Abstract
Integrin transmembrane (TM) and/or cytoplasmic domains play a critical role in integrin bidirectional signaling. Although it has been shown that TM and/or cytoplasmic α and β domains associate in the resting state and separation of these domains is required for both inside-out and outside-in signaling, the role of TM homomeric association remains elusive. Formation of TM homo-oligomers was observed in micelles and bacterial membranes previously, and it has been proposed that homomeric association is important for integrin activation and clustering. This study addresses whether integrin TM domains form homo-oligomers in mammalian cell membranes using cysteine scanning mutagenesis. Our results show that TM homomeric interaction does not occur before or after soluble ligand binding or during inside-out activation. In addition, even though the cysteine mutants and the heterodimeric disulfide-bounded mutant could form clusters after adhering to immobilized ligand, the integrin TM domains do not form homo-oligomers, suggesting that integrin TM homomeric association is not critical for integrin clustering or outside-in signaling. Therefore, integrin TM homo-oligomerization is not required for integrin activation, ligand binding, or signaling.
Keywords: Cell Adhesion, Cell Surface Receptor, Fibrinogen, Integrin, Membrane Proteins, Signal Transduction
Introduction
Integrins are cell adhesion molecules that are essential for many biological functions such as cell migration, survival, and differentiation. These functions are accomplished by integrin bidirectional signaling across the cell membrane. Inside-out activation occurs when specific intracellular molecules such as talin and kindlin bind to the integrin cytoplasmic domain, leading to integrin conformational change and therefore high affinity for extracellular ligands. Then, binding of multimeric extracellular ligands results in outside-in signaling that is critical for many cellular processes. It has been shown that integrin bidirectional signal transduction requires integrin structural change and lateral distribution (clustering) (1, 2).
Integrins are type I transmembrane (TM)2 proteins consisting of two noncovalently associated α and β subunits, each with a large extracellular domain, a single-span TM domain, and a short cytoplasmic domain. Recent structural studies have greatly advanced our understanding of integrin conformational change during inside-out activation (3–9). Even though relatively short, the integrin TM/cytoplasmic domains play a crucial role in this process. It has been shown that the association of α and β subunit TM/cytoplasmic domains is critical for stabilizing integrins in the resting state (10–16). When induced by binding of the β subunit cytoplasmic domain through talin or other intracellular molecules, the TM/cytoplasmic domains separate (17, 18), driving integrin extension and shifting the ligand binding α/β headpiece to an open, high affinity conformation (7, 19). Recently, the structure of the TM/cytoplasmic domains in the resting state was proposed based on Rosetta computational modeling and experimental data using intact integrins on mammalian cell surface (16). In this structure, the αIIb GXXXG motif and its counterparts in the β3 TM domains associate with ridge-in-groove packing, and the αIIb GFFKR motif and the β3 Lys716 in the cytoplasmic segments play a critical role in α/β association. The structures of complex and monomeric α and β subunit TM/cytoplasmic domains have also been solved by NMR (8, 9, 20, 21). These studies have shed light on the structural basis of integrin TM/cytoplasmic domain signaling across the plasma membrane (22).
In contrast to the role of heterodimeric TM/cytoplasmic domain association and dissociation, that of homo-oligomerization of integrin TM domains in integrin signaling remains elusive. In 2001, an NMR study of this region by Li et al. failed to detect heterodimeric association between the α and β subunit TM/cytoplasmic domains using TM/cytoplasmic fragment peptides in micelles. Instead, they observed that the α and β fragments tend to form homodimers and homotrimers, respectively (23). Later, αIIb and β3 TM helices were confirmed to form homo-oligomers in bacterial membranes using the TOXCAT assay (24, 25). The α and β homomeric interactions have been widely studied by computational modeling (26–28). These studies showed that the homo-oligomerization interface and the heterodimerization motifs largely overlap, but it seems that homomeric interaction is less specific than the heterodimeric interaction (25). In 2003, asparagine mutagenesis in the TM region of β3 subunit (with most experiments on the mutation β3G708N) suggested that TM homo-oligomerization contributes to integrin activation and clustering (29). However, the mutation β3G708N, which was reported to enhance trimerization in detergent and increase ligand binding avidity in the transfected CHO cells (29), was later found to activate the integrin by changing ligand binding affinity rather than valence (13). Furthermore, mutations that disrupted homodimerization of integrin TM domains, which also disrupted heterodimerization because the two interfaces overlap, were shown to activate integrins for ligand binding, suggesting that TM domain separation is sufficient to activate integrins (14). Therefore, it was proposed that integrin TM homo-oligomerization is not a critical step for inside-out activation, but instead, it may help to stabilize the integrin in the high affinity state and be important for outside- in signaling (24).
It has been shown that integrin outside-in signaling requires both conformational change and clustering of integrins. However, the mechanism of how integrins transmit these signals across the plasma membrane through the TM/cytoplasmic domains remains unknown. More specifically, it is unclear whether integrin TM homo-oligomerization plays any role in integrin clustering and signaling, even though it has been proposed that it provides structural basis for this process (29, 30). Importantly, although α and β TM homo-oligomerization was found in micelles and bacterial cell membranes (14,23,25,29,31), it has never been observed in mammalian cell membrane using full-length integrins. On the contrary, the TM heterodimeric but not homomeric association of integrin fragments was detected using mini-integrin affinity capture method in mammalian cell membranes (32). In this paper, we tested whether integrin TM domains form homo-oligomers in mammalian cell membranes using disulfide scanning of intact integrin αIIbβ3. Our results show that integrin TM domains do not form homo-oligomers before or after soluble ligand binding or during integrin inside-out and outside-in signaling.
