Abstract
This study shows a significant correlation between functional connectivity, as measured with resting-state functional magnetic resonance imaging (MRI), and neuroanatomical connectivity, as measured with manganese-enhanced MRI, in rats at 10 weeks after unilateral stroke and in age-matched controls. Reduced interhemispheric functional connectivity between the contralesional primary motor cortex (M1) and ipsilesional sensorimotor cortical regions was accompanied by a decrease in transcallosal manganese transfer from contralesional M1 to the ipsilesional sensorimotor cortex after a large unilateral stroke. Increased intrahemispheric functional connectivity in the contralesional sensorimotor cortex was associated with locally enhanced neuroanatomical tracer uptake, which underlines the strong link between functional and structural reorganization of neuronal networks after stroke.
Keywords: brain plasticity, functional connectivity, manganese-enhanced MRI, rats, resting-state functional MRI, stroke
Introduction
Most patients recovering from a stroke exhibit a certain degree of spontaneous restoration of function, which may be related to rearrangement of neuronal networks in adjacent and remote regions with regard to the ischemic lesion. Task- or stimulus-induced functional imaging studies in stroke patients and animal models have provided evidence for shifts of activation patterns in ipsilesional and contralesional sensorimotor cortices in relation to sensorimotor recovery (Calautti and Baron, 2003; Dijkhuizen et al, 2003), which may be based on unmasking or strengthening of existing pathways and/or by formation of new connections (Nudo, 1999; Dancause, 2006). Resting-state functional magnetic resonance imaging (MRI) (rs-fMRI), which assesses the temporal correlation of spontaneous low-frequency blood oxygenation level-dependent (BOLD) signals between brain areas as a measure of functional connectivity (Biswal et al, 1995), has recently showed that patients and rats with a unilateral infarction exhibit initial loss and subsequent restitution of interhemispheric functional connectivity, in parallel to changes in functional status (He et al, 2007; van Meer et al, 2010). Furthermore, increased intrahemispheric functional connectivity in the contralesional primary sensorimotor cortex was observed in rats with large unilateral lesions (van Meer et al, 2010).
The goal of this study was to elucidate whether the restoration or enhancement of interhemispheric and intrahemispheric functional connectivity at chronic stages after unilateral stroke are associated with improved neuroanatomical connectivity. Therefore, we combined rs-fMRI with manganese-enhanced MRI (MEMRI), which allows in vivo mapping of the paramagnetic neuroanatomical tracer manganese (Pautler et al, 1998), at 10 weeks after experimental unilateral stroke in rats, to directly relate measures of functional and structural connectivity in reorganized cortical sensorimotor regions.
Materials and methods
Stroke Model
All animal procedures were approved by the Animal Experiments Committee of the University Medical Center Utrecht and Utrecht University.
A total of 18 male Sprague Dawley rats, weighing 280 to 320 g, were included. Eleven rats underwent transient unilateral focal cerebral ischemia by 90 minutes occlusion of the right middle cerebral artery with an intraluminal filament, as described previously (van Meer et al, 2010). In brief, a 4.0 polypropylene suture (Ethicon, Piscataway, NJ, USA) with a silicon-coated tip was introduced into the right external carotid artery and advanced through the internal carotid artery until a slight resistance was felt, indicating that the middle cerebral artery was occluded. After 90 minutes, the filament was withdrawn from the internal carotid artery to allow reperfusion.
Seven healthy rats served as controls.
