Abstract
Adenosine is an important cerebral vasodilator, but mediating mechanisms are not understood. We investigated the expression of adenosine receptor subtypes in isolated cerebral arterial muscle cells (CAMCs), and their role in adenosine-induced superoxide (O2−) generation and reduction in cerebral arterial tone. Reverse transcriptase-PCR, western blotting, and immunofluorescence studies have shown that CAMCs express transcript and protein for A1, A2A, A2B, and A3 adenosine receptors. Stimulation of CAMCs with adenosine or the A2A agonist CGS-21680 increased the generation of O2− that was attenuated by the inhibition of A2A and A2B adenosine receptor subtypes, or by the peptide inhibitor of nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase gp91ds-tat, or by the mitochondria uncoupler 2,4-dinitrophenol. Application of adenosine or CGS-21680 dilated pressure-constricted cerebral arterial segments that were prevented by the antioxidants superoxide dismutase (SOD) conjugated to polyethylene glycol (PEG) and PEG-catalase or by the A2B adenosine receptor antagonist MRS-1754, or by the mixed A2A and A2B antagonist ZM-241385. Antagonism of the A2A and A2B adenosine receptors had no effect on cerebral vasodilatation induced by nifedipine. These findings indicate that adenosine reduces pressure-induced cerebral arterial tone through stimulation of A2A and A2B adenosine receptors and generation of O2− from NADPH oxidase and mitochondrial sources. This signaling pathway could be one of the mediators of the cerebral vasodilatory actions of adenosine.
Keywords: adenosine, brain ischemia, cerebral hemodynamics, receptors
Introduction
The purine nucleotide, adenosine, causes potent vasodilatation in the cerebral circulation and in other vascular beds (Ibayashi et al, 1991; Ngai and Winn, 1993; Ngai et al, 2001). The effects of adenosine are mediated through activation of surface adenosine receptor subtypes designated as A1, A2A, A2B, and A3, which are widely distributed in neuronal, glial, endothelial, and vascular smooth muscle cells (Ngai and Winn, 1993). The concentration of adenosine rapidly increases after the onset of systemic hypotension and is associated with parallel changes in pial vessel diameter and alterations in cerebral vascular resistance, responses which led to the hypotheses that adenosine is an important metabolic player in cerebral autoregulation (Winn et al, 1985). Conditions such as ischemia, hypoxia, seizures, or neuronal activation rapidly increase the extracellular concentration of adenosine that causes vasodilatation and an increase in cerebral blood flow (Phillis, 1989, 2004; Winn et al, 1979). Such pathologic conditions are also associated with the formation of reactive oxygen species that could have imparted influence on the dynamics of the cerebral circulation (Miller et al, 2005). However, the influence of concomitantly released adenosine on the production of reactive oxygen species under such conditions remained obscure. Adenosine can induce vasodilatation by releasing endogenous factors, such as prostacyclin (PGI2), nitric oxide (NO), endothelium derived hyperpolarizing factor (EDHF), and epoxyeicosatrienoic acids (EETs) (Ikeda et al, 1997; Li et al, 1995; Koehler et al, 2006); whether these mediators, by interacting with adenosine, evoke generation of O2− in cerebral arterial muscle cells (CAMCs) and mitigate pressure-induced cerebral arterial tone is not yet known. In this study, we focused our strategy on the investigation of: (1) the capacity of adenosine to generate O2− and its role in adenosine-induced changes in the diameter of pressure-constricted cerebral arterial segments, (2) expression of adenosine receptor subtype at the transcript and protein levels in freshly isolated cerebral arterial smooth muscle cells, and (3) the specific adenosine receptor subtypes involved and the source of adenosine-induced generation of O2− in isolated CAMCs.
Materials and methods
Effects of Adenosine on Isolated Rat Cerebral Arterial Diameter
Ten-to-twelve-week-old Sprague–Dawley rats were purchased from Harlan (Indianapolis, IN, USA) and housed in the animal facility of the Medical College of Wisconsin for 2 weeks before initiation of experiments. All animals were housed in an environmentally controlled vivarium and had free access to food and water. All experimental protocols were approved by the Animal Care and Use Committee at the Medical College of Wisconsin.
Adult, male Sprague–Dawley rats were anesthetized with sodium pentobarbital (65 mg/kg, intraperitoneally, Anpro Pharmaceutical, Acradia, CA, USA) and the brain was excised and middle cerebral arterial segments (10 to 12 mm in length and 150 to 200 μm outer diameter mentioned hereafter as ‘cerebral arterial segments') were isolated and placed in a myograph perfusion chamber (Living Systems Instrumentation, Burlington, VT, USA), and were incubated at 37°C in physiologic salt solution composed of (in mmol/L): NaCl 141, KCl 4.7, CaCl2 2.5, MgCl2 0.72, KH2PO4 1.7, NaHCO3 25, glucose 11, and HEPES 5 equilibrated to pH 7.4. A bolus of air was passed through the lumen to cause damage to the endothelium of the arterial segments. The isolated arterial segments were cannulated at both ends with glass micropipettes, and secured in place with 8-0 polyethylene suture (Ethicon, Somerville, NJ, USA) using a stereo microscope. Side branches of the arterial segments were tied off with 10-0 polyethylene suture, and superfused with physiologic salt solution aerated with a 21% O2–5% CO2 gas mixture (balance N2), and were maintained at 37°C and pH 7.4. After an equilibration period of 30 minutes at 20 mm Hg and taking control diameter measurements at this pressure, the cannulated arteries were then pressurized to 40 or 120 mm Hg, and the effect of adenosine (1 μmol/L) on diameter was determined before and after treatment with the specific A2B adenosine receptor antagonist N-(4-Cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)phenoxy]-acetamide (MRS-1754, 20 nmol/L) or with the mixed A2A, and A2B adenosine receptor antagonist [4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo-[2,3-a][1,3,5]triazin-5-ylamino]ethyl) phenol (ZM-241385) (100 nmol/L). To evaluate the specificity of the inhibitory actions of the A2A antagonist ZM-241385 and the A2B antagonist MRS-1754, the cerebral vasodilatory action of adenosine (1 μmol/L) was determined after 30 minutes pretreatment of the pressurized cerebral arterial segments with 3, 10, 30, or 100 nmol/L of either ZM-241385 or MRS-1754 by adding to the bath. In further studies, the effects of adenosine or the adenosine A2A agonist 4-[2-[[6-Amino-9-(N-ethyl-b--ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl] benzenepropanoic acid hydrochloride (CGS-21680, 10 μmol/L) on the internal diameter of the pressurized arterial segments was examined before and after treatment with a cocktail or individual preapplication of the cell-permeable superoxide dismutase (SOD) conjugated to polyethylene glycol (PEG-SOD, 100 Units/mL), and catalase conjugated to PEG (PEG-CATL, 100 Units/mL) to dismutate the generated O2− and H2O2, respectively. Furthermore, the effects of the A2B antagonist MRS-1754 (20 nmol/L) and the mixed A2A and A2B ZM-241385 (100 nmol/L) on nifedipine-induced vasodilatation was examined to serve as a control for the A2A and A2B receptors-mediated adenosine-induced vasodilatation. The change in diameter induced by adenosine or its analog CGS-21680 is expressed as the percentage of maximum dilation or as the absolute change in diameter from control.
