Abstract
Elevated extracellular solute concentration (hyperosmotic stress) perturbs cell function and stimulates cell responses by evoking MAPK cascades and activating AP-1 transcription complex resulting in alterations of gene expression, cell cycle arrest, and apoptosis. The results presented here demonstrate that hyperosmotic stress elicited increases in ATF-2 phosphorylation through a novel Polo-like kinase 3 (Plk3) pathway in human corneal epithelial (HCE) cells. We found in hyperosmotic stress-induced HCE cells that Plk3 transferred to the nuclear compartment and was colocalized with ATF-2 in nuclei. Kinase activity of Plk3 was significantly activated by hyperosmotic stimulation. Further downstream, active Plk3 phosphorylated ATF-2 at the Thr-71 site in vivo and in vitro. Overexpression of Plk3 and its mutants enhanced hyperosmotic stress-induced ATF-2 phosphorylation. In contrast, suppression of Plk3 by knocking down Plk3 mRNA effectively diminished the effect of hyperosmotic stress-induced ATF-2 phosphorylation. The effect of hyperosmotic stress-induced activation of Plk3 on ATF-2 transcription factor function was also examined in CRE reporter-overexpressed HCE cells. Our results for the first time reveal that hyperosmotic stress can activate the Plk3 signaling pathway that subsequently regulates the AP-1 complex by directly phosphorylating ATF-2 independent from the effects of JNK and p38 activation.
Keywords: AP-1 Transcription Factor, Epithelial Cell, Protein Kinases, Signal Transduction, Transcription Factors
Introduction
Hyperosmotic stresses are considered as environmental hazards and pathological conditions from increased extracellular hyperosmolarity occurring in diabetes mellitus, uremia, heat shock, fatal burns, infections, and dehydration after exercise. Hyperosmotic stress extracts water out of cells, resulting in cell shrinkage. To restore the volume, the cells undergo a regulatory volume increase occurred within several minutes by uptake inorganic ions and water (1–3). Cell shrinkage and increased ionic strength ultimately alternate cellular architecture compartments, denature proteins, and disturb cell function (4–6). In addition, a persistent increase in hyperosmotic stress induces DNA damage, cell cycle arrest, and apoptosis (5, 7). In the front of the eye, corneal epithelial cells are exposed to these environmental hazards and often injured by hyperosmotic stresses, resulting in delay of wound healing and development of dry eye and other eye diseases (8).
Plk32 is one of the four members in the Polo-like kinase family in mammalian cells. Plk3 shares high homologies with Drosophila Polo kinases (9–12). There is a kinase domain (KD) at the N terminus that phosphorylates downstream proteins at serine/threonine and a Polo-box domain at the C terminus that binds interactive proteins. As the cell cycle progress, Plk3 undergoes substantial changes in abundance, kinase activity, and subcellular distribution. Recent studies indicate that Plk3 is a multifunctional protein and is involved in regulating a variety of molecular and intracellular events that include DNA damage responses, cell cycle controls, and apoptosis (13, 14). Plk3 is rapidly activated upon stress stimulation. These stresses are ionizing radiation, reactive oxygen species, methyl-methane-sulfonate, UV irradiation, and hypoxia (15–17). In previous studies, we found that stress stimulation activates Plk3 to phosphorylate c-Jun protein at Ser-63 and Ser-73, subsequently resulting in activation of apoptotic responses in various cells (16). However, it is still not clear how Plk3 functionally regulates these transcription factors in response to various stress stimulation and how Plk3 interacts with other transcription factors to form heterodimeric AP-1 complexes in addition to the c-Jun protein.
The AP-1 transcription factor complex acts as a central switch to convert extracellular signals into genetic responses and to determine cell proliferation, differentiation, and apoptosis. The AP-1 complex consists of homodimers and heterodimers formed by a group of transcription factors, such as those members in the Jun, Fos, and ATF families (18, 19). The AP-1 complex formed by ATF-2 and c-Jun transcription factors is one of the main components in response to hyperosmotic stress stimulation. Recent studies indicate that the AP-1 complex is formed by heterodimer of ATF-2 and c-Jun instead of ATF-2/ATF-2 or c-Jun/c-Jun homodimers in human cells (20, 21). It has also shown that there are unique structures within these transcription factor proteins, including DNA recognition segments and the basic leucine zipper domain that are responsible for protein-DNA interactions and for forming functional dimers of the AP-1 complex, respectively (21–23).
Upon treating cells with hyperosmotic stresses, the AP-1 transcription complex is activated by the extracellular stimulation mainly through eliciting MAPK cascades, namely the JNKs, and the p38 MAPKs (6, 24–26). Composition, regulation, and function of AP-1 complex are different depending on the cellular context and activation of various MAPKs. In corneal epithelial cells, activation of JNK and p38 result in increase in cell mobility and apoptosis (27–30). In previous studies, we reported that Plk3 is involved in UV irradiation- and hypoxia-induced cell death by activation of c-Jun in cornea epithelial cells (16, 17). In the present study, we investigated whether hyperosmotic stress induces Plk3 activation, which subsequently alters corneal epithelial cell function through activation of ATF-2 in formation of AP-1 complex. Our results revealed that Plk3 is a newly recognized component in signaling pathways to transmit extracellular hyperosmotic stress signals and to regulate ATF-2 in AP-1 complex in addition to the existing kinase cascade pathways.
