Abstract
FTY720 is a novel immunomodulating drug that can be phosphorylated inside cells; its phosphorylated form, FTY720-P, binds to a sphingosine 1-phosphate (S1P) receptor, S1P1, and inhibits lymphocyte egress into the circulating blood. Although the importance of its pharmacological action has been well recognized, little is known about how FTY720-P is released from cells after its phosphorylation inside cells. Previously, we showed that zebrafish Spns2 can act as an S1P exporter from cells and is essential for zebrafish heart formation. Here, we demonstrate that human SPNS2 can transport several S1P analogues, including FTY720-P. Moreover, FTY720-P is transported by SPNS2 through the same pathway as S1P. This is the first identification of an FTY720-P transporter in cells; this finding is important for understanding FTY720 metabolism.
Keywords: ABC Transporter, Immunosupressor, Lipid Transport, Sphingolipid, Transport Drugs, FTY720, Sphingosine 1-Phosphate
Introduction
Sphingosine 1-phosphate (S1P)2 is a bioactive sphingolipid that acts as a ligand for a family of five G protein-coupled receptors (S1P1–5) (1) that are expressed in a variety of tissues, such as brain (2), lymphoid (3), and cancer tissues (4, 5). Through the S1P receptors, S1P regulates diverse cellular functions, such as cell proliferation, migration, actin cytoskeletal rearrangement, and adherens junction assembly (6, 7).
S1P is abundant in plasma, and its cellular source has been assumed to be hematopoietic cells (erythrocytes and platelets) and vascular endothelial cells (8). In immune systems, S1P in plasma plays a major role in regulating the number of circulating lymphocytes with S1P1 (9). We showed that S1P secretion from platelets and erythrocytes was mediated by ATP-dependent and glyburide-sensitive transporters (10, 11). Recently, we identified Spns2 by analyzing the zebrafish mutant ko157, which displays two hearts (12). In zebrafish, Spns2 functioning as an S1P transporter regulates the migration of cardiac progenitors (12). We also identified mammalian orthologues of zebrafish Spns2 and confirmed their ability to export S1P from the cells (12).
FTY720 (fingolimod; 2-amino-2-[2-(4-octyl phenyl)ethyl]-1,3-propanediol) is a novel immunosuppressive agent that shows structural similarity to sphingosine and is intracellularly phosphorylated by a sphingosine kinase (13). FTY720-P, the phosphorylated form of FTY720, has been reported to bind to S1P1 in lymphocytes and/or endothelial cells, which causes the inhibition of lymphocyte trafficking into the circulating blood and the accumulation of lymphocytes in secondary lymphoid organs, such as lymph nodes and Peyer's patches (14–16). Thus, FTY720 is a prodrug that must be phosphorylated to form FTY720-P and circulate in plasma and/or lymph to bind to S1P1 presented at the surface of lymphocytes and/or endothelial cells (9). Although both platelets and erythrocytes synthesize and release S1P (17, 18), FTY720 phosphorylation has been reported to occur in platelets, but not in erythrocytes, because erythrocytes lack the SPHK2 (sphingosine kinase 2) activity that effectively phosphorylates FTY720 (19). Unlike S1P, which is released from platelets by thrombin stimulation, FTY720-P synthesized in platelets is mostly released in a stimulus-independent manner (19).
So far, four ATP-binding cassette (ABC) transporters (ABCA1, ABCB1, ABCC1, and ABCG2) have been reported as possible S1P and/or FTY720-P transporters (20–24). However, there is no direct evidence showing that these ABC transporters function as FTY720-P transporters. In this study, we demonstrated that human SPNS2 is able to export FTY720-P from cells and that neither S1P nor FTY720-P is released from the CHO cells exogenously expressing ABCA1, ABCB1, ABCC1, or ABCG2.
EXPERIMENTAL PROCEDURES
Chemicals
Bovine serum albumin (fatty acid-free) (BSA), sphingosine (Sph), dihydrosphingosine (DH-Sph) and dihydrosphingosine 1-phosphate (DH-S1P) were obtained from Sigma. S1P, C17-sphingosine 1-phosphate (C17-S1P), phytosphingosine 1-phosphate (phyto-S1P), and C17-sphingosine (C17-Sph) were from Avanti. Phytosphingosine (phyto-Sph) was from Biomol. FTY720 and the anti-mouse SPHK1 (sphingosine kinase 1) polyclonal antibody were from Cayman. The anti-rabbit Na+/K+-ATPase α1 subunit mouse monoclonal antibody (clone C464.6) was from Millipore. The anti-HA tag rabbit polyclonal antibody was from MBL. The anti-human ABCA1 (clone AB.H10) and anti-human ABCC1 (clone IU2H10) mouse monoclonal antibodies were from Abcam. The anti-human ABCB1 mouse monoclonal antibody (clone C219) was from Signet, and the anti-human ABCG2 mouse monoclonal antibody (clone BXP-21) was obtained from Kamiya Biomedical Co. All other chemicals were of analytical or HPLC grade and obtained from commercial sources.
