Abstract
Neocortical neurons in vivo receive concurrent synaptic inputs from multiple sources, including feedforward, horizontal, and feedback pathways. Layer 2/3 of the visual cortex receives feedforward input from layer 4 and horizontal input from layer 2/3. Firing of the pyramidal neurons, which carries the output to higher cortical areas, depends critically on the interaction of these pathways. Here we examined synaptic integration of inputs from layer 4 and layer 2/3 in rat visual cortical slices. We found that the integration is sublinear and temporally asymmetric, with larger responses if layer 2/3 input preceded layer 4 input. The sublinearity depended on inhibition, and the asymmetry was largely attributable to the difference between the two inhibitory inputs. Interestingly, the asymmetric integration was specific to pyramidal neurons, and it strongly affected their spiking output. Thus via cortical inhibition, the temporal order of activation of layer 2/3 and layer 4 pathways can exert powerful control of cortical output during visual processing.
INTRODUCTION
In mammalian neocortical circuit, information is transmitted both vertically across layers and horizontally within each layer. In the visual cortex, neurons in layer 2/3 (L2/3) receive strong feedforward input from layer 4 (L4), the main recipient of the thalamic input (Callaway 1998). This pathway is likely to play a key role in shaping the classical receptive field properties of L2/3 neurons (Chisum and Fitzpatrick 2004). Intracortical horizontal connections, which are especially extensive within L2/3 (Gilbert and Wiesel 1979), may serve to amplify the feedforward input (Douglas et al. 1995) and to mediate contextual modulation by stimuli outside of the classical receptive field (Angelucci and Bressloff 2006; Gilbert 1992).
During normal visual processing, the feedforward and horizontal pathways are activated concurrently. The responses of L2/3 neurons must therefore depend on the integration of synaptic inputs from the two pathways. From previous human psychophysical studies, it is well known that the perception of a given visual object is strongly modulated by other stimuli at nearby locations (Hess et al. 2003; Kapadia et al. 2000; Rossi et al. 1996), and these perceptual effects may involve interactions between the feedforward and horizontal pathways in cortical circuits (Angelucci and Bressloff 2006; Gilbert 1992; Polat 1999). In some cases, the relative timing of presentation of center and surround stimuli on a time scale comparable to that of synaptic integration was found to be important for determining the perceptual outcome (Cass and Alais 2006; Polat and Sagi 2006). This suggests that the integration of the feedforward and horizontal inputs may play a part in shaping the temporal properties of visual perception. In the hippocampus, postsynaptic responses were shown to be sensitive to the temporal order of input activation (Ang et al. 2005; Urban and Barrionuevo 1998), suggesting that synaptic integration in mammalian neural circuits may be temporally asymmetric. Characterizing how L2/3 neurons respond to concurrent feedforward (L4) and horizontal (L2/3) inputs is important for understanding not only visual cortical processing, but also experience-dependent development of cortical maps (Miller 1996; Song and Abbott 2001). However, in previous studies, inputs from L4 and L2/3 have been mostly examined in isolation (Feldmeyer et al. 2002, 2006; Hirsch and Gilbert 1991; Lefort et al. 2009), and how the two inputs integrate in postsynaptic cells remains poorly understood.
In this study, we characterized the integration of evoked inputs from L4 and L2/3 in L2/3 cells of visual cortical slices. We found that the two inputs sum sublinearly in a temporally asymmetric manner, with larger responses if L2/3 input preceded L4 input by tens of milliseconds. This asymmetry was specific to the pyramidal neurons, and it depended on the difference in inhibition between the two pathways. Because of this asymmetric synaptic integration, the spiking output of L2/3 showed a marked dependence on the temporal order of activation between the two pathways. This sensitivity to input order is likely to have important implications in both visual processing and experience-dependent cortical plasticity.
METHODS
Slice preparation and recordings
All experiments were approved by the Animal Care and Use Committee at the University of California, Berkeley. Coronal slices (400 μm thick) of primary visual cortex were prepared from Long-Evans rats 2–5 wk of age (Charles River Laboratories). Slices were cut with a vibratome (Serial 1000 Tissue Sectioning System, Ted Pella, Redding, CA) in a chilled solution containing (in mM) 87 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, 75 sucrose, 10 glucose, and 1.3 ascorbic acid (95% O2-5% CO2) and incubated for 20 min at 32°C in the same solution. Slices were stored in an interface chamber at room temperature in artificial cerebrospinal solution containing (in mM) 124 NaCl, 2.5 KCl, 1.5 MgSO4, 1.25 NaH2PO4, 2.5 CaCl2, 26 NaHCO3, and 10 glucose until placed in a submerged chamber for recording at 32–34°C.
Pyramidal cells and interneurons in cortical L2/3 were visually identified by infrared differential interference contrast video microscopy. Whole cell patch-clamp recordings were performed using Multiclamp 700B amplifier (Axon Instruments, Foster City, CA). Data were filtered at 2–10 kHz, sampled at 10 kHz by an Axon 1200 acquisition board (Axon Instruments, Foster City, CA), and analyzed with custom software in Matlab. The internal solution contained (in mM) 120 K-gluconate, 10 HEPES, 0.1 EGTA, 20 KCl, 2 MgCl2, 10 phosphocreatine, 2 Na2ATP, and 0.25 Na GTP. Cells with resting membrane potentials less than −65 mV were excluded from the data analysis. The mean resting potential was −78.6 ± 4.4 (SD) mV for pyramidal cells and −73.0 ± 2.6 (SD) mV for fast-spiking interneurons. Each recorded cell was injected with a constant current through the patch electrode to hold the membrane at −70 mV to ensure consistency across experiments. Input and series resistances were monitored throughout the experiment with somatic current injections; cells with >30% change in input or series resistance were excluded from analysis. For some experiments, the intracellular solution also contained 0.5% neurobiotin. The drugs used were bicuculline and diazepam (both from Sigma-Aldrich, St. Louis, MO).
