Abstract
Many widespread and persistent organic pollutants, e.g., 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), activate the aryl hydrocarbon receptor (AhR), causing it to translocate to the cell nucleus, where it transactivates target genes. AhR's ability to target the blood-brain barrier is essentially unexplored. We show here that exposing isolated rat brain capillaries to 0.05–0.5 nM TCDD roughly doubled transport activity and protein expression of P-glycoprotein, an ATP-driven drug efflux pump and a critical determinant of drug entry into the CNS. These effects were abolished by actinomycin D or cycloheximide or by the AhR antagonists resveratrol and α-naphthoflavone. Brain capillaries from TCDD-dosed rats (1–5 μg/kg, i.p.) exhibited increased transport activity and protein expression of 3 xenobiotic efflux pumps, P-glycoprotein, multidrug resistance-associated protein 2, and breast cancer resistance polypeptide, as well as expression of Cyp1a1 and Cyp1b1, both AhR target genes. Consistent with increased P-glycoprotein expression in capillaries from TCDD-dosed rats, in situ brain perfusion indicated significantly reduced brain accumulation of verapamil, a P-glycoprotein substrate. These findings suggest a new paradigm for the field of environmental toxicology: toxicants acting through AhR to target xenobiotic efflux transporters at the blood-brain barrier and thus reduce brain accumulation of CNS-acting therapeutic drugs.—Wang, X., Hawkins, B. T., Miller, D. S. Aryl hydrocarbon receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier.
Keywords: P-glycoprotein, multidrug resistance-associated protein 2, breast cancer resistance protein, dioxin, brain capillaries
The brain capillary endothelium forms the blood-brain barrier, a selective modulator of solute and fluid exchange between blood and brain (1). This tissue regulates the neuronal extracellular environment and protects the central nervous system (CNS) from neurotoxicants. Barrier function depends primarily on two elements: tight junctions that seal the spaces between cells, and specific transport proteins, expressed in endothelial cell plasma membranes in a polarized manner. These transporters mediate CNS uptake of essential nutrients and ions, regulate fluid balance, and remove potentially toxic metabolic wastes and xenobiotics.
Members of the ABC transporter superfamily, e.g., P-glycoprotein, multidrug resistance-associated proteins (Mrps), and breast cancer resistance protein (Bcrp), are expressed in barrier and excretory tissues where they function as ATP-driven xenobiotic efflux pumps (2). As such, they are major determinants of xenobiotic uptake, distribution and excretion and thus of both xenobiotic toxicity and therapeutic drug efficacy. At the blood-brain barrier, ABC transporters both protect the CNS and impede drug delivery to the brain. P-glycoprotein (Abcb1) is the best studied of these efflux pumps. Its ability to handle a wide range of therapeutic drugs and its high expression in the luminal membrane of brain capillary endothelial cells make it a formidable obstacle to drug entry into the brain. Recent studies show that P-glycoprotein expression and activity at the blood-brain barrier is regulated by multiple distinct signals, including xenobiotic-activated nuclear receptors, inflammation, and oxidative stress (2). In vivo, modulated transporter activity affects drug access to the brain (3, 4). Increased P-glycoprotein expression substantially reduces blood to brain drug transport (drug resistance) and reduced transporter activity increases blood to brain drug transport (improved drug delivery).
The aryl hydrocarbon receptor (AhR), a member of the bHLH-PAS family of DNA-binding proteins, is a cytoplasmic protein that on ligand binding translocates to the nucleus, where it heterodimerizes with AhR nuclear translocator (ARNT) and binds to specific enhancer sequences adjacent to the promoter element of certain genes. AhR ligands include a number of planar aromatic chemicals, many of which are widespread environmental pollutants, e.g., polychlorinated biphenyls (PCBs) and dioxins (5). AhR target genes code for a subset of phase I and phase II drug-metabolizing enzymes and xenobiotic transporters (6–8). The corresponding gene products should act in concert to detoxify xenobiotic substrates and speed their elimination, although enzyme induction by AhR has also been linked to toxicant activation. The involvement of AhR in induction of ABC transporter expression is a recent finding, and work to date has focused to a large extent on mRNA expression levels in liver and intestine (8, 9).
AhR is expressed at the blood-brain barrier, where it can up-regulate expression of target phase I enzymes (7, 10). Recent studies with brain capillary endothelial cells in culture suggest that ABC transporters are AhR targets in that tissue (11). These studies focused largely on changes in transporter mRNA, so it is not clear how AhR activation would affect ABC transporter expression and function in intact brain capillaries and, most importantly, in vivo. In the present study, we demonstrate that exposing isolated rat brain capillaries to subnanomolar concentrations of the high-affinity AhR ligand 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) increases P-glycoprotein protein expression and transport activity. We show similar increases in P-glycoprotein, Mrp2, and Bcrp expression and transport activity in brain capillaries from rats dosed with TCDD. Finally, we use in situ brain perfusion to demonstrate that TCDD dosing selectively tightens the blood-brain barrier to verapamil, a drug that is a P-glycoprotein substrate. These findings suggest a new paradigm for the field of environmental toxicology: toxicants acting through AhR to target blood-brain barrier efflux transporters and reduce the brain accumulation of CNS-acting therapeutic drugs.
MATERIALS AND METHODS
Materials
Mouse polyclonal P-glycoprotein antibody was from Covance (Princeton, NJ, USA), and antibodies against Mrp2, Mrp4, and Bcrp were from Alexis Biochemicals (San Diego, CA, USA). Cyp1a1 antibody was purchased from Xenotech, LLC (Lenexa, KS, USA) and Cyp1b1 antibody was obtained from Aviva System Biology (San Diego, CA, USA). NBD-CSA was custom synthesized (12), and BODIPY-prazosin was purchased from Invitrogen (Eugene, OR, USA). PSC833 was kindly provided by Novartis (Basel, Switzerland). MK571 was obtained from Cayman Chemical (Ann Arbor, MI, USA). KO143 (13) was a kind gift from Dr. Alfred H. Schinkel (Netherlands Cancer Institute, Amsterdam, The Netherlands). Texas red (sulforhodamine 101 free acid), mouse monoclonal β-actin antibody, Ficoll, β-naphthoflavone (BNF), and all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA). TCDD stock solution was prepared by RTI International (Research Triangle Park, NC, USA). [14C]-sucrose (specific activity 300 mCi/mmol) and [3H]-verapamil (specific activity 80 Ci/mmol) were obtained from American Radiolabeled Chemicals (St. Louis, MO, USA). All reagents were of analytical grade or the best available pharmaceutical grade.
