Abstract
We recently reported that c-Jun N-terminal kinase (JNK) is associated with adherens junctions and phosphorylates β-catenin at serine 33/37 and threonine 41. Here, we report that inhibition of JNK led to formation of adherens junctions, which was accompanied by dissociation of α-catenin from the β-catenin/E-cadherin complex and increased association of α-catenin with the cytoskeleton. Conversely, activation of JNK increased binding of α-catenin to β-catenin, which was blocked by the JNK inhibitor SP600125 or JNK siRNA. In addition, inhibition of JNK failed to lead to adherens junction formation in cells where α-catenin was absent or knocked down. Conversely, introduction of α-catenin restored the responsiveness of cells to JNK inhibition and led to cell-cell adhesion. Experiments with domain deletion mutants showed that binding of α-catenin to β-catenin was required for transport of adherens junction complexes to the cell surface, while binding to actin was required for translocation to the cell-cell contact sites. Collectively, our results suggest that JNK affects the association of α-catenin with the adherens junction complex and regulates adherens junctions.—Lee, M.-H., Padmashali, R., Koria, P., Andreadis, S. T. JNK regulates binding of α-catenin to adherens junctions and cell-cell adhesion.
Keywords: β-catenin, junctional actin
Adherens junctions (AJs) are crucial to determining polarity and organization of epithelial cells in 3-dimensional tissues (1). To date, several observations demonstrated that dynamic actin reorganization guides AJ formation (2–4). Initially, prominent filopodia from one cell embed into the membrane of a neighboring cell followed by homophilic binding of E-cadherins to form puncta and eventually an adhesion zipper (5). Over time, actin bundles are formed to stabilize the assembled AJ (6–7). Rho GTPases, including RhoA, Rac1, and Cdc42, which are known to promote stress actin fibers and filopodia are required in AJ formation (8–9). Although the steps of AJ formation have been well documented, the regulatory mechanisms are not completely understood.
Recently, α-catenin in the AJ complex was deemed a compelling target in regulation of cell-cell adhesion because of its ability to control the organization of actin filaments (10–11). Specifically, when α-catenin is released from β-catenin, it forms homodimers that suppress the binding between Arp2/3 and actin filaments, resulting in inhibition of branched actin formation. Subsequently, α-catenin homodimers serve as cross-linkers to compact actin filaments into the bundle structures that stabilize cell-cell adhesion. Previous studies reported that phosphorylation of β-catenin affected its binding to α-catenin (12–14), but the pathways and the biological context where these interactions take place remain to be elucidated.
Recently, we reported that c-Jun N-terminal kinase (JNK) associated with the AJ complex and when activated it phosphorylated β-catenin and led to disruption of AJs and epithelial cell colony dispersion. Conversely, inhibition of JNK's kinase activity promoted AJ formation, even in low Ca2+ medium (15). In this study, we report that the JNK kinase activity was required to maintain the association of α-catenin with the AJ complex. Inhibition of JNK led to dissociation of α-catenin from β-catenin/E-cadherin and the formation of AJs in normal epidermal cells but not in cells lacking α-catenin or when α-catenin was knocked down using shRNA. Exogenous supplementation of α-catenin restored the ability of cells to form AJs, a process that required binding of α-catenin to both of β-catenin and actin. These findings may have wide implications in understanding the process of cell-cell communication, formation of epithelial tissues or cancer metastasis.
MATERIALS AND METHODS
Immunofluorescence and Western blots
Primary keratinocytes were isolated from neonatal human foreskins, as described before (16). ME180 cells were cultured and passed routinely in DMEM supplemented with 10% FBS (Invitrogen, Carlsbad, CA, USA). For blocking JNK experiments, 3 × 105 cells were seeded in 24-well plates and allowed to attach and spread overnight (12–15 h) in low-calcium (50 nM) keratinocyte-SFM medium (Invitrogen). The next day, the cells were left untreated (control) or treated with SP600125 (10 μM) for 30 min, then fixed with 4% formaldehyde and stained. Immunofluorescence was performed as described previously (17–18). The following antibodies were used at the indicated conditions: mouse anti-human E-cadherin (1:125 dilution, 4°C overnight; BD Transduction Laboratories, San Diego, CA, USA), rabbit anti-human β-catenin (1:200 dilution, 4°C overnight; Cell Signaling Technology, Danvers, MA, USA), rabbit anti-human αE-catenin (1:100 dilution, 4°C overnight, Sigma, St. Louis, MO, USA), mouse anti HA-tag (1:100 dilution, 4°C overnight; Cell Signaling Technology) and phalloidin (1:50 dilution, room temperature for 20 min; Invitrogen). Images of the stained cells were acquired using a confocal microscope (LSM 510; Zeiss, Oberkochen, Germany) equipped with a digital camera and Zeiss LSM Image Examiner software.
In Western blots, the following antibodies were used: rabbit anti-human E-cadherin (1:1000 in 5% BSA, Cell Signaling Technology); rabbit anti-human β-catenin (1:1000 in 5% BSA; Cell Signaling Technology); rabbit anti-human αE-catenin (1:1000 in 5% BSA; Sigma); rabbit anti HA-tag (1:1,000 in 2% milk; Sigma); mouse anti Flag (1:1000 in 2% milk; Sigma), or mouse anti-human β-actin (1:2500 in 5% milk; Sigma) overnight at 4°C.