Establishment of SPHK1- or SPHK2-expressing CHO Cells
C-terminal HA-tagged mouse Sphk1 and Sphk2 cDNA were obtained from mouse kidney first-strand cDNA by PCR amplification with specific primers (supplemental Table 1). The amplified fragments were introduced in a TA-cloning vector and confirmed by DNA sequencing. The C-terminal HA-tagged Sphk1 fragment was subcloned into a pcDNA3 vector (pcDNA3/Sphk1) or a pcDNA5/FRT vector (pcDNA5/FRT/Sphk1), and the Sphk2 fragment was subcloned into a pcDNA5/FRT vector (pcDNA5/FRT/Sphk2). The resulting pcDNA3/Sphk1 plasmid was transfected into Flp-In-CHO cells using Lipofectamine 2000 (Invitrogen). After 48 h, the cells were incubated in F-12 medium with 1 mg/ml G418 for 3–4 weeks to obtain Flp-In-CHO/SPHK1 cells constitutively expressing mouse SPHK1 and carrying the Flp-In system. The pcDNA5/FRT/Sphk1 or pcDNA5/FRT/Sphk2 plasmid was transfected into Flp-In-CHO cells. After 48 h, the cells were incubated in F-12 medium with 600 μg/ml hygromycin for 3–4 weeks to obtain CHO/SPHK1 or CHO/SPHK2 cells.
Establishment of Cell Lines Expressing Human SPNS2 (hSPNS2) and ABC Transporters
The coding region of human SPNS2 (NM_001124758), mouse Abca1 (NM_013454), human ABCB1 (NM_000927), mouse Abcc1 (NM_008576), and human ABCG2 (NM_004827) cDNA was amplified from first-strand cDNA of human brain, MEG-01 cells, mouse lung, or mouse brain with gene-specific primers (supplemental Table 1). The resulting PCR fragments were subcloned into a pcDNA5/FRT vector and confirmed by DNA sequencing. All of the constructed expression plasmids were individually transfected into Flp-In-CHO/SPHK1 as described above to establish CHO cell lines stably expressing both SPHK1 and the respective transporter. These cell lines were established by antibiotic selection, and the protein expression of each transporter in all of the established cell lines was confirmed by Western blot analysis using the corresponding specific antibodies.
Transport Assay for Sphingolipids and FTY720-P
The FTY720-P transport assay was performed with the cells either transiently or stably expressing hSPNS2. CHO, CHO/SPHK1, or CHO/SPHK2 cells were transfected with the pEGFP or pEGFP/hSPNS2 vector. Protein expression and localization were confirmed by GFP fluorescence. The transport assay for other sphingolipids was performed with Flp-In-CHO/SPHK1 cells stably expressing each transporter as described previously. The release of sphingolipids, FTY720, and their phosphorylated forms from the cells was measured by o-phthalaldehyde modification followed by HPLC analysis as a modified protocol of Min et al. (25). Briefly, cells were washed with F-12 medium twice and incubated with sphingolipids or FTY720 in the releasing medium (F-12 medium with 1% BSA, 10 mm sodium glycerophosphate, 5 mm sodium fluoride, and 1 mm semicarbazide) at 37 °C. After the incubation, 100-μl aliquots of the medium were transferred to new tubes and subjected to lipid extraction under alkaline chloroform conditions. C17-S1P (30 pmol) was added to each sample as the internal standard. Extracted S1P, its structural analogues, and FTY720-P were dephosphorylated with calf intestinal alkaline phosphatase (30 units) at 37 °C for 90 min. The resulting Sph, its analogues, and FTY720 were extracted with chloroform and dried and resuspended in ethanol. o-Phthalaldehyde modification was performed at room temperature for 1 h or overnight. After centrifugation of the samples, 15 μl of the total sample (135 μl) was analyzed by HPLC (Hitachi) using a Cosmosil 5C 18-AR-II column.
Measurement of the Sphingosine Kinase Activity in the Cell Culture Medium
Cells were washed with F-12 medium twice and incubated with the releasing medium at 37 °C for 2 h. The releasing medium was transferred to microcentrifuge tubes and centrifuged at 10,000 rpm at 4 °C for 5 min to remove the cell debris. 0.2 μCi/ml [3H]Sph (American Radiolabeled Chemicals, Inc.) was added to the supernatant, which was then incubated at 37 °C for 2 h. Lipids were then extracted from the medium and developed on a TLC plate (Merck), which was then quantified with an FLA300 bioimaging analyzer (Fuji Film).
Real-time PCR of First-strand cDNA Prepared from Various Human Tissues
Human major tissue qPCR Array II was obtained from Origene. The real-time quantitative PCR assay was performed with FastStart Master Mix with ROX (Roche Applied Science) using an ABI PRISM 7000 sequence detection system (Applied Biosystems). To detect the human SPNS2 mRNA, the forward primer (ttactggctccagcgtga), the reverse primer (tgatcatgcccaggacag), and Roche Applied Science Universal Probe 27 were used.
Western Blotting
Cells were sonicated in phosphate-buffered saline supplemented with a protease inhibitor mixture (Nacalai Tesque) and centrifuged at 800 × g at 4 °C for 10 min. Subsequently, the membrane fractions were obtained by centrifugation at 100,000 × g at 4 °C for 1 h. Samples were electrophoresed on an SDS-polyacrylamide gel and immunodetected using specific antibodies.
