Abstract
N-methyl-d-aspartate receptors (NMDARs) are critical for establishing, maintaining, and modifying glutamatergic synapses in an activity-dependent manner. The subunit composition, synaptic expression, and some of the properties of NMDARs are regulated by synaptic activity, affecting processes like synaptic plasticity. NMDAR transmission is dynamic, and we were interested in studying the effect of acute low or null synaptic activity on NMDA receptors and its implications for synaptic plasticity. Periods of no stimulation or low-frequency stimulation increased NMDAR transmission. Changes became stable after periods of 20 min of low or no stimulation. These changes in transmission have a postsynaptic origin and are explained by incorporation of GluN2B-containing receptors to synapses. Importantly, periods of low or no stimulation facilitate long-term potentiation induction. Moreover, recovery after a weak preconditioning stimulus that normally blocks subsequent potentiation is facilitated by a nonstimulation period. Thus synaptic activity dynamically regulates the level of NMDAR transmission adapting constantly the threshold for plasticity.
INTRODUCTION
Calcium-permeable N-methyl-d-aspartate–type glutamate receptors (NMDARs) play critical roles during induction of synaptic plasticity, brain development, and pathology (Cull-Candy et al. 2001). Their activation requires near simultaneous binding of glutamate and membrane depolarization, making these receptors molecular coincidence detectors of pre- and postsynaptic activity underlying Hebbian synaptic plasticity (Bliss et al. 2003). The biophysical properties of NMDARs are tightly controlled by multiple allosteric modulators and by the subunit composition of the receptor. NMDARs are heteromultimeric complexes composed of the glycine-binding GluN1 subunit and one or more of the glutamate-binding GluN2 subunits (GluN2 A–D) (Traynelis et al. 2010). In the hippocampus, NMDARs exist mostly as di-heteromeric GluN1/GluN2A or GluN1/GluN2B complexes (Al-Hallaq et al. 2007).
Induction of synaptic plasticity requires postsynaptic activation of NMDARs and the associated calcium influx. The calcium initiates a cascade of biochemical events, leading to long-lasting changes in the synaptic responses mediated by AMPA-type glutamate receptors (AMPARs). The direction of synaptic changes depends on the level of calcium influx into the postsynaptic spine. Thus a low or modest level of calcium influx triggers long-term depression (LTD), whereas a high level of calcium triggers long-term potentiation (LTP). Intermediate levels of calcium influx do not induce plasticity (Cho et al. 2001; Cormier et al. 2001; Cummings et al. 1996).
Importantly, the level of postsynaptic responses required to induce plasticity and to determine its direction, the threshold function, varies as a function of the history of synaptic activity (Abraham and Bear 1996). In vivo and in vitro observations indicate that enhancing or diminishing NMDAR function modifies the threshold to induce synaptic plasticity and the direction of those changes (Cummings et al. 1996; Kirkwood et al. 1996; Philpot et al. 2003, 2007; Quinlan et al. 1999; Yashiro and Philpot 2008). Change in the number of receptors impacts the influx of calcium and therefore determines the direction of plasticity (Cummings et al. 1996). Changes in subunit composition also control synaptic plasticity by changing the interaction of the receptor with signaling molecules (Barria and Malinow 2005) and changing the time course and fractional Ca+2 influx (Sobczyk et al. 2005).
NMDARs have been considered a relatively constant component of the postsynaptic density in contrast to AMPA-type glutamate receptors, which are highly dynamic (Malinow and Malenka 2002). However, the number and subunit composition of NMDARs can be modified by activity-dependent internalization in a clathrin-mediated process (Lavezzari et al. 2004) and by synthesis and degradation (Mu et al. 2003; Rao and Craig 1997). It is also clear that experience and synaptic activity effect a switch in subunit composition of synaptic NMDARs (Bellone and Nicoll 2007; Carmignoto and Vicini 1992; Chavis and Westbrook 2001; Hestrin 1992; Philpot et al. 2001; Quinlan et al. 1999), with consequences for synaptic plasticity (Barria and Malinow 2005). In addition, recent studies in cultured neurons indicate that synaptic NMDARs are mobile and can exchange with extrasynaptic receptors. Subunit composition affects the lateral mobility of NMDARs in the plasma membrane. GluN2B-containing receptors seem to be more mobile and can diffuse to extrasynaptic sites on activation (Groc et al. 2006; Tovar and Westbrook 2002).
We were interested in studying how an acute change in the rate of synaptic activity could affect NMDAR-mediated transmission. Here we report that NMDAR transmission is dynamically regulated and can be modified for a long period, affecting the threshold for synaptic plasticity.
METHODS
Preparation of hippocampal brain slices
Hippocampal slices were prepared using standard protocols from Sprague-Dawley rats 13–17 days old killed according to University of Washington guidelines. Slices were recovered in artificial cerebrospinal fluid (ACSF) with high Mg+2 (in mM: 118 NaCl, 2.5 KCl, 10 MgCl2, 2.5 CaCl2, 26.2 NaHCO3, 1 NaH2PO4, and 2 g/l d-glucose) continuously bubbled with 95% O2-5% CO2. Before placing slices in the recording chamber, slices were cut between CA3 and CA1 to prevent seizure activity when picrotoxin (PTX) was present in the bath.
Electrophysiology
Field recordings were obtained with glass microelectrodes filled with 0.9% NaCl placed in CA1 stratum radiatum. Field potentials were evoked with two bipolar platinum–iridium electrodes (FHC) placed on both sides of the recording electrode on Schaffer collaterals. Slices were perfused at 29°C with low Mg+2 ACSF (0.1 mM Mg+2) or normal Mg+2 ACSF (1.3 mM Mg+2) and 100 μM PTX. Perfused ACSF (3–5 ml/min) was constantly bubbled with O2-CO2, and drugs were applied directly to the bath when necessary at 1:1,000 dilution. No addition of glycine/d-serine supplement was necessary.
