Abstract
Alcohol consumption causes neurocognitive deficits, neuronal injury, and neurodegeneration. At the cellular level, alcohol abuse causes oxidative damage to mitochondria and cellular proteins and interlink with the progression of neuroinflammation and neurological disorders. We previously reported that alcohol inhibits glucose transport across the blood-brain barrier (BBB), leading to BBB dysfunction and neurodegeneration. In this study, we hypothesized that ethanol (EtOH)-mediated disruption in glucose uptake would deprive energy for human astrocytes and neurons inducing neurotoxicity and neuronal degeneration. EtOH may also have a direct effect on glucose uptake in neurons and astrocytes, which has not been previously described. Our results indicate that ethanol exposure decreases the uptake of D-(2-3H)-glucose by human astrocytes and neurons. Inhibition of glucose uptake correlates with a reduction in glucose transporter protein expression (GLUT1 in astrocytes and GLUT3 in neurons). Acetyl-L-carnitine (ALC), a neuroprotective agent, suppresses the effects of alcohol on glucose uptake and GLUT levels, thus reducing neurotoxicity and neuronal degeneration. These findings suggest that deprivation of glucose in brain cells contributes to neurotoxicity in alcohol abusers, and highlights ALC as a potential therapeutic agent to prevent the deleterious health conditions caused by alcohol abuse.
Keywords: Human astrocytes, glucose transporter protein, acetyl-L-carnitine, neurodegeneration
Introduction
Alcohol is the most commonly used and abused drug, with approximately 20 million alcoholics or alcohol abusers in the USA. Alcohol causes more than 100,000 deaths and 297,000 disfiguring injuries each year [1]. The central nervous system (CNS) is one of the major targets of alcohol abuse, which causes several metabolic and neurological disorders [2]. Chronic alcohol abuse causes cognitive impairment with permanent structural damage to the brain. Wernicke-Korsakoff syndrome is one of the most devastating forms of alcohol-associated neurodegeneration; its pathogenesis is mainly related to thiamine deficiency [3, 4]. Free radical mediated damage to mitochondria and other organelles has been well established in association with alcohol-induced neurodegeneration. Alcohol-induced oxidative metabolites enhance mitochondrial membrane permeability, retard ATP production, inhibit lysosomal acidification and enhance lysosomal leakage [5-7]. However, the exact molecular mechanisms underlying this pathological progression remain obscure.
Glucose is the primary energy substrate to CNS for normal brain function. More than 90% of the energy required for brain function is derived from glucose. Limitations in the availability of glucose leads to impaired cognitive abilities, coma or death [8]. Alcohol affects the bio-energy conversion and hence the normal metabolic rate in the brain and other organs of body [9-11]. Transport of glucose across the plasma membrane of BMVECs in the BBB is the first rate-limiting step for glucose metabolism. Glucose is transported across the BBB by facilitative glucose transporter (GLUT) proteins, which supplies glucose to astrocytes, neurons, and other cell types of brain. GLUT1 and GLUT3 are the main glucose transporter proteins in brain [12]. GLUT1 exists in two isoforms having different molecular weights; the 55 kDa isoform is located at the luminal and abluminal membranes of BMVECs, whereas the 45 kDa isoform is expressed in the perivascular end-feet of the surrounding astrocytes [13]. GLUT3 is predominantly express in neurons [14, 15].
Decreased glucose transport and metabolic dysfunction in the CNS by the effects of alcohol has been well documented in cell cultures and animal models [9, 16]. Effects of both acute and chronic ethanol exposure on brain glucose utilization have been reported in rats [17-19]. In rat astrocytes, Singh et al. reported the inhibitory effects of ethanol on hexose uptake [20]. In 1994, William-Hemby and Porrino reported that acute administration of low doses of ethanol increased glucose utilization in specific brain regions, while high doses of ethanol decreases [17]. In 1994, Singh et al. reported that exposure to decreased glucose concentration produced dose-dependent neuronal injury, as indicated by the release of lactate dehydorgenase (LDH) into the culture medium in rat cortical cell cultures [21]. However, the effects of ethanol on brain glucose utilization have not been characterized well at the human brain cellular level. More over, the molecular mechanisms underlying alcohol-mediated impairment on glucose uptake/metabolism in human brain cells remain poorly understood. Recently, we reported the link between glucose deprivation and BBB damage due to alcohol abuse in hBMVECs and a mouse model [22]. In this study, we also found that acetyl-L-carnitine (ALC) exerted neu-roprotective effects by preventing glucose uptake dysfunction at the level of the BBB. ALC is a regulator of mitochondrial function, a neuro-transmitter, and an anti-oxidant [23, 24]. The purpose of the present study was to determine the effects of alcohol on the mechanisms involved in the impairment of glucose uptake and there by hampering of energy requirement for the survival of the brain cells by human neurons and astrocytes. In addition we also explore the therapeutic efficacy of ALC, which can exert neuroprotective effects on neurons and astrocytes after alcohol-induced injury and glucose deprivation.