Animals
All experiments were performed in compliance with the National Institutes of Health animal care and use guidelines and approved by the Animal Care and Use Committee of the National Institute of Environmental Health Sciences. Male retired breeder Sprague-Dawley rats (6–9 mo; Taconic Farms, Germantown, NY, USA) for in vitro experiments, or male Sprague-Dawley adult rats (250–300 g; Charles River Laboratories, Raleigh, NC, USA) for in vivo experiments, were housed in temperature-controlled rooms under a 12-h light-dark cycle and were given ad libitum access to food and water. Animals were euthanized by CO2 inhalation followed by decapitation. For in vivo dosing of adult rats, TCDD dissolved in corn oil (Sigma) was administrated by i.p. injection at a single dose of 1 or 5 μg/kg. Controls received an equal volume of corn oil. After 48 h, rats were euthanized, and brain capillaries were isolated and immediately used for transport experiments and capillary membrane isolation (for subsequent Western blot analysis).
Capillary isolation
Detailed procedures for capillary isolation were described previously (14, 15). Briefly, white matter, meninges, midbrain, choroid plexus, blood vessels, and olfactory lobes were removed from the brains under a dissecting microscope, and brain tissue was homogenized. Tissue was kept in cold PBS (2.7 mM KCl, 1.5 mM KH2PO4, 136.9 mM NaCl, 8.1 mM Na2HPO4, 1 mM CaCl2, 0.5 mM MgCl2, 5 mM d-glucose and 1 mM sodium pyruvate) throughout the isolation procedure. An aliquot of 30% Ficoll was added to an equal volume of brain homogenate, and capillaries were separated from the parenchyma by centrifuging at 5800 g for 20 min. Capillary pellets were washed with 1% BSA in PBS and passed through a syringe column filled with glass beads. Capillaries bound to the glass beads were released by gentle agitation, then washed with PBS and used immediately.
Transport assay
Confocal microscopy-based transport assays with isolated rat brain capillaries have been described previously (14). All experiments were carried out at room temperature in coverslip-bottomed imaging chambers filled with PBS. Protocols for specific experiments are described in respective figure legends. In general, brain capillaries were exposed for 3–4 h to AhR ligands (BNF or TCDD) without or with additional inhibitors. Fluorescent substrates NBD-CSA for P-glycoprotein (14, 15), TX red for Mrp2 (16) and BODIPY-prazosin for Bcrp (17) were added, and luminal substrate accumulation was assessed 1 h later. In some experiments, specific transport inhibitors were included in the incubation medium. To acquire images, the chamber containing the capillaries was mounted on the stage of a Zeiss Model 510 inverted confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany) and imaged through a ×40 water-immersion objective (numeric aperture 1.2) using a 488-nm laser line for NBD-CSA and BODIPY-prazosin or a 543-nm laser line for Texas red. Images were saved to disk and luminal fluorescence was quantitated by Image J software as before (15). Data shown are for a single experiment that is representative of 3–6 replicates.
Western blots
Membranes were isolated from control and ligand-exposed capillaries as described previously (3, 14). Membrane protein was assayed by the Bradford method. An aliquot of the membrane protein was mixed with NuPAGE 4× sample buffer (Invitrogen, Carlsbad, CA, USA), loaded onto 4–12% Bis-Tris NuPAGE gel, electrophoresed, and then transferred to an Immobilon-FL membrane (Millipore, Bedford, MA, USA). The membrane was blocked with Odyssey Blocking Buffer (Li-Cor Biosciences, Lincoln, NE, USA) at room temperature for 1 h and then immunoblotted with antibodies against P-glycoprotein, Mrp2, Bcrp, or Mrp4, using the Odyssey Infrared Imaging System (Li-Cor Biosciences). The membrane was stained with corresponding goat anti-rabbit or goat anti-mouse fluorescence dyes IRDye 680 (or IRDye 800) in PBS with 0.1% Tween 20 at room temperature for 45 min and then imaged using the Odyssey Infrared Imaging System. β-Actin (42 kDa; 1:2000) was used as a loading control; the corresponding secondary antibody was IRDye goat anti-mouse antibody (1:15,000). NewBlot PVDF Stripping Buffer (5×; Li-Cor Biosciences) was used to strip the membranes when needed. The membrane was scanned to ensure complete antibody removal before reprobing.
EMSA
Nuclear protein extract was isolated from control and TCDD-induced brain capillaries using a NE-PER kit (Pierce, Rockford, IL, USA) and by following the manufacturer's instructions. Complementary DNA oligonucleotides 5′-GATCCGGCTCTTCTCACGCAACTCCGAGCTCA-3′ and 5′-TGAGCTCGGAGTTGCGTGAGAAGAGCCGGATC-3′ [dioxin response element (DRE) recognition sequence underscored] were end-labeled with IRDye700 fluorescent dyes by IDT (Coralville, IA, USA) and annealed at 100°C for 5 min (18, 19). The EMSA samples were prepared using the Odyssey Infrared EMSA Buffer Kit (Li-Cor Biosciences), with 5 μg of nuclear protein extract used in each AhR/DRE binding reaction. The binding reaction was incubated at room temperature for 20 min, and the DNA-protein complexes were resolved on a precast, 6% native polyacrylamide gel in 0.5× TBE buffer. The gel was removed from the electrophoresis unit and directly imaged on the Odyssey Infrared Imaging System. To demonstrate competition, a 200× excess of unlabeled specific consensus oligonucleotide was incubated with the nuclear extract for 10 min before the addition of the labeled probe. The gel supershift assay was performed using the above EMSA procedure except for the addition of anti-AhR monoclonal antibody (Thermo Scientific, Rockford, IL, USA) prior to the addition of the fluorescently labeled DRE probe.