Lentiviral expression constructs
Lentiviral vector of Flag-JNK1DN was generated as described before (15). Similarly, HA-αE-catenin was cloned downstream of the CMV promoter between the NheI and SacII sites of pCS-CG lentiviral vector (Addgene, Cambridge, MA, USA), which encodes for EGFP after the internal ribosome entry site (IRES). To generate different domains of αE-catenin, several primers listed in Supplemental Table S1 were used in PCR reactions. Subsequently, the PCR products were inserted into pCS-CG lentiviral vector. The shRNA targeting αE-catenin (5′-GGACCTGCTTTCGGAGTACAT-3′) was generated by cloning downstream of the H1 promoter between the MluI and ClaI sites in pLVTHM lentiviral vector (Addgene). All inserts were verified by sequencing.
Cell extraction and immunoprecipitation
Cells were plated (106 cells/well) in 6-well plates overnight. After the indicated treatment, 0.1% Triton X-100 supplemented with 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 mM β-glycerophosphate, and a cocktail of protease inhibitors (Roche Diagnostics, Indianapolis, IN, USA) was added to the cells for 10 min on ice. After centrifugation (10 min at 4°C), the Triton X-100 soluble supernatant was collected, and the remaining insoluble cell pellets were triturated in SDS sample buffer (Cell Signaling Technology).
For immunoprecipitation, Triton X-100 soluble supernatant (200 μl) was incubated with mouse anti-human β-catenin (1:200; BD Biosciences, San Jose, CA, USA) primary antibodies overnight at 4°C with constant agitation. The next day, protein G-agarose beads (20 μl; Rockland, Gilbertsville, PA, USA) were added to the lysate and incubated at 4°C with constant agitation for 3 h. The beads were spun down at 4°C and washed 5 times with ice-cold RIPA buffer. The pellet was then resuspended in 3× sample buffer, and bound proteins were analyzed using Western blot analysis, as described above.
Statistical analysis
Statistical analysis of the data was performed using a 2-tailed Student's t test (α=0.05) in Microsoft Excel (Microsoft, Redwood, CA, USA). Each experiment was repeated ≥3 times unless indicated otherwise (n=3–4).
RESULTS
Inhibition of JNK induces translocation of E-cadherin/β-catenin to cell-cell contact sites and reorganization of the actin cytoskeleton
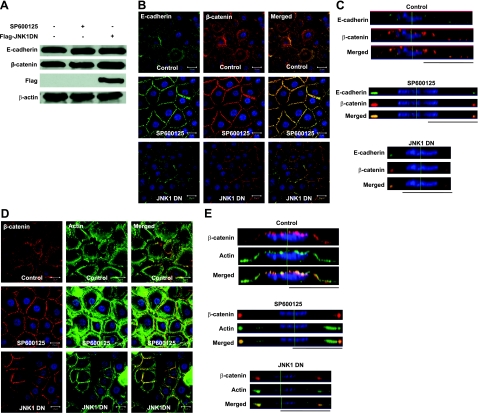
Our recent study showed that JNK associates with the AJ complex and phosphorylates β-catenin (15). Indeed, blocking JNK by a chemical inhibitor (SP600125) or expression of dominant negative JNK1 (JNK1 DN) in human primary keratinocytes induced translocation of E-cadherin and β-catenin to the cell-cell adhesion sites (Fig. 1B) and formation of tight colonies (see Supplemental Movie S1) without altering the amount of either protein (Fig. 1A). Furthermore, the lateral images constructed from confocal z-stack images showed that while in control cells, the majority of E-cadherin and β-catenin molecules were distributed uniformly along the apical side of the cell surface, on JNK inhibition, they concentrated at the cell-cell contact sites (Fig. 1C). These data suggested that inhibition of JNK-induced translocation of E-cadherin and β-catenin from the apical surface to cell-cell contact sites where AJs are formed. Interestingly, JNK inhibition also induced significant reorganization of the actin cytoskeleton. Specifically, actin-rich membrane extensions such as filopodia disappeared, and actin reorganized into thick bundles that aligned parallel to AJs (Fig. 1D, E). Sequential x,y cross-sectional images are presented in Supplemental Fig. S1, and confocal z-stack images showing two adjacent cells are presented in Supplemental Fig. S5A.
Figure 1.
Inhibition of JNK promotes translocation of E-cadherin and β-catenin to the cell-cell adhesion sites and formation of actin bundles. Human primary keratinocytes were treated with 10 μM SP600125 for 30 min or transduced with recombinant lentivirus encoding for flag-JNK1DN. A) Cell lysates were subjected to Western blotting for E-cadherin, β-catenin, Flag, and β-actin. B–E) Confocal (B, D) and lateral images (C, E) of cells stained for β-catenin (red) and E-cadherin (green; B, C) or phalloidin (green; D, E). Cell nuclei were stained with Hoechst (blue). Lateral images were formed by maximum intensity projection from confocal z-stack images. View: ×63. Scale bars = 20 μm.
