Abstract
Alzheimer disease is intimately linked to an excess amount of amyloid-β (Aβ) in the brain. Thus, therapeutic inhibition of Aβ production is an attractive clinical approach to treat this disease. Here we provide the first direct experimental evidence that the treatment of Tg2576 transgenic mice with an inhibitor of β-secretase, GRL-8234, rescues the age-related cognitive decline. We demonstrated that the injected GRL-8234 effectively enters the brain and rapidly decreases soluble Aβ in the brain of Tg2576 mice. The rescue of cognition, which was observed only after long-term inhibitor treatment ranging from 5 to 7.5 mo, was associated with a decrease of brain amyloid-β plaque load. We also found no accumulation of amyloid-β precursor protein after several months of inhibitor treatment. These observations substantiate the idea that Aβ accumulation plays a major role in the cognitive decline of Tg2576 mice and support the concept of Aβ reduction therapy as a treatment of AD.—Chang, W.-P., Huang, X., Downs, D., Cirrito, J. R., Koelsch, G., Holtzman, D. M. Ghosh, A. K., Tang, J. β-Secretase inhibitor GRL-8234 rescues age-related cognitive decline in APP transgenic mice.
Keywords: Alzheimer's disease, Tg2576, memapsin 2
Alzheimer's disease (AD) is the most common neurodegenerative disorder for which no effective therapy is available. Current evidence supports the idea that excess levels of brain Aβ is an important factor in the pathogenesis of the disease (1). Aβ is generated from amyloid-β precursor protein (APP) by two proteases, β-secretase (memapsin 2, BACE1) (2–6) and γ-secretase. Even though the accumulation of brain Aβ can be a consequence of escalated Aβ production or insufficient Aβ degradation and efflux, in theory therapeutic intervention to suppress Aβ production should reduce Aβ levels in all cases. Despite the attractiveness of the “amyloid reduction” approach, among the limited therapeutic targets those including the two secretases are most promising. Presently, the candidate inhibitor drugs for γ-secretase have exhibited toxicity problems (7, 8) rooted in the many physiological functions of this protease. Not only does γ-secretase mediate the Notch cleavage, which is important in cell differentiation, it also functions in the hydrolysis of transmembrane peptide fragments from type I transmembrane proteins in general (7, 8). Another potential problem for the inhibition of γ-secretase is the accumulation of its toxic substrate in the membrane, the fragment derived from C-terminal 99 residues of APP, which has no known alternative processing mechanisms. Such accumulation is consistent with an Aβ rebound after the inhibition period (9).
β-Secretase, however, is devoid of many of these problems. The inhibition of this first step in AD pathogenesis might avoid other harmful late events. Deletion of the β-secretase gene to eliminate Aβ production is not only well tolerated in mice (10–14) but also rescues memory deficits in AD mice (11, 15). Although some β-secretase inhibitors have exhibited the ability to reduce brain Aβ in transgenic AD mice (16), most β-secretase inhibitors developed so far are unsuited for extended in vivo studies (17, 18). Thus, it has not been possible to verify the β-secretase target in an experimental treatment in an AD animal model. Yet, it is important to test the “amyloid reduction” therapeutic strategy in an animal model using experiment conditions similar to those for clinical treatment. We recently reported a potent β-secretase inhibitor GRL-8234 (19). This inhibitor and its derivatives are potential clinical candidates for drug development. Thus, it has many properties needed for a use in long-term Aβ reduction studies in animal models. Tg2576 mice express the Swedish mutant of human APP linked to AD, generate excess brain Aβ and produce amyloid plaques in the brain around 10–12 mo (20, 21). The age-related decline of spatial-reference memory starts at ∼6 mo of age and continues slowly throughout life (22). The rescue of age-related cognitive decline in Tg2576 by GRL-8234 would serve as a stringent end point for this therapeutic model using a β-secretase inhibitor treatment, and the change in Aβ during treatment may also offer insights into the dynamics of Aβ reduction in vivo.
MATERIALS AND METHODS
Brain penetration of GRL-8234
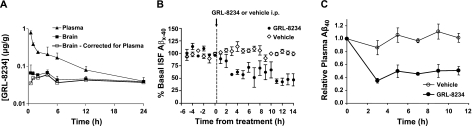
14C-labeled GRL-8234 was synthesized at BioDynamics (Upper Heyford, UK) using the previously described procedure (19). The citrate salt of the compound was obtained to enhance solubility. Compound was administered at a dose of 4 mg (100 μCi of 14C)/kg by intravenous bolus injection in the tail vein of each of 21 male Sprague-Dawley rats. At various intervals, animals in groups of 3 were sacrificed, and blood and brain were collected and homogenized. The radioactivity was determined by scintillation counting. The brain data were corrected for the presence of 4% of plasma (23). The brain penetration of the inhibitor was calculated by the ratio of the area under the curve in 24 h (AUC0–24) values of plasma and brain (see Fig. 2A).
Figure 2.
Inhibitor GRL-8234 enters the brain and inhibits the level of soluble Aβ. A) Amount of GRL-8234 (μg inhibitor/g tissue) in the plasma and brain of rats over a 24-h period following a single intravenous administration of 14C-GRL-8234 (4 mg/kg). At each time point (n=3), plasma and brain were collected for scintillation counting. Level of inhibitor in brain tissue was corrected (open squares) for the presence of 4% plasma (23). Brain penetration of the inhibitor was estimated by AUC to be 16.3% over 24 h. B) Effect of GRL-8234 on interstitial Aβ in Tg2576 mice. Mice (n=4) were intraperitoneally injected with GRL-8234 (33.4 μg/g body weight in vehicle solvent, a 1:1 mixture of 0.5% PEG-300 and 5% glucose), and interstitial Aβ was measured by in vivo microdialysis for 14 h (n=4/group). ISF Aβ levels were reduced by 46.6 ± 1.5% at 3 h after treatment compared to vehicle-treated mice and reduced by 52.3 ± 12.1% at 14 h (P=0.0019 and P=0062, respectively; means±se). C) Plasma Aβ40 of Tg2576 after a single intraperitoneal injection of 8 mg/kg of GRL-8234. Statistical differences were observed at 5 time points from 3 to 11 h (P<0.02, n=6).
