Abstract
Background and Purpose
In focal ischemic cortex, cerebral blood flow autoregulation is impaired, and perfusion passively follows blood pressure variations. Although it is generally agreed that profound hypotension is harmful in acute stroke, the hemodynamic and metabolic impact of increased blood pressure on the ischemic core and penumbra are less well understood. We, therefore, tested whether pharmacologically induced hypertension improves cerebral blood flow and metabolism and tissue outcome in acute stroke using optical imaging with high spatiotemporal resolution.
Methods
Cerebral blood flow, oxyhemoglobin, and cerebral metabolic rate of oxygen were measured noninvasively using simultaneous multispectral reflectance imaging and laser speckle flowmetry during distal middle cerebral artery occlusion in mice. Hypertension was induced by phenylephrine infusion starting 10 or 60 minutes after ischemia to raise blood pressure by 30% for the duration of ischemia; control groups received saline infusion.
Results
Mild induced hypertension rapidly increased cerebral blood flow, oxyhemoglobin, and cerebral metabolic rate of oxygen in both the core and penumbra and prevented the expansion of cerebral blood flow deficit during 1 hour distal middle cerebral artery occlusion. Induced hypertension also diminished the deleterious effects of periinfarct depolarizations on cerebral blood flow, oxyhemoglobin, and cerebral metabolic rate of oxygen without altering their frequency. Consistent with this, mild induced hypertension reduced infarct volume by 48% without exacerbating tissue swelling when measured 2 days after 1 hour transient distal middle cerebral artery occlusion.
Conclusions
Our data suggest that mild induced hypertension increases collateral cerebral blood flow and oxygenation and improves cerebral metabolic rate of oxygen in the core and penumbra, supporting its use as bridging therapy in acute ischemic stroke until arterial recanalization is achieved.
Keywords: cerebral metabolic rate of oxygen, laser speckle flowmetry, middle cerebral artery occlusion, multispectral reflectance imaging, stroke
The treatment of acute stroke is severely limited by the short therapeutic window for currently available treatments such as thrombolysis. Several physiological interventions such as hypothermia and hyperoxia have been tested to delay irreversible ischemic injury and prolong the therapeutic window until more definitive measures can be instituted to achieve reperfusion. Pharmacologically induced hypertension has similarly been tested to improve cerebral blood flow (CBF) and tissue outcome.1-4 Because cerebral autoregulation is impaired after acute ischemic stroke,5-7 CBF passively follows changes in mean arterial pressure (MAP). Although there is general agreement that hypotension during acute stroke is detrimental to perfusion of ischemic brain and tissue outcome,8-10 the impact of hypertension is poorly understood. It is generally recommended that mild to moderate spontaneous elevations in MAP should not be treated during acute stroke.11 On the contrary, pharmacologically induced hypertension seems to improve tissue and functional outcome in experimental focal ischemia in rats,1,12-15 rabbits,2 dogs,16 and monkeys.4,17 Several case reports and small patient series also suggest feasibility and efficacy in human acute ischemic stroke18-31; however, data from larger prospective, randomized trials are not yet available.
Although improved CBF has been shown during induced hypertension in a few studies,1-4,16 the spatiotemporal impact of induced hypertension on collateral CBF, oxygenation, and metabolism in the ischemic core and penumbra is still poorly understood. Recent advances in optical imaging provide a valuable tool to noninvasively monitor CBF, oxygenation, and cerebral metabolic rate of oxygen (CMRO2) with high spatiotemporal resolution.32-34 We used combined laser speckle flowmetry and multispectral reflectance imaging through an intact skull during focal ischemia in mice to test the impact of pharmacologically induced hypertension. Our data show that mild induced hypertension rapidly improves CBF, oxygenation, and CMRO2 in both the core and penumbra and reduces infarct volume.
Materials and Methods
Surgical Preparation
Mice (C57BL/6J, 23 to 28 g; Charles River Laboratories, Wilmington, Mass) were housed under diurnal lighting conditions and allowed food and tap water ad libitum. The care and handling of the animals and experimental protocols were in accord with National Institutes of Health guidelines and approved by the institutional animal care and use committee. Anesthesia was achieved by isoflurane (2% induction, 1% maintenance, in 70% N2O/30% O2). The femoral artery and femoral vein were catheterized for the measurement of MAP and the infusion of phenylephrine or saline. In a subgroup of mice, anesthesia was switched to α-chloralose (50 mg/kg per hour intraperitoneally), which is known to preserve cerebrovascular autoregulatory function.34 The depth of anesthesia was checked by the absence of cardiovascular changes in response to tail pinch. Rectal temperature was kept at 36.8°C to 37.1°C using a thermostatically controlled heating mat (FHC, Brunswick, Maine). Mice were intubated endotracheally, paralyzed (pancuronium bromide, 0.4 mg/kg per hour intraperitoneally), mechanically ventilated (CWE, Ardmore, Pa), placed in a stereotaxic frame (David Kopf, Tujunga, Calif), and allowed to stabilize for 30 minutes after preparation. Arterial blood gases and pH were measured every 20 minutes (Ciba Corning Diagnostics, Medford, Mass). The data were continuously recorded in a computer (AD Instruments, Medford, Mass).
The temporalis muscle was separated from the temporal bone and removed. A burr hole (2 mm diameter) was drilled under saline cooling in the temporal bone overlying the middle cerebral artery (MCA) just above the zygomatic arch. The dura was kept intact and distal MCA was occluded (dMCAO) using a microvascular clip. Reperfusion was achieved by carefully removing the clip after 60 minutes and confirmed in real-time by optical imaging. Mice were excluded if clip placement or removal was associated with arterial injury and hemorrhage or if reperfusion was not achieved. In experiments in which infarct volume was measured, invasive tracheal intubation was not done to reduce morbidity and mortality, and mice were allowed to recover after successful clip removal to be euthanized 48 hours after ischemia. Whole brains were incubated in 2% 2,3,5-triphenyl-2H-tetrazolium chloride for 60 minutes. Brains were cut into 1 mm thick coronal slices, and infarct area at each section was measured. Infarct volume was calculated indirectly by subtracting ipsilateral noninfarcted volume from contralateral hemisphere. The difference between direct and indirect infarct volume was used to measure ischemic brain swelling.
