Abstract
The ATP-driven efflux transporter, breast cancer resistance protein (BCRP), handles many therapeutic drugs, including chemotherapeutics, limiting their ability to cross the blood–brain barrier. This study provides new insight into rapid, nongenomic regulation of BCRP transport activity at the blood–brain barrier. Using isolated brain capillaries from rats and mice as an ex vivo blood–brain barrier model, we show that BCRP protein is highly expressed in brain capillary membranes and functionally active in intact capillaries. We show that nanomolar concentrations of 17-β-estradiol (E2) rapidly reduced BCRP transport activity in the brain capillaries. This E2-mediated effect occurred within minutes and did not involve transcription, translation, or proteasomal degradation, indicating a nongenomic mechanism. Removing E2 after 1 h fully reversed the loss of BCRP activity. Experiments using agonists and antagonists for estrogen receptor (ER)α and ERβ and brain capillaries from ERα and ERβ knockout mice demonstrated that E2 could signal through either receptor to reduce BCRP transport function. We speculate that this nongenomic E2-signaling pathway could potentially be used for targeting BCRP at the blood–brain barrier, in brain tumors, and in brain tumor stem cells to improve chemotherapy of the central nervous system.
Keywords: BCRP, blood–brain barrier, estrogen, nongenomic regulation, transport
Introduction
Despite extensive research, early diagnosis, state-of-the-art neurosurgery, radiation, and chemotherapy, effective treatments for brain tumors are limited, and the diagnosis of brain cancer often remains a death sentence (Buckner et al, 2007). Indeed, for most small molecule chemotherapeutics, failure to eradicate brain tumors as well as brain cancer stem cell remnants is due in large part to the blood–brain barrier and its ATP-driven drug efflux transporters. These transporters reside on the luminal membrane of brain capillaries that make up the barrier providing an obstacle to drug entry. One such transporter is breast cancer resistance protein (BCRP, ABCG2; Eisenblatter and Galla, 2002). Studies show that BCRP restricts brain penetration of the tyrosine kinase inhibitor, imatinib, and of topotecan, another chemotherapeutic drug. Thus, BCRP likely reduces the drugs' effectiveness (Breedveld et al, 2005; de Vries et al, 2007). Furthermore, recent reports indicate that BCRP works in concert with another ATP-driven drug efflux transporter, P-glycoprotein, to prevent brain penetration of two other tyrosine kinase inhibitors, dasatinib and lapatinib (Chen et al, 2009; Lagas et al, 2009; Polli et al, 2009). As a consequence, anticancer drugs that are substrates for transporters do not reach tumor sites in the brain, which limits successful pharmacotherapy, or they only reach tumors at subtherapeutic levels, which can cause drug resistance, and thus, complicate and aggravate the disease (Pal and Mitra, 2006). One strategy for increasing brain levels of certain chemotherapeutics is to manipulate BCRP activity in the brain capillary endothelium. However, mechanisms regulating BCRP at the blood–brain barrier have not been defined.
Ee et al (2004) recently used a human ovarian cancer cell line to identify an estrogen-response element in the promoter region of ABCG2, the gene-encoding BCRP. Other studies show that estrogens regulate BCRP in human breast cancer and placenta cell lines as well as in rat kidney and mouse liver in vivo (Imai et al, 2005; Tanaka et al, 2005; Wang et al, 2008, 2006; Zhang et al, 2006). On the basis of these reports, we hypothesized that estrogens could affect BCRP at the blood–brain barrier. We show here that the estrogen, 17-β-estradiol (E2), signals through both ERα and ERβ to decrease BCRP transport activity in brain capillaries from rat and mouse. These effects were independent of transcription, translation, and proteasomal degradation. Our findings suggest one strategy to bypass BCRP at the blood–brain barrier, which could help improve CNS chemotherapy of brain tumors and brain tumor stem cells.
Materials and methods
Chemicals
The BCRP antibody (BXP-53, rat monoclonal IgG2a, raised against amino-acid residues 221 to 394 of mouse BCRP) and fumitremorgin C (FTC) were obtained from Alexis-Axxora (San Diego, CA, USA). β-Actin antibody was from Abcam (Cambridge, MA, USA; mouse monoclonal IgG1, raised against amino-acid residues 1 to 100 of human β-actin). BODIPY FL prazosin was from Molecular Probes (Eugene, OR, USA); MK571 was from Cayman (Ann Arbor, MI, USA). Lactacystin was obtained from Calbiochem-Novabiochem (La Jolla, CA, USA). Methylpiperidinopyrazole (MPP) and ICI182,780 were from Tocris (Ellisville, MO, USA). All other chemicals were purchased from Sigma (St Louis, MO, USA). Ko143 was a kind gift from Dr Alfred Schinkel (Netherlands Cancer Institute, Amsterdam, The Netherlands; Allen et al, 2002); GF120918 was a kind gift from GlaxoSmithKline (London, UK), and PSC833 was a kind gift from Novartis (Basel, Switzerland).
Animals
All experiments involving animals were conducted in accordance with the AAALAC regulations and the Guides to Animal Use of the University of Minnesota and NIH animal guidelines. Animal protocols were approved by the Institutional Animal Care and Use Committees of the University of Minnesota (IACUC protocol # 0710A17842; PI: Björn Bauer) and NIEHS/NIH (IACUC protocol #LPC99-14; PI: David Miller). Male and female Sprague-Dawley rats (male and female retired breeders: 6 months old, 300 to 500 g average body weight) were purchased from Taconic Farms (Germantown, NY, USA) and from Charles River (Portage, MI, USA). Male and female ERKO-α (estrogen receptor α-deficient, B6.129-Esr1tm1Ksk N10), ERKO-β (estrogen receptor β-deficient, B6.129-Esr2tm1Unc N9), and wild-type mice (C57BL/6 background) were a generous gift from Dr Kenneth Korach (NIEHS, RTP, NC, USA) (Lubahn et al, 1993). Mice were 10 weeks old with an average body weight of female mice of 20 g and an average body weight of male mice of 25 g.
Isolation of Brain Capillaries
Capillaries were isolated from rat and mouse brains as described earlier (Hartz et al, 2008). For each preparation, 10 rats or 20 mice were killed by CO2 inhalation and decapitated. Brains were removed, dissected, and homogenized in ice-cold PBS buffer (2.7 mmol/L KCl, 1.46 mmol/L KH2PO4, 136.9 mmol/L NaCl, 8.1 mmol/L Na2HPO4, 0.9 mmol/L CaCl2, and 0.5 mmol/L MgCl2 supplemented with 5 mmol/L -glucose, 1 mmol/L sodium pyruvate, pH 7.4). The homogenate was mixed with Ficoll (final concentration 15%) and centrifuged at 5800 g for 20 min at 4°C. After resuspending the pellet in 1% BSA, the capillary suspension was passed over a glass bead column. Capillaries adhering to the glass beads were collected by gentle agitation in 1% BSA. Capillaries were washed with PBS and used for transport experiments, western blotting, immunostaining, or RNA isolation.