EXPERIMENTAL PROCEDURES
Culture of Corneal Epithelial Cells
Primary HCE cells and human telomerase-immortalized corneal epithelial cells were cultured in a serum-free keratinocyte medium (defined keratinocyte-SFM; Invitrogen), and a human corneal epithelial (HCE) cell line was grown in Dulbecco's modified Eagle's medium/F-12 (1:1) containing 10% fetal bovine serum and 5 μg/ml insulin in an incubator supplied with 95% air and 5% CO2 at 37 °C. The medium was replaced every 2 days, and the cells were subcultured by treatment of cells with 0.05% trypsin-EDTA. For experiments of hyperosmotic stress stimulation, various concentrations of sorbitol, sucrose, NaCl, and glucose were added to the culture media following a time course. Osmotic pressures of hyperosmotic media were measured by using a vapor pressure osmometer (VAPRO5520).
Gene Transfection and Recombinant Proteins
Various constructs encoding full-length Plk3 (pEGFP-Plk3-FL, amino acids 1–646) and its mutants, including constitutively active Plk3 kinase domain (pEGFP-Plk3-KD, containing amino acids 1–334), inactive Plk3 kinase domain mutant (pEGFP-Plk3T219E, containing the Plk3 kinase domain mutated by substitute of the threonine 219 with a glutamate), and kinase-defective Plk3K52R (a full-length mutant of Plk3 that contains a substitution of the lysine 52 with an arginine), were transfected in corneal epithelial cells for 16–20 h using Lipofectamine reagents (Invitrogen). Transfected cells were subjected to immunoblot analysis and immunocomplex kinase assays. Transfections of JNK1-specfic siRNA (Qiagen; SI02758637), p38-specific siRNA (Qiagen; SI00605157), and Plk3-specific siRNA (Qiagen; SI02223473 and SI02223466) were done by adding respective JNK1-, p38-, and Plk3-specific siRNAs with a final concentration of 25 nm mixed with 12 μl of HiPerFect in 100 μl of serum-free culture medium. Transfection mixtures were incubated for 20 min at room temperature. The mixture was evenly dropped into cultured cells. Transfected cells were cultured under normal growth conditions for 48–84 h before performing experiments. Control cells were transfected with nonsilencing siRNA using the same method as described above. Recombinant fusion proteins including active Plk3 (Plk3a), Plk3 mutant (Plk3 K52R), ATF-2, and ATF-2 mutant (ATF-2T69A/T71A, a generous gift from Dr. Ze'ev Ronai at the Burnham Institute for Medical Research) were expressed using the baculoviral expression system and purified from Sf9 insect cells according to Invitrogen protocol. Briefly, Sf9 cells (ATCC) infected with Plk3 or ATF-2 baculovirus were cultured in Grace's insect cell culture medium. Infected cells were harvested on day 3 and lysed in a lysis buffer (50 mm NaH2PO4, 300 mm NaCl, 1% Nonidet P-40 20 mm imidazole, 1 mm PMSF, 2 μm pepstatin A, 10 units/ml aprotinin). The cell lysates were incubated with nickel-nitrilotriacetic acid-agarose resins for 3 h at 4 °C. Fusion proteins were eluted from nickel-nitrilotriacetic acid resins by using the lysis buffer containing 200 mm imidazole after extensive wash of the resins with the lysis buffer. Fusion proteins were purified by dialyzing in a storage buffer (25 mm Tris, pH7.4, 5 mm EGTA, 2 mm DTT, 0.1% Triton X-100, and 50% glycerol) and stored at 80 °C for subsequent uses. A cAMP response element (CRE) promoter report system was used to determine ATF-2/AP-1 transcription activity following the company's protocol (Promega, WI). Luc2 (Photinus pyralis, the reporter activity) and hRluc (Renilla reniformis, the internal control) activities were determined by a Lumat LB 9507 tube luminometer (Berthold, Germany).
Immunocytochemistry Experiments
Immunostaining
Corneal epithelial cells were grown on glass slides. After different treatments, HCE cells were rinsed twice with PBS, fixed for 15 min in 4% paraformaldehyde, and then permeabilized with PBS, 0.1% Triton X-100 (PBS-T) for 30 min at room temperature. The cells were blocked by incubation with 10% normal horse serum or 10% normal goat serum in PBS-T for 1 h at room temperature, followed by double immunostaining with the corresponding antibodies. The cells were washed with PBS and stained with DAPI. Pictures were captured by a Nikon fluorescent Ti microscope. The image data were analyzed using a Nikon NIS element software program.