Whole cell recordings were obtained from CA1 pyramidal neurons under visual guidance. Whole cell recording pipettes (3–6 MΩ) were filled with intracellular recording solution containing (in mM) 115 cesium methanesulfonate, 20 CsCl, 10 HEPES, 2.5 MgCl2, 2 MgATP, 2 Na2ATP, 0.4 Na3GTP, 10 sodium phosphocreatine, 5 QX-314, and 0.6 EGTA (pH 7.25 and 310 mmol/kg). Series and input resistances were monitored throughout the experiment; data were used only if the resistances changed <10%. The recording chamber was perfused with ACSF containing 119 mM NaCl, 2.5 mM KCl, 4 mM CaCl2, 4 mM MgCl2, 26 mM NaHCO3, 1 mM NaH2PO4, 11 mM glucose, 100 μM PTX (Tocris), and 2 μM 2-chloroadenosine (pH 7.4) and gassed with 5% CO2-95% O2. A higher concentration of Mg+2 in the external solution is used in whole cell configuration to increase blockade of NMDARs at −60 mV. This blockade is removed when holding the membrane potential at +40 mV. Recordings were performed at room temperature (25°C).
Data analysis
The field-recording responses were analyzed by measuring peak amplitude of responses normalized to the baseline response. Data from slices with unsteady baseline responses (>10% change over 10 min) were discarded. Three consecutive points (30 s) were averaged, and these data were included in the ensemble averages of all the slices. Whole cell recordings where analyzed in a similar fashion.
Decay time of excitatory postsynaptic currents (EPSCs) was characterized as the time from the peak of the response to one half its amplitude (time to half decay). Also, a weighted exponential was fit to the falling phase of EPSCs using Clampfit (Molecular Devices).
All results are represented as means ± SE, and statistical comparisons (P) were determined using the Student's t-test. Significance was set at P ≤ 0.05.
RESULTS
Dynamic regulation of NMDAR synaptic transmission
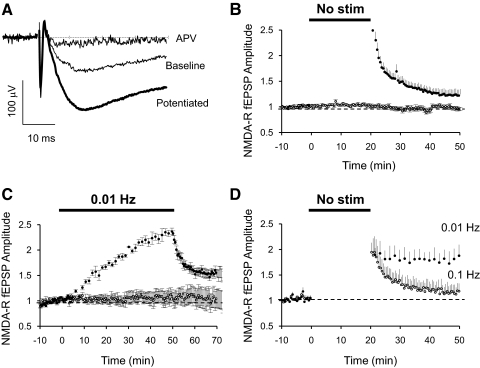
Pharmacologically isolated NMDAR-mediated field excitatory postsynaptic potentials (fEPSPs) were recorded in hippocampal slices perfused with ACSF containing low Mg2+ (Fig. 1A). synaptic activity was controlled by stimulating Schaffer collateral axons in two independent pathways. After obtaining a stable baseline for ≥10 min by stimulating constantly at 0.1 Hz, one pathway was turned off for 20 min. On resuming stimulation, the amplitude of NMDAR fEPSPs was found to be potentiated up to threefold (Fig. 1, A and B). This effect was pathway specific, and the initial potentiation decayed once 0.1 Hz stimulation was resumed. However, the decay was not complete and NMDAR transmission stayed potentiated ∼25% above baseline after 30 min of having resumed stimulation (Fig. 1B). Shorter nonstimulation periods (5 or 10 min) also induced potentiation of NMDAR responses, but they decayed to baseline levels once regular stimulation was resumed (data not shown).
Fig. 1.
Dynamic regulation of N-methyl-d-asparate receptor (NMDAR)-dependent field excitatory postsynaptic potentials (fEPSPs). A: sample traces of isolated NMDAR-dependent fEPSPs (low Mg2+, 2 μM NBQX) before a period of no stimulation (baseline) or after 20 min of no stimulation (potentiated). NMDAR fEPSP is blocked by APV 100 μM. B: potentiation of NMDAR fEPSPs after a no-stimulation period. Normalized peak amplitude of isolated NMDAR-dependent fEPSPs recorded in CA1 region of the hippocampus in artificial cerebrospinal fluid (ACSF) with 0.1 mM Mg2+, 2 μM NBQX, and 100 μM PTX. Field potentials were evoked by stimulation of 2 independent pathways with bipolar electrodes placed on Schaffer collaterals. After obtaining a stable baseline stimulating at 0.1 Hz, stimulation on 1 pathway was suspended for 20 min (●; n = 18). Control pathway was constantly stimulated at 0.1 Hz (○). C: potentiation of NMDAR fEPSPs by low-frequency stimulation. Normalized peak amplitude of isolated NMDAR-dependent fEPSPs as in B. After obtaining a stable baseline by stimulation at 0.1 Hz, 1 pathway was stimulated at 0.01 Hz for 50 min (●; n = 4) and returned to 0.1 Hz. Control pathway was constantly stimulated at 0.1 Hz (○). D: decay of potentiation is activity dependent. Normalized peak amplitude of isolated NMDAR-dependent fEPSPs as in B. Both pathways were potentiated by a nonstimulation period of 20 min. On resuming stimulation, 1 pathway was stimulated at 0.01 Hz (●; n = 3) and the other at 0.1 Hz (○).
Lower-frequency stimulation also caused a slow increase in NMDAR fEPSPs in a pathway-specific manner. After obtaining ≥10 min of stable synaptic transmission by stimulating at 0.1 Hz, one pathway was set to stimulate axons at a frequency of 0.01 Hz. Transmission in this pathway slowly increased (Fig. 1C). As soon as stimulation was returned to 0.1 Hz, NMDAR transmission decayed but stayed potentiated above baseline (Fig. 1C).