Stimulating electrodes pulled from borosilicate theta glass were placed in L4 (directly below the recorded cell) and in L2/3 (off to the side of the recorded cell). Each pathway was stimulated extracellularly (5–200 μA, 0.1 ms) to evoke a 3–20 mV response in the recorded cell. Responses to presynaptic stimulation were measured at 0.2 Hz with randomly interleaved individual and paired stimulation. For paired stimulation, the two pathways were stimulated at ISIs between 0 and 100 ms.
Immunohistochemistry and imaging
After the experiments in which neurobiotin was added to the intracellular solution, slices were fixed overnight in PBS containing 4% paraformaldehyde. For neurobiotin immunohistochemistry, slices were rinsed in PBS three times for 15 min. After rinsing, slices were incubated overnight in PBS solution containing streptavidin-FITC (1:1,000) and 1% Triton-X 100. After incubation, slices were rinsed in PBS three times for 15 min and mounted with Vectorshield (Vector Laboratories, Burlingame, CA). The coverslip was sealed with nail polish. Images were collected with a Leica confocal microscope with a 40× Plan Apo objective (NA 1.0).
Data analysis
For the subthreshhold integration experiments in current clamp, the peak amplitudes of the postsynaptic potentials (PSPs) were measured relative to baseline, averaged over 9–20 sweeps. Linearity of synaptic integration was measured by the difference in response amplitude between the paired stimulation and the linear sum divided by the maximum linear sum for each cell [at 0 ms interstimulus interval (ISI)]. For the suprathreshhold integration experiments in current clamp, stimulation intensity was set to elicit spiking by paired stimulation. The probability of spiking was calculated as the percentage of the 15–20 trials that elicited spiking.
Measurement of synaptic conductances
To prevent spiking and to improve voltage clamp, 3 mM QX-314 (BMD Biosciences, Gibbstown, NJ) was added to the intracellular solution. The electrode capacitance was compensated. The synaptic response for each pathway was measured at three to five different holding potentials (−100 to −20 mV). Conductances were calculated following the methods described previously (Wehr and Zador 2003). Briefly, at each moment during recording, the instantaneous current amplitude was plotted against the holding voltage [corrected for both liquid junction potential (10 mV) and voltage drop across the series resistance]. Total synaptic conductance GT was measured by the slope of the linear fit, and the intercept provided the estimated reversal potential ET. The excitatory and inhibitory conductances (GE and GI) were computed as
where EE (0 mV) and EI (−80 mV) are reversal potentials for excitatory and inhibitory conductances, respectively. These values were similar to the values for EE and EI measured experimentally, which were −7.5 ± 12.9 (n = 4) and −78.8 ± 8.5 (SD) mV (n = 11), respectively (Supplemental Materials).1
Simulation
We used a simple model to reconstruct the voltage traces from excitatory and inhibitory conductances
where V is the membrane potential, Cm is the membrane capacitance of the cell, and GE and GI are the excitatory and inhibitory conductances, respectively, averaged from the population of experiments (Fig. 3D). GR is the resting membrane conductance, EE and EI are the reversal potentials for the excitatory and inhibitory inputs, respectively, and ER is the resting potential. The values of Cm (0.22 nF) and GR (5.6 nS) were measured experimentally by somatic current injection (0.02 nA, 500 ms). We set ER = −70 mV, EE = 0 mV, and EI = −80 mV.
Fig. 3.
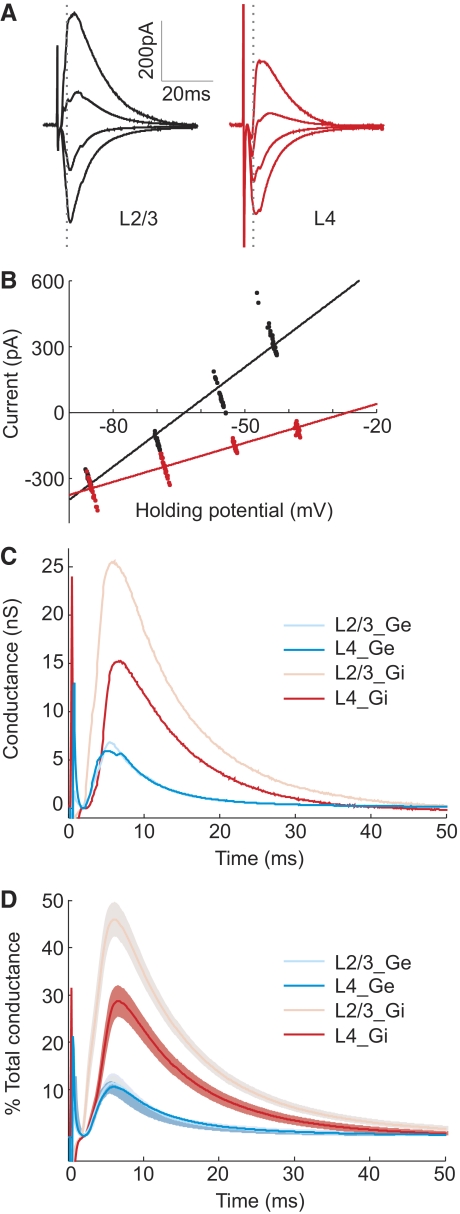
Different amplitudes of L2/3 and L4 inhibitory conductances. A: synaptic currents evoked by extracellular stimulation in L2/3 (black) and L4 (red) recorded at 4 different holding potentials (−85, −70, −55, and 45 mV) with 3 mM QX-314 in recording pipette. Each trace was averaged from 22 trials. B: instantaneous synaptic currents at 3.5 ms after extracellular stimulation (dotted gray lines in A) are plotted against holding potentials. Each point represents data recorded in each trial. Fits were performed using linear regression. C: excitatory and inhibitory conductances evoked by stimulation in each pathway for the cell shown in A and B. D: conductances averaged across 24 experiments. In each experiment, each conductance was normalized by the total peak amplitude conductance before averaging across experiments. Shaded region, ±SE.