Materials and methods
Chemicals and antibodies
Antibodies to GLUT1, GLUT3, glial fibrillary acidic protein (GFAP, astrocyte marker) and neurofilament (NF, neuronal marker) were obtained from Abcam (Cambridge, MA). An anti-α-actin antibody was purchased from Millipore (Billerica, MA). All secondary Alexa Fluor antibodies were from Invitrogen. D-(2-3H)-glucose (5 mCi, 185 MBq) was from PerkinElmer Life and Analytical Sciences (Waltham, MA). Cytochalasin B (CB, glucose transporter protein inhibitor), ALC and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture
Cortical neurons and astrocytes were obtained from our neural tissue core facility, where these cells are routinely isolated from elective abortus specimens of human fetal brain tissue. Tissues were obtained in full compliance with the ethical guidelines of both the National Institutes of Health (NIH) and the University of Nebraska. Briefly, dissociated tissues were incubated with 0.25% trypsin for 30 min, neutralized with 10% fetal bovine serum, and further dissociated by trituration. Cells were cultured on poly-D-lysine pre-coated cover slips and flasks (BD Labware, Bedford, MA). Neurons were maintained in Neurobasal™ Medium containing glutamine (0.5 mM), penicillin (50 μg/ml) and streptomycin (50 μg/ml) in combination with GIBCO™B-27 supplements with antioxidants as described previously [25]. Astrocytes were cultured in DMEM/F-12 media containing HEPES (10 mM), sodium bicarbonate (13 mM; pH 7), 10% fetal bovine serum, penicillin (100 μg/ml) and streptomycin (100 μg/ml) as described [26]. The purity of neuronal populations was assessed by MAP-2 antibodies (Chemicon), while the purity of astrocyte cultures was assessed by GFAP antibodies, resulting in 95-100% enrichment of neurons or astrocytes. Cells were cultured and passaged using routine methods. For glucose uptake and cell viability assays, cells were cultured in 96-well plates (20,000 cells/well). Cells were plated on 12-well plates containing glass cover slips (40,000 cells/well) for immunocyto-chemistry experiments. For protein extractions, astrocytes were cultured in T-75 cm2 culture flasks (1 × 106 cells/flask), and neurons were cultured in 6 well plates (0.2 × 106 cells/well). The cell culture media was changed every third day until cells were confluent (4-6 days for astrocytes; 12-14 days for neurons).
Glucose uptake assay
Following the modified method of Takakura [27] D-(2-3H)-glucose uptake was performed on fully confluent human astrocytes and neurons. Cells were exposed to 50 mM ethanol (EtOH) for 24 hr in the presence or absence of 10 μM CB (10 mM stock dissolved in DMSO) or 100 μM ALC. EtOH concentrations of 50 mM were derived from dose-dependent toxicity assay (5, 10, 20, 50, 100, 200, 500 mM of EtOH for 24 hrs), in which 100 - 500 mM of EtOH showed approximately 10 - 40% cell toxicity after a 24 hr exposure. We used 10 μM CB, as MTT assay analysis showed that concentrations higher than 20 μM were toxic to astrocytes and neurons (data not shown). The cells were then incubated overnight in glucose-free DMEM/F-12 media (for astrocytes) and glucose-free neurobasal media (for neurons) containing equimolar concentrations of D-(2-3H)-glucose (1.0 μCi) and non-radiolabeled glucose. After washing off the excess 3H-glucose with Krebs-Ringer phosphate-HEPES (KRPH) buffer, cellular proteins were precipitated with 10% TCA at 4°C for 15 min. Precipitated proteins were transferred to a 96 well nitrocellulose filter using the Unifilter-96 Harvester as per the manufacturer's instructions (PerkinElmer, Waltham, MA). Using a Beckman 96 well plate reader, radioactivity was measured by β-top counter.