The decay in the amplitude of NMDAR transmission after resuming stimulation at the regular 0.1 Hz could be a phenomenon that itself depends on synaptic activity (Dozmorov et al. 2003). To test this idea, both pathways were potentiated by a 20 min nonstimulation period. Stimulation was resumed at different frequencies on each pathway. On the pathway where stimulation was resumed at 0.1 Hz, the initial potentiation decayed but stabilized above baseline level as before (Fig. 1D, ○). Stimulation of the other pathway was resumed at 0.01 Hz. In this case, NMDAR transmission did not decay and remained potentiated at maximum level (Fig. 1D, ●). Interestingly, the nonstimulation period used to induce potentiation occluded further potentiation by the low-rate stimulation at this pathway.
We conclude that the level of NMDAR transmission is rapidly adjusted by synaptic activity in a pathway-specific manner, but more stable changes can be achieved if the modification in synaptic activity is long enough.
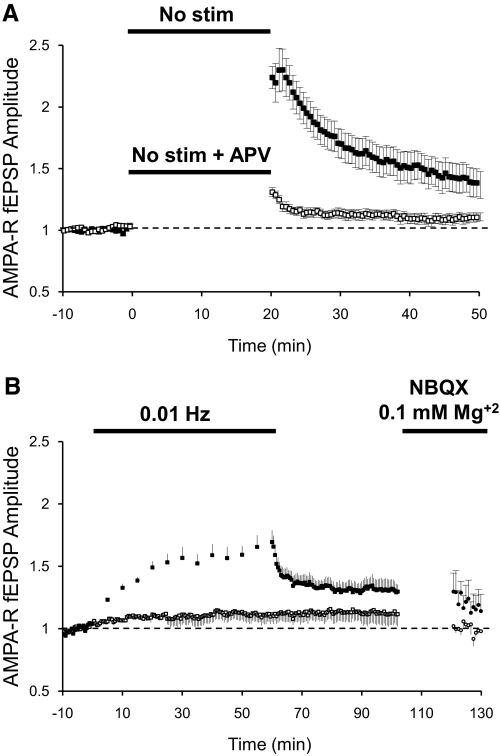
Potentiation by a nonstimulation period or by low-frequency stimulation was also observed when recording AMPAR-mediated fEPSPs in ACSF with normal Mg+2. After obtaining a 10 min baseline stimulating at 0.1 Hz, one pathway was turned off for 20 min. On resuming stimulation, AMPAR transmission was found to be potentiated more than twofold. Initial potentiation quickly declined but stayed potentiated above baseline for ≥30 min (Fig. 2A, ■). Interestingly, this AMPAR potentiation was blocked when 100 μM APV, an NMDAR antagonist, was added to the bath during the nonstimulation period (Fig. 2A, □). Lowering the frequency of stimulation from 0.1 (baseline) to 0.01 Hz also increased AMPAR transmission (Fig. 2B) but to a lesser degree than the potentiation observed with NMDAR fEPSPs. On resuming stimulation at 0.1 Hz, AMPAR responses also decayed but stayed potentiated above baseline levels (Fig. 2B, ■). After AMPAR potentiation reached a stable level, we isolated NMDAR fEPSPs pharmacologically to confirm that, under these conditions, NMDAR transmission had also been potentiated (Fig. 2B, ●).
Fig. 2.
Potentiation of AMPAR-dependent fEPSPs. A: normalized peak amplitude of AMPAR-dependent fEPSPs recorded in CA1 region of the hippocampus in regular ACSF (1.3 mM Mg+2). Field potentials were evoked at 0.1 Hz by stimulation of 2 independent pathways with bipolar electrodes placed on Schaffer collaterals. After obtaining a stable baseline by stimulation at 0.1 Hz, 1 pathway was suspended for 20 min (■; n = 16) or suspended for 20 min in the presence of 100 μM APV (□; n = 8). B: peak amplitude of AMPAR-dependent fEPSPs recorded as in A. After baseline, 1 pathway was stimulated at 0.01 Hz for 60 min and returned to 0.1 Hz (■; n = 4). After potentiation was stabilized, the perfusion solution was exchanged to a solution containing 0.1 mM Mg+2 and 2 μM NBQX to isolate NMDAR-dependent potentials (●).
Potentiation of NMDAR synaptic transmission has a postsynaptic origin
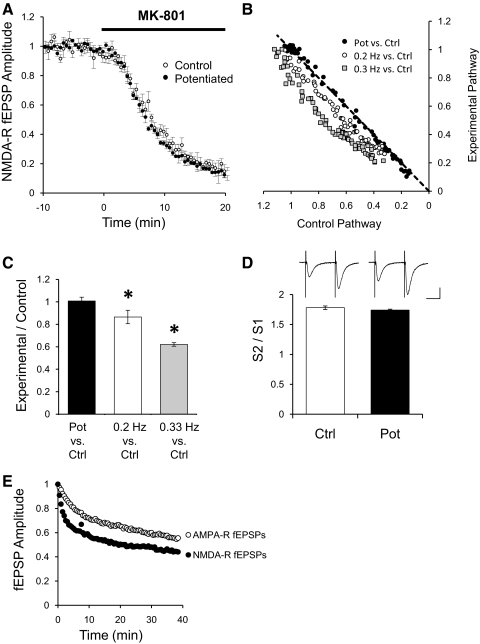
Amplitude changes on NMDAR transmission could be caused by changes in the probability of release of neurotransmitter in the pathway that is silenced for a period of time or that is stimulated at low frequencies. To test this hypothesis, we induced potentiation in one pathway by low-frequency stimulation at 0.01 Hz while constantly stimulating the control pathway at the regular 0.1 Hz. Stimulation at 0.1 Hz was resumed in the experimental pathway and potentiation allowed stabilization as in Fig. 1B. At this point, MK-801, a use-dependent NMDAR blocker, was applied to the bath. If potentiation is caused by an increased probability of release, the potentiated pathway is expected to be blocked faster than the control pathway (Hessler et al. 1993; Rosenmund et al. 1993). As observed in Fig. 3A, control and potentiated pathways were blocked at the same rate. Because the diffusion of the drug into the tissue is the same for both pathways, this experiment suggests that there is no change in the probability of release. To verify that this method is sensitive to detect changes in probability of release, we applied MK-801 while stimulating two independent pathways at different rates. Figure 3B plots the normalized amplitude of NMDAR fEPSPs of the control pathway stimulated at 0.1 Hz versus the amplitude of the potentiated pathway or pathways stimulated at 0.2 Hz, or 0.3 Hz after MK-801 has been applied. Control and potentiated pathways were blocked at the same rate; therefore they follow the line of equality (Fig. 3B, ●). As expected, the pathway stimulated at 0.2 or 0.3 Hz was blocked by MK-801 faster than the respective control pathway stimulated at the regular 0.1 Hz, falling below diagonal (Fig. 3B). Quantification for the population data were done by taking the ratio of the fEPSP amplitude of the experimental pathway over the amplitude of the control pathway at 50% of the total MK-801 blockade (Fig. 3C). As expected for two pathways with similar probability of release, the ratio of the potentiated pathway to the nonpotentiated control pathway is 1 (Fig. 3C, filled bar). The ratio of pathways stimulated at 0.2 or 0.3 Hz over their respective control pathways is <1 (Fig. 3C).
