Abstract
We have previously identified lipocalin 2 (Lcn2) as a cytokine playing a critical role in the regulation of body fat mass, lipid metabolism, and insulin resistance. Lcn2 deficiency reduces PPARγ gene expression in adipocytes. In this study, we investigated the role of Lcn2 in PPARγ activation and function via assessing the insulin sensitization and fatty acid (FA) homeostasis of PPARγ agonist in high-fat diet (HFD)-induced obesity in Lcn2−/− mice. We found that rosiglitazone (Rosi) significantly improved insulin sensitivity in Lcn2−/− mice as effectively as in wild-type (WT) mice; unfed-state levels of blood glucose, free FAs, and triglycerides (TGs) were significantly reduced after a 25-d treatment of Rosi in Lcn2−/− mice. However, Rosi action on fat deposition and FA homeostasis was altered; Rosi-induced body weight and subcutaneous fat gain and liver lipid accumulation were markedly lessened in Lcn2−/− mice. The results of in vivo metabolic labeling showed that Rosi markedly reduced de novo lipogenesis in adipose tissue of Lcn2−/− mice. In brown adipose tissue (BAT), the expression of the genes functioning in TG hydrolysis and mitochondrial oxidation was up-regulated more in Lcn2−/− than in WT mice. Most strikingly, Rosi stimulated significantly higher levels of uncoupling protein-1 expression in BAT, and completely rescued cold intolerance in Lcn2−/− mice. We demonstrate that Lcn2 is a critical selective modulator of PPARγ activation and function in lipid homeostasis and energy expenditure.—Jin, D., Guo, H., Bu, Y. S., Zhang, Y., Hannaford, J., Mashek, D. G., Chen, X. Lipocalin 2 is a selective modulator of peroxisome proliferator-activated receptor-γ activation and function in lipid homeostasis and energy expenditure.
Keywords: rosiglitazone, brown adipose tissue, thermogenesis, insulin sensitization
Lipocalin 2 (LCN2), also known as neutrophil gelatinase-associated lipocalin, siderocalin, and 24p3, is a member of the lipocalin subfamily. Lipocalins are small secreted proteins with a hydrophobic ligand binding pocket; known ligands for lipocalins include fatty acids (FAs), retinol, retinoic acids, steroids, odorants, and pheromones (1). Because of their ability to bind a range of ligands, lipocalins possess a variety of biological functions. For instance, as a component of the innate immune system, LCN2 plays a key role in implementing the acute-phase response (2) and also has a role in the regulation of apoptosis (3).
We and others have recently identified LCN2 as a cytokine that connects obesity and insulin resistance (4–5). Lcn2 expression is strongly stimulated by TNF-α and LPS (lipopolysaccharide) in macrophages and adipocytes (5). Lcn2 expression in adipose tissue, liver, and serum was significantly increased in db/db diabetic and ob/ob mice. Administration of the antidiabetic agent thiazolidinedine (TZD) to genetic obese mice reduces Lcn2 gene expression in adipose tissue, suggesting that LCN2 is important for mediating TZD effects. In addition to TNF-α and TZD, studies in humans have shown that insulin is another critical regulator of Lcn2 expression in omental adipose tissue (6). In our previous in vitro study, the addition of recombinant mouse Lcn2 protein to the culture medium of 3T3-L1 adipocytes significantly induced the expression of PPARγ and the PPARγ target genes adiponectin and lipoprotein lipase (LPL). Knocking down Lcn2 expression reduces PPARγ and LPL expression in 3T3-L1 adipocytes (5).
Most important, we have recently characterized Lcn2 as a critical regulator of energy metabolism, glucose and lipid homeostasis, and insulin resistance in Lcn2-deficient mice (7). In the present study, Lcn2-deficient mice are cold intolerant. They also developed significantly increased body fat mass, exacerbated adipocyte hypertrophy, fatty liver, and insulin resistance on high-fat diet (HFD) feeding (7). In addition, primary adipocytes isolated from Lcn2-deficient mice expressed significantly lower levels of Pparg gene (7). These data together suggest that Lcn2 has an important role in the regulation of adipogenesis, lipogenesis, and insulin resistance; regulating PPARγ activation may be one important mechanism for the role of LCN2 in lipid mechanism and the development of adipose tissue mass.
The antidiabetic agents, TZDs, exert their metabolic effects by lowering blood glucose, improving insulin sensitivity, inducing adipogenesis, and reducing inflammatory response through fully activating PPARγ activity. As a ligand-activated nuclear receptor, PPARγ activity is regulated by the ligand-dependent transactivation and transrepression mechanism involving the recruitment of coactivators or corepressors (8). A variety of ligands are responsible for the activation of PPARγ leading to distinct biological consequences, suggesting that the binding of different ligands may result in differential patterns of PPARγ transcriptional activation. For example, TZDs are known to fully activate PPARγ, causing improved glucose intolerance and insulin sensitivity, which is commonly accompanied by weight gain, fluid retention, hyperphagia, and fat-mass increase (9–10). It is well accepted that white adipose tissue (WAT) is one of the primary targets for the insulin-sensitizing action of TZD, as PPARγ is abundantly expressed in this tissue. TZD increases the transactivation of PPARγ and induces the expression of PPARγ-regulated adipogenic genes, which is associated with the effect of TZDs. However, TZD increases the transrepression of PPARγ, leading to the inhibition of NF-κB transcription activation and cytokine production, and thus contributes to the anti-inflammatory action of TZDs. Previous studies have shown that partial PPARγ agonists selectively activate PPARγ activity, displaying full insulin sensitization, but less adipogenic activity (11–13). These results suggest that adipogenic and insulin-sensitizing activities are independently regulated depending on the patterns of PPARγ transactivation and transrepression.
In addition to WAT, brown adipose tissue (BAT) expresses significantly higher levels of PPARγ, but the activation of PPARγ in BAT plays an opposite role in energy balance. BAT functions to stimulate energy expenditure through dissipating energy as heat, which helps prevent obesity and maintain body temperature in a cold environment. BAT has also been demonstrated to be another important target tissue for TZD effects. For example, TZD treatment resulted in the increase in BAT fat mass in vivo, as well as the induction of BAT adipocyte differentiation in vitro in BAT adipocyte cell line HIB-1B (14). The TZD effects on increasing BAT adiposity and an induced up-regulation of uncoupling proteins (15–17) are indicative of increased energy dissipation, which is one of the important mechanisms for antidiabetic effects of TZD.