Immunocytochemistry
For immunocytochemistry, primary human astrocytes and neurons were cultured on glass cover slips in 12 well plates until 80-100% confluent. Cells were then treated with 50 mM EtOH with and without CB (10 μM) or ALC (100 μM) for 24 hours. Cells were washed with PBS and fixed in ice-cold acetone-methanol (1:1 v/v). Coverslips were blocked for 1 hr at room temperature in a solution containing 3% bovine serum albumin and 0.4% Triton X-100, and were subsequently incubated overnight at 4°C with primary antibodies: mouse anti-GLUT1 (1:250 dilution) and rabbit anti-GFAP (1:150 dilution) for astrocytes; rabbit anti-GLUT3 (1:250 dilution) and mouse anti-NF (1:250 dilution) for neurons. Cells were washed and incubated for 1 hr with secondary antibodies: anti-mouse-IgG Alexa Fluor 594 for GLUT1, anti-rabbit-IgG Alexa Fluor 488 for GFAP, anti-rabbit-IgG Alexa Fluor 594 for GLUT3 and anti-mouse-IgG Alexa Fluor 488 for NF. Cover slips were then mounted on to glass slides with immuno-mount containing DAPI (Invitrogen), and fluorescence microphotographs were captured by fluorescent microscopy (Eclipse TE2000-U, Nikon microscope, Melville, NY) using NIS-Elements (Nikon) software.
Western blotting
Astrocytes cultured in T-75 cm2 flasks and neurons cultured in 6 well plates were lysed with CellLytic-M (Sigma) buffer containing protease inhibitor for 30 min at 4°C, centrifuged at 14000 × g, and cell lysate protein concentrations were estimated by BCA analysis (Thermo Scientific, Rockford, IL). We loaded 20 μg protein/lane and resolved the lysates by SDS-PAGE on 4-15% gradient gels (Thermo Scientific) and then transferred the protein on to a nitrocellulose membrane. After blocking with Superblock T-20 (Thermo Scientific, Rockford, IL) for 1 hr, membranes were incubated overnight with a polyclonal antibody against GLUT1 for astrocytes and GLUT3 for neurons (1:1000, Abcam, Cambridge, MA) at 4°C. After three washes, membranes were incubated for 1 hr with horseradish peroxidase-conjugated secondary antibodies. Immunoreactive bands were detected by West Pico Chemiluminescence Substrate (Thermo Scientific) using an autoradiography developer. Data were quantified using the Gel-pro32 software package (Version 3.1, Media Cybernetics, Marlow, UK) as arbitrary densi-tometry intensity units.
Statistical analysis
Values are expressed as the mean ± SEM. Within an individual experiment, each data point was determined from three to five replicates. Statistical analysis of the data was performed using GraphPad Prism V5 (Sorrento Valley, CA). Comparisons between samples were performed by one-way ANOVA with Dunnett's post-hoc test. Differences were considered significant at P values ≤ 0.05.
Results
EtOH inhibits glucose uptake and GLUT1 expression in human astrocytes
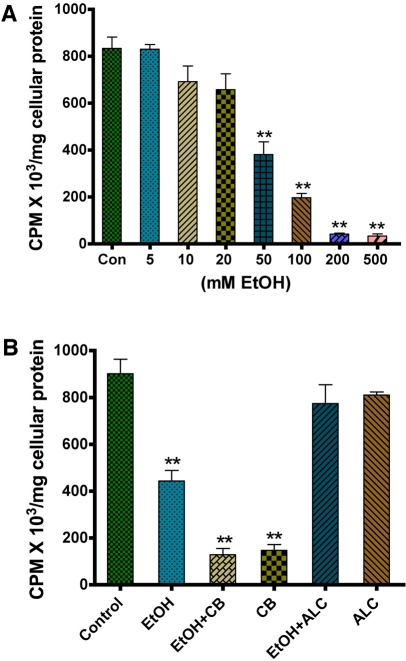
Human astrocytes were cultured with 5-500 mM EtOH for 24 hr, and were assessed for changes in glucose uptake. We found that the glucose uptake was decreased in a dose-dependent fashion with increase in EtOH concentration. However, the significant decrease in glucose uptake was noted from 50 mM to 500 mM EtOH (Figure 1A). To validate the involvement of glucose transporters (GLUTs) in glucose uptake, we studied the effect of CB on glucose uptake in human astrocytes with or without EtOH treatment. We observed that CB alone significantly inhibited glucose uptake, which was further diminished by treatment with EtOH (Figure 1B). This result suggests the direct involvement of GLUT1 in astrocytic glucose transport. Next, we evaluated the effect of ALC (100 μM) on glucose uptake in astrocytes. The concentration of ALC, 100 μM, was derived from dose dependent study of 50-500 μM on cell viability (data not shown). ALC stabilized the rate of glucose uptake in astrocytes after EtOH application, indicating a protective effect of ALC (Figure 1B).
Figure 1.