Optical Imaging
After reflection of scalp laterally, skull surface was covered with a thin layer of mineral oil to prevent drying.34,35 Multispectral reflectance imaging was performed to measure changes in oxyhemoglobin (oxyHb), deoxyhemoglobin, an total Hb (ie, oxyHb+ deoxyhemoglobin).33 Light from a halogen fiberoptic illuminator (Capra Optical, Natick, Mass) was passed through 6 10-nm-wide bandpass filters (560 to 610 nm) placed on a filter wheel (Thorlabs, Newton, NJ), rotating at 3 to 5 revolutions/s. The filtered light was coupled into a fiberoptic bundle (Edmund Scientific, Tonawanda, NY) for cortical illumination. Images were acquired at each band sequentially using a variable magnification objective (×0.75 to ×3; Edmund Optics, Barrington, NJ) and focused either through (infrared laser) or reflected off of (visible light) a dichroic mirror onto 2 CCD cameras (Coolsnap fx, 1300×1030 pixels, Roper Scientific, Trenton, NJ; Cohu 4600, 640×480 pixels, Cohu, San Diego, Calif). The final image size for multispectral reflectance imaging was 433×343 pixels after 3×3 binning. Raw data were collected in sequences of 30 frames at 10 Hz (5 frames/wavelength), and a 5-second delay was added to acquire one sequence approximately every 7.5 seconds. The reflectance image from each wavelength was averaged over the sequence. Each set of multispectral images was converted to changes in hemoglobin oxygenation and volume using a nonlinear fitting procedure based on a Monte Carlo model of light propagation in tissue rather than the traditional modified Beer Lambert relationship; the latter was inaccurate for large hemoglobin concentration changes35a. Briefly, the difference between the intensity changes predicted by the Monte Carlo model for a given set of optical properties (absorption and scattering coefficients), and the measured intensity changes at each time point for all 6 wavelengths was minimized. The fitting parameters were the absorption and scattering coefficients of the tissue. OxyHb and deoxyhemoglobin were assumed to be the only chromophores in the tissue at these wavelengths such that the absorption coefficient was:
where ε is the molar extinction coefficient and C is the concentration of each chromophore. Preischemic baseline concentrations were assumed to be CoHbO=100 μmol/L and CoHbR=40 μmol/L.
Laser speckle flowmetry was used to study the spatiotemporal characteristics of CBF changes during focal cerebral ischemia.34,36 A laser diode (780 nm) was used to diffusely illuminate intact skull. The laser penetration depth is approximately 500 μm. Raw speckle images were used to compute speckle contrast (the ratio of the SD of pixel intensities to their mean), which is a measure of speckle visibility related to the velocity of scattering particles and therefore CBF.37 Ten consecutive raw speckle images were acquired at 15 Hz, processed by computing the speckle contrast using a sliding grid of 7×7 pixels, and averaged to improve signal-to-noise ratio. Speckle contrast images were converted to images of correlation time values, which represent the decay time of the light intensity autocorrelation function. The correlation time is inversely and linearly proportional to the mean blood velocity.37 Relative CBF images were calculated from the ratio of baseline image of correlation time to subsequent images obtained every 7.5 seconds. Multispectral reflectance imaging and laser speckle flowmetry images were coregistered using surface landmarks to ensure complete spatial overlap.
The CMRO2 changes were calculated using the relationship:
where the subscript “0” indicates baseline values; γr and γt are vascular weighting constants, which take into account that the measured changes in hemoglobin are a combination of venous and arterial quantities.38-40
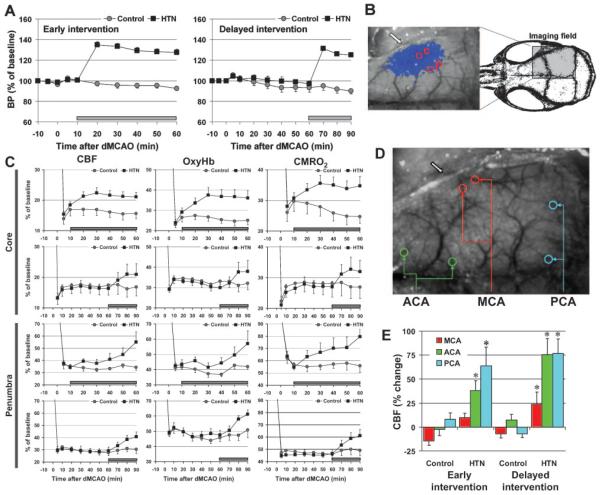
Based on the severity of CBF reduction immediately after dMCAO, 2 regions of interest (250×250 μm) were identified (Figure 1) corresponding to the core (center of severe CBF reduction) and hemodynamic penumbra (steep portion of CBF gradient between the core and nonischemic cortex) for changes in CBF, oxyHb, total Hb, and CMRO2. Collateral CBF was assessed by placing 2 regions of interest each on middle, anterior, or posterior cerebral artery branches (MCA, ACA, PCA, respectively) identified by their anatomic locations, morphology and color. The area (mm2) with severe (0% to 20% residual CBF), moderate (21% to 30%), or mild CBF deficit (31% to 40%)34 was also quantified. Periinfarct depolarizations (PIDs) were identified by the attendant spreading CBF changes previously shown to reliably detect PIDs in focal ischemia.35,41 The impact of PIDs on CBF, oxyHb, and CMRO2 were calculated as percent maximum reduction during each PID compared with the preceding baseline.
Figure 1.