Reverse Transcriptase-Polymerase Chain Reaction
Total RNA was isolated from rat brain capillaries, brain, choroid plexus, placenta, intestine, kidney, and liver using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and further purified using the RNeasy mini kit (QIAGEN, Valencia, CA, USA). Reverse transcription was performed using the GeneAmp RNA PCR kit (Applied Biosystems, Foster City, CA, USA). Polymerase chain reaction (PCR) of reverse transcriptase (RT) products was performed with Taq DNA polymerase from Promega (Madison, WI, USA) using primers for rat BCRP (forward, 5′-CATTCACCAGCCTCGGTATTCCATC-3′ reverse, 5′-CTGGCAATCCATCTGAGCTGATGAC-3′). Primers were custom synthesized by QIAGEN Operon (Alameda, CA, USA).
Western Blotting
Protein expression in tissues was analyzed by western blotting as described earlier (Hartz et al, 2008). Liver, kidney, brain, choroid plexus, and capillaries were homogenized in lysis buffer (Sigma) containing complete protease inhibitor (Roche, Mannheim, Germany). Homogenized samples were centrifuged at 10,000 g for 15 mins; denucleated supernatants were used as brain, choroid plexus, and capillary lysates. Crude membrane fractions from liver, kidney, and brain capillaries were obtained by centrifugation of denucleated supernatants at 100,000 g for 90 mins. Pellets of crude plasma membranes were resuspended in buffer and protein concentrations were determined.
Western blots were performed using the Invitrogen NuPage Bis-Tris electrophoresis and blotting system (Invitrogen, Carlsbad, CA, USA). After protein transfer, blotting membranes were blocked and incubated with primary antibody to BCRP (1:50, 5 μg/mL) or β-actin (1:1000, 1 μg/mL). Membranes were washed and incubated with horseradish peroxidase-conjugated ImmunoPure secondary IgG (1:15,000; Pierce, Rockford, IL, USA) for 1 h. Proteins were detected using SuperSignal West Pico Chemoluminescent Substrate (Pierce). Bands were visualized and recorded using a BioRad Gel Doc 2000 gel documentation system (BioRad, Hercules, CA, USA). Densitometric analysis of BCRP band intensities and digital analysis of the molecular weights for BCRP monomer and dimer were performed with QuantityOne 1-D v4.6.5 software (BioRad). Molecular weight marker RPN800 used for analyses was from GE Healthcare (Piscataway, NJ, USA). In brain capillary membranes, BCRP monomer was identified at 78 kDa and BCRP dimer was identified at 156 kDa. In crude membranes from kidney and liver, BCRP monomer was identified at 72 to 73 kDa and BCRP dimer was identified at 144 to 148 kDa. The difference in BCRP monomer and dimer molecular weight between peripheral tissues (kidney, liver) and brain capillaries is likely due to differences in glycosylation.
Breast Cancer Resistance Protein Transport Assay
The BCRP transport activity was measured as described earlier (Shukla et al, 2009). Freshly isolated capillaries were transferred to glass cover slips and incubated for 1 h at room temperature with modulators in 2 μmol/L BODIPY FL prazosin, a fluorescent BCRP substrate. For each treatment, images of at least 10 capillaries were acquired by confocal microscopy (Zeiss 410 invert laser scanning confocal microscope, × 40 oil immersion objective, numerical aperture × 1.2, 488 nm line of argon laser; Carl Zeiss Inc, Thornwood, NY, USA or Nikon C1 laser scanning confocal microscope unit, Nikon TE2000 inverted microscope, × 40 oil immersion objective, 1.3 numerical aperture, 488 nm line of a Spectra Physics argon laser, model 163C, 515/30 nm band pass filter; Nikon Instruments Inc, Melville, NY, USA). Images were analyzed by measuring luminal BODIPY FL prazosin fluorescence using Scion Image software (Scion Corp., Frederick, MD, USA) and Image J software (NIH, Bethesda, MD, USA) as described earlier (Hartz et al, 2008; Shukla et al, 2009). Specific luminal BODIPY FL prazosin accumulation was determined by taking the difference between total luminal fluorescence and fluorescence in the presence of the metabolic inhibitor, NaCN (1 mmol/L) (Hartz et al, 2008). Sigmoidal dose–response curves and EC50 values were calculated after nonlinear regression analysis (Hill slope fixed to 1) of specific luminal BODIPY FL prazosin fluorescence using GraphPad Prism Software Version 4.01 (GraphPad Software, Inc, La Jolla, CA, USA).
Immunostaining
Freshly isolated rat brain capillaries adhering to glass cover slips were fixed for 15 mins with 3% paraformaldehyde/0.2% glutaraldehyde at room temperature. After washing with PBS, capillaries were permeabilized for 30 mins with 0.1% (v/v) Triton X-100 in PBS and subsequently blocked with 1% BSA in PBS. Capillaries were incubated for 1 h at 37°C with the primary antibody to BCRP (1:20, 12.5 μg/mL). After washing with 1% BSA, capillaries were incubated for 1 h at 37°C with the corresponding Alexa Fluor 488-conjugated secondary IgG (1:1000, 2 μg/mL; Invitrogen, Eugene, OR, USA); negative controls were incubated with secondary antibody only. Nuclei were counterstained with 5 μg/mL propidium iodide for 15 mins. Staining of BCRP was visualized using a Zeiss 510 metalaser scanning confocal microscope (Zeiss 510 NLO laser scanning confocal microscope, × 40 water immersion objective, numerical aperture × 1.2, 488 nm line of argon laser; Carl Zeiss Inc, Thornwood, NY, USA).
Statistical Analysis
Data are presented as mean±s.e.m. One- or two-tailed unpaired Student's t-test was used to evaluate the differences between controls and treated groups; differences were considered to be statistically significant when P<0.05.
Results
Breast Cancer Resistance Protein Expression and Transport Activity in Brain Capillaries
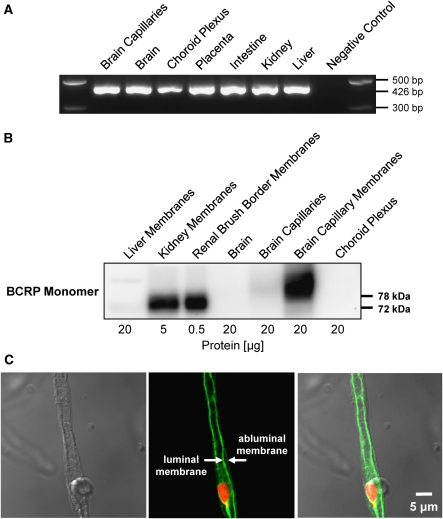
We analyzed BCRP expression in isolated rat brain capillaries using RT-PCR, western blotting, and immunohistochemistry. By RT-PCR, we detected a signal for BCRP mRNA (426 bp amplicon) in all tissues examined, including brain capillaries (Figure 1A). Western blots showed a strong signal for BCRP monomer expression at 72 to 73 kDa in crude kidney membranes and kidney brush border membranes (positive controls, Figure 1B). In agreement with BCRP being a membrane protein, the BCRP protein signal was weak in total brain capillary lysate, but at the same protein loading level capillary plasma membranes showed a strong signal at 78 kDa, indicating localization and enrichment of BCRP protein in the brain capillary endothelial cell membrane. Immunostaining localized BCRP to the luminal membrane of brain capillaries (Figure 1C), which is consistent with earlier work in human, pig, rat, and mouse (Hori et al, 2004; Cooray et al, 2002; Aronica et al, 2005; Tachikawa et al, 2005; Eisenblaetter et al, 2003).