Enzymatic Dispersion of the Cerebral Artery to Single Cells
Adult, 10-to-12-week-old male Sprague–Dawley rats were anesthetized with sodium pentobarbital (65 mg/kg, intraperitoneally), middle cerebral arteries were carefully dissected, and smooth muscle cells were enzymatically dispersed as described previously (Gebremedhin et al, 2008). Briefly, the dissected rat middle cerebral arterial segments were first placed in a vial containing 1 mL solution of bovine serum albumin (0.5 mg/mL) in low-calcium dissociation solution composed of (in mmol/L): 134 NaCl, 5.2 KCl, 1.2 MgSO4.7H2O, 1.18 KH2PO4, 0.05 CaCl2, 24 NaHCO3, 11 glucose, and 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) with pH adjusted to 7.4, for 10 minutes at room temperature (RT) (25°C). The cerebral arterial segments were then transferred to another vial and incubated for 20 minutes in 1 mL solution of dithiothreitol (Sigma, St Louis, MO, USA) (0.5 mg/mL) and papain (Worthington, Freehold, NJ, USA) (60 Units/mL) in low-calcium dissociation solution, and placed in a water-jacketed beaker at 37°C. After 20 minutes incubation, the supernatant layer was removed and replaced with 0.5 mL of dissociation solution containing collagenase type II (240 Units/mL; Worthington), elastase (15 Units/mL), and trypsin inhibitor (0.1 mg/mL, Sigma), and incubated at 37°C and pH 7.4. Supernatant fractions were then collected at 5-minute intervals and diluted to 1 mL with fresh low-calcium dissociation solution. The procedure was repeated by incubating the remaining cerebral arterial tissue with fresh enzyme containing low-calcium dissociation solution. Complete dispersion of the cerebral arterial segments to single smooth muscle cells was usually attained within 30 to 40 minutes after incubation with the dissociation solution. The fractions which contained single CAMCs were pooled and kept at 4°C and used for experiments involving quantitative reverse transcriptase (RT)-PCR, western blot analysis, and immunofluorescence to determine the expression of the transcript and protein of the adenosine receptor subtypes, and for detection and measurement of superoxide generation induced by adenosine receptor stimulation using the superoxide detecting probe hydroethidine (HE).
Quantitative Reverse Transcriptase-PCR Identification of Adenosine Receptor Subtypes in Cerebral Arterial Muscle Cells
Total RNA was isolated from rat CAMCs using TRIzol reagent. Subsequently, 1 μg of total CAMCs RNA was reverse transcribed using a mixture of random and poly-T primers according to the manufacturer's protocol (Invitrogen, Carlsbad, CA, USA). Primers (generously provided by Dr John A Auchampach, Medical College of Wisconsin) designed on the basis of amino-acid homology among humans, rat, and mouse had the following sequence: A1 (forward, 5′-TGGCTCTGCTTGCTATTG-3′ reverse, 5′-GGCTATCCAGGCTTGTTC-3′), A2A (forward-5′ TCAGCCTCCGCCTCAATG-3′ reverse, 5′-CCTTCCTGGTGCTCCTGG-3′), A2B (forward, 5′-TTGGCATTGGATTGACTC-3′ reverse, 5′-TATGAGCAGTGGAGGAAG-3′), and A3AR (forward, 5′-CGACAACACCACGGAGAC-3′ reverse, 5′-GCTTGACCACCCAGATGAC-3′) using Beacon Design software (Bio-Rad, Hercules, CA, USA). PCR amplification (in SYBR Green Supermix) was performed using an iCycler iQ thermocycler (Bio-Rad) for 40 cycles of 25 seconds at 95°C, followed by 45 seconds at an optimized annealing temperature for each adenosine receptor subtype. The cycle threshold, determined as the initial increase in fluorescence above background, was ascertained for each sample. Melt curves were performed upon completion of the cycles to ensure that nonspecific products were absent. For quantification of adenosine receptor transcripts, a standard curve plotting cycle threshold versus copy number was constructed for each receptor subtype by analyzing the 10-fold serial dilutions of plasmids containing the full-length mouse adenosine receptor clones. Adenosine receptor transcript levels were expressed as relative abundance.
Immunofluorescence Microscopy
Freshly isolated CAMCs were rinsed in phosphate-buffered saline (PBS) at RT and fixed with 4% paraformaldehyde for 10 minutes. The CAMCs were rinsed in PBS at RT and incubated in blocking solution (5% bovine serum albumin in PBS) in a humidified chamber for 30 minutes. The adherent CAMCs were then incubated with polyclonal antibodies: anti-A1 (A1R11-A) (Alpha Diagnostics International, San Antonio, TX, USA), anti-A3 (A3R31-A) (Alpha Diagnostics International), anti-A2A (sc-7504, Santa Cruz Biotechnology, Santa Cruz, CA, USA), or anti-A2B (sc-7507, Santa Cruz Biotechnology) antibody overnight at 4°C at the following dilutions: A1: 1/200; A2A: 1/200; A2B: 1/100; A3: 1/200. The CAMCs were then rinsed three times in PBS and incubated with Alexa Fluor or DyLight 488-conjugated secondary antibody produced in rabbit (Invitrogen) or bovine (Jackson ImmunoResearch, West Grove, PA, USA) at a dilution of 1/400 (1 hour; RT). The CAMCs were subsequently rinsed three times in PBS. After rinsing CAMCs in PBS for 10 minutes, cells were mounted with the SlowFade mounting medium (Invitrogen), and images were taken using Nikon E-600 fluorescent microscope (Nikon, Belmont, CA, USA) equipped with fluorescein isothiocyanate filters, with excitation at 492 nm and emission at 520 nm.
Western Blotting
Freshly isolated rat cerebral arteriolar smooth muscle cells (CAMCs) were suspended in 0.5 mL SDS buffer (50 mmol/L Tris, pH 7.0, 2% SDS, 10% glycerol) containing protease inhibitors (2 μg/mL antipain, 1 μg/mL aprotinin, 2 μg/mL leupeptin, 1 mg/mL phenylmethylsulfonyl fluoride), and homogenized using a tissue glass homogenizer. A 50 μL aliquot of homogenate was used for protein determination by the Bio-Rad protein assay method. Laemmli buffer was added to the CAMC homogenate samples, and then placed in boiling water for 5 minutes, followed by chilling on ice. Samples (5 μg protein per well) were loaded onto a 10% acrylamide gel and subjected to SDS-PAGE using the Bio-Rad minigel system. The loaded samples were then electroblotted onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). The membrane was blocked with 5% milk for 1 hour and incubated for 3 hours at RT or at 4°C overnight with the primary antibody anti-A1 or anti-A3 adenosine receptor antibody (catalog nos A1R11-A and A3R31-A; Alpha Dignostics International, respectively, diluted 1:1,000 in PBS containing 0.5% Tween 20,) and anti-A2A adenosine receptor and anti-A2B adenosine receptor antibodies (catalog nos sc-7502 and sc-7507 (Santa Cruz Biotechnology), respectively, diluted 1:500 in PBS containing 0.5% Tween 20). Membranes were washed three times in PBS containing 0.5% Tween 20 solution and then incubated at RT for 1 hour with horseradish peroxidase-conjugated donkey or bovine anti-goat IgG secondary antibody (Biocompare, San Francisco, CA, USA) at 1:5,000 dilution. Membranes were exposed to films, and the signals were detected using a Supersignal Substrate kit (Pierce, Rockford, IL, USA).
Confocal Micoscopy Imaging
Isolated cerebral arterial segments were cannulated and pressurized at 40 mm Hg incubated with the superoxide detecting fluorescent probe HE (1 μmol/L) and the H2O2 detecting fluorescent probe dichlorofluorescien-diacetate (DCF-DA, 5 μmol/L) at 37°C for 5 minutes. The fluorescent probe-loaded cerebral arterial segments were then stimulated with adenosine (1 μmol/L), and then removed from the chamber in 5-minute time intervals, rinsed in physiologic salt solution, and placed on a microscope slide in a spacer between the slide and coverslip in dark to begin taking images. The cerebral arterial segments were imaged using an Olympus microscope (Olympus, Center Valley, PA, USA) at × 20 magnification at an exposure time of 100 milliseconds on Leica TCS SP2 laser (Leica Geosystems Inc., San Ramon, CA, USA) scanning confocal microscopy (HE excitation: 510 nm, emission: 595 nm, and for DCF-DA excitation: 480 nm and emission: 520 nm). Fluorescence intensity was analyzed and averaged by taking the integrated intensity of three identical regions per vessel segment using Metamorph image analysis software (Molecular Devices, Sunnyvale, CA, USA).