In situ brain perfusion
In situ brain perfusion was carried out as described previously (20). Rats were anesthetized by i.p. injection with 1 ml/kg ketamine cocktail (79 mg/ml ketamine, 3 mg/ml zylazine, and 0.6 mg/ml acepromazine) and administered heparin (10 kU/kg). The common carotid arteries were exposed by midline incision at the neck and were perfused with oxygenated Ringer solution (37°C) (in mM): 117 NaCl, 4.7 KCl, 0.8 MgSO4, 24.8 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, and 10 d-glucose, plus 39 mg/ml 170-kDa dextran, 1 mg/ml BSA, and 0.055 mg/ml Evan's Blue) at 3 ml/min. [14C]-sucrose (0.5 μCi/ml) or [3H]-verapamil (0.1 μCi/ml) was infused into the circuit via syringe pump at 0.5 ml/min for 20 min. Samples of perfusate were collected from the cannulae at the end of each experiment. Brains were removed and stripped of meninges, midbrain, and choroid plexuses, and were minced by hand. Tissue and 100-μl perfusate samples were incubated for 2 d with tissue solubilization solution (Hyamine Hydroxide; MP Biomedicals, Santa Ana, CA, USA). Solubilized samples were prepared for scintillation counting by adding 100 μl 30% acetic acid and 4 ml liquid scintillation cocktail (CytoScint ES; MP Biomedicals), and incubation overnight in the dark. Results of scintillation counting were expressed as the ratio of radioactivity in the brain to that of perfusate (Rbr; μl/g):
where Cbrain represents radioactivity in the brain tissue (dpm/g), and Cperfusate represents radioactivity in the perfusate (dpm/μl).
Statistical analyses
Data are expressed as means ± se. Statistical analyses of differences between groups was by 1-way ANOVA (Newman-Keuls multiple-comparison test) using Prism 4.0 software (GraphPad, San Diego, CA, USA). Differences between two means were considered significant when P < 0.05.
RESULTS
In vitro TCDD exposure
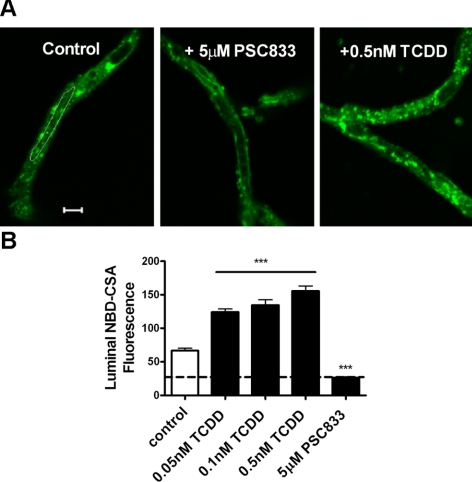
Our laboratory has established a method to measure the transport activity of ATP-driven efflux transporters in freshly isolated brain capillaries using fluorescent substrates, confocal microscopy, and digital image analysis; we have previously shown these capillaries to be viable for ≥8 h (14, 15). Transport activity is measured as the specific accumulation of a fluorescent substrate in capillary lumens. Figure 1A shows representative confocal images of rat brain capillaries incubated to steady state (60 min) with the fluorescent P-glycoprotein substrate NBD-CSA. Fluorescence was concentrated in the luminal space in the controls, and luminal fluorescence was greatly reduced when capillaries were exposed to 5 μM PSC833, a specific inhibitor of P-glycoprotein (Fig. 1A). This concentration of PSC833 causes maximal inhibition of NBD-CSA accumulation in rat brain capillaries. Thus, transport activity (specific transport) is the difference in steady-state luminal fluorescence in the absence and presence of this specific inhibitor, i.e., accumulation above that measured in capillaries exposed to 5 μM PSC833- (Fig. 1B). Previous studies indicated that residual luminal fluorescence represents nonspecific accumulation, likely from diffusive entry plus binding to cellular elements (14, 16).
Figure 1.
Increased P-glycoprotein transport activity in isolated rat brain capillaries following exposure to TCDD. A) Representative confocal images showing luminal accumulation of NBD-CSA, a fluorescent substrate for P-glycoprotein. Luminal fluorescence was reduced by 5 μM PSC833, a specific inhibitor of P-glycoprotein, and was increased following 3 h of exposure to 0.5 nM TCDD. Scale bar = 5 μm. B) Exposing rat brain capillaries to TCDD increased luminal accumulation of NBD-CSA in a concentration-dependent manner. Values are means ± se for 8–12 capillaries from a single preparation (each containing pooled brain tissue from 5–10 rats). ***P < 0.001 vs. control.
Initial experiments with the AhR ligand, BNF, showed concentration-dependent stimulation of P-glycoprotein transport activity in rat brain capillaries that was detectable after 1 h of exposure and maximal after 3 h; BNF was effective in the low micromolar concentration range (not shown). TCDD is a widespread environmental pollutant that is a high-affinity ligand for AhR (21, 22). Exposing rat brain capillaries to 0.05–0.5 nM TCDD for 3 h increased in a concentration-dependent manner luminal accumulation of NBD-CSA (Fig. 1B, P<0.001); increasing the TCDD concentration to 1 nM did not increase transport activity further (not shown). From these data, one can see that concentrations of TCDD well below 0.5 nM increased in transporter activity. In experiments with 6 separate brain capillary preparations, each using pooled brain tissue from 5–10 rats, exposure to 0.5 nM TCDD more than doubled mean specific P-glycoprotein transport activity (145 ± 20% increase, mean ± se; P<0.01).
Figure 2A shows that the increase in P-glycoprotein transport activity caused by TCDD exposure was abolished by actinomycin D and by cyclohexamide, indicating dependence on transcription and translation. Neither actinomycin D nor cyclohexamide by themselves affected P-glycoprotein transport (not shown). Increases in transport activity were also abolished by micromolar concentrations of the AhR antagonists, resveratrol (23) and α-naphthoflavone (α-NF; Fig. 2B, C). The latter chemical can act as both an AhR agonist and antagonist (24). At millimolar concentrations, α-NF stimulates dioxin receptor-ARNT heterodimerization and enhances subsequent binding of the complex to DNA response elements (25). At nanomolar to micromolar concentrations, it affects the formation of the ARNT-DNA complex and prevents transactivation (24).