To test the hypothesis that LCN2 is important for the regulation of PPARγ activation, we examined the effect of LCN2 deficiency on the action of PPARγ agonist TZD and explored the mechanism by which TZD acts in an HFD-induced lipid dysregulation and insulin resistance in the absence of LCN2. Our results showed that the insulin-sensitizing and antidyslipidemia effects of TZD remain effective in Lcn2-deficient mice. Interestingly, in the absence of Lcn2, the Rosi effect on lipogenesis, weight gain, and fat-mass increase was abolished; Rosi markedly reduced [3H] H2O incorporation into FAs in adipose tissue of Lcn2−/− mice. We also found that Rosi exerted its insulin-sensitizing effect through a distinct mechanism involving the enhancement of uncoupling protein 1 (UCP-1)-mediated BAT thermogenesis in Lcn2-deficient mice.
MATERIALS AND METHODS
Animals
Mice used in this study were C57BL/6 wild-type (WT) and Lcn2-null (Lcn2−/−) mice, as described previously (7). Mice were housed in specific pathogen-free facility at the University of Minnesota. Animal handling followed the U.S. National Institutes of Health guidelines, and experimental procedures were approved by the University of Minnesota Animal Care and Use Committee. Mice were allocated into groups (3 or 4 mice/cage) and fed an HFD (fat calories: 60%) obtained from Bio-Serv (F3282; New Brunswick, NJ, USA) or a regular chow diet, with free access to water for all studies.
In the rosiglitazone study, age-matched male WT and Lcn2−/− mice at 3 wk of age were fed an HFD and subjected to oral gavage of rosiglitazone [10 mg/kg body weight (bw)/d] for 25 d after the development of insulin resistance and obesity in response to HFD preloading for 14 wk. Glucose-tolerance tests (GTTs) and insulin-tolerance tests (ITTs) were performed after 14 and 20 d of Rosi gavage, respectively. At 21 wk of age, mice were sacrificed, and blood and tissues were collected after 18 h food deprivation. In the adaptive thermogenesis study, age-matched male WT and Lcn2−/− mice fed a regular chow diet at 10 wk of age were given rosiglitazone (10 mg/kg bw/d) daily by oral gavage for 2 wk. Mice were then exposed to 4°C, with free access to water. Rectal temperature of the mice was measured at the indicated time points using a MicroTherma Thermometer with rectal probe for mice (Braintree Scientific, Braintree, MA, USA). After the final measurement of rectal temperature, mice were killed for tissues and blood collection.
Primary mouse adipose cell isolation
Preparation of isolated adipose cells from WT and Lcn2−/− mice was performed as described previously (18, 19). After mincing, epididymal fat pads were digested with collagenase (2 mg/ml solution) in digestion vials containing Krebs-Ringer bicarbonate HEPES (KRBH) buffer (pH 7.4), 200 nM adenosine, and 3.5% BSA. After a 2-h digestion, adipose cells were separated by centrifugation at 1200 rpm for 10 min and washed twice with KRBH buffer. After the final wash, adipose cells were collected for RNA extraction.
Metabolic studies
During the experimental period of rosiglitazone administration, glucose and insulin tolerance tests were conducted by the intraperitoneal (i.p.) injection of glucose and insulin. Mice were deprived of food overnight (12 h) for GTTs and for 6 h for ITTs. GTTs and ITTs were conducted by intraperitoneal (i.p.) injection of glucose (1 mg/g bw) or insulin (0.75 mU/g bw) with blood collection at 0, 15, 30, 60, 90, and 120 min. Blood glucose was measured using an Ascensia glucometer (Bayer Healthcare, Mishawaka, IN, USA).
Triglyceride (TG) and free fatty acid (FFA) measurement
Serum TG and FFA levels were determined by using commercially available kits (Stanbo Laboratory, Boerne, TX, USA). Liver TG content measurement was described previously (7). Lipid extraction was performed using the Bligh-Dyer method (20). Briefly, frozen liver tissue (100 mg) was homogenized in 1 ml water. Lipid was extracted using chloroform:methanol (2:1). An aliquot of the organic phase was collected and dried with nitrogen, and then dissolved in isopropanol alcohol containing 1% Triton. TG content was determined using commercially available kits (Stanbo Laboratory).
FA synthesis in vivo
The rate of de novo lipogenesis was determined by measuring the amount of newly synthesized FAs present in adipose tissue of 14-wk-old WT and Lcn2−/− mice that were fed an HFD for 8 wk, followed by 14 d of Rosi by oral gavage. One hour after i.p. injection of 1 mCi of [3H]H2O in saline, adipose tissue was removed, and total lipids were extracted from a 100-mg portion of tissue in chlorform:methanol (2:1, v/v) (20). Samples were saponified in ethanolic KOH, and sterols were extracted with petroleum ether. Following acidification with acetic acid, FAs were extracted from the same samples with petroleum ether (21). Aliquots of 3H-labeled FAs were counted in a liquid scintillation counter (LS6000IC; Beckman Coulter, Fullerton, CA, USA). The rate of FA synthesis was calculated as detections per minute (dpm) per whole fat-pad tissue.
Relative quantitative real-time RT-PCR
Total RNAs were extracted from tissues and cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. RT-PCR was performed using SYBR GreenER qPCR SuperMix Universal kit (Invitrogen) with an ABI StepOnePlus real-time PCR Systems (Applied Biosystems, Foster City, CA, USA). Primers specific for the examined genes are shown in Supplemental Table S1. Results are presented as levels of expression relative to that of controls after normalizing to β-actin using the ΔΔCt method. Statistical significance was determined by 2-tailed Student's t test.
Western blot analysis
Tissue samples were homogenized and solubilized in RIPA buffer (Sigma, St. Louis, MO, USA). Protein concentrations of homogenized samples were measured using the bicinchoninic acid method (Pierce Chemical Co., Rockford, IL, USA). Equal amounts of proteins were separated on SDS-PAGE and immunoblotted with anti-PPARγ (Cell Signaling, Danvers, MA, USA) and anti-UCP1 (Abcam, Cambridge, MA, USA) antibodies according to the recommendations of the manufacturers. After incubation with primary antibodies, the membranes were incubated with secondary antibodies conjugated to horseradish peroxidase. ECL Western Blotting Detection Systems (GE Healthcare BioSciences, Piscataway, NJ, USA) were used to detect antibody reactivity.
Statistical analysis
Results are expressed as means ± se. Differences in the parameters between WT and Lcn2−/− mice were evaluated using 1-way ANOVA with a 0.05 2-sided significance level. A value of P < 0.05 was considered significant.