Magnetic Resonance Imaging
Magnetic resonance imaging was performed at 70 days after stroke, when the recovery of sensorimotor function had reached a plateau (van Meer et al, 2010; van der Zijden et al, 2008). During MRI, rats were anesthetized and mechanically ventilated with 1 to 2% isoflurane in air/O2 (2:1). End-tidal CO2 levels were kept within normal range by adjusting ventilation volume and/or rate. Magnetic resonance imaging measurements were conducted on a 4.7-T horizontal bore MR system (Varian, Palo Alto, CA, USA). First, multiecho multislice T2-weighted MRI (repetition time (TR)/TE (echo time)=3600/15 milliseconds; echo train length=12; voxel resolution=0.25 × 0.25 × 1.0 mm3) and gradient echo three-dimensional MRI (TR/TE=6/2.58 milliseconds; flip angle=40° voxel resolution=0.23 × 0.31 × 0.31 mm3) were conducted for assessment of ischemic lesion size and location, and for registration purposes, respectively. Thereafter, for at least 10 minutes, end-tidal isoflurane was reduced to 1%, followed by rs-fMRI with a gradient echo, echo planar imaging sequence (TR/TE=500/19 milliseconds; flip angle=35° voxel resolution=0.5 × 0.5 × 1.5 mm3; 1200 BOLD images). Subsequently, T1-weighted MRI (Look–Locker gradient echo; TR/TE=5000/3.4 milliseconds; inversion time=10 milliseconds; image TR=24 × 8 milliseconds; flip angle=10° voxel resolution=0.5 × 0.5 × 0.5 mm3) was conducted for baseline measurement of premanganese tissue R1.
Two days after the initial MRI experiments, 90 nL of 0.1 mol/L MnCl2 was injected into the left (contralesional) primary motor cortex (M1) (Canals et al, 2008). Postmanganese T1-weighted and gradient echo three-dimensional MRI were conducted 1 day after MnCl2 injection.
Experimental Groups
Stroke group assignments were based on the size and location of ischemic lesions on T2-weighted images at 70 days after stroke. One animal died beforehand because of stroke-induced cachexia. Animals with mostly subcortical tissue damage and intact primary somatosensory and motor cortices were assigned to group SI (n=5), whereas animals with a lesion involving both subcortical and primary somatosensory cortical tissues were assigned to group SII (n=5). One animal from group SI and one animal from group SII were excluded from further analysis because of unsuccessful MnCl2 injection. Age-matched healthy rats were assigned to control group C (n=7).
Data Analysis and Statistics
Gradient echo three-dimensional images were registered nonrigidly to a reference image matched to a three-dimensional model of a rat brain atlas (Paxinos and Watson, 2005). Bilateral sensorimotor cortical regions of interest (ROIs), i.e., the primary and secondary motor cortices (M1, M2), forelimb region of the primary somatosensory cortex (S1fl) and secondary somatosensory cortex (S2), were projected from the atlas onto the rs-fMRI time series and R1 maps.
Preprocessing of rs-fMRI data included motion correction, spatial smoothing, and linear regression, as described previously (van Meer et al, 2010). Subsequently, low-frequency BOLD fluctuations were extracted by applying a band-pass filter with 0.01<f<0.1 Hz. Functional connectivity was calculated as the Fisher-transformed correlation coefficient z′ (ln((1+r)/(1−r))/2) between the mean low-frequency BOLD time-series signal in the left M1 (seed region; 22 mm3) and other cortical sensorimotor ROIs. Group mean whole-brain functional connectivity maps were obtained by voxel-wise calculation of z′ with the mean time-series signal from the left M1 as reference, averaged across subjects.
Manganese enhancement after MnCl2 injection in the left M1 was measured in all ROIs and calculated as the difference in premanganese and postmanganese R1 (ΔR1) (van der Zijden et al, 2008). Group mean ΔR1 maps were obtained by averaging across subjects.
Two-way repeated measures ANOVA (analysis of variance) with factors group and ROI was used to statistically compare intrahemispheric and interhemispheric functional connectivities (z′), as well as ipsilateral and contralateral ΔR1s between groups, followed by post hoc Bonferroni's testing. Pearson's correlation analysis was used to test for correlation between functional (z′) and neuroanatomical connectivity (ΔR1) of the left M1 with other sensorimotor cortical ROIs. Values are shown as mean±s.d. P<0.05 was considered significant.