Fig. 3.
Potentiation does not change probability of release. A: normalized peak amplitude of NMDAR fEPSPs for a control pathway (○; n = 8) or a potentiated pathway (●) after addition of 40 μM MK-801 to the bath. Field potentials were recorded in CA1 region of the hippocampus in ACSF with 0.1 mM Mg+2, 2 μM NBQX, and 100 μM PTX. Field potentials were evoked by stimulation of 2 independent pathways with bipolar electrodes placed on Schaffer collaterals. One pathway was potentiated by stimulating at 0.01 Hz for 50 min and returned to 0.1 Hz. Control pathway was constantly stimulated at 0.1 Hz. After stabilization of the potentiated pathway, MK-801 was added to the bath (40 μM). B: normalized peak amplitude of NMDAR-mediated fEPSPs from the potentiated pathway plotted against the normalized peak amplitude from the control pathway during the application of 40 μM MK-801 (●). Gray squares and open circles are normalized peak amplitude of NMDAR-mediated fEPSPs from a pathway stimulated at 0.2 or 0.3 Hz plotted against their respective control pathway stimulated at 0.1 Hz during the application of 40 μM MK-801. C: quantification of the blockade by 40 μM MK-801 of NMDAR-mediated fEPSPs. Ratio of the fEPSP peak amplitude of the potentiated pathway over the amplitude of the control pathway at 50% of the total MK-801 blockade (filled bar, n = 8). Ratio of the fEPSP peak amplitude from a pathway stimulated at 0.2 Hz (n = 4), or 0.3 Hz (n = 4), over their respective control pathway stimulated at 0.1 Hz at 50% of the total MK-801 blockade. *P < 0.05 Student's t-test. D: paired pulse facilitation of AMPAR-dependent fEPSPs. AMPAR-dependent fEPSPs were recorded in CA1 region of the hippocampus in regular ACSF (1.3 mM Mg+2). Field potentials were evoked at 0.1 Hz by stimulation of 2 independent pathways with bipolar electrodes placed on Schaffer collaterals. After obtaining a stable baseline, 1 pathway was suspended for 20 min to potentiate it. After potentiation, several trials of 2 stimuli 50 ms apart were delivered to the control pathway (open bar) or to the potentiated pathway (filled bar; n = 16), and the ratio of the fEPSPs amplitude calculated. Insets: sample traces from the control pathway or the potentiated pathway. Bars = 20 ms and 0.4 mV. E: normalized decay phase of potentiation for AMPAR- and NMDAR-mediated fEPSPs responses. Decay phases from Figs. 1B and 2A were normalized to the maximal potentiation.
Changes in paired-pulse facilitation should also reflect whether there is a change in the probability of release on the potentiated pathway. To measure paired-pulse facilitation, AMPAR fEPSPs were recorded, and one pathway was potentiated by a nonstimulation period as in Fig. 2A while constantly stimulating the control pathway at 0.1 Hz. After resuming stimulation, we allowed potentiation to stabilize to measure whether the stable potentiation observed was caused by changes in probability of release. Several trials consisting of two stimuli 50 ms apart were delivered to the potentiated pathway and the control pathway. As shown in Fig. 3D, no difference was observed in paired-pulse facilitation in the potentiated pathway compared with the control pathway.
These experiments suggest that the potentiation of NMDAR responses induced by lack or low-frequency stimulation is not caused by changes in the probability of release of neurotransmitter.
These experiments, however, do not test whether the early phase of potentiation observed is caused by changes in the probability of release. On resuming stimulation, the rapid decay in the amplitude of either AMPAR- or NMDAR-mediated field potentials prevented testing the effect of MK-801 or paired-pulse facilitation during this early phase. However, a closer examination of the time course of the decay phase of the potentiation suggests that the early potentiation observed is not caused by a transient increase in probability of release. Figure 3E shows the normalized decay phase of the potentiation for AMPAR- and NMDAR-mediated responses obtained from Figs. 2A and 1B, respectively. If the potentiation is caused by presynaptic changes, it would be expected that the decay phase to be similar for both type of responses. After normalization to the maximal potentiation, a single exponential was fit to the decay phase. AMPAR-mediated field potentials decayed with a tau of 11.5 ± 0.5 m, whereas NMDAR-mediated field potentials decayed significantly faster with a tau of 6.9 ± 0.4 m.
