Abstract
Metastasis accounts for the majority of cancer-related deaths. Accurate prediction of metastatic potential of tumors has been elusive, and the search for clinically useful markers continues. We previously reported that GIV/Girdin triggers tumor cell migration by virtue of a C-terminal guanine-nucleotide exchange factor motif that activates Gαi. Here we identify GIV as a metastasis-related protein whose full-length transcript (GIV-fl) is expressed exclusively in highly invasive colon, breast, and pancreatic carcinoma cells and not in their poorly invasive counterparts. A prospective, exploratory biomarker study conducted on a cohort of 56 patients with stage II colorectal cancer revealed a significant correlation between GIV-fl expression in tumor epithelium and shortened metastasis-free survival. Survival rate for patients with GIV-fl-positive tumors is significantly reduced compared with the patients with GIV-fl-negative tumors [P<0.0001; hazard ratio=0.076; CI=0.052–0.30 (95%)]. At the 5-yr mark, survival is 100% in the GIV-fl-negative group and 62 ± 9% (mean±se; P=6×10−5) in the GIV-fl-positive group. Furthermore, GIV-fl expression predicts a risk of mortality independent of the microsatellite stability status, a well-established prognosticator of colorectal cancers. We conclude that GIV-fl is a novel metastasis-related protein and an independent adverse prognosticator that may serve as a useful adjunct to traditional staging strategies in colorectal carcinoma.—Garcia-Marcos, M., Jung, B. H., Ear, J., Cabrera, B., Carethers, J. M., Ghosh, P. Expression of GIV/Girdin, a metastasis-related protein, predicts patient survival in colon cancer.
Keywords: PI3K-Akt, guanine-nucleotide exchange factor, cell migration, growth factor receptors, cytoskeleton, G protein, microsatellite instability
Metastasis is a multistep, complex process that involves migration of tumor cells to distant locations in the body. Cancer cells that migrate or invade utilize chemotactic receptors to sense growth factor gradients and must amplify PI3K-Akt signaling (1). In human epithelial cancers, the PI3K-Akt signaling pathway is frequently hyperactivated during cancer invasion (2), and progressive enhancement of PI3K-Akt coupled to efficient cell migration is a hallmark of high metastatic potential (3). However, accurate prediction of metastatic potential of tumors using mechanistically identified biological markers has met limited success (4). Despite many recent studies (5) showing that the expression of genes/proteins associated with PI3K-Akt signaling, actin remodeling, motility, and invasion vary among tumors, most of them have failed to make a transition into cancer clinics as biomarkers for prognostication. Thus, the search continues for molecules/markers that are proven to play a critical role during tumor invasion, that have well-characterized mechanisms of action, and that may also serve to stratify the risk of metastasis among cancer patients in clinics.
We recently discovered (6, 7) a molecular complex comprised of a trimeric G-protein-, Gαi-, and Gα-interacting, vesicle-associated protein (GIV or Girdin) that is required for growth factors [EGF (8, 9), IGF (10), VEGF (11), and insulin (6, 9, 12)] to enhance Akt, remodel actin, and trigger cell migration. Subsequently, we reported that GIV is a nonreceptor guanine-nucleotide exchange factor (GEF) for Gαi and that a unique GEF motif in GIV's C terminus (see Fig. 1A) is required for activation of Gαi (6). By activating Gαi and releasing “free” Gβγ, GIV amplifies Akt signaling via the Gβγ-PI3K pathway (6). Recently, we (13) gained insights into how multiple growth factor receptors enhance PI3K-Akt signals via GIV. With the use of epidermal growth factor receptor (EGFR), the prototype member of the growth factor receptor tyrosine kinase family, we demonstrated that GIV's C terminus directly binds the autophosphorylated cytoplasmic tails of EGFR and thereby links G-protein to ligand-activated receptors. When GIV's C terminus is intact, a Gαi-GIV-EGFR signaling complex is assembled, EGFR autophosphorylation is enhanced, and the receptor's association with the plasma membrane (PM) is prolonged. Accordingly, PM-based signals that trigger motility (PI3K-Akt and PLCγ1) are amplified, actin is remodeled, and cell migration is triggered (13). Thus, GIV's C terminus serves as a common platform that links ligand-activated receptors (13) at the leading edge to actin (8), Akt (8, 12), and Gαi (6), 3 components the interplay of which is essential for cell migration.
Figure 1.
GIV-fl is a metastasis-related protein in epithelial carcinomas. A) Functional domains of GIV/Girdin. N-terminal Hook domain interacts with microtubules (41), coiled-coil (CC) domain mediates homodimerization (8), Gα-binding domain (GBD) interacts with α-subunits of Gi (42), and the extreme C terminus interacts with EGFR (13), Akt kinase, and actin (8, 12). The key regulatory component of GIV, i.e., the GEF domain with which GIV interacts with and activates Gαi, is located within the C terminus (6). B) Expression of GIV-fl is induced during metastatic progression of human breast cancer. Lysates of the 21T series of mammary cancer cell lines were analyzed for expression of GIV-fl (as determined by GIV-CTAb, which recognizes the C-terminal 19 aa of hGIV) and actin by immunoblotting (IB) and GIV and GAPDH mRNA by RT-PCR. CT, C terminus; NT, N terminus; GBD, Gα-binding domain; Fw, forward; Rev, reverse. C) GIV-fl is detectable exclusively in the highly metastatic subclone (Ls-LiM6) of colon adenocarcinoma cells derived from the poorly metastatic parent line, Ls-174T. Lysates of Ls-174T and its derivative cell line, Ls-LiM6, were analyzed for expression of GIV-fl, Gαi3, Akt (phospho and total), and actin by immunoblotting and GIV and GAPDH mRNA by RT-PCR as in B. D) GIV-fl is detectable exclusively in highly metastatic variants of pancreatic carcinoma cells. Lysates of pancreatic cancer cell lines with low (L) or high (H) invasive potential were analyzed for expression of GIV-fl and actin by immunoblotting as in B. E) GIV-fl is detectable in the highly metastatic, FG, but not in nonmetastatic, SG, pancreatic adenocarcinoma subclones derived from the parent cell line CO 357. Lysates of Colo 357 and its derivative cell lines, FG and SG, were analyzed for expression of GIV-fl, Gαi3, pan Gβ, and actin by immunoblotting and GIV and GAPDH mRNA by RT-PCR as in B. Numbers under IB bands indicate densitometry values, calculated based on actin loading.