Figure 2.
A) Inhibiting transcription by 1 μM actinomycin or translation by 100 μg/ml cycloheximide blocked the effects of 0.5 nM TCDD on P-glycoprotein transport activity. B, C) TCDD-induced increase in transport activity was abolished when capillaries were pretreated with the specific AhR antagonists, resveratrol (RES; B) or α-naphthoflavone (α-NF; C). Values are means ± se for 8–12 capillaries from a single preparation (pooled brains from 5–10 rats). ***P < 0.001 vs. control.
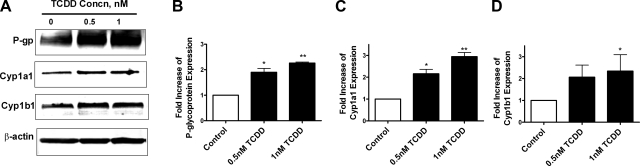
The increase in P-glycoprotein transport activity in brain capillaries exposed to TCDD was accompanied by a concentration-dependent increase in transporter protein expression (Fig. 3A). Cyp1a1 and Cyp1b1 protein expression also increased in capillaries exposed to TCDD (Fig. 3A). The genes encoding these enzymes are well-established AhR targets (26). Measurements of transporter and enzyme protein band intensities on Western blots from 3 separate capillary preparations indicated significant increases with TCDD exposure (Fig. 3B–D; P<0.05).
Figure 3.
Increased protein expression of P-glycoprotein, Cyp1a1, and Cyp1b1 in isolated rat brain capillaries following 3 h of exposure to 0.5–1 nM TCDD in vitro. A) Western blots with β-actin as a loading control. B–D) Measured band intensities in membranes from 3 preparations: P-glycoprotein (B), Cyp1a1 (C), and Cyp1b1 (D). *P < 0.05, **P < 0.01 vs. control.
In vivo TCDD exposure
To determine the effect of in vivo AhR ligand exposure on blood-brain barrier ABC transporter activity and expression, we dosed rats with a single i.p. injection of 1 or 5 μg/kg TCDD and 2 d later isolated brain capillaries and liver membranes. TCDD dose levels were chosen based on published data showing significant induction of hepatic enzymes (26, 27). Capillaries were used to determine transport activity and transporter and enzyme protein expression; liver membranes were used to determine transporter and enzyme protein expression. In these in vivo dosing experiments, we extended the focus to include two other ABC transporters expressed at the blood-brain barrier and in hepatocytes, Mrp2 and Bcrp (2, 28). Previous experiments with rat and mouse brain capillaries showed that transport activity of Mrp2 could be assayed using Texas red (sulforhodamine 101 free acid) as substrate (16), and Bcrp activity could be assayed using BODIPY-prazosin (17, 29). In those studies, MK571 and KO143 proved to be specific inhibitors of Mrp2- and BCRP-mediated transport, respectively. Figure 4 shows that dosing rats with TCDD significantly increased specific P-glycoprotein, Mrp2, and BCRP transport activity in brain capillaries (P<0.001). For P-glycoprotein and Mrp2, increases in transport activity were dose dependent. Consistent with increased transporter activity, Western blots of brain capillary membranes showed increased immunoreactivity for all three transport proteins in membranes isolated from TCDD-exposed rats (Fig. 5). As in the in vitro exposure studies, dosing rats with TCDD increased Cyp1a1 and Cyp1b1 expression in brain capillary membranes (Fig. 5A).
Figure 4.
Increased transport activity of P-glycoprotein, Mrp2, and BCRP in brain capillaries following in vivo dosing of rats with 1 μg/kg or 5 μg/kg TCDD (single i.p. injection, tissues collected 2 d later). A) Representative confocal images showed luminal accumulation of fluorescent substrates NBD-CSA (for P-glycoprotein), TX red (for Mrp2), and BODIPY-prazosin (for Bcrp) in capillaries from control and TCDD-dosed rats. Scale bar = 5 μm. B–D) Dose-dependent increases of transport activity in isolated rat brain capillaries for NBD-CSA (B), Texas Red (C), and BODIPY-prazosin (D). Values are means ± se for 8–12 capillaries from a single preparation (pooled brains from 10 rats). ***P < 0.001 vs. control.
Figure 5.
Up-regulation of transporter and enzyme protein expression in brain capillary (A) and liver (B) membranes following in vivo dosing of rats with 1 μg/kg or 5 μg/kg TCDD (single i.p. injection, tissues collected 2 d later).
TCDD dosing also increased expression of P-glycoprotein, Mrp2, Bcrp, Mrp4, Cyp1a1, and Cyp1b1 in liver membranes (Fig. 5B). Note that liver membranes from TCDD-dosed rats also showed increased protein expression for all three ABC transporters, as well as Cyp1a1 and Cyp1b1. This result was certainly expected for the enzymes, which have been long known to increase protein expression in liver and other tissues in response to AhR ligands (30, 31). For ABC transport proteins in liver, less is known about the consequences of dosing animals with AhR ligands. Although there is compelling evidence that Mrp2 mRNA and protein expression increases after dosing (32), strong corresponding evidence for P-glycoprotein and Bcrp is lacking, since the majority of published studies measured changes in transporter mRNA, not protein. Thus, in vivo exposure to TCDD increased protein expression of ABC transporters and target CYPs in both blood-brain barrier and liver.
EMSA analysis
To determine whether TCDD induction of transporter and enzyme expression in brain capillaries could reflect transcriptional activation, we conducted EMSA assays with capillary nuclear extracts and a fluorescent probe that contained a DRE consensus sequence. Exposing rat brain capillaries to 0.5–1.0 nM TCDD shifted the probe band on the gel to higher mass (Fig. 6A, lanes 3 and 4). Notably, a similar shift was seen when capillary nuclei from rats dosed with 1 or 5 μg/kg TCDD were used in the assay (Fig. 6A, lanes 6 and 7). These gels also show that the intensity of the shifted band increased with in vitro TCDD exposure concentration (Fig. 6A, lanes 2–4) or in vivo TCDD dose (Fig. 6A, lanes 5–7). No such shift was seen when 200-fold excess unlabeled probe was added to the assay (Fig. 6A, lane 8). Finally, when nuclear extract was pretreated with antibody against AhR, the band was shifted further (supershift), confirming specificity of the assay for TCDD-induced movement of AhR into the nucleus (Fig. 6B, lane 3).