RESULTS
LCN2 deficiency does not affect the efficacy of TZD in HFD-induced insulin resistance and dyslipidemia
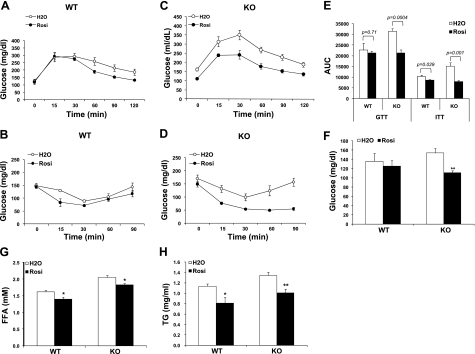
The effect of TZD on HFD-induced insulin resistance in Lcn2−/− mice was evaluated by administrating Rosi to HFD-fed WT and Lcn2−/− mice; GTTs and ITTs were performed after 14 and 20 d of Rosi gavage, respectively. Figure 1A–D illustrates that Rosi treatment effectively reduced glucose intolerance (Fig. 1A, C) and improved insulin sensitivity (Fig. 1B, D) in both WT and Lcn2−/− mice. The results of the GTTs and ITTs, presented as area under the curve (AUC), showed that Rosi effectively exerts its insulin-sensitizing function in Lcn2−/− mice (Fig. 1E). Unfed-state blood glucose levels were also significantly reduced in Lcn2−/− mice after Rosi treatment for 25 d (Fig. 1F). Elevated blood concentrations of FFAs and TGs are important characteristics associated with insulin resistance in HFD-induced obesity. Consistent with our previous report (7), Lcn2−/− mice had higher serum levels of FFAs and TGs than WT mice under the control conditions. Rosi treatment for 25 d led to a significant reduction in serum levels of FFAs and TGs in both WT and Lcn2−/− mice (Fig. 1G, H). These data suggest that Lcn2−/− mice display a normal response to Rosi effect on glucose homeostasis and systemic insulin sensitivity.
Figure 1.
Effect of Rosi on glucose tolerance, insulin sensitivity, and dyslipidemia in Lcn2−/− mice. A, B) Glucose (A) and insulin (B) tolerance tests conduced in WT mice fed an HFD (n=6–8, age=19–20 wk). C, D) Glucose (C) and insulin (D) tolerance tests conduced in Lcn2−/− mice fed an HFD (n=6–8, age=19–20 wk). E) Results of GTTs and ITTs, presented as area under the curve (AUC). F–H) Unfed-state blood glucose levels (F), serum FA level (G) and blood triacylglycerol levels (H). Data are represented as means ± se. KO, Lcn2−/− knockout mice. *P < 0.05; **P < 0.01.
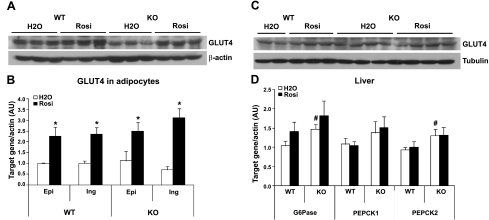
To explore the possible mechanisms for the glucose-lowering effect of Rosi in Lcn2−/− mice, we examined the glucose transporter 4 (GLUT4) protein content in WAT and muscle, Slc2a4 gene expression in inguinal and epididymal adipocytes, and the expression of gluconeogenic genes in the liver. We found that GLUT4 protein content was decreased in WAT of Lcn2−/− mice compared to WT mice; Rosi treatment led to an increase in the GLUT4 protein content in WAT of both WT and Lcn2−/− mice (Fig. 2A). Increased GLUT4 gene expression by Rosi was also observed in epididymal and inguinal adipocytes of WT and Lcn2−/− mice (Fig. 2B). However, GLUT4 protein content in skeletal muscle was not distinguishably changed regardless of genotype and Rosi treatment (Fig. 2C). These results are consistent with the previous reports that TZD was able to restore the reduced GLUT4 protein content in adipose tissue in HFD-induced mice (22) and Zucker obese rats (23).
Figure 2.
Effect of Rosi on GLUT4 protein content, gene expression, and gluconeogenic enzymes in Lcn2−/− mice (n=6). A) GLUT4 protein content in adipose tissue of mice in response to Rosi treatment. B) Gene expression of GLUT4 in epididymal (Epi) and inguinal (Ing) adipocytes from mice without or with Rosi treatment. C) GLUT4 protein content in muscle of mice in response to Rosi treatment. D) Expression levels of gluconeogenic genes in liver of mice in response to Rosi treatment. Gene expression data are represented as means ± se. *P < 0.05 vs. untreated group; #P < 0.05 vs. untreated WT group.
In a previous study, we have shown that Lcn2−/− mice developed more severe fatty liver and had higher expression levels of gluconeogenic genes in the liver as compared to WT mice, indicating hepatic insulin resistance in Lcn2−/− mice. Studies in human patients with type 2 diabetes have reported that the hepatic glucose production in response to TZD treatment was either reduced (24, 25) or unaffected (26) in the patients with type 2 diabetes. In this study, we consistently showed that the expression levels of liver gluconeogenic genes G6p (glucose-6-phosphatase) and ck2 (phosphoenolpyruvate carboxykinase 2) were higher in Lcn2−/− mice compared to WT mice. However, Rosi treatment did not alter the mRNA expression levels of G6p, ck1, and ck2, regardless of LCN2 deficiency (Fig. 2D). These results suggest that the increased glucose uptake, rather than the hepatic glucose production, is the major contributor to the glucose-lowering effects of Rosi in Lcn2−/− mice.
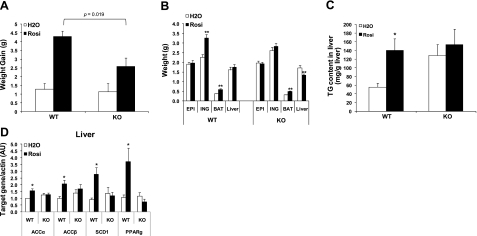
LCN2 deficiency diminishes TZD-induced weight gain, fat-mass increase, and liver lipid accumulation
It has been documented that insulin sensitization with TZD is associated with favored anabolic metabolism, which promotes the uptake and storage of FFAs in the form of triacylglycerol in PPARγ-activated tissues leading to a reduction in circulating FAs and the improvement of insulin resistance. This anabolic metabolic state also results in weight gain, increased subcutaneous fat mass, and liver lipid accumulation. WAT is the main target tissue of PPARγ activation for lipid storage, while BAT is the major thermogenic site for energy expenditure. Consistently, we have shown that Rosi administration significantly increased body weight gain (Fig. 3A), as well as inguinal and BAT fat mass (Fig. 3B) in WT mice when compared with the untreated control mice. In both WT and Lcn2−/− mice, epididymal fat-pad weight was unchanged in response to Rosi treatment. Interestingly, Rosi-induced weight gain and inguinal fat-mass increase were almost completely abolished; instead, liver weight was significantly reduced after Rosi treatment in Lcn2−/− mice (Fig. 3A, B). As previously observed (7), Lcn2−/− mice have elevated liver TG content compared to WT mice in the untreated condition (Fig. 3C). A 25-d treatment of Rosi induced 2-fold increase in liver TG content in WT mice (Fig. 3C), which is similar to a previous report in ob/ob mice (27). However, a Rosi-induced increase in liver TG content was not perceived in Lcn2−/−mice (Fig. 3C). Consistent with TG accumulation in liver, Rosi treatment markedly induced the expression of lipogenic genes, including Acaca, Acacb, Scd1, and Pparg, in the liver of WT mice, while the expression levels of these genes were not significantly altered in response to Rosi treatment in Lcn2−/− mice (Fig. 3D). The failure of Rosi stimulation to induce fat-mass increase in WAT and lipogenic gene expression in the liver suggests that LCN2 is an important mediator for anabolic metabolism from PPARγ activation in adipose tissue and the liver.
