Abstract
Alphaviruses are enveloped arboviruses. The viral envelope is derived from the host cell and is positioned between two icosahedral protein shells (T = 4). Because the viral envelope contains glycoproteins involved in cell recognition and entry, the integrity of the envelope is critical for the success of the early events of infection. Differing levels of cholesterol in different hosts leads to the production of alphaviruses with distinct levels of this sterol loaded in the envelope. Using Mayaro virus, a New World alphavirus, we investigated the role of cholesterol on the envelope of alphavirus particles assembled in either mammalian or mosquito cells. Our results show that although quite different in their cholesterol content, Mayaro virus particles obtained from both cells share a similar high level of lateral organization in their envelopes. This organization, as well as viral stability and infectivity, is severely compromised when cholesterol is depleted from the envelope of virus particles isolated from mammalian cells, but virus particles isolated from mosquito cells are relatively unaffected by cholesterol depletion. We suggest that it is not cholesterol itself, but rather the organization of the viral envelope, that is critical for the biological activity of alphaviruses.
Keywords: Cholesterol, Lipid Raft, Membrane Structure, Virus Entry, Virus Structure, Laurdan Fluorescence, Lipid Lateral Organization, Mayaro Virus
Introduction
Viral infection is a complex process that includes many steps and interactions of viral components with different cellular structures. The entry step is a critical early event of the infection cycle and for enveloped viruses, it relies on the fusion of the viral envelope with either the endosomal or plasma membrane of the cell. Lipid composition, of both the viral envelope and host cell membrane, seems to play an important role in the virus infection cycle.
Mayaro virus (MAYV)3 is a member of the genus Alphavirus in the family Togaviridae. MAYV infection is widespread and endemic in South America, and it results in a dengue-like acute febrile illness (1). Although most cases of human infection are sporadic, the possibility that MAYV can infect and be transmitted by Aedes aegypti has been suggested and may be responsible for an increase in MAYV infection, which is considered one of the major emerging diseases in the neotropics (2). Moreover, imported cases of MAYV infection were reported recently in two European countries (3, 4), revealing the potential for this virus to break geographical barriers and reach non-endemic areas.
Alphaviruses are enveloped arboviruses, maintained in nature by replicating alternately in a hematophagous arthropod, usually a mosquito, and a vertebrate host, usually mammals and birds. The viral envelope is acquired during the process of budding from a cellular membrane and plays an important role during virus entry into the host cell, which is thought to occur by receptor-mediated endocytosis followed by fusion of the viral envelope and the endosomal membrane (5) or by direct penetration from the cell surface in the absence of membrane fusion (6). Although the overall infection cycle in mammalian and mosquito cells is similar, there seem to be some different requirements for cellular factors from the host cell, as is illustrated by cholesterol. For example, the fusion reaction mediated by alphavirus glycoproteins shows a strong dependence on cholesterol in the target membrane, as exemplified by the prototypical alphaviruses Sindbis virus (SINV) and Semliki Forest virus (SFV) when infecting mammalian cells (7, 8). Moreover, the process of budding is also highly dependent on the presence of cholesterol in the mammalian cell membrane (9, 10), resulting in a high loading of this lipid in the viral envelope. However, mosquitoes are cholesterol auxotrophs and contain only low levels of this sterol, typically provided by the blood meal diet in females host cells, and it is still unclear how alphavirus particles overcome the requirement for cholesterol in mosquito cells (11).
Many viruses have been shown to require cholesterol in either the viral envelope or the endosomal/plasma membrane (or both) for infection. The infectivity of both influenza virus type A and human herpesvirus 6 (HHV-6) is notably affected by cholesterol depletion from their envelopes (12, 13). Vaccinia virus (VACV) and varicella-zoster virus (VZV) show a requirement for cholesterol in the plasma membrane for efficient penetration into the host cell (14, 15). Human immunodeficiency virus type 1 (HIV-1) and herpes simplex virus type 1 (HSV-1) entry has also been shown to require cholesterol in both the target and viral membranes (16–21). This cholesterol requirement may involve the participation of raft domains in the infection process. Interestingly, a recent study on the prototypical Alphavirus, SINV, suggested that the amount of cholesterol in the host cell membrane and in virus particles has no effect on the ability of the virus to infect cells, and the dependence of cholesterol for infectivity reflects differences in cell lines and methodologies used in previous works (22).
Although the requirement of host cholesterol for alphavirus infection in mammalian and mosquito cells has been discussed by many groups, little is known about the effect that this sterol may have when incorporated in the envelope of the virus particles produced in these distinct cells. In this work, we aimed to investigate this issue using MAYV as a model. Our results show that, despite the great difference in their cholesterol content, the envelopes of MAYV particles obtained from both mammalian and mosquito cells are highly ordered, as assessed by Laurdan fluorescence. Viral stability and infectivity is severely compromised when the envelope phase is changed by cholesterol depletion using methyl-β-cyclodextrin (MβCD), which occurs only for MAYV isolated from mammalian cells. Because cholesterol depletion from MAYV isolated from mosquito cells led only to a decrease in the lipid-packing of the envelope without affecting its phase, we suggest that a factor other than cholesterol is provided by mosquito cells and accounts for an increased envelope lateral organization of MAYV produced in these cells.