Potentiation increases GluN2B-containing receptors
The increase in amplitude of NMDAR function after a period of low or no synaptic activity could be caused by incorporation of NMDARs to existing or new postsynaptic sites. Several reports indicate that GluN2B-containing receptors are highly mobile in the plane of the plasma membrane (Groc et al. 2006; Tovar and Westbrook 2002; Zhao et al. 2008) and that synaptic activity drives GluN2B-containing receptors away from synapses (Tovar and Westbrook 2002). We reasoned that low or no stimulation could allow a build-up of synaptic NMDARs dominated by GluN2B-containing receptors.
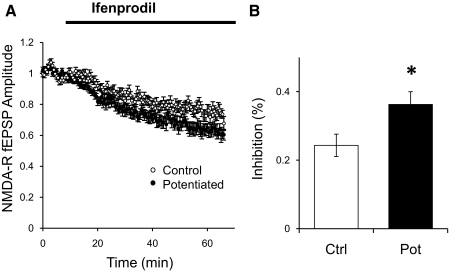
We tested this hypothesis in isolated NMDAR fEPSPs. We induced potentiation in one pathway by low-frequency stimulation (0.01 Hz) or by a 20 min nonstimulation period. After returning to the normal stimulation rate (0.1 Hz) long enough for potentiation of NMDAR synaptic transmission to reach a stable level, ifenprodil was added to the bath. Ifenprodil is an antagonist of GluN2B-containing receptors (Williams 1993). The effect of ifenprodil had a slow onset, and the blockade was relatively modest in both pathways (∼20–40%), possibly because of the slow penetration of ifenprodil into the tissue (Fig. 4A). However, the percentage of blockade in the potentiated pathway was significantly larger than in the control nonpotentiated pathway, supporting the idea that potentiation is caused by addition of GluN2B-containing receptors to existing or new synapses (Fig. 4B).
Fig. 4.
Effect of ifenprodil on NMDAR-mediated fEPSPs after potentiation. A: normalized peak amplitude of NMDAR fEPSPs for a control pathway (○; n = 12) or a pathway potentiated by a low frequency period (●). After resuming stimulation at 0.1 Hz in the experimental pathway and potentiation stabilized, 3 μM ifenprodil was added to the bath. B: percentage of inhibition on NMDAR fEPSPs in the control pathway (open bar) or the potentiated bar (filled bar) measured 50 min after 3 μM ifenprodil. *P < 0.05 Student's t-test.
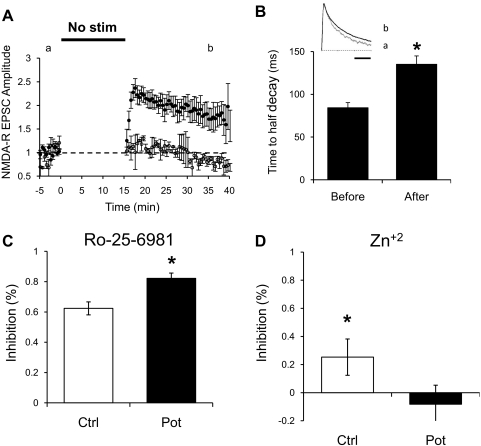
To better examine this possibility, we tested whether a potentiation of NMDAR-mediated synaptic transmission by periods of low or no synaptic activity was also observed in whole cell recordings. We recorded EPSCs from hippocampal CA1 pyramidal while holding the membrane potential at +40 mV. An antagonist of AMPA-type glutamate receptors, NBQX, was added to the bath to isolate NMDAR-mediated EPSCs. Bipolar stimulation electrodes were placed over Schaffer collateral fibers ∼200–300 μm from the targeted CA1 neuron. After obtaining a stable baseline of ≥5 min by stimulating at a constant rate of 0.1 Hz, one pathway was turned off for 15 min, and the membrane potential was held at −60 mV to reduce damage to the cell. On resuming stimulation and returning the membrane potential to +40 mV, a potentiation up to twofold was observed (Fig. 5A). Similar potentiation was also observed when the cell was held at +40 mV during the nonstimulation period (data not shown). The potentiation developed slowly, probably because of the rapid change in the holding potential of the membrane. Similarly to NMDAR fEPSPs, initial potentiation of NMDAR EPSCs decayed, although in a less pronounced manner. These experiments were also repeated with monopolar glass stimulating electrodes to rule out possible artifacts caused by electrochemical reactions occurring at the tip of the bipolar platinum–iridium electrodes. Similar potentiation was observed, and the data were pooled.
Fig. 5.
Potentiation of evoked NMDAR-dependent postsynaptic currents. A: normalized peak amplitude of NMDAR excitatory postsynaptic currents (EPSCs) obtained at +40 mV and in the presence of 2 μM NBQX. Whole cell recordings were obtained from CA1 pyramidal cells and EPSCs evoked every 10 s by stimulation of 2 independent pathways with bipolar electrodes or glass monopolar electrodes placed on Schaffer collaterals. After obtaining a stable baseline by stimulation at 0.1 Hz, 1 pathway was suspended for 15 min and resumed at same stimulation rate (●; n = 10). The control pathway was constantly stimulated at 0.1 Hz; however, amplitude during this period is not shown for clarity (○). During the nonstimulation period, the membrane potential of the cell was held at −60 mV. B: time to half decay of NMDAR-dependent EPSCs from the potentiated pathway before the nonstimulation period or after 15–20 min of resuming stimulation (n = 5). *P < 0.05, Student's t-test. Inset: normalized traces before potentiation (A) and after potentiation (B) from Fig. 5A. Bar = 200 ms. C: effect of Ro-25-6981 on NMDAR-mediated currents. After potentiation was induced in 1 pathway as in A, Ro-25-6981 (1 μM) was bath applied. A larger blockade is seen in the potentiated pathway (filled bar; n = 6) than in the control pathway (open bar). *P < 0.05. D: percent inhibition of isolated NMDAR EPSCs by Zn+2. Potentiation was induced on 1 pathway as in A and then 100 nM Zn+2 was bath applied. In the potentiated pathway, no significant effect was seen (filled bar; n = 6). In the control pathway (open bar), a significant (∼25%) blockade of current was seen. *P < 0.01 Student's t-test.