JNK inhibition dissociates α-catenin from the β-catenin/E-cadherin complex
The commonly accepted dogma is that binding of α-catenin to β-catenin helps docking of the AJs to the actin cytoskeleton, while α-catenin homodimers inhibit lamellipodia formation and strengthen cell-cell adhesion. However, conventional wisdom was recently challenged by studies showing that α-catenin can bind to either β-catenin or to actin but not both at the same time (10). These results prompted us to examine whether binding of α-catenin to β-catenin was dependent on JNK activation.
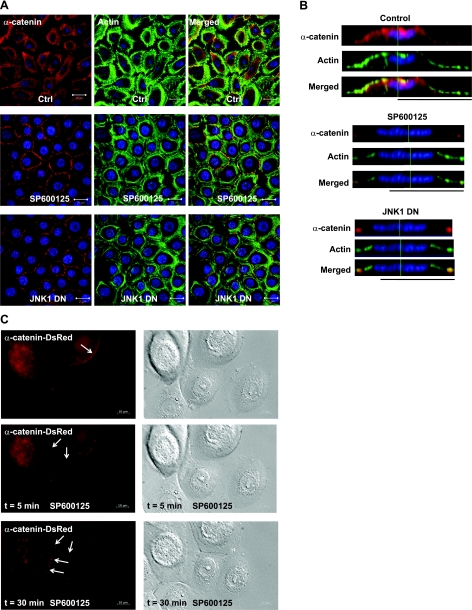
Similar to β-catenin and E-cadherin, immunostaining showed that α-catenin colocalized with junctional actin on inhibition of JNK (Fig. 2A, B). In control cells, α-catenin was distributed on the apical side of the plasma membrane, as well as in the cell cytoplasm, and actin-rich lamellipodia and philopodia protruded from the cell surface. On JNK inhibition, α-catenin localized at the cell junctions, and actin formed bundles parallel to the cell surface right underneath. Overexpression of α-catenin-DsRed fusion protein in primary keratinocytes confirmed these observations and enabled real-time monitoring of α-catenin translocation to the newly formed cell-cell contact sites following JNK inhibition (Fig. 2C and Supplemental Movie S2).
Figure 2.
α-Catenin translocates to cell-cell contact sites on inhibition of JNK. Human primary keratinocytes remained untreated (ctrl), they were treated with SP600125 (SP600125), or they were genetically modified to express dominant-negative JNK1 (JNK1DN). A) Confocal images of the cells stained with α-catenin (red) and actin phalloidin (green). Cell nuclei were stained with Hoechst (blue). Scale bars = 20 μm. B) Lateral images were formed by maximum intensity projection from confocal z-stack images. C) Human primary keratinocytes were transduced with recombinant lentivirus encoding for α-catenin-DsRed. On SP600125 (20 μM) treatment, α-catenin-DsRed-expressing cells were followed using time-lapse microscopy. View: ×63.
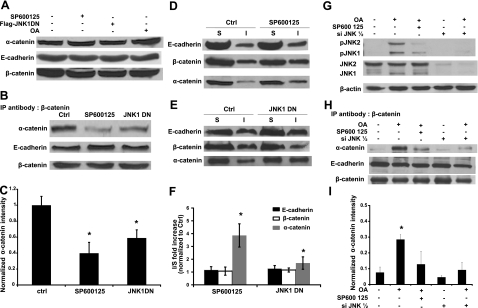
Although the amount of α-catenin, β-catenin, and E-cadherin remained the same on inhibition of JNK (Fig. 3A), immunoprecipitation with anti-β-catenin showed that the association of α-catenin with β-catenin decreased significantly, but β-catenin remained bound to E-cadherin (Fig. 3B, C). This result prompted us to determine whether the association of α-catenin with the actin cytoskeleton was affected by the activity of JNK. Under control conditions, a small fraction of α-catenin proteins was associated with the cytoskeleton (Fig. 3D, E). Interestingly, blocking JNK by SP600125 or JNK1DN increased the amount of α-catenin in the Triton-X-insoluble fraction of cell extracts, while the relative fraction of β-catenin and E-cadherin remained unchanged (Fig. 3F). These results showed that inhibition of JNK enhanced association of α-catenin—but not β-catenin or E-cadherin—with the actin cytoskeleton, suggesting that α-catenin may be binding to actin following its dissociation from β-catenin.
Figure 3.
JNK kinase activity is necessary for the association of α-catenin with β-catenin and E-cadherin. Human primary keratinocytes remained untreated (ctrl), they were treated with SP600125 (SP600125, 10 μM), they were genetically modified to express dominant negative JNK1, or they were treated with okadaic acid (OA, 500 nM). A) Cell lysates were subjected to Western blotting with antibody against α-catenin. E-cadherin and β-catenin were used as loading control. B) Cell lysates were immunoprecipitated with an antibody against β-catenin and subsequently subjected to Western blotting for α-catenin and E-cadherin. β-Catenin was used as loading control. C) Lane intensity of α-catenin was analyzed by Kodak gel documentation software and normalized to β-catenin. D, E) After Triton-X100 extraction, soluble (S) and insoluble (I) fractions were separated and subjected to Western blotting for E-cadherin, β-catenin, and α-catenin. F) For each of the indicated proteins, the ratio of the band intensity of the Triton X-100 insoluble over the soluble fractions after JNK inhibition (SP600125 or JNK1DN) was normalized to the same ratio of control cells. G) A431 or siJNK1/2 A431 cells were treated with okadaic acid (OA, 500 nM) for 30 min in the presence or absence SP600125 (10 μM). Cell lysates were subjected to Western blotting for phosphor JNK and total JNK; β-actin served as a loading control. H) Cells were treated as in G, and cell lysates were immunoprecipitated with antibody against β-catenin and subsequently subjected to Western blotting for α-catenin and E-cadherin. β-Catenin was used as loading control. I) Lane intensity of α-catenin was analyzed by Kodak gel documentation software and normalized to β-catenin. *P < 0.05 vs. control.
