Abstract
Retinoic X receptor (RXR) is a promising target for drug discovery against cancer and metabolic syndromes. Here, we identified a specific RXRα antagonist, danthron, from the traditional Chinese medicine rhubarb. Danthron repressed all tested RXRα-involved response element transcription, including the RXRE, PPRE, FXRE, and LXRE. Results from native PAGE and isothermal titration calorimetry (ITC)-based assays indicated that danthron bound to the tetrameric RXRα-LBD in a specific stoichimetric ratio, and such a binding could influence the corepressor SMRT affinity to the receptor. Additionally, a unique tetrameric structure of the apo-RXRα ligand-binding domain (LBD) was determined, which exhibited a larger tetramer interface and different ligand-binding pocket size compared with the one previously reported. Together with the biochemical and biophysical results, the determined crystal structure of danthron-soaked RXRα-LBD suggested a new mechanism for danthron antagonism to tetrameric RXRα. Moreover, the in vivo efficient improvement of insulin sensitivity by danthron was observed in diet-induced obese (DIO) mice. Thus, our findings were expected to supply new insights into the structural basis of RXRα antagonist for its further potential therapeutic application.
Keywords: Crystal Structure, Drug Action, Metabolic Syndrome, Nuclear Receptors, Protein-Drug Interactions
Introduction
Retinoic X receptor (RXR),4 as a member of nuclear receptors (NRs), plays a central role in NR-regulated signaling pathways, for its obligate heterodimer partnership with almost one-third of the family members, including peroxisome proliferator-activated receptor (PPAR), farnesoid X receptor (FXR), and liver X receptor (LXR) (1). RXR also self-associates into homodimer or homotetramer in the active or auto-repressed state (2). It is involved in a broad spectrum of biological processes such as cell growth, differentiation, metabolism, morphogenesis, and embryogenic development (3, 4). Therefore, RXR has been considered as an important target for drug discovery in the treatment of cancer and metabolic syndromes (5, 6).
In structure, RXR exhibits typical features of nuclear receptor family. It primarily consists of a central DNA-binding domain and a carboxyl-terminal ligand-binding domain (LBD) (7). The multifunctional LBD is responsible for RXR dimerization, tetramerization, and ligand-induced activation (8). It is suggested that RXR exists predominately in inactive homotetramer in the absence of ligand in vivo and dissociates upon ligand binding to form homodimer or heterodimers with other NRs (9). To date, crystal structures of apoRXRα-LBD have been determined both in dimeric and tetrameric conformations (10, 11). The tetramer is formed with two dimers packed in a bottom-to-bottom manner (11). Binding of agonist such as RXR natural ligand 9-cis retinoic acid (9cRA) induces notable conformational changes in which the activation function-2 (AF-2) domain rotates and moves to its active position to seal off the ligand-binding pocket (LBP), thus recruiting coactivators to initiate transcription (12–14). Therefore, ligand-induced dissociation of the tetramer is considered to be the first step for RXR activation, and tetramer formation serves to sequester excess RXR into an inactive pool within the cell (15). However, the structural basis regarding RXR antagonist still remains elusive.
Small molecules that selectively regulate RXR signaling pathways are useful for their therapeutic application (7). RXR agonists have been reported to exhibit glucose-lowering, insulin-sensitizing, and anti-obesity efficiency in animal models of insulin resistance and type 2 diabetes (16). However, undesirable side effects such as hypertriglyceridemia and suppression of the thyroid hormone axis also exist (6). Currently, there have been increasing numbers of reports on RXR antagonists, which are found to decrease body weight, plasma glucose, and insulin levels, while exhibiting few effects on food intake in vivo (17, 18).
In the current work, we identified a novel RXRα antagonist danthron, 1,8-dihydroxyanthraquinone (see Fig. 1A), from the traditional Chinese medicine rhubarb. This natural product repressed all of the tested RXRα-involved response elements transcription, including RXRE, PPRE, FXRE, and LXRE, without binding to the corresponding nuclear receptors, except RXRα. Results from the native PAGE indicated that danthron bound to the tetrameric RXRα-LBD and transformed the 9cRA-induced RXRα-LBD dimer to tetramer. ITC-based assay revealed that danthron bound to RXRα-LBD with a stoichimetric ratio of 1:2 (two ligands bound to one RXRα-LBD tetramer). Furthermore, danthron binding could influnce the affinity of the silencing mediator for retinoid and thyroid receptor (SMRT) to the receptor. In addition, a unique homotetrameric structure of apoRXRα-LBD was discovered, which exhibited a larger tetramer interface and different ligand-binding pocket size, compared with the previously reported one. The danthron-interacting residues in LBP were determined by the crystal structure of danthron-soaked RXRα-LBD. These results have thus suggested a potential mechanism of RXRα-LBD tetramer stabilization by danthron. Furthermore, danthron exhibited an efficient improvement of insulin sensitivity in DIO mice, implying that danthron functioned as an insulin sensitizer in vivo. As a first report on RXRα-antagonist structural characterization, our work was expected to have provided a new insight of a novel antagonistic mode on the inactive RXR tetramer.
FIGURE 1.