EXPERIMENTAL PROCEDURES
Plasmid Construction and Transient Transfection
Plasmids coding for full-length human αIIb and β3 were subcloned into pEF/V5-HisA and pcDNA3.1/Myc-His(+), respectively (33). Amino acid substitution in TM and TM proximal regions to cysteine was made using site-directed mutagenesis with the QuikChange kit (Stratagene). Constructs were transfected into 293T cells (American Type Culture Collection) using a FuGENE transfection kit (Roche Applied Science) according to the manufacturer's instructions.
Immunoprecipitation
Twenty-four hours after transfection, 293T cells were treated with 20 μg/ml 2-bromopalmitate, metabolically labeled with [35S]cysteine/methionine for 1.5 h before adding chase medium containing 500 μg/ml cysteine and 100 μg/ml methionine, and cells were cultured for 17 h overnight (12). Then, cells were detached and suspended in Tris-buffered saline (TBS) (106 cells in 100 μl) supplemented with 5 mm Ca2+, 1 mm Mn2+ with 3 mg/ml fibrinogen or 1 mm Mn2+ with 50 μm RGD peptide and incubated at room temperature for 30 min. Then, saponin was added to a final concentration of 0.02%, and 20 μm CuSO4/100 μm o-phenanthroline was added by 10-fold dilution from stock solutions, and cells were incubated on ice for 10 min. Oxidation was quenched by adding an equal volume of TBS containing Ca2+ and 5 mm N-ethylmaleimide. Cells were lysed by the addition of an equal volume of 2% Triton X-100 and 0.1% Nonidet P-40 in the same buffer for 10 min on ice. Cell lysate was centrifuged and immunoprecipitated with 1 μg of anti-β3 mAb AP3 and protein G-Sepharose at 4 °C for 2 h. After three washes with lysis buffer, precipitated integrin was dissolved into 0.5% SDS sample buffer and subjected to nonreducing 7.5% SDS-PAGE and fluorography (12).
For αIIbW967C mutant, 2 mm DTT was added to the 35S-labeled cells and incubated at 37 °C for 10 min, washed twice with TBS, and then oxidized by Cu-phenanthroline on ice for 10 min. Oxidation was quenched by adding an equal volume of TBS containing Ca2+ and 5 mm N-ethylmaleimide. Cells were lysed, and integrins were immunoprecipitated as described above.
To test whether integrins form TM homomeric disulfide after adhering to the immobilized fibrinogen, 35S-labeled cells were seeded on the surface of 6-well plates precoated with immobilized fibrinogen and incubated at 37 °C for 1 h. After washing twice with TBS, adherent cells were incubated on ice with Cu-phenanthroline and saponin and quenched with 5 mm N-ethylmaleimide. Cells were lysed, and integrins were immunoprecipitated as described above.
Ligand-induced Binding Site (LIBS) Epitope Expression
LIBS epitope expression was measured as described (34). Briefly, transfected cells suspended in HEPES-buffered saline supplemented with 5.5 mm glucose and 1% bovine serum albumin (BSA) were incubated with either 5 mm Ca2+, 3 mg/ml fibrinogen, or 50 μm Gly-Arg-Gly-Asp-Ser-Pro peptide (GRGDSP) in the presence of 1 mm Mn2+ at 37 °C for 15 min, and then 10 μg/ml anti-LIBS1 antibody was added. After incubation on ice for 30 min, cells were washed and stained with FITC-labeled anti-mouse IgG on ice for 30 min. The stained cells were subjected to flow cytometry, and LIBS epitope expression was expressed as the percentage of mean fluorescence intensity of anti-LIBS antibody relative to mean fluorescence intensity of the conformation-independent anti-β3 mAb AP3.
Cell Spreading, Integrin Clustering, and Microscopy
Glass bottom 6-well plates (MatTek Corporation, Ashland, MA) were coated overnight at 4 °C with 20 μg/ml human fibrinogen in phosphate-buffered saline (PBS) at pH 7.4 followed by blocking with 1% BSA at room temperature for 1 h. The transiently transfected HEK293T cells were detached by trypsin/EDTA and washed three times with DMEM. Cells were seeded on fibrinogen-coated dishes. After incubation at 37 °C for 1 h, cells were washed 3 times with PBS and fixed with 3.7% formaldehyde in PBS at room temperature for 10 min or for integrin clustering, 10 μg/ml anti-β3 mAb AP3 was added at room temperature for 30 min followed by staining with 10 μg/ml FITC-conjugated goat anti-mouse IgG for 30 min at room temperature before fixation. 400 nm cytochalasin D was added as control before seeding.
Differential interference contrast imaging was conducted on a Leica TCS SP2 spectral confocal system, coupled to a DM IRE2 inverted microscope with 63× oil objective. For the quantification of cell spreading area, outlines of 100 randomly selected adherent cells were generated, and the number of pixels contained within each of these regions was measured using ImageJ software (National Institutes of Health, Bethesda, MD).