Figure 6.
EMSA assay showing binding of AhR to dioxin response element (DRE) following TCDD exposure in vitro and in vivo. A) EMSA gel. Lane 1: negative control (no nuclear protein); lane 2: control (in vitro exposure); lane 3: 0.5 nM TCDD, 3 h in vitro exposure; lane 4: 1 nM TCDD, 3 h in vitro exposure; lane 5: control (in vivo exposure); lane 6: 1 μg/kg TCDD; lane 7: 5 μg/kg TCDD; lane 8: 5 μg/kg TCDD plus 200× excess of unlabeled probe. B) Supershift with antibody against AhR. Lane 1: negative control; lane 2: 5 μg/kg TCDD; lane 3: 5 μg/kg TCDD plus antibody.
In situ brain perfusion
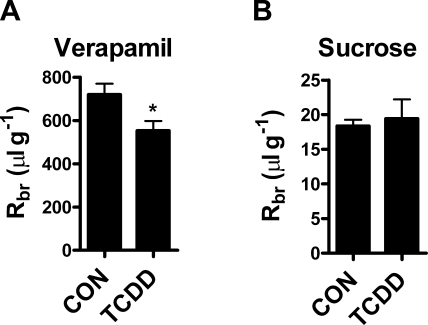
The TCDD-induced increases in brain capillary P-glycoprotein transport activity and protein expression in vivo imply selective strengthening of the blood-brain barrier to P-glycoprotein substrates. To evaluate this possibility, we used in situ brain perfusion to measure brain accumulation of the P-glycoprotein substrate verapamil in control and 5 μg/kg TCDD-dosed rats. In situ brain perfusion is a well-established method for measuring net transport of molecules across the blood-brain barrier (33). One advantage of this approach for the current study is that transport is measured under conditions of constant flow and tracer concentration, thus controlling for hemodynamic changes that might be caused by TCDD dosing. Further, since the tracer is administered directly into the cerebral circulation via the carotid artery, changes in brain distribution reflect alterations in blood-brain barrier function, without having to correct for possible changes in peripheral excretion and metabolism of the test drug.
Verapamil is used frequently as a prototypical P-glycoprotein substrate; knockout of the mdr1a gene (coding for P-glycoprotein) in mice is associated with a 5- to 6-fold increase in brain uptake of verapamil (34). In rats, adding 5 μM CSA to the perfusate increases brain uptake of verapamil ∼5-fold (4, 20). We recently demonstrated that signals that diminish P-glycoprotein-mediated transport activity in isolated brain capillaries also increase brain distribution of verapamil in vivo (4, 20). If TCDD dosing increased P-glycoprotein transport activity at the blood-brain barrier, we would expect to see the opposite effect, that is, reduced brain uptake of verapamil, which has recently been demonstrated following chronic antidepressant treatment (35). Figure 7A shows that TCDD dosing of rats significantly reduced brain distribution of the P-glycoprotein substrate verapamil to 76% of the control value (553±45 ml/g in dosed rats vs. 720±50 ml/g in controls, Fig. 7A). Brain distribution of sucrose (an indicator of tight junction integrity) in TCDD-dosed rats was 19.4 ± 2.8 ml/g, not significantly different from controls (18.4±0.9 ml/g), indicating that neither tight junction-restricted permeability nor vascular volume were altered by TCDD (Fig. 7B). Taken together, our in vivo experiments show that TCDD dosing increased transport activity and protein expression of ABC transporters in rat brain capillaries. Consistent with increased P-glycoprotein activity, TCDD dosing significantly reduced the brain distribution of a prototypical P-glycoprotein substrate.
Figure 7.
Reduced brain uptake of P-glycoprotein substrate verapamil in TCDD-dosed rats. A) Rats received a single injection of TCDD (5 μg/kg). After 2 d, rat brains were perfused via the common carotid arteries with Ringer solution containing either [14C]-sucrose or [3H]-verapamil. TCDD dosing significantly reduced brain distribution of the P-glycoprotein substrate verapamil compared to control, indicating increased P-glycoprotein activity. B) Brain distribution of sucrose in TCDD-treated rats did not differ significantly from controls, indicating that neither tight junction-restricted permeability nor vascular volume was altered by TCDD.
DISCUSSION
The present results establish the blood-brain barrier as a dioxin target tissue. In doing so, they disclose a mechanism through which environmental toxicants alter normal transport function of the barrier, likely increasing neuroprotection, but importantly further restricting delivery of therapeutic drugs to the CNS. Dioxins are widespread and persistent environmental toxicants that are high-affinity ligands for AhR, a ligand-activated xenoreceptor. AhR is expressed in multiple tissues, including brain (36), brain capillaries, and brain capillary endothelial cells (10, 11). TCDD exposure in vitro and in vivo induces in brain capillaries expression of xenobiotic metabolizing enzymes that are prototypical AhR targets, i.e., Cyp1a1 and Cyp1b1 (7, 11, 37), findings confirmed in the present study. A number of adverse neurological effects, such as, cognitive dysfunction and retarded development, have been documented in studies of dioxin-dosed animals and of individuals occupationally exposed to AhR ligands, e.g., dioxins and PCBs (5, 38–40). These chemicals are also developmental neurotoxicants, targeting critical processes in the developing brains of embryos and neonates (41, 42). A recent study indicates disrupted function of blood-brain barrier tight junctions in mice that were dosed with three PCB congeners (75–150 μg/kg). Because such effects were seen with PCBs that were and were not AhR ligands, the authors concluded that barrier disruption was not mediated by the AhR (43).