JNK activation increases the association of α-catenin to the β-catenin/E-cadherin complex
Following previous studies (13, 15, 19), we employed okadaic acid (OA) to activate JNK transiently in A431 cells and examined binding of β-catenin to α-catenin using immunoprecipitation. Under control conditions (10% FBS), A431 cells formed colonies, JNK phosphorylation was minimal, and α-catenin was not associated with β-catenin. Interestingly, OA treatment induced phosphorylation of JNK (Fig. 3G) and increased binding of α-catenin to β-catenin by 3.7 ± 0.03 fold (P<0.05) without affecting binding of β-catenin to E-cadherin (Fig. 3H, I). The association of α-catenin to β-catenin was suppressed significantly by SP600125 or JNK1/2 siRNA (Fig. 3H, I), suggesting that the kinase activity of JNK was necessary for maintaining binding of α-catenin to the β-catenin/E-cadherin complex.
Release of α-catenin from β-catenin is dependent on JNK but independent of actin polymerization
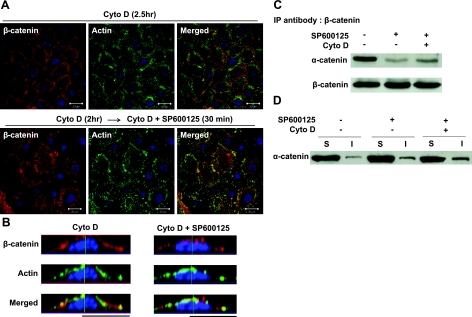
Next, we examined whether JNK-mediated dissociation of α-catenin from β-catenin was dependent on actin polymerization. To this end, prior to SP600125, treatment cells were treated with cytochalasin D (Cyto D), an inhibitor of actin polymerization, which is known to prevent AJ formation (5). As expected, Cyto D treatment disrupted the actin cytoskeleton, as evidenced by the granular appearance of phalloidin actin, and prevented SP600125-induced translocation of β-catenin to the cell-cell adhesion sites (Fig. 4A, B). JNK inhibition decreased the association of α-catenin with β-catenin and increased its association with the actin cytoskeleton, even in Cyto D-treated cells (Fig. 4C, D), suggesting that the interactions of α-catenin with β-catenin and the cell cytoskeleton were dependent on the activity of JNK but independent of actin polymerization.
Figure 4.
Release of α-catenin from β-catenin is independent of actin polymerization. Human primary keratinocytes were pretreated with actin polymerization inhibitor, cytochalasin D (Cyto D; 2 μM), for 2 h. Next, the cells were treated with either vehicle or SP600125 (10 μM) in the presence of Cyto D for 30 min. A) Confocal images of cells stained with β-catenin (red) and actin phalloidin (green). Nuclei were visualized with Hoechst (blue). View: ×63. Scale bars = 20 μm. B) Lateral images were formed by maximum intensity projection from confocal z-stack images. C) Cell lysates were immunoprecipitated with antibody against β-catenin and subsequently subjected to Western blotting for α-catenin. β-Catenin was used as loading control. D) Soluble (S) and insoluble (I) fractions were separated and subjected to Western blotting for α-catenin.
α-Catenin is necessary for JNK-mediated AJ formation
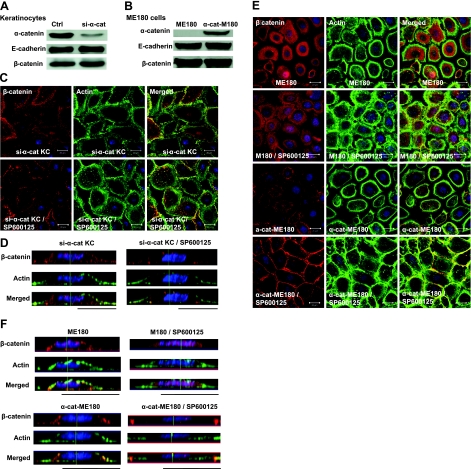
Since JNK inhibition released α-catenin from β-catenin/E-cadherin, we examined whether JNK-mediated AJ formation required α-catenin. To this end, we employed two approaches. First, we knocked down α-catenin in primary human keratinocytes using shRNA encoding recombinant lentivirus. The virus also encoded for EGFP to facilitate sorting of transduced cells, which exhibited more than 70% reduction in the level of α-catenin and were termed si-α-cat KC (Fig. 5A). We also employed a gain-of-function approach by supplying α-catenin exogenously to an epidermoid cervical carcinoma cell line, ME180, which does not normally express α-catenin (Fig. 5B).