Figure 1.
The BCRP expression in isolated rat brain capillaries. (A) RT-PCR of liver, kidney, intestine, placenta, choroid plexus, brain, and brain capillaries for rat Abcg2. The RT-PCR shows a signal for Abcg2 mRNA at 426 bp in all tissues that were examined. (B) Western blot of liver crude membrane, kidney crude membrane, renal brush border membrane, brain homogenate, brain capillary lysate, brain capillary membrane, and choroid plexus for BCRP. The western blot shows protein expression of BCRP monomer (72 to 73 kDa) in kidney membrane, renal brush border membrane (positive control), and brain capillary membrane (78 kDa). (C) Left: Transmitted light image of an isolated rat brain capillary. Middle: Representative image of an isolated brain capillary immunostained for BCRP (green) and counterstained for nuclei (red); the arrows indicate BCRP staining in the luminal membrane and lack of staining in the abluminal membrane. Right: Overlay of fluorescence channel and transmitted light channel. BCRP, breast cancer resistance protein; RT-PCR, reverse transcriptase-polymerase chain reaction.
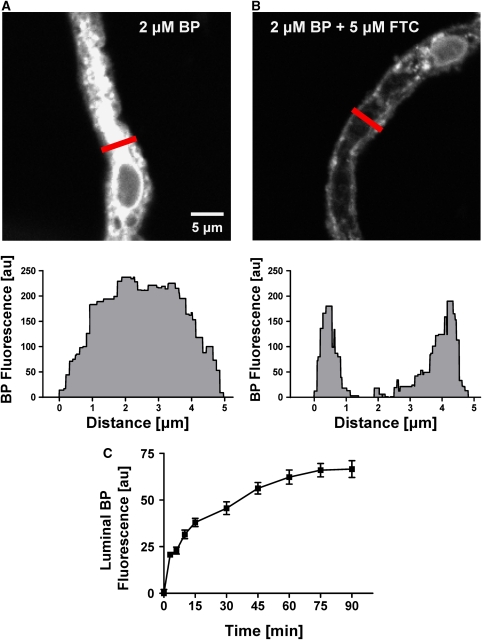
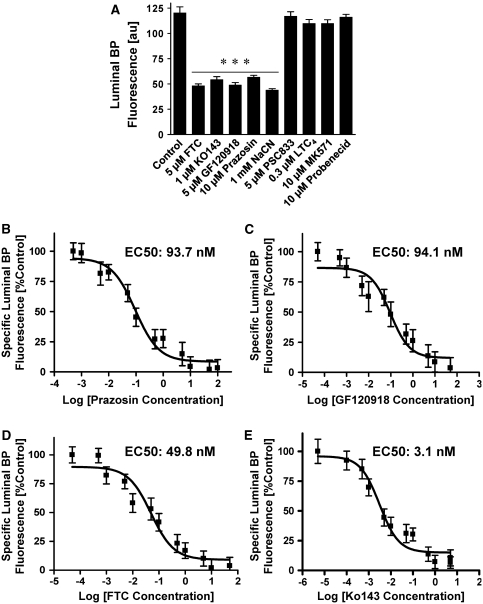
The BCRP functional activity was determined using an assay we recently developed for freshly isolated rat brain capillaries using live tissue confocal microscopy with quantitative, digital image analysis (Shukla et al, 2009). This assay allows real-time monitoring of functionally intact brain capillaries by measuring luminal accumulation of the fluorescent BCRP substrate, BODIPY FL prazosin (BP). Figure 2A (left image) shows a representative confocal image of a capillary incubated in buffer containing 2 μmol/L BODIPY FL prazosin for 60 mins. Fluorescence intensity of BODIPY FL prazosin is low in the incubation medium, moderate in the endothelium, and highest in the capillary lumen, indicating concentrative transport of BODIPY FL prazosin from bath to lumen. Exposing capillaries to 5 μmol/L of the BCRP-specific inhibitor, FTC, substantially blocked luminal BODIPY FL prazosin accumulation (Figure 2B). Figure 2C shows the time course of BODIPY FL prazosin accumulation in brain capillary lumens. Luminal fluorescence increased rapidly and reached steady-state levels after about 60 mins. For the experiments reported here, BODIPY FL prazosin transport was measured at steady state, that is, after 60 mins of incubation with 2 μmol/L BODIPY FL prazosin. Figure 3A shows that luminal fluorescence was reduced by about 60% when metabolism was inhibited by NaCN and when capillaries were exposed to the BCRP inhibitors, FTC, Ko143, and GF120918. A P-glycoprotein inhibitor (PSC833) and Mrp substrates/inhibitors (LTC4, MK571, probenecid) were without effect. Note that roughly 40% of steady-state luminal BODIPY FL prazosin was not affected by BCRP inhibitors, unlabeled prazosin, or NaCN. This component of BODIPY FL prazosin accumulation in brain capillary lumens is likely due to nonspecific binding of the dye to capillaries plus simple diffusion into the luminal space, which is consistent with our findings from transport assays for P-glycoprotein and Mrp2 (Bauer et al, 2008; Hartz et al, 2008).
Figure 2.
The BCRP-mediated BODIPY FL prazosin transport in isolated brain capillaries. (A) Representative image of an isolated rat brain capillary at steady state after 1 h exposure to 2 μmol/L BODIPY FL prazosin. The image analysis of the cross-section (gray) is shown below the image. Note that BODIPY FL prazosin (BP) transport is concentrative from bath (no visible fluorescence) to endothelium (low fluorescence) to capillary lumen (high fluorescence). (B) The BCRP inhibitor, FTC, blocks concentrative BODIPY FL prazosin transport. Analysis of the cross-section (gray) shows fluorescence in the endothelial cells but no accumulation of BODIPY FL prazosin in the capillary lumen. (C) Time course of BODIPY FL prazosin accumulation in capillary lumens. Each data point represents the mean±s.e.m. for 10 to 15 capillaries from a single preparation (pooled tissue from 10 rats). Units are arbitrary fluorescence units (scale 0 to 255). BCRP, breast cancer resistance protein; FTC, fumitremorgin C.
Figure 3.
The BCRP-specific BODIPY FL prazosin transport in isolated brain capillaries. (A) Accumulation of BODIPY FL prazosin (BP) fluorescence in capillary lumens is sensitive to unlabeled prazosin, to the BCRP inhibitors, FTC, Ko143, GF120918, and to the metabolic inhibitor, NaCN. In contrast, the P-glycoprotein-specific inhibitor, PSC833, and the Mrp substrates/inhibitors, LTC4, probenecid and MK571, had no effect on luminal BODIPY FL prazosin accumulation. These data indicate that BODIPY FL prazosin transport in brain capillaries is BCRP specific. (B–E) Nonlinear regression curves (Hill coefficient=1) for prazosin (B; EC50: 93.7 nmol/L), GF120918 (C; EC50: 94.1 nmol/L), FTC (D; EC50: 49.8 nmol/L), and Ko143 (E; EC50: 3.1 nmol/L). Each data point represents the mean±s.e.m. for 10 to 15 capillaries from a single preparation (pooled tissue from 3 to 10 rats). Units are arbitrary fluorescence units (scale 0 to 255). Statistical comparison: ***significantly lower than controls, P<0.001. BCRP, breast cancer resistance protein; FTC, fumitremorgin C.
