Abstract
The ATP-sensitive potassium channel (KATP) regulates insulin secretion in pancreatic β cells. Loss of functional KATP channels because of mutations in either the SUR1 or Kir6.2 channel subunit causes persistent hyperinsulinemic hypoglycemia of infancy (PHHI). We investigated the molecular mechanism by which a single phenylalanine deletion in SUR1 (ΔF1388) causes PHHI. Previous studies have shown that coexpression of ΔF1388 SUR1 with Kir6.2 results in no channel activity. We demonstrate here that the lack of functional expression is due to failure of the mutant channel to traffic to the cell surface. Trafficking of KATP channels requires that the endoplasmic reticulum-retention signal, RKR, present in both SUR1 and Kir6.2, be shielded during channel assembly. To ask whether ΔF1388 SUR1 forms functional channels with Kir6.2, we inactivated the RKR signal in ΔF1388 SUR1 by mutation to AAA (ΔF1388 SUR1AAA). Inactivation of similar endoplasmic reticulum-retention signals in the cystic fibrosis transmembrane conductance regulator has been shown to partially overcome the trafficking defect of a cystic fibrosis transmembrane conductance regulator mutation, ΔF508. We found that coexpression of ΔF1388 SUR1AAA with Kir6.2 led to partial surface expression of the mutant channel. Moreover, mutant channels were active. Compared with wild-type channels, the mutant channels have reduced ATP sensitivity and do not respond to stimulation by MgADP or diazoxide. The RKR → AAA mutation alone has no effect on channel properties. Our results establish defective trafficking of KATP channels as a molecular basis of PHHI and show that F1388 in SUR1 is critical for normal trafficking and function of KATP channels.
ATP-sensitive potassium channels (KATP) couple metabolic signals to cell excitability. They play important roles in many tissues, including regulation of insulin secretion, control of vascular tone, and protection of neurons and muscles from ischemia (1–3). KATP channels are octameric complexes composed of four sulfonylurea receptors (SUR.x) and four inward rectifier potassium channels Kir6.x (4–7). They are regulated by intracellular ATP and ADP. ATP inhibits channel activity whereas ADP, in the presence of Mg2+, antagonizes the inhibitory effect of ATP and stimulates channel activity (8). These gating properties are crucial for the ability of the channel to sense metabolic changes in cells. Thus, in pancreatic β cells, the [ATP]/[ADP] ratio increases in response to increases in blood glucose levels, leading to KATP channel closure, membrane depolarization, activation of voltage-gated Ca2+ channels, and insulin release. Conversely, when blood glucose levels are low, the [ATP]/[ADP] ratio decreases, KATP channels open, and insulin secretion ceases.
Persistent hyperinsulinemic hypoglycemia of infancy (PHHI) is a neonatal metabolic disease characterized by inappropriate insulin hypersecretion and profound hypoglycemia (9, 10). Genetic studies have identified ≈50 PHHI mutations in the KATP channel genes (11, 12). Among these, some introduce premature stop codons that result in nonfunctional truncated proteins (13, 14), and some result in channels that are unable to respond to stimulation by MgADP (15–17). Mutant channels in the latter group, although they are active in excised membrane patches and exhibit normal sensitivity to ATP inhibition, are unable to open in intact cells upon glucose starvation because of reduced or lack of response to MgADP (15–17). Patients bearing these mutations also have poor responses to diazoxide, a potassium channel opener commonly used to treat PHHI, because the same mutations cause parallel decreases in channel response to diazoxide (15, 16). Despite our progress in understanding how certain mutations cause excessive insulin secretion, the molecular defects of KATP channels caused by many PHHI mutations in the channel genes remain unknown.
Correct trafficking and cell surface expression of KATP channels is under the control of a tripeptide endoplasmic reticulum (ER)-retention signal, RKR, present in both the SUR1 and Kir6.2 subunits (18). When expressed independently, the two proteins are retained in the ER because of exposure of the RKR signal. Removal of this retention signal allows the proteins to escape the ER quality control mechanism and express on the cell surface (18, 19). Under normal conditions, SUR1 and Kir6.2 associate with one another to form an octameric channel complex. This association is proposed to shield the ER-retention signal and permit the channel complex to traffic to the cell surface. An anterograde trafficking signal involving the C terminus of SUR1 also has been identified (20). Deletion of as few as 7 aa from the C terminus of SUR1 markedly reduces surface expression of KATP channels (20). Although defective trafficking of KATP channels has been proposed as a potential mechanism by which mutations in the SUR1 and Kir6.2 genes can cause PHHI (20–23), direct evidence is still lacking. We show here that a previously identified PHHI mutation in SUR1, ΔF1388 (23), causes defective trafficking and lack of surface expression of KATP channels. The study provides evidence that defective KATP channel trafficking is a molecular basis of PHHI. Moreover, we show that the trafficking defect caused by the ΔF1388 SUR1 mutation can be partially overcome by inactivation of the RKR ER-retention signal in SUR1. The resulting mutant channel, although it is fully active in the absence of ATP, has decreased ATP sensitivity and does not respond to stimulation by MgADP or diazoxide.
Materials and Methods
Molecular Biology.