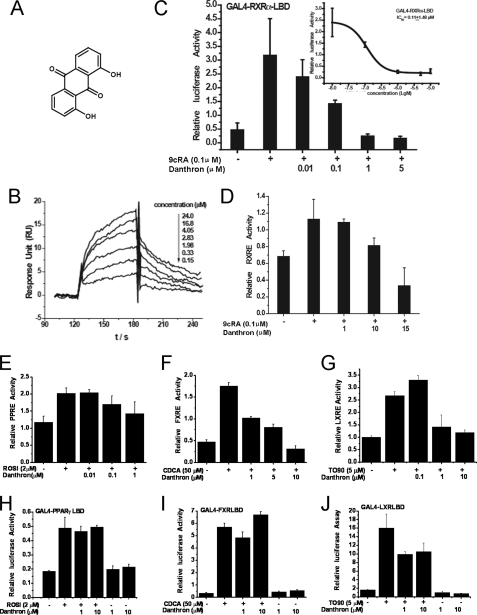
Danthron was a specific RXRα antagonist. A, chemical structure of danthron. B, danthron specifically bound to RXRα-LBD as evaluated by SPR technology-based assay. C, danthron antagonized 9cRA-stimulated transactivation of Gal4-RXRα-LBD in a dose-dependent manner by mammalian one-hybrid assay. The half-maximal inhibitory concentration (IC50) was indicated. D, danthron antagonized the transactivation of RXRE mediated by the RXRα:RXRα homodimer. E, danthron antagonized the transactivation of PPRE mediated by the RXRα:PPARγ heterodimer. F, danthron antagonized the transactivation of FXRE mediated by the RXRα:FXR heterodimer. G, danthron antagonized the transactivation of LXRE mediated by the RXRα:LXRα heterodimer. H, danthron exhibited no activities to PPARγ by mammalian one-hybrid assay. I, danthron exhibited no activities to FXR by mammalian one-hybrid assay. J, danthron exhibited no activities to LXRα by mammalian one-hybrid assay. PPARγ agonist rosiglitazone (ROSI), LXR agonist TO901317 (TO90), and FXR agonist chenodeoxycholic acid (CDCA) were used as positive controls.
EXPERIMENTAL PROCEDURES
Materials
All the cell culture reagents were purchased from Invitrogen. The UAS-TK-Luc reporter was generously donated by Dr. Daniel P. Kelly (Washington University School of Medicine). The fusion constructs of pGAL4-RXRα-LBD, pGAL4-LXRα-LBD, pGAL4-FXR-LBD, and pGAL4-PPARγ-LBD were generated using pGBKT7-RXRα-LBD, pcDNA3.1-LXRα, pET22b-FXR-LBD, and pcDNA3.1-PPARγ (kindly provided by Dr. Gordon Hager, National Cancer Institute) as templates, respectively. The pGL3-pro-RXRE-Luc vector was constructed by inserting four DR1 sequences with XhoI-BglII sites. The pGL3-pro-LXRE-Luc plasmid was kindly provided by Dr. Peter A. Edwards (The Medicine Institute at University of California, Los Angeles), and pGL3-pro-FXRE-Luc vector was kindly provided by Dr. Majlis Hermansson (AstraZeneca, Mölndal, Sweden). The corepressor SMRT peptide was synthesized with the sequence NMGLEAIIRKALMGKY based on the published results (19).
Luciferase Assay
Mammalian one-hybrid test and transactivation experiment of RXRα-involved response element were performed using luciferase assay. HEK293T (human embryonic kidney) cells were cultured in DMEM supplemented with 10% FBS, 50 units/ml penicillin-streptomycin (Sigma) at 37 °C in a humidified atmosphere with 5% CO2. Transient transfection was conducted using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Briefly, 5 h after transfected with luciferase reporter vector and Renilla luciferase vector pRL-SV40 (50 ng/well), cells were incubated with varied concentrations of compounds for another 24 h. Luciferase activities were measured using the Dual-Luciferase Assay System kit (Promega).
Protein Purification
Preparation of RXRα-LBD was performed according to the previously published approach (14). Briefly, the coding sequence of human RXRα-LBD (residues 221–458) was cloned to the vector pET15b, and Escherichia coli strain BL21 (DE3) was used for protein expression. The culture was induced with 0.5 mm isopropyl 1-thio-β-d-galactopyranoside and incubated at 25 °C for 6 h. His-tagged RXRα-LBD was purified with nickel-nitrilotriacetic acid resin (Qiagen), and the tag was then removed by thrombin (Novagen). The protein was further purified with Superdex 200 (Amersham Biosciences) and concentrated to 10 mg/ml.
SPR Technology-based Assay
Binding affinity of danthron toward RXRα-LBD was assayed with a BIACORE 3000 instrument (BIACORE) based on our previous report (20). All agents were purchased from GE Healthcare. Briefly, RXRα-LBD was immobilized onto a CM5 sensor chip according to the standard primary amine-coupling procedures. Danthron was serially diluted and injected into the channels at a flow rate of 20 μl/min for 60 s, followed by disassociation for 120 s. BIAevaluation software (version 3.1; Biacore) was used to determine the equilibrium dissociation constant (KD) of the compound.
Native PAGE Assay
The separating gel was prepared by mixing 4.8 ml of water, 2.7 ml of 30% acrylamide/0.8% bisacrylamide, 2.5 ml 5 m Tris-Cl, pH 8.8, 0.05 ml of 10% (w/v) ammonium persulfate, and 0.005 ml of N,N,N′,N′-tetramethylethylenediamine. The stacking gel was prepared by mixing 3.3 ml of water, 0.67 ml of 30% acrylamide/0.8% bisacrylamide, 1 ml 0.5 m Tris-Cl, pH 6.8, 0.03 ml of 10% (w/v) ammonium persulfate, and 0.005 ml of N,N,N′,N′-tetramethylethylenediamine. Phosphate gel buffer was prepared with 100 mm sodium phosphate and adjusted to pH 6.5. Albumin from bovine serum (66 kDa for monomer and 132 kDa for dimer) was purchased from Sigma and used as a native PAGE marker. A constant current of 30 mA was used for the 5-h polyacrylamide gel electrophoresis. All experiments were performed at 4 °C.