Here, our primary focus was on ATP-driven xenobiotic efflux transporters located on the luminal (blood-facing) plasma membrane of brain capillary endothelial cells. First, we show that exposing isolated rat brain capillaries to subnanomolar concentrations of TCDD for 3 h increased both specific transport activity and protein expression for P-glycoprotein. The increase in P-glycoprotein transport activity was abolished when capillaries were exposed to actinomycin D or cyclohexamide, indicating dependence on transcription and translation. Consistent with TCDD action through AhR, resveratrol and α-NF, both AhR antagonists, abolished the TCDD-induced increases in P-glycoprotein transport activity; EMSA analysis of brain capillary nuclear extracts showed TCDD-induced translocation of AhR to the nucleus. The simplest explanation for these findings is that nuclear translocation and increased transporter expression are directly linked through AhR-ARNT binding to AREs within the promoter region of the transporter gene. However, we cannot rule out the possibility that AhR transactivates P-glycoprotein through an indirect mechanism, e.g., through another transcription factor such as p53 (44).
Second, we show that brain capillaries from TCDD-dosed rats exhibited substantially increased transport activity and protein expression for P-glycoprotein and two other ABC transporters: Mrp2 and Bcrp. For P-glycoprotein, increases in transport activity and protein expression in rats dosed with 5 μg/kg TCDD were comparable to those seen in brain capillaries exposed to 0.5 nM TCDD for 3 h; the latter in vitro exposure maximally increased transport activity. Consistent with transporter up-regulation, TCDD-dosed rats exhibited significantly reduced brain accumulation of verapamil, a drug that is a P-glycoprotein substrate (in situ brain perfusion). TCDD dosing did not change brain uptake of sucrose, a small solute used to monitor changes in blood-brain barrier passive permeability. Thus, dioxin selectively altered transporter-mediated transport across the barrier. In this regard, we previously showed that dosing mice with rifampin, a ligand for the human pregnane-X receptor (PXR), increased blood-brain barrier P-glycoprotein expression by ∼2–3-fold and reduced by 70% the efficacy of injected methadone in an antinociception assay (2). The latter observation was indicative of reduced methadone entry into the brain in response to rifampin dosing and subsequent induction of P-glycoprotein at the blood-brain barrier.
Studies in which genetic and pharmacological tools were used to knock out P-glycoprotein indicate that this transporter is a primary obstacle to entry into the brain for many therapeutic drugs and a near-absolute barrier for some (45, 46). For those CNS-acting drugs that are P-glycoprotein substrates, increased transporter expression is equivalent to selective tightening of the blood-brain barrier, a phenomenon first documented in our study of transporter induction in transgenic mice by human PXR ligands (2). Moreover, for some drugs that are neurotoxicants, e.g., ivermectin and its derivatives (47), and for some endogenous neurotoxicants, e.g., β-amyloid protein (29), P-glycoprotein serves a neuroprotective role by limiting brain accumulation. In contrast to blood-brain barrier P-glycoprotein, which influences brain uptake of a remarkably wide range of therapeutic drugs, Bcrp appears to affect brain uptake of only a few drugs, e.g., topotecan and imatinib (48). Despite the long availability of two strains of Mrp2-null rats (and, more recently, of Mrp2-null mice), we are hard pressed to name any drugs for which Mrp2 is a dominant efflux transporter at the blood-brain barrier. It is likely, however, that, as in liver and kidney (49), Mrp2 contributes to brain to blood transport of potentially toxic endogenous metabolic wastes and xenobiotic metabolites, e.g., glutathione conjugates, which are organic anions.
It is now clear that expression of P-glycoprotein, Mrp2, and Bcrp at the blood-brain barrier is modulated by ligand-activated nuclear receptors from subfamily 1 (PXR and CAR) and subfamily 3 (GR) (2, 50). Certainly, in brain capillaries as in liver (28), these nuclear receptors appear to coordinately up-regulate multiple ABC transporters in addition to drug metabolizing enzymes. Since many ligands for PXR, CAR, and GR are therapeutic drugs, induction of enzymes and efflux transporters is one mechanism that could underlie drug-drug interactions at the blood-brain barrier.
The present results showing coordinated induction of the same three blood-brain barrier efflux transporters by the AhR ligand, TCDD, suggest that interactions between environmental toxicants and drugs should also be of concern with regard to therapeutic drug delivery to the brain. Indeed, given that TCDD (present study) and BNF (unpublished results) caused identical effects in isolated brain capillaries, one would expect other AhR ligands, e.g., other dioxins, dioxin-like PCBs, and many polycyclic aromatic hydrocarbons, to similarly affect barrier function. Certainly, induction of transporter expression at the blood-brain barrier has the potential to increase CNS drug resistance in exposed individuals and contribute to patient-to-patient variability in response to CNS-acting drugs. The extent to which exposure to AhR ligands alters the selective, transporter-dependent blood-brain barrier in the patient population remains to be determined.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health.