Effect of Adenosine on Intracellular O2− Generation in Rat Isolated Cerebral Arterial Muscle Cells
Fluorescent Detection of Superoxide in Cerebral Arterial Muscle Cells
Freshly dissociated rat CAMCs were allowed to adhere on 30-mm glass Petri dishes precoated with 0.01% poly--lysine (Sigma) in 1 mL Dulbecco's PBS (Gibco, Carlsbad, CA, USA), loaded with 1 μmol/L of the superoxide detecting probe HE, which is preferentially oxidized by superoxide to form the fluorescent product 2-hydroxyethidium (2-OH-E+) (Zhao et al, 2003), and its specificity for superoxide over other reactive oxygen species has been confirmed previously (Zhao et al, 2003), and then stimulated with 1 μmol/L adenosine or 10 μmol/L CGS-21680 for 30 minutes at 37°C with or without pretreatment with the specific peptide inhibitor of nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase gp91ds-tat (5 μmol/L) or its control-scrambled gp91 sequence (gp91-scramb-tat, 5 μmol/L). In additional studies, CAMCs were stimulated with either the adenosine A2B antagonist MRS-1754 (20 nmol/L) or with the mixed adenosine A2A and A2B antagonist ZM-241385 (100 nmol/L) (Gessi et al, 2006). The production of superoxide was determined by fluorescent microscopy using Nikon E-600 fluorescent microscope equipped with TRITC (tetramethyl rhodamine isothiocyanate) filters with excitation at 510 nm and emission at 595 nm (Gebremedhin et al, 2008). Fluorescence signal was quantified by measuring the mean pixel value (F) of a manually selected area of a frame of vascular smooth muscle cell image using MetaMorph software (Molecular Devices). The values were exported to Microsoft Excel (Microsoft, San Francisco, CA, USA) and the fluorescence change (ΔF)=F−BF was computed, where BF is the mean of the background somatic cell body fluorescence signals.
Measurement of Adenosine-Induced Intracellular Superoxide Generation by High-Performance Liquid Chromatography
Freshly isolated rat CAMCs were first treated in dark with 1 μmol/L HE prepared in Dulbecco's PBS, and then stimulated with either adenosine (1 μmol/L) or with the A2A adenosine receptor agonist CGS-21680 (10 μmol/L) for 30 minutes in the absence or presence of the specific peptide inhibitor of NADPH-oxidase gp91ds-tat (5 μmol/L) or its control-scrambled gp91 sequence (gp91-scramb-tat, 5 μmol/L), or with the mitochondrial uncoupling agent 2,4-dinitrophenol (100 μmol/L) (Jin et al, 2004) at 37°C, and then washed twice in Dulbecco's PBS in dark. The CAMCs were then lysed in 0.25 mL lyses buffer (0.1% Triton X-100 in Dulbecco's PBS, pH 7.4), and 50 μL of the cell lysate was used to determine the cell protein level as described above. The remaining lysate solution was mixed with 0.5 mL of N-butanol and extracted in dark by vortexing for 1 minute, followed by centrifugation at 10,000 × g for 10 minutes at 24°C. The N-butanol phase was separated and evaporated under stream of nitrogen gas. The dried samples were taken up by adding 0.1 mL of high-performance liquid chromatography (HPLC) grade water. The HE, ethidium (E+) and the superoxide oxidation product of HE 2-OH-E+ were separated on HPLC system equipped with fluorescence and ultraviolet detectors. The mobile phase was H2O/CH3CN (acetonitrile). The stationary phase was a C18 reverse-phase column (Partisil ODS-3,250 × 4.5 mm2, Alltech Association, Deerfield, IL, USA). A volume of 50 μL of the sample was injected into the HPLC system (HP 1100, Agilent Technologies, Palo Alto, CA, USA) with a C18 column (250 × 4.5 mm) equilibrated with 10% CH3CN in 10% trifluoroacetic acid. Hydroethidine, E+, and 2-OH-E+ were separated by a linear increase in CH3CN concentration from 10% to 70% in 46 minutes at a flow rate of 0.5 mL/min. The elution was monitored for hydroethidium and its oxidation products E+ and 2-OH-E+ by a variable ultraviolet detector at 210 and 350 nm and a fluorescence detector with excitation and emission at 510 and 595 nm, respectively. The HPLC peak area for each experiment was normalized to protein concentration and compared as previously described (Gebremedhin et al, 2008; Zhao et al, 2003; Zielonka et al, 2008, 2009).
Drugs and Chemicals
All chemicals were analytical grade and obtained from Sigma. CGS-21680, ZM-241385, and MRS-1754 were obtained from Tocris (Plymouth, PA, USA). Hydroethidine and DCF-DA (Invitrogen).
Statistical Analysis
Data are expressed as mean±s.e.m. Differences between mean values were assessed using Student's t-test or ANOVA (analysis of variance) for multiple comparisons, followed by a Duncan's new multiple range tests. A P-value of <0.05 was considered statically significant.
Results
Expression of Adenosine Receptor Subtypes in Cerebral Arterial Muscle Cells
To determine the expression of adenosine receptor subtypes in CAMCs, we performed quantitative RT-PCR, western blotting, and immunofluorescence analysis of the A1, A2A, A2B, and A3 adenosine receptor mRNA and protein expression in isolated CAMCs of male Sprague–Dawley rats. The results of the RT-PCR analysis of A1, A2A, A2B, and A3 mRNA in CAMCs showed a predominant expression of the A2B adenosine receptor subtype when compared using its threshold cycle (Ct) value that averaged 33.3±0.25 (P<0.05), followed by that of the A2A adenosine receptor subtypes (Ct value 42±0.2) and that of A3 (Ct value 43.2±1.1). The expression of the A1 adenosine receptor subtype in CAMCs is relatively lower than that of the other adenosine receptor subtypes. The low Ct value of the A2B adenosine receptor indicated availabilities of more copies of this subtype in CAMCs as compared with the A2A or A3 adenosine receptor subtypes that have higher Ct count values. These results seem to provide evidence that the transcript for the A2B adenosine receptor is the major subtype expressed in rat CAMCs, followed by the A2A and the A3 adenosine receptor subtypes (Figure 1A). Studies of western blotting using specific antibodies against the A1, A2A, A2B, and A3 detected the presence of protein of expected molecular size for the A1 (∼38 kDa), A2A (∼45 kDa), A2B (∼53 kDa), and A3 (∼52 kDa) adenosine receptor subtypes in isolated CAMCs (Figure 1B). In addition, results of the immunofluorescent studies using polyclonal antibodies specific for the A1, A2A, A2B, and A3 adenosine receptor subtypes confirmed the expression of the protein for these adenosine receptor subtypes as shown by punctate staining pattern at the cell membranes of isolated CAMCs. Furthermore, the expression of the A3 adenosine receptor, but not that of A1, A2A, and A2B subtypes, also seems to be distributed in the cytosole, despite the fact that such a nature of the expression for adenosine receptor subtypes has not been described before (Figure 1C).
Figure 1.
Expression of adenosine receptor subtypes in isolated cerebral arterial muscle cells (CAMCs). (A) Bar graphs showing relative abundance of the mRNA of adenosine receptor subtypes in CAMCs of the rat. (*P<0.05 A2B versus the other adenosine receptor subtypes (n=3 trials using CAMCs dissociated from cerebral arterial segments isolated from 10 rats)). (B) Western blots for the A1, A2A, A2B, and A3 adenosine receptors (5 μg protein per lane) showing expression of the proteins for the respective adenosine receptor subtypes in isolated CAMCs. (C) Representative immunofluorescence and respective bright field images of freshly isolated cerebral arterial smooth muscle cells showing a punctate staining pattern at the membranes of isolated cerebral arterial smooth muscle cells. There was no nonspecific binding of the secondary antibody on the membrane probed by omitting the primary antibodies (two bottom images of the right panel).
Confocal Microscopy Detection of O2− Generation in the Cerebral Arterial Muscle
In an attempt to examine the capacity of adenosine to induce generation of superoxide in CAMCs, the effects of adenosine on superoxide production was determined after stimulation of cerebral arterial segments that were preincubated with the superoxide detecting probe HE (1 μmol/L) for 10 minutes, with 1, 10, 100, or 1,000 nmol/L adenosine for 5 minutes, and images were taken by confocal microscopy and analyzed using MetaMorph software (Molecular Devices). As depicted in Figure 2A, adenosine induced concentration-dependent increase in the intensity of 2-OH-E+, the oxidation product of HE by O2−, fluorescent signal in CAMCs that reached maximum with 1,000 nmol/L adenosine. To examine whether adenosine-generated O2− is dismutated and existed in the form of H2O2, the cerebral arterial segments were also preincubated with the O2− detecting probe HE in the presence of the H2O2 detecting probe DCF-DA (5 μmol/L) as described in the ‘Materials and methods' section. As can be seen in Figure 2B, stimulation with exogenous adenosine (1 μmol/L) first increased the fluorescence intensity of the O2− detecting probe HE within 1 to 5 minutes and upon a delay of >5 minutes, an increase in the fluorescence intensity of the H2O2 detecting fluorescent probe DCF-DA appeared, revealing the formation of H2O2. These findings suggested that stimulation of CAMCs with adenosine evoked the generation of superoxide that gradually dismutated to H2O2 and could have contributed to adenosine-induced increase in diameter of the pressure-constricted cerebral arterial segments (n=5 independent experiments).