EXPERIMENTAL PROCEDURES
Cells and Viruses
Baby hamster kidney (BHK-21) and African green monkey kidney (Vero) cells were cultured as monolayers at 37 °C with 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) (Sigma) supplemented with 10% fetal bovine serum (Cultilab, Campinas, SP, Brazil), 3% tryptose phosphate (Difco, Detroit, MI), 100 units of penicillin/ml, and 100 μg streptomycin/ml (Invitrogen, Carlsbad, CA). Aedes albopictus larvae (C6/36) cells were adherently cultured at 28 °C in Leibovitz (L15) medium (Invitrogen) supplemented with 5% fetal bovine serum, 3% tryptose phosphate, 100 units of penicillin/ml, 100 μg of streptomycin/ml, and 2 mm l-glutamine. MAYV (VR-1277) was obtained from the American Type Culture Collection (Manassas, VA).
Virus Propagation and Purification
Mayaro virus was propagated in BHK-21 cells for 48 h at 37 °C and in C6/36 cells for 72 h at 28 °C. The virus was purified as described previously (23) with some modifications. After propagation, the supernatant was collected and cleared of cellular debris by centrifuging at 8,000 rpm for 20 min at 4 °C in a RPR 12–2 rotor. The supernatant was applied to a 30% sucrose cushion and centrifuged in a Beckman 45 Ti rotor at 32,000 rpm for 1 h 40 min at 4 °C. The pellet was suspended in TNE buffer (10 mm Tris, 100 mm NaCl, 1 mm EDTA), layered onto a discontinuous 5–50% sucrose density gradient and centrifuged at 30,000 rpm for 1 h 30 min at 4 °C in a Beckman SW 41 rotor. Fractions were collected and the fraction containing the virus was identified by reading the optical density at 260 and 280 nm. To avoid the problems caused by freezing and thawing, purified virions were maintained at 4 °C and used within 2 weeks.
Cholesterol Depletion
Cholesterol depletion from the viral envelope was performed by incubating viruses at 100 μg of viral proteins/ml with the indicated concentration of methyl-β-cyclodextrin (MβCD, Sigma) for 30 min at 37 °C. After incubation, cyclodextrin was removed by centrifugation in a YM-100 Microcon filter device (Millipore, Billerica, MA).
Cholesterol Content Measurement
The cholesterol content was determined using an Amplex Red cholesterol assay kit (Molecular Probes, Eugene, OR) according to the manufacturer's instructions. For the measurement of viral envelope cholesterol content, virus (100 μg/ml) was concentrated by centrifugation in a YM-100 Microcon filter device, resuspended in Amplex Red reaction buffer and analyzed on a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA) by reading its absorbance at 544 nm.
Virus Titer Determination
Virus titer was determined by plaque assay in Vero cells. Briefly, confluent monolayers of Vero cells in multiwell plates were infected with serial dilutions of Mayaro virus and incubated for 60 min at 37 °C in a 5% CO2 incubator. After this period, non-adsorbed virus was removed, and a semisolid medium (1.6% carboxymethylcellulose in DMEM) was added. Plates were incubated at 37 °C in 5% CO2 for 48 h. After the removal of the semisolid medium, the cells were stained with 1% crystal violet, and plaques were counted.
Spectrofluorimetric Analysis of Laurdan Fluorescence
The packing level of the Mayaro viral envelope was inferred by means of Laurdan (2-dimethylamino-6-lauroylnaphthalene, Molecular Probes) fluorescence. Briefly, viral samples (100 μg/ml) were incubated with 20 μm Laurdan for 30 min at 4 °C and analyzed on a spectrofluorimeter PC1 (ISS, Champaign, IL). The samples were excited at 360 nm, and the fluorescence emission was scanned from 380 to 600 nm. The assay was carried out at 37 °C, with constant stirring. Generalized polarization (GP) values were calculated as a function of the fluorescence emission intensities at 440 and 490 nm, based on Equations 1 and 2,
where
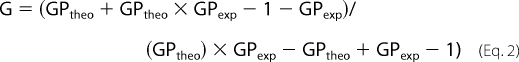 |
GPtheo corresponds to the known GP value of a standard solution of Laurdan in DMSO at 22 °C (0.207), and GPexp corresponds to the experimentally determined GP value of the standard solution (24).
Negative-staining Electron Microscopy
Virus samples were prepared for negative-staining electron microscopy by placing them onto a Formvar-coated hexagonal (300-hex) Cu grid, allowing them to adhere for 1 min, and immediately staining them for 1 s with 2% aqueous uranyl acetate. After a drying period, the grid was viewed on a Morgani electron microscope (FEI Co., Hillsboro, OR) operated at 100 kV. After scanning the negatives, images were processed with Adobe Photoshop (Adobe Systems Inc., Mountain View, CA).