Mild induced hypertension increases CBF and oxygenation and improves CMRO2 in ischemic core and penumbra through pial anterior and posterior cerebral artery collaterals. A, Intravenous phenylephrine infusion (horizontal bar) starting 10 or 60 minutes after dMCAO (hypertension [HTN], n=14 or 6) increased MAP by approximately 30% compared with saline controls (n=14 or 7, P<0.05). B, Speckle contrast image of CBF obtained through intact skull showing the position of imaging field. Superimposed in blue are pixels with residual CBF ≤30% (see “Methods”). The core (c) and penumbra (p) regions of interest (see “Methods” for definition) to quantify the hemodynamic and metabolic parameters are also shown. Block arrows indicate MCA occlusion site. C, Phenylephrine infusion starting 10 or 60 minutes after dMCAO (horizontal bars) rapidly increased CBF, oxyHb, and CMRO2 in both the core and penumbra (P<0.05 versus prephenylephrine baseline for all 3 parameters). D, Speckle contrast image showing the regions of interest used to selectively measure flow changes within ACA and PCA branches and MCA branches distal to the occlusion site. E, CBF within MCA, ACA, and PCA branches at 60 or 90 minutes after dMCAO (early or delayed intervention, respectively), expressed as percent change from baseline immediately before the infusion. Induced hypertension augmented flow within PCA and ACA; flow increase in MCA branches was significantly blunted. In control groups, there was no statistically significant change in flow within any of the measured arteries. *P<0.05 versus baseline, P>0.05 ACA versus PCA.
Experimental Protocol
Imaging started 1 minute before dMCAO and continued for 60 or 90 minutes. In the hypertension group, the MAP was increased by phenylephrine (0.2 mg/mL intravenously) starting 10 (early intervention) or 60 minutes (delayed intervention) after dMCAO and continuing for the duration of ischemia; infusion rate was adjusted to maintain 30% increase in MAP (Figure 1). In the control group, saline was infused. The α-1 adrenergic receptor agonist phenylephrine was chosen over other vasopressors such as angiotensin or mixed adrenergic agonists, because: (1) cerebral vessels lack α-1 receptors and do not constrict in response to phenylephrine; (2) phenylephrine is less arrhythmogenic; and (3) unlike angiotensin, phenylephrine does not impair cerebrovascular endothelial function. A relatively mild increase in MAP of 30% was targeted to limit cardiovascular stress in mice and to test MAP limits compatible with thrombolysis guidelines. Physiological parameters were within normal limits and did not differ among groups at baseline (Table 1). At the end of the infusion period, hypertension group showed mild acidosis; however, there was no correlation between arterial blood gas values and the area of CBF deficit in individual animals (r2=7×10−7 for pH, and 5×10−2 for pCO2 versus area of CBF deficit in early intervention group).
Table 1.
Physiological Parameters
| Control (n=21) |
Hypertension (n=20) |
|||
|---|---|---|---|---|
| Before Saline |
During Saline |
Before Phe |
During Phe |
|
| MAP (mm Hg) | 71±2 | 66±2* | 76±3 | 97±2* |
| pH | 7.37±0.01 | 7.35±0.01 | 7.36±0.01 | 7.28±0.01* |
| pCO2 (mm Hg) | 37±1 | 40±2 | 37±1 | 45±1* |
| pO2 (mm Hg) | 141±3 | 122±4* | 139±4 | 128±6* |
P<0.05 versus baseline.
Phe indicates phenylephrine. Pooled data from early and delayed intervention groups.
Data were expressed as mean±SE and statistically compared using paired or unpaired Student t test and 2-way analysis of variance for repeated measures followed by Fisher’s protected least significant difference test, except when otherwise specified. P<0.05 was considered statistically significant.
Results
Distal MCAO caused an abrupt reduction in CBF and oxyHb concentration in the core and penumbra (Figure 1C). Calculated CMRO2 dropped to 20% to 30% and 45% to 55% of baseline in the core and penumbra, respectively. In the control group, CBF, oxyHb, and CMRO2 remained stably low or gradually worsened in both regions during the next 60 to 90 minutes. In the early intervention group, intravenous phenylephrine infusion 10 minutes after dMCAO rapidly increased CBF, oxyHb, and CMRO2 over prephenylephrine baseline in both the core and penumbra (Figure 1C). In the severely ischemic core, this increase persisted throughout the phenylephrine infusion. In penumbra, CBF and oxyHb increased progressively throughout the infusion period so that 60 minutes after dMCAO, CBF and oxyHb were 60% and 35% higher than control, respectively. The calculated CMRO2 also progressively improved in the penumbra and returned to near normal at 60 minutes (P>0.05 versus baseline; Figure 1C). Delayed onset of phenylephrine infusion 60 minutes after dMCAO also increased CBF, oxyHb, and CMRO2 in both the core and penumbra. The efficacy of delayed intervention to augment CBF, oxyHb, and CMRO2 did not statistically differ from early induced hypertension (Table 2). Interestingly, mild induced hypertension did not alter total Hb concentration, an indirect measure of cerebral blood volume, in either the core or penumbra (8% and 13% increase compared with the control at 60 minutes, respectively, P>0.05).
Table 2.
Impact of Early or Delayed Mild Induced HTN on CBF, oxyHb, and CMRO2 in the Core and Penumbra
| Core |
Penumbra |
|||||||
|---|---|---|---|---|---|---|---|---|
| Early Intervention (10 minutes) |
Delayed Intervention (60 minutes) |
Early Intervention (10 minutes) |
Delayed Intervention (60 minutes) |
|||||
| Control (n=14) |
HTN (n=14) |
Control (n=7) |
HTN (n=6) |
Control (n=14) |
HTN (n=14) |
Control (n=7) |
HTN (n=6) |
|
| CBF | −7±4 | 15±5* | −2±7 | 25±11* | −3±3 | 58±20* | 10±7 | 40±9* |
| OxyHb | −4±8 | 24±7* | −3±3 | 25±12* | 1±7 | 36±21* | 11±6 | 30±6* |
| CMRO2 | −16±4 | 13±9* | −3±5 | 17±7* | −2±5 | 43±16* | 7±5 | 32±7* |
Data are expressed as percent change from prephenylephrine baseline (10 or 60 minutes after dMCAO for early or delayed intervention, respectively) to postphenylephrine (60 or 90 minutes after dMCAO for early or delayed intervention, respectively). Phenylephrine infusion started 10 or 60 minutes after dMCAO and continued for 50 or 30 minutes in early or delayed intervention groups, respectively. Control groups received saline infusion for the same period.