Determination of brain soluble Aβ by in vivo microdialysis
In vivo microdialysis to assess brain interstitial fluid (ISF) Aβ1–x in the hippocampus of awake, freely moving Tg2576 mice, 4 mo of age, was performed in a manner similar to that previously described (24). This technique samples soluble molecules within the extracellular fluid that are smaller than 38 kDa. Tg2576 mice were intraperitoneally injected with either vehicle solvent (50:50 mixture of PEG300 and D5W, v/v) or the mesylate salt of GRL-8234 in the vehicle solvent (33.4 μg/g body weight) at the given time point (see Fig. 2B).
Inhibitor treatment of transgenic mice
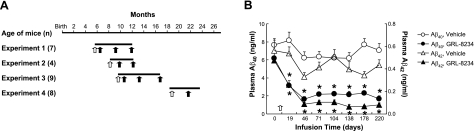
Tg2576 mice with a B6;SJL mixed background were obtained from Taconic Farms (Hudson, NY, USA), and sighted animals were determined by PCR genotyping for presence of the retinal degeneration gene (25). At the beginning of the experiments (see Fig. 3A), mice were implanted subcutaneously at the back just below the neck with Alzet osmotic pumps (model 1002; Durect Corp., Cupertino, CA, USA) containing either vehicle solvent (1:1 mixture of 0.5% PRG-300 and 5% glucose) or a solution of GRL-8234 (synthesized at OrgSyn Laboratory, Chicago, IL, USA, using the previously described procedure; ref. 19) in the same solvent. The pumps had a rate of 4.8 μl/d, which delivered 33.4 μg inhibitor/d/g body weight. Pumps were replaced at the end of their working duration of 21 d. Blood was sampled from the saphenous vein, and plasma was separated by centrifugation. Plasma concentrations of Aβ40 and Aβ42 in each sample were determined by sandwich ELISA (Invitrogen, Carlsbad, CA, USA).
Figure 3.
Effect of GRL-8234 treatment on plasma Aβ levels. A) Experimental schemes. Infusion of inhibitor or vehicle solvent was initiated at surgical implantation of osmotic pump (open arrows) in mice at the age indicated by the scale at top and was continued by a series of pumps, each lasting 21 d, throughout the experimental period (horizontal bar). Cognitive performance of mice was evaluated with Morris water maze at time points indicated (solid arrows). Brain tissues were collected at end of the last cognitive tests. Number of mice (n) used in each control and inhibitor-treatment group is listed for each experiment. B) Effect of GRL-8234 inhibitor treatment on plasma Aβ40 and Aβ42 concentrations in Tg2576 mice. Open arrow indicates time of initial inhibitor or vehicle solvent delivery. Blood samples were collected weekly; only 1/4 of data points are shown. Data are from experiment 1 (A); n = 7/group. *P < 0.05.
Cognitive tests
Spatial reference memory was tested for Tg2576 mice using a modified procedure of the Morris water maze (MWM) (26) at the given time points (see Fig. 3A). In experiments where multiple MWMs were applied, they were separated by >3 mo to minimize the carry over of memory from the previous tests. The status as to whether the mice were receiving inhibitor was masked. Each test was performed in a pool of 138 cm in diameter with an escape platform submerged 1 cm and surrounded by spatial cues. The hidden platform test was performed up to 6 d. Each mouse was given 4 daily 60-s training trials with an intertrial interval of 5 min. During each trial, a mouse was released into the water in randomly chosen quadrants, and the escape latency time was recorded and averaged from 4 daily training trials for each mouse. Probe trials were given as indicated in the figure legends. During these trials, the mice swam with the platform in a retracted position for 60 s; then the platform was raised to its normal position, accessible to the test animal. The crossing times in each quadrant were recorded by a video tracking system, and the annulus crossing index (ACI) and time in quadrant (TQ) were calculated and averaged. The reference-memory test was followed by a cued (visible platform) version of MWM, 4 trials/d for 3 d. The pool contained an escape platform marked by a 15-cm-high black and white pole, and all external cues were blocked. The visible platform test was intended as a check for vision and motor ability. Latency times were similar between both groups in each experiment of all tests. Swim speeds were determined using EthoVision XT software (Noldus Information Technology, Wageningen, The Netherlands).
Spatial working memory was tested using a standard Y maze. Each mouse was placed in the middle of one of the 3 arms with its head directed toward the center and allowed to explore freely in the apparatus for 5 min. The maze was sanitized with ethanol between testing sessions. Latency to exit the start arm and sequence and number of arm visits were recorded. Total arm entry (N) and spontaneous alternation [% alternation/(N − 2)] were calculated.
Analysis of brain Aβ
At the end of each experiment, the mice were anesthetized with avertin, then intracardially perfused with phosphate-buffered saline solution for 3 min, and the brains were harvested. One hemisphere of the brain was stored in liquid nitrogen for the determination of Aβ. The tissues were first homogenized in 25 mM Tris-HCl (pH 7.4) containing 20 mM 3-[(3-cholamidopropyl)dimethylammonic]-1-propane sulfonate (CHAPS), 3 mM EDTA, and 150 mM NaCl, and the centrifuged pellets were extracted by 50 mM Tris-HCl (pH 8.0) containing 5 M guanidine-HCl (16). Aβ40 and Aβ42 were determined for the extracts by sandwich ELISA (Invitrogen). For the determination of Aβ oligomers, the brains from experiment 3 were homogenized and fractionated into extracellular-enriched, intracellular-enriched, and membrane fractions according to the method described by Lesne et al. (22). Fractions were subjected to Western blots with the primary anti-Aβ17–24 antibody (MAB1561; Chemicon, Temecula, CA, USA) or anti-BACE487–501 antibody (195102; Calbiochem, La Jolla, CA, USA) followed by ECL detection system (Pierce, Rockford, IL, USA). The second hemisphere of the brains was used for the determination of Aβ plaques. The brains were fixed for 2 d in 10% buffered formalin, then sectioned into 5-μm slices for immunohistochemical staining using the anti-Aβ39–43 antibody (CM 333 AK; Biocare Medical, LLC, Concord, CA, USA). For experiments 2 and 3, the immunohistochemical staining was performed using a Ventana instrument (Ventana Medical Systems, Inc., Tucson, AZ, USA) and plaques were calculated by IPLab 3.65 Eval software (Scanalytics Inc, Reutlingen, Germany). For experiment 1, the section and staining were handled by NeuroScience Associates, Inc. (Los Angeles, CA, USA), and plaques were calculated by NIS-Elements, BR2.30 (Nikon, Melville, NY, USA). For all data, analysis of variance (ANOVA) and Student's t test were used for statistical analysis.