Immunoprecipitation and Immunocomplex Kinase Assay
Corneal epithelial cells (5 × 107) were rinsed with PBS and incubated in 1 ml of lysis buffer (20 mm Tris, pH 7.5, 137 mm NaCl, 1.5 mm MgCl2, 2 mm EDTA, 10 mm sodium pyrophosphate, 25 mm glycerophosphate, 10% glycerol, 1% Triton X-100, 1 mm sodium vanadate, 1 mm phenylmethylsulfonyl fluoride, 250 μm 4-nitrophenyl-phosphate, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) on ice for 30 min. The cell lysates were spun at 13,000 × g for 10 min at 4 °C and incubated at 4 °C overnight with antibodies against Plk3, JNK, and p38, respectively (Santa Cruz Biotechnology). The immunocomplexes were recovered by incubation with 50 μl of 10% protein A/G-Sepharose (Santa Cruz Biotechnology). The immunocomplex beads were rinsed twice with lysis buffer and once with kinase buffer and then subjected to immunoblotting and kinase assay. To measure activities of Plk3, JNK, and p38, immunocomplex kinase assays were carried out by incubation of immunoprecipitated Plk3, JNK, or p38 with ATF-2 fusion protein in 30 μl of kinase buffer (20 mm HEPES, pH 7.6, 5 mm MgCl2, 10 μm MnCl2, 25 mm glycerophosphate, 1 mm sodium orthovanadate, 2 mm dithiothreitol, 20 μm ATP, and 10 μCi of [γ-32P]ATP) for 30 min at 37 °C. Kinase reactions were terminated by adding an equal volume of 2× Laemmli buffer and boiling for 5 min. Equal volumes of the samples were displayed on 12% SDS-PAGE and visualized by exposure on x-ray films. For cold kinase assay, [γ-32P]ATP was omitted from the kinase buffer. Western blot assay was performed by harvesting 5 × 105 cells in 0.5 ml of lysis buffer containing: 137 mm NaCl, 1.5 mm MgCl2, 2 mm EDTA, 10 mm sodium pyrophosphate, 25 mm β-glycerophosphate, 10% glycerol, 1% Triton X-100, 1 mm sodium orthovanadate, 1 mm phenylmethylsulfonyl fluoride, 10 mg/ml leupeptin, 10 μg/ml aprotinin, and 20 Tris, pH 7.5. Cell lysates were rinsed by ice-cold PBS and precleared by centrifugation at 13,000 × g for 20 min. The samples were denatured by adding an equal volume of 2× Laemmli buffer and boiling for 5 min. The samples were loaded into a 12% SDS-PAGE gel and fractionated by electrophoresis. Proteins in the gel were electrotransferred to PVDF membrane. The membrane was exposed to the blocking buffer (5% nonfat milk in TBS, 0.1% Tween 20 (TBS-T) for 1 h at room temperature and then incubated with respective antibodies overnight at 4 °C. After three washes with TBS-T buffer, the membrane was incubated with HRP-linked secondary antibody for 1 h at room temperature. Expression of proteins was detected with a Western blot detection kit (Santa Cruz Biotechnology).
RESULTS
Hyperosmotic Stress-induced ATF-2 Phosphorylation and Plk3 Activation
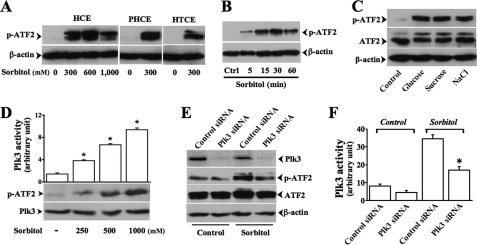
In previous studies, we found that Plk3 is a kinase that is responsive to UV irradiation and hypoxic stimulation. However, the effects of hyperosmotic stress on Plk3 activity and further on downstream events are still unknown. First, the effect of hyperosmotic stress-induced ATF-2 phosphorylation was studied by stimulating various human corneal epithelial cells with increased extracellular osmotic pressures that were created by adding high concentrations of sorbitol up to 1,000 mm. Phosphorylation levels of ATF-2 were markedly increased by 300 mm sorbitol in the culture medium in HCE, primary HCE, and human telomerase-immortalized corneal epithelial cells (Fig. 1A). However, hyperosmotic stress-induced ATF-2 phosphorylation was not further increased when the extracellular sorbitol concentration was further elevated to 1,000 mm. A time course for hyperosmotic stress-induced ATF-2 activation was examined for 60 min after exposure of HCE cells to 300 mm sorbitol (Fig. 1B). ATF-2 was activated by sorbitol within 5 min and reached the peak level at 50 min. The effect of hyperosmotic stress-induced ATF-2 phosphorylation by 300 mm of glucose, sucrose, and NaCl were also tested to compare with the effect of high concentration sorbitol in HCE cells (Fig. 1C). Second, the effect of hyperosmotic stress-induced Plk3 activation on ATF-2 phosphorylation was investigated following increased concentrations of extracellular sorbitol. The results of immunocomplex kinase assays demonstrated that hyperosmotic stress-induced Plk3 activation phosphorylated ATF-2 fusion protein following a increased sorbitol concentrations (Fig. 1D). Using Plk3-specific siRNA Plk3 mRNA, Plk3 mRNA expression was knocked down in HCE cells. The effect of hyperosmotic stress-induced Plk3 activation on ATF-2 phosphorylation was further characterized in Plk3 deficient cells. Knockdown of Plk3 significantly suppressed ATF-2 phosphorylation compared with control siRNA-transfected cells (Fig. 1, E and F). These results suggest that hyperosmotic stress induced Plk3 activation, which may play a role in activation ATF-2 by directly phosphorylating ATF-2 in corneal epithelial cells.