To reconstruct the voltage traces evoked by paired stimuli, the conductances of the two pathways were first summed linearly with the appropriate ISI before the voltage traces were reconstructed as described above.
RESULTS
Integration of L4 and L2/3 inputs in L2/3 pyramidal cells
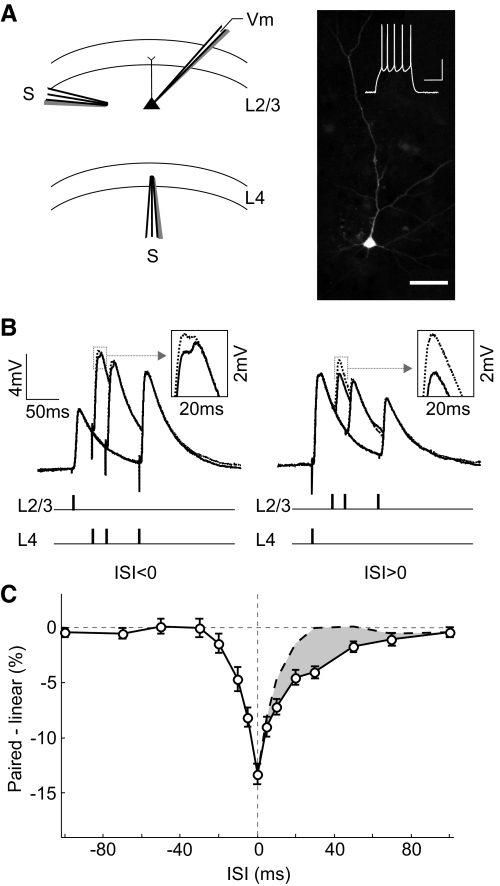
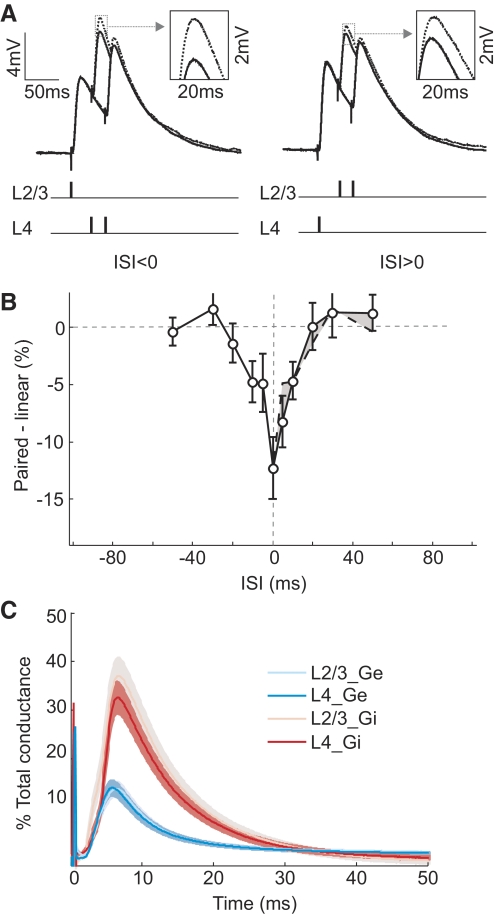
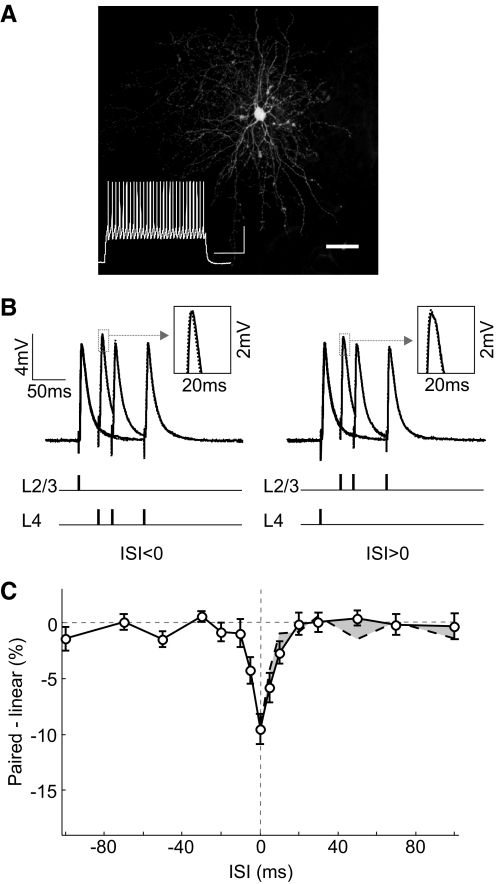
Whole cell recordings were made from L2/3 pyramidal cells in visual cortical slices from rats (P29–P35). One input was activated with a simulating electrode positioned in L4 directly below the recorded cell (activating axons in L4), and another input was activated with an electrode in L2/3 150–200 μm from the recorded cell (activating axons from neighboring pyramidal cells and interneurons in L2/3) (Fig. 1A). To examine the interaction between these inputs, we stimulated the two pathways either separately or in close temporal proximity, with an ISI varying between −100 and 100 ms. We calculated the difference between the measured PSP evoked by paired stimulation (Fig. 1B, solid line) and the linear sum of the responses evoked separately (Fig. 1B, dotted line). We found that L2/3 and L4 inputs integrated sublinearly within a window of ±80 ms (Fig. 1C; n = 58 experiments). Interestingly, this window showed a pronounced asymmetry: whereas the sublinearity for positive ISIs (L4 preceding L2/3) extended to ∼80 ms, for negative ISIs, it existed only within ∼30 ms. As a result, between 10 and 70 ms, a larger response was evoked when the L2/3 input preceded the L4 input (Fig. 1C, shaded area), and this difference peaked at ∼30 ms. This asymmetry was not caused by any systematic difference in evoked PSP amplitude between the two pathways, because when we divided the experiments into two groups based on the relative PSP size (L4 PSP > L2 PSP or L2 PSP > L4 PSP), the asymmetry was observed in both groups (Supplemental Fig. S4).