RESULTS
GRL-8234 reduces brain Aβ in Tg2576 mice
To demonstrate brain penetration of GRL-8234 (properties in Fig. 1), rats were injected intravenously with 14C-radiolabeled inhibitor (4 μg/g), and radioactivity in brain and plasma was measured. At 1 h after injection, about 10% of the radioactivity was found in the brain, and it decreased slowly thereafter, in contrast to a rapid decrease in the plasma (Fig. 2A). After correction for the radioactivity contributed by 4% plasma in the brain (23), the AUCs in Fig. 2A show that 16% of GRL-8234 is present in the brain over a 24-h period. Given the high potency of this inhibitor (Ki=1.8 nM), these compound levels would be expected to effectively inhibit β-secretase activity in vivo. The inhibition of brain Aβ by GRL-8234 was studied by intracerebral microdialysis (24). Three-month-old Tg2576 mice were injected intraperitoneally with the inhibitor (33.4 μg/g), and ISF levels of Aβ40 were measured. ISF Aβ40 was decreased by ∼50% in 3 h. A slower decline to >50% reduction by 12 h occurred (Fig. 2B). These results suggest that GRL-8234 entered the brain and inhibited Aβ production over an extended period. For comparison, we also measured the plasma Aβ40 level of Tg2576 mice after intraperitoneal injection with inhibitor (8 μg/g). A maximal inhibition of ∼50% was seen at 3 h, and the inhibition was maintained through 12 h (Fig. 2C).
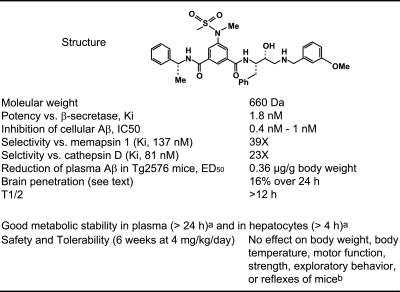
Figure 1.
Summary of the properties of GRL-8234. aUnpublished results. bTested in rotarod, wire hanging, open field, and Irwin tests, respectively.
GRL-8234 treatment rescues age-related cognitive decline
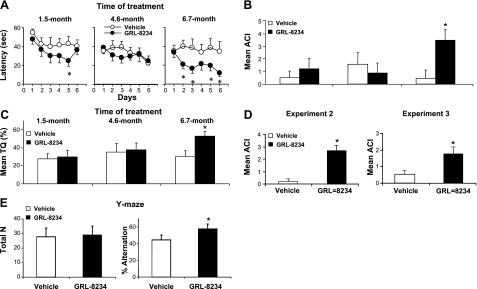
We then asked whether GRL-8234 could rescue the age-related cognitive decline in Tg2576 mice. Long-term inhibitor delivery (33.4 μg/g/d) was achieved with implanted osmotic pumps, and mice were tested for cognitive performance in the MWM at different time points (Fig. 3A). The longitudinal testing is designed to simulate human clinical trials (27) and has been used successfully for therapeutic models on transgenic AD mice (28, 29). During the course of the experiments, mice exhibited no overt toxicity or difference in body weight between the groups during any of our chronic dosing paradigms. In experiment 1, inhibitor infusion started with 5.5-mo-old mice and continued for 6.7 mo (Fig. 3A). Plasma Aβ40 and Aβ42 levels of the treated mice were reduced by 60–80% throughout the experimental period (Fig. 3B). Cognitive performance did not differ significantly between the control and experimental groups at treatment time lengths of 1.5 and 4.6 mo. However, at 6.7 mo, significant differences between the groups were observed (Fig. 4A–C). The escape latency (EL) showed clear separation on the performance of the two groups at 6.7 mo, and the data were statistically different in 4 of 5 d of testing (Fig. 4A). The differences in EL were substantiated by the swim speed analysis, which showed no difference between the two groups (14.5±0.7 and 15.4±0.9 cm/s for vehicle and GRL-8234, respectively) and by two other tests, the ACI and TQ, which both unambiguously showed that a difference between the two groups occurred only for cognitive testing at 6.7 mo (P=0.0004) (Fig. 4B, C). The cognitive rescue was confirmed in two similar experiments using different experimental paradigms. In experiments 2 and 3 (Fig. 3A), strong inhibition of plasma Aβ40 and Aβ42, ranging from 60 to 80%, was again observed. In these experiments, after 4–6 mo of treatment, the EL time of the two groups was statistically significant (in 4 consecutive days in experiments 2 and 3 of 5 d in experiment 3; Supplemental Fig. S1A). A clear statistically significant improvement in ACI of the treated group was observed in both experiments (Fig. 4D). These three experiments demonstrated the rescue of cognitive decline by inhibitor treatment. Notably, the effect was seen only after a treatment of several months. Since cognitive decline in Tg2576 progresses at a slow rate, we asked whether the time needed to demonstrate rescue by the inhibitor could be shortened if the experimental period included the sharpest decline between 5 and 6 mo of age (22). Since the starting age of the mice in experiment 1 was 5.5 mo and missed half of the sharpest decline, we started an additional experiment with mice aged 4.7 mo and carried the inhibitor treatment for 6 wk. Cognitive tests at the end of the experiment showed no significant difference in EL or ACI between the treated and control groups (Supplemental Fig. S1B), confirming that longer treatment time is needed for cognitive rescue.