NMDAR subunit composition determines the kinetics of NMDAR-mediated EPSCs, with GluN2B conferring to NMDARs slower decay kinetics than the GluN2A subunit (Dingledine et al. 1999). Consistent with the hypothesis that the increase in amplitude of NMDAR responses after a period of low or no synaptic activity is caused by incorporation of GluN2B-containing receptors, potentiated NMDAR EPSCs exhibited a slower decay rate. Figure 5B shows the values for the time to half decay of NMDAR EPSCs before and after potentiation. Also, after normalization of the peak amplitude (Fig. 5B, inset), a weighted exponential was fit to the decay phase of the EPSC. The resulting decay tau was 85.8 ± 6.8 ms before potentiation and significantly slower, 116.4 ± 6.4 ms, after potentiation.
In a different set of experiments, we used Ro-25-6981 to confirm that potentiation of NMDAR currents increases the proportion of synaptic GluN2B-containing receptors. Ro-25-6981 is a more potent and selective blocker of GluN2B containing receptors with the same mode of action as ifenprodil (Fischer et al. 1997). After potentiation, Ro-25-6981 was added to the bath. As expected, the kinetics of decay of NMDAR responses became faster. The weighted decay time constant of the potentiated pathway changed from a tau of 136.2 ± 34.5 ms before Ro-25-6981 to 76.8 ± 7.2 ms after blockade of GluN2B-containing receptors. Importantly, the potentiated pathway had a larger fraction of the NMDAR current blocked by Ro-25-6981 than the control pathway (Fig. 5C). In addition, we used a low concentration Zn+2 (100 nM) to block GluN2A-containing receptors (Paoletti et al. 1997). A small blockade of NMDAR currents is observed in the control pathway, consistent with a small percentage of GluN2A present at synapses at this age. The potentiated pathway was not blocked by Zn+2 (Fig. 5D), supporting the idea of a larger population of GluN2B at these synapses.
Together, these experiments argue that potentiation is caused by incorporation of GluN2B-containing receptors into existing or new synapses during the absence or low synaptic activity.
Significance of dynamic adjustment of NMDAR transmission
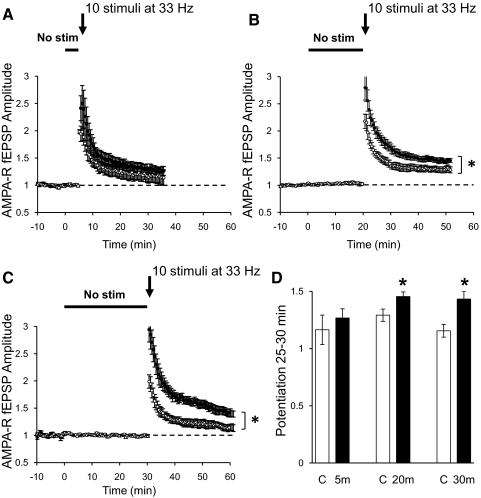
Potentiation of NMDAR transmission should increase Ca+2 influx into the postsynaptic cell, facilitating induction of synaptic plasticity. To test this idea, we monitored AMPAR-mediated synaptic transmission in the CA1 region of hippocampal slices while stimulating two independent pathways. After establishing a baseline, one pathway underwent a silent period of different durations, whereas the second pathway was used as a control and stimulated constantly at 0.1 Hz. Immediately after resuming stimulation, a weak tetanus that by itself produces a small potentiation was delivered to both pathway. No difference in the level of potentiation induced by the tetanus was observed between the control pathway and the pathway that underwent a 5 min nonstimulation period (Fig. 6A). However, the same tetanus induced a significantly larger potentiation in the pathway that underwent a nonstimulation period of 20 or 30 min compared with the potentiation induced in the control pathway (Fig. 6, B and C). This indicates that potentiation of NMDAR transmission by a nonstimulation period reduces the threshold necessary to produce potentiation of glutamatergic synaptic transmission.
Fig. 6.
Nonstimulation period increases synaptic plasticity. A–C: normalized AMPAR-mediated fEPSPs recorded in CA1 region of the hippocampus in regular ACSF with 100 μM PTX. Field potentials were evoked by stimulation of 2 independent pathways with bipolar electrodes placed on Schaffer collaterals. After obtaining a stable baseline stimulating at 0.1 Hz, 1 pathway was turned off for 5 (A, ●; n = 8), 20 (B, ●; n = 7), or 30 min (C, ●; n = 8). Control pathways were constantly stimulated at 0.1 Hz (○). Immediately after the nonstimulation period, both pathways were given a mild tetanus consisting of 10 stimuli at 33 Hz (arrow). D: quantification of the potentiation of AMPAR synaptic transmission by 10 stimuli at 33 Hz tetanus 25–30 min after the tetanus. *P < 0.05, Student's t-test.