The first clue that GIV's C terminus, and more specifically its GEF function, might play a role in tumor invasion came from our finding that GIV-dependent activation of Gαi is essential for Akt enhancement and actin remodeling during tumor cell migration, a set of prometastatic features that are associated with increased tumor invasion (6, 9). We (6) previously demonstrated that these prometastastic features of tumor cells are abolished when the interface between GIV's GEF motif and Gαi is disrupted by selective mutagenesis, thereby defining the “Gαi-GIV interface” as a potential therapeutic target against cancer metastasis. The role of full-length GIV (GIV-fl) in tumor invasion/metastasis in vivo was further substantiated when its depletion was found (10) to markedly impair metastasis in mouse models. In addition, using in vivo murine Matrigel plug assay, Kitamura et al. (11) demonstrated the role of endothelial GIV-fl in VEGF-mediated neoangiogenesis, a prerequisite for tumor progression. Recently, we (13) demonstrated that expression of GIV-fl, and more specifically its C terminus, which contains the key motifs (EGFR binding, Akt/actin binding, and GEF; Fig. 1A), is dysregulated in breast and colorectal tumor cells by alternative splicing. In poorly invasive cancer cells and in preinvasive, early staged colorectal carcinomas, GIV-fl was replaced by a C-terminally truncated variant, GIVΔCT. In highly invasive cancer cells and late-staged invasive carcinomas, GIV-fl is highly expressed. Consequently, only some tumor cells and tumors, but not all, express GIV-fl, which contains a functionally intact C terminus, and GIV-fl expression correlates with metastatic progression. Striking differences in GIV-fl expression have also been reported (10) among primary tumors of other human carcinomas (e.g., breast, colon, lung, and uterine cervical), but the clinical significance of such differential GIV-fl expression remains unknown.
Despite the breadth of information available on the molecular and biological functions of GIV-fl during cancer invasion and angiogenesis (6–15), it remains unexplored whether expression of GIV-fl in some tumors/tumor cells, and not others, might serve as a tool for prognostication. We hypothesized that differential expression of GIV's C terminus in tumors reflects its invasiveness and investigated whether the presence of the critical C terminus can distinguish the highly metastatic from poorly metastatic cancers and thereby prognosticate survival among cancer patients.
MATERIALS AND METHODS
Reagents and antibodies
Unless otherwise mentioned, all chemicals were reagent grade and obtained from Sigma (St. Louis, MO, USA), and cell culture media were purchased from Invitrogen (Carlsbad, CA, USA). Custom-designed primers were from Valugene (San Diego, CA, USA). Affinity-purified anti-GIV/Girdin C terminus (GIV-CTAb; IBL America, Spring Lake Park, MN, USA), raised against the C-terminal 19 aa of GIV (8) was used to visualize GIV-fl in all experiments. Antibodies against pan-Gβ (M-14) and Gαi3 used for immunoblotting were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), mAb against actin was from Sigma, rabbit IgG against phospho-Akt (serine 473) was from Cell Signaling (Beverly, MA, USA), and mAb against total Akt was from BD Biosciences (San Diego, CA, USA). Goat anti-rabbit and goat anti-mouse Alexa Fluor 680 or IRDye 800 F(ab′)2 for immunoblotting were from Li-Cor Biosciences (Lincoln, NE, USA).
Cell culture and lysis
Unless mentioned otherwise, all cell lines used in this work were propagated as per American Type Culture Collection guidelines. The 21T breast cell lines (16N, NT, and MT2) were obtained from Arthur B. Pardee (Dana-Farber Cancer Institute and Harvard Medical School, Boston, MA, USA) and maintained as described previously (3, 16). The Ls-174T and Ls-LiM6 cell lines were obtained from Robert Bresalier (M. D. Anderson Cancer Center, Houston, TX, USA) and grown as described previously (17). Ls-174T is a poorly metastatic colon cancer cell line harvested from a Duke's clinical stage B tumor. Ls-LiM6 is a highly metastatic subclone of Ls-174T cells that was selected by serial passage of Ls-174T through a murine cecum-to-liver metastasis model. All the pancreatic cancer cell lines (18, 19) used in this work were a generous gift from Michael Bouvet (University of California, San Diego). The highly metastatic, fast-growing (FG) and nonmetastatic, slow-growing (SG) variants of Colo 357 parent line were generated using a model of hepatic and pulmonary metastases following splenic injection into the nude mouse (19).
Lysates were prepared by resuspending the cells in lysis buffer (20 mM HEPES pH 7.2, 5 mM Mg-acetate, 125 mM K-acetate, 0.4% Triton X-100, and 1 mM DTT, supplemented with phosphatase (Sigma) and protease (Roche, Indianapolis, IN, USA) inhibitor cocktails, passed through a 28-gauge needle at 4°C, and cleared (1000 g for 10 min) before use in subsequent experiments.
Immunoblotting
Protein samples were separated on 10% SDS-PAGE and transferred to PVDF membranes (Millipore, Billerica, MA, USA). Membranes were blocked with PBS supplemented with 5% nonfat milk and incubated sequentially with primary and secondary antibodies. Infrared imaging with 2-color detection and quantification of Western blots were performed according to the manufacturer's protocols using an Odyssey imaging system (Li-Cor Biosciences).
RT-PCR
RT-PCR was performed exactly as described previously (13). Primers used in this work were designed using Invitrogen's Oligo Perfec Designer and evaluated with NetPrimer from Premierbiosoft (13). GAPDH (Allele Biotechnology and Pharmaceuticals, San Diego, CA, USA) mRNA amplified from the same samples served as an internal loading control. The sequences of various GIV-fl primers are available on request. To rule out contamination due to genomic DNA, RT-PCR-minus reactions were run similarly, only with elimination of the step of cDNA synthesis using reverse transcriptase.
Patient selection and data collection
A total of 56 patients with stage II (Astler-Collier- Duke's B2) colon cancers from the United States and Switzerland (20, 21) were used as a historic cohort for this study. These samples were collected between 1984 and 1989. In a prospective design, all patients were followed from diagnosis until death or until data were censored (and the patient was alive). The primary outcome measured was metastasis-free survival, measured from the date of histological diagnosis of colorectal cancer. Histopathologic staging was confirmed as Duke's B2 based on the absence of tumor invasion into lymph nodes (an average of 11.3 nodes/patient) that were sampled during surgical resection of the primary tumor. In line with the standard of care in the 1980s, most of these patients underwent en bloc hemicolectomy with lymph node resections but did not receive systemic chemotherapy. All tumors were fixed, sliced, stained, scored for histological grading, microdissected, and analyzed as described previously (21).