Figure 4.
Effect of GRL-8234 treatment on cognitive performance of Tg2576 mice. A–D) Effect of β-secretase inhibitor GRL-8234 on cognitive performance of Tg2576 mice. Mice from experiment 1 (n=7; Fig. 3A) were treated with either inhibitor or vehicle solvent and tested for spatial reference memory in MWM at time point in treatment indicated at top. A) Escape latency time for control (open circles) and treated mice (solid circles). *P < 0.05. B) Mean ACI from probe trials. *P < 0.001. C) Mean percentage TQ from probe trials. *P < 0.001. D) Mean ACI from probe trials of experiments 2 (n=4) and 3 (n=9). Data were obtained at the end of each experiment, as shown in A. Probe trials were administered at the beginning of d 3, 5, and 6 of training for experiment 2 and d 3, 5, 7, and 8 of training for experiment 3. *P < 0.001 (left panel); *P = 0.005 (right panel). E) Effect of β-secretase inhibitor GRL-8234 on Y-maze cognitive performance of Tg2576 mice. Mice from experiment 3 (n=9) were tested for spatial working memory in Y maze followed by MWM. Latency to exit the start arm and sequence and number of arm visits were recorded. Total arm entry N and spontaneous alternation, % alternation/(N − 2), were calculated. *P = 0.04.
In experiment 4, we asked whether GRL-8234 could rescue the cognitive decline of Tg2576 mice older than 16 mo (Fig. 3A). Although the reduction of plasma Aβ was similar to other experiments (Supplemental Fig. S1C), no significant difference in cognitive performance was found for either EL or ACI after treatment of 2.7 mo (Supplemental Fig. S1D). After 4.4 mo of treatment, the aging mice in both experimental and control groups had become physically unable to perform additional cognitive tests, and the experiment was terminated.
We have also examined the cognitive performance in a Y-maze test for the mice in experiment 3 after the last MWM test. As shown in Fig. 4E, no significant difference was found between the control and experimental groups on the total arm entry; i.e., 27.7 ± 6.1 and 29.0 ± 4.8, respectively. However, mice treated with GRL-8234 showed cognitive performance improvement, with higher percentage alternation (57.8±3.6) as compared with the control mice (44.6±5.7).
Brain amyloid and APP in GRL-8234-treated mice
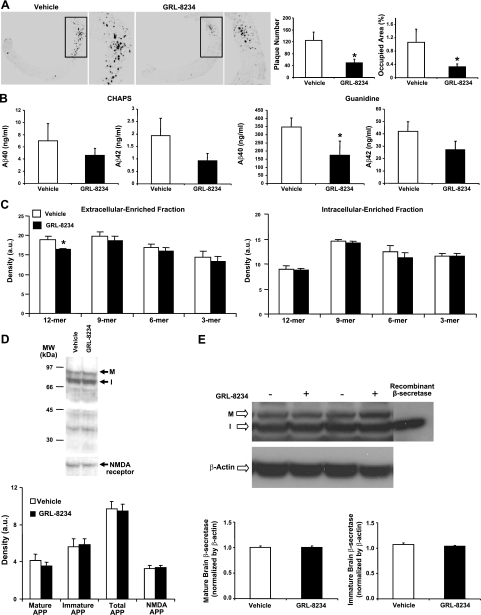
At the termination of the inhibitor experiments, we analyzed brain Aβ in brain sections. Immunohistochemical slides were prepared from one hemisphere of the brains of mice in experiment 1, and amyloid plaques and Aβ were quantitated. We observed ∼65% reduction in both the plaque number (P=0.017) and plaque area (P=0.036) for the treated group as compared to the control group (Fig. 5A). Plaque reduction was confirmed in experiments 2 and 3, in which both plaque number and plaque area were reduced by ∼45–60% in the inhibitor-treated group (see Supplemental Fig. S2A, B). In addition, the treated old mice in experiment 4 had significantly fewer plaques than the control mice (Supplemental Fig. 2C, D), even though no cognitive rescue was observed. We also determined Aβ40 and Aβ42 levels in the second hemisphere of the brains, using a conventional extraction method with CHAPS buffer and a guanidine solution. Although reductions of both Aβ40 and Aβ42 in the range of 40–50% were seen for the treated group (Fig. 5B), only Aβ42 reduction in experiment 1 was statistically significant. As discussed below, we believe that the extraction method might have serious limitations for determining brain amyloid changes. The overall biochemical results, however, are consistent with the histological data that accumulation of brain Aβ was dramatically reduced after chronic treatment with GRL-8234.
Figure 5.