Analysis of Viral RNA Leakage from Samples Treated with MβCD
Mayaro virus (100 μg/ml) was treated with 25 mm MβCD as described above. A control sample was maintained under similar conditions in the absence of the drug. Subsequently, viral samples were centrifuged in Microcon-PCR filter devices (Millipore), resuspended in TE buffer (10 mm Tris-HCl, 1 mm EDTA, pH 7.5) and incubated for 5 min at 4 °C with RiboGreen (Molecular Probes) according to the manufacturer's instructions. The samples were then analyzed with a PC1 spectrofluorimeter (ISS, Champaign, IL) set for excitation at 480 nm and emission scanning from 500 nm to 600 nm.
RESULTS
MAYV Particles Isolated from Mammalian and Mosquito Cells Differ in Their Cholesterol Content but Not in Their Infectivity
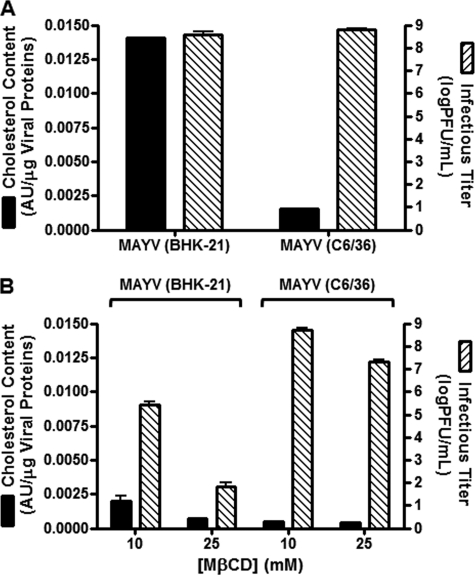
BHK-21 (mammalian) and C6/36 (mosquito) cells were infected with MAYV and the virus progenies were purified and assayed for cholesterol content (relative to total viral protein) and infectious titer (Fig. 1A). Viruses produced in mammalian cells have a 10-fold higher cholesterol content than viruses produced in mosquito cells. It is important to highlight that mosquito cells were grown in culture medium supplemented with fetal bovine serum. No delipidation method was used, and the difference in cholesterol content reflects what is incorporated into the viral envelope with regular host cell metabolism when all the lipids are provided by the serum supplementation used. When analyzed for infectivity using a plaque assay, these virus samples did not significantly differ in their infectious titer. These results show that MAYV infection in mammalian cells produces high cholesterol content particles (HCCPs), while MAYV infection in mosquito cells produces low cholesterol content particles (LCCPs), and despite this difference, both virus progenies are similarly infectious. This suggests that a high cholesterol content in the viral envelope is not essential for the biological activity of MAYV.
FIGURE 1.
Cholesterol content and infectivity of MAYV and the effect of cholesterol depletion from the viral envelope on these parameters. MAYV particles isolated from BHK-21 (HCCPs) and C6/36 (LCCPs) cells were assayed for cholesterol content using a cholesterol assay kit and for infectivity by a plaque assay in Vero cells (A). HCCPs and LCCPs were subjected to cholesterol depletion by 10 or 25 mm MβCD and, after removal of the drug, assayed for cholesterol content and infectivity as before (B). PFU: plaque-forming units. AU: absorbance units.
Depletion of Envelope Cholesterol Has Different Effects on HCCPs and LCCPs
As a first measure of the importance of envelope cholesterol in HCCPs and LCCPs, we next assayed the relative cholesterol depletion promoted by MβCD treatment and its effect on virus infectivity. Purified HCCPs and LCCPs were treated with two different concentrations of MβCD for 30 min (Fig. 1B) and analyzed for cholesterol content and infectious titer as before. To prevent changes in cellular membranes caused by MβCD, it was removed with a filter before virions were added to the cells. This procedure was also performed on control virus samples without affecting their infectious titer (data not shown). Exposure to MβCD resulted in cholesterol depletion to very low levels in both virus samples (to ∼5% of the original cholesterol content of HCCPs when incubated with 25 mm MβCD). However, this led to a large drop in virus infectivity only for HCCPs (7 logs for HCCPs and 1 log for LCCPs with 25 mm MβCD). It is noteworthy that although HCCPs treated with 10 mm MβCD contain almost the same amount of cholesterol as untreated LCCPs (compare Figs. 1, A and B), the infectious titer of the former is 4 logs lower than that of the latter. Infectivity of LCCPs was not affected by exposure to 10 mm MβCD. These results suggest that envelope cholesterol is only important for MAYV infectivity when virions are assembled with a high content of this sterol, as occurs when viruses are produced in mammalian cells.