Cell Adhesion
Cell adhesion on immobilized human fibrinogen was assessed by the measurement of cellular lactate dehydrogenase activity as described (1). Briefly, cells suspended in HEPES-buffered saline supplemented with 5.5 mm glucose, 1% bovine serum albumin, and 1 mm Ca2+ were added into flat bottom 12-well plates (1 × 105 cells/well) that had been precoated with 20 μg/ml fibrinogen and blocked with 1% BSA. After incubation at 37 °C for 1 h, wells were washed three times with HEPES-buffered saline supplemented as indicated above. Remaining adherent cells were lysed with 1% Triton X-100, and lactate dehydrogenase activity was assayed using the Cytotoxicity Detection Kit (lactate dehydrogenase) (Roche Applied Science) according to the manufacturer's instructions. Cell adhesion was expressed as a percentage of bound cells relative to total input cells.
RESULTS
Integrin TM Domains Do Not Form Homo-oligomers before or after Soluble Ligand Binding
Previously, cysteine mutagenesis and heterodimeric disulfide scanning were used to identify the integrin αIIb and β3 TM interface in mammalian cell membrane successfully (12, 16). Here, we used single-cysteine mutations of the αIIb and β3 TM region (αIIb residues 965–995 and β3 residues 691–723; Fig. 1A) and applied a similar method to determine whether the αIIb or β3 TM domains form homo-oligomers during integrin signaling. As suggested by the TOXCAT assay (24, 25) and predicted by computer modeling (26–28), the αIIb TM helix forms homodimers, and the β3 TM helix forms homotrimers, using surfaces that are similar to those in the heterodimeric interface. If this homomeric interface is actually formed in the mammalian cell membrane, we expected that some single-cysteine mutations of these residues would form homodimeric disulfide bonds in the presence of an oxidation catalyst such as Cu-phenanthroline. In addition, 2-bromopalmitate was used to block cysteine palmitoylation, which can inhibit disulfide formation; and saponin was used to increase the permeability of Cu-phenanthroline and the efficiency of disulfide bond formation as described previously (16). To confirm the efficiency of disulfide bond formation in the presence of Cu-phenanthroline, several cysteine pairs (one from α and the other from β) at the TM or TM proximal region, which have been shown to form heterodimeric disulfide bonds (12, 16), were used as controls.
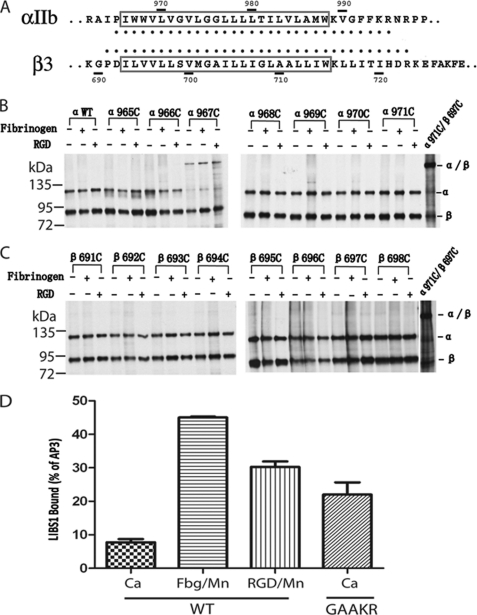
FIGURE 1.
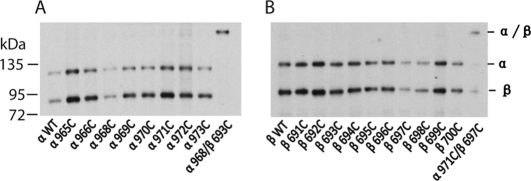
Integrin TM domains do not form homo-oligomers before and after soluble ligand binding. A, sequences of the αIIb and β3 TM regions are shown. Segments predicted as TM are boxed. Residues used for cysteine scanning in this study are indicated by heavy dots. B, lysates from 35S-labeled HEK293T cell transfectants were immunoprecipitated with mAb AP3. Precipitates were subjected to nonreducing 7.5% SDS-PAGE and fluorography. Except for αIIbW967C, none of the cysteine mutants of the αIIb TM regions formed homomeric disulfide bonds before or after soluble ligand binding. C, none of the cysteine mutants of the β3 TM regions formed homomeric disulfide bonds before or after soluble ligand binding. D, exposure of the LIBS1 epitope. LIBS epitope exposure was determined as the percentage of mean fluorescence intensity of anti-LIBS1 antibody relative to nonfunctional anti-β3 mAb AP3. Error bars are S.D.
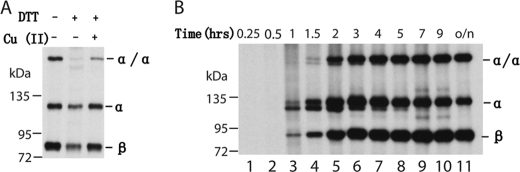
As shown in Fig. 1, the cysteine pair α971C/β697C formed a heterodimeric disulfide bond in the presence of Cu-phenanthroline (Fig. 1B), as described previously (12). By contrast, none of the single-cysteine mutants of αIIb TM regions formed homomeric disulfide bonds under these conditions except αIIbW967C (Figs. 1B and 2). As reported previously (12, 13), αIIbW967C, when co-transfected with wild-type β3, formed a homomeric disulfide bond in the resting state, and therefore, the αIIbβ3 integrin associated to form a disulfide-bridged tetramer (αIIbW967C/β3)2. This residue is located outward, away from the heterodimeric interface or predicted homodimeric interface (12, 13). When DTT was added, the disulfide bond was reduced. After DTT was removed and Cu-phenanthroline was added to catalyze disulfide formation, only a trace amount of homomeric disulfide was formed on the cell surface (Fig. 2A). We further traced the disulfide bond formation of αIIbW967C mutant after 35S labeling by lysing cells and immunoprecipitating the protein at different time points (Fig. 2B). After 30 min of labeling, αIIb precursor was formed. Then after 1 h, mature αIIb subunit was formed, and a trace amount of disulfide-bonded αIIb was observed. In 1.5 h, a significant amount of disulfide-bonded dimeric αIIbW967C was formed. This suggests that the homomeric disulfide bond is formed during biosynthesis and post-translational modification. Because this disulfide bond of the αIIb mutant is formed during biosynthesis and in the resting state in which heterodimeric TM association is not affected, we excluded this mutant for the following studies. None of the other 30 αIIb cysteine mutants formed a homomeric disulfide bond (Fig. 1B and supplemental Fig. 1), and neither did any of the 34 β3 cysteine mutants (Fig. 1C and supplemental Fig. 2). These results suggest that the αIIb and β3 TM helices do not form homomeric associations when αIIbβ3 is in the resting state.