RESULTS
hSPNS2 Is Able to Transport S1P Analogues
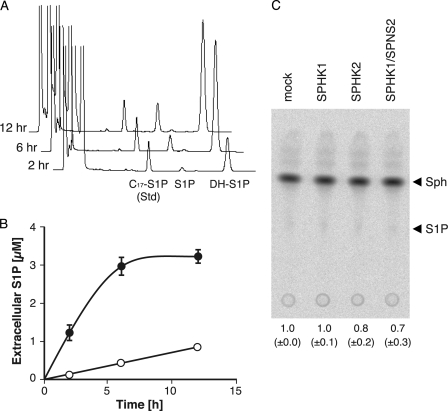
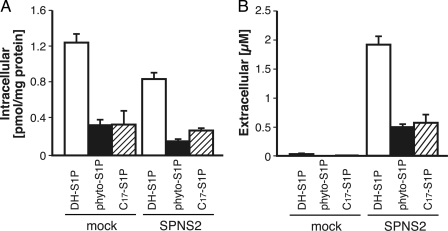
In the HPLC analysis of the endogenous S1P export activity in hSPNS2-expressing cells, a large peak corresponding to DH-S1P in the HPLC profile was observed after the S1P peak in a time-dependent manner (Fig. 1, A and B). This increase of the extracellular S1P and DH-S1P depended on the expression of hSPNS2. Neither S1P nor DH-S1P was synthesized outside cells with the secreted SPHK1 because there was no detectable S1P in the culture medium isolated from the CHO cells expressing SPHK1 alone and no detectable sphingosine kinase activity in the medium isolated from the CHO/SPHK1 cells with or without SPNS2 expression (Fig. 1C) (12). Because SPHK1 selectively uses DH-Sph rather than Sph as a substrate, SPHK1-expressing CHO cells accumulate DH-S1P inside cells and export more DH-S1P than S1P (Fig. 1B and supplemental Fig. 1). This result suggests that hSPNS2 can transport S1P analogues accumulated inside cells. To determine the substrate specificity of hSPNS2, the release of other S1P analogues by hSPNS2 was investigated. When DH-Sph, phyto-Sph, or C17-Sph was added to the culture medium, each lipid was efficiently taken up by the cells and phosphorylated by SPHK1, which resulted in DH-S1P, phyto-S1P, or C17-S1P accumulation inside the cells (Fig. 2A). These phosphorylated S1P analogues were exported from the cells expressing hSPNS2 (Fig. 2B). However, DH-Sph, phyto-Sph, and C17-Sph were not exported from the hSPNS2-expressing cells (supplemental Fig. 2).
FIGURE 1.
hSPNS2-mediated release of endogenous S1P and DH-S1P. Shown are an HPLC diagram (A) and quantitative results (B) of time-dependent secretion of endogenous S1P and DH-S1P from cells. CHO cells expressing SPHK1 and hSPNS2 were incubated with the releasing medium for 2, 6, or 12 h. The releasing medium was collected, and the amounts of secreted S1P and DH-S1P were measured by HPLC. C17-S1P was used as the internal standard. Open and closed circles indicate secreted S1P and DH-S1P, respectively. Experiments were performed more than three times, and error bars indicate the S.D. C, sphingosine kinase activity in culture medium. The activity of SPHK was analyzed by the conversion of [3H]Sph to [3H]S1P. Lipid extracts were separated on a TLC plate, and bands corresponding to [3H]S1P were quantified. The relative amount of [3H]S1P in the medium of CHO/SPHK1 (SPHK1), CHO/SPHK2 (SPHK2), or CHO/SPHK1/hSPNS2 (SPHK1/SPNS2) compared with CHO (mock) cells is indicated at the bottom of the TLC plate image. Experiments were performed more than three times, and values are means with S.D.
FIGURE 2.
DH-S1P, phyto-S1P, and C17-S1P are released from hSPNS2-expressing cells. CHO/SPHK1 (mock) or CHO/SPHK1/hSPNS2 (SPNS2) cells were grown for 2 days and incubated with the releasing medium containing DH-Sph, phyto-Sph, or C17-Sph at 5 μm for 2 h. The cells (A) and releasing medium (B) were collected, and sphingolipid contents were measured by HPLC. C17-S1P was used as the internal standard for measuring DH-S1P and phyto-S1P, whereas phyto-S1P was used as the internal standard for measuring C17-S1P. Experiments were performed more than three times, and error bars indicate the S.D.
hSPNS2 Acts as an FTY720-P Exporter from the Cells
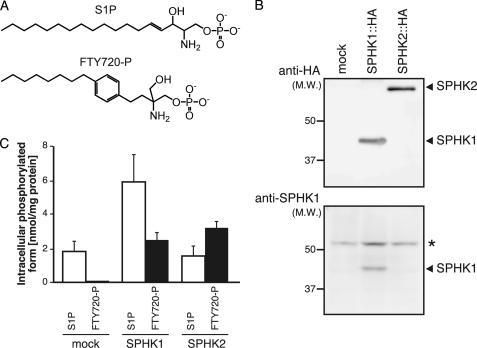
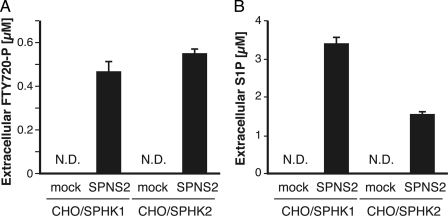
FTY720-P, which is structurally similar to S1P (Fig. 3A), acts as an agonist of S1P receptors (S1P1, S1P3, S1P4, and S1P5). Therefore, it is possible that hSPNS2 also acts as an FTY720-P exporter from cells. There are two sphingosine kinases, SPHK1 and SPHK2, and it has been reported that SPHK2 can phosphorylate FTY720 more effectively than SPHK1 (19, 26, 27). Thus, CHO cells stably expressing HA-tagged mouse SPHK1 (pcDNA5/FRT/SPHK1) or SPHK2 (pcDNA5/FRT/SPHK2) were used for measuring the FTY720-P export from cells. Cells were established as described in “Experimental Procedures,” and HA-tagged mouse SPHK1 and SPHK2 expression was confirmed by Western blotting with an anti-HA or anti-SPHK1 antibody (Fig. 3B). Although Sph phosphorylation was more efficient in CHO cells expressing SPHK1 rather than SPHK2, FTY720 was phosphorylated at a similar level in SPHK1- and SPHK2-expressing cells (Fig. 3C). Both cells were used for measuring the hSPNS2-dependent FTY720-P export. FTY720 was added to the cells, and the amount of FTY720-P released from the cells was measured as described under “Experimental Procedures.” As shown in Fig. 4A, FTY720-P synthesized inside the cells was detected in the medium when hSPNS2 was expressed in either CHO/SPHK1 or CHO/SPHK2 cells. In contrast to S1P, which was predominantly synthesized by SPHK1 and secreted from the SPHK1-expressing cells (Fig. 4B), FTY720-P was comparably secreted from both the SPHK1- and the SPHK2-expressing cells; these results correlated with the intracellular amount of FTY720-P (Figs. 3C and 4A). FTY720-P was not detected in the medium of the cells without hSPNS2 expression, indicating that hSPNS2 is able to export FTY720-P from cells.