Figure 3.
Effect of Rosi on body weight, fat-pad weight, and liver lipid accumulation in Lcn2−/− mice (n=13–16). Body weight gain (A), weight of fat pads and liver (B), liver TG content (C), and expression levels of lipogenic genes in liver (D) in response to Rosi treatment. Gene expression data are represented as means ± se. *P < 0.05, **P < 0.01 vs. untreated group.
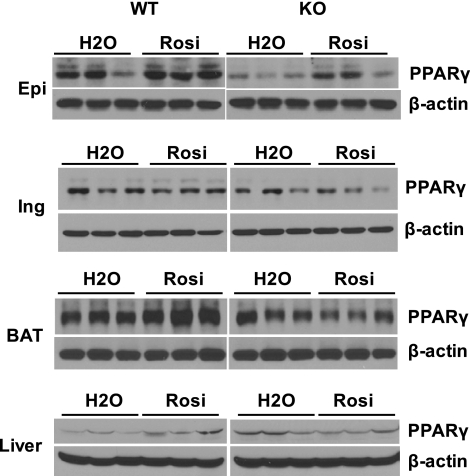
To establish the mechanism for the diminished increase in fat mass and lipid accumulation in the liver in the absence of LCN2, the protein expression levels of PPARγ, the key gene involved in adipogenesis and lipid metabolism, were extensively examined in different adipose depots, BAT, and liver using Western blot analysis. As illustrated in Fig. 4, in the untreated condition, PPARγ protein expression levels were significantly decreased in epididymal adipose tissue but slightly reduced in inguinal tissue and BAT in Lcn2−/− mice compared to WT controls, while Lcn2−/− liver expressed higher levels of PPARγ protein as compared with WT mice (Fig. 4). Notably, the response of these tissues to Rosi-stimulated PPARγ expression was altered in Lcn2−/− mice. Rosi treatment led to a significant increase in PPARγ expression in epididymal adipose tissue, BAT, and liver in WT mice. However, in Lcn2−/− mice, this response was observed only in epididymal adipose tissue but vanished in BAT, inguinal adipose tissue, and liver (Fig. 4). Rosi did not change inguinal adipose tissue PPARγ expression in either WT or Lcn2−/− mice.
Figure 4.
Effect of Rosi treatment on PPARγ protein expression in epididymal tissue (Epi), inguinal tissue (Ing), BAT, and liver in WT and Lcn2−/− mice fed an HFD.
LCN2 deficiency alters the TZD effects on lipogenesis and FA homeostasis in adipose tissue
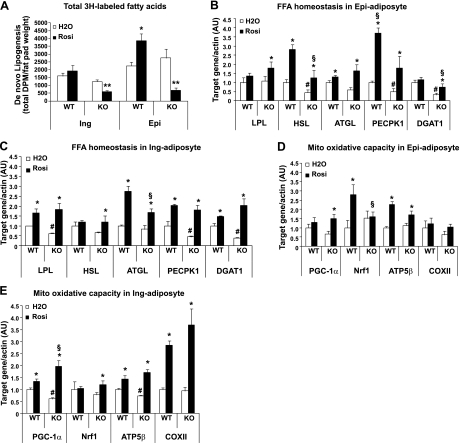
To explore the mechanism behind Rosi lowering FFA and blunting fat-mass increase, we examined the effect that Rosi had on FA homeostasis in Lcn2−/− mice. First, we assessed Rosi effect on de novo lipogenesis by measuring the rate of in vivo incorporation of [3H] H2O into FAs in adipose tissue of HFD-fed WT and Lcn2−/− mice receiving 14 d of Rosi treatment. As illustrated in Fig. 5A, Rosi treatment resulted in a significant increase in 3H-labeled FAs in epididymal adipose tissue and a slight increase in inguinal adipose tissue of WT mice. Most strikingly, in Lcn2−/− mice, Rosi markedly reduced the synthesis of 3H-labeled FAs in epididymal and inguinal adipose tissue, suggesting that Lcn2 deficiency completely blocks or even reverses Rosi-induced increase in adipose tissue de novo lipogenesis.
Figure 5.
Effect of Rosi on adipose tissue de novo lipogenesis and the expression of genes involved in FA homeostasis and mitochondrial function in Lcn2−/− mice (n=6–8). A) Total 3H-labeled FAs in adipose tissue (n=5). B–E) Expression of genes involved in FA homeostasis and mitochondrial function in epididymal (Epi) adipocytes (B, D) and inguinal (Ing) adipocytes (C, E). Data are represented as mean ± se. *P < 0.05, **P < 0.01 vs. untreated group; #P < 0.05 vs. untreated WT group; §P < 0.05 vs. Rosi-treated WT group.
Fatty acid homeostasis in adipose tissue plays a very important role in controlling circulating FFAs and body fat mass. We next sought to elucidate the possible mechanisms for FFA-lowering effects of Rosi in Lcn2−/− mice and examined the expression of genes involved in FA recycling, esterification, and FA oxidation in epididymal and inguinal adipocytes. LPL is a key enzyme regulating circulating TG hydrolysis, while ck1 is important for FA recycling through glyceroneogensis. We showed that the expression levels of LPL and ck1 genes in inguinal (Fig. 5C) and ck1 gene in epididymal adipocytes (Fig. 5B) were significantly reduced in Lcn2−/− mice when compared with those in WT mice. Rosi administration to Lcn2−/− mice was able to significantly restore LPL expression to the level in WT epididymal (Fig. 5B) and inguinal adipocytes (Fig. 5C). In Lcn2−/− inguinal adipocytes, Rosi was also able to bring the decreased ck1 mRNA expression back to the level comparable to that in WT inguinal adipocytes (Fig. 5C). However, in Lcn2−/− epididymal adipocytes, Rosi only raised ck1 expression to 50% of the level in WT epididymal adipocytes (Fig. 5B).