FLAG epitope (DYKDDDDK) was inserted at the N terminus of the hamster SUR1 cDNA by sequential overlap extension PCR. Constructs containing point mutations were prepared by using the QuickChange site-directed mutagenesis kit (Stratagene). Epitope tag and mutations were confirmed by DNA sequencing. All SUR1 and SUR1-Kir6.2 fusion constructs were in pECE vector, and mouse Kir6.2 cDNA was in pCMV6b vector (15).
Immunofluorescence Staining.
COSm6 cells were plated in 6-well tissue culture plates, transfected with Lipofectamine (GIBCO/BRL) or Fugene (Roche) according to the manufacturer's directions, and analyzed 48 h posttransfection. For cell surface staining, COSm6 cells transiently transfected with various FLAG-tagged SUR1 constructs (with or without Kir6.2) were incubated with anti-FLAG M2 mouse mAb (diluted to 10 μg/ml in OptiMEM containing 1% BSA; Sigma) for 1 h at 4°C. The cells were washed with ice-cold PBS and incubated with Cy3-conjugated donkey anti-mouse secondary antibodies (Jackson ImmunoResearch) for 30 min at 4°C. After 3 × 5-min washes in ice-cold PBS, cells were viewed immediately by using a Leica fluorescent microscope. For total cellular staining of FLAG-tagged SUR1, cells were fixed with cold (−20°C) methanol for 5 min. Fixed cells were incubated with the anti-FLAG M2 mAb (10 μg/ml in PBS containing 1% BSA) at room temperature for 1 h, washed in PBS, incubated with Cy3-conjugated donkey anti-mouse secondary antibodies for 30 min at room temperature, and washed again in PBS before viewing.
Immunoblotting.
COS1 cells were plated onto 35-mm culture dishes and transiently transfected with FLAG-tagged SUR1 constructs in the presence or absence of Kir6.2 by using Fugene. Cells coexpressing SUR1 and Kir6.2 were transfected with 0.6 μg SUR1 and 0.1 μg Kir6.2 per 35-mm dish; cells expressing SUR1 alone were transfected with 1 μg SUR1 per 35-mm dish. Cells were lysed 48 h posttransfection in 20 mM Hepes, pH 7.0/5 mM EDTA/150 mM NaCl/1% Nonidet P-40 with Complete protease inhibitors (Roche). Proteins in the cell lysates were separated by SDS/PAGE (10%), transferred to nitrocellulose, analyzed by M2 anti-FLAG antibody (Sigma) followed by horseradish peroxidase-conjugated anti-mouse secondary antibodies (Amersham Pharmacia), and visualized by chemiluminescence (Super Signal West Femto; Pierce).
Patch-Clamp Recordings.
COSm6 cells were transfected by using Lipofectamine or Fugene and plated onto coverslips. The cDNA for the green fluorescent protein was cotransfected with SUR1 and Kir6.2 to facilitate identification of positively transfected cells. Patch-clamp recordings were made 36–72 h posttransfection. All experiments were performed at room temperature as described (7, 15, 16, 24). Micropipettes were pulled from thin-walled glass (WPI Instruments, Waltham, MA) on a horizontal puller (Sutter Instruments, Novato, CA). Electrode resistance was typically 0.5–1 MΩ when filled with K-INT solution (below). Inside-out patches were voltage-clamped with an Axopatch 1D amplifier (Axon Instruments, Foster City, CA). The standard bath (intracellular) and pipette (extracellular) solution (K-INT) had the following composition: 140 mM KCl/10 mM K-Hepes/1 mM K-EGTA, pH 7.3. ATP was added as the potassium salt. All currents were measured at a membrane potential of −50 mV (pipette voltage = +50 mV), and inward currents at this voltage are shown as upward deflections. Data were analyzed by using PCLAMP 7 software (Axon Instruments). Off-line analysis was performed by using Microsoft excel programs. Data were presented as mean ± SEM. Microsoft solver was used to fit ATP-dose–response curves by a least-squares algorithm.
Results
ΔF1388 SUR1 Prevents Trafficking of KATP Channels to the Cell Surface.
Previous studies have demonstrated that ΔF1388 SUR1, when coexpressed with Kir6.2 in COS cells, resulted in no KATP channel activity (16, 23). The lack of functional expression could be due to lack of channel expression on the cell surface or dysfunction of properly assembled and targeted channels. To distinguish these possibilities, we first determined whether the ΔF1388 SUR1 mutant protein is expressed on the cell surface.