FIGURE 2.
The homomeric disulfide bond of the αIIbW967C was formed during biosynthesis. A, formation of the homomeric disulfide bond after DTT treatment. The 35S-labeled cells were treated with or without 2 mm DTT at 37 °C for 10 min. After washing with TBS, DTT-treated cells were incubated with or without Cu-Phenanthroline. B, tracing the formation of disulfide bond of αIIb W967C mutant after labeling. The cells were lysed at different periods of time after 35S labeling, and integrin αIIbβ3 was immunoprecipitated and subjected to 7.5% nonreducing SDS-PAGE. The additional bands of the α and α/α are probably due to the precursors of the αIIb subunit.
To determine whether the α and β TM domains form homo-oligomers after soluble ligand binding, we used Mn2+ to activate integrin αIIbβ3 followed by binding of soluble fibrinogen and ligand-mimetic RGD peptide. It has been shown that ligand binding induces integrin conformational changes that expose the LIBS epitopes, which are located at the interfaces between the headpiece and tailpiece and between the α leg and β leg so that they are buried in the bent conformation but exposed in the extended conformation (35, 36). We used anti-β3 LIBS mAb LIBS1 (37) to determine the conformational states of the αIIbβ3 in the presence of Mn2+ with fibrinogen or RGD. The LIBS1 bound poorly to wild-type αIIbβ3 in Ca2+ (Fig. 1D), suggesting that it is in the bent conformation. The binding significantly increased when fibrinogen with Mn2+ or RGD with Mn2+ was added (Fig. 1D), suggesting that these ligands bound to integrins, and they stabilized integrins in the more extended conformation. Disulfide scanning was used to test whether α and β TM domains form homomeric interface after integrin bound to soluble fibrinogen and RGD peptide under the same condition (Fig. 1, B and C, and supplemental Figs. 1 and 2), and no homomeric disulfide formation was observed for any mutants except the αW967C as described above, suggesting that after ligand binding, even though integrins are stabilized in the more extended state and probably with two separating legs and TM/cytoplasmic tails, homo-oligomerization does not occur for TM/cytoplasmic tails in mammalian cell membrane.
Integrin TM Domains Do Not Form Homo-oligomers during Inside-out Signaling
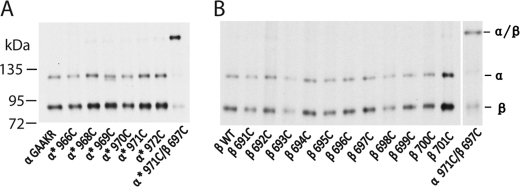
We designed experiments to test whether the integrin TM region forms homo-oligomers during inside-out activation. It is well known that the GFFKR motif in the αIIb cytoplasmic domain is crucial for maintaining integrin in the resting state. When the two phenylalanines of the GFFKR motif are mutated to alanines (GAAKR mutant), integrins are activated to bind ligands with high affinity. Thus, this GAAKR mutant can be used to mimic integrin inside-out activation (12). We confirmed that the mutant bound fibrinogen constitutively; in addition, it bound LIBS1 much better than the wild-type in the Ca2+ condition (Fig. 1D), indicating that the mutation shifts integrin toward more extended conformation, well mimicking inside-out signaling. We made six single-cysteine mutants using this GAAKR construct and co-transfected them with the wild-type β3 integrin. None of the αIIb integrin cysteine mutants formed a homomeric disulfide bond (Fig. 3). Similar results were obtained when 34 β3 single-cysteine mutants were co-transfected with the αIIb GAAKR mutant; none of these mutants formed a homomeric disulfide bond (Fig. 3). These results strongly suggest that during integrin inside-out activation across the mammalian cell membrane, TM homomeric association does not occur. Therefore, the TM homo-oligomerization does not play any role in this process.
FIGURE 3.
Integrin TM domains do not form homo-oligomers during inside-out activation. Samples were processed as in Fig. 1 and subjected to nonreducing 7.5% SDS-PAGE and fluorography. A, cysteine mutations of the αIIb GAAKR mutant were co-transfected with wild-type β3 integrin. No homomeric disulfide bond was formed for any of the αIIb cysteine mutants. B, cysteine mutations of the β3 TM region were co-transfected with the αIIb GAAKR mutant. No homomeric disulfide bond was formed for any of the β3 cysteine mutants.