Results
Functional Connectivity
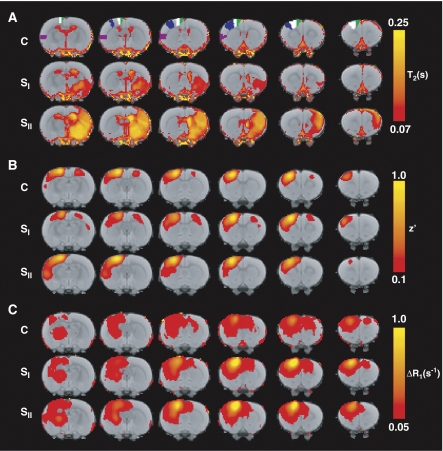
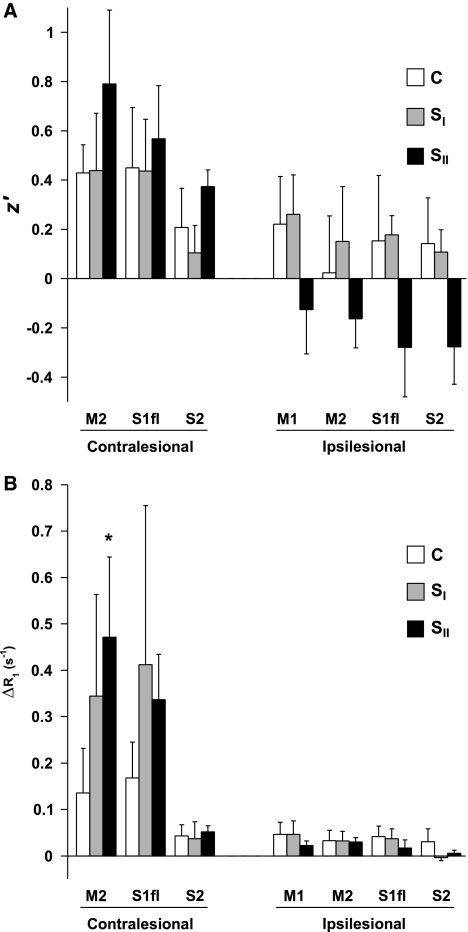
Figure 1A shows averaged brain T2 maps for the different groups. Prolonged T2, indicative of ischemic tissue damage, is evident in mainly subcortical regions in SI rats, and in both subcortical and primary somatosensory cortical areas in SII rats. Figure 1B shows averaged maps of resting-state functional connectivity with the left (contralesional) M1. In control rats, intrahemispheric and interhemispheric connectivity between M1 and ipsilateral and contralateral sensorimotor cortices was clearly apparent. At 10 weeks after stroke, functional connectivity of contralesional M1 was noticeably altered depending on the lesion extent in the opposite hemisphere. A significant increase in intrahemispheric functional connectivity of contralesional sensorimotor cortical ROIs with contralesional M1 was found in SII rats as compared with controls and SI animals (Figure 2A). Conversely, in the same group, a significant reduction in interhemispheric functional connectivity was detected between contralesional M1 and ipsilesional sensorimotor cortical ROIs as compared with groups C and SI (Figure 2A).
Figure 1.
(A) Color-coded maps of thresholded mean T2, (B) z′ (with the left (contralesional) M1 as seed region), and (C) ΔR1 (1 day after MnCl2 injection into the left (contralesional) M1), for groups C, SI, and SII, overlaid on a T2-weighted MRI template of consecutive coronal rat brain slices. The left ROIs M1 (primary motor cortex) (white), M2 (secondary motor cortex) (green), S1fl (forelimb region of the primary somatosensory cortex) (blue), and S2 (secondary somatosensory cortex) (purple) are delineated on the rat brain template. MRI, magnetic resonance imaging; ROI, region of interest.
Figure 2.
(A) Intrahemispheric and interhemispheric z′ of the left (contralesional) M1 with left (contralesional) M2, S1fl, and S2, and right (ipsilesional) M1, M2, S1fl, and S2, respectively, for groups C, SI, and SII. (B) Intrahemispheric and interhemispheric manganese-induced ΔR1 (per second) in left (contralesional) M2, S1fl, and S2, and right (ipsilesional) M1, M2, S1fl, and S2, respectively, at 1 day after MnCl2 injection in the left (contralesional) M1, for groups C, SI, and SII. There was a significant group effect (F(2)=4.45; P=0.036) of increased intrahemispheric z′ in contralesional ROIs for group SII as compared with groups C and SI. Besides, a significant group effect (F(2)=10.53; P=0.002) of decreased interhemispheric z′ in ipsilesional ROIs was also found for group SII as compared with groups C and SI. A significant group × ROI effect (F(2)=5.02; P=0.004) of increased ΔR1 in contralesional ROIs was found for groups SI and SII. A significant group × ROI effect (F(2)=5.23; P<0.001) of decreased ΔR1 in ipsilesional ROIs was found for group SII. *P<0.05 versus equivalent ROI in group C. ROI, region of interest.