Fig. 1.
Integration of L2/3 and L4 inputs in L2/3 pyramidal neurons. A: left: schematic illustration of experimental setup. Right: fluorescence image of a neurobiotin-filled pyramidal neuron; scale bar, 50 μm. Inset: regular spiking pattern of this cell evoked by current injection (240 pA, 500 ms); scales, 30 mV, 200 ms. B: solid line, postsynaptic potentials (PSPs) evoked by paired stimuli at 3 different interstimulus intervals (ISIs; 30, 50, and 100 ms), each averaged from 10 trials. Dotted line, linear sum of PSPs evoked by separate stimuli in each pathway. Schematic below indicates stimulation time in each pathway. Inset: enlarged view of PSP peak in the dotted box. C: integration window, difference between the peak amplitude of paired response and that of the linear sum was plotted against ISI (n = 58). Dashed line, mirror image of data at ISI <0 shown on the right side for comparison. Shaded area, difference between the dashed and solid curve. Error bar, ±SE.
Role of inhibition in sublinear summation
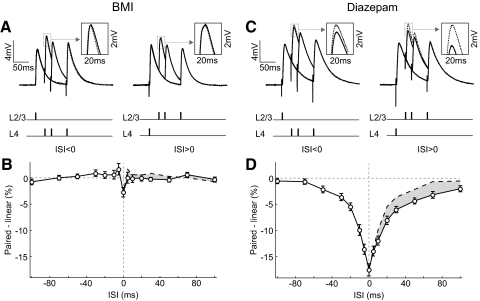
Previous studies suggested that inhibition may contribute to the sublinearity of synaptic integration (Fuentealba et al. 2004; Spruston 2008). To examine the role of inhibition in the integration of the L4 and L2/3 pathways in cortical L2/3, we blocked inhibition by bath application of bicuculline (2 or 3 μM), a GABAA receptor antagonist. At this concentration, bicuculline did not markedly affect the PSP shape (Fig. 2A). However, the sublinearity at both negative and positive ISIs was eliminated (Fig. 2B). Conversely, to enhance the activity of GABAA receptors, we bath applied diazepam (30 μM), an allosteric modulator that augments GABAA receptor–mediated postsynaptic inhibition (Macdonald and Barker 1978). Diazepam also did not markedly affect the PSP shapes (Fig. 2C), but both the amplitude and the width of the sublinearity window were increased (Fig. 2D). The difference between the integration at negative and positive ISIs was also observed over a longer time window, ≤100 ms (shaded area). Together, these experiments indicate that the sublinear integration depends critically on GABAA receptor–mediated inhibition.
Fig. 2.
Role of GABAA receptor–mediated inhibition in sublinear integration. A: paired PSPs (solid) and linear sums (dotted) at 3 different ISIs (30, 50, and 100 ms) in the presence of 2 or 3.3 μM bicuculline (BMI). Each trace was averaged from 14 trials. Inset: enlarged view of PSP peak in the dotted box. B: integration window in the presence of BMI (n = 56). Error bar, ±SE. C: paired PSPs and linear sums at 3 different ISIs (30, 50, and 100 ms) in the presence of 30 μM diazepam. Each trace was averaged from 10 trials. D: integration window in the presence of diazepam (n = 42). Error bar, ±SE.
Difference in inhibition between the two pathways
Although inhibition seems to be required for the sublinearity, the asymmetry could be caused by differences between the two pathways in either excitation or inhibition. To distinguish these possibilities, we measured both the excitatory and inhibitory inputs from each pathway. The evoked currents were measured under voltage clamp at several holding potentials (Fig. 3A). At each time point, the instantaneous excitatory and inhibitory conductances were computed from the linear regression of the I-V plots (Fig. 3B; see methods). As shown in Fig. 3C for the example cell and Fig. 3D for the population average, the excitatory inputs were similar between L2/3 (L2/3_Ge) and L4 (L4_Ge) pathways. However, the inhibitory input of L2/3 pathway (L2/3_Gi) was significantly larger than that of L4 pathway [L4_Gi; ratio, 249 ± 50% (SE); P = 0.007, paired t-test, n = 24].