ITC Technology-based Assay
The thermodynamic properties of danthron binding to RXRα-LBD, corepressor SMRT peptide binding to apo-, or danthron-bound RXRα-LBD were determined using a VP-ITC titration calorimeter (MicroCal) in phosphate buffer at 25 °C. The heat of dilution was obtained by injecting danthron or SMRT into the same buffer and subtracted from the reaction before the fitting process.
Crystallization and Data Collection
RXRα-LBD crystals grew in the condition of 100 mm Tris-Cl, pH 6.5, 10% PEG4000, 5% glycerol. The complex crystal was obtained by soaking the RXRα-LBD crystal in the danthron-containing mother liquor with a mass ratio of 1:50. Diffraction data were collected at BL17U of Shanghai Synchrotron Radiation Facility in China and integrated with HKL2000 (21).
Structure Determination and Refinement
Phasing was performed by MOLREP (22). Structure refinement was carried out by Refmac5 (22) and CNS (23). Model building was performed by COOT (24). The quality of the final model was checked by PROCHECK (25). The statistics of the data collection and structure refinement were summarized in Table 1. All of the structure figures were prepared by PyMOL (26). Atomic coordinates and structure factors have been deposited to the Protein Data Bank under accession codes 3NSP and 3NSQ.
TABLE 1.
Data collection and structure refinement statistics
| Apo-RXRα-LBD | RXRα-LBD-danthron | |
|---|---|---|
| Data collection | ||
| Space group | P21212 | P21212 |
| Cell dimensions | ||
| a, b, c (Å) | 114.83, 100.27, 47.16 | 115.03, 99.92, 47.20 |
| α, β, γ | 90°, 90°, 90° | 90°, 90°, 90° |
| Resolution (Å) | 29.72-2.90 (3.00-2.90)a | 14.88-2.60 (2.69-2.60) |
| Rsym or Rmergeb | 0.158 (0.682) | 0.128 (0.344) |
| I/σI | 4.7 (2.0) | 4.8 (2.0) |
| Completeness (%) | 90.7 (96.2) | 95.5 (95.7) |
| Redundancy | 9.3 (3.8) | 4.5 (4.3) |
| Refinement | ||
| Resolution (Å) | 15.00-2.90 | 14.88-2.60 |
| No. of reflections | 11,445 | 16,495 |
| Rwork/Rfreec | 0.197/0.225 | 0.234/0.257 |
| No. of atoms | 3,019 | 3,113 |
| Protein | 2,985 | 2,992 |
| Ligand | 0 | 18 |
| Water | 34 | 103 |
| B-factors (Å2) | 24.7 | 38.7 |
| Protein | 25.3 | 24.8 |
| Ligand | 41.9 | |
| Water | 21.2 | 22.3 |
| r.m.s.d.d | ||
| Bond lengths (Å) | 0.011 | 0.010 |
| Bond angles | 1.376° | 1.235° |
| Ramachandran plot (%) | ||
| Most favored regions | 97.0 | 97.1 |
| Allowed regions | 3.0 | 2.9 |
a Values in parentheses are for highest-resolution shell.
b Rsym or Rmerge = ΣhΣi|Ihi − 〈h〉|/ΣhΣiIhi, where Ihi and 〈Ih〉 are the i-th and mean measurement of the intensity of reflection h, respectively.
c Rwork/Rfree = Σh|Fo.h − Fc.h|/ΣhFo.h, where Fo.h and Fc.h are the observed and calculated structure factor amplitudes, respectively.
d r.m.s.d., root mean square deviation.
Insulin Tolerance Test
C57/BL6 male mice were obtained from Shanghai SLAC Laboratory Animal Co., Ltd. Mice were fed with a high fat diet for 3 months and treated with danthron (5 mg/kg) or vehicle orally for 8 weeks. The animals were then fasted for 6 h and then given intraperitoneal injection of insulin at 1.5 units/kg. Blood samples were analyzed at 15, 30, 45, 60, 90, and 120 min using Accu-Chek active blood sugar test meter (Roche Diagnostics). All of the animals received humane care, and the procedures in these experiments were performed according to the institutional ethical guidelines.
RESULTS
Danthron Was a Specific RXRα Antagonist
According to the SPR technology-based assay, danthron dose-dependently bound to RXRα-LBD with a KD value of 6.2 μm (Fig. 1B). To further explore the effect of danthron on the transactivation of RXRα, a mammalian one-hybrid test was employed. As shown in Fig. 1C, danthron inhibited 9cRA-induced RXRα transactivation by IC50 at 0.11 μm. Moreover, danthron repressed the transactivation of RXRE mediated by the RXRα:RXRα homodimer (Fig. 1D). Also, we examined the selectivity of danthron to several kinds of RXRα-involved heterodimers. The results showed that danthron dose-dependently repressed the transactivation of the relevant RXRα-involved response elements, including PPRE, FXRE, and LXRE, which are mediated by RXRα:PPARγ, RXRα:FXR, and RXRα:LXRα heterodimers, respectively (Fig. 1, E–G). However, danthron exhibited no activities to these nuclear receptors in mammalian one-hybrid test (Fig. 1, H–J). These results thus suggested that danthron exerted its transrepression activities on the RXRα homodimer and RXRα-involved heterodimers just through targeting RXRα, further addressing its selectivity to RXRα over other nuclear receptors.