Integrin TM Domains Do Not Form Homo-oligomers after Adhering to Immobilized Fibrinogen
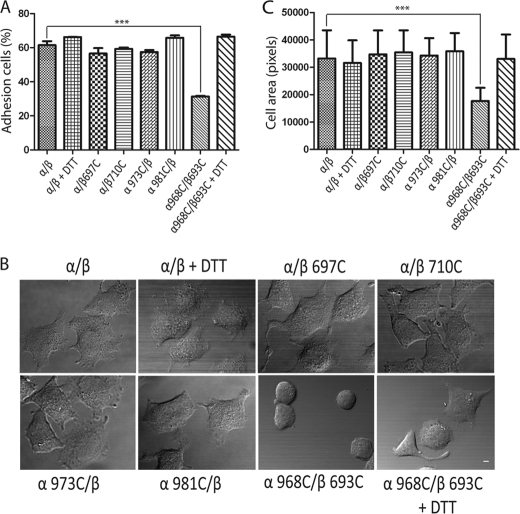
After binding to immobilized ligands, integrins on the mammalian cell surface will transmit outside-in signals. We carried out a series of experiments to address whether TM homomers form during this process. We randomly chose two αIIb and two β3 cysteine mutants and used them for cell adhesion and cell spreading assay. HEK293T cells transiently transfected with wild-type and mutant αIIbβ3 were seeded on fibrinogen-precoated dish surfaces at 37 °C for 1 h. The amount of adherent cells was assessed by quantifying the cellular lactate dehydrogenase activity. All selected mutants could adhere to the immobilized fibrinogen similarly to the wild-type receptor, whereas the disulfide-bounded mutant (α968C/β693C) showed much less adhesion, and DTT treatment which was used to disrupt the disulfide bond was able to recover its adhesion to the similar level to the wild-type with DTT (Fig. 4A). To test whether the single-cysteine mutations could affect cell spreading, HEK293T transient transfectants were coated on the immobilized fibrinogen at 37 °C for 1 h, followed by fixation and microscopic analysis. Cells transfected with all single-cysteine mutants demonstrated cell spreading to a level similar to those transfected with the wild-type receptor. By contrast, the disulfide-bounded mutant (α968C/β693C) showed no spreading, and DTT treatment restored cell spreading (Fig. 4, B and C). These results confirmed that dissociation of the TM heterodimers is required for integrin outside-in signaling as described previously using CHO cell transfectants (1). We also chose four cysteine mutants (αG972C, αG976C, βL705C, and βL712C), which were proposed to be critical for the homomeric interactions (25, 31), and used them for the cell spreading assay. Cells transfected with all mutants showed cell spreading similar to those transfected with the wild-type integrin (data not shown). Taken together, our results indicate that the single-cysteine mutations in this region do not affect the integrin adhesion to immobilized fibrinogen, nor do they affect outside-in signaling.
FIGURE 4.
Cell adhesion and spreading of randomly selected TM cysteine mutants. A, adhesion of HEK293T transfectants in the presence of 1 mm Ca2+ to surfaces coated with 20 μg/ml fibrinogen. The amount of bound cells was determined by measuring lactate dehydrogenase activity as described under “Experimental Procedures.” Data are representative of three independent experiments, each in triplicate. B, differential interference contrast images of HEK293T transfectants after adhering to immobilized fibrinogen at 37 °C. The images are representative of three independent experiments. Scale bar, 10 μm. C, quantification of the areas of adhering/spreading cells as described under “Experimental Procedures.” Error bars are S.D. ***, p < 0.001.
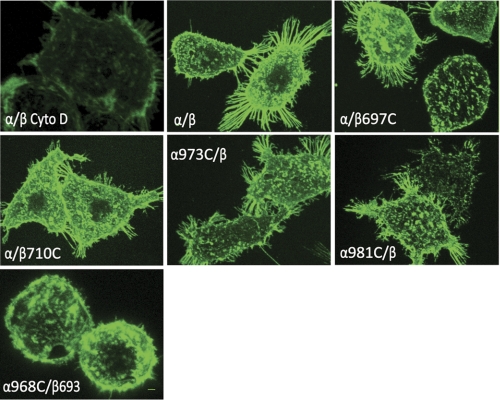
It is widely believed that lateral association (i.e. “clustering”) of integrin heterodimers, which occurs as a consequence of multivalent ligand binding (38, 39), plays a major role in outside-in signaling (40–42) (for review, see Ref. 43). It was also shown that ligand binding can directly lead to and stabilize separation of integrin cytoplasmic domains (44), and this integrin conformational change is critical for outside-in signaling as well (1). However, it remains unclear whether the integrin TM homomeric interaction after the TM helix separation is critical for integrin clustering. To assess formation of integrin clustering, HEK293T transfectants were allowed to adhere to fibrinogen-coated substrates followed by fixation and staining with fluorescent anti-αIIbβ3 antibodies. Under these conditions, wild-type integrins and all cysteine mutants could be readily detected in clustered patterns (Fig. 5). Interestingly, although the disulfide-bounded α968C/β693C mutant is defective for cell spreading, it formed similar cell clusters as others, in contrast to the negative control in which the actin filament disruptor cytochalasin D prevented integrin clustering (Figs. 4 and 5).
FIGURE 5.
Integrin clustering of selected TM cysteine mutants. Confocal microscopy studies of integrin clustering on the cell surface are shown. Cells expressing αIIbβ3 wild type and mutants were seeded on fibrinogen-coated surface for 1 h at 37 °C in the presence or absence of cytochalasin D (Cyto D). Attached cells were then stained with anti-β3 mAb AP3 in the presence or absence of cytochalasin D for 30 min at room temperature, followed by staining with FITC-conjugated goat anti-mouse IgG for 30 min at room temperature. After fixation, cells were subjected to confocal microscopy.