FIGURE 3.
Phosphorylation of Sph and FTY720 in SPHK1- or SPHK2-expressing cells. A, chemical structures of S1P and FTY720-P are indicated. B, expression of SPHK1 and SPHK2. Cell lysates prepared from CHO/mock (mock), CHO/SPHK1 (SPHK1::HA), or CHO/SPHK2 (SPHK2::HA) cells were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Western blot analysis was performed using anti-HA and anti-SPHK1 antibodies. Both SPHK1 and SPHK2 stably expressed in CHO cells were tagged at the C terminus with the HA epitope. *, bands not derived from the tagged SPHK1/2 proteins. C, S1P and FTY720-P synthesis in SPHK1- or SPHK2-overexpressing cells. CHO (mock), CHO/SPHK1 (SPHK1), or CHO/SPHK2 (SPHK2) cells were incubated with the releasing medium containing 5 μm Sph or FTY720 for 2 h. The cells were collected, and the S1P and FTY720-P contents were measured by HPLC. C17-S1P was used as the internal standard. Experiments were performed more than three times, and error bars indicate the S.D.
FIGURE 4.
FTY720-P is released from hSPNS2-expressing cells. CHO/SPHK1 or CHO/SPHK2 cells were transfected with pEGFP (mock) or pEGFP/hSPNS2 (SPNS2). 24 h after transfection, the cells were incubated with releasing medium containing 5 μm FTY720 (A) or Sph (B) for 2 h. The releasing medium was collected, and the amounts of released FTY720-P (A) and S1P (B) were measured by HPLC as described under “Experimental Procedures.” Experiments were performed more than three times, and error bars indicate the S.D. N.D., not detected.
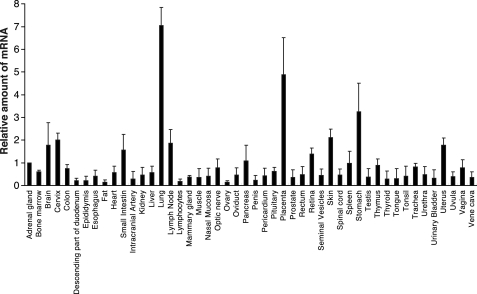
Tissue Distribution of SPNS2 mRNA in Human Tissues
FTY720-P has been reported to be synthesized in platelets; however, so far, it is not clear whether these cells indeed contribute to the supply of FTY720-P in vivo (28). Because hSPNS2 acts as an FTY720-P transporter, hSPNS2-expressing tissues and cells are good candidates for FTY720-producing cells. Real-time PCR analysis of mRNAs isolated from various human tissues demonstrated that the human SPNS2 mRNA is abundant in lung and placenta and moderately expressed in many other tissues (Fig. 5). However, human hematopoietic and vascular tissues showed relatively low contents of the SPNS2 mRNA (Fig. 5). Furthermore, the SPNS2 mRNA is poorly transcribed in the megakaryoblast cell line, MEG-01, and the erythromyeloblastoid leukemia cell line, K562, by quantitative real-time PCR analysis (less than 1% of the SPNS2 mRNA in the adrenal gland). The mRNA of human SPHK2 is abundant in the kidney, liver, and brain and is detected at a relatively lower level in other tissues (29). Moreover, FTY720-P synthesis has been observed in lymph nodes, Peyer's patches, and spleen (30). Although the tissue distribution pattern of SPNS2 and SPHK2 in human tissues is different, SPNS2 is detected in most SPHK2-expressing tissues (Fig. 5). This result suggested that, in addition to platelets, FTY720-P might also be synthesized and secreted from these tissues (19).
FIGURE 5.
Tissue distribution of human SPNS2 mRNAs. Quantitative real-time PCR was performed with first strand cDNA synthesized from various human tissue mRNAs. Experiments were performed more than three times, and error bars indicate the S.D.
ABC Transporters Could Not Promote S1P or FTY720-P Release from CHO Cells
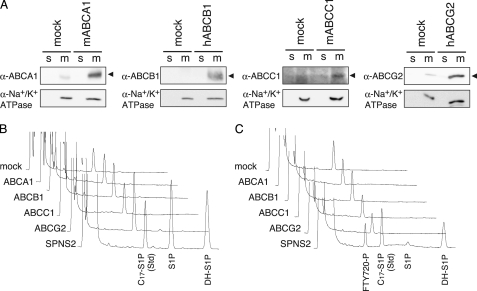
ABC transporters ABCA1, ABCB1, ABCC1, and ABCG2 have been reported to contribute to the release of S1P and/or FTY720-P from astrocytes, mast cells, fibroblasts, or breast cancer cells (20–24). To examine whether these ABC transporters by themselves are able to transport S1P and/or FTY720-P, each ABC transporter was stably expressed in CHO/SPHK1 cells, and the release of S1P or FTY720-P from the cells was investigated. The expression of each transporter was confirmed by measuring its activity and Western blotting with the corresponding specific antibody (Fig. 6A and supplemental Fig. 3). Although functional transporters were successfully expressed in CHO/SPHK1 cells, S1P or FTY720-P secretion was not detected in the medium of these cells (Fig. 6, B and C), which suggested that these ABC transporters are not sufficient to function as S1P or FTY720-P transporters when they are exogenously expressed in CHO cells.