To track the expression of SUR1, the N terminus of the protein was tagged with a FLAG epitope. The N terminus of SUR1 is predicted to lie on the extracellular face of the plasma membrane (Fig. 1; refs. 25 and 26), allowing access to anti-FLAG antibodies without permeabilizing cells. The FLAG-tagged SUR1, when coexpressed with Kir6.2, gives rise to channels with properties indistinguishable from the untagged channels (not shown). Cell surface expression of wild-type and ΔF1388 SUR1 was monitored by immunofluorescent labeling of the FLAG epitope. Labeling was performed with living cells at 4°C to prevent endocytosis of the antibodies, which might give rise to intracellular staining. Although clear fluorescent signal in the plasma membrane of cells coexpressing FLAG-tagged wild-type SUR1 (FLAG-WT SUR1) and Kir6.2 was observed, no signal was detected in cells expressing FLAG-tagged ΔF1388 SUR1 (FLAG-ΔF1388 SUR1) and Kir6.2 (Fig. 2A Upper). The lack of surface expression of FLAG-ΔF1388 SUR1 is not due to poor transfection efficiency of the mutant SUR1 construct or lack of biosynthesis of the mutant protein. Staining of cells fixed and permeablized with methanol shows that the percentage of cells expressing FLAG-ΔF1388 SUR1 is comparable to that of cells expressing FLAG-WT SUR1 (17.06 ± 1.03 vs. 17.01 ± 1.30%; n = 8). There is, however, a clear difference in the staining pattern. Whereas FLAG-ΔF1388 SUR1 shows strong perinuclear staining that is consistent with accumulation of the protein in the ER, FLAG-WT SUR1 has less perinuclear and more membranous staining (Fig. 2B). Immunoblot analysis of SUR1 (Fig. 2C) showed that the total steady-state protein levels are similar for all SUR1 constructs. The wild-type SUR1 was resolved into two major bands when coexpressed with Kir6.2. These two bands correspond to the mature complex glycosylation and immature core glycosylation forms reported previously (5, 18, 26). In contrast, the ΔF1388 SUR1 was detected as a single lower band whether or not Kir6.2 was coexpressed, consistent with the mutant protein being trapped in the ER. Although faint surface staining was observed in cells expressing FLAG-ΔF1388AAA (see below), no mature, complex glycosylated band was detected for this construct by immunoblotting. This may reflect differences in the sensitivity of the two procedures in detecting surface SUR1.
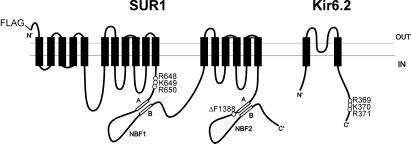
Figure 1.
Location of the ΔF1388 mutation and the RKR ER-retention signals in SUR1 and Kir6.2. The topology of SUR1 shown is by Tusnady et al. (25). The ΔF1388 mutation is in the NBF2. The RKR motif in SUR1 is in a cytoplasmic segment right before the first nucleotide binding fold (NBF1). The RKR motif in Kir6.2 is near the C terminus (18).
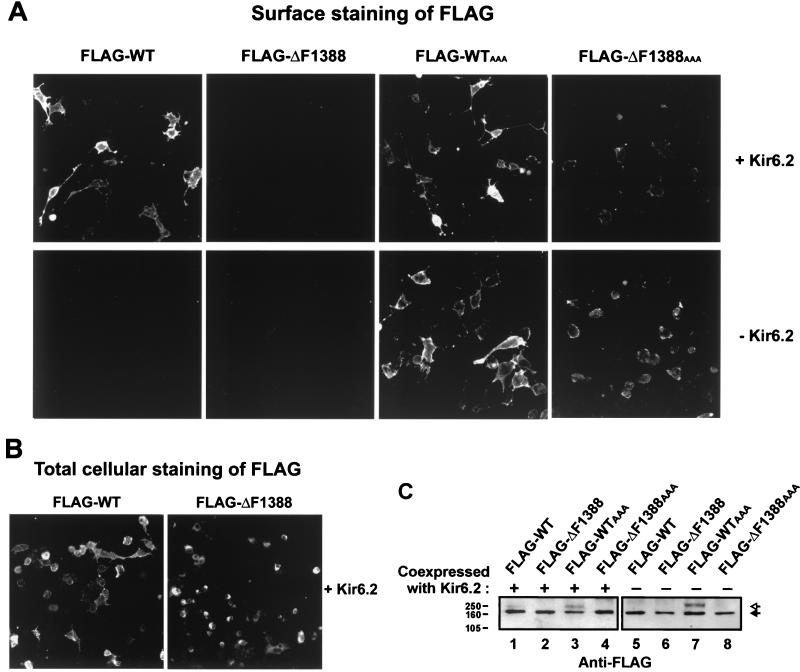
Figure 2.
Lack of surface expression of ΔF1388 SUR1 mutant KATP channels. (A) COSm6 cells transiently transfected with FLAG-wild type (WT) SUR1, FLAG-ΔF1388 SUR1, or FLAG-ΔF1388 SUR1AAA, in the presence (Upper) or absence (Lower) of Kir6.2, were immunostained for the FLAG epitope. When coexpressed with Kir6.2, surface staining was clearly observed with FLAG-WT SUR1 but not with FLAG-ΔF1388 SUR1. Mutating the RKR sequence to AAA in SUR1 (FLAG-ΔF1388AAA SUR1) partially overcomes the trafficking defect caused by the ΔF1388 mutation and allows surface expression of the mutant protein both in the presence (Upper) and in the absence (Lower) of Kir6.2. (B) COSm6 cells transiently expressing Kir6.2 and FLAG-WT SUR1 or FLAG-ΔF1388 SUR1 were fixed with methanol and immunostained for the FLAG epitope. The FLAG-WT SUR1 and the FLAG-ΔF1388 SUR1 showed equivalent levels of staining, suggesting the wild type and the mutant protein are expressed at similar levels. A strong perinuclear staining pattern indicative of ER accumulation was observed with FLAG-ΔF1388 SUR1, whereas less perinuclear and more membrane staining was seen with FLAG-WT SUR1. (C) FLAG-WT, FLAG-ΔF1388, FLAG-WTAAA, and FLAG-ΔF1388AAA SUR1 constructs were expressed in the presence or absence of Kir6.2 and detected by immunoblotting with antibody to the FLAG tag. Solid arrow indicates core glycosylated SUR1; open arrow denotes complex glycosylated SUR1 seen with FLAG-WT coexpressed with Kir6.2 (lane 1) and FLAG-WTAAA (lanes 3 and 7). Molecular mass markers (kDa) are indicated at left. Note that the amount of DNA used for transfection in the two blots were different (see Materials and Methods), so intensities of bands cannot be compared between blots.