Figure 5.
α-Catenin is necessary for JNK-mediated regulation of AJs. A, B) Human primary keratinocytes were transduced with recombinant lentivirus encoding for shRNA targeting the α-catenin mRNA (si-α-cat; A); ME180 cells lacking α-catenin were transduced with full-length a-catenin (α-cat-M180; B). Amounts of E-cadherin, β-catenin, and α-catenin were detected using Western blotting. C–F) Confocal (C, E) and lateral images (D, F) of si-α-cat cells (C, D) or ME180 and α-cat-M180 cells (E, F) treated with either vehicle (ctrl) or SP600125 (10 μM) for 30 min, then stained with β-catenin (red) and actin phalloidin (green); nuclei were stained with Hoechst (blue). Lateral images were formed by maximum-intensity projection from confocal z-stack images. View: ×63. Scale bars = 20 μm.
Compared to control cells where β-catenin was mainly localized on the apical surface, in si-α-cat KC and ME180 cells, β-catenin was found mostly in the cytoplasm. Most important, SP600125 treatment failed to induce translocation of β-catenin to cell-cell adhesion sites and actin reorganization in si-α-cat KC (Fig. 5C, D) or ME180 cells (Fig. 5E, F). Conversely, introduction of α-catenin into ME180 cells (α-cat-ME180) restored the sensitivity of cells to SP600125 causing translocation of β-catenin to cell-cell adhesion sites, as well as actin reorganization (Fig. 5E, F and Supplemental Fig. S5B). As expected, these effects were inhibited by Cyto D (Supplemental Fig. S2).
Binding of α-catenin to both β-catenin and actin is required for JNK-mediated AJ formation
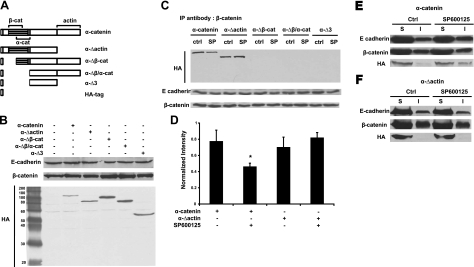
Next, we examined which of the α-catenin domains may be necessary for AJ formation on JNK inhibition. To this end, we engineered deletion mutants of α-catenin lacking the binding domains of actin (α-Δactin), β-catenin (α-Δβ-cat), β/α-catenin (α-Δβ/α-cat), or all three (α-Δ3) and expressed them in ME180 cells using recombinant lentivirus (Fig. 6A, B). Immunoprecipitation showed that wild-type (wt) α-catenin and α-Δactin bound to β-catenin, while deletion mutants lacking the β-catenin binding domain did not (Fig. 6C). In contrast to wt α-catenin, α-Δactin did not respond to SP600125 treatment as it remained associated with β-catenin (Fig. 6C, D) and did not associate with the Triton-X insoluble fraction (Fig. 6E, F). None of the other mutants was found in the Triton-X insoluble fraction (Supplemental Fig. S3).
Figure 6.
Binding of α-catenin to β-catenin and actin is required for JNK-mediated AJ formation. A) Schematics of different domains of α-catenin. ME180 cells were transduced with recombinant lentivirus encoding for different domains of α-catenin as indicated, each fused with HA tag to facilitate detection. B) Cell lysates were subjected to Western blotting for detecting total amount of E-cadherin, β-catenin, and HA-tagged α-catenin domains. C) ME180 cells expressing different domains of α-catenin were treated with either vehicle (ctrl) or SP600125 (10 μM) for 30 min. Cell lysates were immunoprecipitated with antibody against β-catenin and subsequently subjected to Western blotting for HA tag and E-cadherin. β-Catenin was used as loading control. D) Each band intensity in C was normalized to the corresponding β-catenin and plotted as mean ± sd of 3 independent experiments. E, F) ME180 cells expressing α-catenin (E) or α-Δactin (F) were treated with either vehicle or SP600125 (10 μM) for 30 min. Triton-X100 soluble (S) and insoluble (I) fractions were subjected to Western blotting for E-cadherin, β-catenin, and HA tag.
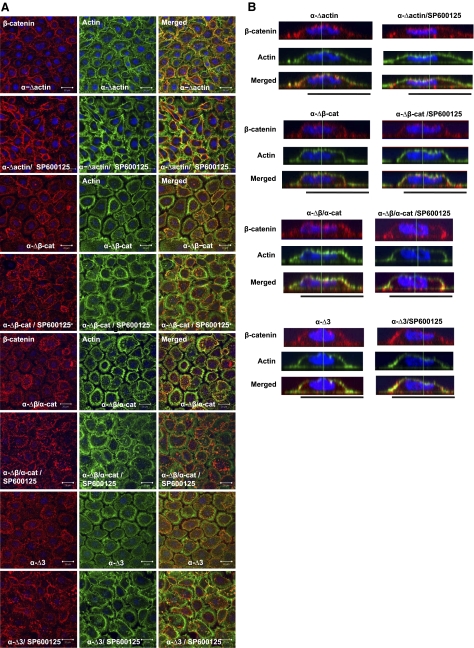
As shown above, expression of α-catenin restored the response of ME180 cells to JNK inhibition, leading to AJ formation and peripheral actin reorganization into bundles underneath the newly formed AJs (Fig. 5E). In contrast, none of the α-catenin mutants lacking the β-catenin or actin binding sites enabled actin reorganization or β-catenin translocation to cell-adhesion sites (Fig. 7 and Supplemental Fig. S5C). In cells expressing the α-Δactin mutant, β-catenin appeared to form rings but even in the presence of SP600125, cells extended abundant actin filopodia, some of which contained β-catenin at their tips (Fig. 7A, α-Δactin). The lateral confocal z stacks show that β-catenin was localized on the cell surface but failed to translocate to cell-cell contact sites upon JNK inhibition (Fig. 7B).