Detailed dose–response studies showed that prazosin reduced luminal BODIPY FL prazosin fluorescence in a concentration-dependent manner and that luminal fluorescence was maximally blocked by inhibitor concentrations as low as 0.25 μmol/L Ko143, 1 μmol/L FTC, and 5 μmol/L GF120918 (data not shown). Figures 3B–3E show sigmoid dose–response curves for all four compounds; nonlinear regression analyses (Hill coefficient=1) yielded EC50 values of 93.7 nmol/L for prazosin, 94.1 nmol/L for GF120918, 49.8 nmol/L for FTC, and 3.1 nmol/L for Ko143 (Figure 3). The same ranking of EC50 values for GF120918, FTC, and Ko143 have been reported earlier in BCRP-overexpressing cell cultures (Henrich et al, 2007; Pick et al, 2008). However, due to the lower BCRP expression levels in brain capillaries compared with relatively high BCRP levels in these engineered overexpressing systems, the EC50 values calculated in this study are about 20- to 80-fold lower than those reported earlier. Together, these data show that concentrative, specific, and ATP-driven bath-to-lumen transport of BODIPY FL prazosin in brain capillaries was mediated by BCRP.
Breast Cancer Resistance Protein Expression and Transport Activity at the Blood–Brain Barrier is Gender Independent
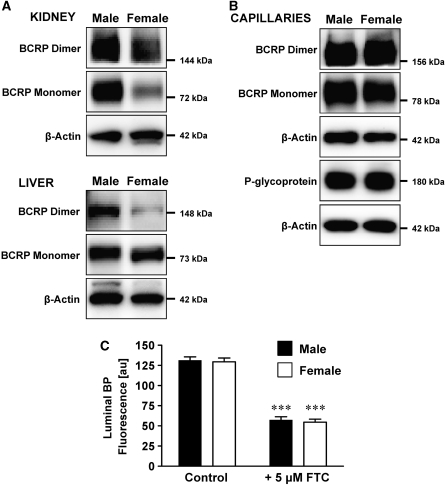
The BCRP is a ‘half transporter' that exists as monomer, dimer, and tetramer; however, it is the BCRP dimer that is functionally active (Xu et al, 2004). We therefore analyzed plasma membranes for BCRP dimer in addition to the monomer. Lower BCRP expression levels for females compared with males have been reported in kidney, liver, and intestine for human, rat, and mouse (Merino et al, 2005; Tanaka et al, 2005). The western blots in Figure 4A confirm these findings. In kidney, we found that BCRP monomer was significantly reduced, and BCRP dimer was slightly reduced in female rats compared with male rats (48%±1% (n=2 western blots) and 77%±2% (n=3 western blots), respectively, compared with 100% male controls; determined by densitometric analysis; BCRP levels were normalized to β-actin). In liver tissue from female rats, BCRP monomer was unchanged, but BCRP dimer was significantly reduced compared with male rats (34%±12% compared with 100% male controls (n=4 western blots); determined by densitometric analysis; BCRP levels were normalized to β-actin). However, no data are available on gender-dependent expression of BCRP at the blood–brain barrier. Figure 4B shows that there was no difference in the expression of BCRP and P-glycoprotein in brain capillaries isolated from male and female rats and this was confirmed by densitometric analysis (n=4 for BCRP monomer, n=3 for BCRP dimer, n=3 for P-glycoprotein). Consistent with this, we found no gender difference in BCRP transport activity in brain capillaries (Figure 4C).
Figure 4.
The BCRP expression and function in isolated rat brain capillaries is gender independent. (A) In female rats, BCRP monomer and dimer expression in kidney and BCRP dimer expression in liver are reduced compared with male rats. (B) BCRP and P-glycoprotein expression in brain capillaries from male and female rats is gender independent. For all western blots, β-actin was used as protein loading control. (C) BCRP-mediated transport of BODIPY FL prazosin (BP) into the lumens of capillaries from male and female rats is gender independent in untreated control capillaries and FTC-treated capillaries. For luminal BODIPY FL prazosin fluorescence, each data point represents the mean±s.e.m. for 10 to 15 capillaries from a single preparation (pooled tissue from 10 rats). Units are arbitrary fluorescence units (scale 0 to 255). Statistical comparison: ***significantly lower than controls, P<0.001. BCRP, breast cancer resistance protein; FTC, fumitremorgin C.
E2 Decreases Breast Cancer Resistance Protein Transport Activity
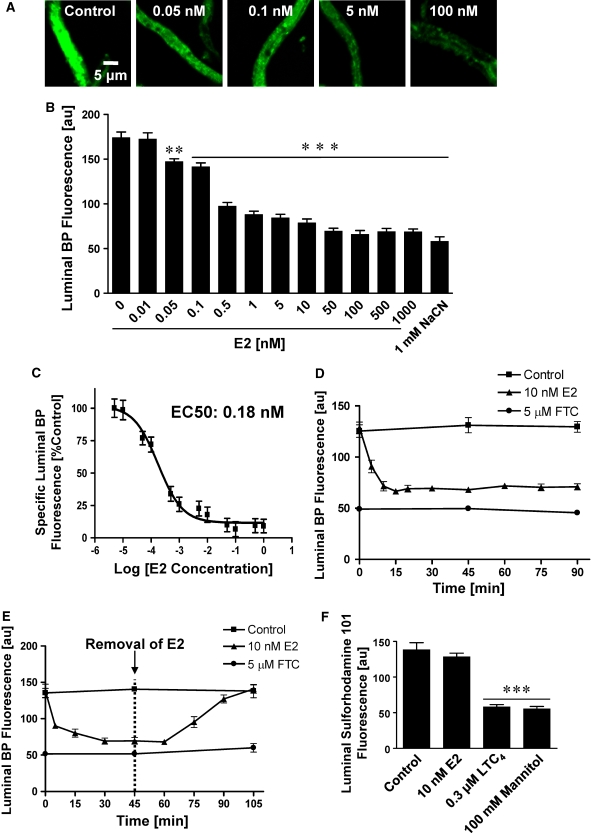
To determine whether blood–brain barrier BCRP is modulated by estrogen, we exposed freshly isolated brain capillaries from male rats to 0.01 to 1000 nmol/L 17-β-estradiol (E2) for 1 h and then measured BCRP transport activity. Control capillaries (Figure 5A, left image) showed high luminal BODIPY FL prazosin fluorescence, but capillaries exposed to 17-β-estradiol showed a concentration-dependent decrease in luminal fluorescence (Figures 5A and 5B). With a calculated EC50 value of 0.18 nmol/L (nonlinear regression analyses; Hill coefficient=1), 17-β-estradiol proved to be more potent in reducing BCRP transport function than the specific BCRP inhibitors GF120918, FTC, and Ko143 (Figures 3B–3E and 5C). Additional experiments indicated that E2 had the same effect on BCRP-mediated transport in capillaries from female rats (data not shown).