Inactivation of the RKR Motif in SUR1 Allows Partial Expression of ΔF1388 Mutant Channels.
Although ΔF1388 SUR1 causes failure of KATP channels to traffic to the cell surface, it still may form functional channels with Kir6.2. If so, molecular or pharmacological manipulations that can overcome the trafficking defect may have therapeutic values. Both SUR1 and Kir6.2 contain a RKR ER-retention signal that prevents the individual subunit from exiting the ER in the absence of the other subunit. Upon proper assembly of the two subunits, the ER-retention signals become shielded to allow trafficking of the channel complex to the plasma membrane (18). Similar arginine-framed ER-retention signals have been identified in cystic fibrosis transmembrane conductance regulator (CFTR) (27). Inactivation of these arginine-framed ER-retention signals in CFTR can partially overcome the trafficking defect caused by a cystic fibrosis-associated CFTR mutation ΔF508 (27). We asked whether similar manipulations in SUR1 could overcome the trafficking defect caused by the ΔF1388 mutation.
The RKR motif in SUR1 is located in a cytoplasmic loop between the putative 11th transmembrane domain and the first nucleotide-binding fold (Fig. 1). When we inactivated the RKR signal sequence in FLAG-ΔF1388 SUR1 by mutating it to AAA (FLAG-ΔF1388 SUR1AAA), we observed surface labeling in cells cotransfected with FLAG-ΔF1388 SUR1AAA and Kir6.2 (Fig. 2A Upper). However, the fluorescent signal was clearly weaker compared with that of cells expressing FLAG-WT SUR1 or FLAG-SUR1AAA channels (Fig. 2A Upper), suggesting that inactivation of the RKR signal only partially overcomes the trafficking defect. Similarly, cells transfected with FLAG-ΔF1388 SUR1AAA alone also showed surface expression level that is lower than cells transfected with FLAG-WT SUR1AAA alone (Fig. 2A Lower).
ΔF1388 SUR1AAA and Kir6.2 Form KATP Channels with Altered Physiological Properties.
Surface expression of FLAG-ΔF1388 SUR1AAA allowed us to analyze the interaction between the mutant SUR1 and Kir6.2 at the functional level by patch-clamp recordings. Potassium currents that were readily inhibited by ATP were recorded from cells coexpressing FLAG-ΔF1388 SUR1AAA and WT Kir6.2 (Fig. 3B), demonstrating that the mutant SUR1 can form functional channels with Kir6.2. Comparison of KATP current densities from cells coexpressing Kir6.2 and WT SUR1, SUR1AAA, or ΔF1388 SUR1AAA by using inside-out patch-clamp recordings shows that the expression level of ΔF1388 SUR1AAA mutant channels is 16.8 ± 4.9% (n = 35) that of WT channels. Mutation of RKR to AAA alone (SUR1AAA channels) caused an increase in the level of expression (162.1 ± 23.2% that of WT channels, n = 37), consistent with that reported by Zerangue et al. (18).
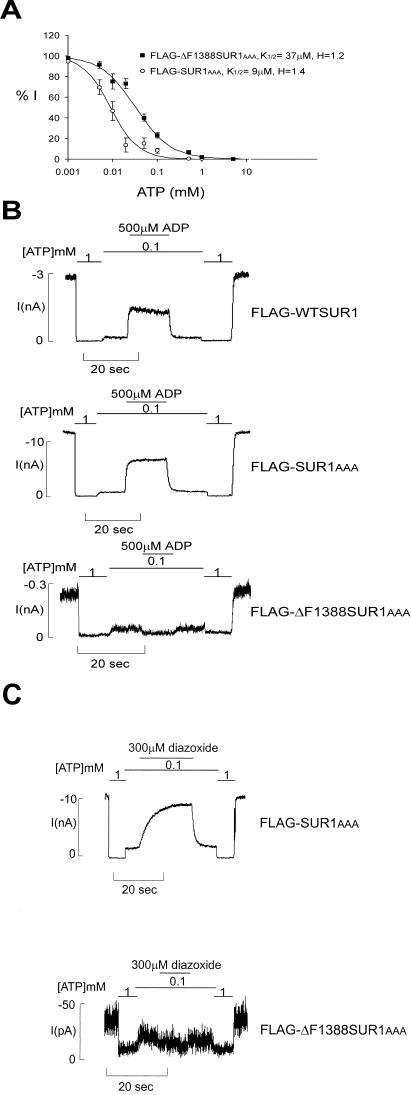
Figure 3.