Effect of GRL-8234 on Aβ and APP in brains of Tg2576 mice. A) Effect of inhibitor treatment on amyloid-β plaques. Brains from mice in experiment 1 were fixed, sliced, and immunohistochemically stained with anti-Aβ39–43 monoclonal antibody. Left panels: representative stainings for control (vehicle; left) and treated mice (GRL-8234, right). Low-power (×40) and high-power (×200) views show a reduction in amyloid plaque deposits. Right panels: average plaque numbers (left) and average plaque occupied area in percentage of total area (right) in cortex of the brain in vehicle- and GRL-8234-treated mice (n=7). *P = 0.017 (left); *P = 0.036 (right). B) Amount of Aβ40 (left graphs) and Aβ42 (right graphs) in extract of Tg2576 mouse brains at end of experiment 1. One hemisphere of each mouse was extracted for soluble Aβ using a buffer containing CHAPS (left panels) and for insoluble Aβ using a buffer containing guanidine (right panels). Amounts of Aβ40 and Aβ42 from both homogenates were determined by sandwich ELISA. Open and solid bars represent data from control and inhibitor-treated mice, respectively (n=7). *P = 0.05. C) Brain Aβ oligomer species from extracellular-enriched fractions in control (open bars) and treated Tg2576 mice (solid bars). One brain hemisphere from mice in experiment 3 was homogenized; extracellular-enriched (left panel) and intracellular-enriched (right panel) fractions were prepared, and Aβ oligomer species were determined by densitometry reading of bands in Western blots. Only the decrease of the 12-mer in the inhibitor-treated group was significant (P=0.007, n=9). D) Brain full-length APP in vehicle- and GRL-8234-treated Tg2576 mice from experiment 3. Membrane fraction of brain homogenate was prepared and extracted with 3% SDS in 50 mM Tris-HCl buffer (pH 7.4) and analyzed by Western blot. Top panel: representative pattern from each group; M and I indicate bands of mature and immature APP, respectively. Bands at bottom are from a separate Western blot of the membrane marker: ε2 subunit of N-methyl d-aspartate (NMDA) receptor. Bottom panel: average density of APP species and NMDA receptor, as determined from Western blot bands. Amounts of APP from the two groups were not statistically different (n=9). E) Brain β-secretase in intracellular-enriched fractions of vehicle- and GRL-8234-treated Tg2576 mice from experiment 3. Top panel: representative pattern of β-secretase from each group; M and I indicate bands of mature and immature β-secretase, respectively, with a single band for recombinant mature β-secretase. Bands at bottom are from a separate Western blot of the control β-actin blot. Bottom panel: average density of β-secretase as determined from Western blot bands. Amounts of mature or immature β-secretase from vehicle- and GRL-8234-treated mice are not statistically different (P=0.214 and P=0.497, respectively; n = 9).
To determine whether the rescue of cognitive decline is accompanied by a change of Aβ oligomer species, we prepared subcellular fractions from mouse brains of experiment 3 and determined Aβ oligomers according to a method described by Lesne et al. (22). We found little change in the overall patterns of Aβ oligomers in the extracellular-enriched fraction and intracellular-enriched fraction (Fig. 5C), except a 15% reduction of the amount of Aβ dodecamer in the extracellular-enriched fraction of the inhibitor-treated group as compared to the control group (P=0.007, n=9, Fig. 5C). The membrane-enriched fraction contained Aβ oligomers too low to be determined quantitatively.
A potential risk of long-term therapeutic use of a β-secretase inhibitor is the accumulation of the substrate APP. We therefore analyzed brain APP in Tg2576 mice from experiment 3 after 6.5 mo of treatment with GRL-8234. As shown in Fig. 5D, we found no difference between control and treated groups in mature APP, immature APP, and total APP, which indicated that inhibitor treatment did not cause an accumulation of APP.
To be certain that the decrease of brain Aβ in inhibitor-treated mice was not due to an altered expression of β-secretase, we performed Western blotting for β-secretase in brain homogenates of experiment 3. As shown in Fig. 5E, the level of β-secretase was indistinguishable between these two groups, indicating that the observed Aβ reduction and cognitive rescue was not due to a reduction in the expression of β-secretase.
DISCUSSION
To the best of our knowledge, this is the first time an inhibitor specifically designed to lower Aβ production targeting β-secretase has been applied to APP transgenic mice on a long-term basis and resulted in the rescue of age-related cognitive decline. A γ-secretase inhibitor, DAPT, was show to enhance contextual fear conditioning of Tg2576 mice after a single injection 3 h before the test (28). Since Aβ modulates synaptic activity as part of its normal physiological functions (29), the interpretation of a short-term cognitive test might be complicated by the difficulty of segregating the physiological and toxic effects of Aβ. Also, Aβ oligomer-induced synaptic loss was observed only after several days (30); a short-term cognitive effect within a few hours might be influenced significantly by cognitive enhancing or suppressing effects of the inhibitor. It is unclear whether such cognitive change is a model for disease modification in AD. However, it is well established that Aβ is responsible for a component of age-related cognitive decline seen in some types of human APP transgenic mice (26, 31), and the rescue of such cognitive decline with long-term inhibitor-induced Aβ reduction is the closest available model for treating human AD. Our evidence suggests that the observed cognitive rescue mainly resulted from the inhibition of Aβ production in the brain. Not only did the inhibitor reach the brain effectively, it significantly reduced amyloid plaque load at the end of the experiments. Further, it acutely lowered Aβ in the ISF space of the brain following a single injection of the inhibitor. In contrast to a fast decline of interstitial Aβ following the injection of γ-secretase inhibitor LY411,575, which reached maximal inhibition within 1 h (32), the time for interstitial Aβ decrease to reach bottom was 4–7 h (Fig. 2B). Since the injected GRL-8234 required 4 h to reach the highest point in the brain (Fig. 2A), the relative slow interstitial Aβ decline for GRL-8234 appears to be mainly caused by the rate of brain penetration of the inhibitor. It is interesting that after the inhibitor reached the highest concentration in rat brain in 4 h (Fig. 2A), the interstitial Aβ of AD mice continued to decrease for another 20% during the period from 4 to 12 h (Fig. 2B). The observed kinetic difference would appear too large to have been caused by the species difference between rats and the transgenic mice. Among the potential contributing factors are the nonspecific protein binding of the inhibitor in the brain and the possible presence of a preformed APP C99 pool that could still produce Aβ by γ-secretase for a period after inhibitor administration. The presence of an APP C99 pool can also contribute to the difference in the rate of decline for the response of interstitial Aβ to LY411,575 and GRL-8234. Since Aβ in Tg2576 mice is primarily produced in the brain (33), the Aβ reduction in plasma may reflect a reduced efflux of Aβ from the brain which is known to be a rapid process (34). The observations that Aβ reduction at 3 h and its level persisted over 12 h after inhibitor injection are similar in the brain and plasma (Fig. 2B, C) is consistent with the above assumption.