Figure 2.
Confocal microscopy detection of adenosine-induced O2− and H2O2 generation. (A) Application of exogenous adenosine induced concentration-dependent increase in the generation of O2− as determined by the change in average intensity of the hydroethidine (HE) fluorescence signal under equal time interval in cerebral arterial segments that started at 10 nmol/L and significantly increased by subsequent graded increase in adenosine concentration and reached maximum at 1 μmol/L. (B) Confocal microscopy images of cerebral arterial segment showing adenosine (1 μmol/L) induced O2− generation and gradual formation of H2O2 as determined by the combined use of the O2− detecting probe HE, and the H2O2 detecting probe dichlorofluorescien-diacetate (DCF-DA) (n=8, *P<0.05).
Effects of Exogenous Adenosine on the Diameter of Pressurized Cerebral Arterial Segments
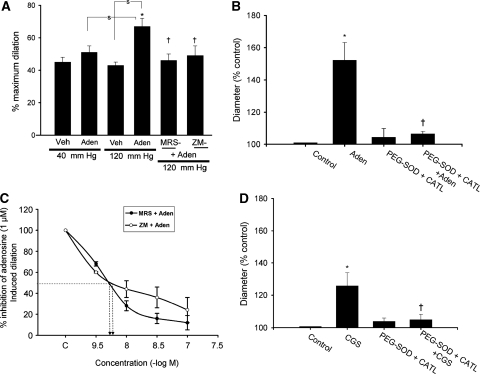
In isolated, endothelium removed, and cannulated middle cerebral arterial segments, increases in intraluminal pressure to 40 or 120 mm Hg induced a decrease in the internal diameter by 11%±1% and by 27%±3%, respectively, of the resting diameter (185±15 μm, n=6) measured at 20 mm Hg. Stimulation of the pressurized cerebral arterial segments with 1 μmol/L adenosine caused an increase in the diameter of the cannulated cerebral arterial segment at 40 mm Hg (n=6) or at 120 mm Hg (n=6, P<0.05) expressed as the percentage of maximum dilation induced by a combination of the direct relaxant papaverine (10 μmol/L) and Ca2+-free bath solution (Figure 3A). This adenosine-induced increase in diameter was attenuated after treatment of the cerebral arterial segments with the specific A2B adenosine receptor antagonist MRS-1754 (20 nmol/L) or with the mixed A2A and A2B adenosine receptor antagonist ZM-241385 (100 nmol/L) (Figure 3A). Pretreatment of the pressurized cerebral arterial segments with either ZM-241385 or MRS-1754 had no direct effect on the control arterial diameter (data not shown). In additional studies, effects of the A2A adenosine receptor antagonist ZM-241385 and the A2B adenosine receptor antagonist MRS-1574 at concentrations of 3, 10, 30, and 100 nmol/L were separately determined on the ability of adenosine (1 μmol/L) to induce an increase in the diameter of pressurized (120 mm Hg) cerebral arterial segments to examine their specificity. As depicted in Figure 3C, pretreatment of cerebral arterial segments with either ZM-241385 or MRS-1754 for 30 minutes at concentrations of 3, 10, 30, and 100 nmol/L reduced the adenosine-induced increase in diameter in a concentration-dependent manner. The concentrations of the A2A adenosine receptor antagonist ZM-241385 and that of the A2B adenosine receptor antagonist MRS-1754 required to induce halfway reduction of the maximal adenosine evoked an increase in diameter approximated to 6.5 and 6.7 nmol/L, respectively. The maximal inhibition of the adenosine evoked an increase in diameter of the cerebral arterial segments by pretreatment with either the A2A or A2B adenosine receptor antagonists were not significantly different (n=4 arterial segments obtained from 4 rats for each group, P>0.05). In contrast, pretreatment of CAMCs with either MRS-1754 (20 nmol/L) or ZM-241385 (100 nmol/L) had no significant influence on the increase in diameter of pressurized (120 mm Hg) cerebral arterial segments induced by the L-type Ca2+ channel blocker nifedipine (1 μmol/L, Figure 4B, n=5, P>0.05) ruling out a possible direct influence of these adenosine receptor antagonists on adenosine-induced cerebral vasodilatation. These findings indicate that stimulation of the A2A and A2B adenosine receptor subtypes in the cerebral arterial muscle are specific for the increase in diameter of the cerebral arterial segments induced by exogenous application of adenosine. However, given the prevalence of differences in size, receptor distribution, and ion channel type expression, which greatly influence reactivity of the cerebral vasculature, the findings of these studies using cerebral arterial segments of 150 to 200 μm diameter in size may not generally represent the role of smaller cerebral arterioles that are known to have greater influence on cerebral vascular resistance.
Figure 3.
Adenosine receptor subtypes and oxidants mediate adenosine-induced increase in diameter of pressure constricted cerebral arterial segments. (A) Role of adenosine receptor subtypes and oxidants in adenosine-induced increase in diameter of pressure constricted cerebral arterial segments. Bar graphs depicting the effects of exogenously applied adenosine (1 μmol/L) or vehicle on the diameter of cerebral arterial segments pressurized at 40 or 120 mm Hg before and after treatment with the A2B adenosine receptor antagonist MRS-1754 (20 nmol/L) or with the mixed A2A and A2B adenosine receptor antagonist ZM-241385 (100 nmol/L). Adenosine caused an increase in the diameter of the cerebral arterial segments pressurized at either 40 or 120 mm Hg that was significantly attenuated by the A2B adenosine receptor antagonist MRS-1754 and by the mixed A2A and A2B adenosine receptor antagonist ZM-241385. *Denotes the significant increase (P<0.05), and † represents the significant attenuation (P<0.05) of the adenosine-induced increase in the diameter of the arterial segments pressurized at 120 mm Hg by pretreatment with either MRS-1754 or ZM-241385 (n=6 for all groups studied). (C) Concentration-dependent inhibition of the adenosine (1 μmol/L)-induced increase in the diameter of pressurized cerebral arterial segments by the A2A adenosine receptor antagonist ZM-241385 (○) and by the A2B adenosine receptor antagonist MRS-1754 (•). (B and D) Effects of the antioxidants superoxide dismutase (SOD) conjugated to polyethylene glycol (PEG-SOD) and PEG-catalase on adenosine and CGS-21680 induced an increase in the diameter of pressure-constricted rat cerebral arterial segments. Preincubation of cannulated and pressurized cerebral arterial segments with a mixture of PEG-SOD (100 Units/mL) and PEG-catalase (100 Units/mL) had no significant effect on the control diameter, but significantly attenuated the increase in the diameter induced (panel B) by adenosine (1 μmol/L) and (panel C) by CGS-21680 (10 μmol/L). *Denotes the significant difference from control (P<0.05), and †denotes the significant difference from the increase in diameter induced by adenosine (panel A) or by CGS-21680 (panel B) (n=5 for each group).