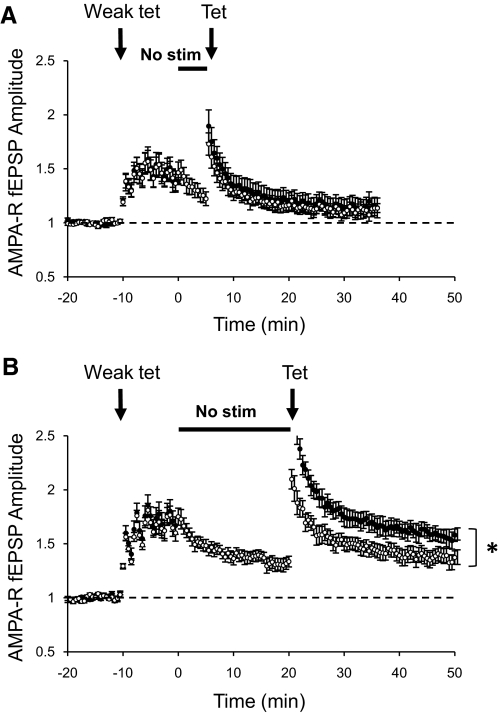
Another important feature of synaptic plasticity is the fact that a weak preconditioning stimulus can block the induction of subsequent potentiation by a stronger tetanus. This blockade is temporary, and plasticity can be induced after a recovery period (Huang et al. 1992). Here we tested the idea that a nonstimulation period could relieve that block of potentiation by increasing NMDAR transmission. To test this, a weak preconditioning stimulus was delivered to both pathways and then one pathway was turned off for 5 or 20 min while the second pathway was constantly stimulated at the regular 0.1 Hz (control pathway). At the end of the nonstimulation period, a tetanus that induces small potentiation was delivered to both pathways. No potentiation was observed in the pathway that received only the preconditioning stimulus (control pathway) or that received the preconditioning stimulus followed by 5 min of nonstimulation period (Fig. 7A). When a pathway underwent a preconditioning stimulus followed by a period of 20 min of constant stimulation before the tetanus, a small potentiation was induced (Fig. 7B, ○), indicating a small recovery from the preconditioning stimulus. However, when a nonstimulation period of 20 min followed the preconditioning stimulus, a larger potentiation was induced (Fig. 7B, ●). These experiments indicate that a silent period allows synaptic plasticity to recover faster from the blockade produced by previous activation of synapses.
Fig. 7.
Nonstimulation allows faster recovery after a weak preconditioning tetanus. A and B: normalized peak amplitude of AMPAR-mediated fEPSPs evoked by stimulation of 2 independent pathways with bipolar electrodes placed on Schaffer collaterals. After acquiring a stable baseline, a weak preconditioning tetanus was delivered to both pathways (Weak tet = 4 stimuli at 30 Hz, 6 times every 2 min). Immediately after the weak tetanus, 1 pathway was suspended for 5 (A, ●; n = 8) or 20 min (B, ●; n = 10). Control pathways were constantly stimulated at the regular rate of 0.1 Hz (○). Immediately after the nonstimulation period, a stronger tetanus was delivered to both pathways (Tet = 10 stimuli at 30 Hz). *P < 0.05 Student's t-test.
DISCUSSION
In this study, we showed that NMDAR function can be enhanced in minutes when synapses undergo a period of nonstimulation or a period of low activity compared with baseline. This increase in NMDAR function can be long-lasting, modifying the plastic properties of synapses.
Use-dependent depression of NMDAR responses has been reported (Dozmorov et al. 2004), as well as several mechanisms that produce a use-dependent downregulation of NMDAR function. Use-dependent activation of calcineurin enhances a type of NMDAR desensitization (Krupp et al. 2002; Tong et al. 1995), and calcium/calmodulin mediates calcium-dependent inactivation of the receptor (Krupp et al. 1999). In addition, NMDARs can be internalized (Roche et al. 2001) or move away from synapses (Tovar and Westbrook 2002) in an activity-dependent manner. Thus it is not surprising that a reduction in the level of synaptic activity enhances NMDAR function. This could be a mechanism for synapses to adjust NMDAR function, dynamically reflecting recent synaptic activity.
Biochemical experiments show that, in hippocampus, NMDARs exist mostly as diheteromeric GluN1/GluN2A or GluN1/GluN2B complexes with a small percentage existing as triheteromers composed of GluN1/GluN2A/GluN2B (Al-Hallaq et al. 2007). Although these biochemical experiments do not assess the synaptic composition of NMDARs, experiments using specific GluN2B blockers suggest that a large fraction of synaptic receptors are diheteromers GluN1/GluN2B. These receptors are replaced by GluN2A-containing receptors in an activity-dependent manner or after induction of LTP (Barria and Malinow 2002; Bellone and Nicoll 2007; Yashiro and Philpot 2008).
Activity-dependent internalization and lateral movements on the plasma membrane depend on the NR2 subunit composition of NMDARs. GluN2B-containing receptors are more mobile than GluN2A-containing receptors (Groc et al. 2006; Zhao et al. 2008) and are responsible for partial recovery of NMDAR currents after irreversible blockade of synaptic NMDARs (Tovar and Westbrook 2002; Zhao et al. 2008). Also, GluN2B subunits contain a binding site for the AP2 clathrin adaptor protein responsible for the activity-dependent internalization of NMDARs (Lavezzari et al. 2004). Our data show that low or no synaptic activity for a short period of time increases the proportion of GluN2B-containing receptors at synapses, affecting the kinetics of evoked EPSCs and rendering NMDAR synaptic transmission more sensitive to ifenprodil or Ro-25-6981. An increase in GluN2B-containing receptors facilitates synaptic plasticity because of their ability to interact with CaMKII, an interaction necessary for LTP induction (Barria and Malinow 2005; Foster et al. 2010). Also, it lengthens the NMDAR EPSCs (Monyer et al. 1994) and increases Ca+2 influx (Sobczyk et al. 2005). Thus the cell can respond locally to relative changes in the level of synaptic activity, constantly adapting the threshold for synaptic plasticity.
We suggest that, on stimulation, NMDARs move away from synapses or are internalized until equilibrium is reached. Nonstimulation periods, or low-frequency stimulation (respect to baseline), allow receptors to move or recycle back into synapses.
The increase observed in NMDAR function can be translated into an increase in AMPAR-mediated synaptic transmission, the workhorse of excitatory transmission. A nonstimulation period also increases AMPAR-mediated transmission in a manner that depended on NMDAR activation. It is clear that synaptic potentiation of AMPAR-mediated transmission depends on the levels of NMDAR activation and calcium influx (Cho et al. 2001; Cormier et al. 2001; Cummings et al. 1996); therefore it is not surprising that an increase in NMDAR function after a period of low-frequency stimulation primes that pathway for larger potentiation by a protocol that normally does not induce much change. Moreover, low-frequency or no stimulation speeds recovery after a preconditioning stimulus that normally blocks the ability to undergo potentiation for a period of time (Huang et al. 1992).
