Abstract
Various proteases in the brain contribute to ischemic brain injury. We investigated the involvement of the asparaginyl endopeptidase legumain after experimental stroke. On the basis of gene array studies and in situ hybridizations, we observed an increase of legumain expression in the peri-infarct area of rats after transient occlusion of the middle cerebral artery (MCAO) for 120 mins with a maximum expression at 24 and 48 h. Immunohistochemical analyses revealed the expression of legumain in Iba1+ microglial cells and glial fibrillary acidic protein-positive astrocytes of the peri-infarct area in mice after MCAO. Post-stroke recovery was also studied in aged legumain-deficient mice (45 to 58 weeks old). Legumain-deficient mice did not show any differences in physiologic parameters compared with respective littermates before, during MCAO (45 mins), and the subsequent recovery period of 8 days. Moreover, legumain deficiency had no effect on mortality, infarct volume, and the neurologic deficit determined by the rotating pole test, a standardized grip strength test, and the pole test. However, a reduced number of invading CD74+ cells in the ischemic hemisphere indicates an involvement in post-stroke inflammation. We conclude that legumain is not essential for the functional deficit after MCAO but may be involved in mechanisms of immune cell invasion.
Keywords: astrocyte, CD74, cerebral ischemia, legumain, microglia, poststroke inflammation
Introduction
After experimental ischemic stroke, activated intra- and extracellular proteases including calpains (Bevers and Neumar, 2008), cathepsins (Wen et al, 2008), and matrix metalloproteinases (Rosell and Lo, 2008) contribute to acute and delayed brain damage. Multiple actions of these enzymes attribute to cleavage of extracellular matrix proteins (Fukuda et al, 2004) and to other cell death mechanisms (Jourquin et al, 2003). Lysosomal cystein proteases and matrix metalloproteinases may also regulate post-stroke inflammation by processing proinflammatory cytokines (Amantea et al, 2007) and modulation of leukocyte infiltration into the brain parenchyma (Campbell et al, 2004). However, delayed upregulation of proteases may be involved in tissue re-organization and re-modeling, suggesting a dual function dependent on the spatial and temporal activation patterns (Zhao et al, 2006).
The asparaginyl endopeptidase legumain is a unique late endosomal cysteine protease expressed in many mammalian tissues, including the brain (Chen et al, 1998, Shirahama-Noda et al, 2003). Legumain is activated under acidic conditions in an autocatalytic process (Li et al, 2003) and it specifically hydrolyzes the carbonyl side of a surface asparagine residue of the substrate sequence (Chen et al, 1997). Several legumain substrates have been identified, including the extracellular matrix protein fibronectin (Morita et al, 2007), progelatinase (Chen et al, 2001), cathepsins H, B, L (Shirahama-Noda et al, 2003), and α-thymosin (Sarandeses et al, 2003). The intracellular localization at cell membranes (Liu et al, 2003), the involvement in secretion from monocytes (Clerin et al, 2008), and an increased migratory and invasive activity of legumain-overexpressing tumor cells (Liu et al, 2003) indicate that legumain may be important for cell migration. Legumain has been proposed to be involved in processing antigens for class II major histocompatibility complex (MHC) presentation (Manoury et al, 1998) though it has not been confirmed (Maehr et al, 2005). Hence, legumain-deficient mice show no differences in processing of the invariant chain or maturation of class II MHC products. Moreover, the presentation of ovalbumin and myelin oligodendrocyte glycoprotein, two antigens that contain asparagine residues, to primary T cells was not affected in legumain-deficient mice (Maehr et al, 2005).
We earlier showed an increased expression of various proteases in the peri-infarct region after experimental stroke (Rickhag et al, 2006). Upregulation in late-expression clusters suggests an involvement in mechanisms secondary to acute neurotoxic cascades. The aim of this investigation was to specify the expression of legumain in different areas of the lesioned hemisphere after middle cerebral artery occlusion (MCAO). We further tested the hypothesis if legumain deficiency affects brain damage and post-stroke recovery after MCAO. Moreover, brain-derived molecular cues have not been identified to regulate the invasion of peripheral immune cells after stroke. On the basis of previous studies (Manoury et al, 1998; Maehr et al, 2005), we hypothesize that legumain is involved in attraction of those cells into the ischemic hemisphere.
Materials and methods
Transient Middle Cerebral Artery Occlusion in Rat
All animal experiments were carried out with the approval of the Malmö-Lund ethical committee. Transient MCAO was induced as described previously (Rickhag et al, 2008). In brief, male Wistar rats (325 to 350 g, HsdBrlHan; Harlan, Scandinavia, Denmark) were housed under diurnal light conditions and were fasted for 12 h before surgery. During surgery, physiologic parameters (arterial blood pressure and gases after tail artery cannulation and insertion of a catheter (SIMS Portex, Hythe, UK) for rectal body temperature) were measured and controlled within physiologic limits. Moreover, body temperature was measured after 1 and 2 h of occlusion and after 1 and 2 h of recirculation. Rats were anesthetized (initial 4% fluothane in N2O/O2 (70:30), during surgery 2% fluothane in N2O/O2; AstraZeneca, Lund, Sweden) and the right common carotid artery and external carotid artery were occluded permanently, the internal carotid artery was exposed and ligated. A nylon filament (top diameter 0.3 to 0.4 mm) was introduced into the internal carotid artery through a small incision into the distal end of the common carotid artery and pleaded up to occlude the origin of the MCA for 2 h. The filament was withdrawn and awake rats were placed in a cooling box. At 2 h after occlusion, a neurologic score was assessed and only rats showing rotational asymmetry and dysfunctional limb placement were included into the study. Rats were killed at different time points after tMCAO (3, 6, 12, 24, and 48 h) and fixative perfused brains were processed for immunohistochemical analyses. The same procedure was performed in sham-operated animals but no filament was introduced into the internal carotid artery.