Structural Connectivity
Manganese-enhanced MRI showed clear manganese accumulation in the ipsilateral sensorimotor cortex, caudate putamen, and thalamus in all groups at 1 day after injection in the left (contralesional) M1 (Figure 1C). There were no significant group differences in total brain uptake of manganese, as measured from mean ΔR1 in the whole rat brain tissue (ΔR1=0.038±0.020 per second, ΔR1=0.032±0.021 per second, and ΔR1=0.040±0.008 per second, for groups C, SI, and SII, respectively). At 10 weeks after stroke, manganese enhancement was increased in the contralesional sensorimotor cortex. A group × ROI interaction effect of significantly elevated manganese build-up in contralesional sensorimotor cortical ROIs was found for SI and SII rats (Figure 2B). Increased manganese enhancement was particularly observed in contralesional M2 of SII animals.
Manganese accumulation was relatively low in the right (ipsilesional) hemisphere, contralateral to the injection site. Still, SII rats showed a significant interaction effect of reduced manganese build-up in ipsilesional sensorimotor cortical ROIs (Figure 2B).
Correlation Between Functional and Structural Connectivity
Functional connectivity (z′) and manganese build-up (ΔR1) from the left (contralesional) M1 to intrahemispheric cortical sensorimotor ROIs (M2, S1fl, and S2) correlated significantly for all groups together (r=0.31, P=0.02). We found a trend for a correlation between z′ and ΔR1 in right (ipsilesional) cortical sensorimotor ROIs (M1, M2, S1fl, and S2) for the pooled groups (r=0.18, P=0.08).
Discussion
In this study, we combined rs-fMRI with MEMRI to elucidate the relationship between functional and neuroanatomical brain connectivity of the contralesional sensorimotor cortex in the chronic phase after experimental unilateral stroke.
Intrahemispheric Connectivity
In line with our previous study (van Meer et al, 2010), we found a significant increase in intrahemispheric functional connectivity within the contralesional sensorimotor cortex in rats with a relatively large unilateral infarction in the opposite (ipsilesional) sensorimotor network. In addition, we detected increased accumulation of the neuronal tracer manganese within the contralesional sensorimotor cortex of rats with large unilateral ischemic lesions, which suggests that the increase in functional connectivity is related to stronger local neuroanatomical association. The ipsilateral concentration of manganese after injection in the contralesional sensorimotor cortex may be explained by enhanced local neuronal connectivity and diminished axonal transport to remote (contralateral) regions (see below). An apparent regional linkage between functional and structural connectivity is supported by the observed significant correlation between synchronized low-frequency BOLD signals (z′) and manganese-induced ΔR1 in intrahemispheric sensorimotor regions when data from all experimental groups were combined.
The poststroke increase in neuroanatomical and functional connectivity in the contralesional cortex may be brought about by remodeling of neuronal elements, i.e., axonal sprouting, synaptogenesis, and dendritic growth, which has been detected in various animal stroke models (reviewed in Nudo, 1999; Dancause, 2006). Similarly, in chronic human stroke patients, elevated activation responses to sensory stimulation of the unaffected hand have been found in an area of the contralesional somatosensory cortex with increased cortical thickness (Schaechter et al, 2006). Although not the subject of this study, we speculate that reorganization of the contralesional cortical tissue may be induced by increased compensatory use of the unaffected limbs and/or altered input from the affected ipsilesional cortex.
