PubMed] [Google Scholar]
- Bogduk N, Macintosh JE, Pearcy MJ. A universal model of the lumbar back muscles in the upright position. Spine 17: 897–913, 1992 [DOI] [PubMed] [Google Scholar]
- Brown MC, Engberg I, Matthews PBC. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol 192: 773–800, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumagne S, Cordo P, Lysens R, Verschueren S, Swinnen S. The role of paraspinal muscle spindles in lumbosacral position sense in individuals with and without low back pain. Spine 25: 989–994, 2000 [DOI] [PubMed] [Google Scholar]
- Brumagne S, Lysens R, Swinnen S, Verschueren S. Effect of paraspinal muscle vibration on position sense of the lumbosacral spine. Spine 24: 1328–1331, 1999 [DOI] [PubMed] [Google Scholar]
- Cao D-Y, Khalsa PS, Pickar JG. Dynamic responsiveness of lumbar paraspinal muscle spindles during vertebral movement in the cat. Exp Brain Res 197: 369–377, 2009a [DOI] [PubMed] [Google Scholar]
- Cao D-Y, Pickar JG. The effect of the velocity of vertebral actuation in muscle history protocols on sensory signaling from lumbar paraspinal muscle spindles. Soc Neurosci Abstr 80.4, 2009a [Google Scholar]
- Cao D-Y, Pickar JG. Thoracolumbar fascia does not influence proprioceptive signaling from lumbar paraspinal muscle spindles in the cat. J Anat 215: 417–424, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D-Y, Pickar JG, Ge W, Ianuzzi A, Khalsa PS. Position sensitivity of feline paraspinal muscle spindles to vertebral movement in the lumbar spine. J Neurophysiol 101: 1722–1729, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholewicki J, Crisco JJ, Oxland TR, Yamamoto I, Panjabi MM. Effects of posture and structure on three-dimensional coupled rotations in the lumbar spine: a biomechanical analysis. Spine 21: 2421–2428, 1996 [DOI] [PubMed] [Google Scholar]
- Cholewicki J, McGill SM. Lumbar posterior ligament involvement during extremely heavy lifts estimated from fluoroscopic measurements. J Biomech 25: 17–28, 1992 [DOI] [PubMed] [Google Scholar]
- Cholewicki J, McGill SM. Mechanical stability of the in vivo lumbar spine: implications for injury and chronic low back pain. Clin Biomech 11: 1–15, 1996 [DOI] [PubMed] [Google Scholar]
- Crisco JJ, Panjabi MM, Yamamoto I, Oxland TR. Euler stability of the human ligamentous lumbar spine: Part II. Experiment. Clin Biomech 7: 27–32, 1992 [DOI] [PubMed] [Google Scholar]
- Crowe A, Matthews PBC. The effects of stimulation of static and dynamic fusimotor fibres on the response to stretching of the primary endings of muscle spindles. J Physiol 174: 109–131, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbaba R, Taylor A, Ellaway PH, Rawlinson S. Classification of longissimus lumborum muscle spindle afferents in the anaesthetized cat. J Physiol 571: 489–498, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn WO, Marsh BS. Muscular force at different speeds of shortening. J Physiol 85: 277–297, 1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gade VK, Wilson SE. Position sense in the lumbar spine with torso flexion and loading. J Appl Biomech 23: 93–102, 2007 [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Kinethesia: roles for afferent signals and motor commands. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Neural Control of Movement. Bethesda, MD: Am. Physiol. Soc, 1996, sect. 12, pt. 1, p. 128–172 [Google Scholar]
- Ge W, Long CR, Pickar JG. Vertebral position alters paraspinal muscle spindle responsiveness in the feline spine: effect of positioning duration. J Physiol 569: 655–665, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W, Pickar JG. Time course for the development of muscle history in lumbar paraspinal muscle spindles arising from changes in vertebral position. Spine J 8: 320–328, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granata KP, Orishimo KF, Sanford AH. Trunk muscle coactivation in preparation for sudden load. J Electromyogr Kinesiol 11: 247–254, 2001 [DOI] [PubMed] [Google Scholar]
- Gregory JE, Morgan DL, Proske U. Aftereffects in the responses of cat muscle spindles. J Neurophysiol 56: 451–461, 1986 [DOI] [PubMed] [Google Scholar]
- Gregory JE, Morgan DL, Proske U. Changes in size of the stretch reflex of cat and man attributed to aftereffects in muscle spindles. J Neurophysiol 58: 628–640, 1987 [DOI] [PubMed] [Google Scholar]