The relative long time of several months of inhibitor treatment required to achieve the cognitive rescue likely reflects the known slowness in cognitive decline in Tg2576 (22). We have considered whether insoluble Aβ had been deposited in the brain of AD mice before the inhibitor treatment and the slow solubilization of the insoluble Aβ during the treatment is the reason for the long-time requirement in cognitive rescue. However, this seems unlikely, as substantial Aβ plaques remained in the brain when cognitive difference was observed. Lesne et al. (22) demonstrated that the cognitive performance of Tg2576 mice, as measured by TQ, fluctuated much more along the longitudinal time points than the differences between TQ values of the wild-type and transgenic mice. We observed a similar longitudinal fluctuation, which appears to obscure the age-dependent decline of TQ values of the untreated group (Fig. 4A). However, the differences in cognitive performance between the control and treated groups are verified with different parameters (latency, ACI and TQ) and are consistent with the rescue of cognitive decline by inhibitor treatment. To assess the extent of cognitive rescue, we have compared the age-related decline of the mean TQ of Tg2576 (22) to the increase of this value from treatment observed in our studies. The rate of TQ decline for Tg2576 from 5 to 9 mo of age is ∼2.1%/mo and from 5 to 16 mo of age is ∼1.4%/mo (22). The mean TQ gain by inhibitor treatment for all three experiments is 2.86%/mo treatment (P=0.002, n=20). The relative high value of TQ gain from our experiments suggests that the inhibitor treatment restored a significant part of age-related cognitive decline in Tg2576 and could be to a level near that of the wild-type mice at the equivalent ages. We did not extensively test cognitive rescue by GRL-8234 using other cognitive tests, because MWM tests are best characterized in Tg2576 and are by far the most stringent tests. Also, because of the tightness in the schedule of MWM studies, only limited windows were available for other cognitive tests. However, a single Y-maze test at the end of experiment 3 did produce statistically significant difference, which confirmed a general cognitive improvement in the treated mice.
The cognitive improvement was accompanied by a significant reduction of brain Aβ. Based on the interstitial Aβ and the plaque load, the reduction of soluble Aβ by the inhibitor was ∼50%. Although we observed a similar reduction of brain Aβ in treated mice using a conventional extraction method, most of these data failed to reach statistical significance. We have found that the conventional extraction methods consistently give large ses and overestimate soluble Aβ in mouse brain. While the interstitial Aβ was inhibited by more than half with a single inhibitor injection, soluble Aβ extracted by CHAPS buffer had a mean reduction of 15% and was not statistically significant after daily injection of 16 d. A possible problem is the resolubilization of the freshly deposited Aβ by rigorous homogenization in CHAPS buffer. In addition, the estimation of insoluble Aβ by the guanidine extract would include Aβ deposited prior and during the experimental period, and thus cannot accurately reflect the acute decrease in new soluble Aβ generation, which is predominantly cell secreted Aβ, from the inhibitor treatment. In view of these limitations, we believe that the Aβ reduction in our studies is better represented by the interstitial Aβ (acute effect) and the plaque counts (chronic effect), which were highly reproducible. The fact that plaques were present in the treated groups suggests that Tg2576 mice had a robust Aβ production system, which could still form plaques even after a reduction of Aβ formation by 50%, at least when treatments were started at these ages tested. An interesting conclusion from these observations with potential clinical implication is that a significant cognitive rescue can be achieved with partial reduction of Aβ and plaque loads. The observation that the overall patterns of Aβ oligomers were similar in the brain of control and inhibitor-treated groups (Fig. 5C) was not unexpected. Although the production of Aβ was inhibited by GRL-8234, we had no reason to expect the inhibitor would influence the oligomerization process of Aβ. In addition, the method for the fractionation and oligomer analysis was originally used for young mice with no brain amyloid plaques (22). In our experiment, all the brains contained β-amyloid plaques. This posts a question whether freshly deposited β-amyloid may be solubilized in the homogenization step and thus influences the oligomer patterns. At the present, such possibility can not be completely excluded.
Since the age-related cognitive decline in Tg2576 is a model for the preclinical pathogenesis of AD, the current results might lend insights to the strategy of AD treatment. The success in cognitive rescue observed here substantiates that Aβ reduction is a logical clinical approach and that Aβ is responsible for the cognitive decline. A previous study reported that the deletion of both copies of β-secretase genes rescued the cognitive decline in a transgenic AD mice model, but the cognitive rescue was not observed for the mice with the deletion of one of the two copies of β-secretase gene (15). These observations imply a potential therapeutic dilemma with the use of β-secretase inhibitor drugs: a complete inhibition of β-secretase in the treatment risks blocking necessary functions of the protease, yet a partial inhibition might not be sufficient to modify the progression of the disease. The concern on the physiological consequences of a total suppression of β-secretase activity is reasonable in view that various phenotypes have been observed in the absence of β-secretase gene (14, 15, 35, 36). In this regard, our current results are particularly encouraging as the cognitive rescue was observed with a reduction of Aβ by about two-thirds. The observation of cognitive rescue in younger mice suggests that the application of β-secretase inhibitor drugs to AD patients would have a chance of success at a relative early stage of the pathogenesis. We also observed a significant reduction of amyoid-β plaque loads in old mice treated with the inhibitor, suggesting that some benefit may be derived in therapeutic treatment of late-stage AD patients with β-secretase inhibitor drugs. The finding that GRL-8234 did not cause an accumulation of APP after a long-term treatment implies that the alternative APP processing by α-secreatase is functioning at a sufficient capacity in vivo to compensate for β-secretase inhibition over 4 mo. It seems probable that this high-capacity compensatory mechanism might also be present in long-term AD treatment with β-secretase inhibitors. All these results lend support for the reduction of β-amyloid as a clinical strategy and encourage the further substantiation of β-secretase inhibitor drugs in human patients.