Transient Middle Cerebral Artery Occlusion in Mouse
Studies were performed on age-matched male legumain wild-type and knockout mice with a C57BL/6J background (44 to 45 and 55 to 66 weeks old) (Shirahama-Noda et al, 2003). Transient MCAO occlusion was induced as essentially described by Nygren and Wieloch (2005). Briefly, anesthesia was induced by inhalation of 3% isoflurane in N2O/O2 (70:30) and maintained by inhalation of 2% isoflurane in N2O/O2 (70:30) through a face mask during the initial phase of surgery. During surgery, body temperature was maintained at 37°C using a heating pad and controlled by a temperature probe inserted into the rectum. Regional cerebral blood flow in the MCA was controlled by a flexible optical fiber connected to a laser Doppler (PeriFlux System 5000; Perimed, Jarfalla, Sweden) mounted on the skull of the right hemisphere 4 mm lateral from midline and 2 mm posterior from bregma. A silicon-coated filament (Prolene 6-0; Johnson & Johnson, New Brunswick, NJ, USA) was introduced into the internal carotid artery through an incision in the external carotid artery. The filament was advanced until it blocked the origin of the MCA. Placement was confirmed by a reduction in laser Doppler flow and isoflurane concentration was decreased to 1.5% during MCAO. We have only included mice with adequate occlusion, which was considered by (1) regional cerebral blood flow reduction by at least 70% immediately after filament placement, (2) sustained reduction of regional cerebral blood flow during occlusion time of 45 mins, and (3) completely recovered regional cerebral blood flow within 5 mins after the filament was removed.
Carbon Black Perfusion
Legumain-deficient mice and respective wild-type littermates were perfused with carbon black as described previously (Berry et al, 1975). Briefly, animals were anesthetized with 2% isoflurane and perfused with NaCl, followed by 4% formaldehyde. Finally, animals were perfused with a carbon black solution containing 40 mL encre de Chine India ink (Panduro Hobby AB, Malmö, Sweden), 20 mL of a 25% mannitol solution, and 7 g gelatin dissolved in 120 mL water. Brains were dissected immediately and kept at 4°C, overnight, in a fixative. Pictures were acquired with a MicroPublisher 3.3 RTV CCD camera (QImaging, Surrey, BC, Canada) under standard conditions.
Post-Surgical Management of Mice
After surgery, mice were transferred to heating cages and checked regularly during the first 48 h after surgery. The cage temperature was regulated to maintain the body temperature for 2 days. Body temperature was measured every hour for the first 5 postoperative hours, at 24 h, and further every 24 h for 5 days. Body weight was controlled every 24 h after MCAO for 5 consecutive days. Glucose solution (5%, intraperitoneally) was injected after a standardized protocol to prevent weight loss after MCAO.
Rotating Pole Test
Rotating pole test was performed in mice as described previously (Ruscher et al, 2009). An elevated wooden pole (750 mm above ground, diameter 15 mm, length 1,500 mm) was rotating at 0 or 3 or 10 r.p.m. to the right or left, respectively. Mice were trained to traverse the pole to reach a platform on the opposite side at any rotation, 12 h before surgery. After MCAO, mice were tested at days 2 and 8 using a score from 0 to 6: 0, the mouse falls off the pole; 1, the mouse tries to walk forward but falls off; 2, the front paws embrace the pole while crossing and the mouse manages to reach the platform; 3, the mouse is jumping with its hind legs or slipping with its feet while crossing; 4, the mouse traverses the pole with more than eight slips; 5, the mouse crosses the pole with three to seven slips; 6, the mouse crosses the pole fast with zero to two slips. All tests were recorded and evaluated by an experienced person masked to the study. A healthy mouse performs with a score of 5 to 6.
Pole Test
Mice were placed head upward on the top of a vertical wooden pole with superficial circular notches every 5 mm (diameter: 8 mm, length: 500 mm). The time to turn to the head-down position (tturn) and the total time to turn and reach the floor with all four paws (ttotal) was taken in five trials. If the mouse did not turn but descended in the head-up or lateral position, the value of the ttotal was attributed to the tturn. The maximum time to perform the test was set to 120 secs. The best performance was used for the statistical analyses. The pole test was performed 1 day before and at 2 and 8 days after MCAO.
Grip Strength Test
Forelimb strength was measured using the Grip Strength Test Meter GS3 (Bioseb, Chaville, France). Mice voluntarily gripped a grid with either the healthy or the paralyzed forelimb and pulled it backward. The maximum strength out of three trials was taken for analysis.
Infarct Size Measurement
Coronal brain sections (thickness 30 μm) were stained for the neuronal specific antigen NeuN (dilution 1:1,000; Millipore, Hampshire, UK). Infarcted areas, the nonlesioned area of the infarcted hemisphere, and the nonlesioned contralateral hemisphere were outlined and the infarct volume was calculated as described previously (Ruscher et al, 2009).
In Situ Hybridization Histochemistry
Wistar rats were subjected to MCAO and killed at various time points of recovery (3, 6, 12, 24, 48 h, n=5 for each time point). Isolated brains were cut on a cryostat in coronal sections and mounted on Superslide glasses (SuperFrost Plus; Menzel-Gläser, Braunschweig, Germany). After fixation for 15 mins with 4% paraformaldehyde, sections were rinsed in phosphate-buffered saline and dehydrated in ethanol. An oligonucleotide specific for legumain mRNA (Rickhag et al, 2006) was 3′-labeled with α-[35S]dATP using terminal deoxynucleotidyl transferase (Amersham Biosciences, Uppsala, Sweden). Hybridization was performed in a humidified chamber for 18 h at 42°C using a hybridization cocktail containing 50% formamide and 107 c.p.m./mL of labeled oligonucleotide. After hybridization, the sections were washed at 55°C in 1 × SSC for 3 × 15 mins followed by 1 × SSC at room temperature for 15 mins. Further, the sections were rinsed in water and dehydrated in ethanol. The radioactive slides were then exposed to BioMax MR films (Kodak, Kista, Sweden) and the intensity from the autoradiograms was photographed.
Immunohistochemistry
Brain sections (thickness 30 μm) from 4% paraformaldehyde-perfused animals were washed in phosphate-buffered saline and quenched (3% H2O2, 10% methanol) for 15 mins. After blocking with 2% normal horse serum in phosphate-buffered saline supplemented with 0.25% Triton X-100 for 60 mins, the sections were incubated with the following primary antibodies: polyclonal rabbit anti-legumain (dilution 1:400; Shirahama-Noda et al, 2003), polyclonal sheep anti-legumain (dilution 1:400; R&D Systems, Minneapolis, MN, USA), monoclonal anti-NeuN (dilution 1:2,000; Millipore), and CD74 (dilution 1:600; BD Biosciences Pharmingen, San Jose, CA, USA) at 4°C overnight followed by a secondary biotinylated horse anti-goat antibody (dilution 1:400; Vector Laboratories, CA, Burlingame, USA). Visualization was achieved through the Vectorstain ABC Elite kit (Vector Laboratories) using 3,3-diaminobenzidine/H2O2. Omission of the primary antibody served as a negative control.