Microsatellite analysis
The microsatellite instability (MSI) status of tumors had been previously determined (20, 22, 23) using the National Cancer Institute-recommended panel of 5 microsatellite markers (BAT25, BAT26, D5S346, D2S123, and D17S250; ref. 24). With the use of this approach, tumors were classified as either MSI-high (H) or microsatellite stable (MSS) depending on whether they had inactivated MMR genes. To detect mutant alleles, MMR genes were interrogated using radiolabeled primers in gel-shift assays exactly as described previously (20). The presence of additional PCR products/bands from tumor DNA, but not observed in DNA from normal tissue from same patient, was scored as positive for instability at that locus. In keeping with National Cancer Institute consensus on MSI (24), MSI-H were defined as those with ≥2 of 5 markers of novel MMR alleles compared with matched nontumor DNA, whereas MSS tumors had no detectable mutations in either of the 5 markers listed.
Immunohistochemistry
Immunohistochemistry (IHC) was performed as described previously (13, 20). Briefly, slides containing colon cancer tissue were deparaffinized in xylene and rehydrated in graded alcohols to water. Slides were immersed in sodium citrate buffer (pH 6.0) and heated in a microwave 4 times, 4 min each, for antigen retrieval. Slides were then processed using a Dako Signal Catalyzed Amplification (CSA) System (Dako Corp., Carpinteria, CA, USA). Endogenous peroxidase activity was blocked by incubation with H2O2. Ten percent goat serum was added for 15 min to block nonspecific protein binding. Rabbit GIV-CTAb (anti-Girdin; 6 μg/ml; IBL America) was incubated overnight and then rinsed with PBS. Biotinylated anti-rabbit IgG was added for 15 min, followed by incubation with peroxidase-labeled streptavidin for 15 min at room temperature. Sections were washed with PBS, incubated with DAB and H2O2 for 1 min, lightly counterstained with hematoxylin, dehydrated in graded alcohols, cleared in xylene, and coverslipped. Staining was scored as 0 = absent, 1+ = moderately positive, and 2+ = strongly positive by 3 independent observers masked to patient outcome and stage. Epithelial and stromal components of tumors were identified by staining duplicate slides in parallel with hematoxylene and eosin and visualizing by light microscopy.
Statistical analysis
Statistical analyses were done using the software Matlab (The MathWorks, Inc., Natick, MA, USA). We evaluated Kaplan-Meier curves with the log-rank test. All P values reported are 2-sided, and values of P < 0.05 were considered to indicate significance. All graphical data presented in this work was prepared using GraphPad (GraphPad Software, Inc., San Diego, CA, USA).
Ethics statement
The studies involving human tissues collected from U.S. and Swiss hospitals were performed under Institutional Review Board approval at each participating institution. Written consent was obtained from each participant in the study. This study was approved by the University of California, San Diego, Human Subjects Institutional Review Board (project 080602).
RESULTS
GIV-fl is a metastasis-related protein whose expression increases during metastatic progression
Previously, we observed differences in the levels of GIV-fl expression among cancer cells of diverse genetic background (9, 13). To minimize genetic diversity, we now examined GIV-fl expression during metastatic progression of cancer in the isogenic background of the 21T series of human mammary cells (16N, NT, and MT2) (3, 16). These cells were derived by successive biopsies from a single patient with breast cancer in which 16N is from the normal breast, NT from the primary tumor (invasive ductal carcinoma), and MT2 from the metastatic pleural effusions. Expression of GIV-fl protein was analyzed using an antibody against the C terminus of GIV, GIV-CTAb (8, 13), which recognizes the full-length protein by immunoblotting, and GIV-fl message was qualitatively assessed using oligoneucleotide primer pairs that amplify the C-terminal half of GIV by RT-PCR (13). We found that GIV-fl expression (protein and mRNA) increased during metastatic progression (Fig. 1B), coinciding with the previously reported (3) progressive increase in Akt activity and invasiveness of these cell lines. That the highly invasive NT and MT2 cell lines express higher levels of GIV mRNA compared with 16N was also validated by real-time quantitative RT-PCR (data not shown). Next, we investigated paired colon cancer cell lines, Ls-174T and Ls-LiM6, that differ only with respect to expression of metastasis-related proteins (17). The Ls-LiM6 line was established in a mouse metastasis model by selecting clones with high liver-metastasizing ability from poorly metastatic Ls-174T parent cells. Prior studies (4) using this model have confirmed that metastases are a result of “selection” of preexisting highly metastatic clones from the parent tumor. We found that highly metastatic Ls-LiM6 cells expressed GIV-fl mRNA and protein, whereas the poorly metastatic Ls174T parent cells did not (Fig. 1C). As in the case of the 21T breast cancer cells (3), GIV-fl expression coincided with increasing Akt activity during colon cancer metastasis (Fig. 1C).
We also found that among pancreatic cancer cells of diverse genetic background (18), GIV-fl is detectable exclusively in those with high metastatic potential (Fig. 1D and Table 1). To investigate GIV-fl expression among isogenic pancreatic cancer cells, we analyzed the Colo series of cell lines, Colo-FG and SG, which were established in mouse models of tumor metastasis by selecting cells with high or low liver/lung-metastasizing ability, respectively, from the Colo-357 parental line (19). Once again, highly metastatic Colo-FG cells expressed GIV-fl mRNA and protein, whereas the poorly metastatic Colo-SG cells did not (Fig. 1E). Thus, GIV-fl behaves as a signature protein of metastasis in that it is increasingly expressed in the cells that undergo selective enrichment in the liver and lungs during the course of metastatic progression in breast, colon, and pancreatic carcinomas. A comprehensive analysis of GIV-fl protein expression pattern among 20 cancer cell lines, across 3 human neoplasms (Table 1), confirms that GIV-fl is a bona fide metastasis-related protein in that its expression distinguishes those cells with the highest metastatic/invasive potential.
Table 1.