We then further examined the ramification of LCN2 deficiency on Rosi-induced FA esterification/TAG synthesis in adipocytes. Diacylglycerol O-acyltransferase 1 (DGAT1) is a rate-limiting enzyme responsible for the final step of TG synthesis, and PPARγ activation leads to the up-regulation of Dgat1 expression in adipocytes and WAT (28–29). In Lcn2−/− mice, Dgat1 gene expression was markedly down-regulated in both epididymal (Fig. 5B) and inguinal adipocytes (Fig. 5C) compared to WT mice. Rosi treatment significantly augmented Dgat1 gene expression in inguinal but not in epididymal adipocytes in WT mice, which supports the report that TZD induces fat gain selectively in subcutaneous fat depots. In the absence of LCN2, Rosi treatment restored Dgat1 expression significantly in Lcn2−/− inguinal adipocytes (Fig. 5B), but only 50% in epididymal adipocytes (Fig. 5C). The above data of ck1 and Dgat1 gene expression patterns suggest that Rosi-stimulated FA recycling and FA esterification (TAG synthesis) remain intact in Lcn2−/− inguinal adipocytes but was impaired in epididymal adipocytes.
We next determined whether Lcn2−/− mediates the effects of Rosi on the expression of genes involved in TG hydrolysis and FA oxidation in adipocytes, which are important pathways in controlling FA/TG homeostasis in adipocytes. As shown in Fig. 5B, C, hormone-sensitive lipase (Lipe) gene expression was significantly down-regulated in Lcn2−/− epididymal and inguinal (marginally decreased) adipocytes compared to WT adipocytes. After Rosi treatment, Lipe and adipose TG lipase (Pnpla2) gene expression was significantly up-regulated in Lcn2−/− epididymal and inguinal adipocytes. However, Rosi did not affect Lipe gene expression in WT inguinal adipocytes (Fig. 5C). In addition, in Lcn2−/− mice, the expression of genes having key roles in mitochondrial biogenesis and FA oxidation, including peroxisome proliferator-activated receptor-γ coactivator 1α (Ppargcla), nuclear respiratory factor 1(Nrf1), ATP synthase β subunit (Atp5β), and cytochrome-c oxidase subunits II (mt-Co2) were markedly up-regulated in inguinal adipocytes in response to Rosi treatment, similar to that observed in WT mice; the expression levels of Pparcla were even higher than those in WT mice (Fig. 5E). A significant increase in Pparcla and Atp5β gene expression by Rosi was also observed in Lcn2−/− epididymal adipocytes (Fig. 5D). These results indicate that LCN2 deficiency does not alter the stimulatory effects of Rosi on FA recycling and esterification, mitochondrial biogenesis, and FA oxidation, which are significant contributors to the FFA-lowering effect of Rosi in WT mice.
LCN2 deficiency markedly enhances TZD-induced BAT thermogenic capacity and energy expenditure
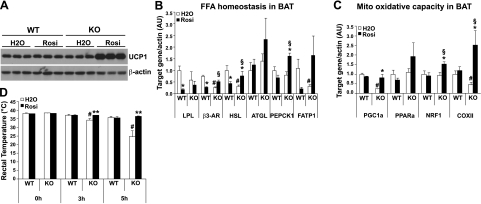
BAT plays an important role in the regulation of energy expenditure via a thermogenic mechanism, particularly in small mammals. TZD stimulation of BAT thermogenesis or energy expenditure has not been well determined, as in some cases, TZD administration to rodents and humans failed to induce functional thermogenesis (13, 30). To explore the mechanism for TZD effects on the improvement of systemic insulin resistance and the reduction in circulating FAs in Lcn2−/− mice, BAT thermogenesis was examined in mice treated with or without Rosi. Our results showed that the protein expression levels of UCP-1, a key thermogenic regulator, were not significantly changed in BAT of HFD-fed WT mice after Rosi administration for 25 d, while Rosi treatment markedly induced the expression of BAT UCP-1 protein in Lcn2−/− mice fed a HFD (Fig. 6A).
Figure 6.
Effect of Rosi on the expression of UCP-1 protein and mitochondrial genes in BAT and thermogenic function of BAT in Lcn2−/− mice. A) UCP-1 protein expression in response to Rosi in BAT; results represent 3 mice from one of 2 independent experiments conducted on 2 independent sets of mice (n=5–8). B, C) Expression of genes in involved in FA homeostasis (B) and mitochondrial function (C) in response to Rosi in BAT (n=6). D) Effect of Rosi on adaptive thermogenesis in Lcn2−/− mice. Body temperature of Lcn2−/− mice and WT mice with or without Rosi treatment (n=5) exposed to 4°C. Data are represented as means ± se. *P < 0.05, **P < 0.01 vs. untreated group; #P < 0.05 vs. untreated WT group; §P < 0.05 vs. Rosi-treated WT group.
We next assessed the oxidative capacity of BAT by examining the expression of key genes controlling FA availability and β-oxidation, as well as mitochondrial biogenesis and function. Interestingly, Rosi differentially regulated the expression of genes involved in FA homeostasis, such as Lipe, Lpl, ck1, β3 adrenergic receptor (Adrb3), and FA transport protein 1 (Slc27a1), in BAT in WT and Lcn2−/− mice (Fig. 6B). For example, the expression of all these genes was down-regulated by Rosi in WT BAT, but up-regulated in Lcn2−/− BAT (Fig. 6B). This suggests that Rosi promotes anabolic lipid metabolism in WT BAT, while in Lcn2−/− BAT, Rosi increases the intracellular pool of FAs by stimulating the release of FAs from the intracellular TG, increasing FA recycling, and increasing FA uptake from the circulation.
We further hypothesized that Rosi enhances the mitochondrial oxidative capacity to utilize the increased amount of intracellular FAs in BAT, which may account for the effect of Rosi to lower circulating FFAs and improve insulin sensitivity in Lcn2−/− mice. Our results showed that the expression levels of the genes responsible for mitochondrial biogenesis and FA oxidation, including Ppargcla, Ppara, Nrf1, and mt-Co2, were significantly increased in BAT of LCN2−/− mice treated with Rosi for 25 d compared to untreated mice (Fig. 6C), whereas Rosi treatment did not alter the expression of these genes in BAT of HFD-fed WT mice. Finally, the thermogenic function of BAT was evaluated in mice after Rosi treatment. Mice were exposed to 4°C for 5 h, and rectal temperature was measured every 30 min. As reported previously (7), body temperature of Lcn2−/− mice with no treatment significantly dropped within 5 h compared with WT mice (Fig. 6D). Strikingly, Lcn2−/− mice with Rosi treatment were able to maintain body temperature at the level similar to WT mice (Fig. 6D). In accordance with the higher levels of UCP-1 protein expression, these results proved that Rosi increases the FA availability and β-oxidation capacity of BAT in Lcn2−/− mice.