Interhemispheric Connectivity
In agreement with our previous study (van Meer et al, 2010), interhemispheric functional connectivity between contralesional M1 and ipsilesional sensorimotor cortical ROIs in rats with large subcortical and cortical infarctions was significantly decreased as compared with controls and rats with mainly subcortical tissue injury. This reflects desynchronization of signaling between the bilaterally homologous sensorimotor fields, which may be a direct result of extensive damage to the ipsilesional sensorimotor cortex. The observed negative z′ values in SII animals even suggest anticorrelations, but more likely point toward minimal correlation between interhemispheric signals, as discussed in our previous paper (van Meer et al, 2010). In line with the reduction in interhemispheric functional connectivity, we detected a significant decline in transcallosal manganese transport toward ipsilesional sensorimotor ROIs in SII rats. This seems to be in contrast with an earlier study in which we found an increase in transhemispheric manganese transfer at 10 weeks after stroke in the same rat model (van der Zijden et al, 2008). However, in that study, MnCl2 was injected into the perilesional sensorimotor cortex, and elevated levels of manganese were measured in subcortical areas of the contralesional hemisphere. Furthermore, in this study, we used a much smaller MnCl2 dose to prevent potential manganese-induced neurotoxicity (Canals et al, 2008), but which could have reduced the sensitivity to detect tracer accumulation in remote regions, opposite to the injection site. Evidently, the choice of an optimal dose for MEMRI experiments is not straightforward, as it depends on multiple factors and may vary contingent on the research question.
Correlation Between Functional and Structural Connectivity
Our study shows parallel changes in functional and structural connectivity in the ipsilesional and contralesional cortical sensorimotor network after experimental stroke in rats. Furthermore, we detected a significant overall correlation between rs-fMRI-based functional connectivity and MEMRI-based neuroanatomical association in the contralesional (left) hemisphere for all ROIs and animal groups pooled together. Our findings in the anesthetized rat brain corroborate the concept that synchronization of low-frequency BOLD fluctuations are closely associated with structural connectivity, as previously reported for monkey (Vincent et al, 2007) and human brains (Damoiseaux and Greicius, 2009).
Acknowledgments
We thank Gerard van Vliet, Annette van der Toorn, and René Zwartbol for technical assistance.
The authors declare no conflict of interest.
References
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke. 2003;34:1553–1566. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- Canals S, Beyerlein M, Keller AL, Murayama Y, Logothetis NK. Magnetic resonance imaging of cortical connectivity in vivo. Neuroimage. 2008;40:458–472. doi: 10.1016/j.neuroimage.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- Dancause N. Vicarious function of remote cortex following stroke: recent evidence from human and animal studies. Neuroscientist. 2006;12:489–499. doi: 10.1177/1073858406292782. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM, Singhal AB, Mandeville JB, Wu O, Halpern EF, Finklestein SP, Rosen BR, Lo EH. Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: a functional magnetic resonance imaging study. J Neurosci. 2003;23:510–517. doi: 10.1523/JNEUROSCI.23-02-00510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Recovery after damage to motor cortical areas. Curr Opin Neurobiol. 1999;9:740–747. doi: 10.1016/s0959-4388(99)00027-6. [DOI] [PubMed] [Google Scholar]
- Pautler RG, Silva AC, Koretsky AP. In vivo neuronal tract tracing using manganese-enhanced magnetic resonance imaging. Magn Reson Med. 1998;40:740–748. doi: 10.1002/mrm.1910400515. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.2005The Rat Brain in Stereotaxic Coordinates5th ed.Elsevier Academic Press: Burlington, MA, USA [Google Scholar]
- Schaechter JD, Moore CI, Connell BD, Rosen BR, Dijkhuizen RM. Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain. 2006;129:2722–2733. doi: 10.1093/brain/awl214. [DOI] [PubMed] [Google Scholar]
- van der Zijden JP, Bouts MJ, Wu O, Roeling TA, Bleys RL, van der Toorn A, Dijkhuizen RM. Manganese-enhanced MRI of brain plasticity in relation to functional recovery after experimental stroke. J Cereb Blood Flow Metab. 2008;28:832–840. doi: 10.1038/sj.jcbfm.9600576. [DOI] [PubMed] [Google Scholar]
- van Meer MP, van der Marel K, Wang K, Otte WM, El Bouazati S, Roeling TA, Viergever MA, Berkelbach van der Sprenkel JW, Dijkhuizen RM. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J Neurosci. 2010;30:3964–3972. doi: 10.1523/JNEUROSCI.5709-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]