FIGURE 6.
S1P-releasing assay of cells expressing ABC transporters. A, expression of ABC transporters in the cells transfected with each transporter individually. Membrane (m) and soluble fractions (s) prepared from CHO/SPHK1 cells (mock) or CHO/SPHK1 cells stably expressing mABCA1, hABCB1, mABCC1, or hABCG2 were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Western blot analysis was performed with specific antibodies for each individual ABC transporter as described under “Experimental Procedures.” A ubiquitously expressed membrane protein, Na+/K+-ATPase, was used as the loading control of each membrane fraction. B and C, S1P or FTY720-P secretion from ABC transporter-expressing cells. CHO/SPHK1 cells (mock) or CHO/SPHK1 cells stably expressing mABCA1, hABCB1, mABCC1, hABCG2, or hSPNS2 were incubated with releasing medium containing 5 μm Sph (B) or FTY720 (C) for 2 h. The releasing medium was collected, and the amounts of released phosphorylated sphingolipids were measured by HPLC. Each diagram is a typical chromatogram of the sphingolipids released from the cells overexpressing an individual ABC transporter. C17-S1P was used as the internal standard. Experiments were performed more than three times.
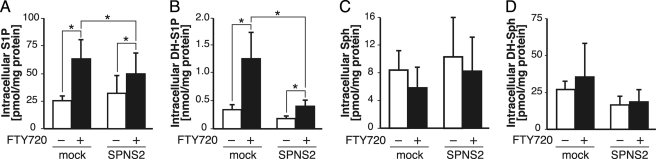
FTY720 Affects the Contents of Intracellular Sphingolipids
When hSPNS2 was expressed in cells with SPHK1 or SPHK2, FTY720-P was synthesized and exported from the cells. It has been reported that FTY720 inhibits S1P lyase (SPL) and ceramide synthase (31, 32). SPL irreversibly degrades S1P to hexadecenal and ethanolamine phosphate, and ceramide synthase converts DH-Sph to dihydroceramide (33, 34). It was reported that treatment of human pulmonary artery endothelial cells with FTY720 caused DH-S1P accumulation inside the cells and decreased the amount of S1P, which suggested that FTY720 predominantly inhibits the function of ceramide synthase rather than that of SPL in human pulmonary artery endothelial cells (32). As shown in Fig. 7, when CHO cells overexpressing SPHK1 were treated with FTY720, the amounts of both intracellular DH-S1P and S1P were increased about 4- and 2-fold, respectively, although the amounts of Sph and DH-Sph were not changed. These results suggest that FTY720 inhibits both SPL and ceramide synthase activities in SPHK1-overexpressing CHO cells. This increase of S1P and DH-S1P was partially suppressed by hSPNS2 expression in the cells as a result of its role in exporting these phosphorylated sphingolipids from the cells (Fig. 7, A and B). However, the intracellular amounts of Sph and DH-Sph did not change with hSPNS2 expression, which supported the previous observation that hSPNS2 only transports phosphorylated sphingolipids (Fig. 7, C and D, and supplemental Fig. 2).
FIGURE 7.
FTY720 affects the intracellular contents of sphingolipids. CHO/SPHK1 (mock) and CHO/SPHK1-expressing hSPNS2 (SPNS2) cells were incubated with the releasing medium for 2 h in the presence (+) or absence (−) of 5 μm FTY720. The cells were collected, and the amounts of each sphingolipid (S1P (A), DH-S1P (B), Sph (C), and DH-Sph (D)) in the cells were measured by HPLC. Experiments were performed more than three times, and error bars indicate the S.D. *, p < 0.001.
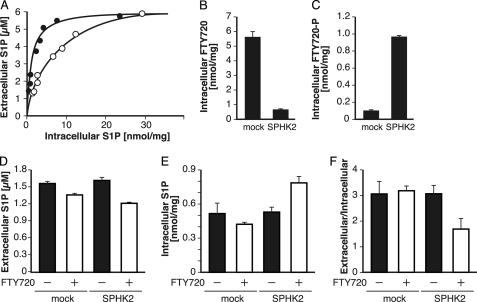
FTY720-P Competitively Inhibits the S1P Transport by hSPNS2
When the amount of Sph added to the medium was increased, the amounts of intracellular S1P and the S1P released from the cells were also increased (Fig. 8). This result indicates that transport of S1P by hSPNS2 depends on a concentration gradient between the inside and outside of the cells. In Fig. 2, we showed that S1P structural analogues were transported by hSPNS2. Thus, it is interesting to investigate whether hSPNS2 transports FTY720-P through the same mechanism of S1P. To address this question, S1P transport activity was measured in the absence or presence of FTY720-P (Fig. 8). To accumulate the intracellular concentration of FTY720-P, 5 μm FTY720 was added to the medium. Actual amounts of the FTY720-P and S1P inside the cells were determined by quantification with HPLC after the lipids were extracted from the cells. In the presence of FTY720 or FTY720-P inside the cells, S1P transport activity significantly decreased (Fig. 8A), indicating that FTY720 or FTY720-P acts as a competitive inhibitor of S1P transport by hSPNS2. To clarify which form acts as a competitive inhibitor, we used CHO and CHO/SPHK2 cells, in which phosphorylation of Sph occurs to a similar extent because FTY720 was effectively phosphorylated only in CHO/SPHK2 cells (Fig. 3C). The intracellular amount of FTY720 in CHO cells was higher than that of CHO/SPHK2 cells, but FTY720-P levels were higher in CHO/SPHK2 cells than in CHO cells (Fig. 8, B and C). By adding FTY720 to the culture medium, although S1P secretion was slightly inhibited in both the CHO and CHO/SPHK2 cells, the amount of secreted S1P was decreased more in CHO/SPHK2 cells than in CHO cells (Fig. 8B). As shown in Fig. 8E, the intracellular amount of S1P was increased in CHO/SPHK2 cells that had been treated with FTY720. Because S1P secretion increased depending on the S1P concentration inside the cells, the ratio of extracellular to intracellular amount of S1P indicated that an inhibition of S1P transport activity was observed only in CHO/SPHK2 cells (Fig. 8F). These results indicate that FTY720-P, but not FTY720, acts as a competitive inhibitor of the S1P transport of hSPNS2, which strongly suggests that both S1P and FTY720-P share the common exporting system of hSPNS2 (Fig. 9).