REFERENCES
- 1. Abbott N. J., Patabendige A. A., Dolman D. E., Yusof S. R., Begley D. J. (2009) Structure and function of the blood-brain barrier. Neurobiol. Dis. 37, 13–25 [DOI] [PubMed] [Google Scholar]
- 2. Miller D. S. (2010) Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol. Sci. 31, 246–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bauer B., Yang X., Hartz A. M., Olson E. R., Zhao R., Kalvass J. C., Pollack G. M., Miller D. S. (2006) In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol. Pharmacol. 70, 1212–1219 [DOI] [PubMed] [Google Scholar]
- 4. Rigor R. R., Hawkins B. T., Miller D. S. (2010) Activation of PKC isoform beta(I) at the blood-brain barrier rapidly decreases P-glycoprotein activity and enhances drug delivery to the brain. J. Cereb. Blood Flow Metab. 30, 1373–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. White S. S., Birnbaum L. S. (2009) An overview of the effects of dioxins and dioxin-like compounds on vertebrates, as documented in human and ecological epidemiology. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 27, 197–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deng L., Lin-Lee Y. C., Claret F. X., Kuo M. T. (2001) 2-acetylaminofluorene up-regulates rat mdr1b expression through generating reactive oxygen species that activate NF-κB pathway. J. Biol. Chem. 276, 413–420 [DOI] [PubMed] [Google Scholar]
- 7. Granberg L., Ostergren A., Brandt I., Brittebo E. B. (2003) CYP1A1 and CYP1B1 in blood-brain interfaces: CYP1A1-dependent bioactivation of 7,12-dimethylbenz (a) anthracene in endothelial cells. Drug Metab. Dispos. 31, 259–265 [DOI] [PubMed] [Google Scholar]
- 8. Jigorel E., Le Vee M., Boursier-Neyret C., Parmentier Y., Fardel O. (2006) Differential regulation of sinusoidal and canalicular hepatic drug transporter expression by xenobiotics activating drug-sensing receptors in primary human hepatocytes. Drug Metab. Dispos. 34, 1756–1763 [DOI] [PubMed] [Google Scholar]
- 9. Han Y., Sugiyama Y. (2006) Expression and regulation of breast cancer resistance protein and multidrug resistance associated protein 2 in BALB/c mice. Biol. Pharm. Bull. 29, 1032–1035 [DOI] [PubMed] [Google Scholar]
- 10. Dauchy S., Dutheil F., Weaver R. J., Chassoux F., Daumas-Duport C., Couraud P. O., Scherrmann J. M., De Waziers I., Decleves X. (2008) ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood-brain barrier. J. Neurochem. 107, 1518–1528 [DOI] [PubMed] [Google Scholar]
- 11. Dauchy S., Miller F., Couraud P. O., Weaver R. J., Weksler B., Romero I. A., Scherrmann J. M., De Waziers I., Decleves X. (2009) Expression and transcriptional regulation of ABC transporters and cytochromes P450 in hCMEC/D3 human cerebral microvascular endothelial cells. Biochem. Pharmacol. 77, 897–909 [DOI] [PubMed] [Google Scholar]
- 12. Schramm U., Fricker G., Wenger R., Miller D. S. (1995) P-glycoprotein-mediated secretion of a fluorescent cyclosporin analogue by teleost renal proximal tubules. Am. J. Physiol. Renal Physiol. 268, F46–F52 [DOI] [PubMed] [Google Scholar]
- 13. Allen J. D., van Loevezijn A., Lakhai J. M., van der Valk M., van Tellingen O., Reid G., Schellens J. H., Koomen G. J., Schinkel A. H. (2002) Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol. Cancer Ther. 1, 417–425 [PubMed] [Google Scholar]
- 14. Hartz A. M., Bauer B., Fricker G., Miller D. S. (2004) Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol. Pharmacol. 66, 387–394 [DOI] [PubMed] [Google Scholar]
- 15. Miller D. S., Nobmann S. N., Gutmann H., Toeroek M., Drewe J., Fricker G. (2000) Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol. Pharmacol. 58, 1357–1367 [DOI] [PubMed] [Google Scholar]
- 16. Bauer B., Hartz A. M., Lucking J. R., Yang X., Pollack G. M., Miller D. S. (2008) Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase-II metabolizing enzyme, GSTpi, at the blood-brain barrier. J. Cereb. Blood Flow Metab. 28, 1222–1234 [DOI] [PubMed] [Google Scholar]
- 17. Shukla S., Zaher H., Hartz A., Bauer B., Ware J. A., Ambudkar S. V. (2009) Curcumin inhibits the activity of ABCG2/BCRP1, a multidrug resistance-linked ABC drug transporter in mice. Pharm. Res. 26, 480–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Joiakim A., Mathieu P. A., Palermo C., Gasiewicz T. A., Reiners J. J., Jr. (2003) The Jun N-terminal kinase inhibitor SP600125 is a ligand and antagonist of the aryl hydrocarbon receptor. Drug Metab. Dispos. 31, 1279–1282 [DOI] [PubMed] [Google Scholar]
- 19. Minsavage G. D., Park S. K., Gasiewicz T. A. (2004) The aryl hydrocarbon receptor (AhR) tyrosine 9, a residue that is essential for AhR DNA binding activity, is not a phosphoresidue but augments AhR phosphorylation. J. Biol. Chem. 279, 20582–20593 [DOI] [PubMed] [Google Scholar]
- 20. Hawkins B. T., Sykes D. B., Miller D. S. (2010) Rapid, reversible modulation of blood-brain barrier P-glycoprotein transport activity by vascular endothelial growth factor. J. Neurosci. 30, 1417–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gasiewicz T. A., Bauman P. A. (1987) Heterogeneity of the rat hepatic Ah receptor and evidence for transformation in vitro and in vivo. J. Biol. Chem. 262, 2116–2120 [PubMed] [Google Scholar]
- 22. Reyes H., Reisz-Porszasz S., Hankinson O. (1992) Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science 256, 1193–1195 [DOI] [PubMed] [Google Scholar]
- 23. Casper R. F., Quesne M., Rogers I. M., Shirota T., Jolivet A., Milgrom E., Savouret J. F. (1999) Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol. Pharmacol. 56, 784–790 [PubMed] [Google Scholar]
- 24. Wilhelmsson A., Whitelaw M. L., Gustafsson J. A., Poellinger L. (1994) Agonistic and antagonistic effects of alpha-naphthoflavone on dioxin receptor function. Role of the basic region helix-loop-helix dioxin receptor partner factor Arnt. J. Biol. Chem. 269, 19028–19033 [PubMed] [Google Scholar]
- 25. Santostefano M., Merchant M., Arellano L., Morrison V., Denison M. S., Safe S. (1993) alpha-Naphthoflavone-induced CYP1A1 gene expression and cytosolic aryl hydrocarbon receptor transformation. Mol. Pharmacol. 43, 200–206 [PubMed] [Google Scholar]