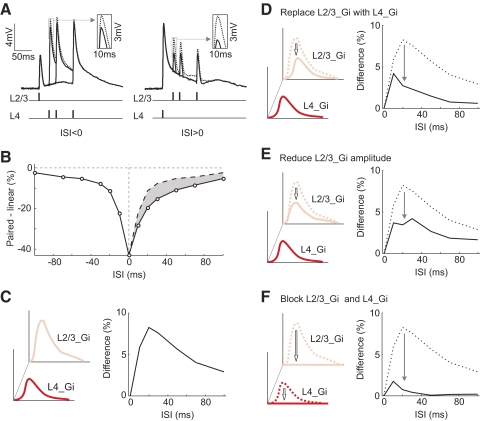
We tested whether this difference in inhibition can account for the temporal asymmetry using simulations. The voltage traces were reconstructed from the excitatory and inhibitory conductances of each pathway shown in Fig. 3D (see methods). For the responses to paired stimulation, the conductances from the two pathways were summed at given ISIs before the voltage traces were reconstructed. These paired responses were then compared with the linear sums of the individual responses to determine the linearity of synaptic integration (Fig. 4A). As shown in Fig. 4B, the integration window for the reconstructed responses was sublinear and temporally asymmetric, qualitatively similar to the experimentally measured window (Fig. 1C). The difference in response amplitude between the positive and negative ISIs peaked at ∼20 ms (Fig. 4C). Note that the voltage-gated conductances, which could prolong the duration of the synaptic responses, were not included in this simple simulation. This could explain why the simulated voltage traces (Fig. 4A) were shorter in duration than the measured responses (Fig. 1B). Although these shorter responses are likely to shorten the time window for integration, they should not affect the temporal asymmetry qualitatively.
Fig. 4.
Simulation of voltage traces from excitatory and inhibitory conductances. A: reconstructed voltage traces using the measured conductances shown in Fig. 3D. B: integration window for reconstructed responses. Shaded area, difference between the dashed (ISI < 0) and solid curve (ISI > 0). C: difference curve. Left: schematic illustration of L2/3_Gi and L4_Gi. Right: difference curve corresponding to shaded area in B. D: equalizing L2/3_Gi and L4_Gi. Left: schematic illustration of replacing L2/3_Gi with L4_Gi. Right: difference curve before (dotted) and after (solid) L2/3_Gi replacement. E: similar to D, with L2/3_Gi amplitude reduced to L4_Gi amplitude, but with the original L2/3_Gi waveform retained. F: similar to D, with L2/3_Gi and L4_Gi blocked by setting both to 0.
To test whether the asymmetry is caused by the difference in inhibition between the two pathways, we equalized their inhibitory conductances by replacing L2/3_Gi with L4_Gi (Fig. 4D). The asymmetry was greatly reduced, indicating that the difference in inhibition plays an important role. We tested whether the asymmetry is primarily caused by the amplitude difference between the two inhibitory inputs by scaling the amplitude of L2/3_Gi to that of L4_Gi (Fig. 4E). This manipulation reduced the asymmetry to a level similar to that in Fig. 4D, indicating that the asymmetric is largely caused by the amplitude difference. In addition, setting both L2/3_Gi and L4_Gi to 0, which corresponds to the experiment of blocking GABAA receptors (Fig. 2, A and B), almost completely eliminated the asymmetry (Fig. 4F), consistent with the experimental result. Together, these simulation results suggest that the temporal asymmetry depends critically on the amplitude difference between L4 and L2/3 inhibitory inputs.
Developmental change of synaptic integration
The inhibitory circuit in the cortex undergoes profound changes between the first and fifth week of life (Gonchar et al. 2007; Huang et al. 2007; Luhmann and Prince 1991). Given the importance of cortical inhibition in shaping the integration window (Figs. 2 and 4), the developmental changes suggest that the integration window may be different across ages. To test this prediction, we measured the integration window in younger animals (P13–P24). The PSP shape (Fig. 5A) was not markedly different from that in the older animals (Fig. 1B). However, although the sublinearity is still present, the asymmetry is largely absent (Fig. 5B), indicating that synaptic integration of the two pathways is indeed developmentally regulated. When we measured the excitatory and inhibitory conductances of each pathway in these young animals, we found that the inhibitory inputs from L4 and L2/3 pathway were not significantly different (Fig. 5C; P = 0.5, paired t-test, n = 20). This provides further support to our finding that the difference in inhibition between the two pathways plays a critical role in the temporally asymmetric integration.
Fig. 5.
Synaptic integration in young animals (P13–P24). A: paired PSPs and linear sums at 2 different ISIs (30 and 50 ms). Each trace was averaged from 10 trials. B: integration window in young animals (n = 31). Error bar, ±SE. C: conductances in young animals, averaged from 20 experiments. Shaded region, ±SE.
Temporal asymmetry of spiking output
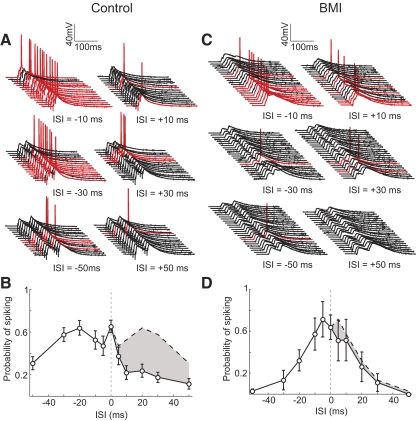
To test the functional consequence of this asymmetric integration, we applied stronger stimulation in the two pathways to measure suprathreshhold responses (Fig. 6A). Although stimulating each pathway separately did not evoke spiking, paired stimulation at short intervals elicited spiking in many trials. We found that the probability of spiking was higher when L2/3 was activated before L4, consistent with the asymmetry in the subthreshhold integration window. Compared with the subthreshold responses, the asymmetry was significantly enhanced in the spiking response (Fig. 6B), presumably because of the spike threshold nonlinearity. At 20–30 ms, the difference in spiking between the negative and positive ISIs was greater than twofold. Bath application of bicuculline greatly diminished the asymmetry in the spiking output (Fig. 6, C and D), similar to that found for the subthreshhold responses (Fig. 2, A and B). Because L2/3 neurons carry the output of V1, the temporal asymmetry in the spiking response may have important impacts on the transmission of visual information to higher visual areas.