HCCPs and LCCPs Share a Similar High Lipid-packing in Their Envelopes but Only HCCPs Are Disturbed by Cholesterol Depletion
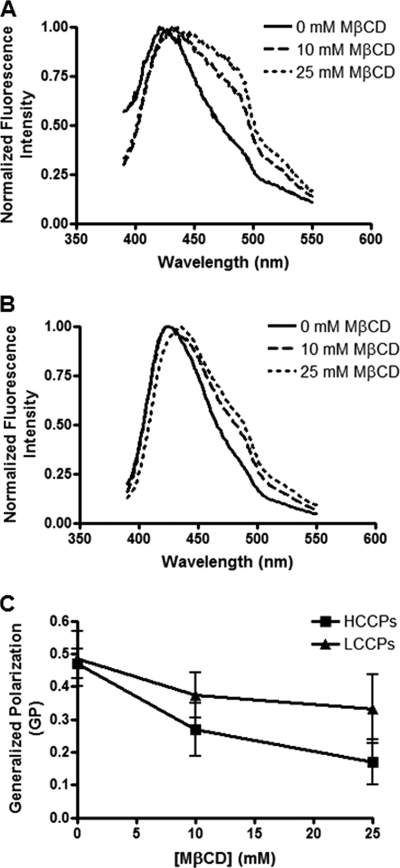
Given the differences in cholesterol content between HCCP and LCCP envelopes, we next assessed the lateral organization of these lipid bilayers as well as the effect of cholesterol depletion on this organization by Laurdan fluorescence analysis (Fig. 2). Laurdan is a fluorescent lipophilic probe that detects changes in membrane phase properties through its sensitivity to the polarity of its environment in the lipid bilayer (25). Variations in the access of water molecules to the membrane due to an altered phase state cause shifts in the Laurdan fluorescence emission spectrum. For example, the Laurdan fluorescence emission spectrum is blue-shifted with increasing membrane condensation, as a consequence of the exclusion of water molecules from the lipid bilayer (26). Quantification of membrane order is achieved by computing the generalized polarization (GP) value, which can theoretically range from −1, corresponding to the most fluid regions, to +1, corresponding to the most condensed ones (24). Lipid phase boundaries for GP values have previously been estimated using liposomes with lipid compositions similar to those of cellular membranes. In such liposomes, GP values below −0.05 and above 0.55 represent membranes in fluid and gel phases, respectively, while GP values between −0.05 and 0.25 and between 0.25 and 0.55 represent liquid-disordered/nonraft and liquid-ordered/raft domains, respectively (27). Recently, Laurdan fluorescence was used to characterize the lipid-packing of the envelope of HIV-1 (28).
FIGURE 2.
Lateral organization of the MAYV envelope and the effect of cholesterol depletion on this parameter. HCCPs (A) and LCCPs (B) were treated with 0, 10 or 25 mm MβCD and, after removal of the drug, incubated with Laurdan. Samples were then analyzed on a spectrofluorimeter at 37 °C using an excitation wavelength of 360 nm and scanning the fluorescence emission from 390 to 550 nm. Generalized polarization (GP) values from these samples were calculated as a function of the fluorescence emission intensities at 440 and 490 nm, as described under “Experimental Procedures” (C).
As shown in Fig. 2, A and B, cholesterol depletion from viral envelopes with MβCD led to a red-shift in the Laurdan fluorescence emission maximum for both HCCPs and LCCPs, suggesting a positive influence of cholesterol in viral envelope organization as expected. Surprisingly, despite the difference in cholesterol content, the envelopes of HCCPs and LCCPs both had a Laurdan GP value of ∼0.48 (Fig. 2C), which corresponds to membranes in a liquid-ordered phase state. Upon cholesterol depletion with 10 mm MβCD, Laurdan GP values dropped to ∼0.27 for HCCPs and ∼0.37 for LCCPs, thus the phase state of the envelopes of both types of particles was not affected. However, when virions were exposed to 25 mm MβCD, Laurdan GP values dropped to ∼0.17 for HCCPs and ∼0.33 for LCCPs. Although the final Laurdan GP value still corresponds to a liquid-ordered membrane phase state for LCCPs, it represents a transition to a liquid-disordered state for HCCPs. As membrane phase state is influenced by temperature, it is important to highlight that Laurdan GP values for both HCCPs and LCCPs were measured at 37 °C. These results indicate that although both HCCPs and LCCPs share a liquid-ordered envelope phase state, cholesterol is responsible for the maintenance of this property only in HCCPs, suggesting that a different molecular factor plays this function in LCCPs.