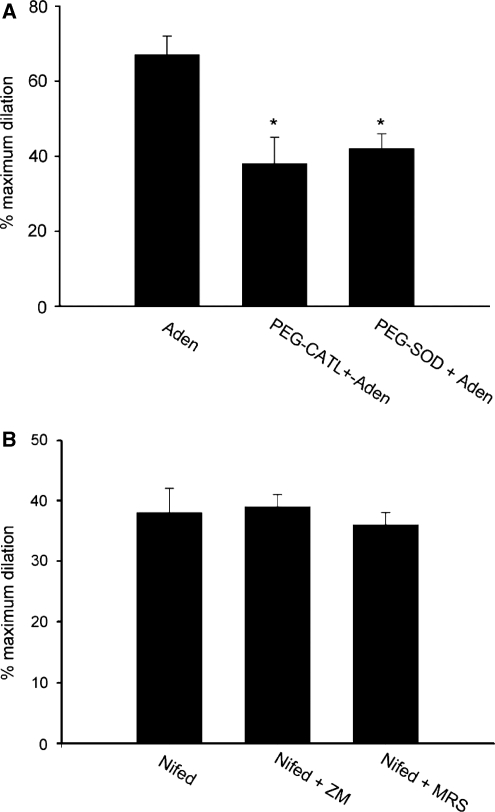
Figure 4.
Adenosine-induced cerebral dilation is mediated by superoxide production and the A2A and A2B adenosine receptor subtypes. (A) Effects of pretreatment with the antioxidant PEG-SOD (100 mU/mL) or PEG-catalase (100 mU/mL) on the vasodilatory effects of adenosine in pressurized cerebral arterial segments. Both PEG-SOD and PEG-catalase prevented the ability of adenosine (1 μmol/L) to increase the diameter of pressurized cerebral arterial segments to the same extent (n=6 for each group, *P<0.05 compared with the control adenosine response). (B) Lack of effect of the antagonists of A2A (ZM-241385, 100 nmol/L) and A2B (MRS-1754, 20 nmol/L) adenosine receptor on the dihydropyridine L-type Ca2+ channel blocker nifedipine (1 μmol/L)-induced increase in diameter of pressurized (120 mm Hg) cerebral arterial segments (n=4 for each group). These results revealed that the actions of A2A and A2B antagonist are specific for the adenosine-induced cerebral vasodilatation.
Adenosine- and the A2A Agonist CGS-21680-Induced Increase in Diameter of Pressure Constricted Cerebral Arterial Segments is Prevented by Pretreatment With PEG-SOD and PEG-catalase
To examine whether the generated O2− and its dismutation product H2O2 contributed to the adenosine-induced increase in diameter of the pressure-constricted cerebral arterial segments, the effects of pretreatment of arterial segments with a cocktail or individual preapplication of the cell-permeable SOD PEG-SOD and the H2O2 dismutase PEG-catalase (PEG-CATL) for 20 minutes on control diameter and on the ability of adenosine to increase the diameter of the cerebral arterial segments was determined. Pretreatment of the pressurized cerebral arterial segments with a cocktail of both antioxidants or with either PEG-SOD or PEG-catalase alone, had no significant effect on control diameter, but prevented the ability of adenosine to induce an increase in diameter of the pressurized (120 mm Hg) cerebral arterial segments to the same extent (Figures 3B and 4A), indicating that the contribution of O2− and its dismutation product H2O2 starts early on to increase the diameter after stimulation of the cerebral arterial segments by adenosine. In additional studies, we also examined the effects of a selective A2A adenosine receptor agonist CGS-21680 (10 μmol/L) on the diameter of the pressure-constricted cerebral arterial segments. As illustrated in Figure 3D, stimulation of the pressure-constricted (120 mm Hg) cerebral arterial segments with 10 μmol/L CGS-21680 induced an increase in diameter that was attenuated in the presence of a cocktail of the antioxidants PFG-SOD and PEG-catalase showing that the increase in cerebral arterial diameter induced by the A2A adenosine receptor agonist CGS-21680 is also mediated by O2− and H2O2 (n=4, *denotes the difference from control diameter and †denotes the difference from diameter response to adenosine at P<0.05).
Localization of Membrane Targets for Adenosine-Induced Superoxide Generation
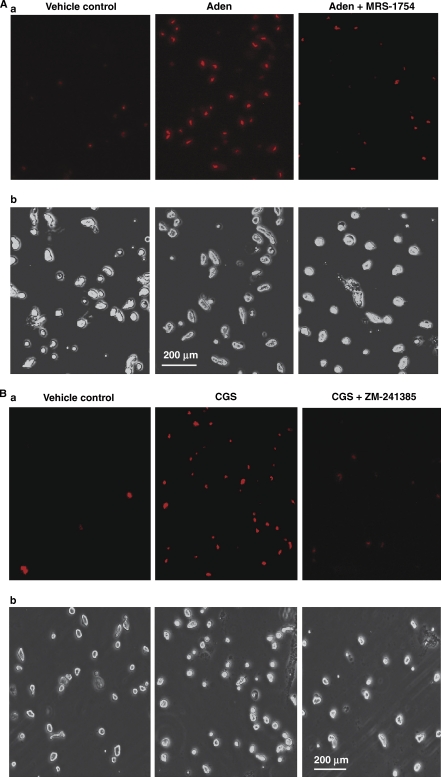
Although extracellular adenosine has been previously shown to induce vasodilatation by activation of the A2A and A2B adenosine receptor subtypes in the brain and in other vascular beds (Ibayashi et al, 1991; Ngai and Winn, 1993; Ngai et al, 2001), the role of O2− and the receptor subtype mediating the adenosine-induced O2− generation in CAMCs has not been delineated. We examined the roles of A2A and A2B adenosine receptor subtypes in the adenosine- and the A2A receptor agonist CGS-21680-induced generation of O2− in isolated CAMCs preincubated with the O2− detecting probe HE (1 μmol/L) for 10 minutes, with or without pretreatment of CAMCs with the specific A2B adenosine receptor antagonist MRS-1754 (20 nmol/L) or with the mixed A2A and A2B adenosine receptor antagonist ZM-241385 (100 nmol/L). Pretreatment with either MRS-1754 or ZM-241385 did not change basal fluorescent signal (data not shown), but prevented the ability of 1 μmol/L adenosine to increase O2− generation as measured by fluorescent microscopy (Figure 5Aa), whereas pretreatment with ZM-241385 alone attenuated the CGS-21680 induced increased intensity of the HE fluorescence in CAMCs (Figure 5Ba).
Figure 5.
Fluorescent microscopy determination of the role of A2A and A2B adenosine receptors in O2− generation in cerebral arterial muscle cells (CAMCs) loaded with the O2− detecting probe hydroethidine (HE). HE-loaded CAMCs were preincubated for 20 minutes with the vehicle control or (A) with or without the A2B adenosine receptor antagonist (MRS-1754, 20 nmol/L), followed by a 30-minute incubation period with adenosine (1 μmol/L) or (B) in the absence and presence of the A2A and A2B adenosine receptor antagonist ZM-241385 (100 nmol/L), followed by 30 minutes incubation with or without treatment with CGS-21680 (10 μmol/L). a and b represent fluorescent and bright field micrograph images of CAMCs for both A and B, respectively.