Fig. 6.
Dependence of spike output on ISI. A: responses to multiple trials of paired stimulation at 3 different ISIs (10, 30, and 50 ms) in an example cell, showing trials with (red) and without (black) spiking. B: probability of spiking plotted against ISI (n = 19). Error bar, ±SE. C: similar to A, for an experiment performed in the presence of 3.3 μM. BMI. D: probability of spiking vs. ISI in the presence of BMI (n = 4). Error bar, ±SE.
Cell type specificity of temporal asymmetry
In addition to the pyramidal neurons in L2/3, neighboring interneurons also receive inputs from both L2/3 and L4. We measured the integration of L2/3 and L4 inputs in the fast-spiking interneurons, identified on the basis of their spiking patterns (McCormick et al. 1985) and nonpyramidal morphology (Dumitriu et al. 2007) (Fig. 7A). The PSPs evoked in the fast-spiking neurons were shorter in duration than in the pyramidal cells (Fig. 7B), and sublinearity of integration of the two inputs was found only at short ISIs (±10 ms; Fig. 7C). Importantly, the window was largely symmetric, indicating that the asymmetry is specific to the pyramidal neurons.
Fig. 7.
Integration window in fast-spiking cells. A: fluorescence image of a neurobiotin-filled nonpyramidal neuron; scale bar, 50 μm. Inset: fast spiking pattern of this cell evoked by current injection (580 pA, 500 ms); scales, 30 mV, 100 ms. B: paired PSPs and linear sums at 3 different ISIs (30, 50, and 100 ms) in a fast-spiking cell. Each trace was averaged from 10 trials. C: integration window for fast-spiking cells (n = 17). Error bar, ±SE.
DISCUSSION
In this study, we found that the integration of L2/3 and L4 inputs in L2/3 of rat visual cortex is sublinear within a temporal window of ±100 ms. The marked asymmetry of the window indicates that, in addition to the interval, the temporal order of activation between the inputs also plays a critical role in determining the postsynaptic response. The sublinearity of integration depends on GABAA receptor–mediated inhibition, and the asymmetry can be largely attributed to the difference in the strength of inhibition between the two pathways. In support of this finding, we showed that the integration is temporally symmetric at an early developmental stage, when inhibitory inputs from the two pathways are similar. Furthermore, the asymmetry in mature visual cortex strongly affects the spiking output, and it is specific to the projection (pyramidal) neurons, indicating that the asymmetry is an important mechanism for shaping the output of the visual cortex.
Sublinear integration
Sublinear integration is widely observed in sensory circuits. In the auditory (Wehr and Zador 2005) and somatosensory (Ego-Stengel et al. 2005; Higley and Contreras 2007; Shimegi et al. 1999) cortices, multiple sensory inputs arriving closely in time generally evoke responses that are smaller than the linear sum of the individually evoked responses. Although this is partly because of the nonlinear interactions between inputs earlier in the sensory pathway (Higley and Contreras 2007), synaptic depression within the cortical circuit also contributes to the sublinearity (Wehr and Zador 2005). In hippocampal pyramidal neurons, voltage-gated potassium channels have been shown to contribute to sublinear integration of inputs targeting different dendritic locations (Margulis and Tang 1998; Urban and Barrionuevo 1998), and the degree of sublinearity can be affected by changes in dendritic active conductances (Frick et al. 2004; Wang et al. 2003).
In this study, we found that blocking GABAA receptors largely eliminated the sublinear interaction in visual cortical L2/3, suggesting that inhibition plays a key role in this circuit, presumably through shunting of the excitatory inputs (Koch and Poggio 1985). Note that previous studies of input integration were often performed under blockade of inhibition (Nettleton and Spain 2000) or with stimulation that recruits little inhibition (Cash and Yuste 1999; Yoshimura et al. 2000). On the other hand, sensory stimuli in vivo are known to cause substantial activation of inhibitory inputs (Borg-Graham et al. 1998; Wehr and Zador 2003). Our finding suggests that such inhibitory activity can exert strong regulation of synaptic integration during sensory processing.
Temporal asymmetry
In the hippocampus, asymmetric temporal integration has been shown to depend on the activation of potassium channels (Urban and Barrionuevo 1998), whereas in our study, the difference in the strength of inhibition between the two pathways seems to be a key mechanism. We note that, for cortical neurons, membrane potentials in the dendrites are poorly controlled by voltage clamp at the soma, which may result in significant distortions in the measurement of amplitude and kinetics of the excitatory and inhibitory synaptic conductances (Williams and Mitchell 2008). However, this is unlikely to account for the large difference in the strength of inhibition between the L4 and L2/3 pathways we observed. Instead, the difference is likely to reflect the activation of different inhibitory circuits. Stimulation in L2/3 may recruit local inhibitory neurons such as basket cells, which constitute ∼50% of the interneurons. These cells specifically target the perisomatic region of the postsynaptic neurons (Markram et al. 2004), exerting strong control of the postsynaptic membrane potential (Spruston 2008). Other interneurons such as large basket, Martinotti, bitufted, double bouquet, and bipolar cells have been observed in L4 but can extend their axons across layers (Markram et al. 2004); thus they could be activated by L4 stimulation. In addition, a disynaptic inhibitory circuit has been suggested in somatosensory cortex that could mediate inhibition of L2/3 neurons evoked by L4 activation (Helmstaedter et al. 2008). Consistent with this disynaptic circuit, we found that L4 but not L2/3 inhibitory input was markedly reduced by pharmacological blockade of excitation (Supplemental Fig. S3). Recruitment of the disynaptic inhibitory circuit in L4 is likely to be less efficient than the recruitment of direct inhibition from L2/3, which could account for the difference in the amplitude of inhibition from the two pathways. In principle, stimuli in L2/3 and L4 can activate axons of passage from other layers. For example, L2/3 neurons send axons to deeper layers, which could be antidromically activated by L4 stimulation. However, this is unlikely with the stimulation intensities used in our study, because we never observed antidromically evoked spiking when recording from neighboring L2/3 neurons. The age-dependent change in the relative strengths of inhibition of the two pathways (Figs. 3D and 5C) may be caused by the difference in the time course of maturation of these inhibitory circuits (Gonchar et al. 2007; Huang et al. 2007; Luhmann and Prince 1991). The different inhibitory circuits in these pathways may also allow various neuromodulators, which are known to modulate the activity of specific inhibitory neurons (Gulledge et al. 2007; Xiang et al. 1998), to regulate the temporal window of synaptic integration in a behaviorally relevant manner.