Immunofluorescence
Sections were processed as described for immunohistochemistry. For colocalization of proteins, the following antibodies were used: polyclonal sheep anti-legumain (dilution 1:200; R&D Systems Europe, Abingdon, UK), polyclonal rabbit anti-legumain (dilution 1:200; Shirahama-Noda et al, 2003), monoclonal mouse anti-NeuN (dilution 1:1,000; Millipore, Solna, Sweden), monoclonal directly Cy3-conjugated anti-GFAP (dilution 1:5,000; Sigma-Aldrich, MO, USA), and biotinylated polyclonal rabbit anti-Iba1 (dilution 1:500; Millipore). After overnight incubation at 4°C, cells were incubated with appropriate secondary antibodies (Cy3, Cy5; conjugated donkey anti-mouse antibody and biotinylated horse anti-sheep antibody, both diluted at 1:200; Jackson ImmunoResearch Laboratories, Suffolk, UK). Sections exposed to the secondary biotinylated donkey anti-sheep antibody were further incubated with an Alexa 488 streptavidin conjugate (dilution 1:300) at room temperature for 60 mins. Fluorescent signals were visualized using a confocal microscopy system (LSM510; Zeiss, Jena, Germany).
Western Blotting
Protein (10 μg) was separated on a 10% SDS polyacrylamide gel. Blocking was performed onto polyvinyldifluoride membranes using blocking buffer (20 mmol/L Tris, 136 mM NaCl (pH 7.6), 0.1% Tween 20, 5% nonfat dry milk), and detected using primary polyclonal antibody against the legumain (dilution 1:2,000; R&D Systems). After incubation at 4°C overnight, the membrane was incubated with a secondary anti-sheep biotin-linked antibody (Dako A/S, Glostrup, Denmark) and a horseradish-peroxidase-linked anti-biotin antibody (dilution 1:3,000; Cell Signaling, Danvers, MA, USA). Signals were visualized by exposing the membrane to a CCD camera (LAS1000; Fujifilm, Tokyo, Japan) using a chemiluminescence kit (Millipore).
Statistical Analysis
Unless otherwise stated, all data are presented as medians with the 25th and 75th percentiles. Data from the rotating pole test were analyzed by the Mann–Whitney U-test and P<0.05 was considered statistically significant. All other statistical analyses were performed as stated in the figure legends.
Results
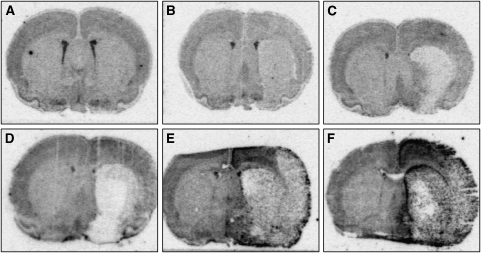
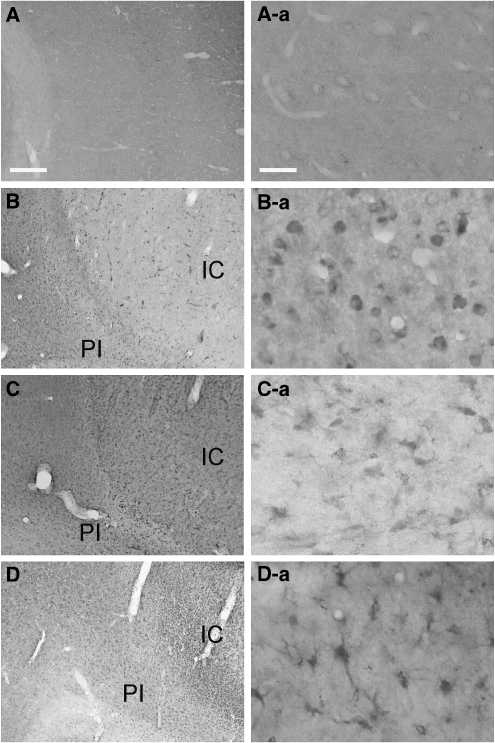
We have used in situ hybridizations to study the spatiotemporal expression of legumain in the ischemic hemisphere after experimental stroke. As shown in Figure 1, a basal expression was detected in sham-operated animals and in the noninfarcted brain areas immediately after MCAO for 120 mins. Signal intensity was markedly decreased in the infarct core compared with corresponding regions of the nonischemic hemisphere. At 3 h after occlusion, an increase of legumain RNA was observed in the parietal peri-infarct area, whereas the signal further decreased in the infarct core, particularly in the striatum. During the following hours, legumain mRNA was further elevated in layers of the peri-infarcted motor cortex (Figure 1D) with a maximum expression at 24 and 48 h after MCAO (Figure 1E and 1F). At 24 h, legumain expression was spread throughout the noninfarcted areas of the ischemic hemisphere. An accumulation was observed toward the infarct core in the striatum, in the parietal peri-infarct cortex, and a legumain-expressing zone was found at the brain surface. In these regions maximum expression was found at 48 h after MCAO. Accordingly, we found a delayed increase in legumain immunoreactivity in the ischemic hemisphere (Figure 2). Although legumain immunoreactivity was present in round cells at 2 days after tMCAO, it appeared in cytoplasmic globules of ramified cells of the peri-infarct area at 4 and 7 days (Figure 2). No specific immunoreactivity was detectable in sham-operated rats. Collectively, the data show a time-dependent upregulation of legumain within peri-infarct areas after transient MCAO.
Figure 1.
Transcriptional regulation of legumain after middle cerebral artery occlusion (MCAO). Representative coronal sections (bregma −1.8 to −1.6) showing legumain mRNA expression in a sham-operated rat (A) and at 3 h (B), 6 h (C), 12 h (D), 24 h (E), and 48 h (F) after transient MCAO for 120 mins, respectively (n=5 each time point).
Figure 2.
Legumain expression in rat after transient middle cerebral artery occlusion (MCAO). Phase contrast micrograph (A) and higher magnification (A-a) showing weak immunoreactivity in neuron-like cells and microvessels in the neocortex of a sham-operated rat. (B) Legumain immunoreactivity in the ischemic hemisphere 2 days after transient MCAO (120 mins) with (B-a) an increased immunoreactivity in microglia like cells inside and around the ischemic core. (C) Legumain immunoreactivity 4 days after occlusion with higher number of ramified cells in the peri-infarct area in C-a. (D) Legumain immunoreactivity accumulates in astrocyte like cells in the peri-infarct region (D-a). IC, ischemic core, PI, peri-infarct area, scale bars: 100 μm, magnifications 12.5 μm.