FIGURE 1.
Hyperosmotic stress-induced ATF-2 phosphorylation through activation of Plk3. A, increases in ATF-2 phosphorylation following increased sorbitol concentrations in various human corneal epithelial cells. PHCE, primary HCE; HTCE, human telomerase-immortalized corneal epithelial. B, high concentration sorbitol-induced ATF-2 phosphorylation following a time course. Ctrl, control. C, effects of high concentrations of glucose (300 mm), sucrose (300 mm), and NaCl (300 mm) on ATF-2 phosphorylation. D, hyperosmotic dose-dependent activation of Plk3. E, effect of knocking down Plk3 mRNA on hyperosmotic stress-induced ATF-2 phosphorylation at 15 min. F, suppression of hyperosmotic stress-induced Plk3 activation by knocking down Plk3 mRNA with specific siRNA. Plk3 activity was determined by immunocomplex kinase assay, and ATF-2 fusion protein was served as the substrate. *, significant difference at p < 0.05 (n = 4).
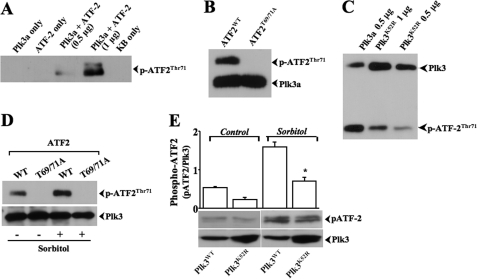
Effect of Active and Inactive Plk3 on Hyperosmotic Stress-induced ATF-2 Phosphorylation
ATF-2 fusion proteins were purified and used as substrates to test the effect of Plk3 kinase on ATF-2 phosphorylation. The constitutively activated Plk3 protein Plk3a was purified and used to directly phosphorylate ATF-2 at Thr-71 (Fig. 2A). However, Plk3a was not able to phosphorylate the mutated ATF-2 fusion protein (ATF-2T69A/T71A) in which two threonine residues (Thr-69 and Thr-71) were replaced by alanine residues (Fig. 2B). The other Plk3 mutant (Plk3K52R) that was kinase-defective by substituting lysine 52 with arginine showed a very weak effect on phosphorylation of ATF-2 fusion protein (Fig. 2C). In addition, Plk3 that was immunoprecipitated from hyperosmotic stress-induced HCE cells phosphorylated the wild type ATF-2 fusion protein at the residue of Thr-71 but failed to interact with ATF-2T69A/T71A mutant protein (Fig. 2D). Transfection of cDNA encoding kinase-defective Plk3K52R in HCE cells resulted in a significant suppression of hyperosmotic stress-induced ATF-2 phosphorylation (Fig. 2E). The Plk3 kinase assay experiments demonstrated the direct interaction of Plk3 and ATF-2 proteins and altered Plk3 activity affected phosphorylation of ATF-2 in vitro and in hyperosmotic stress-induced cells.
FIGURE 2.
Activation of Plk3 by hyperosmotic stress resulting in ATF-2 phosphorylation. A, determination of phosphorylation site in ATF-2 catalyzed by purified and activated Plk3 kinase (Plk3a) fusion protein in vitro. B, activated Plk3a fusion protein failed to phosphorylate ATF-2 mutant (ATF-2T69A/T71A), in which Thr-69 and Thr-71 were substituted by alanine residues. C, comparing the effect of activated Plk3a and kinase-defective Plk3K52R fusion proteins on ATF-2 phosphorylation. D, comparing the effect of hyperosmotic stress-activated Plk3 in HCE cells on phosphorylation of ATF-2 and ATF-2T69A/T71A. E, effect of hyperosmotic stress-activated Plk3wt and Plk3K52R on phosphorylation of ATF-2 in transfected HCE cells. *, a significant difference between Plk3wt and Plk3K52R transfected HCE cells (p < 0.05, n = 3).
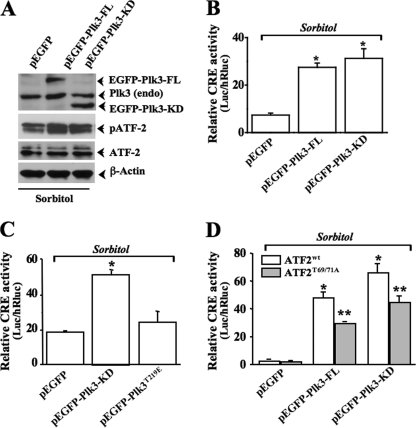
Effect of Altered Plk3 Activity on ATF-2 Transcription Function
To increase Plk3 activity, HCE cell were transfected with cDNAs encoding the active kinase domain of Plk3 (Plk3-KD) and the full-length Plk3 (Plk3-FL). Transfection of cells with Plk3-FL and Plk3-KD enhanced hyperosmotic stress-induced ATF-2 phosphorylation (Fig. 3A). The cells that were transfected with the expression vector containing an enhanced green fluorescent protein (pEGFP) were served as the controls (Fig. 3A). To study the effect of Plk3 activity on ATF-2 function, HCE cells were cotransfected with the CRE reporter. Overexpression of either Plk3-FL or Plk3-KD significantly enhanced hyperosmotic stress-induced CRE reporter activities, respectively (Fig. 3B). Transfection of HCE cells with Plk3T219E mutant that was defective in kinase activity resulted in a reversed effect of Plk3-KD on hyperosmotic stress-induced CRE transcription activation (Fig. 3C). In addition, increases in hyperosmotic stress-induced activity of the CRE reporter by overexpression of Plk3-FL and Plk3-KD were significantly diminished by cotransfecting HCE cells with negative mutant of ATF-2T69A/T71A (Fig. 3D). These results indicate that hyperosmotic stress-induced Plk3 activation affects the ability of ATF-2 to regulate transcription probably through phosphorylation of the ATF-2 protein.