GIV-fl expression
| Carcinoma | Cell line | Origin | Invasiveness | GIV-fl |
|---|---|---|---|---|
| Colon | Ls174T | Duke's B, primary | Low | UD |
| HT29pa | Primary | Low | UD | |
| Ls-LiM6 | Liver metastases | High | D | |
| DLD1a | Duke's C, invasive | High | D | |
| HCT116a | Duke's D, metastatic | High | D | |
| LoVo | Lymph node, invasive | High | D | |
| Breast | MCF7b | Pleural effusion | Low | UD |
| Hs578-Tb | Invasive ductal | High | D | |
| MDA-MB 231b | Pleural effusion | High | D | |
| 21T-NT | Invasive ductal | High | D | |
| 21T-MT2 | Pleural effusion | High | D | |
| Pancreas | BxPC-3 | Primary | Low | UD |
| Mia-PaCa-2 | Primary | Low | UD | |
| Panc-1 | Primary | Low | UD | |
| Colo-357 SG | Variant, Colo-357 | Low | UD | |
| AsPC-1 | Ascites | High | D | |
| Capan 1 | Liver metastasis | High | D | |
| CF PAC | Liver metastasis | High | D | |
| Colo 357 | Lymph node | High | D | |
| Colo-357 FG | Variant, Colo-357 | High | D |
GIV-fl expression distinguishes the highly invasive from poorly invasive cancer cells in breast, colon, and pancreatic carcinomas. Cancer cell lines of variable invasive potential, as determined by their ability to metastasize in murine models (3, 43, 44), were analyzed for GIV-fl expression using GIV-CTAb by immunoblot and/or by RTPCR. The GIV-fl-status was classified as detectable wherever GIV-fl mRNA and/or protein were consistently detected, regardless of whether the level of expression was low or high. This comprehensive table was generated from results presented in this work and our previously published (9) and unpublished work (present study). UD, undetectable; D, detectable.
Ref. 9.
Ref. 13.
Cancer cells expressing GIV-fl are selectively enriched within human liver metastases
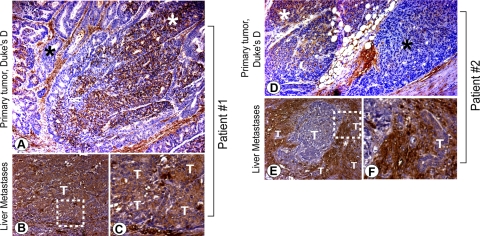
According to the clonal selection theory (4, 25), cells destined to metastasize are present within the tumor even at early stages of growth, and metastatic progression is marked by the ability of these cells to selectively survive the multistep process, disseminate successfully, and establish macrometastasis elsewhere. In keeping with this hypothesis, our results using isogenic cell lines (Fig. 1C, E and Table 1) demonstrate that GIV-fl is expressed by the clone that is “fittest” for successful development of metastasis in animal models. To investigate whether such selection occurs in humans, we compared the pattern of GIV-fl expression in the primary tumor and synchronous liver metastases from patients with metastatic (Duke's stage D) colorectal cancer. We found that despite the heterogeneity in GIV-fl expression observed in primary tumors (Fig. 2A, D), metastases are invariably and homogeneously positive for GIV-fl (Fig. 2B, C, E, F). This finding is consistent with our observations in mouse models of tumor dissemination (Fig. 1): both indicate that cancer progression is accompanied by selective enrichment of GIV-fl-expressing clones within distant metastases.
Figure 2.
Duke's stage D colorectal carcinomas illustrate heterogeneity in GIV-fl expression in primary tumors (A, D) but homogeneity in GIV-fl expression in synchronous liver metastases (B, C, E, F). Expression of GIV-fl was analyzed by IHC using GIV-CTAb on primary tumors and their paired synchronous liver metastases in patients presenting with colorectal cancer of Duke's clinical stage D. Representative findings in 2 such patients (A–C and D–F, respectively) are displayed (n=8). Images in C, F are a higher magnification of the boxed regions in B, E. Epithelia of the primary tumors stain heterogeneously for GIV-fl (A, D; positive and negative regions denoted by white and black stars, respectively), whereas their respective synchronous liver metastases stain homogeneously for GIV-fl. T, tumor epithelium.
Abundance of GIV-fl in tumors prognosticates survival among colorectal cancer patients
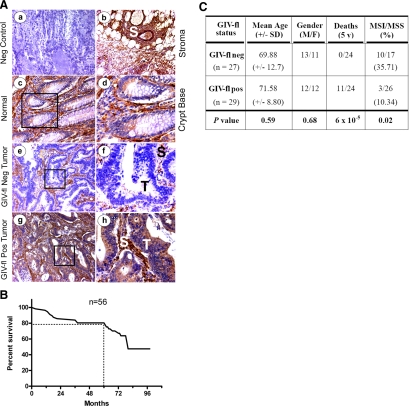
Next, we asked whether expression of GIV-fl distinguishes the highly invasive tumors that carry poor prognosis from poorly invasive tumors that carry a good prognosis. Because an ideal prognosticator identifies at-risk patients even at early stages when tumor invasion is undetectable by histopathology (26), we tested the prognosticating potential of GIV-fl on a cohort of 56 patients with colorectal cancer of Duke's stage B2, where lymph node invasion has not yet occurred (Fig. 3). We explored the potential of GIV-fl as a biomarker by IHC and used a simplified, binary scoring system. Staining was scored as negative (0) or positive (1+/2+), indicating the absence or presence of GIV's C terminus in tumor epithelia. Because IHC findings are highly dependent on the specificity and sensitivity of the antibodies, we used GIV-CTAb, which has previously been validated for detection of GIV-fl in vitro and in situ (10, 11). We found that the expression of GIV-fl in the epithelia of these 56 tumors was variable (Fig. 3A), but the stroma invariably stained homogeneously and strongly positive in normal tissue and carcinomas alike. Of the 56 patients, 21 (37.5%) died during a mean follow-up period of 5.99 ± 1.2 yr; an overall survival of 80% at 5 yr (Fig. 3B, C). This is similar to previously reported survival rates in Duke's stage B (27), thus validating the characteristics of our cohort. Survival rate for patients whose tumor epithelium stained positive (1+ or 2+) for GIV-fl (Fig. 4A) was significantly reduced compared with the patients with tumors negative for GIV-fl [P<0.0001; hazard ratio=0.076; CI=0.052–0.30 (95%); Fig. 4B]. At the 5-yr mark, survival was 100% in the GIV-fl-negative group and 62 ± 9% (mean±se; P=6×10−5) in the GIV-fl-positive group. Among GIV-fl-positive tumors, a trend was observed that higher GIV-fl expression (2+ vs. 1+) correlated with worse prognosis, although this did not reach significance (Fig. 4A, C). Unpaired t test revealed that the GIV-fl-negative and GIV-fl-positive subgroups were otherwise similar with respect to mean age at diagnosis and gender ratio (Fig. 3C). These results demonstrate that GIV can prognosticate survival in patients with colorectal cancer irrespective of their noninvasive clinical stage.