EtOH inhibits glucose uptake in human astrocytes. A. Dose dependent effects of EtOH on D-(2-3H)-glucose uptake by human astrocytes over a 24 hr time period. B. Effects of the GLUT inhibitor CB (10 μM) and ALC (100 μM) on glucose uptake by astrocytes following 50 mM EtOH treatment for 24 hr. **Statistically significant, p<0.01, compared with controls.
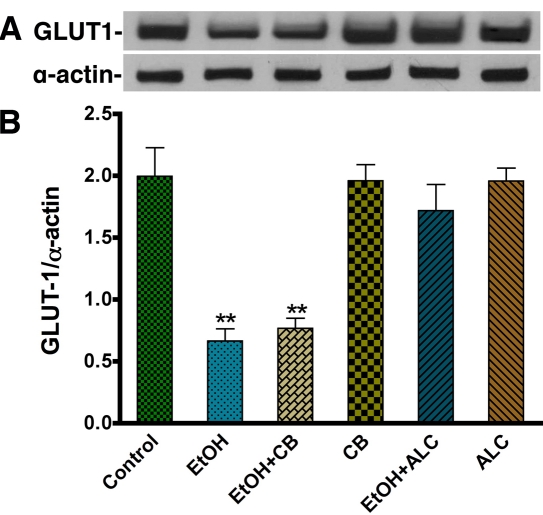
Next, we studied the expression of GLUT1 by immunocytochemistry. In agreement with glucose uptake data, 50 mM EtOH visibly decreased the expression of GLUT1 protein (Figure 2A-D), whereas co-administration of ALC with EtOH blunted the effects of EtOH on GLUT1 protein expression in astrocytes (Figure 2E-F). ALC alone did not change the GLUT1 expression versus control. Since EtOH has significant effects on functional glucose uptake and the expression of GLUT1 by immunocytochemistry analysis, we confirmed the effects of EtOH on GLUT1 protein level by Western blot analysis in primary human astrocytes. In agreement with the uptake assays and immunofluorescence analyses, Western blot analysis also confirmed a significant decrease in GLUT1 expression in 50 mM EtOH compared to untreated cultures (Figure 3A-B). In a similar fashion, ALC attenuated the reduction of GLUT1 protein exerted by 50 mM EtOH (Figure 3A-B). Unlike the GLUT1 isoform found in BMVECs (55 kDa isoform; [22], the GLUT1 protein we detected in astrocytes exhibited a distinct 45 kDa isoform. We also noted that, unlike the glucose uptake findings, CB did not decrease GLUT1 protein levels. This indicates that CB acts pharmacologically as an antagonist of GLUT1 function, rather than modifying GLUT1 protein expression levels (Figure 3A-B).
Figure 2.
EtOH down regulates the expression of GLUT1 in human astrocytes. A-F. Immunocytochemistry of GLUT1 (red) merged with GFAP (green) and DAPI (blue) in human astrocytes. The expression of GLUT1 is shown in control (A-B), 50 mM EtOH (C-D), and 50 mM EtOH+100 μM ALC (E-F). Mouse anti-GLUT1 and rabbit anti-GFAP were used as primary antibodies. An anti-mouse-IgG Alexa Fluor 594 was used to visualize GLUT1 and an anti-rabbit-IgG Alexa Fluor 488 was used to image GFAP. Scale bar = 10 μm in all panels.
Figure 3.
Western blot analysis of GLUT1 in EtOH treated human astrocytes. A. Effects of EtOH on GLUT1 protein levels (45 kDa) in human astrocytes. The effects of CB and ALC for 24 hr on GLUT1 expression are also shown in the band pattern. B. Results are expressed as the ratio of GLUT1 to α-actin bands, and presented as mean values (± SEM; n = 5). (**Statistically significant, p<0.01 compared with controls).