FIGURE 3.
Effects of altered Plk3 activity on hyperosmotic stress-induced ATF-2 transcription activity. A, effects of overexpressing wild type Plk3 (pEGFP-Plk3-FL) and active Plk3 kinase domain (pEGFP-Plk3-KD) on hyperosmotic stress-induced ATF-2 phosphorylation. B, effects of overexpressing pEGFP-Plk3-FL and pEGFP-Plk3-KD on hyperosmotic stress-induced CRE reporter activity. C, effects of overexpressing pEGFP-Plk3-KD and pEGFP-Plk3T219E mutant on hyperosmotic stress-induced CRE reporter activity. D, effects of overexpressing pEGFP-Plk3-FL and pEGFP-Plk3-KD on hyperosmotic stress-induced CRE reporter activity in ATF-2 or ATF-2T69A/T71A cotransfected cells. * and **, significant differences between control and pEGFP-Plk3-FL/pEGFP-Plk3-KD transfected cells and between ATF-2/ATF-2T69A/T71A cotransfected cells, respectively (p < 0.05, n = 6).
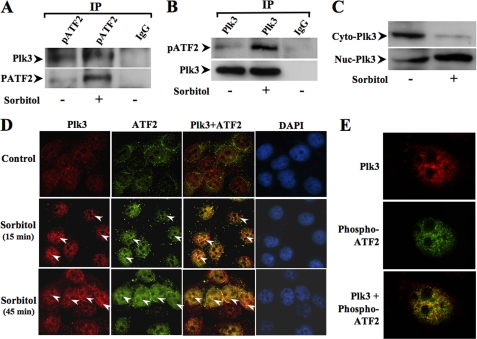
Interactions of Plk3 and ATF-2 in Hyperosmotic Stress-induced Cells
To substantiate the notion that Plk3 mediates hyperosmotic stress-induced ATF-2 activation, we performed further experiments to show that there are interactions between Plk3 and ATF-2 proteins in hyperosmotic stress-induced cells. Hyperosmotic stress-induced interaction between Plk3 and ATF-2 was detected by coimmunoprecipitation and Western analysis. The results of coimmunoprecipitation experiments demonstrated bidirectional effects that anti-phospho-ATF-2 antibody pulled down Plk3 and in turn that anti-Plk3 antibody pulled down phospho-ATF-2 in hyperosmotic stress-induced HCE cells (Fig. 4, A and B). The distribution of Plk3 in the cytosolic and nuclear compartments was measured by Western blot before and after hyperosmotic stimulation. There was an obvious increase in the Plk3 protein level in cell nuclei in response to sorbitol treatment (Fig. 4C). Activation of Plk3 and ATF-2 could be colocalized in the nuclei of hyperosmotic stress-induced HCE cells detected by immunostaining and microscopy imaging. Both Plk3 and ATF-2 were activated to form immune active clusters within 15 and 45 min upon sorbitol stimulation, and both active Plk3 and ATF-2 were colocalized in cell nuclei that were indicated by DAPI nuclear staining (Fig. 4D). The interaction between Plk3 and ATF-2 in the nuclei of hyperosmotic stress-induced HCE cells was more clearly demonstrated by using an anti-phospho-ATF-2 antibody and the higher magnification in immunostaining experiments (Fig. 4E). In addition, Plk3 expression was not detected in Plk3 knockdown cells in control immunostaining experiments (data not shown). The results of immunocytochemistry experiments indicate that there is indeed a protein-protein interaction between Plk3 and ATF-2 that can be colocalized in the nuclei, which is dependent on hyperosmotic stress stimulation.
FIGURE 4.