Danthron Stabilized RXRα-LBD Tetramer and Influenced Corepressor SMRT Binding to Receptor
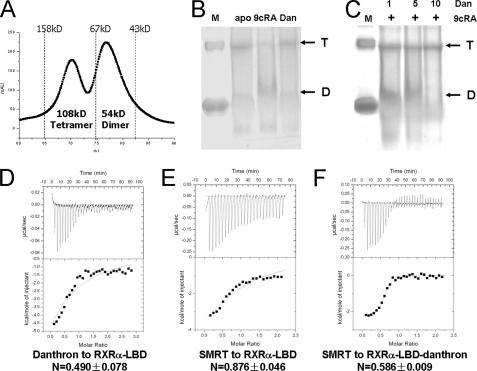
Currently, a few complex structures of antagonist-bound NRs have been reported, including the estrogen receptor (27), PPAR (19), glucocorticoid receptor (28), and retinoic acid receptor (29). All of these structures revealed a conserved dimeric fold, in which antagonists induced H12 adopting an alternative position to recruit corepressor. Here, we wondered whether danthron could induce dissociation of tetrameric RXRα-LBD to dimer. The results from size exclusion chromatogram and native PAGE assays suggested that purified RXRα-LBD existed in equilibrium of tetramer and dimer (Fig. 2, A and B). As an agonist, 9cRA changed this equilibrium to dimer (Fig. 2B), in accord with the crystal structure of 9cRA-induced dimeric RXRα-LBD (12). Interestingly, danthron was found to induce this equilibrium preferring to tetramer as indicated by the fact that the danthron-bound RXRα-LBD was mostly in tetrameric form (Fig. 2B). Moreover, danthron inhibited the 9cRA-induced RXRα-LBD dimer formation to tetramerization in a dose-dependent manner (Fig. 2C). These results thus suggested that danthron binding could stabilize RXRα-LBD tetramer. Different from other nuclear receptor antagonists that bound to dimeric NRs, danthron played its role on tetrameric RXRα.
FIGURE 2.
Danthron stabilized the RXRα-LBD tetramer and changed corepressor binding to the receptor. A, purified RXRα-LBD was in equilibrium of tetramers and dimers as indicated by size exclusion chromatogram assay. B, results from native PAGE showed that apo-RXRα-LBD existed in both tetramer (T) and dimer (D), whereas 9cRA-induced RXRα-LBD was mostly in dimer, and danthron-bound RXRα-LBD was mostly in tetramer. Albumin from bovine serum (66 kDa for monomer and 132 kDa for dimer) was used as marker (M). C, incubation of 9cRA-bound RXRα-LBD with an equal amount or 5- or 10-fold of danthron showed the transformation of RXRα-LBD from dimer to tetramer in a dose-dependent manner. D, ITC results showed that danthron (Dan) bound to RXRα-LBD in a molecular ratio of 1:2. E, ITC results showed that corepressor SMRT peptide bound to apo-RXRα-LBD in a stoichimetric ratio of 1:1. F, ITC results showed that corepressor SMRT peptide bound to danthron-bound RXRα-LBD in a stoichimetric ratio of 1:2.
To further clarify the stoichimetric ratio of danthron binding to RXRα-LBD, ITC experiment was performed. The KD value of danthron bound to RXRα-LBD by ITC experiment was determined at 7.5 μm (Fig. 2D), in a good agreement with the SPR result. However, danthron was found to bind to the receptor in a ratio of 1:2 (Fig. 2D), different from the previously published reports on the equal ratio of receptor-ligand binding (12–14).
It was postulated that binding of the corepressor like the SMRT to nuclear receptors could be stabilized by antagonists (30, 31). Here, we evaluated the potential effects of danthron binding on SMRT peptide affinity to RXRα-LBD by ITC-based assay. As indicated in Fig. 2, E and F, SMRT peptide bound to unliganded RXRα-LBD in a ratio of 1:1, whereas danthron binding changed this ratio to 1:2. Therefore, the biochemical and biophysical results from the native PAGE and ITC experiments showed that danthron bound to the tetrameric RXRα-LBD in a specific stoichimetric ratio, and danthron binding could stabilize this tetramer and influenced the corepressor SMRT affinity to RXRα-LBD.