Figure 7.
Binding of α-catenin to β-catenin and actin is required for JNK-mediated AJ formation. A) ME180 cells expressing different domains of α-catenin, as indicated, were treated with either vehicle (ctrl) or SP600125 (10 μM) for 30 min. Confocal images of cells stained with β-catenin (red) and actin phalloidin (green); nuclei were stained with Hoechst (blue). View: ×63. Scale bars = 20 μm. B) Lateral images were formed by maximum intensity projection from confocal z-stack images.
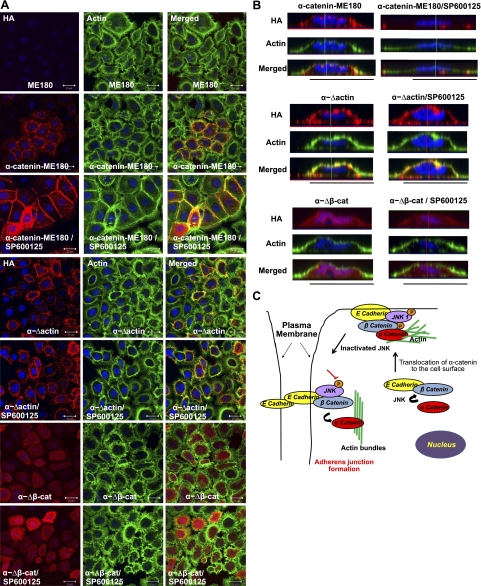
Finally, we investigated the localization of exogenously expressed α-catenin mutants using immunostaining with an antibody against the HA tag. This experiment confirmed that while wt α-catenin and α-Δactin were found on the apical side of the cell membrane, mutants lacking the β-catenin binding domain were localized predominantly in the cytoplasm (Fig. 8A, B and Supplemental Figs. S4 and S5D), suggesting that binding to β-catenin may be necessary for positioning α-catenin to the cell surface. On the other hand, the α-Δactin mutants failed to form AJs in response to SP600125 treatment despite correct localization on the cell surface prior to JNK inhibition (Fig. 8B), suggesting that binding to actin may be necessary for transporting the AJ complex to the cell-cell adhesion sites.
Figure 8.
Requirement for different domains for localization of α-catenin to β-catenin to the cell surface or cell adhesion sites. A) Confocal images of the ME180 cells transduced with full-length α-catenin or α-catenin lacking the actin (α-Δactin) or β-catenin (α-Δβ-cat) domains, each fused with HA tag to facilitate detection. Cells were stained for HA tag (red) and actin phalloidin (green). Cell nuclei were stained with Hoechst (blue). View: ×63. Scale bars = 20 μm. B) Lateral images were formed by maximum-intensity projection from confocal z-stack images. C) Model for JNK-mediated cell adhesion. Binding of α-catenin to β-catenin requires JNK and is necessary for transportation of α-catenin to the cell surface. Upon JNK inhibition, the AJ complex translocates to cell-cell adhesion sites; α-catenin leaves the AJ complex and associates with actin, possibly contributing to actin-bundle formation and strengthening cell adhesion.
DISCUSSION
In this study, we report our findings that actin reorganization and α-catenin are necessary for JNK-mediated regulation of AJs. Several lines of evidence point to this conclusion: inhibition of JNK resulted in formation of AJs, dissociation of α-catenin from the β-catenin/E-cadherin complex, and increased association of α-catenin with the cytoskeleton; JNK activation enhanced binding of α-catenin to β-catenin/E-cadherin significantly; inhibition of JNK did not lead to AJ formation in ME180 cells lacking α-catenin or in primary keratinocytes in which α-catenin was knocked down using siRNA; introduction of α-catenin into ME180 cells restored responsiveness to JNK inhibition, leading to translocation of β-catenin and E-cadherin to the cell-cell adhesion sites and AJ formation; and binding of α-catenin to β-catenin was necessary for translocation of α-catenin to the apical side of the cell membrane, while binding of α-catenin to actin was necessary for translocation of AJ to cell-cell contact sites on JNK inhibition.