Cholesterol Depletion Affects the Physical Stability of HCCPs More Than LCCPs
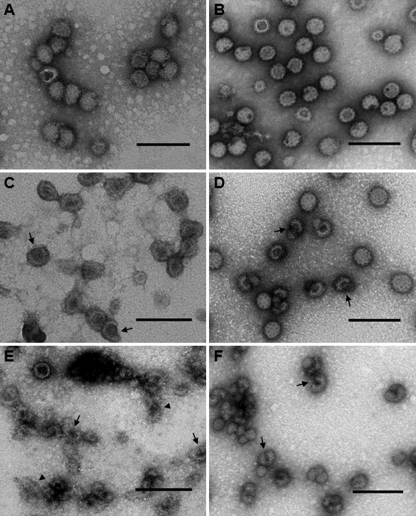
To examine the effects of cholesterol depletion on the physical stability of MAYV particles, MβCD-treated and mock-treated HCCPs and LCCPs were subjected to negative-staining electron microscopy (Fig. 3). As shown in Fig. 3, A and B, mock-treated HCCPs and LCCPs are similar in their physical stability. However, HCCPs appear to be slightly larger in diameter than LCCPs, which is consistent to what was recently shown for SINV produced from mammalian and mosquito cells (29). Upon treatment with 10 or 25 mm MβCD to deplete cholesterol from the viral envelopes, this stability is disturbed and the overall integrity of some of the virus particles is compromised (Fig. 3, C–F). These effects are more pronounced in HCCPs than in LCCPs, and are evident in the formation of breaks in the viral envelope, which results in the nucleocapsid leaking into the surrounding environment (arrows in Fig. 3, C–F). Treatment with 25 mm MβCD led to an almost complete dissolution of the envelopes of HCCPs, giving rise to amorphous material, presumably membrane debris (arrowheads in Fig. 3E). On the other hand, LCCPs treated with the same concentration of MβCD, resulted in less disturbed membrane structures (Fig. 3F). These results indicate that the physical stability of HCCPs is more sensitive to cholesterol depletion than that of LCCPs. The effects of cholesterol depletion on the physical stability of HCCPs were further analyzed by atomic force microscopy (supplemental Fig. S1), revealing a high correlation to the data obtained by negative-staining electron microscopy. Again, physical destabilization of HCCPs with leakage of their nucleocapsids into the environment was observed when virus particles were treated with 10 or 25 mm MβCD, also leading to the deposition of amorphous materials on the mica surface when treated with the highest MβCD concentration (supplemental Fig. S1).
FIGURE 3.
Effect of cholesterol depletion on MAYV structure as assessed by negative-staining electron microscopy. HCCPs (A, C, and E) and LCCPs (B, D, and F) were treated with 0 (A and B), 10 (C and D), or 25 (E and F) mm MβCD, prepared for negative staining and then analyzed by transmission electron microscopy. Arrows in panels C–F point to breaks in the viral envelope from which nucleocapsids leaked into the surrounding environment, while arrowheads in panel E indicate probable membrane debris from severely damaged virus particles. Bars: 100 nm.
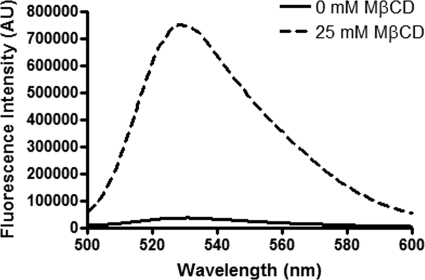
To confirm that cholesterol depletion disrupts the integrity of HCCP envelope, exposing the nucleocapsid, we performed a fluorescence assay with RiboGreen. RiboGreen is a fluorescent dye used in the detection of nucleic acids, including both RNA and DNA. This probe is almost non-fluorescent when free in solution, but it shows a large increase in fluorescence intensity when bound to nucleic acids (30). Because alphavirus nucleocapsids are fenestrated, the viral RNA genome becomes accessible to small molecules once the envelope is partially or totally disrupted (5). Thus, HCCPs were treated with 25 mm MβCD or mock-treated and then assayed for RiboGreen binding by spectrofluorimetry (Fig. 4). As expected, due to the barrier imposed by the viral envelope, mock-treated HCCPs showed very low fluorescence intensity (solid line in Fig. 4). A large increase in fluorescence intensity was observed when virus particles were treated with 25 mm MβCD (dashed line in Fig. 4), thus confirming the disturbance of HCCP envelope integrity upon cholesterol depletion.
FIGURE 4.
Accessibility of MAYV RNA genome upon cholesterol depletion from viral envelope. HCCPs were treated with 0 or 25 mm of MβCD and, after removal of the drug, incubated with RiboGreen. Samples were then analyzed on a spectrofluorimeter using an excitation wavelength of 480 nm and scanning the fluorescence emission from 500 to 600 nm.
DISCUSSION
In the work presented here, rather than focusing on the requirement of cholesterol from host cells for the infection process of alphaviruses, we aimed to investigate the role of cholesterol when this sterol is loaded in the alphavirus envelope, using MAYV as a model. Due to the arboviral nature of alphaviruses, we decided to carry out this study with MAYV isolated from cells from its two typical hosts: mammal and mosquito. Our initial results confirmed a striking difference between the cholesterol content of alphavirus particles produced in these different host cells, as was shown recently for SINV (22). Because we observed that MAYV infection in mammalian cells gives rise to progeny virus particles with a cholesterol content in their envelopes 10-fold higher than envelopes of viruses isolated from infected mosquito cells, we called the former high cholesterol content particles (HCCPs) and the latter low cholesterol content particles (LCCPs).
The high cholesterol content in HCCP envelopes can be explained by the well-established requirement of this lipid for efficient alphavirus budding from the plasma membrane of mammalian cells (31), which constitutively synthesize this steroid. On the other hand, the low level of cholesterol present in LCCP envelopes is as expected, as mosquito cells are cholesterol auxotrophs (11). Thus, all cholesterol found in LCCPs has an exogenous origin, typically the blood-meal diet in vivo and the serum supplementation in vitro. It has previously been shown that alphaviruses are able to replicate in vitro in mosquito cells completely devoid of cholesterol (22) and in vivo in male mosquitoes (32), which feed on plant sap and therefore do not ingest cholesterol during feeding. However, we decided to use mosquito cells that were supplied cholesterol in the culture medium because these viruses naturally infect female mosquitoes, which feed on animal blood and thus provide cholesterol molecules to their cells.