We then further determined, under the same conditions, whether integrin TM domains formed homo-oligomers in the plasma membrane. HEK293T cells transfected with a variety of αIIb and β3 cysteine mutants were seeded on a fibrinogen substrate at 37 °C for 1 h. After cells fully adhered and spread on immobilized fibrinogen, Cu-phenanthroline was added to catalyze disulfide bond formation. Four cysteine mutation pairs, α968C/β693C, α971C/β697C, α972C/β697C, and α955C/β723C, were used as controls to confirm the efficiency of oxidation. Under this condition, four cysteine pairs formed disulfides efficiently. By contrast, none of the αIIb or β3 TM cysteine mutations formed a homomeric disulfide bond (Figs. 6 and supplemental Fig. 3). Because these integrins adhered to the immobilized fibrinogen, clustered on the cell surface and transmitted outside-in signaling leading to cell spreading, the results suggest that homomeric association of the integrin TM domains is not important for integrin functions. Similar results were obtained when CHO cells were used (data not shown), suggesting that integrin TM/cytoplasmic domains do not form homomeric interaction during integrin outside-in signaling in mammalian cells.
FIGURE 6.
Integrin TM domains do not form homo-oligomers after cells adhering to the immobilized fibrinogen. Samples were processed as in Fig. 1 and subjected to nonreducing 7.5% SDS-PAGE and fluorography. A, none of the αIIb cysteine mutants formed a homomeric disulfide bond after adhering to the immobilized fibrinogen. B, none of the β3 cysteine mutants formed a homomeric disulfide bond after adhering to the immobilized fibrinogen.
DISCUSSION
It has been shown that TM heterodimeric association stabilizes integrins in the resting state; when integrins are activated by intracellular signals, two TM/cytoplasmic tails separate, leading to conformational change of the ligand binding extracellular regions, resulting in high affinity for ligands (5, 8, 9, 12, 13, 16–18, 45). Therefore, equilibrium between the dissociated monomers and associated heterodimers of the TM domains is critical for integrin inside-out activation. Based on the observation that integrin TM helices formed homo-oligomers using recombinant peptides in detergent (23, 29, 31), the GALLEX assay in bacteria (45), the TOXCAT assay in bacteria (24, 25), and the computational modeling (26–28), TM homomeric association has been suggested to be important for integrin activation (14, 29, 31). Mutagenesis studies on the proposed TM homomeric interface residues showed that these mutations could lead to integrin activation, suggesting that heterodimer dissociation is sufficient to activate integrins (14). Therefore, a push-pull model was proposed in which after dissociation of the TM heterodimer, integrins are stabilized by TM homomeric association (14).
Here, our experiments on mammalian cell transfectants using full-length integrin αIIbβ3 showed that the αIIb and β3 TM domains do not form any homomeric association in the resting state, nor do they form after soluble ligand binding. The only mutant that formed a homomeric disulfide is αIIbW967C, as reported previously (12, 13). We found that this homomeric disulfide was formed during biosynthesis and in the integrin resting state, and dissociation of the TM heterodimer is not required for its formation. Therefore, we do not think that the same homomeric association occurs during integrin inside-out activation or outside-in signaling. We found that except for this mutant, none of the other 30 αIIb and 34 β3 cysteine mutants formed a homomeric disulfide bond before or after binding to soluble ligands. In addition, during integrin inside-out activation, the integrin TM domains do not form any homomeric association that can be detected by disulfide scanning. Integrins must be regulated to be activated and deactivated quickly in mammalian cells, even within seconds. It has been shown that detachment of integrin from ligands in the trailing edge is critical for integrin functions (46), and mutations that constitutively active integrins result in malfunctions (47–50). Therefore, it is unlikely that integrin TM domains form more stable homomeric association during inside-out signaling.
We further showed that TM homomers do not form upon to binding to ligands on a substrate and subsequent integrin clustering. Although integrin clustering is known to participate in integrin signaling pathways (40, 51–53), the exact mechanisms of integrin clustering remain elusive. One mechanism proposes that the integrin TM homo-oligomerization promotes integrin clustering (29). Studies have established that extracellular ligand binding triggers integrin conformational changes that promote oligomer assembly through TM fragments (54–56). In this study, we observed that after adhering to immobilized fibrinogen integrins clustered on the cell surface, but we did not detect any integrin TM homomeric interaction under this condition. Interestingly, the heterodimeric disulfide-bonded integrin formed clusters, suggesting that even the dissociation of integrin α/β TM domains is not required for integrin clustering. In addition, no evidence has been shown that the integrin extracellular domains form homomeric interaction, and therefore, it is unlikely that they induce integrin clustering during signaling. Taken together, our study suggests that integrin clustering is likely induced by bringing several integrins physically close together by multimeric ligands, and the TM/cytoplasmic domain homomeric association is not required for this process. Additional evidence is needed to define the mechanisms of integrin clustering further.