EtOH impairs glucose uptake and GLUT3 expression in human neurons
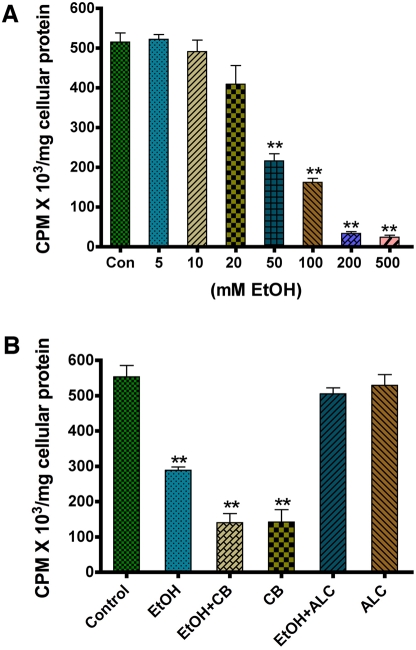
To evaluate whether EtOH disrupts the glucose uptake process in neuronal cells, we treated primary human neuronal cultures with 50 mM EtOH for 24 hr. Cells were used for protein extraction and analysis of changes in GLUT3 protein levels or subjected to glucose assay in glucose free media. The concentration of 50 mM EtOH was selected from the dose-dependent experiments (5-200 mM of EtOH for 24 hrs) on cell viability. Concentrations up to 100 mM did not cause neuronal toxicity at 24 hr exposure (data not shown), however glucose uptake was decreased in a dose-dependent manner, starting at a concentration of 5 mM EtOH (Figure 4A). The significant effect was started from 50 mM EtOH (Figure 4A). We also assessed the role of CB and ALC on glucose uptake. As expected, the GLUT inhibitor CB inhibited the rate of glucose uptake in the presence or absence of EtOH, while ALC effectively protected the adverse effects of EtOH on glucose uptake (Figure 4B).
Figure 4.
Effect of EtOH on glucose uptake in human neurons. A. Dose dependent effects of EtOH (24 hr treatment) on D-(2-3H)-glucose uptake by human neurons. B. Effects of the GLUT inhibitor CB (10 μM) and ALC (100 μM) on glucose uptake by neurons following 50 mM EtOH treatment for 24 hr. **Statistically significant, p<0.01, compared with controls.
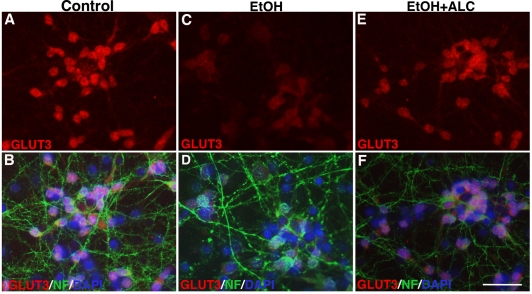
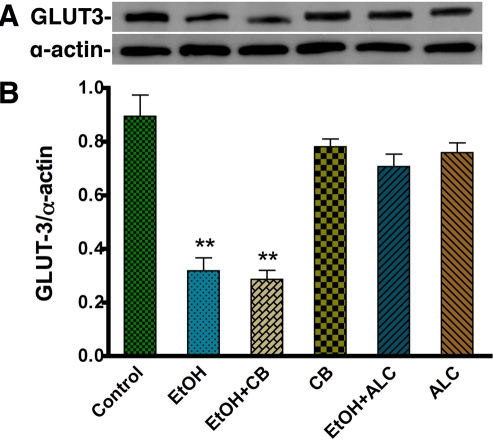
Next, we evaluated the expression of GLUT3, a potential molecular mechanism behind EtOH-induced decrease in glucose uptake in human neurons. Immunocytochemistry analysis shows a substantial decrease in GLUT3 expression in 50 mM EtOH treated human neurons compared with untreated cells (Figure 5A-D). We found that primary human neuronal cells expressed only GLUT3 and not GLUT1 protein (data not shown). We also observed that GLUT3 was localized specifically in neuronal cell bodies, but not in the neurofilaments (Figure 5A). Differences in the cellular localization of glucose transporters may reflect specializations in each cell type for their differing metabolic demands. EtOH-induced decreases in GLUT3 protein expression were further confirmed by Western blot analyses, which demonstrated a significant decrement in the 45 kDa GLUT3 isoform following neuronal exposure to 50 mM EtOH (Figure 6A-B). CB did not alter GLUT3 expression, reiterating its function as a pharmacological antagonist of glucose transporter function. Similar to the findings in astrocytes, the protective effect of ALC on GLUT3 protein levels was also observed. ALC alone did not affect GLUT3 protein levels, but rather it attenuated EtOH-dependent decreases in GLUT3 expression in neuronal cultures (Figure 5E-F, 6A-B). Taken together, our results suggest that EtOH impairs the glucose uptake by inhibiting the expression of GLUT in human astrocytes and neurons.
Figure 5.
EtOH down regulates GLUT3 expression in human neurons. Immunocy-tochemistry of GLUT1 (red) merged with NF (green) and DAPI (blue) in control (A-B), 50 mM EtOH (C-D), and 50 mM EtOH+100 μM ALC (G-H). Rabbit anti-GLUT3 and mouse anti-NF were used as primary antibodies. An anti-rabbit-IgG Alexa Fluor 594 and an anti-mouse-IgG Alexa Fluor 488 were used to visualize GLUT 3 and NF, respectively. Scale bar = 5 μm in all panels.