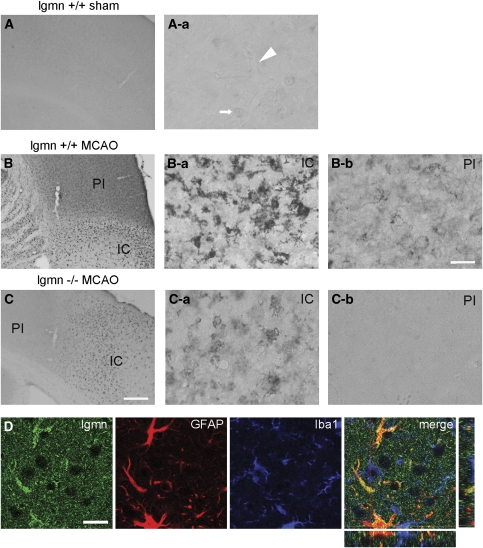
At 3 days after MCAO, legumain immunoreactivity was also increased in glia-like cells of the peri-infarct area in legumain wild-type mice (Figure 3). Immunoreactivity in the peri-infarct area was abolished by serial dilution of the primary antibody (data not shown). No immunoreactivity was found in legumain-deficient mice in this region. In addition, legumain immunoreactivity accumulated in microglia-like cells of the infarct core in wild-type mice but also in legumain-deficient mice. Although legumain immunoreactivity was more pronounced in wild-type animals, it could not be abolished by serial dilution of the primary antibody (data not shown) in both genotypes. The infarct core was devoid of legumain-positive cells by omission of the primary antibody. In addition, recombinant legumain added to the primary antibody solution clearly decreased legumain immunoreactivity in the infarct core (data not shown). In addition, further testing of legumain antibodies revealed no legumain-specific band in western blots from legumain-deficient mice (data not shown). Further immunofluorescence analyses from the peri-infarct area confirmed an expression of legumain in fibrillary acidic protein-positive (GFAP+) astrocytes and Iba1+ microglial cells (Figure 3).
Figure 3.
Legumain (lgmn) expression in mouse after transient middle cerebral artery occlusion (tMCAO). Phase-contrast micrograph (A) and higher magnification (A-a) showing weak lgmn immunoreactivity in microvessels (arrowhead) and associated cells (arrow) in the neocortex of a sham-operated mouse. (B) lgmn immunoreactivity in the hemisphere ipsilateral to the ischemic lesion 72 h after tMCAO with (B-a) an increased unspecific immunoreactivity in microglia-like cells inside the ischemic core (IC) and in (B-b) astrocyte-like and microglial-like cells of the peri-infarct (PI) area. (C) lgmn immunoreactivity in the ischemic hemisphere of an lgmn-deficient mouse with (C-a) an unspecific immunoreactivity of microglial-like cells of the IC and (C-b) no immunoreactivity in the PI area. (D) lgmn (green, AF488)/glial fibrillary acidic protein (GFAP) (red, Cy3)/Iba1 (blue, Cy5) costaining in the PI area 72 h after MCAO for 45 mins. Scale bars: (A–C) 100 μm, magnifications: 12.5 μm, (D) 20 μm.
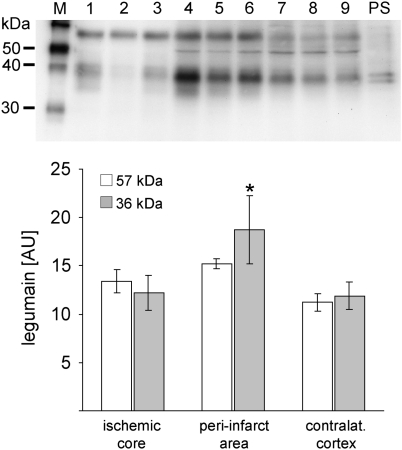
We also performed western blot analyses to evaluate whether legumain is processed into its 36 kDa active form. As shown in Figure 4, an increase of pro-legumain (57 kDa band) was found in the ischemic hemisphere compared with the contralateral cortex. However, no difference was observed between the ischemic core and the peri-infarct area. More importantly and compared with the other regions, active legumain (the 36-kDa band in Figure 4) was significantly increased in the peri-infarct area. In addition, we found a legumain-specific band of around 50 kDa in samples from the peri-infarct area and the contralateral cortex, but not in the ischemic core region, indicating that active legumain might have been processed through a 46/47-kDa intermediate as described before (Li et al, 2003). Results corroborate that active legumain is increased in glial cell populations in the peri-infarct area of the ischemic hemisphere after MCAO.
Figure 4.
Legumain processing in the peri-infarct area after transient middle cerebral artery occlusion (MCAO). Western blot of samples from the infarct core (lane 1 to 3), the peri-infarct area (lane 4 to 6), and the contralateral neocortex (lane 7 to 9) from ischemic legumain wild-type mice 72 h after transient MCAO (45 mins) and densitometric analysis (mean±s.d., *P<0.05). Each lane represents an individual animal. Abbreviations: M, marker lane, PS, legumain positive control (kidney extract).
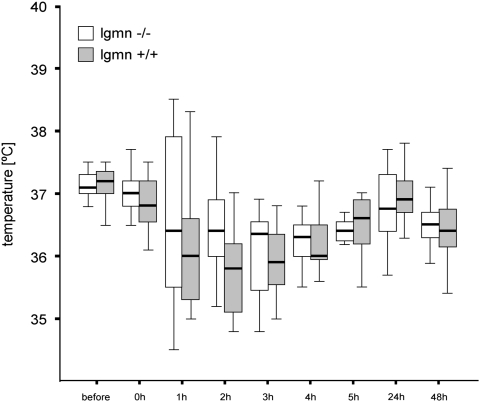
We further investigated the brain damage in legumain knockout mice after MCAO. In total, we studied 16 wild-type and 14 knockout mice. Out of these, seven wild-type and five knockout animals were excluded because of death or lack of recirculation after MCAO. The average age was 55.8±10.4 weeks (mean±s.d.) in wild-type and 53.3±9.3 weeks in the knockout group. The body weight did not differ significantly between the study groups (wild-type mice: 36.5±3.7 g, knockout mice 32.0±4.0 g). Postoperative temperature was maintained around 37°C during MCAO and around 36°C for the first 48 h after MCAO to prevent hypothermia that significantly affects the stroke outcome (Auer, 2001). During and within the first 24 h after MCAO, we could not detect any temperature differences between knockout and wild-type mice (Figure 5).
Figure 5.
Poststroke temperature in legumain (lgmn)-deficient mice. Rectal body temperature was measured immediately before and at the indicated time points after transient middle cerebral artery occlusion (45 mins) by fine lubricated probe (−/− n=11; +/+ n=17). Note that no difference in body temperature was observed between legumain-deficient and wild-type littermates at all time points studied.