Apo-RXRα-LBD Exhibited a Unique Homotetrameric Conformation
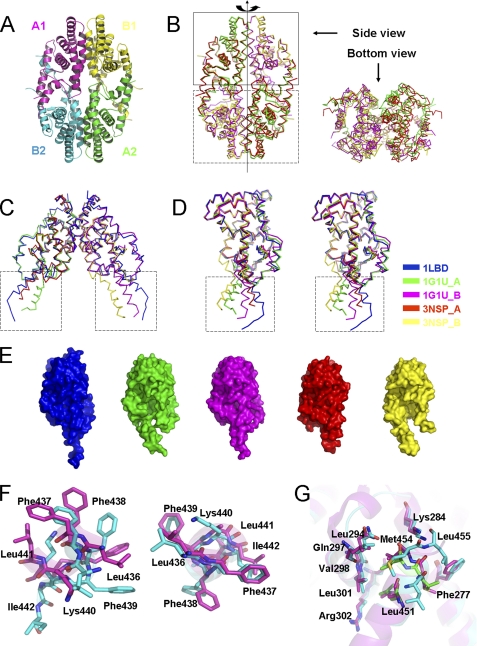
To further elucidate the structural basis of danthron as a specific RXRα antagonist, we tried co-crystallization of RXRα-LBD with danthron but failed to get the crystals either in the presence or absence of corepressor SMRT peptide. Unexpectedly, we determined a unique homotetramer structure of apo-RXRα-LBD. As shown in Fig. 3A, the analyzed crystal structure of apo-RXRα-LBD generally adopted a similar homotetrameric packing with two symmetric dimers combined in a bottom-to-bottom fashion compared with the previously reported one (11). However, superposition of these two tetrameric structures gave different packing modes (Fig. 3B). The two upper dimers overlapped well, whereas the lower dimer in our structure rotated along the tetramer axis (Fig. 3B). Further alignment among the symmetric dimers in these two tetramers and the previously reported RXRα-LBD dimer (10, 11) showed a conserved core structure and variable C-terminal helices H11 and H12 (Fig. 3C). Moreover, unlike the dimeric RXRα-LBD with two identical chains, chains A and B in these two tetramers exhibited different conformations (Fig. 3D). It seemed that C-terminal swinging conformations existed in the apo-RXRα-LBD. As shown in Fig. 3D, in chain B of our structure, H11 moved apart from the core LBD and was shortened by half a turn, whereas H12 moved 9.5Å horizontally and rotated 60° counterclockwise. Chain A also behaved similar movement and rotation, but to a lesser extent. Although H11 and H12 in different structures seemed to be in a swinging manner, their conformations were actually not flexible, considering that H12 positioned itself into the groove between H3′ and H4′ from its neighbor dimer (Fig. 3A). The changes in C-terminal conformation for the apo-RXRα-LBD resulted in different sizes of LBP (Fig. 3E). The previously reported tetrameric RXRα-LBD had a slightly larger pocket on one side and a smaller one on the other compared with the dimeric RXRα-LBD (10, 11). However, chain B in our structure had an even larger pocket size, and chain A had the smallest size. It seemed that as the LBP in one side was enlarged, the pocket in the other may adjust itself by shrinking.
FIGURE 3.
Apo-RXRα-LBD exhibited a unique homotetrameric conformation. A, overall structure of the current tetrameric RXRα-LBD shown in a ribbon diagram. Chains A and B and their symmetric chains were indicated. B, superposition of the current tetrameric RXRα-LBD (red and yellow) with the previously reported tetramer (Protein Data Bank code 1G1U, in green and magenta) showed different dimer packing. Upper dimers in the closed box adopted a similar conformation, but lower dimers in the dashed box did not overlap well due to its rotation along the tetrameric axis. C, superposition of the previous dimeric RXRα-LBD (Protein Data Bank code 1LBD) with the symmetric dimers from the current and previous tetramer showed identical dimer packing and C-terminal swinging conformation (in dashed box). D, superposition of chains from the current structure, previous RXRα-LBD dimer and tetramer in stereo view showed a conserved core structure and C-terminal swinging conformation (in dashed box). E, comparison of the ligand-binding pocket among the five chains mentioned above. Colors in C, D, and E were indicated. F, conformational changes of the residues were shown on the H11/H11 interface. G, conformational changes of the residues were shown on the AF-2/coactivator binding-site surface. The current structure was in cyan, and the previous tetramer was colored in magenta. Conserved residues in coactivator SRC-1 were shown in green.
In addition, there were significant conformational changes on the tetramer interface, comparing our apo-RXRα-LBD structure with the reported structure (11). In the published structure, the H3/H3 interface was composed of the residues 264–269 of chain B and the residues 269–273 of chain A, the H11/H11 interface was constituted of Leu436 and Phe439 from each chain, and the AF-2/coactivator binding site interface was made up of Leu451, Met45, and Leu455 with helices 3 and 4 (11). Our results revealed that Leu436 and Phe439 in chain A and Phe438 in chain B constituted the H11/H11 interface (Fig. 3F). H11 of chain A also made additional hydrophobic interactions with Arg302 in H4 and Trp305 in H5 of chain B. The AF-2 residues 451LMEML455 in chain A resembled the LXXLL motif of coactivators (underlined residues indicate a conserved sequence (11)) and interacted with Phe277, Lys284, and Val298 in chain B (Fig. 3G). These conformational changes in our structure made the two symmetric dimers adhere to each other burying 1475.8Å2 of their surface, much larger than the previously reported tetramer (1242.8Å2) (11). These results thus indicated that our determined apo-RXRα-LBD might be in a more stable state.
Crystal Structure of Danthron-bound RXRα-LBD
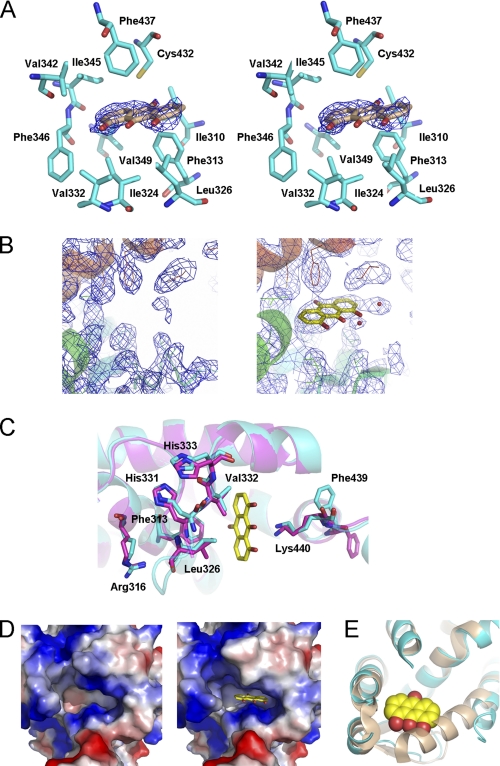
Although we failed to get the co-crystal of RXRα-LBD with danthron, the determined danthron-soaked crystal structure of tetrameric RXRα-LBD seemed to be valuable for elucidation of the ligand interacting information in the LBP. As shown in Fig. 4, A and B, the clear electron density was indicative of danthron existence. In addition, the binding stoichimetric ratio observed in the structure was in good accordance with the ITC results, where two ligands bound to one tetrameric RXRα-LBD.