Despite the differences in cholesterol content, we have shown that these virus progenies share similar high infectious titers and, surprisingly, very similar envelope lipid-packing. Interestingly, the envelope phase state of both HCCPs and LCCPs, inferred based on generalized polarization (GP) values computed from Laurdan fluorescence, is equivalent to that of raft domains in cellular plasma membranes. Determination of membrane organization through Laurdan GP has an intrinsic advantage: this lipophilic probe distributes equally into fluid or condensed membranes and does not associate with specific fatty acids or phospholipid head groups (33). Therefore, GP values reflect the overall membrane structure and not a specific lipid or protein composition (26). High lipid-packing in viral envelopes was previously reported for influenza virus and HIV-1 (28, 34), which are believed to select raft domains during budding from the plasma membrane (35, 36). Additionally, similar to what we observed for MAYV, Scheiffele et al. (34) showed that the SFV envelope is highly ordered. Together, previous work and the work presented here suggest that highly ordered viral envelopes may be a general characteristic of Alphavirus genus members and that, perhaps they too select highly ordered domains on cellular membranes for budding. The selection of ordered domains from insect cells for virus budding may seem surprising, however, we show here for the first time that the envelopes of alphavirus particles grown in insect cells show levels of membrane organization comparable to that observed in cholesterol enriched envelopes obtained from mammalian cells.
Consistent with our suggestion that viruses in insect cells select ordered domains for viral budding, it was previously shown that the alphavirus envelope glycoproteins E1 and E2 undergo post-translational processing, with the addition of palmitic acid (5). This palmitoylation is important for transporting E1 and E2 to the cellular membrane and for the virus particle budding process (37). Palmitoylation of proteins is an address label to send them to highly ordered membrane regions, such as the lipid rafts previously mentioned (38). E2 palmitoylation also provides a lipid anchor to other saturated chain fatty acids and cholesterol in the membrane during viral budding, increasing viral stability as has been proposed for hemagglutinin (HA) of influenza virus (39). Thus, it is likely that the high degree of order found in the envelope of MAYV is a characteristic that remains from these highly ordered membrane regions.
Although we cannot exclude that the high density of viral glycoproteins embedded in the alphavirus envelope alone could account for the maintenance of this membrane in an ordered state, our data highlight the role of cholesterol in maintaining this order. Upon cholesterol depletion, HCCP envelopes undergo a transition to a liquid-disordered phase state, while LCCP envelopes remain in a liquid-ordered state. Previous studies indicate that the increased microviscosity of alphavirus membranes appears to result from the interaction of viral proteins with cellular lipids selected during virus budding (40, 41). Our data suggest that when this organization is severely affected, the particle becomes unstable and more susceptible to disassembly. A large drop in infectivity is only observed when the phase state of the viral envelope is disturbed, suggesting that the existence of lateral membrane organization in virus particles is important for virus-cell interactions because of its importance for the particle physical stability. These highly ordered structures might be related to the maintenance of viral envelope glycoproteins in functional conformations or provide a rigid structure to protect the virion nucleocapsid from the extracellular milieu. Our data are consistent with recent work by Hafer et al. (22) who suggested that biochemical differences in the SINV envelope composition could induce instability of the metastable virus structure leading to loss of infectivity. Destabilization of the physical structure of virus particles upon cholesterol depletion with β-cyclodextrins has also been reported for influenza virus type A, HIV-1, and simian immunodeficiency virus (SIV) (20, 42), all of which were isolated from mammalian host cells. It is important to point out that the envelope of alphaviruses is largely inaccessible to the outside environment, because it is underneath an icosahedral protein shell (5). Despite this, we were able to efficiently remove cholesterol from this structure.
Because MβCD treatment changed the envelope phase state only in HCCPs, likely some factor other than cholesterol acts in maintaining the envelope of LCCPs in a highly ordered state. Interestingly, in addition to a lower content of cholesterol, alphaviruses assembled in mosquito cells were also previously shown to have more ethanolamine-based phospholipids than those assembled in mammalian cells (71.9% versus 23.2%, respectively) (43, 44). Perhaps, these phospholipids could provide an additional resistance to the viral envelope, as they can form highly condensed regions in biological membranes (45). Consistent with this idea, the formation of solid-ordered phases is related to a high content of phosphatidylethanolamine and phospholipids that have a low affinity for cholesterol and a high temperature of gel-fluid phase transition (46–48). Recently, it was shown that a solid-ordered phase state could be achieved at a low temperature in the envelope of influenza viruses with a reduced cholesterol content and an increased concentration of phosphatidylethanolamine, and that these conditions would be important for viral transmission (39). Thus, an interesting parallel can be traced: maybe alphaviruses produced from mosquito cells have a highly ordered envelope due to the high content of ethanolamine-based phospholipids, low content of cholesterol and lower temperature (28 °C), which could facilitate the transmission of these viruses by arthropod vectors.