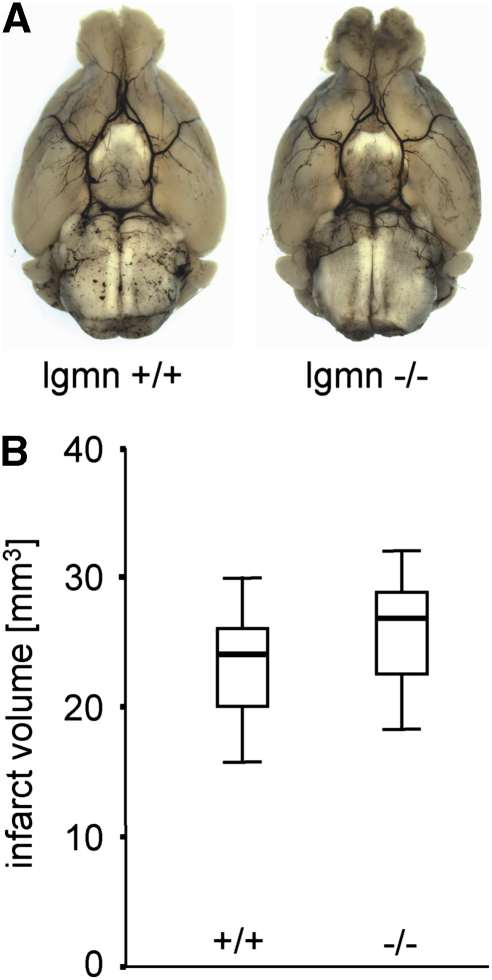
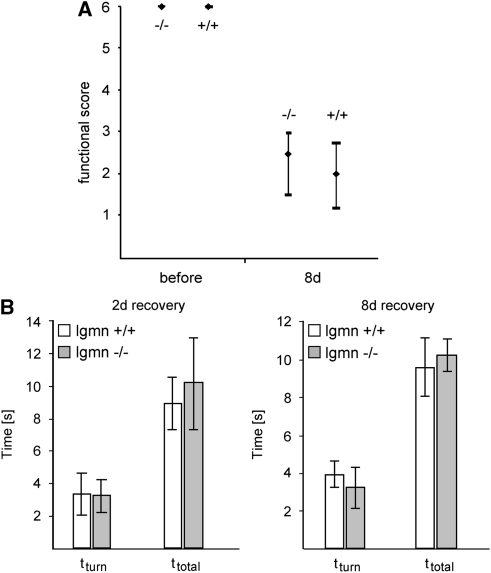
Legumain-deficient mice did not show abnormalities of the larger brain arteries and their branches (Figure 6A). In particular, we found a normal configuration of the circle of Willis with normal branching of the anterior and posterior cerebral arteries. Despite being elongated, the middle cerebral arteries and ramifying branches showed a normal anatomic configuration (Figure 6A). At 8 days after tMCAO, animals were perfused in standard procedure and infarct sizes were evaluated from serial coronal sections and stained for neuronal nuclear protein (NeuN). The infarct size did not differ between the groups with 23.9 mm3 (25th percentile: 20.8 mm3; 75th percentile: 25.7 mm3; n=6) found in legumain wild-type mice and 26.8 mm3 (25th percentile: 22.5 mm3; 75th percentile: 28.8 mm3; n=7) found in legumain-deficient mice (Figure 6B). Moreover, we could not detect significant differences in behavioral tests after tMCAO. Using the rotating pole test, a sensitive approach to test sensorimotor and balance function, we found a similar spontaneous recovery in legumain-deficient and wild-type mice (Figure 7A). No test score differences were also found in the pole test (Figure 7B). We also used a standardized grip test to measure the maximum strength of the paralyzed (left) and nonparalyzed (right) front paw before and after tMCAO. No differences were observed between wild-type (left: 75.9±16.3 g, right: 84.9±21.8 g) and knockout mice (left: 86.6±3.8 g, right: 85.4±4.5 g) before tMCAO. At 8 days of recovery, grip strength was generally decreased (wild-type: left 61.9±15.1 g, right 65.7±17.3 g; knockout: left 52.2±20.9 g, right 59.2±18.8 g), but also this test did not reveal a difference between wild-type and knockout mice. The results of our study support the notion that legumain deficiency has no significant impact on functional outcome after tMCAO.
Figure 6.
Brain arteries and infarct volumes in legumain-deficient mice. (A) Circle of Willis and major arteries of an aged (58 weeks) legumain-deficient mouse and a wild-type mouse. (B) Infarct volume from legumain-deficient mice (−/− n=7) and respective wild-type littermates (+/+ n=6).
Figure 7.
Functional recovery in legumain (lgmn)-deficient mice after transient middle cerebral artery occlusion. Evaluation of sensorimotor function in lgmn-deficient (−/−, n=8) and wild-type littermate (+/+, n=8) mice 8 days after brain ischemia. (A) Animals were tested on the rotating pole at 10 turns per minute to the left. Similar results were obtained with 10 turns to the right (data not shown). (B) The pole test shows the time to turn to the head-down position (tturn) and the total time to turn and reach the floor with all four paws (ttotal) at 2 and 8 days of recovery. Behavioral assessment did not show significant differences between deficient and wild-type mice.
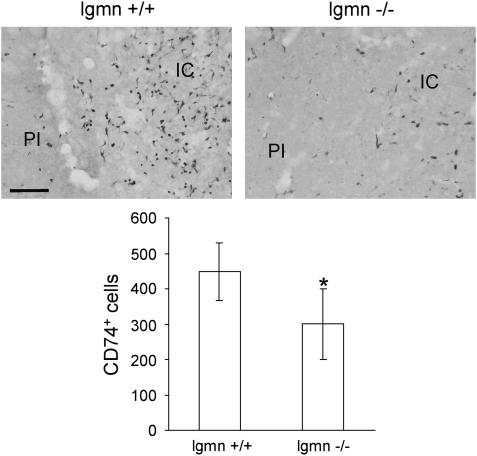
However, legumain may contribute to an invasion of inflammatory cells toward the ischemic core. We determined the number of antigen-presenting CD74+ cells in the ischemic hemisphere of legumain wild-type and deficient mice 8 days after MCAO. As shown in Figure 8, CD74+ cells accumulate in the ischemic core but also in the peri-infarct area of legumain wild-type mice (448±81 cells, n=6). Reduced numbers of those cells were found in the ischemic hemisphere of legumain-deficient mice (301±100 cells, n=7). Interestingly, a decreased number of CD74+ cells in legumain-deficient mice was mainly detected in the ischemic core. This suggests that legumain may be involved in mechanisms of post-stroke tissue inflammation and reorganization in the ischemic core and the peri-infarct area after MCAO.