Functional implications
The dependence of postsynaptic spike probability on the temporal order of L2/3 and L4 inputs is likely to be important for visual processing. In human psychophysical studies, the visibility of a target is improved by the presence of collinear flankers (Kapadia et al. 2000), and in parallel electrophysiological studies, collinear stimuli were found to enhance the firing rates of V1 neurons (Chisum et al. 2003; Kapadia et al. 2000). The integration of temporally overlapping horizontal and feedforward inputs may account for these interactions. Notably, the facilitatory interaction was greater when the flankers were presented before rather than after the target (Cass and Alais 2006; Polat and Sagi 2006). This may be related to our finding that L2/3 pyramidal neurons are more likely to fire when L2/3 pathway is activated before L4 pathway (Fig. 6, A and B).
In addition to visual processing, timing of activation of the two pathways may also be critical for experience-dependent modification of cortical circuits. For example, during sensory deprivation, the order of L4 and L2/3 spiking activity is reversed in vivo (Celikel et al. 2004). This change in the order of spiking activity may underlie the deprivation-induced modification of L4 to L2/3 connections through spike timing–dependent plasticity (Allen et al. 2003). Here we have shown that the probability of postsynaptic spiking is highly sensitive to the temporal order of activation of the two layers, and this sensitivity depends on cortical inhibition (Fig. 6). Inhibitory circuits are known to play important roles in regulating experience-dependent cortical plasticity (Hensch et al. 1998; Huang et al. 1999). Our finding provides a novel mechanism by which input timing can act through cortical inhibition to influence experience-dependent cortical modifications.
GRANTS
This work was supported by a National Institutes of Health Grant R01 EY-018861.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank C. Y. Li for helpful discussions and C. D. Meliza for data acquisition software.
REFERENCES
- Allen CB, Celikel T, Feldman DE. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci 6: 291–299, 2003 [DOI] [PubMed] [Google Scholar]
- Ang CW, Carlson GC, Coulter DA. Hippocampal CA1 circuitry dynamically gates direct cortical inputs preferentially at theta frequencies. J Neurosci 25: 9567–9580, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci A, Bressloff PC. Contribution of feedforward, lateral and feedback connections to the classical receptive field center and extra-classical receptive field surround of primate V1 neurons. Prog Brain Res 154: 93–120, 2006 [DOI] [PubMed] [Google Scholar]
- Borg-Graham LJ, Monier C, Fregnac Y. Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature 393: 369–373, 1998 [DOI] [PubMed] [Google Scholar]
- Callaway EM. Local circuits in primary visual cortex of the macaque monkey. Annu Rev Neurosci 21: 47–74, 1998 [DOI] [PubMed] [Google Scholar]
- Cash S, Yuste R. Linear summation of excitatory inputs by CA1 pyramidal neurons. Neuron 22: 383–394, 1999 [DOI] [PubMed] [Google Scholar]
- Cass J, Alais D. The mechanisms of collinear integration. J Vis 6: 915–922, 2006 [DOI] [PubMed] [Google Scholar]
- Celikel T, Szostak VA, Feldman DE. Modulation of spike timing by sensory deprivation during induction of cortical map plasticity. Nat Neurosci 7: 534–541, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisum HJ, Fitzpatrick D. The contribution of vertical and horizontal connections to the receptive field center and surround in V1. Neural Netw 17: 681–693, 2004 [DOI] [PubMed] [Google Scholar]
- Chisum HJ, Mooser F, Fitzpatrick D. Emergent properties of layer 2/3 neurons reflect the collinear arrangement of horizontal connections in tree shrew visual cortex. J Neurosci 23: 2947–2960, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Koch C, Mahowald M, Martin KA, Suarez HH. Recurrent excitation in neocortical circuits. Science 269: 981–985, 1995 [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Cossart R, Huang J, Yuste R. Correlation between axonal morphologies and synaptic input kinetics of interneurons from mouse visual cortex. Cereb Cortex 17: 81–91, 2007 [DOI] [PubMed] [Google Scholar]
- Ego-Stengel V, Mello e Souza T, Jacob V, Shulz DE. Spatiotemporal characteristics of neuronal sensory integration in the barrel cortex of the rat. J Neurophysiol 93: 1450–1467, 2005 [DOI] [PubMed] [Google Scholar]
- Feldmeyer D, Lubke J, Sakmann B. Efficacy and connectivity of intracolumnar pairs of layer 2/3 pyramidal cells in the barrel cortex of juvenile rats. J Physiol 575: 583–602, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D, Lubke J, Silver RA, Sakmann B. Synaptic connections between layer 4 spiny neurone-layer 2/3 pyramidal cell pairs in juvenile rat barrel cortex: physiology and anatomy of interlaminar signalling within a cortical column. J Physiol 538: 803–822, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci 7: 126–135, 2004 [DOI] [PubMed] [Google Scholar]
- Fuentealba P, Crochet S, Timofeev I, Steriade M. Synaptic interactions between thalamic and cortical inputs onto cortical neurons in vivo. J Neurophysiol 91: 1990–1998, 2004 [DOI] [PubMed] [Google Scholar]