FIGURE 8.
Effect of FTY720-P on the S1P release mediated by hSPNS2. A, inhibition of S1P release from cells with FTY720 treatment. CHO/SPHK1-expressing hSPNS2 cells were incubated with releasing medium containing different concentrations of Sph for 2 h in the presence or absence of 5 μm FTY720. The releasing medium and cells were collected, and the amounts of sphingolipids were measured by HPLC. Closed and open circles indicate S1P released from untreated and FTY720-treated cells, respectively. B–F, the CHO (mock) and CHO/SPHK2 (SPHK2) cells were incubated with releasing medium containing 5 μm Sph for 2 h in the presence (+) or absence (−) of 3 μm FTY720. The contents of intracellular FTY720 (B), intracellular FTY720-P (C), extracellular S1P (D), and intracellular S1P (E) were measured by HPLC. F shows the ratio of the values in D to those in E. Experiments were performed more than three times, and error bars indicate the S.D.
FIGURE 9.
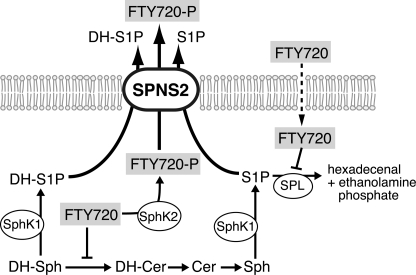
Schematic illustration of the sphingolipid metabolic pathway in CHO cells expressing SPHK1/2 and hSPNS2. S1P and FTY720-P are synthesized through the phosphorylation of Sph and FTY720 by SPHK1 and/or SPHK2, respectively. S1P and FTY720-P are exported through SPNS2. Intracellularly, FTY720 inhibits the conversion of DH-Sph into DH-Cer and S1P into hexadecenal.
DISCUSSION
S1P is a secreted sphingolipid mediator that activates S1P receptors on target cells. Recently, we have identified the zebrafish S1P transporter Spns2 (zSpns2) that regulates S1P export from cells. Because disruption of zSpns2 causes two hearts quite similar to the phenotype of a zebrafish S1P2 mutant, the regulation of S1P secretion is essential for the biological function of S1P (12). Although zebrafish Spns2 plays an important role in cardiac development, the molecular mechanism of S1P transport by zSpns2 remains unclear. In this study, we revealed the substrate specificity of hSPNS2.
FTY720 induces lymphocytopenia by inhibiting lymphocyte egress from secondary lymphoid tissues; however, it does not impair lymphocyte activation and proliferation (35). FTY720 is a new class of immunosuppressors that has been approved in Russia and will be approved in the United States as a novel oral therapeutic drug for multiple sclerosis. Conversion of FTY720 to FTY720-P is essential for the effects of this drug. FTY720 is intracellularly phosphorylated by SPHK2, and FTY720-P is localized in intracellular compartments (36, 37). FTY720-P acts as a ligand for S1P receptors (S1P1, S1P3, S1P4, and S1P5) expressed at the cell surface (30, 38). The release of FTY720-P, which is produced inside the cells, to the extracellular space to be recognized by the S1P receptors is crucial. However, the kind of molecule that regulates the secretion of FTY720-P from cells is not fully understood. We found that hSPNS2 transports not only S1P but also phosphorylated sphingolipids, such as DH-S1P, phyto-S1P, and C17-S1P (Fig. 2), which suggests that FTY720-P is also recognized by hSPNS2 as a substrate. When FTY720 was added to the medium of CHO/SPHK1 or CHO/SPHK2 cells, FTY720 was intracellularly phosphorylated to FTY720-P, and FTY720-P was secreted into the culture medium if the cells were also expressing hSPNS2 (Figs. 3 and 4). Furthermore, FTY720-P competitively inhibited S1P transport, which indicated that both S1P and FTY720-P share a common transport mechanism (Fig. 9). When 5 μm FTY720 was added to the culture medium, S1P transport activity was partially inhibited at lower S1P concentrations (Fig. 8A). In the clinical trial for multiple sclerosis, 0.5 and 1.25 mg of FTY720 were administered and significantly improved the relapse rate, the risk of disability progression, and MRI (39). Blood FTY720 peak concentration was reported as 0.65 ng/ml (2 nm) at 36 h following the administration of 1 mg of FTY720 (40). This FTY720 concentration is 2,500 times lower than that of our in vitro assay condition, which suggested that S1P transport activity should not be affected by FTY720 treatment at this low concentration.