Figure 6.
Western blot analysis for GLUT3 expression in EtOH treated human neurons. A-B. Effects of EtOH (24 hr treatment) on GLUT3 (45 kDa) protein levels in human neurons. Bar graph shows the results, and are expressed as the ratio of GLUT3 to that of α-actin bands, and presented as mean values (± SEM; n = 5). **Statistically significant, p<0.01, compared with controls.
Discussion
Glucose is the indispensable energy source for the brain, which is transported from circulation to the brain through the BBB by GLUT1. GLUT1 delivers glucose to astrocytes via the astrocytic 45 kDa GLUT1 isoform, and to neurons via the neuronal GLUT3 protein [15]. There are additional glycolytic products of the glucose metabolism such as lactate and pyruvates are transported into neurons by endothelial and astrocytic monocarboxylate transporter1 (MCT1) and out of the neurons by neuronal MCT2 [28]. Interference of these shuttling processes is expected to disrupt the regulation of glucose metabolism and energy utilization in the brain. Our findings emphasize that alcohol inhibits glucose uptake in human brain astrocytes and neurons, and ALC acts as a neuroprotective agent to prevent the effects of alcohol on glucose uptake. In the present study, the concentration of EtOH for our experiments was determined from dose-and time-dependent effects on glucose uptake. The concentration of glucose was based on cellular glucose saturation uptake, and the appropriate dose of CB was determined by cell viability assay. The concentration of glucose used in glucose-free media was justified by our cellular glucose saturation uptake study. Note that the cells were exposed to EtOH, but not starved with glucose. Thus, the saturation of glucose uptake was less than 1.0 mM, which was within the 3.4 mM intracellular concentration of glucose in endothelial cells under physiological conditions [29].
We propose that the loss of GLUT function can adversely affect the rate of glucose uptake and utilization by glial and neuronal cells. CB, an inhibitor of GLUT1 and GLUT3, decreased the rate of glucose uptake without affecting GLUT expression levels, as indicated by immunocytochemistry and Western blot analyses. These results show that CB inhibited glucose uptake and transport by modulating the active binding sites of GLUTs and not by affecting protein synthesis or degrading membrane-embedded GLUT proteins. However, EtOH altered glucose uptake as well as GLUTs protein levels.
We recently demonstrated that alcohol affects BBB integrity, which may potentially cause neurodegeneration by limiting the glucose supply to the CNS [22]. Therefore, inhibition of glucose uptake by ethanol (EtOH) in astrocytes and neurons indicates that EtOH can directly deprive these cell types of glucose, critical for maintaining a high metabolic rate. The implications of these findings reiterate the pathophysiological effects of alcohol abuse on the CNS. In our experiments, we found that neuronal cells were more sensitive to EtOH exposure than astrocytes. One reason for the different effects could be attributed to the fetal origin and the adaptive nature of these cell types. Fetal neurons are very sensitive to exogenous stress agents, whereas glial cells like astrocytes are better adaptive to environmental stress. It remains open for further investigation whether neurons and astrocytes derived from adult brain tissues are less sensitive to EtOH insults. Another possible explanation may be that astrocytes are also better adapted to utilize non-carbohydrate substrates such as fatty acids as an alternative source of bio-energy under high-stress conditions. Such was the case when glucose delivery to astrocytes was limited by EtOH exposure. This argument was supported by the fact that ALC protected neurons and astrocytes from the adverse effects of EtOH [30]: the primary function of ALC is transporting the long-chain fatty acids into the mitochondria for oxidation to generate ATP. It also acts as a precursor for neurotransmission. Thus, it is often referred to as anti-oxidant because ALC maintains the functional integrity of the mitochondria by enhancing the b -oxidation of fatty acids for energy production [23]. These activities emphasize the therapeutic potential of ALC. Another interesting observation was that ALC prevented the decrease in glucose uptake by stabilizing GLUT1 and GLUT3 protein levels after EtOH treatment. Glucose uptake and transport are possible only when the GLUTs are enzymatically glycosylated by acetylglucosamine. We hypothesize that ALC may stabilize the glycosylation state of astrocytic GLUT1 and neuronal GLUT3 by donating acetyl groups to glucosamine, as to enhance the activity of acetylglucosamine. Thus, acetylglucosamine would be free to glycosylate GLUT1 or GLUT3 even in the presence of EtOH. GLUT1 and GLUT3 are the principal glucose transporters that facilitate the transport of glucose in the brain. Therefore, it is critical that GLUT1 and GLUT3 are functioning correctly in the plasma membrane to meet the high metabolic demands of astrocytes and neurons [28, 31].