FLAG-ΔF1388 SUR1AAA mutant channels have reduced ATP sensitivity and do not respond to MgADP or diazoxide. (A) Dose–response curves of FLAG-SUR1AAA and FLAG-ΔF1388 SUR1AAA channels to ATP inhibition. The K1/2 is estimated by fitting the data points to the Hill equation {Irel = 1/[1 + ([ATP]/K1/2)H]}, with Irel being the current relative to the current in the absence of ATP. Each data point represents the average of 8–9 patches, with the error bar being the SEM. H = 1.4 and 1.2 for FLAG-SUR1AAA and FLAG-ΔF1388 SUR1AAA channels, respectively. (B) Representative current traces recorded from inside-out membrane patches containing FLAG-wild type (WT) SUR1, FLAG-SUR1AAA, or FLAG-ΔF1388 SUR1AAA channels. Currents were recorded at −50 mV. Inward currents are shown as upward deflections. Patches were exposed to differing concentrations of ATP and ADP, as indicated by the bars above the records. Free [Mg2+] was maintained at 1 mM in all ATP-containing solutions. (C) Representative current traces showing the lack of response to diazoxide of FLAG-ΔF1388 SUR1AAA channels, in contrast to FLAG-SUR1AAA channels. Patches were exposed to differing concentrations of ATP and diazoxide, as indicated by the bars above the records. Free [Mg2+] was maintained at 1 mM in all ATP-containing solutions.
A number of PHHI-associated SUR1 mutations located in the second nucleotide-binding fold (NBF2) of the protein have been shown to cause reduced KATP channel response to MgADP and diazoxide (15–17). Because F1388 is also in NBF2 of SUR1, we examined in detail the physiological properties of FLAG-ΔF1388 SUR1AAA channels. Channel open probability (Po) was estimated by using noise analysis (24). There is no significant difference between the Po of WT channels (0.46 ± 0.01, n = 5) and the Po of ΔF1388 SUR1AAA channels (0.47 ± 0.05, n = 7). ATP dose–response experiments showed that FLAG-ΔF1388 SUR1AAA mutant channels have reduced sensitivity to ATP inhibition, with a half-maximal inhibitory concentration, K1/2, of 37 μM (Fig. 3A), whereas FLAG-SUR1AAA channels have ATP sensitivity (K1/2 of 9 μM) similar to that of WT channels (≈10 μM; ref. 24). In WT channels, MgADP antagonizes the inhibitory effects of ATP and stimulates channel activity. However, this stimulatory effect is completely absent in FLAG-ΔF1388 SUR1AAA mutant channels (Fig. 3B). The mutant channel also lacks response to diazoxide (Fig. 3C). The lack of response is not due to the RKR-to-AAA mutation because FLAG-SUR1AAA channels have a normal response to MgADP (Fig. 3B) and diazoxide (Fig. 3C).
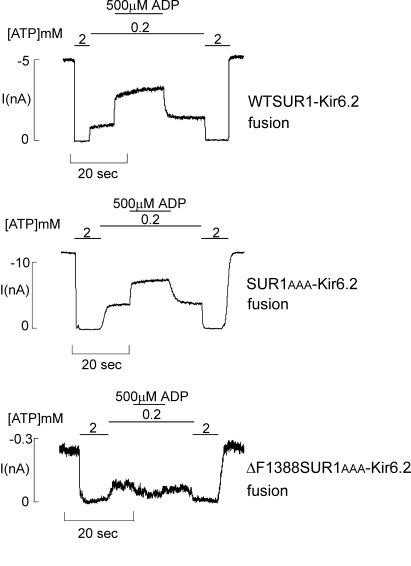
The stimulatory effect of MgADP on KATP channel activity is conferred by the SUR1 subunit (15, 19, 28, 29). Channels formed by ΔC25 Kir6.2 (a mutant Kir6.2 in which the C-terminal 25 aa including the RKR signal have been removed; refs. 18 and 19) in the absence of SUR1 have reduced ATP sensitivity (K1/2 ∼ 100 μM) and do not respond to MgADP or diazoxide (19, 30). One can argue that the altered ATP sensitivity and MgADP response we observed with the ΔF1388SUR1AAA mutant channel is a result of weak association between the mutant SUR1 and Kir6.2 or even temporary dissociation between the two subunits after reaching cell surface. In this scenario, although ΔF1388 SUR1AAA can assemble with Kir6.2 and traffic to the cell surface, the interaction between the two subunits is so weak that there is little or no “functional” coupling between the two subunits. To test this possibility, we used a ΔF1388 SUR1AAA-Kir6.2 fusion construct in which ΔF1388 SUR1AAA was physically linked to Kir6.2 through a hexaglycine linker (5, 7) to force physical association between the two subunits and to ensure an octameric channel stoichiometry. Analysis of channels formed by the mutant fusion protein shows that similar to channels formed by expressing ΔF1388 SUR1AAA and Kir6.2 as individual subunits, channels formed by the mutant fusion protein also fail to respond to MgADP stimulation (Fig. 4). In contrast, SUR1AAA–Kir6.2 fusion channels have a positive response to stimulation by MgADP (Fig. 4). These results provide convincing evidence that the ΔF1388 mutation not only causes defective trafficking of the channel complex, it also affects sensitivity of the channel to ATP, MgADP, and diazoxide.
Figure 4.