P<0.05, 2-way analysis of variance for repeated measures.
HTN indicates hypertension.
To test whether ACA and PCA collaterals contribute to CBF augmentation during induced hypertension, we performed selective CBF measurements within regions of interest placed over identifiable branches of respective arteries (Figure 1D). This analysis showed that induced hypertension at 10 or 60 minutes after dMCAO augmented CBF within both PCA and ACA branches, but not within MCA branches immediately distal to the occlusion (Figure 1E).
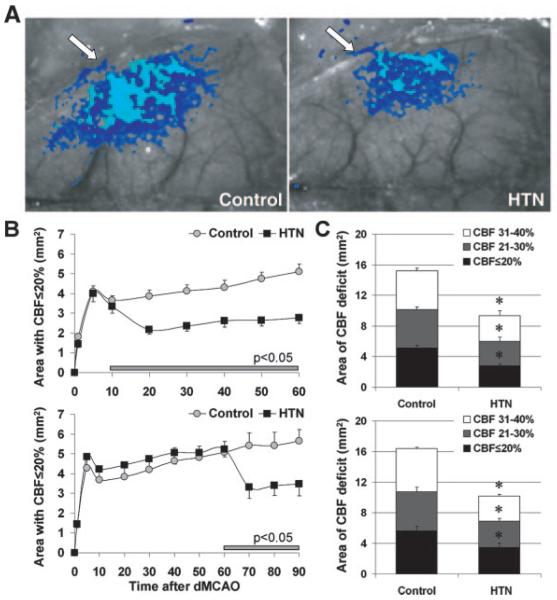
Mild induced hypertension at 10 or 60 minutes after dMCAO rapidly reduced the area of severe CBF deficit (ie, residual CBF ≤20%) by almost half (Figure 2A–B). The areas of moderate (21% to 30%) or mild CBF deficit (31% to 40%) were also reduced by induced hypertension (Figure 2C). Because isoflurane impairs CBF autoregulation, we repeated these experiments under α-chloralose anesthesia (n=5 and 4 control and hypertension, respectively), which is known to better preserve cerebrovascular physiology.34 Induced hypertension 10 minutes after dMCAO reduced the area of severe CBF deficit by 39% under α-chloralose anesthesia (versus 46% under isoflurane, P>0.05), suggesting that the benefit is not dependent on anesthetic choice.
Figure 2.
Mild induced hypertension decreases the area of hypoperfusion and preserves CBF. A, Representative speckle contrast images taken 60 minutes after dMCAO are shown from control and hypertension (HTN) groups. Superimposed in light and dark blue are pixels with CBF ≤20% and 21% to 30%, respectively. B, Time course of the area with CBF ≤20% in control and early (upper) or delayed (lower) hypertension groups. Phenylephrine infusion (intravenously, horizontal bar) rapidly reduced the area of severe CBF deficit and prevented the gradual expansion typically observed in control group (P<0.05 versus control). C, Composite bar graph showing the areas of severe, moderate, and mild CBF deficits (ie, residual CBF ≤20%, 21% to 30%, and 31% to 40%, respectively) in control and early (upper) or delayed (lower) hypertension groups, 60 or 90 minutes after dMCAO, respectively, showing 30% to 50% reduction. *P<0.05 versus control.
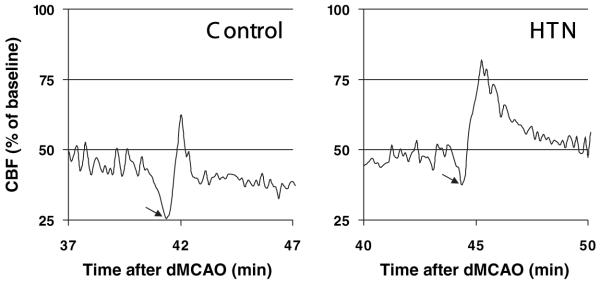
PIDs negatively impact CBF and oxygenation, and their suppression improves perfusion in ischemia.35,42 In control mice, spontaneous PIDs were detected at a frequency of 2.4±0.5/h; each PID transiently reduced CBF (Figure 3, arrow), oxyHb concentration, and CMRO2 in both the core and penumbra (Table 3). Mild induced hypertension did not alter the frequency of PIDs (2.4±0.6/h), but significantly reduced the magnitude of transient reductions in CBF, oxyHb, and CMRO2 during each PID (Figure 3; Table 3).
Figure 3.
Mild induced hypertension ameliorates the hypoperfusion during periinfarct depolarizations. Representative CBF tracings from the penumbra showing the transient hypoperfusion (arrows) during PIDs in control and hypertension (HTN) groups. Induced hypertension reduced the magnitude and duration of hypoperfusion during PIDs, thereby ameliorating the negative impact of PIDs on tissue oxygenation and CMRO2 (Table 3).
Table 3.
Impact of Induced Hypertension on Vasoconstrictive Coupling During PIDs in the Core and Penumbra
| Core |
Penumbra |
|||
|---|---|---|---|---|
| Control | Hypertension | Control | Hypertension | |
| CBF | −26±3% | −12±2%* | −37±4% | −19±3%* |
| OxyHb | −22±3% | −12±3%* | −60±6% | −32±4%* |
| CMRO2 | −20±2% | −9±2%* | −23±5% | −10±2%* |
Data are expressed as average percent change in measured parameters during a PID (see Figure 3 for representative tracings of CBF change during PIDs). All PIDs occurring during saline or phenylephrine infusion (starting 10 minutes after dMCAO) were averaged within each experiment.
P<0.05 versus control in both core and penumbra; n=14 each.