In conclusion, the present study demonstrates that EtOH exposure caused deleterious effects on glucose uptake and glucose transporter proteins in primary human astrocytes and neurons. Our data suggest that the deprivation of glucose for bio-fuel production in the brain is a key mechanism for neuronal degeneration in alcohol abusers. Furthermore, these changes in glucose transport and transporter expression were attenuated by ALC, highlighting this reagent as potentially beneficial in combating alcohol-associated neurocognitive decline.
Acknowledgments
This work was supported in part by NIH/NIAAA grant AA016403-01A2 (to JH) and by UNMC Faculty Retention Fund.
Glossary
Abbreviations:
- ALC
Acetyl-L-carnitine
- BBB
Blood brain barrier
- CB
Cytochalasin B
- EtOH
Alcohol
- GFAP
Glial fibrillary acidic protein
- GLUT
Glucose transporter protein
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
- NF
Neurofilament
References
- 1.WHO, editor. Geneva: WHO Statistical Information System (WHOSIS); 2007. [Google Scholar]
- 2.Oscar-Berman M, Marinkovic K. Alcoholism and the brain overview. Alcohol Res Health. 2003;27:125–33. [PMC free article] [PubMed] [Google Scholar]
- 3.Harper C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? J Neuropathol Exp Neurol. 1998;57:101–10. doi: 10.1097/00005072-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Harper C, Dixon G, Sheedy D, Garrick T. Neuro-pathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:951–61. doi: 10.1016/S0278-5846(03)00155-6. [DOI] [PubMed] [Google Scholar]
- 5.Kurose I, Higuchi H, Kato S, Miura S, Watanabe N, Kamegaya Y, Tomita K, Takaishi M, Horie Y, Fukuda M, Mizukami K, Ishii H. Oxidative stress on mitochondria and cell membrane of cultured rat hepatocytes and perfused liver exposed to ethanol. Gastroenterology. 1997;112:1331–43. doi: 10.1016/s0016-5085(97)70147-1. [DOI] [PubMed] [Google Scholar]
- 6.Donohue TM, Jr, McVicker DL, Kharbanda KK, Chaisson ML, Zetterman RK. Ethanol administration alters the proteolytic activity of hepatic lysosomes. Alcohol Clin Exp Res. 1994;18:536–41. doi: 10.1111/j.1530-0277.1994.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 7.Donohue TM, Curry-McCoy TV, Nanji AA, Kharbanda KK, Osna NA, Radio SJ, Todero SL, White RL, Casey CA. Lysosomal leakage and lack of adaptation of hepatoprotective enzyme contribute to enhanced susceptibility to ethanol-induced liver injury in female rats. Alcohol Clin Exp Res. 2007;31:1944–52. doi: 10.1111/j.1530-0277.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 8.Sieber FE, Traystman RJ. Special issues: glucose and the brain. Crit Care Med. 1992;20:104–14. [PubMed] [Google Scholar]
- 9.Singh SP, Snyder AK, Eman S. Effects of ethanol on hexose uptake by cultured rat brain cells. Alcohol Clin Exp Res. 2006;14:741–5. doi: 10.1111/j.1530-0277.1990.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 10.Van Horn CG, Cunningham CC. Contributions of dietary carbohydrate and ethanol to alterations in liver glycogen levels and glycolytic activity. Alcohol. 1999;19:139–44. doi: 10.1016/s0741-8329(99)00030-0. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham CC, Coleman WB, Spach PI. The effects of chronic ethanol consumption on hepatic mitochondrial energy metabolism. Alcohol Alcohol. 1990;25:127–36. doi: 10.1093/oxfordjournals.alcalc.a044987. [DOI] [PubMed] [Google Scholar]
- 12.Leybaert L. Neurobarrier coupling in the brain: a partner of neurovascular and neurometabolic coupling? J Cereb Blood Flow Metab. 2005;25:2–16. doi: 10.1038/sj.jcbfm.9600001. [DOI] [PubMed] [Google Scholar]
- 13.Yeh WL, Lin CJ, Fu WM. Enhancement of glucose transporter expression of brain endothelial cells by vascular endothelial growth factor derived from glioma exposed to hypoxia. Mol Pharmacol. 2008;73:170–7. doi: 10.1124/mol.107.038851. [DOI] [PubMed] [Google Scholar]
- 14.Silverman M. Structure and function of hexose transporters. Annu Rev Biochem. 1991;60:757–94. doi: 10.1146/annurev.bi.60.070191.003545. [DOI] [PubMed] [Google Scholar]