Even though the TM separation is not required for integrin clustering, it is critical for integrin outside-in signaling (1). We showed that all cysteine mutants could adhere and spread on the fibrinogen-coated surface, suggesting that they could transmit signals into the cells after binding to immobilized ligands. However, we did not observe any TM homomeric disulfide formation in the same condition, indicating that the TM homomeric association does not occur during integrin outside-in signaling. Based on the previous FRET studies (44), we assume that ligand-induced TM separation is likely coupled to cytoplasmic domain separation, implying that α-β cytoplasmic domain interactions somehow constrain or inhibit kinase activation (1, 13, 17, 44, 57). One possibility is that separation of TM and cytoplasmic domains induced by multimeric ligand binding is required for kinases or other intracellular proteins to bind integrin cytoplasmic tails. On the other hand, some studies have shown that certain tyrosine kinases, such as Src, associate constitutively with integrin αIIbβ3 in platelets, and platelet adhesion to fibrinogen causes a rapid increase in Src activation (58). Thus, another possibility is that the association of the integrin TM and cytoplasmic domains somehow constrains the activity of integrin tail-bound kinases, whereas dissociation of the TM and cytoplasmic domains releases these constraints. In either case, binding of multimeric ligands brings integrins together and at the same time, induces dissociation of the TM/cytoplasmic domains, resulting in kinase autophosphorylation and activation. The current study strongly suggests that during this process, the TM homomeric association does not play any role in kinase activation.
In conclusion, our study shows that the integrin TM homomeric association does not occur in mammalian cell membranes before or after soluble ligand binding, during inside-out activation or outside-in signaling. Therefore, conformational change induced by intracellular signals or extracellular ligands, more specifically, separation of TM and cytoplasmic domains, but not the homomeric association, is critical for integrin bidirectional signaling.
Supplementary Material
This work was supported by American Heart Association Grant 10GRNT3960011 and Louisiana Board of Regents Grant LEQSF(2009-12)-RD-A07.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
- TM
- transmembrane
- LIBS
- ligand-induced binding site.
REFERENCES
- 1. Zhu J., Carman C. V., Kim M., Shimaoka M., Springer T. A., Luo B. H. (2007) Blood 110, 2475–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Askari J. A., Tynan C. J., Webb S. E., Martin-Fernandez M. L., Ballestrem C., Humphries M. J. (2010) J. Cell Biol. 188, 891–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xiong J. P., Stehle T., Diefenbach B., Zhang R., Dunker R., Scott D. L., Joachimiak A., Goodman S. L., Arnaout M. A. (2001) Science 294, 339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xiong J. P., Stehle T., Zhang R., Joachimiak A., Frech M., Goodman S. L., Arnaout M. A. (2002) Science 296, 151–155 [DOI] [PubMed] [Google Scholar]
- 5. Vinogradova O., Velyvis A., Velyviene A., Hu B., Haas T., Plow E., Qin J. (2002) Cell 110, 587–597 [DOI] [PubMed] [Google Scholar]
- 6. Xiao T., Takagi J., Coller B. S., Wang J. H., Springer T. A. (2004) Nature 432, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu J., Luo B. H., Xiao T., Zhang C., Nishida N., Springer T. A. (2008) Mol. Cell 32, 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lau T. L., Kim C., Ginsberg M. H., Ulmer T. S. (2009) EMBO J. 28, 1351–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang J., Ma Y. Q., Page R. C., Misra S., Plow E. F., Qin J. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 17729–17734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hughes P. E., Diaz-Gonzalez F., Leong L., Wu C., McDonald J. A., Shattil S. J., Ginsberg M. H. (1996) J. Biol. Chem. 271, 6571–6574 [DOI] [PubMed] [Google Scholar]
- 11. Lu C., Takagi J., Springer T. A. (2001) J. Biol. Chem. 276, 14642–14648 [DOI] [PubMed] [Google Scholar]
- 12. Luo B. H., Springer T. A., Takagi J. (2004) PLoS Biol. 2, e153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luo B. H., Carman C. V., Takagi J., Springer T. A. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 3679–3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li W., Metcalf D. G., Gorelik R., Li R., Mitra N., Nanda V., Law P. B., Lear J. D., Degrado W. F., Bennett J. S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 1424–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Partridge A. W., Liu S., Kim S., Bowie J. U., Ginsberg M. H. (2005) J. Biol. Chem. 280, 7294–7300 [DOI] [PubMed] [Google Scholar]
- 16. Zhu J., Luo B. H., Barth P., Schonbrun J., Baker D., Springer T. A. (2009) Mol. Cell 34, 234–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anthis N. J., Haling J. R., Oxley C. L., Memo M., Wegener K. L., Lim C. J., Ginsberg M. H., Campbell I. D. (2009) J. Biol. Chem. 284, 36700–36710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moser M., Legate K. R., Zent R., Fässler R. (2009) Science 324, 895–899 [DOI] [PubMed] [Google Scholar]
- 19. Wegener K. L., Campbell I. D. (2008) Mol. Membr. Biol. 25, 376–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lau T. L., Partridge A. W., Ginsberg M. H., Ulmer T. S. (2008) Biochemistry 47, 4008–4016 [DOI] [PubMed] [Google Scholar]