Figure 8.
Analysis of CD74 positive cells in the ischemic hemisphere of legumain (lgmn) wild-type and deficient mice after transient middle cerebral artery occlusion (tMCAO). Quantification of CD74+ cells from the ischemic hemisphere of lgmn wild-type (n=6) and deficient (n=7) mice 8 days after tMCAO for 45 mins (mean±s.d., P<0.05, Student's t-test). Abbreviations: IC, infarct core, PI, peri-infarct area. Scale bar: 100 μm.
Discussion
The aim of this study was to investigate the expression of the late endosomal asparaginyl endopeptidase legumain in the ischemic rodent brain and to evaluate the functional outcome of legumain-deficient mice after experimental stroke. We present three important findings: (1) legumain expression is increased in the peri-infarct area of rats after transient MCAO and is upregulated in microglial cells and reactive astrocytes; (2) legumain deficiency does not affect infarct volume or neurologic outcome in aged mice after MCAO; and (3) the number of CD74+ cells in the ischemic hemisphere is significantly reduced in legumain-deficient mice, indicating an altered inflammatory response in the ischemic hemisphere.
Legumain Expression in the Brain after Middle Cerebral Artery Occlusion
Legumain is differentially expressed in various mammalian tissues (Chen et al, 1998; Shirahama-Noda et al, 2003). In line with results from a previous gene array study, we show here an upregulation of legumain expression and elevated legumain protein levels in the rat brain. Legumain mRNA increased in the peri-infarct region from 3 h and onwards and followed a delayed time pattern similar to that for other proteins, including proteases such as cathepsin B (Rickhag et al, 2006). Although legumain was weakly expressed in microvessels and adjacent cells in sham-operated mice, microglial cells and astrocytes strongly expressed legumain in the peri-infarct area after MCAO.
Macrophage-specific legumain was proposed to be released in atherosclerotic plaques and promote inflammatory cell invasion, contributing to the progression of atherosclerosis (Clerin et al, 2008). Our in situ hybridization and immunohistochemistry study findings that legumain is upregulated after MCAO were confirmed by western blot, and in addition we show that legumain is processed into the active 36 kDa form (Li et al, 2003) particularly in the peri-infarct area. This result and the delayed dot-like accumulation of immunoreactivity in branches of astroglial cells but also in microglia of the peri-infarct area suggest that brain-derived legumain may be secreted (Clerin et al, 2008) and may function as a chemoattractant for invading inflammatory cells. However, we cannot exclude that legumain is secreted by invading peripheral monocytes but also by resident microglia and may be taken up by astroglial cells.
Glial cells produce both pro- and anti-inflammatory cytokines that may modulate the entry and migration of inflammatory cells in the CNS parenchyma (John et al, 2003). In addition, it was shown that legumain increases the in vitro migratory and invasive activity of tumor cells (Liu et al, 2003). Little is known about secreted glial molecular guidance cues for cell migration toward the peri-infarct tissue and the ischemic core region (Sofroniew, 2005). However, our observation of a reduced number of legumain antigen-presenting CD74+ cells in the ischemic core and adjacent peri-infarct area of legumain-deficient mice supports the idea that legumain is involved in cell migration toward the lesion side after MCAO. Further studies will clarify the effect of different legumain levels on cell migration and expression of proinflammatory cytokines in the ischemic hemisphere.
Potential Function of Legumain in Tissue Re-organization
This study was also conducted to evaluate a putative function of legumain after MCAO. However, legumain deficiency did not affect the infarct volume compared with the respective littermates. In addition, behavioral tests showed no difference between the two genotypes. Low legumain levels in the early phase after MCAO when damage is developing and delayed upregulation may explain why legumain is not directly involved in the mechanisms of neurotoxicity or neuroprotection. More likely, the spatial and temporal distribution of legumain-positive microglial and astroglial cells in the peri-infarct area suggests an involvement in the mechanisms of wound healing and tissue reorganization. Recently, a number of studies have brought to attention the importance of the extracellular matrix turnover in these mechanisms (Rosell and Lo, 2008). In particular, late inhibition of matrix metalloproteinase-9, colocalized with markers of neurovascular remodeling, increases ischemic brain injury and impairs functional recovery 2 weeks after experimental stroke onset (Zhao et al, 2006). Legumain has been shown to regulate extracellular matrix turnover through the degradation of fibronectin (Morita et al, 2007). Extracellular fibronectin may be taken up by caveolin-mediated endocytosis and degraded intracellularly in legumain-enriched lysosomes (Sottile and Chandler, 2005). Hence, legumain-positive astrocytes of the peri-infarct area that are enriched in submembraneous globular structures indicate such a mechanism for extracellular matrix proteins.
The type II transmembrane glycoprotein CD74 (invariant chain, Ii) is associated with the MHC class II α- and β-chains and functions as a chaperone in transport of αβIi (invariant chain) complexes from the endoplasmic reticulum to endocytic compartments (Wolf and Ploegh, 1995). CD74 further prevents peptide binding in the endoplasmic reticulum, and contributes to peptide editing in the MHC class II compartment (Stumptner-Cuvelette and Benaroch, 2002). In addition to its function as a chaperone molecule, CD74 has a role in cell signaling. Recently, CD74 was reported to be a high-affinity binding protein for the proinflammatory cytokine, macrophage migration-inhibitory factor (Leng et al, 2003). Except for an accumulation in neurofibrillary tangles (Bryan et al, 2008), CD74-positive cells have not been reported in the normal brain and under pathologic conditions.
A reduced number of CD74+ cells indicate an altered inflammatory response in the ischemic core and in the adjacent peri-infarct area of legumain-deficient mice. However, we also observed CD74+ cells in the ischemic hemisphere of deficient mice, indicating that legumain (together with other molecules) indirectly contributes to mechanisms of CD74+ cell invasion after MCAO. It has to be determined whether low levels of legumain are associated with MHC class II processing as previously shown in endotoxin-tolerant monocytes (Wolk et al, 2005). However, it has to be determined whether brain-derived glia can express and process MHC class II proteins on an ischemic insult. Our study results indicate that brain-derived legumain contributes to CD74+ cell infiltration in the ischemic hemisphere after experimental stroke indirectly by modification of molecules involved in mechanisms of immune cell attraction.