Figure 3.
Characteristics of the cohort of patients with colorectal carcinoma analyzed in this study. A) GIV-fl expression varies among colorectal adenocarcinomas within the same stage. Paraffin-embedded human colon samples [normal (a–d) or cancers of Duke's clinical stage B2 (e–h)] were analyzed for GIV-fl by IHC using GIV-CTAb. Normal colon epithelium stained negative for GIV-fl other than at crypt bases. Stroma (S) consistently stained strongly positive (b–h) in both normal and cancer tissues alike, whereas tumor epithelia showed variable staining (e–h). Representative fields from normal colon crypts (c, d), tumors negative for GIV-fl (e, f), and positive for GIV-fl (g, h) are displayed. Staining was scored as negative or positive by 3 independent observers masked to the disease stage with >95% congruency. Images in d, f, g are a higher magnification of the boxed regions in c, e, g. B) Kaplan-Meier survival curve for the cohort of patients with Duke's stage B colorectal cancer analyzed in this study. Kaplan-Meier plot was generated using the survival data (y axis) of patients in the Duke's B cohort plotted against the duration of follow-up (x axis). Overall survival in this cohort at 5 yr is ∼80%. C) Characteristics of 56 patients with Duke's B2 colorectal cancer evaluated for GIV-fl expression. Mean age, age at diagnosis of colon cancer; M, male; F, female; Death, number of patients who died within 5-yr follow-up period; MSS, colorectal cancer with MSS; MSI, cancer with high-frequency MSI. Age at diagnosis and gender were unknown for 8/56 patients, whereas information on survival and MSI status was available for all subjects. Total number of patients who received a 5-yr follow up was 24 in each group. We used an unpaired Student's t test to compare all variables (mean age at diagnosis, gender ratio M/F, deaths within 5 yr and MSI:MSS ratio).
Figure 4.
Stratification of colorectal tumors by abundance of GIV-fl expression as determined by IHC. A) Intensity of GIV-fl staining varied among tumors. Paraffin-embedded human colon cancer samples of Duke's clinical stage B2 were analyzed for GIV-fl as in Fig. 3A. Staining of the tumor epithelium was scored as 0 = absent (a), 1+ = moderately positive (b), 2+ = strongly positive (c) by 3 independent observers masked to patient outcome and stage. Images are representative of tumors in each scoring category. B) Kaplan-Meier survival curves for patients with colorectal cancer stratified according to GIV-fl expression status. GIV-fl expression was determined in 56 patients with Duke's B2 colon cancers as in Fig. 3A. Staining was scored as negative or positive by 3 independent observers masked to patient outcome and stage with >95% congruency. Log-rank analysis of the survival curve shows that survival of patients with colorectal cancer where tumor epithelium stained negative for GIV-fl protein (GIV-fl neg) was significantly better than that of patients with cancers where tumor epithelia stained positive GIV-fl (GIV-fl pos) by IHC using GIV-CTAb (P<0.001). C) Kaplan-Meier survival curves for patients with colorectal cancer, stratified according to intensity of GIV-fl expression. GIV-fl expression was determined in 56 patients with Duke's B2 colon cancers and scored as in A. Log-rank analysis of the survival curve shows a trend that among GIV-fl-positive tumors, higher GIV-fl staining (2+ vs. 1+) correlated with worse prognosis, but it did not reach significance (P=0.69).
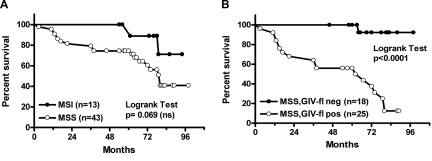
Next, we compared the prognostic value of GIV-fl status to that of microsatellite DNA stability status, a well-established prognosticator used in the clinical setting for colorectal cancers. MSS status was previously determined (21) in this cohort of patients using the NCI-recommended markers (24). Consistent with prior studies (28), patients with high-frequency MSI in tumors had better survival than those with MSS tumors. Although prognostication using MSI status failed to reach significance (P=0.069; Fig. 5A), we noted that a significantly greater percentage of GIV-fl-negative tumors also harbor MSI compared with GIV-fl-positive tumors (35 vs. 10%; P=0.02); the latter are largely MSS (Fig. 3C). Furthermore, survival within the subgroup of patients with MSS tumors was effectively predicted based on their GIV-fl status (Fig. 5B), thus validating the strength of GIV-fl as a novel marker that prognosticates independent of the tumor microsatellite status.
Figure 5.
Prognostic value of GIV-fl expression is independent of tumor MSS status. A) Kaplan-Meier survival curves for patients with colorectal cancer, stratified according to microsatellite status alone. Patients with Duke's B2 colorectal cancer were classified according to microsatellite status of their cancer (24). Log-rank analysis of the Kaplan-Meier curves shows that despite a trend that patients with tumors that feature MSI had favorable survival vs. those with MSS, microsatellite status alone is an insufficient predictor of survival (P=0.069). B) Kaplan-Meier survival curves for patients with colorectal cancer, stratified according to microsatellite status in combination with GIV-fl expression status. Patients with Duke's B2 colorectal cancer were classified according to microsatellite and GIV-fl expression status of their tumor. Log-rank analysis of the survival curve shows that when GIV-fl expression (as in Fig. 4B) was applied in tandem with microsatellite status, patients with MSS tumors (n=43) whose tumor epithelium stained negative for GIV-fl (GIV-fl neg) had better survival than patients whose tumor epithelium stained positive (GIV-fl pos) (P<0.0001).