- 15.Maher F, Vannucci SJ, Simpson IA. Glucose transporter proteins in brain. FASEB J. 1994;8:1003–11. doi: 10.1096/fasebj.8.13.7926364. [DOI] [PubMed] [Google Scholar]
- 16.Handa RK, DeJoseph MR, Singh LD, Hawkins RA, Singh SP. Glucose transporters and glucose utilization in rat brain after acute ethanol administration. Metab Brain Dis. 2000;15:211–22. doi: 10.1007/BF02674530. [DOI] [PubMed] [Google Scholar]
- 17.Williams-Hemby L, Porrino LJ. Low and moderate doses of ethanol produce distinct patterns of cerebral metabolic changes in rats. Alcohol Clin Exp Res. 1994;18:982–8. doi: 10.1111/j.1530-0277.1994.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 18.Vina JR, Salus JE, DeJoseph MR, Pallardo F, Towfighi J, Hawkins RA. Brain energy consumption in ethanol-treated, Long-Evans rats. J Nutr. 1991;121:879–86. doi: 10.1093/jn/121.6.879. [DOI] [PubMed] [Google Scholar]
- 19.Grunwald F, Schrock H, Biersack HJ, Kuschinsky W. Changes in local cerebral glucose utilization in the awake rat during acute and chronic administration of ethanol. J Nucl Med. 1993;34:793–8. [PubMed] [Google Scholar]
- 20.Singh LD, Singh SP, Handa RK, Ehmann S, Snyder AK. Effects of ethanol on GLUT1 protein and gene expression in rat astrocytes. Metab Brain Dis. 1996;11:343–57. doi: 10.1007/BF02029495. [DOI] [PubMed] [Google Scholar]
- 21.Singh SP, Ehmann S, Snyder AK. Ethanol and glucose-deprivation neurotoxicity in cortical cell cultures. Metabolism. 1994;43:1108–13. doi: 10.1016/0026-0495(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 22.Muneer PM, Alikunju S, Szlachetka AM, Haorah J. Inhibitory effects of alcohol on glucose transport across the blood-brain barrier leads to neurodegeneration: preventive role of acetyl-L: -carnitine. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-2076-4. DOI: 10.1007/s00213-010-2076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagen TM, Ingersoll RT, Wehr CM, Lykkesfeldt J, Vinarsky V, Bartholomew JC, Song MH, Ames BN. Acetyl-L-carnitine fed to old rats partially restores mitochondrial function and ambulatory activity. Proc Natl Acad Sci U S A. 1998;95:9562–6. doi: 10.1073/pnas.95.16.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagen TM, Liu J, Lykkesfeldt J, Wehr CM, Ingersoll RT, Vinarsky V, Bartholomew JC, Ames BN. Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. Proc Natl Acad Sci U S A. 2002;99:1870–5. doi: 10.1073/pnas.261708898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haorah J, Ramirez SH, Floreani N, Gorantla S, Morsey B, Persidsky Y. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic Biol Med. 2008;45:1542–50. doi: 10.1016/j.freeradbiomed.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Floreani NA, Rump TJ, Muneer PM, Alikunju S, Morsey BM, Brodie MR, Persidsky Y, Haorah J. Alcohol-Induced Interactive Phosphorylation of Src and Toll-like Receptor Regulates the Secretion of Inflammatory Mediators by Human Astrocytes. J Neuroimmune Pharmacol. doi: 10.1007/s11481-010-9213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takakura Y, Kuentzel SL, Raub TJ, Davies A, Baldwin SA, Borchardt RT. Hexose uptake in primary cultures of bovine brain microvessel endothelial cells. I. Basic characteristics and effects of D-glucose and insulin. Biochim Biophys Acta. 1991;1070:1–10. doi: 10.1016/0005-2736(91)90139-y. [DOI] [PubMed] [Google Scholar]
- 28.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–91. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pardridge WM, Boado RJ. The Blood-Brain Barrier. In: Pardridge WM, editor. New York: Raven Press; 1993. pp. 395–440. [Google Scholar]
- 30.Rump TJ, Muneer PM, Szlachetka AM, Lamb A, Haorei C, Alikunju S, Xiong H, Keblesh J, Liu J, Zimmerman MC, Jones J, Donohue TM, Jr, Persidsky Y, Haorah J. Acetyl-L-carnitine protects neuronal function from alcohol-induced oxidative damage in the brain. Free Radic Biol Med. 2010;49:1494–504. doi: 10.1016/j.freeradbiomed.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab. 2008;295:E242–53. doi: 10.1152/ajpendo.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]