We used aged (approximately 1-year-old) mice in this study. All animals showed severe impairment and, in contrast to young animals, recovery was markedly delayed in all mice independent from genotype. We also observed a slight increase in mortality compared with previous studies using young mice (Nygren and Wieloch, 2005), which can be most likely attributed to age as similar observations have been made in aged rats subjected to transient MCAO (Badan et al, 2003). Neuronal degeneration, glial scar formation, and post-stroke inflammation contributing to stroke recovery are significantly altered in the aged rodent brain (for a review, see Petcu et al, 2008). Delayed activation may explain that no differences were detected in behavioral tests in legumain-deficient and wild-type mice 8 days after MCAO.
Previous studies have shown an increased body temperature in legumain-deficient mice (Chan et al, 2009). High body temperature is associated with poorer outcome after experimental and clinical stroke (Dietrich et al, 1990; Zaremba, 2004). In this study, we did not detect any temperature differences between legumain wild-type and deficient mice. This discrepancy could be due to the small number of animals included in the study reported by Chan et al. Further, temperature differences obtained in younger animals may be ameliorated in aged mice similar to body weights as shown previously (Manoury et al, 1998; Shirahama-Noda et al, 2003). It has also been shown that legumain-deficient mice develop symptoms resembling a hemophagocytic syndrome (Chan et al, 2009) accompanied by increased serum levels for the proteohormone erythropoietin. Apart from a possible relevance for an increased reactive hematopoiesis (Chan et al, 2009), it remains speculative whether elevated erythropoietin levels exert any function in the brain of legumain-deficient mice after MCAO in particular in view of its neuroprotective actions in other models of experimental stroke (Ruscher et al, 2002).
Conclusion
In summary, legumain expression level and activity are increased in the peri-infarct area of rats after transient MCAO and are upregulated in microglial cells and reactive astrocytes. Legumain does not appear to be involved in the acute stage of neurodegeneration but may influence the immune response during stroke.
Acknowledgments
We thank Kerstin Beirup and Carin Sjölund for excellent technical assistance.
The authors declare no conflict of interests.
References
- Amantea D, Russo R, Gliozzi M, Fratto V, Berliocchi L, Bagetta G, Bernardi G, Corasaniti MT. Early upregulation of matrix metalloproteinases followingreperfusion triggers neuroinflammatory mediators in brain ischemia in rat. Int Rev Neurobiol. 2007;82:149–169. doi: 10.1016/S0074-7742(07)82008-3. [DOI] [PubMed] [Google Scholar]
- Auer RN. Non-pharmacologic (physiologic) neuroprotection in the treatment of brain ischemia. Ann NY Acad Sci. 2001;939:271–282. doi: 10.1111/j.1749-6632.2001.tb03635.x. [DOI] [PubMed] [Google Scholar]
- Badan I, Buchhold B, Hamm A, Gratz M, Walker LC, Platt D, Kessler C, Popa-Wagner A. Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery. J Cereb Blood Flow Metab. 2003;23:845–854. doi: 10.1097/01.WCB.0000071883.63724.A7. [DOI] [PubMed] [Google Scholar]
- Berry K, Wisniewski HM, Svarzbein L, Baez S. On the relationship of brain vasculature to production of neurological deficit and morphological changes followingacute unilateral common carotid artery ligation in gerbils. J Neurol Sci. 1975;25:75–92. doi: 10.1016/0022-510x(75)90188-4. [DOI] [PubMed] [Google Scholar]
- Bevers MB, Neumar RW. Mechanistic role of calpains in postischemic neurodegeneration. J Cereb Blood Flow Metab. 2008;28:655–673. doi: 10.1038/sj.jcbfm.9600595. [DOI] [PubMed] [Google Scholar]
- Bryan KJ, Zhu X, Harris PL, Perry G, Castellani RJ, Smith MA, Casadesus G. Expression of CD74 is increased in neurofibrillary tangles in Alzheimer's disease. Mol Neurodegener. 2008;3:13. doi: 10.1186/1750-1326-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SJ, Finlay M, Clements JM, Wells G, Miller KM, Perry VH, Anthony DC. Reduction of excitotoxicity and associated leukocyte recruitment by a broad-spectrum matrix metalloproteinase inhibitor. J Neurochem. 2004;89:1378–1386. doi: 10.1111/j.1471-4159.2004.02441.x. [DOI] [PubMed] [Google Scholar]
- Chan CB, Abe M, Hashimoto N, Hao C, Williams IR, Liu X, Nakao S, Yamamoto A, Zheng C, Henter JI, Meeths M, Nordenskjold M, Li SY, Hara-Nishimura I, Asano M, Ye K. Mice lacking asparaginyl endopeptidase develop disorders resembling hemophagocytic syndrome. Proc Natl Acad Sci USA. 2009;106:468–473. doi: 10.1073/pnas.0809824105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JM, Dando PM, Rawlings ND, Brown MA, Young NE, Stevens RA, Hewitt E, Watts C, Barrett AJ. Cloning, isolation, and characterization of mammalian legumain, an asparaginyl endopeptidase. J Biol Chem. 1997;272:8090–8098. doi: 10.1074/jbc.272.12.8090. [DOI] [PubMed] [Google Scholar]
- Chen JM, Dando PM, Stevens RA, Fortunato M, Barrett AJ. Cloning and expression of mouse legumain, a lysosomal endopeptidase. Biochem J. 1998;335:111–117. doi: 10.1042/bj3350111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JM, Fortunato M, Stevens RA, Barrett AJ. Activation of progelatinase A by mammalian legumain, a recently discovered cysteine proteinase. Biol Chem. 2001;382:777–783. doi: 10.1515/BC.2001.093. [DOI] [PubMed] [Google Scholar]
- Clerin V, Shih HH, Deng N, Hebert G, Resmini C, Shields KM, Feldman JL, Winkler A, Albert L, Maganti V, Wong A, Paulsen JE, Keith JC, Jr, Vlasuk GP, Pittman DD. Expression of the cysteine protease legumain in vascular lesions and functional implications in atherogenesis. Atherosclerosis. 2008;201:53–66. doi: 10.1016/j.atherosclerosis.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Busto R, Valdes I, Loor Y. Effects of normothermic versus mild hyperthermic forebrain ischemia in rats. Stroke. 1990;21:1318–1325. doi: 10.1161/01.str.21.9.1318. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL, Jr, del Zoppo GJ. Focal cerebral ischemia induces active proteases thatdegrade microvascular matrix. Stroke. 2004;35:998–1004. doi: 10.1161/01.STR.0000119383.76447.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GR, Lee SC, Brosnan CF. Cytokines powerful regulators of glial cell activation. Neuroscientist. 2003;9:10–22. doi: 10.1177/1073858402239587. [DOI] [PubMed] [Google Scholar]