- 26. Harrigan J. A., McGarrigle B. P., Sutter T. R., Olson J. R. (2006) Tissue specific induction of cytochrome P450 (CYP) 1A1 and 1B1 in rat liver and lung following in vitro (tissue slice) and in vivo exposure to benzo(a)pyrene. Toxicol. In Vitro 20, 426–438 [DOI] [PubMed] [Google Scholar]
- 27. Drahushuk A. T., McGarrigle B. P., Tai H. L., Kitareewan S., Goldstein J. A., Olson J. R. (1996) Validation of precision-cut liver slices in dynamic organ culture as an in vitro model for studying CYP1A1 and CYP1A2 induction. Toxicol. Appl. Pharmacol. 140, 393–403 [DOI] [PubMed] [Google Scholar]
- 28. Klaassen C. D., Aleksunes L. M. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol. Rev. 62, 1–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hartz A. M., Miller D. S., Bauer B. Restoring blood-brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer's disease. Mol. Pharmacol. 77, 715–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ko H. P., Okino S. T., Ma Q., Whitlock J. P., Jr. (1996) Dioxin-induced CYP1A1 transcription in vivo: the aromatic hydrocarbon receptor mediates transactivation, enhancer-promoter communication, and changes in chromatin structure. Mol. Cell. Biol. 16, 430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nebert D. W., Roe A. L., Dieter M. Z., Solis W. A., Yang Y., Dalton T. P. (2000) Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem. Pharmacol. 59, 65–85 [DOI] [PubMed] [Google Scholar]
- 32. Johnson D. R., Klaassen C. D. (2002) Regulation of rat multidrug resistance protein 2 by classes of prototypical microsomal enzyme inducers that activate distinct transcription pathways. Toxicol. Sci. 67, 182–189 [DOI] [PubMed] [Google Scholar]
- 33. Smith Q. R., Allen D. D. (2003) In situ brain perfusion technique. Methods Mol. Med. 89, 209–218 [DOI] [PubMed] [Google Scholar]
- 34. Dagenais C., Zong J., Ducharme J., Pollack G. M. (2001) Effect of mdr1a P-glycoprotein gene disruption, gender, and substrate concentration on brain uptake of selected compounds. Pharm. Res. 18, 957–963 [DOI] [PubMed] [Google Scholar]
- 35. De Klerk O. L., Bosker F. J., Willemsen A. T., van Waarde A., Visser A. K., de Jager T., Dagyte G., den Boer J. A., Dierckx R. A., Meerlo P. (2009) Chronic stress and antidepressant treatment have opposite effects on P-glycoprotein at the blood-brain barrier: an experimental PET study in rats. J. Psychopharmacol 24, 1237–1242 [DOI] [PubMed] [Google Scholar]
- 36. Kainu T., Gustafsson J. A., Pelto-Huikko M. (1995) The dioxin receptor and its nuclear translocator (Arnt) in the rat brain. Neuroreport 6, 2557–2560 [DOI] [PubMed] [Google Scholar]
- 37. Filbrandt C. R., Wu Z., Zlokovic B., Opanashuk L., Gasiewicz T. A. (2004) Presence and functional activity of the aryl hydrocarbon receptor in isolated murine cerebral vascular endothelial cells and astrocytes. Neurotoxicology 25, 605–616 [DOI] [PubMed] [Google Scholar]
- 38. Mandal P. K. (2005) Dioxin: a review of its environmental effects and its aryl hydrocarbon receptor biology. J. Comp. Physiol. B 175, 221–230 [DOI] [PubMed] [Google Scholar]
- 39. Bertazzi P. A., Bernucci I., Brambilla G., Consonni D., Pesatori A. C. (1998) The Seveso studies on early and long-term effects of dioxin exposure: a review. Environ. Health Perspect. 106(Suppl. 2), 625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hong S. J., Grover C. A., Safe S. H., Tiffany-Castiglioni E., Frye G. D. (1998) Halogenated aromatic hydrocarbons suppress CA1 field excitatory postsynaptic potentials in rat hippocampal slices. Toxicol. Appl. Pharmacol. 148, 7–13 [DOI] [PubMed] [Google Scholar]
- 41. Collins L. L., Williamson M. A., Thompson B. D., Dever D. P., Gasiewicz T. A., Opanashuk L. A. (2008) 2,3,7,8-Tetracholorodibenzo-p-dioxin exposure disrupts granule neuron precursor maturation in the developing mouse cerebellum. Toxicol. Sci. 103, 125–136 [DOI] [PubMed] [Google Scholar]
- 42. Nayyar T., Zawia N. H., Hood D. B. (2002) Transplacental effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the temporal modulation of Sp1 DNA binding in the developing cerebral cortex and cerebellum. Exp. Toxicol. Pathol. 53, 461–468 [DOI] [PubMed] [Google Scholar]
- 43. Seelbach M., Chen L., Powell A., Choi Y. J., Zhang B., Hennig B., Toborek M. (2010) Polychlorinated biphenyls disrupt blood-brain barrier integrity and promote brain metastasis formation. Environ. Health Perspect. 118, 479–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mathieu M. C., Lapierre I., Brault K., Raymond M. (2001) Aromatic hydrocarbon receptor (AhR).AhR nuclear translocator- and p53-mediated induction of the murine multidrug resistance mdr1 gene by 3-methylcholanthrene and benzo(a)pyrene in hepatoma cells. J. Biol. Chem. 276, 4819–4827 [DOI] [PubMed] [Google Scholar]
- 45. Agarwal S., Sane R., Gallardo J. L., Ohlfest J. R., Elmquist W. F. (2010) Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J. Pharmacol. Exp. Ther. 334, 147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen Y., Agarwal S., Shaik N. M., Chen C., Yang Z., Elmquist W. F. (2009) P-glycoprotein and breast cancer resistance protein influence brain distribution of dasatinib. J. Pharmacol. Exp. Ther. 330, 956–963 [DOI] [PubMed] [Google Scholar]
- 47. Geyer J., Gavrilova O., Petzinger E. (2009) Brain penetration of ivermectin and selamectin in mdr1a, b P-glycoprotein- and bcrp-deficient knockout mice. J. Vet. Pharmacol. Ther. 32, 87–96 [DOI] [PubMed] [Google Scholar]
- 48. Noguchi K., Katayama K., Mitsuhashi J., Sugimoto Y. (2009) Functions of the breast cancer resistance protein (BCRP/ABCG2) in chemotherapy. Adv. Drug Deliv. Rev. 61, 26–33 [DOI] [PubMed] [Google Scholar]
- 49. Zhou S. F., Wang L. L., Di Y. M., Xue C. C., Duan W., Li C. G., Li Y. (2008) Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr. Med. Chem. 15, 1981–2039 [DOI] [PubMed] [Google Scholar]
- 50. Wang X., Sykes D. B., Miller D. S. (2010) Constitutive androstane receptor-mediated upregulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. Mol. Pharmacol. 78, 376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]