DISCUSSION
LCN2, as a member of the lipocalin subfamily with a unique hydrophobic binding pocket, is postulated to serve as an efficient transporter for lipophilic molecules. However, the specific ligands for LCN2 are not well characterized. It is reasonable to hypothesize that LCN2 transports the ligands controlling the activation of nuclear receptors, including PPARγ. In the absence of LCN2, mice developed insulin resistance, dyslipidemia, and fatty liver disease, and showed decreased adipose PPARγ expression (7). These observations led to the hypothesis that LCN2 is important for the full action of PPARγ agonists in the improvement of the diabetic state. To test the hypothesis, we examined the effect and mechanism of TZD on insulin sensitization, lipogenesis, and energy utilization in Lcn2−/− mice. We found that TZD could still effectively improve insulin resistance in Lcn2−/− mice. However, LCN2 deficiency significantly abolished TZD-induced weight gain, fat-mass increase, and liver lipid accumulation, but markedly augmented BAT thermogenesis and energy utilization through increasing mitochondrial biogenesis and oxidative capacity.
As full PPARγ agonists, TZDs improve systemic insulin sensitivity and induce adipocyte differentiation and lipogenesis, which promotes lipid storage and inevitably leads to weight gain and increased fat mass. Several studies in humans have demonstrated that TZD treatment stimulates fat gain selectively in subcutaneous regions (31, 32); the mechanism behind this has been associated with increased stimulation of the FA uptake and esterification, as well as relatively lower rates of FA oxidation in the subcutaneous fat depot compared to the epididymal fat depot (33). In addition, TZD treatment increases liver TG content in HFD-induced obese models (27) or causes hepatocirrhosis in humans (34), which significantly restricts the clinical use of TZDs. Many efforts have been made to determine alternative agents that could reduce or abolish these side effects, but maintain the antidiabetic effects. Several new non-TZD selective partial agonists of PPARγ have been developed having effective antidiabetic action but diminished weight gain and fat-mass increase. MBX-102, one of the partial PPARγ agonists, exhibits full therapeutic activity without stimulating weight gain and lipid accumulation; which is shown to be associated with its weak transactivation but strong transrepression activity of PPARγ (12). These studies suggest that PPARγ activity could be selectively activated in different tissues. Indeed, PPARγ activation is regulated via a complicated transactivation and transrepression mechanism involving a variety of ligands, coactivators, and corepressors (8). Because LCN2 serves as a putative transporter for hydrophobic ligands, it is likely that LCN2 is critical for the regulation of PPARγ activation.
In this study, we found that in the absence of LCN2, Rosi treatment could maintain a full insulin-sensitizing effect and antidyslipidemic action but significantly diminish weight gain and subcutaneous fat-mass increase in HFD-induced obesity. Consistent with the increased adipogenesis and liver lipid accumulation, Rosi treatment also increased adipose tissue de novo lipogenesis and the expression of adipogenic/lipogenic genes in adipose tissue (data not shown) and the liver of WT mice. However, in Lcn2−/− mice, Rosi treatment led to a marked reduction in adipose tissue de novo lipogenesis; Rosi-induced lipid accumulation and lipogenic gene expression in the liver vanished. These results suggest that the decreased lipogenesis in Rosi-treated Lcn2−/− mice may be a significant contributor to the decreased body weight and fat gain.
Elevation of circulating FAs is a critical contributor to insulin resistance. Promoting lipid storage in adipose tissue is believed to be an important mechanism for the antidiabetic and FA-lowering effects of TZDs. In the present study, Rosi significantly decreased serum levels of FFAs and TGs in Lcn2−/− mice as effectively as in WT mice, without significantly increasing fat mass. ck1 and Dgat1 are the PPARγ target genes encoding two important enzymes with activities that modulate FFA and TG levels in the circulation and adipose tissue via regulating glyceroneogenesis and FA esterification, respectively. The up-regulation of ck1 and Dgat1 expression in adipose tissue has been associated with fat depot expansion and improvement of insulin sensitivity in mice (29). TZD treatment induces ck1 expression, leading to the suppression of FA release from adipocytes (35); increased ck1 expression in adipose tissue has been reported to contribute to the FA-lowering effect of TZD in type 2 diabetes (36). As a PPARγ target gene, the Dgat1 expression was also up-regulated in adipose tissue in response to the TZD activation of PPARγ (28–29). Lower expression levels of Dgat1 in adipose tissue were observed in subjects with impaired glucose tolerance compared to normal glucose-tolerant subjects (28). Overexpression of Dgat1 in WAT resulted in increased fat mass but protected mice from HFD-induced insulin resistance (37). Our results showed that epididymal and inguinal adipocytes expressed significantly lower levels of Lpl, ck1, and Dgat1 genes in Lcn2−/− mice compared to WT mice. The effectiveness of Rosi-stimulated expression of ck1 and Dgat1 was blunted in epididymal adipocytes but remains intact in inguinal adipocytes in the absence of LCN2. These data suggest that the effect of LCN2 deficiency on Rosi action in FA homeostasis in adipose tissue is depot specific.
In addition to FA uptake (recycling) and TG synthesis, the rates of TG hydrolysis and FA oxidation are important regulators of TG levels and FA homeostasis in adipocytes. TZD stimulation of FA uptake and oxidation in adipose tissue has been previously reported in type 2 diabetic patients (38). Our results have suggested that Rosi similarly induces the expression of genes involved in TG hydrolysis, mitochondrial biogenesis, and FA oxidation in WT and Lcn2−/− inguinal and epididymal adipocytes, with the exception that Lcn2−/− inguinal adipocytes expressed significantly higher levels of Ppargcla than WT inguinal adipocytes. The data suggest that Rosi-increased mitochondrial biogenesis and FA oxidation in WAT are still effective in Lcn2−/− mice, which is another important mechanism for the FFA-lowering effect of Rosi in Lcn2−/− mice.
In contrast to the role of WAT as an energy storage site, the main function of BAT is to expend energy as a means to control body weight and produce heat through nonshivering thermogenesis during the cold conditions through uncoupling proteins. However, the concept of UCP1 as an important target for antidiabetes treatment is controversial. For example, in obese Zucker rats and obese KKAy mice, TZD treatment reversed insulin resistance but was unable to induce UCP1 expression in BAT and increase energy expenditure (13, 30). Consistent with these observations, our results showed that Rosi failed to significantly induce UCP1 expression in BAT in WT mice. Noticeably, in Lcn2−/− mice, Rosi dramatically enhanced UCP1 protein expression levels, suggesting that Rosi increases UCP1-mediated thermogenesis and energy expenditure in BAT in Lcn2−/− mice. The following evidence further supports this finding. First, we showed that Rosi significantly up-regulated the expression of genes controlling the FA availability for oxidation, such as Adrb3, Lipe, ck1, and Slc27a1, in BAT in Lcn2−/− mice compared to unaltered or reduced expression of these genes in WT mice. Second, the expression of genes involved in mitochondrial biogenesis and oxidative function, such as Ppargcla, Ppara, Nrf1, and mt-Co2, were also up-regulated by Rosi in BAT of Lcn2−/− mice. These data suggest that Rosi promotes more FA uptake and higher TG hydrolysis activity to make more FAs available for oxidation, which is coupled with increased mitochondrial oxidative capacity in BAT in Lcn2−/− mice when compared with WT mice. Finally, we proved that Rosi enhances BAT thermogenesis in Lcn2−/− mice at the functional level. Rosi treatment completely rescued the cold intolerance of Lcn2−/− mice. All these data together demonstrate that the improved energy (FA) utilization and increased energy expenditure in BAT are the main mechanism for the FFA-lowering and insulin-sensitizing effects of Rosi in Lcn2−/− mice, which is distinct from the anabolic mechanism by which Rosi exerts its effects in WT mice.