FIGURE 4.
Crystal structure of danthron-bound RXRα-LBD. A, electron density of danthron (contoured at 1.0σ level) was shown in stereo view. Hydrophobic residues in LBP were indicated in cyan sticks. B, 2Fo − Fc maps in LBP of apo- and danthron-soaked RXRα-LBD structures revealed a clear electron density of danthron. C, danthron binding induced conformational changes of LBP. Danthron-bound RXRα-LBD was in cyan, apo-RXRα-LBD was in magenta, and danthron was in yellow. D, danthron induced changes on the electrostatic surface of LBP. E, N terminus of H3 in danthron-bound RXRα-LBD structure (in cyan) failed to bend toward the ligand as it did in 9cRA-induced activation state (Protein Data Bank code 1FBY, in wheat), due to the steric hindrance from danthron.
Danthron was found to bind to the hydrophobic LBP by interacting with Phe313 in H5, Ile324 and Leu326 in S1, Val332 in S2, Val342 and Phe346 in H7, and Phe437 in H11 (Fig. 4A). Danthron binding was further stabilized by forming hydrogen bonds with Lys440 (Fig. 4C). Interaction with danthron made Lys440 closer to the compound and induced an overturning of Phe439 in H11. Mutation of the conserved residue Phe439 on the tetramer interface was reported to abolish the ability of RXR to form tetramer (32). Upon danthron binding to RXRα-LBD, Phe439 might play an essential role in stabilizing this homotetramer. The residues involved in the hydrophobic interaction also adjusted themselves after danthron binding. As shown in Fig. 4C, the most significant conformational changes occurred on the β-sheets S1 and S2. To approach danthron, Leu326 in S1 and Val332 in S2 moved toward the ligand. This movement made the neighbor residues His331 and His333 alter their conformations to get the whole β-sheet closer to danthron. Phe313 and Arg316 in H5 also shifted greatly. Arg316 was the essential residue in the active RXRα-LBD structure by forming hydrogen bonds with RXR agonist 9cRA (12). All of these conformational changes of the hydrophobic residues led to the changes on the LBP electrostatic surface (Fig. 4D). Because danthron was an anthraquinone type of compound with strong hydrophobic features in structure, changes on the LBP electrostatic surface were expected to facilitate danthron binding to the pocket. Moreover, the N-terminal part of H3 failed to bend toward the ligand as it did in the agonist-induced activation state, due to the steric hindrance from danthron (Fig. 4E). Although the LBP on both sides were open to solvent, danthron was found to bind to merely two chains of the tetramer as indicated in our determined crystal structure of danthron-soaked RXRα-LBD. The pocket of chain A was shrunken and not large enough for danthron binding. The binding stoichimetric ratio in the structure is in good accord with the ITC results (Fig. 2D).
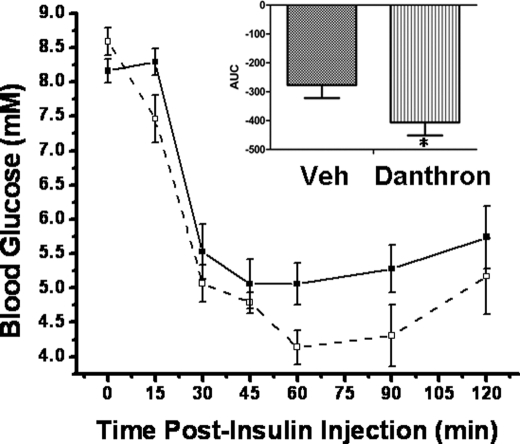
Danthron Improved Insulin Sensitivity in DIO Mice
The insulin tolerance test result showed that danthron-treated diet-induced obesity mice exhibited lower glucose levels after insulin challenge, compared with the control vehicle-treated group (Fig. 5). Our results thereby suggested that danthron functioned as an insulin sensitizer in vivo.
FIGURE 5.
Danthron improved insulin sensitivity in vivo. In the insulin tolerance test, danthron-treated DIO mice showed lower glucose levels after insulin challenge, compared with the vehicle (Veh)-treated group. The area under curve of danthron-treated DIO mice exhibited a significant difference from that of the control group. Significant difference at p < 0.05; *, n = 5. (■, vehicle-treated group; □, danthron-treated group).
DISCUSSION
Structural comparison of our determined apo-RXRα-LBD structure with the previously reported tetramer (11) clearly showed that the conformational changes of C-terminal H12 in our structure were more substantial, which resulted in a larger tetramer interface and different LBP size (Fig. 3). It is tentatively suggested that our unique crystal packing might possibly attribute to the different crystallization conditions. As indicated, our crystals grew in a lower pH value of 6.5, different from that of 7.5 for the previous tetramer (11). Interestingly, dimeric RXRα-LBD crystal grew in a neutral pH of 7.0 (10). The physiological significance of these different conformations might be related to the competence of RXR for different ligands. RXR is known to distribute widely in various tissues with variable intracellular pHs (16). Therefore, RXR might probably adopt different conformations to accept different ligands, consequently forming heterodimers with different nuclear receptors to mediate different genes transcription, which was in accordance to the central position of RXR in the signaling pathways (1).