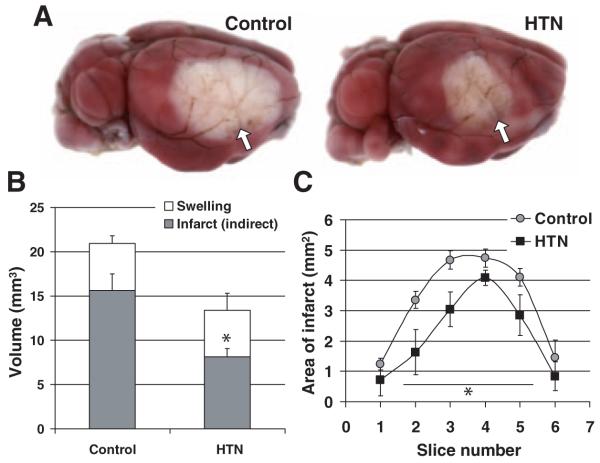
Mild induced hypertension during 1 hour dMCAO concentrically reduced infarct volume by 48% when assessed after 48 hours (n=7 and 5 control and hypertension, respectively; Figure 4); although the absolute volume of ischemic swelling was not exacerbated by induced hypertension during dM-CAO, it tended to be larger in proportion to the total infarct volume in the hypertension group (28±4% versus 36±6%, P=0.23).
Figure 4.
Mild induced hypertension decreases infarct volume. A, Representative topical 2,3,5-triphenyl-2H-tetrazolium chloride-stained brains from control and hypertension (HTN) groups demonstrating the cortical infarct 48 hours after 1 hour transient dMCAO. Block arrows show the dMCAO site. B, Phenylephrine infusion (n=5) during dMCAO (HTN) reduced infarct volume by 48% compared with saline controls (n=7) without increasing ischemic tissue swelling. C, Infarct areas (direct) were decreased in both anterior and posterior slice levels, suggesting concentric reduction (*P<0.05 versus control).
Discussion
This is the first detailed spatiotemporal investigation of the hemodynamic and metabolic impact of pharmacologically increased systemic blood pressure on the ischemic core and penumbra. Using high spatiotemporal resolution laser speckle flowmetry and multispectral reflectance imaging simultaneously, we found that increasing MAP by 30% 10 minutes after dMCAO improved CBF, oxygenation, and CMRO2 in both the core and penumbra; delayed intervention 60 minutes after dMCAO was equally efficacious. Consistent with a marked hemodynamic and metabolic improvement during induced hypertension, infarct volume was reduced by almost 50% without significantly exacerbating ischemic swelling. The concentric improvement in CBF and infarct volume suggested that raising MAP augments pial collateral flow from all 3 divisions of internal carotid artery, including the MCA branches proximal to the occlusion.
Although increased morbidity and mortality due to prolonged phenylephrine infusions in mice precluded testing longer treatment times in this study, prolonged vasopressor infusions are feasible and part of standard care in the clinical setting, for example, in neurocritical care of patients with subarachnoid hemorrhage-induced delayed vasospasm. Several anecdotal reports and small nonrandomized series have suggested that induced hypertension may improve cerebral perfusion and neurological deficits in acute stroke. For example, MAP elevation by 50% using phenylephrine acutely improved mean transit time on perfusion-weighted MRI and neurological deficits in a patient with embolic anterior cerebral artery A2 branch occlusion19; cerebral blood volume was not altered by this treatment, which is consistent with our findings. In a study of 19 patients with acute large MCA strokes, a 30% elevation of MAP caused a 35% increase in mean ipsilateral MCA flow velocities; the contralateral increase was only 17%, presumably due to intact cerebral autoregulation.20 In another small, randomized study, the volume of perfusion deficit on MRI significantly decreased from day 1 to 3 in patients who received induced hypertension treatment.26
Our understanding of the mechanisms by which hypertension improved tissue outcome has been limited. Although induced hypertension has reduced infarct size in several studies,1-3,12,43 its impact on cerebral perfusion has been assessed in only a few studies.1,2,4,16 In α-chloralose-anesthetized baboons, a 30% increase in blood pressure enhanced CBF by almost 60% when measured by hydrogen clearance method and partially restored cortical somatosensory evoked potentials.4 In pentobarbital-anesthetized dogs, induced hypertension augmented CBF in all animals studied.16 When measured using [14C]-iodoantipyrine autoradiography 15 minutes after proximal MCAO in rats, the volume of severely ischemic tissue (CBF ≤15 mL/100 g per minute) was decreased by a 30% increase in MAP.1 Our 2-dimensional analysis of CBF deficit using laser speckle flowmetry showed that the area of CBF deficit shrinks in a concentric manner on induced hypertension (Figure 2A). We, therefore, hypothesized that hypertension increases perfusion in the ischemic cortex through pial collaterals from ACA and PCA branches and confirmed this by making selective measurements from these collaterals; MCA flow immediately distal to the occlusion did not significantly increase, confirming complete occlusion. These data suggest that pial collaterals are the major source of CBF augmentation by induced hypertension in this dMCAO model.
The multispectral reflectance imaging demonstrated in real-time that improved CBF translates into increased oxygen delivery and higher CMRO2, 2 critical determinants of tissue viability. Moreover, oxygen metabolism progressively increases in penumbra over time and is improved even in the severely ischemic core. It should be noted, however, that the relative increase in CBF exceeded the increase in CMRO2, perhaps suggesting irreversible impairment in oxygen utilization in a subset of cells. Induced hypertension has previously been reported to reduce tissue lactate levels in focal ischemia,44 providing indirect evidence for utilization of increased oxygen delivery by ischemic brain metabolism. Consistent with this observation, induced hypertension decreased infarct volume by almost 50%. The volume of ischemic tissue swelling was not increased by hypertension, suggesting that any potential increase in edema formation by induced hypertension was offset by a net reduction in infarct volume. Consistent with these findings, published experimental data suggest that induced hypertension does not increase ischemic brain edema or intrainfarct hematoma formation and may be safe even when administered after reperfusion.12-15,45-48 We also found that the deleterious effect of PIDs on CBF and oxygenation were ameliorated by induced hypertension, providing an additional mechanism for metabolic improvement.