ΔF1388 SUR1AAA–Kir6.2 fusion channels also lack response to MgADP. Patches containing fusion channels were exposed to differing concentrations of ATP and MgADP, as indicated by the bars above the records. Free [Mg2+] was maintained at 1 mM in all ATP-containing solutions. Currents were recorded at −50mV. Inward currents are shown as upward deflections. The higher ATP concentration used (200 μM) was necessary to achieve sufficient inhibition of channel activity, because the fusion channels have lower ATP sensitivity (K1/2 ∼ 50 μM) compared with normal wild-type channels formed by SUR1 and Kir6.2 as individual subunits (K1/2 ∼ 10 μM) (5, 7).
Discussion
Mutations in KATP channels of pancreatic β cells are the major cause of the recessive form of PHHI (11, 12). The molecular consequences of the known PHHI mutations include protein truncation because of premature stop codons and reduction or abolition of channel response to MgADP (15–17). Our study of a single amino acid deletion mutation in SUR1, ΔF1388, provides direct evidence that, in addition, defective trafficking of KATP channels is also an underlying molecular mechanism of the disease. Future genetic and functional studies are expected to reveal more disease-associated mutant KATP channels that have trafficking defects.
Defective trafficking of a number of ion channels are known to cause human diseases. For example, the most prevalent cystic fibrosis-causing mutation of CFTR, ΔF508, results in mistrafficking of the channel protein (31). Several mutations in the human ether-a-go-go potassium channels identified in congenital long QT syndrome (OMIM 152427) have processing and trafficking defects (32). Studies of ΔF508 CFTR have shown that the defective trafficking is a consequence of its structural misfolding (33–35). SUR1 shares structural similarity with CFTR. They both belong to the ATP-binding cassette (ABC) transporter family and contain two intracellular NBFs that are highly conserved (36). Intriguingly, both F508 of CFTR and F1388 of SUR1 are located in the NBFs. It will be interesting to determine whether ΔF1388 SUR1 also adopts a misfolded conformation. One of the hallmarks of CFTR ΔF508 is that the mutant protein is rapidly degraded by the proteasome-dependent pathway (37), resulting in reduced cellular protein levels. We did not observe significant differences in the steady-state protein levels between wild-type SUR1 and ΔF1388 mutant SUR1. However, our immunoblots provide only a rough assessment of total protein levels; more sensitive kinetic measurements may be required to see whether the degradation rates of wild-type SUR1 and ΔF1388 SUR1 differ. Comparative studies of ΔF508 CFTR and ΔF1388 SUR1 mentioned above will provide insight into the role of these phenylalanine residues in the folding and function of the NBFs and ABC transporters.
Various strategies have been developed to correct protein misfolding. These include temperature reduction, the use of generic chemical chaperones such as glycerol, and the use of specific ligands that bind to the protein (38–41). Recently, it was shown in CFTR that inactivation of arginine-framed ER-retention signals partially overcomes the trafficking defect caused by the ΔF508 mutation (27). We demonstrate here that inactivation of RKR in SUR1 also partially overcomes the trafficking defect caused by the ΔF1388 mutation. Although the mechanism by which inactivation of RKR signal allows partial surface expression of ΔF1388 SUR1 is not yet clear, we speculate that inactivation of the retention signal may allow some misfolded mutant protein that is destined to degradation to escape the ER quality control mechanism. Interestingly, in the case of CFTR, multiple arginine-framed signals are present, and the extent of rescue of the ΔF508 mutant was greater when all four arginine-framed signals were inactivated simultaneously than when they were inactivated individually (27). It would be important to search for additional arginine-framed signals in SUR1 and examine how they affect the trafficking of the wild-type and the ΔF1388 mutant SUR1/KATP channel complex.
Functional study of the ΔF1388 SUR1AAA mutant channel reveals that the ΔF1388 mutation not only affects the trafficking but also the gating properties of KATP channels. In this respect, the ΔF1388 SUR1 mutation is again reminiscent of the ΔF508 CFTR mutation, which also alters the gating of the chloride channel (42). The ΔF1388 SUR1AAA mutant channel expressed on the cell surface has reduced ATP sensitivity, and, similar to several SUR1 NBF2 mutants characterized previously, it does not respond to MgADP or diazoxide (15–17). The changes in gating properties are not due to mutation of the RKR sequence to AAA, because channels formed by coexpressing Kir6.2 and SUR1AAA without the ΔF1388 mutation exhibit normal ATP sensitivity and response to MgADP and diazoxide. The currents we reported here are unlikely to arise from partially assembled channel complexes because expression of a ΔF1388 SUR1AAA–Kir6.2 fusion construct, which forces the correct 4 SUR1:4 Kir6.2 octameric stoichiometry, also gives rise to channels that do not respond to MgADP. It is not yet clear how ΔF1388 affects the function of NBF2 to alter channel response to nucleotides. Bienengraeber et al. (43) recently have demonstrated that the NBF2 of SUR2A harbors an ATPase activity that determines the ATP sensitivity of KATP channels. Mutations in the NBF2 that reduce the ATPase activity cause an increase in sensitivity of KATP channels to ATP. It is possible that ΔF1388 also alters the ATPase activity at NBF2. Another potential mechanism is that ΔF1388 abolishes the cooperative nucleotide binding between the two NBFs. This mechanism has been shown to be responsible for the reduced channel sensitivity to MgADP in another PHHI-associated SUR1 mutation R1420C, also located in NBF2 (17). The lack of response of ΔF1388 mutant channels to MgADP indicates that even if the trafficking defect of the ΔF1388 channel could be corrected, the channels would not be physiologically functional because they would not be able to sense changes in glucose levels.