Our results, combined with previous studies using partial PPARγ agonists, have clearly demonstrated that lipogenesis/adipogenesis and insulin sensitivity are differentially regulated by PPARγ activation; PPARγ activation requires ligands and coactivator binding, suggesting that LCN2 may interfere with PPARγ activation at the level of the recruitment of coactivators/corepressors. It is likely that in the absence of LCN2, PPARγ is only partially or selectively activated by TZD. Our results also indicate that increasing adipogenesis and fat storage in adipose tissue are not tightly correlated with the insulin-sensitizing action of TZD or insulin sensitivity in many other situations; however, increasing energy utilization, fat oxidation, and lowering FFA levels are more important for insulin sensitization.
In summary, we have described here that in the absence of LCN2, Rosi improves insulin sensitivity through a distinct mechanism; Rosi exerts its full insulin sensitization activity, with reduced fat gain. LCN2 deficiency impairs Rosi effect on lipogenesis, but increases Rosi-induced FA uptake and FA oxidation in BAT. Enhanced BAT thermogenesis, increased FA oxidation in adipose tissue, and blunted lipogenesis in the liver and adipose tissue together contribute to Rosi effects on insulin sensitization and lowering circulating FAs, with reduced fat gain in Lcn2−/− mice. Our data suggest that LCN2 is an important regulator for the activation of PPARγ that controls adipogenesis and lipogenesis in adipose tissue and liver. In this fashion, LCN2 is a very attractive marker for the developing and screening of antidiabetic drugs.
Supplementary Material
Acknowledgments
The project described was supported by grant R01DK080743 (to X.C.) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the National Institutes of Health. The authors thank Wendy Wright for assistance in mouse breeding and colony maintenance and Dr. David A. Bernlohr (Department of Biochemistry, Molecular Biology, and Biophysics, University of Minnesota) for providing the critical comments on the manuscript.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Akerstrom B., Flower D. R., Salier J. P. (2000) Lipocalins: unity in diversity. Biochim. Biophys. Acta 1482, 1–8 [DOI] [PubMed] [Google Scholar]
- 2. Liu Q., Nilsen-Hamilton M. (1995) Identification of a new acute phase protein. J. Biol. Chem. 270, 22565–22570 [DOI] [PubMed] [Google Scholar]
- 3. Devireddy L. R., Teodoro J. G., Richard F. A., Green M. R. (2001) Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science 293, 829–834 [DOI] [PubMed] [Google Scholar]
- 4. Yan Q. W., Yang Q., Mody N., Graham T. E., Hsu C. H., Xu Z., Houstis N. E., Kahn B. B., Rosen E. D. (2007) The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 56, 2533–2540 [DOI] [PubMed] [Google Scholar]
- 5. Zhang J., Wu Y., Zhang Y., Leroith D., Bernlohr D. A., Chen X. (2008) The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol. Endocrinol. 22, 1416–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tan B. K., Adya R., Shan X., Syed F., Lewandowski K. C., O'Hare J. P., Randeva H. S. (2009) Ex vivo and in vivo regulation of lipocalin-2, a novel adipokine, by insulin. Diabetes Care 32, 129–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guo H., Jin D., Zhang Y., Wright W., Bazuine M., Brockman D. A., Bernlohr D. A., Chen X. (2010) Lipocalin 2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes 59, 1376–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenfeld M. G., Lunyak V. V., Glass C. K. (2006) Sensors and signals: A coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 20, 1405–1428 [DOI] [PubMed] [Google Scholar]
- 9. Rennings A. J., Smits P., Stewart M. W., Tack C. J. (2006) Fluid retention and vascular effects of rosiglitazone in obese, insulin-resistant, nondiabetic subjects. Diabetes Care 29, 581–587 [DOI] [PubMed] [Google Scholar]
- 10. Guan Y., Hao C., Cha D. R., Rao R., Lu W., Kohan D. E., Magnuson M. A., Redha R., Zhang Y., Breyer M. D. (2005) Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat. Med. 11, 861–866 [DOI] [PubMed] [Google Scholar]
- 11. Reginato M. J., Bailey S. T., Krakow S. L., Minami C., Ishii S., Tanaka H., Lazar M. A. (1998) A potent antidiabetic thiazolidinedione with unique peroxisome proliferator-activated receptor gamma-activating properties. J. Biol. Chem. 273, 32679–32684 [DOI] [PubMed] [Google Scholar]
- 12. Gregoire F. M., Zhang F., Clarke H. J., Gustafson T. A., Sears D. D., Favelyukis S., Lenhard J., Rentzeperis D., Clemens L. E., Mu Y., Lavan B. E. (2009) MBX-102/JNJ39659100, a novel peroxisome proliferator-activated receptor-ligand with weak transactivation activity retains antidiabetic properties in the absence of weight gain and edema. Mol. Endocrinol. 23, 975–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fukui Y., Masui S., Osada S., Umesono K., Motojima K. (2000) A new thiazolidinedione, NC-2100, which is a weak PPAR-gamma activator, exhibits potent antidiabetic effects and induces uncoupling protein 1 in white adipose tissue of KKAy obese mice. Diabetes 49, 759–767 [DOI] [PubMed] [Google Scholar]
- 14. Tai T. A., Jennermann C., Brown K. K., Oliver B. B., MacGinnitie M. A., Wilkison W. O., Brown H. R., Lehmann J. M., Kliewer S. A., Morris D. C., Graves R. A. (1996) Activation of the nuclear receptor peroxisome proliferator-activated receptor gamma promotes brown adipocyte differentiation. J. Biol. Chem. 271, 29909–29914 [DOI] [PubMed] [Google Scholar]
- 15. Rabelo R., Camirand A., Silva J. E. (1997) 3′,5′-cyclic adenosine monophosphate-response sequences of the uncoupling protein gene are sequentially recruited during darglitazone-induced brown adipocyte differentiation. Endocrinology 138, 5325–5332 [DOI] [PubMed] [Google Scholar]