- Gregory JE, Morgan DL, Proske U. Aftereffects in the responses of cat muscle spindles and errors of limb position sense in man. J Neurophysiol 59: 1220–1230, 1988 [DOI] [PubMed] [Google Scholar]
- Hall LA, McCloskey DI. Detections of movements imposed on finger, elbow and shoulder joints. J Physiol 335: 519–533, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DK. Tension due to interaction between the sliding filaments in resting striated muscle: the effect of stimulation. J Physiol 199: 637–684, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald DA, Moseley GL, Hodges PW. The lumbar multifidus: does the evidence support clinical beliefs? Man Ther 11: 254–263, 2006 [DOI] [PubMed] [Google Scholar]
- Macintosh JE, Bogduk N. The anatomy and function of the lumbar back muscles and their fascia. In: Physical Therapy of the Low Back, edited by Twomey LT, Taylor JR. New York: Churchill Livingstone, 1987, p. 103–134 [Google Scholar]
- Matthews PBC. Mammalian Muscle Receptors and Their Central Actions. Baltimore, MD: Williams & Wilkins, 1972 [Google Scholar]
- McMahon TA. Muscles, Reflexes, and Locomotion. Princeton, NJ: Princeton Univ. Press, 1984 [Google Scholar]
- Moorhouse KM, Granata KP. Role of reflex dynamics in spinal stability: intrinsic muscle stiffness alone is insufficient for stability. J Biomech 40: 1058–1065, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjabi MM. The stabilizing system of the spine. Part 1. Function, dysfunction, adaptation, and enhancement. J Spinal Disord 5: 383–389, 1992 [DOI] [PubMed] [Google Scholar]
- Pickar JG, Cao D-Y, Ge W. The responsiveness of lumbar paraspinal muscle spindles is affected by the history of vertebral position along 3 orthogonal axes of vertebral motion. Soc Neurosci Abstr 180.10, 2008 [Google Scholar]
- Pickar JG, Ge W. Classification of muscle spindles in the lumbar spine of the anesthetized cat. Soc Neurosci Abstr 650.3, 2006 [Google Scholar]
- Prochazka A. Proprioceptive feedback and movement regulation. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Neural Control of Movement. Bethesda, MD: Am. Physiol. Soc, 1996, sect. 12, pt. 1, p. 89–127 [Google Scholar]
- Proske U. What is the role of muscle receptors in proprioception? Muscle Nerve 31: 780–787, 2005 [DOI] [PubMed] [Google Scholar]
- Proske U, Morgan DL. Do cross-bridges contribute to the tension during stretch of passive muscle? J Muscle Res Cell Motil 20: 433–442, 1999 [DOI] [PubMed] [Google Scholar]
- Proske U, Morgan DL, Gregory JE. Thixotropy in skeletal muscle and in muscle spindles: a review. Prog Neurobiol 41: 705–721, 1993 [DOI] [PubMed] [Google Scholar]
- Scott JJ. Classification of muscle spindle afferents in the peroneus brevis muscle of the cat. Brain Res 509: 62–70, 1990 [DOI] [PubMed] [Google Scholar]
- Soltys JS, Wilson SE. Directional sensitivity of velocity sense in the lumbar spine. J Appl Biomech 24: 244–251, 2008 [DOI] [PubMed] [Google Scholar]
- Taylor JL, McCloskey DI. Proprioceptive sensation in rotation of the trunk. Exp Brain Res 81: 413–416, 1990 [DOI] [PubMed] [Google Scholar]
- Wilder DG, Pope MH, Frymoyer JW. The biomechanics of lumbar disc herniation and the effect of overload and instability. J Spinal Disord 1: 16–32, 1988 [PubMed] [Google Scholar]
- Willigenburg NW, Kingma I, van Dieen JH. How is precision regulated in maintaining trunk posture? Exp Brain Res 203: 39–49, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Granata KP. Reposition sense of lumbar curvature with flexed and asymmetric lifting postures. Spine 28: 513–518, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Gregory JE, Proske U. The effects of muscle conditioning on movement detection thresholds at the human forearm. Brain Res 735: 125–130, 1996 [DOI] [PubMed] [Google Scholar]
- Wise AK, Gregory JE, Proske U. The responses of muscle spindles to small, slow movements in passive muscle and during fusimotor activity. Brain Res 821: 87–94, 1999 [DOI] [PubMed] [Google Scholar]
- Wood SA, Gregory JE, Proske U. The influence of muscle spindle discharge on the human H reflex and the monosynaptic reflex in the cat. J Physiol 497: 279–290, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]