DISCUSSION
Here we introduce GIV-fl as a metastasis-related protein, the expression of which effectively identifies those cancers that carry a “high-invasion–poor-prognosis” signature. GIV-fl meets the criteria for metastasis-related protein because the full-length protein with an intact C terminus is detected exclusively in highly invasive carcinoma cells and not in their poorly invasive counterparts. Our inability to detect GIV-fl either using C-terminal antibodies by immunoblotting or using C-terminal primers by RT-PCR is in accordance with our recent discovery (13) that in poorly invasive cancer cells and in noninvasive early tumors, GIV-fl is alternative spliced and replaced by a truncated variant (GIVΔCT) that lacks its C terminus. As a direct consequence of this tumor-specific splicing event, expression of GIV's C terminus is restricted to only some cancer cells and tumors and correlates with the tumor's clinical stage; i.e., noninvasive tumors of early stages are largely comprised of cells that express GIVΔCT, and with advancing clinical stages, invasive tumors are increasingly comprised of cells expressing GIV-fl (13). Because the presence of GIV's C terminus distinguishes tumor cells with highest invasiveness/metastatic potential from others, we conclude that alternative splicing of GIV's C terminus is one of the key mechanisms that impart the “metastasis-related” property to the GIV-fl gene.
We demonstrate that GIV-fl effectively prognosticates survival in a cohort of patients in whom the tumor is of an intermediate stage and prognostication is otherwise extremely challenging. The intermediate-stage cohort was chosen based on our recent observations (13) that GIV-fl is expressed in ∼50% of these tumors, whereas it is present in all late-stage, invasive tumors and is absent in all early stage, noninvasive tumors. We found that expression of GIV-fl in tumor epithelia negatively correlates with the duration of metastasis-free survival. These results portray the clinical significance of prior observations (10) that carcinoma cells expressing GIV-fl metastasize efficiently, whereas cells without GIV-fl (depleted by shRNA) fail to invade or to form distant metastases in a mouse model of cancer progression. Because the presence of GIV-fl in tumors predates and accurately predicts metastatic progression, GIV-fl qualifies as a mechanistically identified marker for prognostication. Intriguingly, stroma associated with the tumor or with normal tissue consistently showed strong staining that did not vary with the disease stage or survival. This is in agreement with prior reports by us and others (9, 10, 13) that GIV-fl is expressed at levels severalfold higher in cells of mesenchymal origin as compared with epithelia. Because the tumor stroma is a key factor in driving cancer progression (29), it remains to be seen how stromal GIV-fl influences the expression and/or function of GIV-fl in the tumor epithelia during metastasis. Because GIV is key player in signal transduction, increases in GIV-fl within tumors would be expected to affect the various receptor-initiated signaling pathways that it is known to regulate (13). Although such direct correlative analysis has not yet been performed in tumors in situ, it is worth noting that independent studies have documented an increased phosphorylation of Akt (30) and expression of PLCγ1 (31), 2 signaling pathways driven by GIV-fl (13), during metastatic progression of colorectal carcinoma. Finally, it would also be important to investigate whether detection of more specific pool of post-translationally modified GIV (like Akt-phosphorylated GIV-pSer 1416; ref. 8) provides a further layer of refinement to the strength or accuracy of prognostication by GIV-fl.
We found that prognostication by GIV-fl status is in agreement with, but independent of, the tumor microsatellite status; prognostic value of the latter has been unequivocally established through multiple studies (32). We found that the noninvasive tumors that are GIV-fl-negative are also more likely to harbor MSI, whereas GIV-fl-positive tumors are largely MSS. Thus, GIV-fl status and MSS status are in agreement with each other, because MSI tumors tend to grow larger in size but are noninvasive and carry a good prognosis, much like GIV-fl negative tumors, whereas MSS tumors tend to metastasize early and are associated with poor survival (23, 32, 33), much like GIV-fl positive tumors. This internal consistency observed between GIV-fl expression, MSI status, and tumor characteristics (size vs. invasiveness) not only suggests an intertwined relationship but also supports the proposed role of GIV's C-terminal GEF function in balancing tumor growth and invasion during oncogenic progression (13), i.e., absence of GIV-fl promotes mitosis and aids tumor growth, whereas the presence of GIV-fl triggers migration and aids tumor invasion. GIV-fl status also prognosticates within the cohort of patients with MSS tumors, indicating that the accuracy and strength of prognostication using GIV-fl status is observed independent of the tumor microsatellite status. Whether microsatellite instability directly affects GIV-fl expression or vice versa remains to be seen. We conclude that GIV-fl status offers a powerful, new prognostic subclassification for colorectal cancer at early stages. By these criteria, GIV-fl fundamentally differs from other late-stage tissue-based prognostic markers that have been declined for clinical use (26).
Because GIV-fl behaved like a metastasis-related protein also in breast and pancreatic carcinoma cells, we checked whether GIV-fl (also known as KIAA1212/ccDc88a) appeared as a signature gene among prognosticators identified during genome-wide array-based screening of other cancers and found that this is indeed the case in a variety of cancers, e.g., esophageal, breast, endometrial, and colon (34, 35). In breast cancers, induction of GIV-fl expression was recognized among the 500-gene classifier that identified tumors with wild-type p53 that unexpectedly carried a poor prognosis, similar to those with p53 mutations. Whether GIV-fl can serve as a biomarker for prognostication in other carcinomas is a question that warrants further clinical trials. Finally, activating mutations of trimeric G-protein α-subunits, Gαs (36, 37), Gαi (37), Gαo (38), and Gαq (39), have been reported in certain human adenomas and carcinomas, but it has not been possible to exploit such knowledge in the realm of cancer prognostication. By demonstrating that the nonreceptor GEF, GIV-fl prognosticates survival, we have translated the significance of G-protein activation in cancers to a clinically useful prognostication biomarker.
In summary, we show that detection of GIV's C terminus can serve as an attractive, conserved, and convenient target for development as a metastasis biomarker for carcinomas. Together with the fact that we recently identified GIV's C-terminal GEF motif as a potential therapeutic target in our armamentarium against cancer metastasis (6, 40), the current work promises an opportunity for the practice of personalized medicine. Patients with poor prognosis-bearing, GIV-fl-positive tumors could qualify for an adjuvant therapy in the form of inhibitors of this critical G-protein-dependent signaling pathway.
Acknowledgments
The authors thank Marilyn G. Farquhar for scientific mentoring, advice, and thoughtful comments during preparation of this work. The authors thank Samuel B. Ho (University of California, San Diego) for the generous gift of paired tumor tissue samples from patients with Duke's D colorectal cancer with synchronous liver metastases and Michael Lam (University of California, San Diego) for advice and technical help with the real-time quantitative RT-PCR.