- 16. Camirand A., Marie V., Rabelo R., Silva J. E. (1998) Thiazolidinediones stimulate uncoupling protein-2 expression in cell lines representing white and brown adipose tissues and skeletal muscle. Endocrinology 139, 428–431 [DOI] [PubMed] [Google Scholar]
- 17. Matsuda J., Hosoda K., Itoh H., Son C., Doi K., Hanaoka I., Inoue G., Nishimura H., Yoshimasa Y., Yamori Y., Odaka H., Nakao K. (1998) Increased adipose expression of the uncoupling protein-3 gene by thiazolidinediones in wistar fatty rats and in cultured adipocytes. Diabetes 47, 1809–1814 [DOI] [PubMed] [Google Scholar]
- 18. Xiang C. C., Wu Y. J., Ma L., Ding L., Lisinski I., Brownstein M. J., Cushman S. W., Chen X. (2007) Characterisation of insulin-resistant phenotype of cultured rat primary adipose cells. Diabetologia 50, 1070–1079 [DOI] [PubMed] [Google Scholar]
- 19. Weber T.M., Joost H.G., Simpson I.A., Cushman S.W. (1988) The Insulin Receptor, A. R. Liss, New York [Google Scholar]
- 20. Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 21. Dietschy J. M., Spady D. K. (1984) Measurement of rates of cholesterol synthesis using tritiated water. J. Lipid Res. 25, 1469–1476 [PubMed] [Google Scholar]
- 22. Lessard S. J., Rivas D. A., Chen Z. P., Bonen A., Febbraio M. A., Reeder D. W., Kemp B. E., Yaspelkis B. B., 3rd, Hawley J. A. (2007) Tissue-specific effects of rosiglitazone and exercise in the treatment of lipid-induced insulin resistance. Diabetes 56, 1856–1864 [DOI] [PubMed] [Google Scholar]
- 23. Kramer D., Shapiro R., Adler A., Bush E., Rondinone C. M. (2001) Insulin-sensitizing effect of rosiglitazone (BRL-49653) by regulation of glucose transporters in muscle and fat of zucker rats. Metabolism 50, 1294–1300 [DOI] [PubMed] [Google Scholar]
- 24. Miyazaki Y., Glass L., Triplitt C., Matsuda M., Cusi K., Mahankali A., Mahankali S., Mandarino L. J., DeFronzo R. A. (2001) Effect of rosiglitazone on glucose and non-esterified fatty acid metabolism in type II diabetic patients. Diabetologia 44, 2210–2219 [DOI] [PubMed] [Google Scholar]
- 25. Gastaldelli A., Miyazaki Y., Pettiti M., Santini E., Ciociaro D., Defronzo R. A., Ferrannini E. (2006) The effect of rosiglitazone on the liver: decreased gluconeogenesis in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 91, 806–812 [DOI] [PubMed] [Google Scholar]
- 26. Miyazaki Y., Mahankali A., Matsuda M., Glass L., Mahankali S., Ferrannini E., Cusi K., Mandarino L. J., DeFronzo R. A. (2001) Improved glycemic control and enhanced insulin sensitivity in type 2 diabetic subjects treated with pioglitazone. Diabetes Care 24, 710–719 [DOI] [PubMed] [Google Scholar]
- 27. Chao L., Marcus-Samuels B., Mason M. M., Moitra J., Vinson C., Arioglu E., Gavrilova O., Reitman M. L. (2000) Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. J. Clin. Invest. 106, 1221–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ranganathan G., Unal R., Pokrovskaya I., Yao-Borengasser A., Phanavanh B., Lecka-Czernik B., Rasouli N., Kern P. A. (2006) The lipogenic enzymes DGAT1, FAS, and LPL in adipose tissue: Effects of obesity, insulin resistance, and TZD treatment. J. Lipid. Res. 47, 2444–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim J. Y., van de Wall E., Laplante M., Azzara A., Trujillo M. E., Hofmann S. M., Schraw T., Durand J. L., Li H., Li G., Jelicks L. A., Mehler M. F., Hui D. Y., Deshaies Y., Shulman G. I., Schwartz G. J., Scherer P. E. (2007) Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117, 2621–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burkey B. F., Dong M., Gagen K., Eckhardt M., Dragonas N., Chen W., Grosenstein P., Argentieri G., de Souza C. J. (2000) Effects of pioglitazone on promoting energy storage, not expenditure, in brown adipose tissue of obese fa/fa Zucker rats: Comparison to CL 316,243. Metab. Clin. Exp. 49, 1301–1308 [DOI] [PubMed] [Google Scholar]
- 31. Mori Y., Murakawa Y., Okada K., Horikoshi H., Yokoyama J., Tajima N., Ikeda Y. (1999) Effect of troglitazone on body fat distribution in type 2 diabetic patients. Diabetes Care 22, 908–912 [DOI] [PubMed] [Google Scholar]
- 32. Smith S. R., De Jonge L., Volaufova J., Li Y., Xie H., Bray G. A. (2005) Effect of pioglitazone on body composition and energy expenditure: A randomized controlled trial. Metabolism 54, 24–32 [DOI] [PubMed] [Google Scholar]
- 33. Laplante M., Festuccia W. T., Soucy G., Gelinas Y., Lalonde J., Berger J. P., Deshaies Y. (2006) Mechanisms of the depot specificity of peroxisome proliferator-activated receptor gamma action on adipose tissue metabolism. Diabetes 55, 2771–2778 [DOI] [PubMed] [Google Scholar]
- 34. Kohlroser J., Mathai J., Reichheld J., Banner B. F., Bonkovsky H. L. (2000) Hepatotoxicity due to troglitazone: Report of two cases and review of adverse events reported to the united states food and drug administration. Am. J. Gastroenterol. 95, 272–276 [DOI] [PubMed] [Google Scholar]
- 35. Tordjman J., Chauvet G., Quette J., Beale E. G., Forest C., Antoine B. (2003) Thiazolidinediones block fatty acid release by inducing glyceroneogenesis in fat cells. J. Biol. Chem. 278, 18785–18790 [DOI] [PubMed] [Google Scholar]
- 36. Cadoudal T., Blouin J. M., Collinet M., Fouque F., Tan G. D., Loizon E., Beale E. G., Frayn K. N., Karpe F., Vidal H., Benelli C., Forest C. (2007) Acute and selective regulation of glyceroneogenesis and cytosolic phosphoenolpyruvate carboxykinase in adipose tissue by thiazolidinediones in type 2 diabetes. Diabetologia 50, 666–675 [DOI] [PubMed] [Google Scholar]
- 37. Koliwad S. K., Streeper R. S., Monetti M., Cornelissen I., Chan L., Terayama K., Naylor S., Rao M., Hubbard B., Farese R. V., Jr. (2010) DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J. Clin. Invest. 120, 756–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boden G., Homko C., Mozzoli M., Showe L. C., Nichols C., Cheung P. (2005) Thiazolidinediones upregulate fatty acid uptake and oxidation in adipose tissue of diabetic patients. Diabetes 54, 880–885 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.