Our experiments with ME180 cells lacking α-catenin, as well as α-catenin knockdown in primary keratinocytes, showed that α-catenin was required for AJ formation on inhibition of JNK. In addition, α-catenin mutants demonstrated that binding of α-catenin to β-catenin and actin was necessary for AJ formation. First, α-catenin lacking the β-catenin binding domain did not localize to the apical cell surface but remained in the cytoplasm suggesting that binding to β-catenin might be required for transporting α-catenin to the cell surface. The corollary would be that the AJ complexes are recruited to adhesion sites from the cell surface and not from the cytoplasm. On the other hand, α-catenin lacking the actin binding domain (aa 695–906) was found on the apical cell surface but remained associated with β-catenin and failed to translocate to cell adhesion sites on SP600125 treatment, suggesting that interaction of α-catenin with actin might be necessary for AJ formation. Currently, it is not clear whether α-catenin interacts with actin directly or via other actin-binding molecules such as ZO-1, which is known to bind the same domain (aa 695–906; Supplemental Table S1) (20). It is worth noting that this result is different from a previous study showing that residues 1–643 of α-catenin were sufficient for imparting adhesion activity in E-cadherin/α-catenin fusion molecules (20). However, the latter system may not represent a physiologically relevant behavior as the E-cadherin/α-catenin fusion protein caused cell adhesion, even in the presence of cytochalasin D (20) or pervanadate (21). In addition, the E-cadherin/α-catenin fusion bypassed the requirement for β-catenin and therefore, may not be subjected to JNK-mediated regulation of α-catenin/β-catenin binding. Collectively, these data suggest that binding to β-catenin is required for localizing α-catenin to the cell surface, while binding to actin may be necessary for transporting the AJ complex to cell adhesion sites, possibly through reorganization of the actin cytoskeleton.
Interestingly, after JNK inhibition α-catenin was found in the triton-X insoluble fraction even when cells were treated with cyto D, which disrupts actin microfilaments. However, previous studies have shown that even after cyto D treatment, a significant amount of filamentous actin remained in the cells (22). Indeed, our data showed that cyto D-treated cells were stained strongly with fluorescent phalloidin, which binds to filamentous but not monomeric actin (22), suggesting that a significant amount of α-catenin might still be bound to filamentous actin, even after cyto D treatment. Alternatively, free α-catenin may colocalize with other triton-X inextractable structures such as caveolae (23), which are associated with the actin cytoskeleton and AJs components (24–26). More experiments are required to address this hypothesis.
Inhibition of JNK by SP600125 or expression of JNK1DN released α-catenin from the AJ complex and increased the association of α-catenin with the actin cytoskeleton. On the other hand, phosphorylation of JNK by OA treatment led to the increased association of α-catenin with β-catenin, which was reversed in the presence of SP600125 or JNK1/2 siRNA. These results suggest that JNK1 activation may be critical for maintaining the association of α-catenin with the AJ complex. Recent studies demonstrated that when it does not bind to β-catenin, α-catenin forms homodimers that bind to actin and block actin polymerization by inhibiting the Arp2/3 complex (10, 27).
Combining these data with our current findings, we propose the following model for JNK-mediated cell adhesion (Fig. 8C). When JNK is phosphorylated, α-catenin is bound to β-catenin in the AJ complex and localizes at the apical side of the cell surface. Conversely, when the kinase activity of JNK is blocked, α-catenin leaves the AJ complex and associates with the actin cytoskeleton, possibly contributing to actin bundle formation (10). Actin bundles may stabilize AJs by restricting membrane movement, thereby enabling formation of multiple bonds that eventually strengthen cell adhesion. This model agrees with force spectroscopy measurements, showing that the presence of α-catenin strengthened intercellular adhesion (28–29). Taken together, our data suggest that JNK may coordinate cell-cell adhesion with the underlying cytoskeleton in a swift and dynamic manner by regulating binding of α-catenin to the AJ complex or to the actin cytoskeleton.
Supplementary Material
Acknowledgments
This work was supported by grants from the U.S. National Institutes of Health (R01 EB000876) and National Science Foundation (BES-0354626) to S.T.A.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Lee M., Vasioukhin V. (2008) Cell polarity and cancer—cell and tissue polarity as a non-canonical tumor suppressor. J. Cell Sci. 121, 1141–1150 [DOI] [PubMed] [Google Scholar]
- 2. Chu Y. S., Thomas W. A., Eder O., Pincet F., Perez E., Thiery J. P., Dufour S. (2004) Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J. Cell Biol. 167, 1183–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brevier J., Montero D., Svitkina T., Riveline D. (2008) The asymmetric self-assembly mechanism of adherens junctions: a cellular push-pull unit. Phys. Biol. 5, 16005. [DOI] [PubMed] [Google Scholar]