Previously, we used the CHO cells expressing SPHK1 together with zSpns2 or hSPNS2 to measure the S1P transport activity of zSpns2 and hSPNS2 (12). Using this system, however, only the S1P analogues that were synthesized through phosphorylation by sphingosine kinase and accumulated inside cells to form a concentration gradient across the plasma membrane were applicable to the transport assay. To investigate the possibility that other sphingolipids are transported by hSPNS2, it is necessary to measure the uptake of these compounds into the inside-out vesicles prepared from hSPNS2-expressing cells. The ABC transporters ABCA1, ABCB1, ABCC1, and ABCG2 have been reported to transport S1P and ABCB1 for FTY720-P (20–24). When the expressions of ABCA1 in astrocytes, ABCC1 in mast cells or fibroblasts, and ABCG2 in breast cancer cells were suppressed by transporter-specific siRNAs, the S1P release from these cells was significantly decreased (20, 21, 23, 24). However, the overexpression of ABCA1, ABCB1, ABCC1, or ABCG2 in the SPHK1-expressing CHO cells did not promote the secretion of S1P and FTY720-P from cells (Fig. 6, B and C). Furthermore, Lee et al. (41) reported that the S1P concentration in the blood plasma of ABCA1, ABCC1, or ABCA7 knock-out mice was similar to that of wild type mice. We also observed that S1P secretion from platelets and erythrocytes was not altered in ABCA7 knock-out mice (supplemental Fig. 4). One explanation is that functional compensation with other ABC transporters would occur in ABC transporter knock-out mice. Another explanation is that modification(s) of ABC transporters or other factor(s) is required for transporting S1P and/or FTY720-P in CHO cells with exogenous expression of transporters. We emphasize that hSPNS2 is sufficient to export S1P and FTY720-P from the cells by itself.
The present study demonstrated that hSPNS2 is able to release FTY720-P from cells in vitro. Although FTY720/FTY720-P did not inhibit the S1P transport activity of SPNS2 at lower concentrations, the transport activity of FTY720-P from the cells is likely to be important for estimating the pharmacological effects of FTY720. The SPNS2 activity in individuals could affect the efficacy and regulation of FTY720. Given the current understanding of the pharmacological function of FTY720 in the circulating system of lymphocytes, further analysis of the physiological function of SPNS2 is required.
Supplementary Material
Acknowledgments
We thank Chiho Maeda and Kazuya Yokoyama for technical assistance. We also thank Mamiko Onoe for generation of ABCA7-KO mice.
This study was supported by in part by the Management Expenses Grants for National Universities Corporations; a grant-in-aid for Scientific Research (S) (to A. Y.) and grants-in-aid for Scientific Research on Innovation and Scientific Research (C) (to T. N.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; and the Takeda Science Foundation (to T. N. and A. Y.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Figs. 1–4.
- S1P
- sphingosine 1-phosphate
- Sph
- sphingosine
- DH
- dihydro
- ABC
- ATP-binding cassette
- SPL
- S1P lyase
- hSPNS2
- human SPNS2
- zSpns2
- zebrafish Spns2
- Cer
- ceramide.
REFERENCES
- 1. Anliker B., Chun J. (2004) J. Biol. Chem. 279, 20555–20558 [DOI] [PubMed] [Google Scholar]
- 2. Glickman M., Malek R. L., Kwitek-Black A. E., Jacob H. J., Lee N. H. (1999) Mol. Cell. Neurosci. 14, 141–152 [DOI] [PubMed] [Google Scholar]
- 3. Gräler M. H., Bernhardt G., Lipp M. (1998) Genomics 53, 164–169 [DOI] [PubMed] [Google Scholar]
- 4. Yamashita H., Kitayama J., Shida D., Yamaguchi H., Mori K., Osada M., Aoki S., Yatomi Y., Takuwa Y., Nagawa H. (2006) J. Surg. Res. 130, 80–87 [DOI] [PubMed] [Google Scholar]
- 5. Balthasar S., Samulin J., Ahlgren H., Bergelin N., Lundqvist M., Toescu E. C., Eggo M. C., Törnquist K. (2006) Biochem. J. 398, 547–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kluk M. J., Hla T. (2002) Biochim. Biophys. Acta 1582, 72–80 [DOI] [PubMed] [Google Scholar]
- 7. Taha T. A., Argraves K. M., Obeid L. M. (2004) Biochim. Biophys. Acta 1682, 48–55 [DOI] [PubMed] [Google Scholar]
- 8. Hla T., Venkataraman K., Michaud J. (2008) Biochim. Biophys. Acta 1781, 477–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosen H., Goetzl E. J. (2005) Nat. Rev. Immunol. 5, 560–570 [DOI] [PubMed] [Google Scholar]
- 10. Kobayashi N., Nishi T., Hirata T., Kihara A., Sano T., Igarashi Y., Yamaguchi A. (2006) J. Lipid Res. 47, 614–621 [DOI] [PubMed] [Google Scholar]
- 11. Kobayashi N., Kobayashi N., Yamaguchi A., Nishi T. (2009) J. Biol. Chem. 284, 21192–21200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kawahara A., Nishi T., Hisano Y., Fukui H., Yamaguchi A., Mochizuki N. (2009) Science 323, 524–527 [DOI] [PubMed] [Google Scholar]