- Jourquin J, Tremblay E, Decanis N, Charton G, Hanessian S, Chollet AM, Le DT, Khrestchatisky M, Rivera S. Neuronal activity-dependent increase of net matrix metalloproteinase activity is associated with MMP-9 neurotoxicity after kainate. Eur J Neurosci. 2003;18:1507–1517. doi: 10.1046/j.1460-9568.2003.02876.x. [DOI] [PubMed] [Google Scholar]
- Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, Delohery T, Chen Y, Mitchell RA, Bucala R. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DN, Matthews SP, Antoniou AN, Mazzeo D, Watts C. Multistep autoactivation of asparaginyl endopeptidase in vitro and in vivo. J Biol Chem. 2003;278:38980–38990. doi: 10.1074/jbc.M305930200. [DOI] [PubMed] [Google Scholar]
- Liu C, Sun C, Huang H, Janda K, Edgington T. Overexpression of legumain in tumors is significant for invasion/metastasis and a candidate enzymatic target for prodrug therapy. Cancer Res. 2003;63:2957–2964. [PubMed] [Google Scholar]
- Maehr R, Hang HC, Mintern JD, Kim YM, Cuvillier A, Nishimura M, Yamada K, Shirahama-Noda K, Hara-Nishimura I, Ploegh HL. Asparagine endopeptidase is not essential for class II MHC antigen presentation but is required for processing of cathepsin L in mice. J Immunol. 2005;174:7066–7074. doi: 10.4049/jimmunol.174.11.7066. [DOI] [PubMed] [Google Scholar]
- Manoury B, Hewitt EW, Morrice N, Dando PM, Barrett AJ, Watts C. An asparaginyl endopeptidase processes a microbial antigen for class II MHC presentation. Nature. 1998;396:695–699. doi: 10.1038/25379. [DOI] [PubMed] [Google Scholar]
- Morita Y, Araki H, Sugimoto T, Takeuchi K, Yamane T, Maeda T, Yamamoto Y, Nishi K, Asano M, Shirahama-Noda K, Nishimura M, Uzu T, Hara-Nishimura I, Koya D, Kashiwagi A, Ohkubo I. Legumain/asparaginyl endopeptidase controls extracellular matrix remodeling through the degradation of fibronectin in mouse renal proximal tubular cells. FEBS Lett. 2007;581:1417–1424. doi: 10.1016/j.febslet.2007.02.064. [DOI] [PubMed] [Google Scholar]
- Nygren J, Wieloch T. Enriched environment enhances recovery of motor function after focal ischemia in mice, and downregulates the transcription factor NGFI-A. J Cereb Blood Flow Metab. 2005;25:1625–1633. doi: 10.1038/sj.jcbfm.9600157. [DOI] [PubMed] [Google Scholar]
- Petcu EB, Sfredel V, Platt D, Herndon JG, Kessler C, Popa-Wagner A. Cellular and molecular events underlying the dysregulated response of the aged brain to stroke: a mini-review. Gerontology. 2008;54:6–17. doi: 10.1159/000112845. [DOI] [PubMed] [Google Scholar]
- Rickhag M, Deierborg T, Patel S, Ruscher K, Wieloch T. Apolipoprotein D is elevated in oligodendrocytes in the peri-infarct region after experimental stroke: influence of enriched environment. J Cereb Blood Flow Metab. 2008;28:551–562. doi: 10.1038/sj.jcbfm.9600552. [DOI] [PubMed] [Google Scholar]
- Rickhag M, Wieloch T, Gido G, Elmer E, Krogh M, Murray J, Lohr S, Bitter H, Chin DJ, von Schack D, Shamloo M, Nikolich K. Comprehensive regional and temporal gene expression profiling of the rat brain during the first 24 h after experimental stroke identifies dynamic ischemia-induced gene expression patterns, and reveals a biphasic activation of genes in surviving tissue. J Neurochem. 2006;96:14–29. doi: 10.1111/j.1471-4159.2005.03508.x. [DOI] [PubMed] [Google Scholar]
- Rosell A, Lo EH. Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol. 2008;8:82–89. doi: 10.1016/j.coph.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, Priller J, Dirnagl U, Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22:10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscher K, Johannesson E, Brugiere E, Erickson A, Rickhag M, Wieloch T. Enriched environment reduces apolipoprotein E (ApoE) in reactive astrocytes and attenuates inflammation of the peri-infarct tissue after experimental stroke. J Cereb Blood Flow Metab. 2009;29:1796–1805. doi: 10.1038/jcbfm.2009.96. [DOI] [PubMed] [Google Scholar]
- Sarandeses CS, Covelo G, az-Jullien C, Freire M. Prothymosin alpha is processed to thymosin alpha 1 and thymosin alpha 11 by a lysosomal asparaginyl endopeptidase. J Biol Chem. 2003;278:13286–13293. doi: 10.1074/jbc.M213005200. [DOI] [PubMed] [Google Scholar]
- Shirahama-Noda K, Yamamoto A, Sugihara K, Hashimoto N, Asano M, Nishimura M, Hara-Nishimura I. Biosynthetic processing of cathepsins and lysosomal degradation are abolished in asparaginyl endopeptidase-deficient mice. J Biol Chem. 2003;278:33194–33199. doi: 10.1074/jbc.M302742200. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- Sottile J, Chandler J. Fibronectin matrix turnover occurs through a caveolin-1-dependent process. Mol Biol Cell. 2005;16:757–768. doi: 10.1091/mbc.E04-08-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumptner-Cuvelette P, Benaroch P. Multiple roles of the invariant chain in MHC class II function. Biochim Biophys Acta. 2002;1542:1–13. doi: 10.1016/s0167-4889(01)00166-5. [DOI] [PubMed] [Google Scholar]
- Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, Han F, Fukunaga K, Qin ZH. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy. 2008;4:762–769. doi: 10.4161/auto.6412. [DOI] [PubMed] [Google Scholar]
- Wolf PR, Ploegh HL. How MHC class II molecules acquire peptide cargo: biosynthesis and trafficking through the endocytic pathway. Annu Rev Cell Dev Biol. 1995;11:267–306. doi: 10.1146/annurev.cb.11.110195.001411. [DOI] [PubMed] [Google Scholar]
- Wolk K, Grutz G, Witte K, Volk HD, Sabat R. The expression of legumain, an asparaginyl endopeptidase thatcontrols antigen processing, is reduced in endotoxin-tolerant monocytes. Genes Immun. 2005;6:452–456. doi: 10.1038/sj.gene.6364224. [DOI] [PubMed] [Google Scholar]
- Zaremba J. Hyperthermia in ischemic stroke. Med Sci Monit. 2004;10:RA148–RA153. [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]