Determining interaction between Plk3 and ATF-2 in hyperosmotic stress-induced cells. A, detection of Plk3 and ATF-2 interaction by immunoprecipitation (IP) using anti-phospho-ATF-2 (pAFT-2) antibody. Plk3 was detected by Western analysis. B, detection of Plk3 and ATF-2 interaction by immunoprecipitation using anti-Plk3 antibody. ATF-2 was detected by Western analysis using anti-pATF-2 antibody. C, detection of Plk3 distribution in the cytosolic and nuclear compartments with/without hyperosmotic stimulation. D, coimmunolocalization of Plk3 and ATF-2 in hyperosmotic stress-induced cells. E, high amplification view (60×) to show colocalization of Plk3 and phospho-ATF-2 in the nucleus of the hyperosmotic stress-induced cell. Coimmunolocalization experiments were done by immunostaining and double staining of hyperosmotic stress-induced human corneal epithelial cells using antibodies specific to Plk3, ATF-2, and phospho-ATF-2. Photo images were taken by using a Nikon fluorescent microscope at 20× and 60×, respectively. The data were analyzed with a Nikon software program.
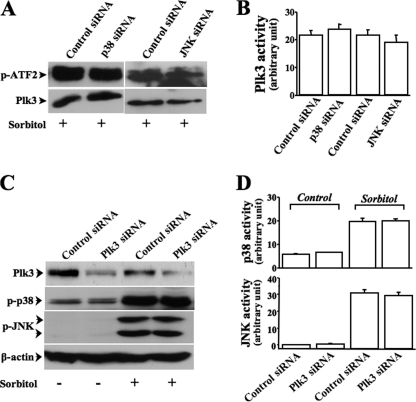
Differentiation of Plk3 Effect from JNK and p38
To further separate the effect of Plk3 on ATF-2 phosphorylation from the effect of JNK/p38 in vivo in hyperosmotic stress-induced cells, we examined the effect of hyperosmotic stress on ATF-2 activation by inhibiting JNK/p38 or Plk3 pathways in HCE cells. Expressions of Plk3, JNK, and p38 were individually suppressed by knocking down mRNA expressions in cells transfected with respective siRNAs. We found that knockdown of JNK and p38 individually did not affect hyperosmotic stress-induced Plk3 activation and its enzymatic ability to phosphorylate ATF-2 fusion protein (Fig. 5, A and B). In the mean time, knockdown of Plk3 had no significant effect on hyperosmotic stress-induced JNK and p38 activation, suggesting that cross-talk may not exist between Plk3 and JNK/p38 proteins in response to hyperosmotic stimulation (Fig. 5, C and D). These results further support the notion that Plk3 plays an important role in hyperosmotic stress-induced ATF-2 activation in parallel to the effects of JNK and p38 signaling pathways.
FIGURE 5.
Effects of suppressing JNK/p38 on hyperosmotic stress-induced Plk3 activation. A, effect of knocking down JNK or p38 mRNAs on hyperosmotic stress-induced Plk3 expression and ATF-2 activation. B, effect of knocking down JNK or p38 mRNAs on hyperosmotic stress-induced Plk3 activity. C, effect of knocking down Plk3 mRNA on hyperosmotic stress-induced JNK and p38 phosphorylation. D, effect of knocking down Plk3 mRNA on hyperosmotic stress-induced JNK and p38 activities. Phosphorylation of JNK/p38 was measured by using anti-phospho-JNK and anti-phospho-p38 antibodies, respectively. The activities of JNK, p38, and Plk3 were determined in three sets of independent experiments by immunocomplex kinase assays, and ATF-2 fusion protein was used as the substrate.
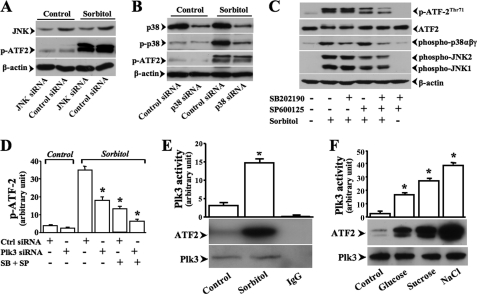
Next, we found that suppression of JNK and p38 activities by knocking down JNK and p38 mRNAs using specific siRNAs could not totally prevent hyperosmotic stress-induced ATF-2 phosphorylation, indicating that there is likely an alternative signaling pathway that mediates hyperosmotic stress-induced ATF-2 activation in addition to JNK and p38 pathways. (Fig. 6, A and B). The relevance of Plk3 in hyperosmotic stress-induced ATF-2 phosphorylation was further characterized by double inhibitions of JNK and p38 kinases. Experiments were performed by suppression of JNK and p38 using inhibitors of SP600125 and SB202190, respectively (Fig. 6C). Hyperosmotic stress-induced ATF-2 phosphorylation in the absence and presence of both SP600125 and SB202190 was also measured in Plk3 knocking down cells. Inhibition of JNK and p38 with both SP600125 and SB202190 in Plk3 knocking down cells resulted in further suppression of hyperosmotic stress-induced ATF-2 phosphorylation (Fig. 6D). To dissect the effect of hyperosmotic activated Plk3 on ATF-2 phosphorylation, JNK and p38 proteins from cell lysates were removed using an immunodepletion procedure through a three-step immunoprecipitation. After immunodepletion procedures, JNK and p38 activities in lysates were markedly diminished to nondetectable levels (data not shown). In the final step, immunoprecipitated Plk3 from hyperosmotic stress-induced HCE cells significantly phosphorylated ATF-2 in the absence of JNK and p38 (Fig. 6E). The effect of hyperosmotic stress-induced Plk3 activation on phosphorylation of ATF-2 was also observed in high concentrations of glucose-, sucrose-, and NaCl-treated cells by immunocomplex assays (Fig. 6F). These results demonstrate for the first time that hyperosmotic stress stimulation induces Plk3 activation in the absence of JNK and p38 activities to phosphorylate ATF-2.