Although observational studies and small clinical trials in carefully selected patients suggest that induced hypertension is feasible and probably safe and effective, larger studies of unselected patients indicate that high blood pressure during acute ischemic stroke is associated with poor outcome,49-51 higher risk of early recurrence,49 hemorrhagic transformation,52 and malignant brain swelling53 in acute stroke. Clearly not all patients will benefit from induced hypertension. Patients with stroke with an identifiable blood pressure threshold above which neurological deficits improve19 are likely to benefit as may patients with significant diffusion–perfusion MRI mismatch. Our data suggest that improved cerebral perfusion and metabolism (eg, perfusion-weighted MRI and MR spectroscopy for tissue lactate) may provide a more sensitive measure than acute improvement in neurological examination for patient selection.
Summary
Our data strongly suggest that when instituted early after vascular occlusion, mild pharmacologically induced hypertension increases cerebral perfusion and oxygen delivery, improves CMRO2, and reduces infarct volume in this distal cortical branch occlusion model; the efficacy of induced hypertension in more proximal arterial occlusions (eg, filament MCAO) or when collateral channels are limited (eg, contralateral stenosis or occlusion), however, needs further testing. Improved understanding of the dynamics of collateral perfusion and risk factors for edema and hemorrhage will be critical to determine the usefulness of induced hypertension in acute stroke management.
Acknowledgments
Sources of Funding
Supported by the National Institutes of Health (P01NS055104, R01EB00790, P50NS10828, and PO1NS35611).
Footnotes
Disclosures
None.
References
- 1.Drummond JC, Oh YS, Cole DJ, Shapiro HM. Phenylephrine-induced hypertension reduces ischemia following middle cerebral artery occlusion in rats. Stroke. 1989;20:1538–1544. doi: 10.1161/01.str.20.11.1538. [DOI] [PubMed] [Google Scholar]
- 2.Smrcka M, Ogilvy CS, Crow RJ, Maynard KI, Kawamata T, Ames A., III Induced hypertension improves regional blood flow and protects against infarction during focal ischemia: time course of changes in blood flow measured by laser Doppler imaging. Neurosurgery. 1998;42:617–624. doi: 10.1097/00006123-199803000-00032. discussion 624–625. [DOI] [PubMed] [Google Scholar]
- 3.Chileuitt L, Leber K, McCalden T, Weinstein PR. Induced hypertension during ischemia reduces infarct area after temporary middle cerebral artery occlusion in rats. Surg Neurol. 1996;46:229–234. doi: 10.1016/0090-3019(95)00453-x. [DOI] [PubMed] [Google Scholar]
- 4.Hope DT, Branston NM, Symon L. Restoration of neurological function with induced hypertension in acute experimental cerebral ischaemia. Acta Neurol Scand Suppl. 1977;64:506–507. [PubMed] [Google Scholar]
- 5.Symon L, Crockard HA, Dorsch NW, Branston NM, Juhasz J. Local cerebral blood flow and vascular reactivity in a chronic stable stroke in baboons. Stroke. 1975;6:482–492. doi: 10.1161/01.str.6.5.482. [DOI] [PubMed] [Google Scholar]
- 6.Olsen TS, Larsen B, Herning M, Skriver EB, Lassen NA. Blood flow and vascular reactivity in collaterally perfused brain tissue. Evidence of an ischemic penumbra in patients with acute stroke. Stroke. 1983;14:332–341. doi: 10.1161/01.str.14.3.332. [DOI] [PubMed] [Google Scholar]
- 7.Symon L, Branston NM, Strong AJ. Autoregulation in acute focal ischemia. An experimental study. Stroke. 1976;7:547–554. doi: 10.1161/01.str.7.6.547. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira-Filho J, Silva SC, Trabuco CC, Pedreira BB, Sousa EU, Bacellar A. Detrimental effect of blood pressure reduction in the first 24 hours of acute stroke onset. Neurology. 2003;61:1047–1051. doi: 10.1212/01.wnl.0000092498.75010.57. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed N, Nasman P, Wahlgren NG. Effect of intravenous nimodipine on blood pressure and outcome after acute stroke. Stroke. 2000;31:1250–1255. doi: 10.1161/01.str.31.6.1250. [DOI] [PubMed] [Google Scholar]
- 10.Adams H, Adams R, Del Zoppo G, Goldstein LB. Guidelines for the early management of patients with ischemic stroke: 2005 guidelines update a scientific statement from the Stroke Council of the American Heart Association/American Stroke Association. Stroke. 2005;36:916–923. doi: 10.1161/01.STR.0000163257.66207.2d. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein LB. Blood pressure management in patients with acute ischemic stroke. Hypertension. 2004;43:137–141. doi: 10.1161/01.HYP.0000113297.76013.51. [DOI] [PubMed] [Google Scholar]
- 12.Cole DJ, Drummond JC, Osborne TN, Matsumura J. Hypertension and hemodilution during cerebral ischemia reduce brain injury and edema. Am J Physiol. 1990;259:H211–217. doi: 10.1152/ajpheart.1990.259.1.H211. [DOI] [PubMed] [Google Scholar]
- 13.Cole DJ, Schell RM, Drummond JC, Patel PM, Marcantonio S. Focal cerebral ischemia in rats: effect of phenylephrine-induced hypertension during reperfusion. J Neurosurg Anesthesiol. 1992;4:78–84. doi: 10.1097/00008506-199204000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Cole DJ, Matsumura JS, Drummond JC, Schell RM. Focal cerebral ischemia in rats: effects of induced hypertension, during reperfusion, on CBF. J Cereb Blood Flow Metab. 1992;12:64–69. doi: 10.1038/jcbfm.1992.8. [DOI] [PubMed] [Google Scholar]