Figure 5.
E2 decreases BCRP transport activity. (A) Representative images of isolated rat brain capillaries incubated for 1 h with 2 μmol/L BODIPY FL prazosin with or without E2. Luminal steady-state accumulation of BODIPY FL prazosin decreased with increasing concentrations of E2. (B) Analysis of confocal images reveals a concentration-dependent decrease of luminal BODIPY FL prazosin fluorescence in capillaries exposed to E2. (C) Nonlinear regression curve for E2 (Hill coefficient=1); EC50: 0.18 nmol/L. (D) Rapid decrease of BCRP transport activity in capillaries exposed to E2. Capillaries were loaded with 2 μmol/L BODIPY FL prazosin for 60 mins to steady state. After adding 10 nmol/L E2 (time 0 min), BCRP transport activity decreased within 15 mins. (E) E2 effects on BCRP transport activity are reversible. Capillaries were loaded for 60 mins to steady state with 2 μmol/L BODIPY FL prazosin. When 10 nmol/L E2 was added to the buffer (time 0 on graph), BCRP activity decreased rapidly; BCRP activity recovered completely when E2 was removed (time point 45 mins). (F) E2 had no effect on luminal accumulation of sulforhodamine 101, an Mrp2-specific substrate, whereas mannitol, a tight junction opener, and the Mrp2 inhibitor, LTC4, both decreased Mrp2-mediated sulforhodamine 101 accumulation in brain capillaries. These data indicate that E2 did not alter tight junction permeability, but specifically reduced luminal BODIPY FL prazosin fluorescence through decreased BCRP transport activity. Each data point represents the mean±s.e.m. for 7 to 10 capillaries from a single preparation (pooled tissue from 10 rats). Units are arbitrary fluorescence units (scale 0 to 255). Statistical comparison: **significantly lower than controls, P<0.01, ***significantly lower than controls, P<0.001.
To determine the time course of E2 action on BCRP, we preloaded capillaries to steady state with 2 μmol/L BODIPY FL prazosin and then added 10 nmol/L E2. In control capillaries, luminal fluorescence was unchanged over the entire time course. In contrast, E2 rapidly decreased luminal BODIPY FL prazosin fluorescence. Within 15 mins, accumulation was reduced by 47%. Luminal fluorescence remained at this reduced level over the remaining 75 mins of the time course (Figure 5D). Removing E2 after 45 mins of exposure resulted in a rapid increase of luminal BODIPY FL prazosin to control levels (Figure 5E). Thus, E2 rapidly and reversibly reduced BCRP function.
The E2-mediated decrease in luminal BODIPY FL prazosin fluorescence could have resulted from reduced BCRP transport function or from opening of tight junctions leading to leakage of BODIPY FL prazosin out of capillary lumens. If E2 affected blood–brain barrier tight junction integrity, one would also expect decreased luminal fluorescence of substrates that are transported into the lumen by other ATP-driven efflux pumps such as Mrp2. We previously showed that sulforhodamine 101, an organic anion, is transported into rat brain capillary lumens by Mrp2 (Bauer et al, 2008). Figure 5F shows that the Mrp inhibitor, LTC4, and the osmotic tight junction disruptor, mannitol, both reduced luminal fluorescence of sulforhodamine 101. Importantly, E2 had no effect on Mrp2-mediated accumulation of sulforhodamine 101 in capillary lumens. Thus, E2 did not alter tight junction permeability, but specifically reduced luminal BODIPY FL prazosin fluorescence through decreased BCRP transport activity.
Mechanism of E2-Mediated Decrease of Breast Cancer Resistance Protein Transport Function
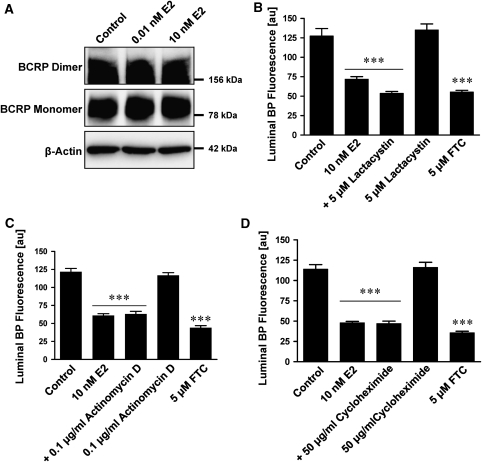
Despite well-documented, long-term, genomic effects of 17-β-estradiol involving regulation of gene expression, there is now substantial evidence for nongenomic E2 effects in several cell types (Meyer et al, 2009; Raz et al, 2008). Such nongenomic effects of steroid hormones are rapid, reversible, and independent of gene transcription, protein synthesis, or proteasomal degradation (Boonyaratanakornkit and Edwards, 2007; Simoncini and Genazzani, 2003). We used these criteria to determine whether E2 could signal through a nongenomic pathway in isolated rat brain capillaries. Capillaries exposed to E2 for 1 h showed no change in protein expression of BCRP monomer or dimer (Figure 6A). Inhibiting proteasomal degradation with lactacystine, a selective inhibitor of the 20S proteasome, did not reverse the E2-mediated decrease of BCRP transport function (Figure 6B). In addition, inhibitors of transcription (actinomycin D) and protein translation (cycloheximide) did not affect E2-mediated downregulation of BCRP function (Figures 6C and 6D). These findings are consistent with E2 acting on BCRP in brain capillaries through a nongenomic-signaling pathway (Simoncini and Genazzani, 2003).
Figure 6.
E2 decreases BCRP transport activity through nongenomic signaling. (A) One hour exposure of brain capillaries to E2 does not affect BCRP monomer or dimer protein expression. β-Actin was used as protein loading control. (B) Lactacystin, a proteasome inhibitor, does not block E2-mediated reduction of BODIPY FL prazosin fluorescence in capillary lumens, indicating that proteasomal degradation is not involved in BCRP downregulation caused by E2. Inhibition of transcription with actinomycin D (C) and inhibition of translation with cycloheximide (D) did not reverse BCRP downregulation by E2. This indicates that E2-mediated downregulation of BCRP is independent of transcription and translation. For B, C, and D, FTC was used as a positive control for BCRP inhibition. Each data point represents the mean±s.e.m. for 10 to 15 brain capillaries from a single preparation (pooled tissue from 10 rats). Units are arbitrary fluorescence units (scale 0 to 255). Statistical comparison: ***significantly lower than controls, P<0.001. BCRP, breast cancer resistance protein; FTC, fumitremorgin C.