- 13. Brinkmann V. (2007) Pharmacol. Ther. 115, 84–105 [DOI] [PubMed] [Google Scholar]
- 14. Chiba K., Yanagawa Y., Masubuchi Y., Kataoka H., Kawaguchi T., Ohtsuki M., Hoshino Y. (1998) J. Immunol. 160, 5037–5044 [PubMed] [Google Scholar]
- 15. Budde K., Schütz M., Glander P., Peters H., Waiser J., Liefeldt L., Neumayer H. H., Böhler T. (2006) Clin. Transplant. 20, 17–24 [DOI] [PubMed] [Google Scholar]
- 16. Thangada S., Khanna K. M., Blaho V. A., Oo M. L., Im D. S., Guo C., Lefrancois L., Hla T. (2010) J. Exp. Med. 207, 1475–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yatomi Y., Ruan F., Hakomori S., Igarashi Y. (1995) Blood 86, 193–202 [PubMed] [Google Scholar]
- 18. Hänel P., Andréani P., Gräler M. H. (2007) FASEB J. 21, 1202–1209 [DOI] [PubMed] [Google Scholar]
- 19. Anada Y., Igarashi Y., Kihara A. (2007) Eur. J. Pharmacol. 568, 106–111 [DOI] [PubMed] [Google Scholar]
- 20. Sato K., Malchinkhuu E., Horiuchi Y., Mogi C., Tomura H., Tosaka M., Yoshimoto Y., Kuwabara A., Okajima F. (2007) J. Neurochem. 103, 2610–2619 [DOI] [PubMed] [Google Scholar]
- 21. Mitra P., Oskeritzian C. A., Payne S. G., Beaven M. A., Milstien S., Spiegel S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 16394–16399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Honig S. M., Fu S., Mao X., Yopp A., Gunn M. D., Randolph G. J., Bromberg J. S. (2003) J. Clin. Invest. 111, 627–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takabe K., Kim R. H., Allegood J. C., Mitra P., Ramachandran S., Nagahashi M., Harikumar K. B., Hait N. C., Milstien S., Spiegel S. (2010) J. Biol. Chem. 285, 10477–10486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nieuwenhuis B., Lüth A., Chun J., Huwiler A., Pfeilschifter J., Schäfer-Korting M., Kleuser B. (2009) J. Mol. Med. 87, 645–657 [DOI] [PubMed] [Google Scholar]
- 25. Min J. K., Yoo H. S., Lee E. Y., Lee W. J., Lee Y. M. (2002) Anal. Biochem. 303, 167–175 [DOI] [PubMed] [Google Scholar]
- 26. Paugh S. W., Payne S. G., Barbour S. E., Milstien S., Spiegel S. (2003) FEBS Lett. 554, 189–193 [DOI] [PubMed] [Google Scholar]
- 27. Billich A., Bornancin F., Dévay P., Mechtcheriakova D., Urtz N., Baumruker T. (2003) J. Biol. Chem. 278, 47408–47415 [DOI] [PubMed] [Google Scholar]
- 28. Kihara A., Igarashi Y. (2008) Biochim. Biophys. Acta 1781, 496–502 [DOI] [PubMed] [Google Scholar]
- 29. Liu H., Sugiura M., Nava V. E., Edsall L. C., Kono K., Poulton S., Milstien S., Kohama T., Spiegel S. (2000) J. Biol. Chem. 275, 19513–19520 [DOI] [PubMed] [Google Scholar]
- 30. Brinkmann V., Davis M. D., Heise C. E., Albert R., Cottens S., Hof R., Bruns C., Prieschl E., Baumruker T., Hiestand P., Foster C. A., Zollinger M., Lynch K. R. (2002) J. Biol. Chem. 277, 21453–21457 [DOI] [PubMed] [Google Scholar]
- 31. Bandhuvula P., Tam Y. Y., Oskouian B., Saba J. D. (2005) J. Biol. Chem. 280, 33697–33700 [DOI] [PubMed] [Google Scholar]
- 32. Berdyshev E. V., Gorshkova I., Skobeleva A., Bittman R., Lu X., Dudek S. M., Mirzapoiazova T., Garcia J. G., Natarajan V. (2009) J. Biol. Chem. 284, 5467–5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kihara A., Ikeda M., Kariya Y., Lee E. Y., Lee Y. M., Igarashi Y. (2003) J. Biol. Chem. 278, 14578–14585 [DOI] [PubMed] [Google Scholar]
- 34. Pewzner-Jung Y., Ben-Dor S., Futerman A. H. (2006) J. Biol. Chem. 281, 25001–25005 [DOI] [PubMed] [Google Scholar]
- 35. Brinkmann V. (2004) Yonsei Med. J. 45, 991–997 [DOI] [PubMed] [Google Scholar]
- 36. Igarashi N., Okada T., Hayashi S., Fujita T., Jahangeer S., Nakamura S. (2003) J. Biol. Chem. 278, 46832–46839 [DOI] [PubMed] [Google Scholar]
- 37. Maceyka M., Sankala H., Hait N. C., Le Stunff H., Liu H., Toman R., Collier C., Zhang M., Satin L. S., Merrill A. H., Jr., Milstien S., Spiegel S. (2005) J. Biol. Chem. 280, 37118–37129 [DOI] [PubMed] [Google Scholar]
- 38. Mandala S., Hajdu R., Bergstrom J., Quackenbush E., Xie J., Milligan J., Thornton R., Shei G. J., Card D., Keohane C., Rosenbach M., Hale J., Lynch C. L., Rupprecht K., Parsons W., Rosen H. (2002) Science 296, 346–349 [DOI] [PubMed] [Google Scholar]
- 39. Kappos L., Radue E. W., O'Connor P., Polman C., Hohlfeld R., Calabresi P., Selmaj K., Agoropoulou C., Leyk M., Zhang-Auberson L., Burtin P. (2010) N. Engl. J. Med. 362, 387–401 [DOI] [PubMed] [Google Scholar]
- 40. Kovarik J. M., Schmouder R., Barilla D., Wang Y., Kraus G. (2004) Br. J. Clin. Pharmacol. 57, 586–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee Y. M., Venkataraman K., Hwang S. I., Han D. K., Hla T. (2007) Prostaglandins Other Lipid Mediat. 84, 154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.