- Gilbert CD. Horizontal integration and cortical dynamics. Neuron 9: 1–13, 1992 [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature 280: 120–125, 1979 [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Wang Q, Burkhalter A. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front Neuroanat 1: 3, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Park SB, Kawaguchi Y, Stuart GJ. Heterogeneity of phasic cholinergic signaling in neocortical neurons. J Neurophysiol 97: 2215–2229, 2007 [DOI] [PubMed] [Google Scholar]
- Helmstaedter M, Staiger JF, Sakmann B, Feldmeyer D. Efficient recruitment of layer 2/3 interneurons by layer 4 input in single columns of rat somatosensory cortex. J Neurosci 28: 8273–8284, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282: 1504–1508, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RF, Hayes A, Field DJ. Contour integration and cortical processing. J Physiol Paris 97: 105–119, 2003 [DOI] [PubMed] [Google Scholar]
- Higley MJ, Contreras D. Cellular mechanisms of suppressive interactions between somatosensory responses in vivo. J Neurophysiol 97: 647–658, 2007 [DOI] [PubMed] [Google Scholar]
- Hirsch JA, Gilbert CD. Synaptic physiology of horizontal connections in the cat's visual cortex. J Neurosci 11: 1800–1809, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Di Cristo G, Ango F. Development of GABA innervation in the cerebral and cerebellar cortices. Nat Rev Neurosci 8: 673–686, 2007 [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98: 739–755, 1999 [DOI] [PubMed] [Google Scholar]
- Kapadia MK, Westheimer G, Gilbert CD. Spatial distribution of contextual interactions in primary visual cortex and in visual perception. J Neurophysiol 84: 2048–2062, 2000 [DOI] [PubMed] [Google Scholar]
- Koch C, Poggio T. Models of the Visual Cortex. New York: Wiley, 1985 [Google Scholar]
- Lefort S, Tomm C, Floyd Sarria JC, Petersen CC. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron 61: 301–316, 2009 [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Prince DA. Postnatal maturation of the GABAergic system in rat neocortex. J Neurophysiol 65: 247–263, 1991 [DOI] [PubMed] [Google Scholar]
- Macdonald R, Barker JL. Benzodiazepines specifically modulate GABA-mediated postsynaptic inhibition in cultured mammalian neurones. Nature 271: 563–564, 1978 [DOI] [PubMed] [Google Scholar]
- Margulis M, Tang CM. Temporal integration can readily switch between sublinear and supralinear summation. J Neurophysiol 79: 2809–2813, 1998 [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5: 793–807, 2004 [DOI] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol 54: 782–806, 1985 [DOI] [PubMed] [Google Scholar]
- Miller KD. Receptive fields and maps in the visual cortex: models of ocular dominance and orientation columns. In: Models of Neural Networks, III, edited by Domany E, van Hemmen J, Schulten K. New York: Springer-Verlag, p. 55–78, 1996 [Google Scholar]
- Nettleton JS, Spain WJ. Linear to supralinear summation of AMPA-mediated EPSPs in neocortical pyramidal neurons. J Neurophysiol 83: 3310–3322, 2000 [DOI] [PubMed] [Google Scholar]
- Polat U. Functional architecture of long-range perceptual interactions. Spat Vis 12: 143–162, 1999 [DOI] [PubMed] [Google Scholar]
- Polat U, Sagi D. Temporal asymmetry of collinear lateral interactions. Vision Res 46: 953–960, 2006 [DOI] [PubMed] [Google Scholar]
- Rossi AF, Rittenhouse CD, Paradiso MA. The representation of brightness in primary visual cortex. Science 273: 1104–1107, 1996 [DOI] [PubMed] [Google Scholar]
- Shimegi S, Ichikawa T, Akasaki T, Sato H. Temporal characteristics of response integration evoked by multiple whisker stimulations in the barrel cortex of rats. J Neurosci 19: 10164–10175, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Abbott LF. Cortical development and remapping through spike timing-dependent plasticity. Neuron 32: 339–350, 2001 [DOI] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci 9: 206–221, 2008 [DOI] [PubMed] [Google Scholar]
- Urban NN, Barrionuevo G. Active summation of excitatory postsynaptic potentials in hippocampal CA3 pyramidal neurons. Proc Natl Acad Sci USA 95: 11450–11455, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Xu NL, Wu CP, Duan S, Poo MM. Bidirectional changes in spatial dendritic integration accompanying long-term synaptic modifications. Neuron 37: 463–472, 2003 [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426: 442–446, 2003 [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron 47: 437–445, 2005 [DOI] [PubMed] [Google Scholar]
- Williams SR, Mitchell SJ. Direct measurement of somatic voltage clamp errors in central neurons. Nat Neurosci 11: 790–798, 2008 [DOI] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science 281: 985–988, 1998 [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Sato H, Imamura K, Watanabe Y. Properties of horizontal and vertical inputs to pyramidal cells in the superficial layers of the cat visual cortex. J Neurosci 20: 1931–1940, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.