We cannot discard the hypothesis that in our assays for cholesterol depletion MβCD also extracted other lipids from the viral envelope. Although MβCD may also interact with membrane phospholipids, it is important to highlight that this compound has a much stronger effect on membrane cholesterol than on phospholipids, as cholesterol molecules are easily lodged inside cyclodextrin rings. Indeed, a number of studies reported only minimal release of phospholipids in comparison with cholesterol from cell membranes under exposure to cyclodextrins (49). Furthermore, MβCD treatment led to minimal effects on LCCPs and strong effects on HCCPs. If a general extraction of lipids with similar efficiencies for all lipid classes was the case, a more pronounced effect on LCCPs would be expected.
Altogether, our work shows for the first time that lateral organization is present in the MAYV envelope, independent of cholesterol. Envelope lipid-packing is critical for virus particle stability and infectivity. In particles isolated from mammalian cells, cholesterol is important for the maintenance of their structure, most likely because of its role in the formation of raft-like lipid domains (47). On the other hand, when particles are isolated from insect cells, cholesterol is not important for envelope lateral organization. Because insect cells were grown under the same serum supplementation used for mammalian cells and no delipidation method was used, our data suggest that they provide an additional factor for membrane ordering.
Previous studies have suggested that virus particles may be interesting models to study lipid-lipid and lipid-protein interactions because they represent small populations of cellular membranes modified during virus particle assembly. We believe that in addition to this, enveloped virus particles may also help to shed light on the mechanisms of stabilization of macromolecular assemblies through membrane lateral organization and the implication of this organization for the function of proteins present in specialized portions of cellular membranes.
Supplementary Material
Acknowledgment
We thank Emerson R. Gonçalves for competent technical assistance.
The work was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Fundação Universitária José Bonifácio (FUJB), Instituto Milênio de Biologia Estrutural em Biomedicina e Biotecnologia (IMBEBB), Instituto Nacional de Ciência e Tecnologia em Biologia Estrutural e Bioimagem (INCTBEB), and Programa de Apoio a Núcleos de Excelência (PRONEX).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- MAYV
- Mayaro virus
- SINV
- Sindbis virus
- SFV
- Semliki Forest virus
- HHV-6
- human herpesvirus 6
- VACV
- vaccinia virus
- VZV
- varicella-zoster virus
- HIV-1
- human immunodeficiency virus type 1
- HSV-1
- herpes simplex virus type 1
- MβCD
- methyl-β-cyclodextrin
- HCCPs
- high cholesterol content particles
- LCCPs
- low cholesterol content particles
- GP
- generalized polarization.
REFERENCES
- 1. Tesh R. B., Watts D. M., Russell K. L., Damodaran C., Calampa C., Cabezas C., Ramirez G., Vasquez B., Hayes C. G., Rossi C. A., Powers A. M., Hice C. L., Chandler L. J., Cropp B. C., Karabatsos N., Roehrig J. T., Gubler D. J. (1999) Clin. Infect. Dis. 28, 67–73 [DOI] [PubMed] [Google Scholar]
- 2. Vasconcelos P. F., Travassos da Rosa A. P., Rodrigues S. G., Travassos da Rosa E. S., Dégallier N., Travassos da Rosa J. F. (2001) Cad. Saúde Pública 17, 155–164 [DOI] [PubMed] [Google Scholar]
- 3. Receveur M. C., Grandadam M., Pistone T., Malvy D. (2010) Euro Surveill. 15, P11–19563 [PubMed] [Google Scholar]
- 4. Hassing R. J., Leparc-Goffart I., Blank S. N., Thevarayan S., Tolou H., van Doornum G., van Genderen P. J. (2010) J. Infect. 61, 343–345 [DOI] [PubMed] [Google Scholar]
- 5. Strauss J. H., Strauss E. G. (1994) Microbiol. Rev. 58, 491–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paredes A. M., Ferreira D., Horton M., Saad A., Tsuruta H., Johnston R., Klimstra W., Ryman K., Hernandez R., Chiu W., Brown D. T. (2004) Virology 324, 373–386 [DOI] [PubMed] [Google Scholar]
- 7. Phalen T., Kielian M. (1991) J. Cell Biol. 112, 615–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smit J. M., Bittman R., Wilschut J. (1999) J. Virol. 73, 8476–8484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marquardt M. T., Phalen T., Kielian M. (1993) J. Cell Biol. 123, 57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu Y. E., Cassese T., Kielian M. (1999) J. Virol. 73, 4272–4278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clayton R. B. (1964) J. Lipid Res. 15, 3–19 [PubMed] [Google Scholar]
- 12. Sun X., Whittaker G. R. (2003) J. Virol. 77, 12543–12551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang H., Li Y., Sadaoka T., Tang H., Yamamoto T., Yamanishi K., Mori Y. (2006) J. Gen. Virol. 87, 277–285 [DOI] [PubMed] [Google Scholar]