Supplementary Material
Acknowledgments
The authors thank Dr. Michelle Nicolle for comments and suggestions; Dr. J. Donald Capra for critical reading of the manuscript; Dr. Geoffrey Bilcer for arranging the synthesis of the inhibitor; Dr. William Sonntag, Dr. Matthew Mitschelen, and Julie Farley for the use of facility and advice; and Huining Da and Darby Kinsey for excellent technical assistance.
This work was supported in part by U.S. National Institutes of Health (NIH) grant AG-18933 and an Alzheimer Association Pioneer Award (to J.T.) and NIH COBRE/IDeA grant P20 RR 15577-07 (to W.-P.C.). J.T. is holder of the J. G. Puterbaugh Chair in Biomedical Research at the Oklahoma Medical Research Foundation.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Selkoe D. J., Schenk D. (2008) Alzheimer's disease: molecular understanding predicts amyloid-based therapeutics. Annu. Rev. Pharmacol. Toxicol. 43, 545–584 [DOI] [PubMed] [Google Scholar]
- 2. Lin X., Koelsch G., Wu S., Downs D., Dashti A., Tang J. (2000) Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc. Natl. Acad. Sci. U. S. A. 97, 1456–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yan R., Bienkowski M. J., Shuck M. E., Miao H., Tory M. C., Pauley A. M., Brashier J. R., Stratman N. C., Mathews W. R., Buhl. A. E., Carter D. B., Tomasselli A. G., Parodi L. A., Heinrikson R. L., Gurney M. E. (1999) Membrane-anchored aspartyl protease with Alzheimer's disease beta-secretase activity. Nature 402, 533–537 [DOI] [PubMed] [Google Scholar]
- 4. Vassar R., Bennett B. D., Babu-Khan S., Kahn S., Mendiaz E. A., Denis P., Teplow D. B., Ross S., Amarante P., Loeloff R., Luo Y., Fisher S., Fuller J., Edenson S., Lile J., Jarosinski M. A., Biere A. L., Curran E., Burgess T., Louis J. C., Collins F., Treanor J., Rogers G., Citron M. (1999) Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286, 735–741 [DOI] [PubMed] [Google Scholar]
- 5. Hussain I., Powell D., Howlett D. R., Tew D. G., Meek T. D., Chapman C., Gloger I. S., Murphy K. E., Southan C. D., Ryan D. M., Smith T. S., Simmons D. L., Walsh F. S., Dingwall C., Christie G. (1999) Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol. Cell. Neurosci. 14, 419–427 [DOI] [PubMed] [Google Scholar]
- 6. Sinha S., Anderson J. P., Barbour R., Basi G. S., Caccavello R., Davis D., Doan M., Dovey H. F., Frigon N., Hong J., Jacobson-Croak K., Jewett N., Keim P., Knops J., Lieberburg I., Power M., Tan H., Tatsuno G., Tung J., Schenk D., Seubert P., Suomensaari S. M., Wang S., Walker D., Zhao J., McConlogue L., John V. (1999) Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature 402, 537–540 [DOI] [PubMed] [Google Scholar]
- 7. Vetrivel K. S., Zhang Y. W., Xu H., Thinakaran G. (2006) Pathological and physiological functions of presenilins. Mol. Neurodegener. 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wolfe M. S. (2008) Gamma-secretase inhibition and modulation for Alzheimer's disease. Curr. Alzheimer. Res. 5, 158–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barten D. M., Meredith J. E., Jr., Zaczek R., Houston J. G., Albright C. F. (2006) Gamma-secretase inhibitors for Alzheimer's disease: balancing efficacy and toxicity. Drugs R D 7, 87–97 [DOI] [PubMed] [Google Scholar]
- 10. Cai H., Wang Y., McCarthy D., Wen H., Borchelt D. R., Price D. L., Wong P. C. (2001) BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat. Neurosci. 4, 233–234 [DOI] [PubMed] [Google Scholar]
- 11. Ohno M., Sametsky E. A., Younkin L. H., Oakley H., Younkin S. G., Citron M., Vassar R., Disterhoft J. F. (2004) BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer's Disease. Neuron 41, 27–33 [DOI] [PubMed] [Google Scholar]
- 12. Luo Y., Bolon B., Kahn S., Bennett B. D., Babu-Khan S., Denis P., Fan W., Kha H., Zhang J., Gong Y., Martin L., Louis J. C., Yan Q., Richards W. G., Citron M., Vassar R. (2001) Mice deficient in BACE1, the Alzheimer's beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat. Neurosci. 4, 231–232 [DOI] [PubMed] [Google Scholar]
- 13. Roberds S. L., Anderson J., Basi G., Bienkowski M. J., Branstetter D. G., Chen K. S., Freedman S. B., Frigon N. L., Games D., Hu K., Johnson-Wood K., Kappenman K. E., Kawabe T. T., Kola I., Kuehn R., Lee M., Liu W., Motter R., Nichols N. F., Power M., Robertson D. W., Schenk D., Schoor M., Shopp G. M., Shuck M. E., Sinha S., Svensson K. A., Tatsuno G., Tintrup H., Wijsman J., Wright S., McConlogue L. (2001) BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: implications for Alzheimer's disease therapeutics. Hum. Mol. Genet. 10, 1317–1324 [DOI] [PubMed] [Google Scholar]
- 14. Harrison S. M., Harper A. J., Hawkins J., Duddy G., Grau E., Pugh P. L., Winter P. H., Shilliam C. S., Hughes Z. A., Dawson L. A., Gonzalez M. I., Upton N., Pangalos M. N., Dingwall C. (2003) BACE1 (beta-secretase) transgenic and knockout mice: identification of neurochemical deficits and behavioral changes. Mol. Cell. Neurosci. 24, 646–655 [DOI] [PubMed] [Google Scholar]
- 15. Laird F. M., Cai H., Savonenko A. V., Farah M. H., He K., Melnikova T., Wen H., Chiang H. C., Xu G., Koliatsos V. E., Borchelt D. R., Price D. L., Lee H. K., Wong P. C. (2005) BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J. Neurosci. 25, 11693–11709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang W. P., Koelsch G., Wong S., Downs D., Da H., Weerasena V., Gordon B., Devasamudram T., Bilcer G., Ghosh A. K., Tang J. (2004) In vivo inhibition of Abeta production by memapsin 2 (beta-secretase) inhibitors. J. Neurochem. 89, 1409–1416 [DOI] [PubMed] [Google Scholar]