Potential sources of O2− Generated by the Stimulation of Adenosine A2A and A2B Receptor Subtypes in Cerebral Arterial Muscle Cells
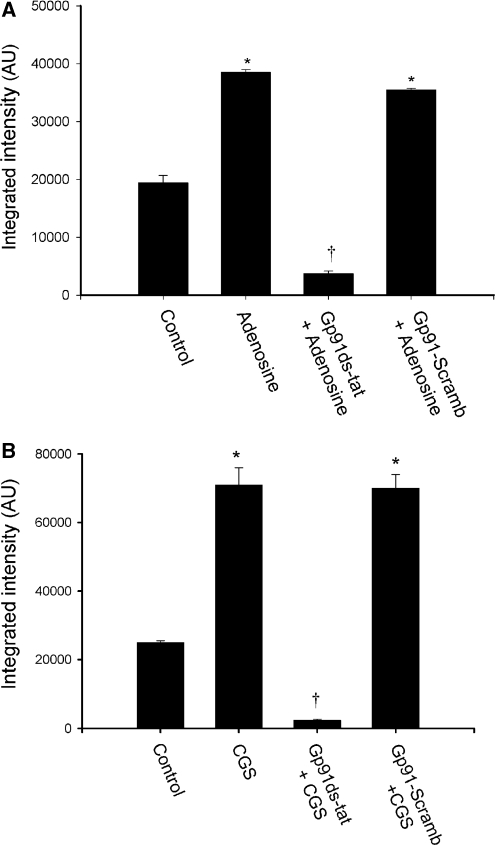
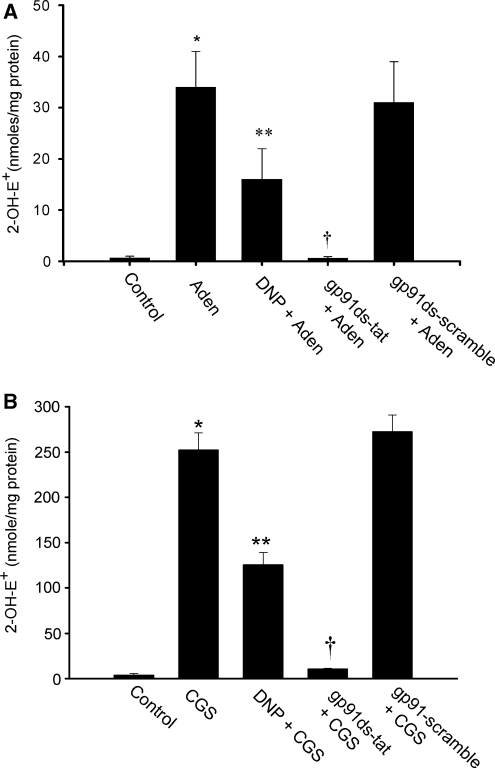
The oxidant O2− could be released from different sources in response to various stimuli and impact cerebral vascular function (Miller et al, 2006). These studies show that stimulation of the A2A and A2B adenosine receptors on CAMCs evoke generation of O2−. However, the potential source(s) of the released O2− after stimulation of these adenosine receptor subtypes are not known. We investigated the possible sources of O2− generated by stimulation of isolated CAMCs with adenosine or CGS-21680 using fluorescent microscopy and fluorescent HPLC analysis methods of O2−detection. As depicted in Figures 6A and 6B, stimulation of CAMCs, preloaded with the O2− detecting probe HE, with either adenosine or CGS-21680 caused an increase in the intensity of the HE fluorescent signal (n=5, *P<0.05). Preincubation of CAMCs with the specific peptide inhibitor of NADPH-oxidase gp91ds-tat attenuated the A2A and A2B adenosine receptor stimulation evoked increase in intensity of the HE fluorescent signal, while not being affected by the control gp91-scrambled-tat peptide, showing the contribution of NADPH oxidase to the A2A and A2B adenosine receptor stimulation-induced generation of O2− in CAMCs (Figures 6A and 6B). In fluorescent HPLC analysis studies, we examined whether mitochondrial and NADPH-oxidase sources of O2− production contributed to the adenosine- or CGS-21680-induced generation of O2−in CAMCs. As depicted in Figures 7A and 7B, stimulation of the HE-loaded CAMCs with adenosine or CGS-21680 increased the concentration of the oxidation product of HE, 2-OH-E+, normalized to milligram of cellular protein. As shown in this figure preincubation of HE-loaded CAMCs with either the mitochondrial uncoupling agent 2,4-dinitrophenol or the peptide inhibitor of NADPH-oxidase gp91ds-tat resulted in attenuation of the adenosine- or the CGS-21680-induced increase in O2−generation in CAMCs, which was not affected by the gp91-scramb-tat peptide (*P<0.02, **P<0.05, †P<0.04, n=5 to 6 for each group). Taken together, these findings suggest that the mitochondria and NADPH oxidase are important sources of O2− generated by stimulation of the A2A and A2B adenosine receptors in CAMCs.
Figure 6.
Fluorescent microscopy detection of adenosine receptor stimulation-induced O2− generation. Detection of superoxide generation level using hydroethidine (HE) staining in freshly dissociated cerebral arterial muscle cells (CAMCs) after treatment with vehicle control or with and without the presence of the specific peptide nicotinamide adenine dinucleotide phosphate (NADPH) inhibitor gp91ds-tat (5 μmol/L) or the scrambled control peptide gp91-scramb-tat (5 μmol/L), followed by stimulation of the cells with (A) adenosine (1 μmol/L) or (B) with the A2A adenosine receptor agonist CGS-21680 (10 μmol/L) (panel B) for 30 minutes. Stimulation of the CAMCs with either adenosine (panel A) or CGS-21680 (panel B) significantly increased the average HE fluorescent intensity, which was significantly attenuated by previous treatment of the cells with gp91ds-tat but not with the scrambled peptide. Data are representative of four independent experiments. * Denotes the significant increase (P<0.05) in adenosine or CGS-21680 induced increase in HE fluorescence intensity, and † represents the significant attenuation (P<0.05) of the intensity of the HE fluorescence signal by pretreatment with gp91ds-tat. (n=4 to 5 experiments for each group).
Figure 7.
Fluorescence high-performance liquid chromatography (HPLC) determination of the level of the oxidation product of the O2− detecting probe hydroethidine (HE) by O2−, 2-hydroxy-ethidium (2-OH-E+), in freshly dissociated cerebral arterial muscle cells (CAMCs) after treatment with vehicle alone or stimulation with either (A) adenosine (1 μmol/L) or (B) the A2A adenosine receptor agonist CGS-21680 (10 μmol/L) before and after treatment of the CAMCs with the mitochondria uncoupling agent 2,4-dinitrophenol (DNP, 100 μmol/L) or the specific peptide inhibitor of nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase gp91ds-tat (5 μmol/L) or with the scrambled control peptide gp91-scramb-tat (5 μmol/L). Stimulation of CAMCs with either adenosine (panel A) or CGS-21680 (panel B) significantly increased concentrations of 2-OH-E+ normalized to milligram protein of CAMCs. Pretreatment of the CAMCs with either DNP or gp91ds-tat, but not the scrambled peptide gp91-scramb-tat, significantly reduced the concentration of 2-OH-E+ stimulated by adenosine or CGS-21680 (n=4 separate experiments for each group, * denotes P<0.05 compared with control level of 2-OH-E+ to that after stimulation with adenosine or CGS-21680; ** and † represents significant difference from the adenosine or CGS-21680-induced increase in 2-OH-E+ concentration (P<0.05) after treatment with DNP and gp91ds-tat, respectively).
Discussion
The results of these studies obtained using multiple detection methods showed that isolated CAMCs express transcript and protein for the A1, A2A, A2B, and A3 adenosine receptor subtypes. The findings also showed that stimulation of the A2A and A2B adenosine receptor subtypes by exogenous adenosine induces generation of O2− and its dismutation product H2O2, and an increase in the diameter of pressure-constricted rat cerebral arterial segments. This capacity of exogenous adenosine to evoke adenosine receptor-dependent generation of O2− is confirmed by the use of confocal microscopy, fluorescent microscopy, and fluorescent HPLC analysis methods of O2− detection, and by the use of antioxidants PEG-SOD and PEG-catalase, and specific peptide inhibitors of NADPH-oxidase, as well as a mitochondrial uncoupler. A notable implication of these new findings is that the oxidants O2− and H2O2 released by adenosine may regulate cerebral arterial tone under normal physiologic conditions. In contrast to our present observations, previous studies have reported that adenosine decreases the plasma membrane content of the flavocytochrome b components p22phox and gp91phox of the NADPH–oxidase complex in neutrophils, a finding that may correlate well with the inhibition of generation of the reactive oxygen species O2− (Swain et al, 2003). Such contrasting actions by adenosine as compared with the present observations may suggest that this nucleoside could evoke differential regulation of O2− production in different cell types that might be related to possible differences in the distribution and expression of adenosine receptor subtypes.
Adenosine is released under normal physiologic situations and this release exceedingly increases under conditions of hypoxia and ischemia (Winn et al, 1979, 1981), and has been considered to be a potential regulator of cerebral blood flow (Phillis, 1989, 2004; Winn et al, 1979, 1981, 1980). However, the contribution of adenosine-induced generation of O2− and H2O2 to the regulation of the cerebral circulation under such conditions has not been previously described. Therefore, it is possible that the observed adenosine-induced oxidant-mediated increase in cerebral arterial diameter observed in this study could be a critically important response in that it may have the potential to promote tissue perfusion and enhance delivery of oxygen and nutrients to tissues with increased energy demand. Furthermore, as adenosine is a known endogenous regulator of cerebral blood flow in health (Phillis, 1989; Phillis et al, 1979), its capacity to generate O2− and H2O2 could also be considered as a possible indicator of the involvement of the generated oxidants in the regulation of cerebral blood flow even under normal physiologic circumstances.