This work was supported by awards from the Burroughs Wellcome Fund and American Gastroenterology Association to P.G.; U.S. National Institutes of Health (NIH) grants K08-DK-074019 and R01-DK-067287 to B.H.J and J.M.C, respectively; and the University of California, San Diego, Digestive Diseases Research Development Center, U.S. Public Health Service grant DK-080506. M.G.-M. was supported by Basque Government Postdoctoral Fellowship BFI06.300 and Susan G. Komen Postdoctoral Fellowship KG080079. J.E. is a recipient of a McNair Student Research Scholarship. The authors thank Erin P. Forry for help with tissue culture, and she was supported by NIH grant CA100768 to Marilyn Farquhar.
REFERENCES
- 1. Muller A., Homey B., Soto H., Ge N., Catron D., Buchanan M. E., McClanahan T., Murphy E., Yuan W., Wagner S. N., Barrera J. L., Mohar A., Verástegui E., Zlotnik A. (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 [DOI] [PubMed] [Google Scholar]
- 2. Larue L., Bellacosa A. (2005) Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 24, 7443–7454 [DOI] [PubMed] [Google Scholar]
- 3. Qiao M., Iglehart J. D., Pardee A. B. (2007) Metastatic potential of 21T human breast cancer cells depends on Akt/protein kinase B activation. Cancer Res. 67, 5293–5299 [DOI] [PubMed] [Google Scholar]
- 4. Fidler I. J. (2003) The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat. Rev. Cancer 3, 453–458 [DOI] [PubMed] [Google Scholar]
- 5. Qiao M., Sheng S., Pardee A. B. (2008) Metastasis and AKT activation. Cell Cycle 7, 2991–2996 [DOI] [PubMed] [Google Scholar]
- 6. Garcia-Marcos M., Ghosh P., Farquhar M. G. (2009) GIV is a nonreceptor GEF for G alpha i with a unique motif that regulates Akt signaling. Proc. Natl. Acad. Sci. U. S. A. 106, 3178–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia-Marcos M., Ghosh P., Ear J., Farquhar M. G. A structural determinant that renders Gαi sensitive to activation by GIV/Girdin is required to promote cell migration. J. Biol. Chem. 285, 12765–12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Enomoto A., Murakami H., Asai N., Morone N., Watanabe T., Kawai K., Murakumo Y., Usukura J., Kaibuchi K., Takahashi M. (2005) Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev. Cell 9, 389–402 [DOI] [PubMed] [Google Scholar]
- 9. Ghosh P., Garcia-Marcos M., Bornheimer S. J., Farquhar M. G. (2008) Activation of Galphai3 triggers cell migration via regulation of GIV. J. Cell Biol. 182, 381–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang P., Enomoto A., Jijiwa M., Kato T., Hasegawa T., Ishida M., Sato T., Asai N., Murakumo Y., Takahashi M. (2008) An actin-binding protein Girdin regulates the motility of breast cancer cells. Cancer Res. 68, 1310–1318 [DOI] [PubMed] [Google Scholar]
- 11. Kitamura T., Asai N., Enomoto A., Maeda K., Kato T., Ishida M., Jiang P., Watanabe T., Usukura J., Kondo T., Costantini F., Murohara T., Takahashi M. (2008) Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate Girdin. Nat. Cell Biol. 10, 329–337 [DOI] [PubMed] [Google Scholar]
- 12. Anai M., Shojima N., Katagiri H., Ogihara T., Sakoda H., Onishi Y., Ono H., Fujishiro M., Fukushima Y., Horike N., Viana A., Kikuchi M., Noguchi N., Takahashi S., Takata K., Oka Y., Uchijima Y., Kurihara H., Asano T. (2005) A novel protein kinase B (PKB)/AKT-binding protein enhances PKB kinase activity and regulates DNA synthesis. J. Biol. Chem. 280, 18525–18535 [DOI] [PubMed] [Google Scholar]
- 13. Ghosh P., Beas A., Bornheimer S. J., Garcia-Marcos M., Ear J., Forry E. P., Johannson C. J., Jung B., Cabrera B. L., Carethers J. M., Farquhar M. G. (2010) A Gi-GIV molecular complex binds epidermal growth factor receptor and determines whether cells migrate or proliferate. Mol. Biol. Cell. 21, 2338–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Enomoto A., Ping J., Takahashi M. (2006) Girdin, a novel actin-binding protein, and its family of proteins possess versatile functions in the Akt and Wnt signaling pathways. Ann. N. Y. Acad. Sci. 1086, 169–184 [DOI] [PubMed] [Google Scholar]
- 15. Weng L., Enomoto A., Ishida-Takagishi M., Asai N., Takahashi M. Girding for migratory cues: roles of the Akt substrate Girdin in cancer progression and angiogenesis. Cancer Sci. 101, 836–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Band V., Zajchowski D., Swisshelm K., Trask D., Kulesa V., Cohen C., Connolly J., Sager R. (1990) Tumor progression in four mammary epithelial cell lines derived from the same patient. Cancer Res. 50, 7351–7357 [PubMed] [Google Scholar]
- 17. Bresalier R. S., Hujanen E. S., Raper S. E., Roll F. J., Itzkowitz S. H., Martin G. R., Kim Y. S. (1987) An animal model for colon cancer metastasis: establishment and characterization of murine cell lines with enhanced liver-metastasizing ability. Cancer Res. 47, 1398–1406 [PubMed] [Google Scholar]
- 18. Grzesiak J. J., Bouvet M. (2006) The alpha2beta1 integrin mediates the malignant phenotype on type I collagen in pancreatic cancer cell lines. Br. J. Cancer 94, 1311–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vezeridis M. P., Meitner P. A., Tibbetts L. M., Doremus C. M., Tzanakakis G., Calabresi P. (1990) Heterogeneity of potential for hematogenous metastasis in a human pancreatic carcinoma. J. Surg. Res. 48, 51–55 [DOI] [PubMed] [Google Scholar]
- 20. Jung B., Doctolero R. T., Tajima A., Nguyen A. K., Keku T., Sandler R. S., Carethers J. M. (2004) Loss of activin receptor type 2 protein expression in microsatellite unstable colon cancers. Gastroenterology 126, 654–659 [DOI] [PubMed] [Google Scholar]