- 4. Scott J. A., Shewan A. M., den Elzen N. R., Loureiro J. J., Gertler F. B., Yap A. S. (2006) Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts. Mol. Biol. Cell 17, 1085–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vasioukhin V., Bauer C., Yin M., Fuchs E. (2000) Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 100, 209–219 [DOI] [PubMed] [Google Scholar]
- 6. Cavey M., Rauzi M., Lenne P. F., Lecuit T. (2008) A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature 453, 751–756 [DOI] [PubMed] [Google Scholar]
- 7. Zhang J., Betson M., Erasmus J., Zeikos K., Bailly M., Cramer L. P., Braga V. M. (2005) Actin at cell-cell junctions is composed of two dynamic and functional populations. J. Cell Sci. 118, 5549–5562 [DOI] [PubMed] [Google Scholar]
- 8. Vaezi A., Bauer C., Vasioukhin V., Fuchs E. (2002) Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev. Cell 3, 367–381 [DOI] [PubMed] [Google Scholar]
- 9. Noren N. K., Niessen C. M., Gumbiner B. M., Burridge K. (2001) Cadherin engagement regulates Rho family GTPases. J. Biol. Chem. 276, 33305–33308 [DOI] [PubMed] [Google Scholar]
- 10. Drees F., Pokutta S., Yamada S., Nelson W. J., Weis W. I. (2005) Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 123, 903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pokutta S., Weis W. I. (2007) Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu. Rev. Cell Dev. Biol. 23, 237–261 [DOI] [PubMed] [Google Scholar]
- 12. Piedra J., Miravet S., Castano J., Palmer H. G., Heisterkamp N., Garcia de Herreros A., Dunach M. (2003) p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin interaction. Mol. Cell. Biol. 23, 2287–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Serres M., Grangeasse C., Haftek M., Durocher Y., Duclos B., Schmitt D. (1997) Hyperphosphorylation of beta-catenin on serine-threonine residues and loss of cell-cell contacts induced by calyculin A and okadaic acid in human epidermal cells. Exp. Cell. Res. 231, 163–172 [DOI] [PubMed] [Google Scholar]
- 14. Bek S., Kemler R. (2002) Protein kinase CKII regulates the interaction of beta-catenin with alpha-catenin and its protein stability. J. Cell Sci. 115, 4743–4753 [DOI] [PubMed] [Google Scholar]
- 15. Lee M. H., Koria P., Qu J., Andreadis S. T. (2009) JNK phosphorylates beta-catenin and regulates adherens junctions. FASEB J. 23, 3874–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koria P., Andreadis S. T. (2006) Epidermal morphogenesis: the transcriptional program of human keratinocytes during stratification. J. Invest. Dermatol. 126, 1834–1841 [DOI] [PubMed] [Google Scholar]
- 17. Tian J., Lei P., Laychock S. G., Andreadis S. T. (2008) Regulated insulin delivery from human epidermal cells reverses hyperglycemia. Mol. Ther. 16, 1146–1153 [DOI] [PubMed] [Google Scholar]
- 18. Liu J. Y., Peng H. F., Andreadis S. T. (2008) Contractile smooth muscle cells derived from hair-follicle stem cells. Cardiovasc. Res. 79, 24–33 [DOI] [PubMed] [Google Scholar]
- 19. Avdi N. J., Malcolm K. C., Nick J. A., Worthen G. S. (2002) A role for protein phosphatase-2A in p38 mitogen-activated protein kinase-mediated regulation of the c-Jun NH(2)-terminal kinase pathway in human neutrophils. J. Biol. Chem. 277, 40687–40696 [DOI] [PubMed] [Google Scholar]
- 20. Imamura Y., Itoh M., Maeno Y., Tsukita S., Nagafuchi A. (1999) Functional domains of alpha-catenin required for the strong state of cadherin-based cell adhesion. J. Cell Biol. 144, 1311–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ozawa M., Kemler R. (1998) Altered cell adhesion activity by pervanadate due to the dissociation of alpha-catenin from the E-cadherin-catenin complex. J. Biol. Chem. 273, 6166–6170 [DOI] [PubMed] [Google Scholar]
- 22. Atkinson S. J., Hosford M. A., Molitoris B. A. (2004) Mechanism of actin polymerization in cellular ATP depletion. J. Biol. Chem. 279, 5194–5199 [DOI] [PubMed] [Google Scholar]
- 23. Stickney J. T., Bacon W. C., Rojas M., Ratner N., Ip W. (2004) Activation of the tumor suppressor merlin modulates its interaction with lipid rafts. Cancer Res. 64, 2717–2724 [DOI] [PubMed] [Google Scholar]
- 24. Thomsen P., Roepstorff K., Stahlhut M., van Deurs B. (2002) Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol. Biol. Cell 13, 238–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu Y., Liao H. L., Niu X. L., Yuan Y., Lin T., Verna L., Stemerman M. B. (2003) Low density lipoprotein induces eNOS translocation to membrane caveolae: the role of RhoA activation and stress fiber formation. Biochim. Biophys. Acta 1635, 117–126 [DOI] [PubMed] [Google Scholar]
- 26. Orlichenko L., Weller S. G., Cao H., Krueger E. W., Awoniyi M., Beznoussenko G., Buccione R., McNiven M. A. (2009) Caveolae mediate growth factor-induced disassembly of adherens junctions to support tumor cell dissociation. Mol. Biol. Cell 20, 4140–4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pokutta S., Drees F., Yamada S., Nelson W. J., Weis W. I. (2008) Biochemical and structural analysis of alpha-catenin in cell-cell contacts. Biochem. Soc. Trans. 36, 141–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bajpai S., Correia J., Feng Y., Figueiredo J., Sun S. X., Longmore G. D., Suriano G., Wirtz D. (2008) α-Catenin mediates initial E-cadherin-dependent cell-cell recognition and subsequent bond strengthening. Proc. Natl. Acad. Sci. U. S. A. 105, 18331–18336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bajpai S., Feng Y., Krishnamurthy R., Longmore G. D., Wirtz D. (2009) Loss of alpha-catenin decreases the strength of single E-cadherin bonds between human cancer cells. J. Biol. Chem. 284, 18252–18259 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.