The ability to induce synaptic plasticity varies as a function of age and experience (Abraham and Bear 1996). The threshold function that defines whether LTD or LTP occurs can be adjusted by enhancing or diminishing NMDAR function (Cummings et al. 1996; Kirkwood et al. 1996; Philpot et al. 2003, 2007; Quinlan et al. 1999; Yashiro and Philpot 2008). This work showed that NMDAR synaptic transmission can be regulated dynamically by relatively low levels of stimulation. This regulation is rapid and local, tuning synapses to the recent history of glutamate release and priming or damping further synaptic changes. This could be a general paradigm to optimize the level of synaptic plasticity, adapting it to the levels of activity of specific synaptic inputs.
GRANTS
This work was supported by PHS NRSA T32 GM07270 from NIGMS (A. C. Gambrill and G. P. Storey) and NIH-NINDS R01NS060756 (A. Barria).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19: 126–130, 1996 [DOI] [PubMed] [Google Scholar]
- Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci 27: 8334–8343, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron 35: 345–353, 2002 [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 48: 289–301, 2005 [DOI] [PubMed] [Google Scholar]
- Bellone C, Nicoll RA. Rapid bidirectional switching of synaptic NMDA receptors. Neuron 55: 779–785, 2007 [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL, Morris RG. Introduction. Long-term potentiation and structure of the issue. Philos Trans R Soc Lond B Biol Sci 358: 607–611, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science 258: 1007–1011, 1992 [DOI] [PubMed] [Google Scholar]
- Chavis P, Westbrook G. Integrins mediate functional pre- and postsynaptic maturation at a hippocampal synapse. Nature 411: 317–321, 2001 [DOI] [PubMed] [Google Scholar]
- Cho K, Aggleton JP, Brown MW, Bashir ZI. An experimental test of the role of postsynaptic calcium levels in determining synaptic strength using perirhinal cortex of rat. J Physiol 532: 459–466, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier RJ, Greenwood AC, Connor JA. Bidirectional synaptic plasticity correlated with the magnitude of dendritic calcium transients above a threshold. J Neurophysiol 85: 399–406, 2001 [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 11: 327–335, 2001 [DOI] [PubMed] [Google Scholar]
- Cummings JA, Mulkey RM, Nicoll RA, Malenka RC. Ca2+ signaling requirements for long-term depression in the hippocampus. Neuron 16: 825–833, 1996 [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev 51: 7–61, 1999 [PubMed] [Google Scholar]
- Dozmorov M, Li R, Xu HP, Jilderos B, Wigstrom H. Slowly developing depression of N-methyl-D-aspartate receptor mediated responses in young rat hippocampi. BMC Neurosci 5: 26, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozmorov M, Niu YP, Xu HP, Xiao MY, Li R, Sandberg M, Wigstrom H. Active decay of composite excitatory postsynaptic potentials in hippocampal slices from young rats. Brain Res 973: 44–55, 2003 [DOI] [PubMed] [Google Scholar]
- Fischer G, Mutel V, Trube G, Malherbe P, Kew JN, Mohacsi E, Heitz MP, Kemp JA. Ro 25–6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther 283: 1285–1292, 1997 [PubMed] [Google Scholar]
- Foster KA, McLaughlin N, Edbauer D, Phillips M, Bolton A, Constantine-Paton M, Sheng M. Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation. J Neurosci 30: 2676–2685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci USA 103: 18769–18774, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler NA, Shirke AM, Malinow R. The probability of transmitter release at a mammalian central synapse. Nature 366: 569–572, 1993 [DOI] [PubMed] [Google Scholar]
- Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature 357: 686–689, 1992 [DOI] [PubMed] [Google Scholar]
- Huang YY, Colino A, Selig DK, Malenka RC. The influence of prior synaptic activity on the induction of long-term potentiation. Science 255: 730–733, 1992 [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MC, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature 381: 526–528, 1996 [DOI] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. Interactions of calmodulin and alpha-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors. J Neurosci 19: 1165–1178, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. Calcineurin acts via the C-terminus of NR2A to modulate desensitization of NMDA receptors. Neuropharmacology 42: 593–602, 2002 [DOI] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci 24: 6383–6391, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25: 103–126, 2002 [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12: 529–540, 1994 [DOI] [PubMed] [Google Scholar]
- Mu Y, Otsuka T, Horton AC, Scott DB, Ehlers MD. Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron 40: 581–594, 2003 [DOI] [PubMed] [Google Scholar]
- Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci 17: 5711–5725, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Cho KK, Bear MF. Obligatory role of NR2A for metaplasticity in visual cortex. Neuron 53: 495–502, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Espinosa JS, Bear MF. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. J Neurosci 23: 5583–5588, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron 29: 157–169, 2001 [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci USA 96: 12876–12880, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Craig AM. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron 19: 801–812, 1997 [DOI] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci 4: 794–802, 2001 [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Clements JD, Westbrook GL. Nonuniform probability of glutamate release at a hippocampal synapse. Science 262: 754–757, 1993 [DOI] [PubMed] [Google Scholar]
- Sobczyk A, Scheuss V, Svoboda K. NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. J Neurosci 25: 6037–6046, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong G, Shepherd D, Jahr CE. Synaptic desensitization of NMDA receptors by calcineurin. Science 267: 1510–1512, 1995 [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron 34: 255–264, 2002 [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R, Sibley D. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62: 405–496, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol 44: 851–859, 1993 [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology 55: 1081–1094, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Peng Y, Xu Z, Chen R-q, Gu Q-h, Chen Z, Lu W. Synaptic metaplasticity through NMDA receptor lateral diffusion. J Neurosci 28: 3060–3070, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]