- 21. Jung B., Smith E. J., Doctolero R. T., Gervaz P., Alonso J. C., Miyai K., Keku T., Sandler R. S., Carethers J. M. (2006) Influence of target gene mutations on survival, stage and histology in sporadic microsatellite unstable colon cancers. Int. J. Cancer 118, 2509–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carethers J. M., Hawn M. T., Greenson J. K., Hitchcock C. L., Boland C. R. (1998) Prognostic significance of allelic lost at chromosome 18q21 for stage II colorectal cancer. Gastroenterology 114, 1188–1195 [DOI] [PubMed] [Google Scholar]
- 23. Gervaz P., Cerottini J. P., Bouzourene H., Hahnloser D., Doan C. L., Benhattar J., Chaubert P., Secic M., Gillet M., Carethers J. M. (2002) Comparison of microsatellite instability and chromosomal instability in predicting survival of patients with T3N0 colorectal cancer. Surgery 131, 190–197 [DOI] [PubMed] [Google Scholar]
- 24. Boland C. R., Thibodeau S. N., Hamilton S. R., Sidransky D., Eshleman J. R., Burt R. W., Meltzer S. J., Rodriguez-Bigas M. A., Fodde R., Ranzani G. N., Srivastava S. (1998) A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 58, 5248–5257 [PubMed] [Google Scholar]
- 25. Talmadge J. E. (2007) Clonal selection of metastasis within the life history of a tumor. Cancer Res. 67, 11471–11475 [DOI] [PubMed] [Google Scholar]
- 26. Duffy M. J., van Dalen A., Haglund C., Hansson L., Holinski-Feder E., Klapdor R., Lamerz R., Peltomaki P., Sturgeon C., Topolcan O. (2007) Tumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur. J. Cancer 43, 1348–1360 [DOI] [PubMed] [Google Scholar]
- 27. IMPACT-B2 (1999) Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) J. Clin. Oncol. 17, 1356–1363 [PubMed] [Google Scholar]
- 28. Gryfe R., Kim H., Hsieh E. T., Aronson M. D., Holowaty E. J., Bull S. B., Redston M., Gallinger S. (2000) Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N. Engl. J. Med. 342, 69–77 [DOI] [PubMed] [Google Scholar]
- 29. Li H., Fan X., Houghton J. (2007) Tumor microenvironment: the role of the tumor stroma in cancer. J. Cell. Biochem. 101, 805–815 [DOI] [PubMed] [Google Scholar]
- 30. Saglam O., Garrett C. R., Boulware D., Sayegh Z., Shibata D., Malafa M., Yeatman T., Cheng J. Q., Sebti S., Coppola D. (2007) Activation of the serine/threonine protein kinase AKT during the progression of colorectal neoplasia. Clin. Colorectal Cancer 6, 652–656 [DOI] [PubMed] [Google Scholar]
- 31. Noh D. Y., Lee Y. H., Kim S. S., Kim Y. I., Ryu S. H., Suh P. G., Park J. G. (1994) Elevated content of phospholipase C-gamma 1 in colorectal cancer tissues. Cancer 73, 36–41 [DOI] [PubMed] [Google Scholar]
- 32. Walther A., Houlston R., Tomlinson I. (2008) Association between chromosomal instability and prognosis in colorectal cancer: a meta-analysis. Gut 57, 941–950 [DOI] [PubMed] [Google Scholar]
- 33. Popat S., Hubner R., Houlston R. S. (2005) Systematic review of microsatellite instability and colorectal cancer prognosis. J. Clin. Oncol. 23, 609–618 [DOI] [PubMed] [Google Scholar]
- 34. Esteller M. (2007) Novel Tumour Suppressor, World Intellectual Property Organization; (http://www.wipo.int) WO/2007/122369 [Google Scholar]
- 35. Miller L. (2006) U.S. Patent 20060074565 and World Intellectual Property Organization WO/2007/122369 and WO/2007/013671
- 36. Landis C. A., Masters S. B., Spada A., Pace A. M., Bourne H. R., Vallar L. (1989) GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature 340, 692–696 [DOI] [PubMed] [Google Scholar]
- 37. Lyons J., Landis C. A., Harsh G., Vallar L., Grunewald K., Feichtinger H., Duh Q. Y., Clark O. H., Kawasaki E., Bourne H. R., McCormick F. (1990) Two G protein oncogenes in human endocrine tumors. Science 249, 655–659 [DOI] [PubMed] [Google Scholar]
- 38. Kan Z., Jaiswal B. S., Stinson J., Janakiraman V., Bhatt D., Stern H. M., Yue P., Haverty P. M., Bourgon R., Zheng J., Moorhead M., Chaudhuri S., Tomsho L. P., Peters B. A., Pujara K., Cordes S., Davis D. P., Carlton V. E., Yuan W., Li L., Wang W., Eigenbrot C., Kaminker J. S., Eberhard D. A., Waring P., Schuster S. C., Modrusan Z., Zhang Z., Stokoe D., de Sauvage F. J., Faham M., Seshagiri S. (2010) Diverse somatic mutation patterns and pathway alterations in human cancers. Nature 466, 869–873 [DOI] [PubMed] [Google Scholar]
- 39. Van Raamsdonk C. D., Bezrookove V., Green G., Bauer J., Gaugler L., O'Brien J. M., Simpson E. M., Barsh G. S., Bastian B. C. (2009) Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 457, 599–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garcia-Marcos M., Ghosh P., Ear J., Farquhar M. G. (2010) A structural determinant that renders G alpha(i) sensitive to activation by GIV/girdin is required to promote cell migration. J. Biol. Chem. 285, 12765–12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Simpson F., Martin S., Evans T. M., Kerr M., James D. E., Parton R. G., Teasdale R. D., Wicking C. (2005) A novel hook-related protein family and the characterization of hook-related protein 1. Traffic 6, 442–458 [DOI] [PubMed] [Google Scholar]
- 42. Le-Niculescu H., Niesman I., Fischer T., DeVries L., Farquhar M. G. (2005) Identification and characterization of GIV, a novel Galpha i/s-interacting protein found on COPI, endoplasmic reticulum-Golgi transport vesicles. J. Biol. Chem. 280, 22012–22020 [DOI] [PubMed] [Google Scholar]
- 43. Flatmark K., Maelandsmo G. M., Martinsen M., Rasmussen H., Fodstad O. (2004) Twelve colorectal cancer cell lines exhibit highly variable growth and metastatic capacities in an orthotopic model in nude mice. Eur. J. Cancer 40, 1593–1598 [DOI] [PubMed] [Google Scholar]
- 44. Thompson E. W., Paik S., Brunner N., Sommers C. L., Zugmaier G., Clarke R., Shima T. B., Torri J., Donahue S., Lippman M. E., Martin G. R., Dickson R. B. (1992) Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J. Cell. Physiol. 150, 534– 544 [DOI] [PubMed] [Google Scholar]