RXR was previously expected to exhibit similar antagonistic features to other nuclear receptors (7). However, in our work, the specific RXR antagonist danthron employs an alternative antagonistic mechanism on RXRα. By interacting with the residues on the essential helices H5 and H11, and β-sheets S1 and S2, danthron stabilizes RXRα-LBD in its inactive tetramer state, where the essential residue Phe439 on the tetramer interface might play an important part. Mutation of this conserved residue Phe439 on the tetramer interface was reported to abolish the ability of RXR to form tetramer (32). RXR exists predominately in inactive homotetramer in vivo, which is different from the other NRs (9); thus, it was not surprising to find an antagonist acting on this inactive tetramer. Because the agonist-induced dissociation of the tetramer is considered to be the first step of RXR activation, danthron plays its role at the initial stage. Danthron arrests RXRα from rearrangement for transactivation, and the autorepression by homotetramer maintains RXRα in its inactive state. Therefore, the antagonism on the tetrameric RXR makes danthron distinguished from other antagonists on dimeric NRs.
In addition, our results from ITC-based assay for the first time indicated that the corepressor SMRT peptide had a binding affinity to the unliganded RXRα-LBD in vitro, although the mechanism involved in RXR repression by this corepressor is still not revealed. Moreover, binding of danthron was found to affect the corepressor SMRT affinity to the receptor.
Cell-based assays showed that danthron could inhibit RXR capability to transactivate reporter constructs driven by response elements for RXRα homodimer and all of the tested RXRα-containing heterodimers. Also, reversion of 9cRA-induced RXRα-LBD dimerization to tetramerization by danthron was observed in native PAGE (Fig. 2C). These results suggested that danthron-bound RXRα-LBD, as an inactive tetramer for transactivation, had no activity on the RXR-regulated gene transcriptions. Therefore, by stabilizing the tetrameric RXRα-LBD, danthron takes its effects on repressing all of the RXRα-involved transcriptions.
Danthron is an anthraquinone-type of compound isolated from the traditional Chinese medicine rhubarb. Rhubarb extracts have been proved to process promising antidiabetic and anticancer properties, although their detailed mechanisms remain unclear (33). One of the rhubarb extracts is rhein, which has an additional carboxyl group at the C3 site of danthron. Rhein was proved effective in the treatment of experimental diabetic nephropathy (34). Emodin, another main component of rhubarb, is an analog of danthron with the C3 and C6 sites substituted for methyl and hydroxyl groups, respectively. It was reported to have the competency to activate PPARγ (35), and emodin-treated diabetic mice showed improved glucose tolerance and insulin sensitivity (36). Possessing a similar chemical structure to other components from rhubarb, danthron was thereby expected to display similar biological activities. Our insulin tolerance test experiment in DIO mice treated with danthron has revealed that this natural product functioned as an efficient insulin sensitizer in vivo.
Our results are expected to have provided new insights into the structural basis of RXR antagonists for their potential therapeutic application. The ability to improve insulin sensitivity has made danthron a promising lead compound for the development of antidiabetic drugs and supplied useful information for understanding the pharmacological mechanisms of rhubarb.
Acknowledgment
We thank BL17U of Shanghai Synchrotron Radiation Facility in China for data collection.
This work was supported by the State Key Program of Basic Research of China (Grants 2010CB912501, 2007CB914304, and 2009CB918502), the National Natural Science Foundation of China (Grants 30925040, 30890044, and 10979072), Key New Drug Creation and Manufacturing Program (2009ZX09301-001), the Science Foundation of Shanghai (Grant 08431902900), the E-Institutes of Shanghai Municipal Education Commission (Grant E09013), and the Foundation of Chinese Academy of Sciences (Grants KSCX2-YW-R-168 and SCX1-YW-02-2).
The atomic coordinates and structure factors (codes 3NSP and 3NSQ) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- RXR
- retinoic X receptor
- SPR
- surface plasmon resonance
- ITC
- isothermal titration calorimetry
- LBD
- ligand-binding domain
- DIO
- diet-induced obese
- SMRT
- silencing mediator for retinoid and thyroid receptor
- NR
- nuclear receptor
- PPAR
- peroxisome proliferator-activated receptor
- FXR
- farnesoid X receptor
- LXR
- liver X receptor
- AF-2
- activation function-2
- 9cRA
- 9-cis retinoic acid
- LBP
- ligand-binding pocket
- RXRE
- RXR-response element
- PPRE
- PPAR-response element
- FXRE
- FXR-response element
- LXRE
- LXR-response element.