In summary, two molecular consequences arise from the ΔF1388 mutation in SUR1: (i) defective trafficking of KATP channels, resulting in lack of channel expression on the cell surface, and (ii) changes in channel response to ATP and MgADP. These results suggest that F1388 provides structural elements critical for the correct trafficking and function of KATP channels. Although a potential interpretation of our findings is that the ΔF1388 mutation does not directly affect the trafficking of the channel and that intracellular retention of the mutant channel results from ER quality-control mechanisms that prevent the exit of dysfunctional channels, we do not favor this interpretation because a number of other PHHI mutant KATP channels that do not respond to MgADP and are physiologically nonfunctional have surface expression levels equivalent to that of wild-type channels (15, 16).
Acknowledgments
We are grateful to Dr. William Skach and Dr. Gary Banker for stimulating discussions and comments on the manuscript. Mouse Kir6.2 cDNA was kindly provided by Dr. S. Seino. This work was supported by a Career Development Award from the American Diabetes Association (to S.-L.S.), grants from the Medical Research Foundation of Oregon (to S.-L.S.) and the March of Dimes Birth Defects Foundation (to S.-L.S.), by Grants DK57699 (to S.-L.S.) and HL 41656 (to C.A.V.) from the National Institutes of Health, and by an American Heart Association Western Affiliate Grant-in-Aid (to C.A.V.).
Abbreviations
- PHHI
persistent hyperinsulinemic hypoglycemia of infancy
- CFTR
cystic fibrosis transmembrane conductance regulator
- ER
endoplasmic reticulum
- NBF2
second nucleotide-binding fold
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ashcroft S J, Ashcroft F M. Cell Signalling. 1990;2:197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- 2.Nichols C G, Lederer W J. Am J Physiol. 1991;261:H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- 3.Terzic A, Jahangir A, Kurachi Y. Am J Physiol. 1995;269:C525–C545. doi: 10.1152/ajpcell.1995.269.3.C525. [DOI] [PubMed] [Google Scholar]
- 4.Inagaki N, Gonoi T, Clement J P, IV, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 5.Clement J P, IV, Kunjilwar K, Gonzalez G, Schwanstecher M, Panten U, Aguilar-Bryan L, Bryan J. Neuron. 1997;18:827–838. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- 6.Inagaki N, Gonoi T, Seino S. FEBS Lett. 1997;409:232–236. doi: 10.1016/s0014-5793(97)00488-2. [DOI] [PubMed] [Google Scholar]
- 7.Shyng S-L, Nichols C G. J Gen Physiol. 1997;110:655–664. doi: 10.1085/jgp.110.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunne M J, Petersen O H. FEBS Lett. 1986;208:59–62. doi: 10.1016/0014-5793(86)81532-0. [DOI] [PubMed] [Google Scholar]
- 9.Aynsley-Green A, Polak J M, Bloom S R, Gough M H, Keeling J, Ashcroft S J H, Turner R C, Boum J D. Arch Dis Child. 1981;56:496–508. doi: 10.1136/adc.56.7.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landau H, Schiller M, editors. Pediatric Surgery of the Liver, Pancreas and Spleen. Philadelphia: Saunders; 1991. pp. 187–201. [Google Scholar]
- 11.Permutt M A, Nestorowicz A, Glaser B. Diabetes Rev. 1996;4:347–355. [Google Scholar]
- 12.Sharma N, Crane A, Gonzalez G, Bryan J, Aguilar-Bryan L. Kidney Int. 2000;57:803–808. doi: 10.1046/j.1523-1755.2000.00918.x. [DOI] [PubMed] [Google Scholar]
- 13.Dunne M J, Kane C, Shepherd R, Sanchez J, James R, Johnson P, Aynsley-Green A, Lu S, Clement J P, IV, Lindley K J. N Engl J Med. 1997;336:703–706. doi: 10.1056/NEJM199703063361005. [DOI] [PubMed] [Google Scholar]
- 14.Nestorowicz A, Inagaki N, Gonoi T, Schoor K P, Wilson B A, Glaser B, Landau H, Stanley C A, Thornton P S, Seino S, et al. Diabetes. 1997;46:1743–1748. doi: 10.2337/diab.46.11.1743. [DOI] [PubMed] [Google Scholar]
- 15.Nichols C G, Shyng S-L, Nestorowicz A, Glaser B, Clement J, IV, Gonzalez G, Aguilar-Bryan L, Permutt A M, Bryan J P. Science. 1996;272:1785–1787. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- 16.Shyng S-L, Ferrigni T, Shepard J, Nestorowicz A, Glaser B, Permutt M A, Nichols C G. Diabetes. 1998;47:1145–1151. doi: 10.2337/diabetes.47.7.1145. [DOI] [PubMed] [Google Scholar]