Despite the fact that the A2A and A2B adenosine receptor subtypes are expressed and functional in CAMCs, their role in regulating cerebral arterial caliber through generation of O2− is not known. In this study, we found that pretreatment of isolated CAMCs with the specific antagonist of the A2B adenosine receptor subtype (MRS-1754) or with the mixed A2A and A2B adenosine receptor antagonist (ZM-241385) significantly attenuated generation of O2− induced by adenosine or by the synthetic adenosine analog and selective A2A adenosine receptor agonist CGS-21680. Results of these studies also showed that pharmacological inhibition of the A2A and A2B adenosine receptor subtypes attenuated the adenosine-induced increase in the diameter of pressure-constricted cerebral arterial segments. Taken at face value, these findings may suggest that the A2A and A2B adenosine receptor subtypes could be potential mediators of adenosine-induced O2− generation and reduction in pressure-induced cerebral arterial tone. Such functional responses to adenosine could be expected to be additive effects of stimulation of the A2A and A2B adenosine receptor subtypes in that the inhibition of both of these adenosine receptor subtypes was found to attenuate adenosine-induced O2− generation and an increase in arterial diameter to the same extent. The observed mediation of the adenosine-induced increase in diameter of cerebral arteries by the A2A and A2B adenosine receptor subtypes is consistent with the previously reported involvement of these adenosine receptor subtypes in the vasodilatory response of different arterial beds, including the cerebral circulation to adenosine (Ibayashi et al, 1991; Winn et al, 1985). Our present finding that activation of these adenosine receptor subtypes by adenosine promotes oxidant generation in CAMCs may bolster the functional influences of these adenosine receptor subtypes by integrating the vascular actions of the generated oxidants to the previously known adenosine receptor-induced cerebral vasodilation mediated by other released factors, such as NO, PGI2, and EETs or EDHF (You et al, 2005). Despite identification of the existence of transcripts and protein for the A1 and A3 adenosine receptor subtypes in CAMCs, further investigation of the functional role of these adenosine receptor subtypes was not performed in this study.
To date, other than pharmacological studies (Edvinsson and Fredholm, 1983), there is no clear evidence indicative of the expression of adenosine receptor subtypes in isolated CAMCs of the rat. Therefore, one of the core findings of these studies is the detection of transcript and protein expression of the A1, A2A, A2B, and A3 adenosine receptor subtypes in isolated CAMCs using quantitative RT-PCR, western blotting, and immunofluorescence techniques. As our method of isolation of pure CAMCs is well developed and has been routinely validated by the expression of the smooth muscle α-actin, contamination by other cell types could not be considered as a possible confounding issue of the present observations (Gebremedhin et al, 1998). At this stage, we presume that these new findings may lead to the development of adenosine receptor-based therapeutic targets for the diagnosis and treatments of various cerebrovascular disorders.
At present, there is no evidence in the literature for a defined role of adenosine A2A and A2B receptor subtypes in mediating the generation of O2− that could have a signaling role in CAMCs in response to stimulation by adenosine or its analog CGS-21680. In an attempt to bridge this gap, we performed studies using subtype-specific adenosine receptor antagonists, and found that both adenosine A2A and A2B receptor subtypes mediate adenosine-induced generation of O2− and increase in cerebral arterial diameter.
One of the physiologically important aspect of the present findings is that adenosine could be regarded as a cerebral vasodilator that could also act through generation of the oxidants O2− and H2O2. Given that the extracellular concentration of adenosine increases under conditions of hypoxia and ischemia, and functions to promote tissue perfusion and delivery of oxygen and nutrients, it is possible that such actions of adenosine could be mediated by oxidants generated by adenosine receptor stimulation under physiologic or pathophysiological conditions. Furthermore, pertinent to this effect is also the possibility that adenosine generated O2− and its dismutation product H2O2 could participate in mediating functional hyperemic response of the brain that follows neuronal activation-induced adenosine release from different brain cell types and regulates cerebral blood flow (Anderson and Nedergaard, 2003; Harder et al, 1998). Indeed, such response of the cerebral circulation to adenosine is also well known to be mediated by endogenously released factors, including NO, PGI2, EETs, or EDHF as mentioned above (You et al, 2005). However, the impact and interaction of these endogenous mediatory factors on the generation of O2− by adenosine in CAMCs is not yet known, and remains to be a challenge that needs to be reckoned.
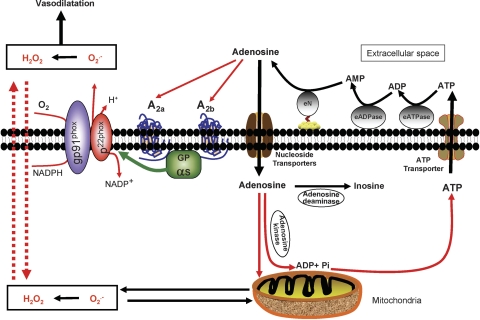
It has been previously suggested that NADPH oxidase and the mitochondria are the major sources of O2− generation in the brain (Miller et al, 2005, 2006), but it is not clear whether these sources were used by adenosine to generate O2− from CAMCs. Results of our present investigations showed that the gp91phox component of the NADPH–oxidase complex and the mitochondria appear to be major sources of O2−, as evidenced by inhibition of adenosine-induced generation of O2− by the specific peptide inhibitor of NADPH-oxidase gp91ds-tat but not by the inactive control peptide gp91-scaramb-tat (Rey et al, 2001; Zhang et al, 2006) and the mitochondrial uncoupler 2,4-dinitrophenol (Jin et al, 2004) in CAMCs. We also found that the peptide inhibitor of the NADPH-oxidase gp91ds-tat markedly prevented the ability of adenosine to increase the diameter of pressure-constricted cerebral arterial segments indicative of mediation of this response by NADPH oxidase. Such involvement of NADPH oxidase in the adenosine-induced cerebral arterial modulation and O2− generation in the brain vasculature could be supported by earlier findings by other investigators who suggested NADPH oxidase as a major source of oxidant production in the brain and regulator of cerebral vascular function (Miller et al, 2005). On the basis of the results of these studies, we propose the concept depicted in Figure 8 that NADPH-oxidase and the mitochondria could be major sources of adenosine-induced generation of O2− and its dismutation product H2O2 through activation of surface adenosine A2A and A2B receptor subtypes in CAMCs that signal the increase in diameter of cerebral arterial segments induced by this nucleoside. It is important to highlight the fact that despite the existing ambiguity regarding the effects of oxidants such as O2− and H2O2 as related to tissue injury or damage, we propose that the beneficial physiologic actions of basal endogenous O2− and H2O2 levels or that generated by physiologic agonists such as adenosine to act as normal modulators of the cerebral arterial muscle reactivity and thus functions of the cerebral circulation.
Figure 8.
Schematic representation of possible mechanisms of adenosine-induced generation of O2− and H2O2 in rat isolated cerebral arterial muscle cells. Extracellular adenosine through activation of G protein-coupled A2A and A2B adenosine receptors stimulates nicotinamide adenine dinucleotide phosphate (NADPH) oxidase to generate O2− and then H2O2. Extracellular adenosine can also enter the cell through nucleotide transporters by a diffusion-limited process to be either hydrolyzed by adenosine deaminase to inosine or act directly on the mitochondria to release O2− and H2O2. Once in the cell, adenosine could also be phosphorylated by adenosine kinase to form AMP, ADP, and finally ATP, which will be transported by the ATP transporter to the extracellular space where it is acted upon by different ectonucleotidases (eN) to the precursor molecules ADP and AMP to generate extracellular adenosine that serves as an extracellular signal molecule by stimulating the G protein-coupled A2A and A2B adenosine receptors on CAMCs to release O2− and H2O2 that can enter the cytosole and stimulate generation of O2− and H2O2 from the mitochondria by the mechanism of reactive oxygen species (ROS)-induced ROS release (Zorov et al, 2006), which could also contribute to the adenosine-induced reduction in cerebral arterial tone.