- 15.Drummond JC, Oh YS, Cole DJ. The influence of phenylephrine-induced hypertension during focal cerebral ischemia on the formation of brain edema. J Neurosurg Anesthesiol. 1991;3:4–11. doi: 10.1097/00008506-199103000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Brawley BW, Strandness DE, Jr, Kelly WA. The physiologic response to therapy in experimental cerebral ischemia. Arch Neurol. 1967;17:180–187. doi: 10.1001/archneur.1967.00470260070008. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi S, Nehls DG, Kieck CF, Vielma J, DeGirolami U, Crowell RM. Beneficial effects of induced hypertension on experimental stroke in awake monkeys. J Neurosurg. 1984;60:151–157. doi: 10.3171/jns.1984.60.1.0151. [DOI] [PubMed] [Google Scholar]
- 18.Mistri AK, Robinson TG, Potter JF. Pressor therapy in acute ischemic stroke: systematic review. Stroke. 2006;37:1565–1571. doi: 10.1161/01.STR.0000222002.57530.05. [DOI] [PubMed] [Google Scholar]
- 19.Chalela JA, Dunn B, Todd JW, Warach S. Induced hypertension improves cerebral blood flow in acute ischemic stroke. Neurology. 2005;64:1979. doi: 10.1212/01.WNL.0000156360.70336.18. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz S, Georgiadis D, Aschoff A, Schwab S. Effects of induced hypertension on intracranial pressure and flow velocities of the middle cerebral arteries in patients with large hemispheric stroke. Stroke. 2002;33:998–1004. doi: 10.1161/01.str.0000014584.17714.2e. [DOI] [PubMed] [Google Scholar]
- 21.Marzan AS, Hungerbuhler HJ, Studer A, Baumgartner RW, Georgiadis D. Feasibility and safety of norepinephrine-induced arterial hypertension in acute ischemic stroke. Neurology. 2004;62:1193–1195. doi: 10.1212/01.wnl.0000118303.45735.04. [DOI] [PubMed] [Google Scholar]
- 22.Duke BJ, Breeze RE, Rubenstein D, Tranmer BI, Kindt GW. Induced hypervolemia and inotropic support for acute cerebral arterial insufficiency: an underused therapy. Surg Neurol. 1998;49:51–54. doi: 10.1016/s0090-3019(97)00353-4. discussion 54–57. [DOI] [PubMed] [Google Scholar]
- 23.Hillis AE, Barker PB, Beauchamp NJ, Winters BD, Mirski M, Wityk RJ. Restoring blood pressure reperfused Wernicke’s area and improved language. Neurology. 2001;56:670–672. doi: 10.1212/wnl.56.5.670. [DOI] [PubMed] [Google Scholar]
- 24.Rordorf G, Koroshetz WJ, Ezzeddine MA, Segal AZ, Buonanno FS. A pilot study of drug-induced hypertension for treatment of acute stroke. Neurology. 2001;56:1210–1213. doi: 10.1212/wnl.56.9.1210. [DOI] [PubMed] [Google Scholar]
- 25.Hillis AE, Kane A, Tuffiash E, Ulatowski JA, Barker PB, Beauchamp NJ, Wityk RJ. Reperfusion of specific brain regions by raising blood pressure restores selective language functions in subacute stroke. Brain Lang. 2001;79:495–510. doi: 10.1006/brln.2001.2563. [DOI] [PubMed] [Google Scholar]
- 26.Hillis AE, Ulatowski JA, Barker PB, Torbey M, Ziai W, Beauchamp NJ, Oh S, Wityk RJ. A pilot randomized trial of induced blood pressure elevation: effects on function and focal perfusion in acute and subacute stroke. Cerebrovasc Dis. 2003;16:236–246. doi: 10.1159/000071122. [DOI] [PubMed] [Google Scholar]
- 27.Hillis AE, Wityk RJ, Beauchamp NJ, Ulatowski JA, Jacobs MA, Barker PB. Perfusion-weighted MRI as a marker of response to treatment in acute and subacute stroke. Neuroradiology. 2004;46:31–39. doi: 10.1007/s00234-002-0918-4. [DOI] [PubMed] [Google Scholar]
- 28.Bogoslovsky T, Happola O, Salonen O, Lindsberg PJ. Induced hypertension for the treatment of acute MCA occlusion beyond the thrombolysis window: case report. BMC Neurol. 2006;6:46. doi: 10.1186/1471-2377-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wise G, Sutter R, Burkholder J. The treatment of brain ischemia with vasopressor drugs. Stroke. 1972;3:135–140. doi: 10.1161/01.str.3.2.135. [DOI] [PubMed] [Google Scholar]
- 30.Meier F, Wessel G, Thiele R, Gottschild D, Brandstatt H. Induced hypertension as an approach to treating acute cerebrovascular ischaemia: possibilities and limitations. Exp Pathol. 1991;42:257–263. doi: 10.1016/s0232-1513(11)80079-4. [DOI] [PubMed] [Google Scholar]
- 31.Olsen TS. Should induced hypertension or hypotension ever be used in the treatment of stroke? Acta Med Scand Suppl. 1983;678:113–120. doi: 10.1111/j.0954-6820.1984.tb08669.x. [DOI] [PubMed] [Google Scholar]
- 32.Ruth B. Blood flow determination by the laser speckle method. Int J Microcirc Clin Exp. 1990;9:21–45. [PubMed] [Google Scholar]
- 33.Dunn AK, Devor A, Bolay H, Andermann ML, Moskowitz MA, Dale AM, Boas DA. Simultaneous imaging of total cerebral hemoglobin concentration, oxygenation, and blood flow during functional activation. Opt Lett. 2003;28:28–30. doi: 10.1364/ol.28.000028. [DOI] [PubMed] [Google Scholar]
- 34.Ayata C, Dunn AK, Gursoy OY, Huang Z, Boas DA, Moskowitz MA. Laser speckle flowmetry for the study of cerebrovascular physiology in normal and ischemic mouse cortex. J Cereb Blood Flow Metab. 2004;24:744–755. doi: 10.1097/01.WCB.0000122745.72175.D5. [DOI] [PubMed] [Google Scholar]