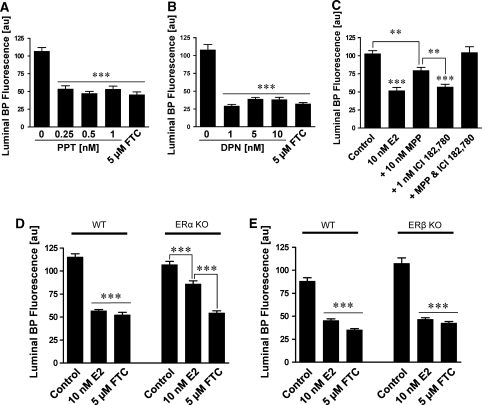
E2 acts through two classical receptors: estrogen receptor α (ERα) and estrogen receptor β (ERβ). We used two strategies, pharmacological tools and ER-null mice, to determine through which of these receptors E2 signaled rapid downregulation of BCRP function in brain capillaries. Exposing isolated rat brain capillaries for 1 h to the ERα agonist, propylpyrazoletriol, or the ERβ agonist, diarylpropionitrile, significantly decreased BCRP transport activity (Figures 7A and 7B). Blocking ERβ with ICI182,780 did not affect E2-mediated downregulation of BCRP activity and blocking ERα with MPP only partially reversed BCRP downregulation (Figure 7C). However, blocking both receptors with MPP and ICI182,780 in combination completely reversed the E2 effect on BCRP activity (Figure 7C). These data suggest that E2 can signal BCRP downregulation through either receptor.
Figure 7.
E2 signals through ERα and ERβ in isolated brain capillaries. Propylpyrazoletriol, an ERα agonist (A), and diarylpropionitrile, an ERβ agonist (B), decrease luminal BODIPY FL prazosin fluorescence. This indicates that activation of both ERα and ERβ causes downregulation of BCRP transport function in rat brain capillaries. (C) ICI182,780, an ERβ antagonist, does not reverse the E2 effect on luminal BODIPY FL prazosin fluorescence. Methylpiperidinopyrazole, an ERα antagonist, partially reverses the E2 effect. Brain capillary exposure to both methylpiperidinopyrazole (ERα antagonist) and ICI182,780 (ERβ antagonist) completely blocks the E2 effect on luminal BODIPY FL prazosin fluorescence. These data indicate that E2 can signal BCRP downregulation through either ERα or ERβ. (D and E) E2 downregulates BCRP transport activity in brain capillaries from wild-type and ERα (D) and ERβ (E) knockout mice. Note that the E2 effect was only partial ERα knockout mice, which is in agreement with the data shown in (C). In A, B, D, and E, FTC was used as positive control for BCRP inhibition. Each data point represents the mean±s.e.m. for 10 to 15 brain capillaries from a single preparation (pooled tissue from 3 to 10 rats or 20 mice). Units are arbitrary fluorescence units (scale 0 to 255). Statistical comparison: **significantly lower than controls, P<0.01; ***significantly lower than controls, P<0.001. BCRP, breast cancer resistance protein; ER, estrogen receptor; FTC, fumitremorgin C.
To confirm these findings, we conducted experiments with brain capillaries isolated from male and female ERα and ERβ knockout and wild-type C57BL/6 control mice (these KO mice were constructed on a C57BL/6 background; Lubahn et al, 1993). Figures 7D and 7E show that in capillaries from male wild-type mice, E2 reduced BCRP transport activity. In capillaries from male KO mice, E2 significantly decreased BCRP transport function despite the lack of receptor. We found similar effects in capillaries from female wild-type, ERα and ERβ knockout mice (data not shown). Note that E2 had a partial inhibitory effect in brain capillaries from both male and female ERα knockout mice, which is in agreement with the data obtained from pharmacologically blocking ERα with MPP (Figure 7C). Thus, both ERs, ERα and ERβ, can be involved in signaling E2-induced rapid downregulation of BCRP transport function.
Discussion
The BCRP is an ABC ‘half transporter' that limits the efficacy of cancer chemotherapy and, therefore, has been the center of many studies investigating its regulation. These studies mainly focused on transcriptional regulation of the transporter, and it was discovered that activation of the nuclear receptor, aryl hydrocarbon receptor, induces BCRP expression (Ebert et al, 2005). Other groups analyzed the promoter of the human ABCG2 gene-encoding BCRP and found response elements for hypoxic stimuli and peroxisome proliferator-activated receptor γ (Robey et al, 2009). Estrogen has also been shown to regulate BCRP in various tissues (Imai et al, 2005; Tanaka et al, 2005; Wang et al, 2008, 2006; Zhang et al, 2006) and Ee et al (2004) recently discovered an estrogen-response element in the human ABCG2 promoter region. This study provides new insights into the regulation of BCRP. It shows E2-induced, rapid and reversible loss of BCRP transport activity at the blood–brain barrier with E2 signaling through both ERα and ERβ to decrease BCRP transport function. This E2-driven effect did not involve transcription, translation, or proteasomal degradation, indicating E2 action through a nongenomic mechanism. Several aspects of our study require further discussion.
We show here that basal BCRP activity and expression levels were the same in brain capillaries from male and female rats. In contrast, in kidney and liver, we (present study) and others found that BCRP is expressed at much lower levels in female rats compared with male rats (Merino et al, 2005; Tanaka et al, 2005). This observation may be explained by the high expression levels of the enzyme, aromatase, which converts testosterone to E2 in male brain tissue, but that is at best poorly expressed in kidney and liver. In addition, the gender-divergent BCRP expression pattern in peripheral tissues is thought to result from the suppressive effect of E2 in female animals.
We show that E2 downregulated BCRP transport activity in brain capillaries. This E2 effect on BCRP activity could result from direct E2–BCRP interaction, from E2 signaling through ERs, or from both. Regarding the first possibility, a steroid-binding element has been identified in one transmembrane domain of the BCRP protein (Ee et al, 2004). It has also been shown in cell culture that hormones including E2 altered ATPase activity upon binding to BCRP, but it was not tested whether BCRP still mediated transport (Velamakanni et al, 2008). Although we cannot fully exclude a direct effect of E2 on BCRP, the nanomolar concentrations of E2 that were effective in our experiments are too low to suggest competitive inhibition given that the fluorescent transporter substrate concentration was in the micromolar range. This is strong evidence for E2 acting through signaling rather than directly interacting with the transporter.
Prior characterization of ERα and ERβ knockout mice has revealed that both receptor subtypes have unique but also overlapping functions in vivo (Liu et al, 2008). In human breast cancer cell lines, ERα activation is associated with transcriptional upregulation and posttranscriptional downregulation of BCRP (Imai et al, 2005; Zhang et al, 2006). In human placenta cell lines, ERβ activation is involved in transcriptional up- and downregulation of BCRP (Wang et al, 2008; Wang et al, 2006). Thus, both ERα and ERβ are involved in E2 regulation of BCRP, but the signaling pathways (ERα and/or ERβ) and the effects on BCRP (up- or downregulation) seem to be tissue specific. Consistent with this, our experiments with ER agonists and antagonists, and with ER knockout mice strongly suggest that both ERs are involved in the rapid (minutes) E2-mediated downregulation of BCRP transport activity in brain capillaries. However, the existence of several receptor subtypes for both ERα and ERβ makes E2 signaling potentially more complex and further studies are needed to fully elucidate the range of receptors involved in E2-mediated BCRP downregulation at the blood–brain barrier.