In conclusion, our present findings show that adenosine through stimulation of the A2A and A2B adenosine receptor subtypes induces generation of O2− and its dismutation product H2O2 in CAMCs that could account for its cerebral vasodilatory action. As adenosine is formed and released into the extracellular space under both physiologic and pathophysiological conditions, its ability to generate the oxidants O2− and H2O2 may not be regarded as a damaging influence alone, but also as an important compensatory physiologic mechanism in the regulation of the functions of cerebral circulation.
Acknowledgments
We thank Aaron John Schuett for excellent technical assistance. We would like also to thank Dr Jacek Zielonka of the Free Radical Research Center of the Medical college of Wisconsin directed by Dr Balaraman Kalyanaraman for the fluorescent HPLC assay studies and Dr Zhang Xeufeng for the help with the immunofluorescence staining studies.
The authors declare no conflict of interest.
References
- Anderson CM, Nedergaard M. Astrocyte-mediated control of cerebral microcorculation. Trends Neurosci. 2003;26:340–344. doi: 10.1016/S0166-2236(03)00141-3. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Fredholm BB. Characterization of adenosine receptors in isolated cerebral arteries of cat. Br J Pharmacol. 1983;80:631–637. doi: 10.1111/j.1476-5381.1983.tb10052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhin D, Lange AR, Narayanan J, Aebly MR, Jacobs ER, Harder DR. Cerebral arterial smooth muscle cells express P450 4A2 enzyme and produce 20-HETE which enhances L-type Ca2+ current. J Physiol (Lond) 1998;507:771–781. doi: 10.1111/j.1469-7793.1998.771bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhin D, Yamaura K, Harder DR. Role of 20-HETE in the hypoxia-induced activation of Ca2+-activated K+ channel currents in rat cerebral arterial muscle cells. Am J Physiol (Heart Circ Physiol) 2008;294:H107–H120. doi: 10.1152/ajpheart.01416.2006. [DOI] [PubMed] [Google Scholar]
- Gessi S, Varani K, Merighi S, Leung E, Mac Lennan S, Baraldi PG, Borea PA. Novel selective antagonist radioligands for the pharmacological study of A2b adenosine receptors. Purinergic Signal. 2006;2:583–588. doi: 10.1007/s11302-006-9019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder DR, Alkayed NJ, Lange AR, Gebremedhin D, Roman RJ. Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke. 1998;29:229–234. doi: 10.1161/01.str.29.1.229. [DOI] [PubMed] [Google Scholar]
- Ibayashi S, Ngai AC, Meno JR, Winn HR. Effects of topical adenosine analogs and forskolin on rat pial arterioles in vivo. J Cereb Blood Flow Metab. 1991;11:72–76. doi: 10.1038/jcbfm.1991.8. [DOI] [PubMed] [Google Scholar]
- Ikeda U, Kurosaki K, Ohya K, Shimada K. Adenosine stimulates nitric oxide synthesis in vascular smooth muscle cells. Cardiovasc Res. 1997;35:168–174. doi: 10.1016/s0008-6363(97)00068-0. [DOI] [PubMed] [Google Scholar]
- Jin Y, McEwen ML, Nottingham SA, Maragos WF, Dragicevic NB, Sullivan PG, Springer JE. The mitochondrial uncoupling agent 2,4-dinitrophenol improves mitochondrial function, attenuates oxidative damage, and increases white matter sparing in the contused spinal cord. J Neurotrauma. 2004;21:1396–1404. doi: 10.1089/neu.2004.21.1396. [DOI] [PubMed] [Google Scholar]
- Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol. 2006;100:307–317. doi: 10.1152/japplphysiol.00938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JM, Fenton RA, Cutler BS, Dobson JG., Jr Adenosine enhances nitric oxide production by vascular endothelial cells. Am J Physiol Cell Physiol. 1995;269:C519–C523. doi: 10.1152/ajpcell.1995.269.2.C519. [DOI] [PubMed] [Google Scholar]
- Miller AA, Drummond GR, Schmidt HHHW, Sobey CG. NADPH-oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ Res. 2005;97:1055–1062. doi: 10.1161/01.RES.0000189301.10217.87. [DOI] [PubMed] [Google Scholar]
- Miller AA, Drummond GR, Sobey CG. Reactive oxygen species in the cerebral circulation: are they all bad. Antioxid Redox Signal. 2006;8:1113–1120. doi: 10.1089/ars.2006.8.1113. [DOI] [PubMed] [Google Scholar]
- Ngai AC, Coyne EF, Meno JR, West GA, Winn HR. Receptor subtypes mediating adenosine-induced dilation of cerebral arterioles. Am J Physiol Heart Circ Physiol. 2001;280:H2329–H2335. doi: 10.1152/ajpheart.2001.280.5.H2329. [DOI] [PubMed] [Google Scholar]
- Ngai AC, Winn HR. Effects of adenosine and its analogs on isolated intracerebral arterioles. Extraluminal and intraluminal application. Circ Res. 1993;73:448–457. doi: 10.1161/01.res.73.3.448. [DOI] [PubMed] [Google Scholar]
- Phillis JW. Adenosine in the control of the cerebral circulation. Cerebrovasc Brain Metab Rev. 1989;1:26–54. [PubMed] [Google Scholar]
- Phillis JW. Adenosine and adenine nucleotides as regulators of cerebral blood flow: roles of acidosis, cell swelling, and KATP channels. Crit Rev Neurobiol. 2004;16:237–270. doi: 10.1615/critrevneurobiol.v16.i4.20. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Edstrom JP, Kostopoulos GK, Kirkpatrick JR. Effects of adenosine and adenine nucleotides on synaptic transmission in the cerebral cortex. Can J Physiol Pharmacol. 1979;157:289–312. doi: 10.1139/y79-194. [DOI] [PubMed] [Google Scholar]
- Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2− and systolic blood pressure in mice. Circ Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- Swain SD, Siemsen DW, Nelson LK, Sipes KM, Hanson AJ, Quinn MT. Inhibition of the neutrophil NADPH oxidase by adenosine is associated with increased movement of flavocytochrome b between subcellular fractions. Inflammation. 2003;27:45–58. doi: 10.1023/a:1022639228723. [DOI] [PubMed] [Google Scholar]
- Winn HR, Morii S, Berne RM. The role of adenosine in autoregulation of cerebral blood flow. Ann Biomed Eng. 1985;13:321–328. doi: 10.1007/BF02584250. [DOI] [PubMed] [Google Scholar]
- Winn HR, Rubio R, Berne RM. Brain adenosine production in the rat during 60 seconds of ischemia. Circ Res. 1979;45:486–492. doi: 10.1161/01.res.45.4.486. [DOI] [PubMed] [Google Scholar]
- Winn HR, Rubio R, Berne RM. Brain adenosine concentration during hypoxia in rats. Am J Physiol. 1981;241:H235–H242. doi: 10.1152/ajpheart.1981.241.2.H235. [DOI] [PubMed] [Google Scholar]
- Winn HR, Welsh JE, Rubio R, Berne RM. Brain adenosine production in rat during sustained alteration in systemic blood pressure. Am J Physiol. 1980;239:H636–H641. doi: 10.1152/ajpheart.1980.239.5.H636. [DOI] [PubMed] [Google Scholar]
- You J, Golding EM, Bryan RM., Jr Arachidonic acid metabolites, hydrogen peroxide, and EDHF in cerebral arteries. Am J Physiol Heart Circ Physiol. 2005;289:H1077–H1083. doi: 10.1152/ajpheart.01046.2004. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kho AL, Anilkumar N, Chibber R, Pagano PJ, Shah AM, Cave AC. Glycated proteins stimulate reactive oxygen species production in cardiac myocytes: involvement of Nox2 (gp91phox)-containing NADPH oxidase. Circulation. 2006;113:1235–1243. doi: 10.1161/CIRCULATIONAHA.105.581397. [DOI] [PubMed] [Google Scholar]
- Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Rad Biol Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- Zielonka J, Hardy M, Kalyanaraman B. HPLC study of oxidation products of hydroethidine in chemical and biological systems: ramifications in superoxide measurements. Free Radic Biol Med. 2009;46:329–338. doi: 10.1016/j.freeradbiomed.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc. 2008;3:8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]