- 17.Matsuo M, Trapp S, Tanizawa Y, Kioka N, Amachi T, Oka Y, Ashcroft F M, Ueda K. J Biol Chem. 2000;275:41184–41191. doi: 10.1074/jbc.M006503200. [DOI] [PubMed] [Google Scholar]
- 18.Zerangue N, Schwappach B, Jan Y N, Jan L Y. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 19.Tucker S J, Gribble F M, Zhao C, Trapp S, Ashcroft F M. Nature (London) 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- 20.Sharma N, Crane A, Clement J P, IV, Gonzalez G, Babenko A P, Bryan J, Aguilar-Bryan L. J Biol Chem. 1999;274:20628–20632. doi: 10.1074/jbc.274.29.20628. [DOI] [PubMed] [Google Scholar]
- 21.Kane C, Shepherd R M, Squires P E, Johnson P R V, James R F L, Milla P J, Aynsley-Green A, Lindey K J, Dunne M J. Nat Med. 1996;2:1344–1347. doi: 10.1038/nm1296-1344. [DOI] [PubMed] [Google Scholar]
- 22.Nestorowicz A, Glaser B, Wilson B A, Shyng S-L, Nichols C G, Stanley C A, Thornton P S, Permutt M A. Hum Mol Genet. 1998;7:1119–1128. doi: 10.1093/hmg/7.7.1119. [DOI] [PubMed] [Google Scholar]
- 23.Nestorowicz A, Wilson B A, Schoor K P, Inoue H, Glaser B, Landau H, Stanley C A, Thornton P S, Clement J P, IV, Bryan J, et al. Hum Mol Genet. 1996;5:1813–1822. doi: 10.1093/hmg/5.11.1813. [DOI] [PubMed] [Google Scholar]
- 24.Shyng S-L, Ferrigni T, Nichols C G. J Gen Physiol. 1997;110:141–153. doi: 10.1085/jgp.110.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tusnady G E, Bakos E, Varadi A, Sarkadi B. FEBS Lett. 1997;402:1–3. doi: 10.1016/s0014-5793(96)01478-0. [DOI] [PubMed] [Google Scholar]
- 26.Raab-Graham K F, Cirilo L J, Boettcher A A, Radeke C M, Vandenberg C A. J Biol Chem. 1999;274:29122–29129. doi: 10.1074/jbc.274.41.29122. [DOI] [PubMed] [Google Scholar]
- 27.Chang X, Cui L, Hou Y, Jensen T J, Aleksandrov A A, Mengos A, Riordan J R. Mol Cell. 1999;4:137–142. doi: 10.1016/s1097-2765(00)80196-3. [DOI] [PubMed] [Google Scholar]
- 28.Gribble F M, Tucker S J, Ashcroft F M. EMBO J. 1997;16:1145–1152. doi: 10.1093/emboj/16.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shyng S-L, Ferrigni T, Nichols C G. J Gen Physiol. 1997;110:643–654. doi: 10.1085/jgp.110.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enkvetchakul D, Loussouarn G, Makhina E, Shyng S-L, Nichols C G. Biophys J. 2000;78:2334–2348. doi: 10.1016/S0006-3495(00)76779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng S H, Gregory R J, Marshall J, Paul S, Souza D W, White G A, O'Riordan C R, Smith A E. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Z, Gong Q, Epstein M L, January C T. J Biol Chem. 1998;273:21061–21066. doi: 10.1074/jbc.273.33.21061. [DOI] [PubMed] [Google Scholar]
- 33.Qu B H, Strickland E, Thomas P J. J Bioenerg Biomembr. 1997;29:483–490. doi: 10.1023/a:1022439108101. [DOI] [PubMed] [Google Scholar]
- 34.Zhang F, Kartner N, Lukacs G L. Nat Struct Biol. 1998;5:180–183. doi: 10.1038/nsb0398-180. [DOI] [PubMed] [Google Scholar]
- 35.Denning G M, Anderson M P, Amara J F, Marshall J, Smith A E, Welsh M J. Nature (London) 1992;358:761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- 36.Higgins C F. Cell. 1995;82:693–696. doi: 10.1016/0092-8674(95)90465-4. [DOI] [PubMed] [Google Scholar]
- 37.Jensen T J, Loo M A, Pind S, Williams D B, Goldberg A L, Riordan J R. Cell. 1995;83:129–135. doi: 10.1016/0092-8674(95)90241-4. [DOI] [PubMed] [Google Scholar]
- 38.Brown C R, Hong-Brown L Q, Biwersi J, Verkman A S, Welch W J. Cell Stress Chaperones. 1996;1:117–125. doi: 10.1379/1466-1268(1996)001<0117:ccctmp>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato S, Ward C L, Krouse M E, Wine J J, Kopito R R. J Biol Chem. 1996;271:635–638. doi: 10.1074/jbc.271.2.635. [DOI] [PubMed] [Google Scholar]
- 40.Loo TW, Clarke D M. J Biol Chem. 1997;272:709–712. doi: 10.1074/jbc.272.2.709. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Z, Gong Q, January C T. J Biol Chem. 1999;274:31123–31126. doi: 10.1074/jbc.274.44.31123. [DOI] [PubMed] [Google Scholar]
- 42.Dalemans W, Barbry P, Champigny G, Jallat S, Dott K, Dreyer D, Crystal R G, Pavirani A, Lecocq J-P, Lazdunski M. Nature (London) 1991;354:526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- 43.Bienengraeber M, Alekseev A E, Abraham M R, Carrasco AJ, Moreau C, Vivaudou M, Dzeja P P, Terzic A. FASEB J. 2000;14:1943–1952. doi: 10.1096/fj.00-0027com. [DOI] [PubMed] [Google Scholar]