- 17. Ghosh A. K., Kumaragurubaran N., Hong L., Koelsh G., Tang J. (2008) Memapsin 2 (beta-secretase) inhibitors: drug development. Curr. Alzheimers Res. 5, 121–131 [DOI] [PubMed] [Google Scholar]
- 18. Ghosh A. K., Gemma S., Tang J. (2008) Beta-secretase as a therapeutic target for Alzheimer's disease. Neurotherapeutics 5, 399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ghosh A. K., Kumaragurubaran N., Hong L., Kulkarni S., Xu X., Miller H. B., Reddy D. S., Weerasena V., Turner R., Chang W., Koelsch G., Tang J. (2008) Potent memapsin 2 (beta-secretase) inhibitors: design, synthesis, protein-ligand X-ray structure, and in vivo evaluation. Bioorg. Med. Chem. Lett. 18, 1031–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., Yang F., Cole G. (1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 [DOI] [PubMed] [Google Scholar]
- 21. Irizarry M. C., McNamara M., Fedorchak K., Hsiao K., Hyman B. T. (1997) APPSW transgenic mice develop age-related A-beta deposits and neuropil abnormalities, but no neuronal loss in CA1. J. Neuropathol. Exp. Neuol. 56, 965–973 [DOI] [PubMed] [Google Scholar]
- 22. Lesne S., Koh M. T., Kotilinek L., Kayed R., Glabe C. G., Yang A., Gallagher M., Ashe K. H. (2006) A specific amyloid-beta protein assembly in the brain impairs memory. Nature 440, 352–357 [DOI] [PubMed] [Google Scholar]
- 23. Remy C., Tropres I., Peoc'h M., Farion R., Decorps M. (2000) In vivo NMR imaging of microvasularization in normal rat brain and in rat brain tumors. Proc. Int. Soc. Mag. Reson. Med. 8, 100 [Google Scholar]
- 24. Cirrito J. R., May P. C., O'Dell M. A., Taylor J. W., Parsadanian M., Cramer J. W., Audia J. E., Nissen J. S., Bales K. R., Paul S. M., DeMattos R. B., Holtzman D. M. (2003) In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J. Neurosci. 23, 8844–8853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garcia M. F., Gordon M. N., Hutton M., Lewis J., McGowan E., Dickey C. A., Morgan D., Arendash G. W. (2004) The retinal degeneration (rd) gene seriously impairs spatial cognitive performance in normal and Alzheimer's transgenic mice. Neuroreport 15, 73–77 [DOI] [PubMed] [Google Scholar]
- 26. Chen G., Chen K. S., Knox J., Inglis J., Bernard A., Martin S. J., Justice A., McConlogue L., Games D., Freedman S. B., Morris R. G. (2000) A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer's disease. Nature 408, 975–979 [DOI] [PubMed] [Google Scholar]
- 27. Whitehouse P. J., Kittner B., Roessner M., Rossor M., Sano M., Thal L., Winblad B. (1998) Clinical trial designs for demonstrating disease-course-altering effects in dementia. Alzheimer Dis. Assoc. Disord. 12, 281–294 [DOI] [PubMed] [Google Scholar]
- 28. Comery T. A., Martone R. L., Aschmies S., Atchison K. P., Diamantidis G., Gong X., Zhou H., Kreft A. F., Pangalos M. N., Sonnenberg-Reines J., Jacobsen J. S., Marquis K. L. (2005) Acute gamma-secretase inhibition improves contextual fear conditioning in the Tg2576 mouse model of Alzheimer's disease. J. Neurosci. 25, 8898–8902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kamenetz F., Tomita T., Hsieh H., Seabrook G., Borchelt D., Iwatsubo T., Sisodia S., Malinow R. (2003) APP processing and synaptic function. Neuron 37, 925–937 [DOI] [PubMed] [Google Scholar]
- 30. Shankar G. M., Bloodgood B. L., Townsend M., Walsh D. M., Selkoe D. J., Sabatini B. L. (2007) Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 27, 2866–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Westerman M. A., Cooper-Blacketer D., Mariash A., Kotilinek L., Kawarabayashi T., Younkin L. H., Carlson G. A., Younkin S. G., Ashe K. H. (2002) The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer's disease. J. Neurosci. 22, 1858–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Han B. H., Zhou M. L., Abousaleh F., Brendza R. P., Dietrich H. H., Koenigsknecht-Talboo J., Cirrito J. R., Milner E., Holtzman D. M., Zipfel G. J. (2008) Cerebrovascular dysfunction in amyloid precursor protein transgenic mice: contribution of soluble and insoluble amyloid-beta peptide, partial restoration via gamma-secretase inhibition. J. Neurosci. 28, 13542–13550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kawarabayashi T., Younkin L. H., Saido T. C., Shoji M., Ashe K. H., Younkin S, G. (2001) Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J. Neurosci. 21, 372–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. DeMattos R. B., Bales K. R., Cummins D. J., Paul S. M., Holtzman D. M. (2002) Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer's disease. Science 295, 2264–2267 [DOI] [PubMed] [Google Scholar]
- 35. Dominguez D. (2005) Phenotypic and biochemical analysis of BACE1- and BACE2-deficient mice. J. Biochem. Chem. 280, 30797–30806 [DOI] [PubMed] [Google Scholar]
- 36. Hu X., Zhou X., He W., Yang J., Xiong W., Wong P., Wilson C. G., Yang R. (2010) BACE1 deficiency causes altered neuronal activity and neurodegeneration. J. Neurosci. 30, 8819–8829 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.