- 21. Lau T. L., Dua V., Ulmer T. S. (2008) J. Biol. Chem. 283, 16162–16168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang W., Luo B. H. (2010) J. Cell. Biochem. 109, 447–452 [DOI] [PubMed] [Google Scholar]
- 23. Li R., Babu C. R., Lear J. D., Wand A. J., Bennett J. S., DeGrado W. F. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 12462–12467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li R., Gorelik R., Nanda V., Law P. B., Lear J. D., DeGrado W. F., Bennett J. S. (2004) J. Biol. Chem. 279, 26666–26673 [DOI] [PubMed] [Google Scholar]
- 25. Zhu H., Metcalf D. G., Streu C. N., Billings P. C., Degrado W. F., Bennett J. S. (2010) J. Mol. Biol. 401, 882–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gottschalk K. E., Adams P. D., Brunger A. T., Kessler H. (2002) Protein Sci. 11, 1800–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gottschalk K. E., Kessler H. (2004) Structure 12, 1109–1116 [DOI] [PubMed] [Google Scholar]
- 28. Lin X., Tan S. M., Law S. K., Torres J. (2006) Proteins 63, 16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li R., Mitra N., Gratkowski H., Vilaire G., Litvinov R., Nagasami C., Weisel J. W., Lear J. D., DeGrado W. F., Bennett J. S. (2003) Science 300, 795–798 [DOI] [PubMed] [Google Scholar]
- 30. Li R., Bennett J. S., Degrado W. F. (2004) Biochem. Soc. Trans. 32, 412–415 [DOI] [PubMed] [Google Scholar]
- 31. Parthasarathy K., Lin X., Tan S. M., Law S. K., Torres J. (2008) Protein Sci. 17, 930–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim C., Lau T. L., Ulmer T. S., Ginsberg M. H. (2009) Blood 113, 4747–4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takagi J., Petre B. M., Walz T., Springer T. A. (2002) Cell 110, 599–611 [DOI] [PubMed] [Google Scholar]
- 34. Luo B. H., Springer T. A., Takagi J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 2403–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beglova N., Blacklow S. C., Takagi J., Springer T. A. (2002) Nat. Struct. Biol. 9, 282–287 [DOI] [PubMed] [Google Scholar]
- 36. Takagi J., Springer T. A. (2002) Immunol. Rev. 186, 141–163 [DOI] [PubMed] [Google Scholar]
- 37. Du X., Gu M., Weisel J. W., Nagaswami C., Bennett J. S., Bowditch R., Ginsberg M. H. (1993) J. Biol. Chem. 268, 23087–23092 [PubMed] [Google Scholar]
- 38. Kim M., Carman C. V., Yang W., Salas A., Springer T. A. (2004) J. Cell Biol. 167, 1241–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buensuceso C., de Virgilio M., Shattil S. J. (2003) J. Biol. Chem. 278, 15217–15224 [DOI] [PubMed] [Google Scholar]
- 40. Hato T., Pampori N., Shattil S. J. (1998) J. Cell Biol. 141, 1685–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miyamoto S., Akiyama S. K., Yamada K. M. (1995) Science 267, 883–885 [DOI] [PubMed] [Google Scholar]
- 42. Miyamoto S., Teramoto H., Coso O. A., Gutkind J. S., Burbelo P. D., Akiyama S. K., Yamada K. M. (1995) J. Cell Biol. 131, 791–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shattil S. J., Newman P. J. (2004) Blood 104, 1606–1615 [DOI] [PubMed] [Google Scholar]
- 44. Kim M., Carman C. V., Springer T. A. (2003) Science 301, 1720–1725 [DOI] [PubMed] [Google Scholar]
- 45. Schneider D., Engelman D. M. (2004) J. Biol. Chem. 279, 9840–9846 [DOI] [PubMed] [Google Scholar]
- 46. Sáchez-Madrid F., del Pozo M. A. (1999) EMBO J. 18, 501–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Semmrich M., Smith A., Feterowski C., Beer S., Engelhardt B., Busch D. H., Bartsch B., Laschinger M., Hogg N., Pfeffer K., Holzmann B. (2005) J. Exp. Med. 201, 1987–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Park E. J., Mora J. R., Carman C. V., Chen J., Sasaki Y., Cheng G., von Andrian U. H., Shimaoka M. (2007) J. Clin. Invest. 117, 2526–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Imai Y., Park E. J., Peer D., Peixoto A., Cheng G., von Andrian U. H., Carman C. V., Shimaoka M. (2008) Blood 112, 5007–5015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Park E. J., Peixoto A., Imai Y., Goodarzi A., Cheng G., Carman C. V., von Andrian U. H., Shimaoka M. (2010) Blood 115, 1572–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kornberg L. J., Earp H. S., Turner C. E., Prockop C., Juliano R. L. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 8392–8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kornberg L., Earp H. S., Parsons J. T., Schaller M., Juliano R. L. (1992) J. Biol. Chem. 267, 23439–23442 [PubMed] [Google Scholar]
- 53. Whitlock B. B., Gardai S., Fadok V., Bratton D., Henson P. M. (2000) J. Cell Biol. 151, 1305–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hantgan R. R., Paumi C., Rocco M., Weisel J. W. (1999) Biochemistry 38, 14461–14474 [DOI] [PubMed] [Google Scholar]
- 55. Hantgan R. R., Lyles D. S., Mallett T. C., Rocco M., Nagaswami C., Weisel J. W. (2003) J. Biol. Chem. 278, 3417–3426 [DOI] [PubMed] [Google Scholar]
- 56. Hantgan R. R., Gibbs W., Stahle M. C., Aster R. H., Peterson J. A. (2004) Biochim. Biophys. Acta 1700, 19–25 [DOI] [PubMed] [Google Scholar]
- 57. Ye F., Hu G., Taylor D., Ratnikov B., Bobkov A. A., McLean M. A., Sligar S. G., Taylor K. A., Ginsberg M. H. (2010) J. Cell Biol. 188, 157–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Obergfell A., Eto K., Mocsai A., Buensuceso C., Moores S. L., Brugge J. S., Lowell C. A., Shattil S. J. (2002) J. Cell Biol. 157, 265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.