- 14. Chung C. S., Huang C. Y., Chang W. (2005) J. Virol. 79, 1623–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hambleton S., Steinberg S. P., Gershon M. D., Gershon A. A. (2007) J. Virol. 81, 7548–7558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Campbell S. M., Crowe S. M., Mak J. (2001) J. Clin. Virol. 22, 217–227 [DOI] [PubMed] [Google Scholar]
- 17. Guyader M., Kiyokawa E., Abrami L., Turelli P., Trono D. (2002) J. Virol. 76, 10356–10364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Viard M., Parolini I., Sargiacomo M., Fecchi K., Ramoni C., Ablan S., Ruscetti F. W., Wang J. M., Blumenthal R. (2002) J. Virol. 76, 11584–11595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campbell S. M., Crowe S. M., Mak J. (2002) AIDS 16, 2253–2261 [DOI] [PubMed] [Google Scholar]
- 20. Graham D. R., Chertova E., Hilburn J. M., Arthur L. O., Hildreth J. E. (2003) J. Virol. 77, 8237–8248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bender F. C., Whitbeck J. C., Ponce de Leon M., Lou H., Eisenberg R. J., Cohen G. H. (2003) J. Virol. 77, 9542–9552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hafer A., Whittlesey R., Brown D. T., Hernandez R. (2009) J. Virol. 83, 9113–9121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mezencio J. M., Rebello M. A. (1993) Mem. Inst. Oswaldo Cruz 88, 299–304 [DOI] [PubMed] [Google Scholar]
- 24. Gaus K., Zech T., Harder T. (2006) Mol. Membr. Biol. 23, 41–48 [DOI] [PubMed] [Google Scholar]
- 25. Parasassi T., de Stasio G., D'Ubaldo A., Gratton E. (1990) Biophys. J. 57, 1179–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris F. M., Best K. B., Bell J. D. (2002) Biochim. Biophys. Acta 1565, 123–128 [DOI] [PubMed] [Google Scholar]
- 27. Dietrich C., Bagatolli L. A., Volovyk Z. N., Thompson N. L., Levi M., Jacobson K., Gratton E. (2001) Biophys. J. 80, 1417–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lorizate M., Brügger B., Akiyama H., Glass B., Müller B., Anderluh G., Wieland F. T., Kräusslich H. G. (2009) J. Biol. Chem. 284, 22238–22247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. He L., Piper A., Meilleur F., Myles D. A., Hernandez R., Brown D. T., Heller W. T. (2010) J. Virol. 84, 5270–5276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones L. J., Yue S. T., Cheung C. Y., Singer V. L. (1998) Anal. Biochem. 265, 368–374 [DOI] [PubMed] [Google Scholar]
- 31. Lu Y. E., Kielian M. (2000) J. Virol. 74, 7708–7719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dhileepan K., Azuolas J. K., Gibson C. A. (1996) J. Med. Entomol. 33, 180–182 [DOI] [PubMed] [Google Scholar]
- 33. Bagatolli L. A., Sanchez S. A., Hazlett T., Gratton E. (2003) Methods Enzymol. 360, 481–500 [DOI] [PubMed] [Google Scholar]
- 34. Scheiffele P., Rietveld A., Wilk T., Simons K. (1999) J. Biol. Chem. 274, 2038–2044 [DOI] [PubMed] [Google Scholar]
- 35. Takeda M., Leser G. P., Russell C. J., Lamb R. A. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 14610–14617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brügger B., Glass B., Haberkant P., Leibrecht I., Wieland F. T., Kräusslich H. G. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 2641–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ivanova L., Schlesinger M. J. (1993) J. Virol. 67, 2546–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smotrys J. E., Linder M. E. (2004) Annu. Rev. Biochem. 73, 559–587 [DOI] [PubMed] [Google Scholar]
- 39. Polozov I. V., Bezrukov L., Gawrisch K., Zimmerberg J. (2008) Nat. Chem. Biol. 4, 248–255 [DOI] [PubMed] [Google Scholar]
- 40. Sefton B. M., Gaffney B. J. (1974) J. Mol. Biol. 90, 343–358 [DOI] [PubMed] [Google Scholar]
- 41. Moore N. F., Barenholz Y., Wagner R. R. (1976) J. Virol. 19, 126–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barman S., Nayak D. P. (2007) J. Virol. 81, 12169–12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luukkonen A., Kaariainen L., Renkonen O. (1976) Biochim. Biophys. Acta 450, 109–120 [DOI] [PubMed] [Google Scholar]
- 44. Luukkonen A., von Bonsdorff C. H., Renkonen O. (1977) Virology 78, 331–335 [DOI] [PubMed] [Google Scholar]
- 45. Polozov I. V., Gawrisch K. (2004) Biophys. J. 87, 1741–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huster D., Arnold K., Gawrisch K. (1998) Biochemistry 37, 17299–17308 [DOI] [PubMed] [Google Scholar]
- 47. Silvius J. R. (2003) Biochim. Biophys. Acta 1610, 174–183 [DOI] [PubMed] [Google Scholar]
- 48. Niu S. L., Litman B. J. (2002) Biophys. J. 83, 3408–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zidovetzki R., Levitan I. (2007) Biochim. Biophys. Acta 1768, 1311–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.