REFERENCES
- 1. Germain P., Chambon P., Eichele G., Evans R. M., Lazar M. A., Leid M., De Lera A. R., Lotan R., Mangelsdorf D. J., Gronemeyer H. (2006) Pharmacol. Rev. 58, 760–772 [DOI] [PubMed] [Google Scholar]
- 2. Kersten S., Dong D., Lee W., Reczek P. R., Noy N. (1998) J. Mol. Biol. 284, 21–32 [DOI] [PubMed] [Google Scholar]
- 3. Mangelsdorf D. J., Kliewer S. A., Kakizuka A., Umesono K., Evans R. M. (1993) Recent Prog. Horm. Res. 48, 99–121 [DOI] [PubMed] [Google Scholar]
- 4. Giguère V. (1994) Endocr. Rev. 15, 61–79 [DOI] [PubMed] [Google Scholar]
- 5. Tanaka T., De Luca L. M. (2009) Cancer Res. 69, 4945–4947 [DOI] [PubMed] [Google Scholar]
- 6. Pinaire J. A., Reifel-Miller A. (2007) PPAR Res. 2007, 94156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Lera A. R., Bourguet W., Altucci L., Gronemeyer H. (2007) Nat. Rev. Drug Discov. 6, 811–820 [DOI] [PubMed] [Google Scholar]
- 8. Gronemeyer H., Gustafsson J. A., Laudet V. (2004) Nat. Rev. Drug Discov. 3, 950–964 [DOI] [PubMed] [Google Scholar]
- 9. Kersten S., Kelleher D., Chambon P., Gronemeyer H., Noy N. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 8645–8649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bourguet W., Ruff M., Chambon P., Gronemeyer H., Moras D. (1995) Nature 375, 377–382 [DOI] [PubMed] [Google Scholar]
- 11. Gampe R. T., Jr., Montana V. G., Lambert M. H., Wisely G. B., Milburn M. V., Xu H. E. (2000) Genes Dev. 14, 2229–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Egea P. F., Mitschler A., Rochel N., Ruff M., Chambon P., Moras D. (2000) EMBO J. 19, 2592–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egea P. F., Mitschler A., Moras D. (2002) Mol. Endocrinol 16, 987–997 [DOI] [PubMed] [Google Scholar]
- 14. Nahoum V., Pérez E., Germain P., Rodríguez-Barrios F., Manzo F., Kammerer S., Lemaire G., Hirsch O., Royer C. A., Gronemeyer H., de Lera A. R., Bourguet W. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 17323–17328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kersten S., Pan L., Noy N. (1995) Biochemistry 34, 14263–14269 [DOI] [PubMed] [Google Scholar]
- 16. Altucci L., Leibowitz M. D., Ogilvie K. M., de Lera A. R., Gronemeyer H. (2007) Nat. Rev. Drug Discov. 6, 793–810 [DOI] [PubMed] [Google Scholar]
- 17. Sakaki J., Kishida M., Konishi K., Gunji H., Toyao A., Matsumoto Y., Kanazawa T., Uchiyama H., Fukaya H., Mitani H., Arai Y., Kimura M. (2007) Bioorg Med. Chem. Lett. 17, 4804–4807 [DOI] [PubMed] [Google Scholar]
- 18. Yotsumoto T., Naitoh T., Kanaki T., Tsuruzoe N. (2005) Metabolism 54, 573–578 [DOI] [PubMed] [Google Scholar]
- 19. Xu H. E., Stanley T. B., Montana V. G., Lambert M. H., Shearer B. G., Cobb J. E., McKee D. D., Galardi C. M., Plunket K. D., Nolte R. T., Parks D. J., Moore J. T., Kliewer S. A., Willson T. M., Stimmel J. B. (2002) Nature 415, 813–817 [DOI] [PubMed] [Google Scholar]
- 20. Zhang L., Liu W., Hu T., Du L., Luo C., Chen K., Shen X., Jiang H. (2008) J. Biol. Chem. 283, 5370–5379 [DOI] [PubMed] [Google Scholar]
- 21. Otwinowski Z., Minor W. (1997) Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 22. Collaborative Computational Project Number 4 (1994) Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 15299374 [Google Scholar]
- 23. Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 [DOI] [PubMed] [Google Scholar]
- 24. Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 25. Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. (1993) J. Appl. Cryst. 26, 283–291 [Google Scholar]
- 26. DeLano W. L. (2002) The PyMOL Molecular Graphics System, DeLano Scientific LLC, San Carlos, CA [Google Scholar]
- 27. Brzozowski A. M., Pike A. C., Dauter Z., Hubbard R. E., Bonn T., Engström O., Ohman L., Greene G. L., Gustafsson J. A., Carlquist M. (1997) Nature 389, 753–758 [DOI] [PubMed] [Google Scholar]
- 28. Schoch G. A., D'Arcy B., Stihle M., Burger D., Bär D., Benz J., Thoma R., Ruf A. (2010) J. Mol. Biol. 395, 568–577 [DOI] [PubMed] [Google Scholar]
- 29. Bourguet W., Vivat V., Wurtz J. M., Chambon P., Gronemeyer H., Moras D. (2000) Mol. Cell 5, 289–298 [DOI] [PubMed] [Google Scholar]
- 30. Chen J. D., Evans R. M. (1995) Nature 377, 454–457 [DOI] [PubMed] [Google Scholar]
- 31. Jackson T. A., Richer J. K., Bain D. L., Takimoto G. S., Tung L., Horwitz K. B. (1997) Mol. Endocrinol. 11, 693–705 [DOI] [PubMed] [Google Scholar]
- 32. Kersten S., Reczek P. R., Noy N. (1997) J. Biol. Chem. 272, 29759–29768 [DOI] [PubMed] [Google Scholar]
- 33. Huang Q., Lu G., Shen H. M., Chung M. C., Ong C. N. (2007) Med. Res. Rev. 27, 609–630 [DOI] [PubMed] [Google Scholar]
- 34. Gao Q., Qin W. S., Jia Z. H., Zheng J. M., Zeng C. H., Li L. S., Liu Z. H. (2010) Planta Med. 76, 27–33 [DOI] [PubMed] [Google Scholar]
- 35. Liu Y., Jia L., Liu Z. C., Zhang H., Zhang P. J., Wan Q., Wang R. (2009) Exp. Mol. Med. 41, 648–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xue J., Ding W., Liu Y. (2010) Fitoterapia 81, 173–177 [DOI] [PubMed] [Google Scholar]