FIGURE 6.
Differentiation of Plk3 effect from JNK and p38 on ATF-2 phosphorylation in hyperosmotic stress-induced cells. A, effect of knocking down JNK mRNA on hyperosmotic stress-induced ATF-2 phosphorylation. B, effect of knocking down p38 mRNA on hyperosmotic stress-induced ATF-2 phosphorylation. C, effects of blocking JNK and p38 with SP600125 and SB202190, respectively, on hyperosmotic stress-induced ATF-2 phosphorylation. D, effects of knocking down Plk3 in the absence and presence of SP600125 and SB202190 on hyperosmotic stress-induced ATF-2 phosphorylation. E, effect of hyperosmotic stress on Plk3 activity. Plk3 activity was determined by immunocomplex kinase assay, and ATF-2 fusion protein was used as the substrate. An immunodepletion procedure was used to remove JNK and p38 in cell lysates. F, effects of high concentrations of high concentrations of glucose (300 mm), sucrose (300 mm), and NaCl (300 mm) on Plk3 activities. *, a significant difference between control and hyperosmotic stress-induced cells (p < 0.05, n = 3).
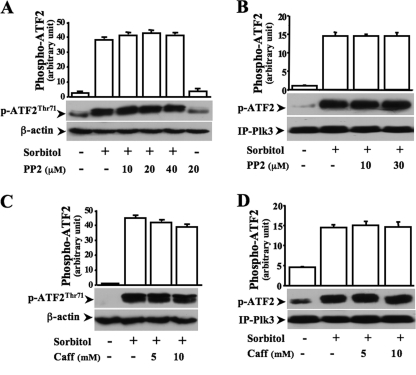
Effects of PP2 and Caffeine on Hyperosmotic Stress-induced ATF-2 Activation
It has been shown that hyperosmotic stress induces activation of Src and can also cause DNA damage. It has been shown that PP2 and caffeine are effective inhibitors for suppression of Src activity and for inhibition of ATM/ATR that is activated in response to DNA damages, respectively. To study the possible involvement of Plk3 in these pathways, various concentrations of PP2 were applied to hyperosmotic stress-induced cells. The results showed that PP2 had no effect on either hyperosmotic stress-induced ATF-2 phosphorylation in vivo or Plk3 kinase activity in vitro (Fig. 7, A and B). In addition, blockade of DNA damage-induced activation of ATM/ATR with 5–10 mm caffeine did not affect hyperosmotic stress-induced ATF-2 phosphorylation in vivo and had no inhibitory effect on hyperosmotic stress-induced Plk3 activity either (Fig. 7, C and D). The results obtained from inhibitions of Src and ATM/ATR in hyperosmotic stress-induced cells suggest that the effect of hyperosmotic stress-induced Plk3 activation on ATF-2 phosphorylation is independent from Src and ATM/ATR pathways.
FIGURE 7.
Effect of inhibiting Src and ATM/ATR on hyperosmotic stress-induced Plk3 and ATF-2 activation. A, effect of inhibiting Src with PP2 on hyperosmotic stress-induced ATF-2 phosphorylation. B, effect of inhibiting Src with PP2 on hyperosmotic stress-induced Plk3 activity. C, effect of inhibiting ATM/ATR with caffeine (Caff) on hyperosmotic stress-induced ATF-2 phosphorylation. D, effect of inhibiting ATM/ATR with caffeine on hyperosmotic stress-induced Plk3 activity. Phosphorylation of ATF-2 was measured by Western analysis using anti-phospho-ATF-2 (at site of threonine 71) antibody. Plk3 activity was determined in three sets of independent experiments by immunocomplex kinase assays.
DISCUSSION
Hyperosmotic stress is one of the environmental stresses that can delay the corneal epithelial wound healing process, resulting in compromising physiological function of the cornea. In various cell types, hyperosmotic stress activates stress-induced MAPK cascades including JNK and p38 signaling pathways (5, 8, 31, 32). Cells respond to hyperosmotic stress through activation of signaling pathways, resulting in further activation of downstream transcription factors, such as ATF-2. We found that hyperosmotic stresses that were generated by increased concentrations of different extracellular solutes including sorbitol, sucrose, glucose, and NaCl indeed activated ATF-2 in human corneal epithelial cells. However, suppression of JNK and p38 by knocking down JNK and p38 mRNA or by applications of inhibitors could not completely prevent hyperosmotic stress-induced ATF-2 phosphorylation as would be expected, which suggests that there are other players in the hyperosmotic stress-induced pathways to activate ATF-2 in human corneal epithelial cells (Fig. 1). To answer this possibility, we explored hyperosmotic stress-induced alternative pathways and revealed a novel pathway involving Plk3. By using a three-step immunoprecipitation/immunodepletion procedure, we were able to remove activities of both JNK and p38 from hyperosmotic stress-induced cells. We found in human corneal epithelial cells that Plk3 activated by high concentrations of sorbitol, sucrose, glucose, and NaCl is able to phosphorylate ATF-2 that is an important transcription factor to form heterodimers of AP-1 complex. In fact, Plk3 phosphorylates ATF-2 in the absence of JNK/p38 activities at the specific site of Thr-71 in hyperosmotic stress-induced human corneal epithelial cells.