Several studies show that E2 can signal through nongenomic mechanisms (Boonyaratanakornkit and Edwards, 2007; Simoncini and Genazzani, 2003). Compared with genomic signaling where cellular responses take hours due to the time required for protein synthesis of estrogen-regulated genes (O'Lone et al, 2004), nongenomic E2 signaling is rapid and occurs within minutes or even seconds. Nongenomic E2 signaling is independent of transcription and translation (Simoncini and Genazzani, 2003), but can be mediated through PI3K/AKT, ERK, PLC, p42/44 MAP kinase, or CREB (Honda et al, 2001; Migliaccio et al, 1996; Zhou et al, 1996). For example, in human neuroblastoma cells, nongenomic estrogen signaling increased ERK1/2 phosphorylation within 5 to 10 mins (Watters et al, 1997), and in cortical and hippocampal neurons, such signaling increased phosphorylation of PI3K/AKT and CREB (Honda et al, 2001). In agreement with these findings, our preliminary data (not shown) also suggest involvement of PI3K/AKT in E2 signaling to BCRP. Moreover, we show here that E2 rapidly reduced BCRP transport function within 15 mins and that this effect was fully reversible and independent from transcription, translation, and proteasomal degradation. Thus, our findings are consistent with an E2-mediated, nongenomic-signaling mechanism. They show for the first time that nongenomic E2 signaling involving both ERα and ERβ occurs at the blood–brain barrier.
Our results showing an estrogen effect on BCRP in brain capillaries imply that estrogens could have a substantial effect on barrier function. The brain produces E2, controls E2 release, and responds to E2, and thus, is a major target of physiologic estrogen action. In addition, xenoestrogens such as the pesticides, DDT, and bisphenol A, enter the body through air, drinking water, and food, or are ingested as therapeutic drugs such as oral contraceptives and antiestrogens used to treat breast cancer, menopausal symptoms, or osteoporosis. Clearly, blood–brain barrier BCRP can be exposed to both endogenous estrogens and xenoestrogens that could both alter tissue estrogen homeostasis and impact blood–brain barrier BCRP function. On the other hand, estrogens are used to reduce stroke damage and delay onset of Alzheimer's disease, and our data suggest that estrogens could potentially also be used therapeutically to reduce BCRP transport activity to improve brain delivery of chemotherapeutics to brain tumors. This is especially important given emerging evidence that BCRP and P-glycoprotein work in concert at the blood–brain barrier (Chen et al, 2009; de Vries et al, 2007; Lagas et al, 2009; Polli et al, 2009). Indeed, for certain drugs, clinically valid strategies that target both P-glycoprotein and BCRP may be required to dramatically improve CNS drug delivery. In this regard, drug-resistant brain cancer stem cell remnants pose a tremendous challenge to neurooncologists because they often regenerate into larger and more aggressive tumors after surgical removal of the primary tumor (Cheshier et al, 2009). It is now clear that BCRP is highly expressed in brain cancer stem cells, and increasing evidence indicates that BCRP contributes to drug resistance in these cells (Patrawala et al, 2005). However, it should be noted that E2 signaling to BCRP may be altered in the context of brain cancer or other CNS disorders involving inflammation. For example, von Wedel-Parlow et al (2009) reported that the inflammatory mediators, TNF-α and IL-1β, affected BCRP protein and function in porcine brain capillary endothelial cells and we previously showed that endothelin-1, a peptide released in brain disease, significantly decreased BCRP protein expression in isolated rat brain capillaries (Bauer et al, 2007). Thus, it remains to be seen whether targeting the E2-signaling pathway described in this study will be a practical approach to increasing the efficacy of chemotherapeutics that are BCRP substrates through downregulation of BCRP at the blood–brain barrier and in brain cancer stem cells.
Acknowledgments
The authors thank Jonathan Lucking, Sylvia Notenboom, and Nathan Lewis for technical assistance, and Emily Madole for editorial assistance. We thank Dr Alfred Schinkel, The Netherlands Cancer Institute, Division of Experimental Therapy, Amsterdam, for providing Ko143.
The authors declare no conflict of interest.
References
- Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–425. [PubMed] [Google Scholar]
- Aronica E, Gorter JA, Redeker S, van Vliet EA, Ramkema M, Scheffer GL, Scheper RJ, van der Valk P, Leenstra S, Baayen JC, Spliet WG, Troost D. Localization of breast cancer resistance protein (BCRP) in microvessel endothelium of human control and epileptic brain. Epilepsia. 2005;46:849–857. doi: 10.1111/j.1528-1167.2005.66604.x. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Lucking JR, Yang X, Pollack GM, Miller DS. Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase-II metabolizing enzyme, GSTpi, at the blood-brain barrier. J Cereb Blood Flow Metab. 2008;28:1222–1234. doi: 10.1038/jcbfm.2008.16. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Miller DS. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol. 2007;71:667–675. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Edwards DP. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin Reprod Med. 2007;25:139–153. doi: 10.1055/s-2007-973427. [DOI] [PubMed] [Google Scholar]
- Breedveld P, Pluim D, Cipriani G, Wielinga P, van Tellingen O, Schinkel AH, Schellens JH. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res. 2005;65:2577–2582. doi: 10.1158/0008-5472.CAN-04-2416. [DOI] [PubMed] [Google Scholar]
- Buckner JC, Brown PD, O′Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007;82:1271–1286. doi: 10.4065/82.10.1271. [DOI] [PubMed] [Google Scholar]
- Chen Y, Agarwal S, Shaik NM, Chen C, Yang Z, Elmquist WF. P-glycoprotein and breast cancer resistance protein influence brain distribution of dasatinib. J Pharmacol Exp Ther. 2009;330:956–963. doi: 10.1124/jpet.109.154781. [DOI] [PubMed] [Google Scholar]
- Cheshier SH, Kalani MY, Lim M, Ailles L, Huhn SL, Weissman IL. A neurosurgeon's guide to stem cells, cancer stem cells, and brain tumor stem cells. Neurosurgery. 2009;65:237–249. doi: 10.1227/01.NEU.0000349921.14519.2A. [DOI] [PubMed] [Google Scholar]
- Cooray HC, Blackmore CG, Maskell L, Barrand MA. Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport. 2002;13:2059–2063. doi: 10.1097/00001756-200211150-00014. [DOI] [PubMed] [Google Scholar]
- de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O. P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res. 2007;13:6440–6449. doi: 10.1158/1078-0432.CCR-07-1335. [DOI] [PubMed] [Google Scholar]
- Ebert B, Seidel A, Lampen A. Identification of BCRP as transporter of benzo[a]pyrene conjugates metabolically formed in Caco-2 cells and its induction by Ah-receptor agonists. Carcinogenesis. 2005;26:1754–1763. doi: 10.1093/carcin/bgi139. [DOI] [PubMed] [Google Scholar]