- 35.Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata C. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab. 2006;26:1018–1030. doi: 10.1038/sj.jcbfm.9600252. [DOI] [PubMed] [Google Scholar]
- 35a.Jones PB, Shin HK, Boas DA, Hyman BT, Moskowitz MA, Ayata C, Dunn AK. Simultaneous multispectral reflectance imaging and laser speckle flowmetry of cerebral blood flow and oxygen metabolism in focal cerebral ischemia. J Biomed Opt. 2008;13 doi: 10.1117/1.2950312. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunn AK, Bolay H, Moskowitz MA, Boas DA. Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab. 2001;21:195–201. doi: 10.1097/00004647-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Briers JD. Laser Doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiol Meas. 2001;22:R35–66. doi: 10.1088/0967-3334/22/4/201. [DOI] [PubMed] [Google Scholar]
- 38.Dunn AK, Devor A, Dale AM, Boas DA. Spatial extent of oxygen metabolism and hemodynamic changes during functional activation of the rat somatosensory cortex. Neuroimage. 2005;27:279–290. doi: 10.1016/j.neuroimage.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 39.Jones M, Berwick J, Johnston D, Mayhew J. Concurrent optical imaging spectroscopy and laser-Doppler flowmetry: the relationship between blood flow, oxygenation, and volume in rodent barrel cortex. Neuroimage. 2001;13:1002–1015. doi: 10.1006/nimg.2001.0808. [DOI] [PubMed] [Google Scholar]
- 40.Mayhew J, Johnston D, Berwick J, Jones M, Coffey P, Zheng Y. Spectroscopic analysis of neural activity in brain: increased oxygen consumption following activation of barrel cortex. Neuroimage. 2000;12:664–675. doi: 10.1006/nimg.2000.0656. [DOI] [PubMed] [Google Scholar]
- 41.Strong AJ, Bezzina EL, Anderson PJ, Boutelle MG, Hopwood SE, Dunn AK. Evaluation of laser speckle flowmetry for imaging cortical perfusion in experimental stroke studies: quantitation of perfusion and detection of peri-infarct depolarisations. J Cereb Blood Flow Metab. 2006;26:645–653. doi: 10.1038/sj.jcbfm.9600240. [DOI] [PubMed] [Google Scholar]
- 42.Shin HK, Dunn AK, Jones PB, Boas DA, Lo EH, Moskowitz MA, Ayata C. Normobaric hyperoxia improves cerebral blood flow and oxygenation, and inhibits peri-infarct depolarizations in experimental focal ischaemia. Brain. 2007;130:1631–1642. doi: 10.1093/brain/awm071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cole DJ, Drummond JC, Shapiro HM, Hertzog RE, Brauer FS. The effect of hypervolemic hemodilution with and without hypertension on cerebral blood flow following middle cerebral artery occlusion in rats anesthetized with isoflurane. Anesthesiology. 1989;71:580–585. doi: 10.1097/00000542-198910000-00017. [DOI] [PubMed] [Google Scholar]
- 44.Aspey BS, Ehteshami S, Hurst CM, McCoy AL, Harrison MJ. The effect of increased blood pressure on hemispheric lactate and water content during acute cerebral ischaemia in the rat and gerbil. J Neurol Neurosurg Psychiatry. 1987;50:1493–1498. doi: 10.1136/jnnp.50.11.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drummond JC, Oh YS, Cole DJ. Does phenylephrine-induced hypertension during focal cerebral ischemia aggravate brain edema? J Neurosurg Anesthesiol. 1989;1:120–121. doi: 10.1097/00008506-198906000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Cole DJ, Drummond JC, Ruta TS, Peckham NH. Hemodilution and hypertension effects on cerebral hemorrhage in cerebral ischemia in rats. Stroke. 1990;21:1333–1339. doi: 10.1161/01.str.21.9.1333. [DOI] [PubMed] [Google Scholar]
- 47.Patel PM, Drummond JC, Cole DJ. Induced hypertension during restoration of flow after temporary middle cerebral artery occlusion in the rat: effect on neuronal injury and edema. Surg Neurol. 1991;36:195–201. doi: 10.1016/0090-3019(91)90112-m. [DOI] [PubMed] [Google Scholar]
- 48.Patel PM, Drummond JC, Cole DJ, Giamela R, Steinauer J. Delayed institution of hypertension during focal cerebral ischemia: effect on brain edema. Acta Neuropathol (Berl) 1991;81:339–344. doi: 10.1007/BF00305878. [DOI] [PubMed] [Google Scholar]
- 49.Leonardi-Bee J, Bath PM, Phillips SJ, Sandercock PA. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke. 2002;33:1315–1320. doi: 10.1161/01.str.0000014509.11540.66. [DOI] [PubMed] [Google Scholar]
- 50.Schrader J, Luders S, Kulschewski A, Berger J, Zidek W, Treib J, Einhaupl K, Diener HC, Dominiak P. The access study: evaluation of acute candesartan cilexetil therapy in stroke survivors. Stroke. 2003;34:1699–1703. doi: 10.1161/01.STR.0000075777.18006.89. [DOI] [PubMed] [Google Scholar]
- 51.Willmot M, Leonardi-Bee J, Bath PM. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension. 2004;43:18–24. doi: 10.1161/01.HYP.0000105052.65787.35. [DOI] [PubMed] [Google Scholar]
- 52.Fagan SC, Bowes MP, Lyden PD, Zivin JA. Acute hypertension promotes hemorrhagic transformation in a rabbit embolic stroke model: effect of labetalol. Exp Neurol. 1998;150:153–158. doi: 10.1006/exnr.1997.6756. [DOI] [PubMed] [Google Scholar]
- 53.Krieger DW, Demchuk AM, Kasner SE, Jauss M, Hantson L. Early clinical and radiological predictors of fatal brain swelling in ischemic stroke. Stroke. 1999;30:287–292. doi: 10.1161/01.str.30.2.287. [DOI] [PubMed] [Google Scholar]