- Ee PL, Kamalakaran S, Tonetti D, He X, Ross DD, Beck WT. Identification of a novel estrogen response element in the breast cancer resistance protein (ABCG2) gene. Cancer Res. 2004;64:1247–1251. doi: 10.1158/0008-5472.can-03-3583. [DOI] [PubMed] [Google Scholar]
- Eisenblatter T, Galla HJ. A new multidrug resistance protein at the blood-brain barrier. Biochem Biophys Res Commun. 2002;293:1273–1278. doi: 10.1016/S0006-291X(02)00376-5. [DOI] [PubMed] [Google Scholar]
- Eisenblatter T, Huwel S, Galla HJ. Characterisation of the brain multidrug resistance protein (BMDP/ABCG2/BCRP) expressed at the blood-brain barrier. Brain Res. 2003;971:221–231. doi: 10.1016/s0006-8993(03)02401-6. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Block ML, Hong JS, Miller DS. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. FASEB J. 2008;22:2723–2733. doi: 10.1096/fj.08-106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich CJ, Robey RW, Bokesch HR, Bates SE, Shukla S, Ambudkar SV, Dean M, McMahon JB. New inhibitors of ABCG2 identified by high-throughput screening. Mol Cancer Ther. 2007;6:3271–3278. doi: 10.1158/1535-7163.MCT-07-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Shimohama S, Sawada H, Kihara T, Nakamizo T, Shibasaki H, Akaike A. Nongenomic antiapoptotic signal transduction by estrogen in cultured cortical neurons. J Neurosci Res. 2001;64:466–475. doi: 10.1002/jnr.1098. [DOI] [PubMed] [Google Scholar]
- Hori S, Ohtsuki S, Tachikawa M, Kimura N, Kondo T, Watanabe M, Nakashima E, Terasaki T. Functional expression of rat ABCG2 on the luminal side of brain capillaries and its enhancement by astrocyte-derived soluble factor(s) J Neurochem. 2004;90:526–536. doi: 10.1111/j.1471-4159.2004.02537.x. [DOI] [PubMed] [Google Scholar]
- Imai Y, Ishikawa E, Asada S, Sugimoto Y. Estrogen-mediated post transcriptional down-regulation of breast cancer resistance protein/ABCG2. Cancer Res. 2005;65:596–604. [PubMed] [Google Scholar]
- Lagas JS, van Waterschoot RA, van Tilburg VA, Hillebrand MJ, Lankheet N, Rosing H, Beijnen JH, Schinkel AH. Brain accumulation of dasatinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by elacridar treatment. Clin Cancer Res. 2009;15:2344–2351. doi: 10.1158/1078-0432.CCR-08-2253. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gao H, Marstrand TT, Strom A, Valen E, Sandelin A, Gustafsson JA, Dahlman-Wright K. The genome landscape of ERalpha- and ERbeta-binding DNA regions. Proc Natl Acad Sci USA. 2008;105:2604–2609. doi: 10.1073/pnas.0712085105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino G, van Herwaarden AE, Wagenaar E, Jonker JW, Schinkel AH. Sex-dependent expression and activity of the ATP-binding cassette transporter breast cancer resistance protein (BCRP/ABCG2) in liver. Mol Pharmacol. 2005;67:1765–1771. doi: 10.1124/mol.105.011080. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Haas E, Prossnitz ER, Barton M. Non-genomic regulation of vascular cell function and growth by estrogen. Mol Cell Endocrinol. 2009;308:9–16. doi: 10.1016/j.mce.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- O'Lone R, Frith MC, Karlsson EK, Hansen U. Genomic targets of nuclear estrogen receptors. Mol Endocrinol. 2004;18:1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- Pal D, Mitra AK. MDR- and CYP3A4-mediated drug-herbal interactions. Life Sci. 2006;78:2131–2145. doi: 10.1016/j.lfs.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- Pick A, Muller H, Wiese M. Structure-activity relationships of new inhibitors of breast cancer resistance protein (ABCG2) Bioorg Med Chem. 2008;16:8224–8236. doi: 10.1016/j.bmc.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Polli JW, Olson KL, Chism JP, John-Williams LS, Yeager RL, Woodard SM, Otto V, Castellino S, Demby VE. An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the cent. Drug Metab Dispos. 2009;37:439–442. doi: 10.1124/dmd.108.024646. [DOI] [PubMed] [Google Scholar]
- Raz L, Khan MM, Mahesh VB, Vadlamudi RK, Brann DW. Rapid estrogen signaling in the brain. Neurosignals. 2008;16:140–153. doi: 10.1159/000111559. [DOI] [PubMed] [Google Scholar]
- Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, Bates SE. ABCG2: a perspective. Adv Drug Deliv Rev. 2009;61:3–13. doi: 10.1016/j.addr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S, Zaher H, Hartz A, Bauer B, Ware JA, Ambudkar SV. Curcumin inhibits the activity of ABCG2/BCRP1, a multidrug resistance-linked ABC drug transporter in mice. Pharm Res. 2009;26:480–487. doi: 10.1007/s11095-008-9735-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncini T, Genazzani AR. Non-genomic actions of sex steroid hormones. Eur J Endocrinol. 2003;148:281–292. doi: 10.1530/eje.0.1480281. [DOI] [PubMed] [Google Scholar]
- Tachikawa M, Watanabe M, Hori S, Fukaya M, Ohtsuki S, Asashima T, Terasaki T. Distinct spatio-temporal expression of ABCA and ABCG transporters in the developing and adult mouse brain. J Neurochem. 2005;95:294–304. doi: 10.1111/j.1471-4159.2005.03369.x. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Slitt AL, Leazer TM, Maher JM, Klaassen CD. Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem Biophys Res Commun. 2005;326:181–187. doi: 10.1016/j.bbrc.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Velamakanni S, Janvilisri T, Shahi S, van Veen HW. A functional steroid-binding element in an ATP-binding cassette multidrug transporter. Mol Pharmacol. 2008;73:12–17. doi: 10.1124/mol.108.038299. [DOI] [PubMed] [Google Scholar]
- von Wedel-Parlow M, Wolte P, Galla HJ. Regulation of major efflux transporters under inflammatory conditions at the blood-brain barrier in vitro. J Neurochem. 2009;111:111–118. doi: 10.1111/j.1471-4159.2009.06305.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Unadkat JD, Mao Q. Hormonal regulation of BCRP expression in human placental BeWo cells. Pharm Res. 2008;25:444–452. doi: 10.1007/s11095-007-9432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhou L, Gupta A, Vethanayagam RR, Zhang Y, Unadkat JD, Mao Q. Regulation of BCRP/ABCG2 expression by progesterone and 17beta-estradiol in human placental BeWo cells. Am J Physiol Endocrinol Metab. 2006;290:E798–E807. doi: 10.1152/ajpendo.00397.2005. [DOI] [PubMed] [Google Scholar]
- Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- Xu J, Liu Y, Yang Y, Bates S, Zhang JT. Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J Biol Chem. 2004;279:19781–19789. doi: 10.1074/jbc.M310785200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhou G, Wang H, Zhang X, Wei F, Cai Y, Yin D. Transcriptional upregulation of breast cancer resistance protein by 17beta-estradiol in ERalpha-positive MCF-7 breast cancer cells. Oncology. 2006;71:446–455. doi: 10.1159/000108594. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]