To further characterize the hyperosmotic stress-induced ATF-2 phosphorylation through activation of Plk3 pathway, cDNAs encoding full-length Plk3 and Plk3 mutants were introduced in hyperosmotic stress-induced cells. The results of transfection experiments showed that full-length Plk3 and constitutive active Plk3 kinase domain mutant enhanced ATF-2 phosphorylation in hyperosmotic stress-induced cells, whereas kinase domain silencing Plk3 mutant had no effect on hyperosmotic stress-induced ATF-2 phosphorylation, although the exogenously expressed protein of kinase-domain silencing Plk3 mutant was markedly increased in the transfected cells. In addition, suppression of Plk3 mRNA expression with specific siRNA clearly inhibited hyperosmotic stress-induced ATF-2 phosphorylation. These results provide further evidence that hyperosmotic stress-induced ATF-2 phosphorylation was indeed mediated by the Plk3 pathway. Furthermore, protein interaction between Plk3 and ATF-2 is verified by two-step experiments. First, using respective antibodies in coimmunoprecipitation experiments can bi-directionally pull down both Plk3 and ATF-2 proteins. Second, both Plk3 and ATF-2 proteins can be colocalized in the nuclei of hyperosmotic stress-induced human corneal epithelial cells. Thus, we demonstrate for the first time that hyperosmotic stress-activated Plk3 is an important event upstream of ATF-2 to phosphorylate ATF-2 in the formation of AP-1 complex that determines cell differentiation and apoptosis in human corneal epithelial cells (17).
The effect of hyperosmotic stress on the activation of Plk3 subsequently resulting in ATF-2 phosphorylation is consistent with previous findings indicating that Plk3 is an important mediator of the cellular response to genotoxic stress. Furthermore, the effect of hyperosmotic stress-activated Plk3 on ATF-2 function was examined by using the AP-1-specific CRE reporter. Activated Plk3 phosphorylated ATF-2 and increased CRE promoter activity. Transfection of a mutant of ATF-2 (ATF-2T69A/T71A) effectively suppressed the effect of hyperosmotic stress-activated Plk3 on CRE promoter activity (Fig. 3). The study results provide new evidence to reveal that Plk3 plays a novel role to mediate the effect of hyperosmotic stress on AP-1 activation in addition to the previous findings that Plk3 is involved in UV irradiation and hypoxic stress-induced signaling pathway to activate c-Jun, the other member in formation of the AP-1 complex (16, 17). In addition, the findings of the present study have physiological significance because corneal epithelium forms the outer layer of the eye and is exposed to harsh environmental conditions including hyperosmotic stresses. It is no doubt that the finding of hyperosmotic stress-induced Plk3 activation that phosphorylates ATF-2 and elicits AP-1 transcription activity provides new insight of the novel mechanisms. These mechanisms involve hyperosmotic stress-induced molecular responses that alter expression levels of the important genes to determine human corneal epithelial cell fate.
The other responses that cells respond to hyperosmotic stress include activation of sodium-potassium cotransporter, ion channels, and water channels, leading to regulation volume increase. In these processes, numerous proteins and signaling pathways could be activated by hyperosmotic stresses (5). The regulation of ATF-2 phosphorylation in response hyperosmotic stress is mostly studied through activation of the p38 pathway (33–35). It has been shown that growth factors can induce ATF-2 phosphorylation through activation of Src and the p38 signaling pathway (36). We tested the effect of inhibiting Src on hyperosmotic stress-induced phosphorylation of ATF-2 with PP2, a potent inhibitor of Src (5, 37, 38). PP2 had no effect on either hyperosmotic stress-induced AFT-2 phosphorylation or Plk3 activity, suggesting that Plk3-activated ATF-2 is independent of the Src-mediated pathway. In addition, hyperosmotic stress could induce a DNA double-strand break and activate ATF-2 through activation of ATM/ATR (39, 40). It has been shown that caffeine is an effective inhibitor to suppress ATM/ATR activity in various cell types including corneal epithelial cells (41). In our study, application of caffeine to human corneal epithelial cells did not have inhibitory effects on either hyperosmotic stress-induced ATF-2 phosphorylation or Plk3 activity, suggesting that hyperosmotic stress-induced Plk3 activation that subsequently phosphorylates ATF-2 is an independent event from ATM/ATR activity (Fig. 7). These data support the notion that hyperosmotic stress-induced activation of Plk3 is one of the novel signaling pathways that lead to ATF-2 phosphorylation in human corneal epithelial cells.
This work was supported, in whole or in part, by National Institutes of Health Grants EY 018343 (to L. L.) and CA074229 (to W. D.).
- Plk
- Polo-like kinase
- HCE
- human corneal epithelial
- KD
- kinase domain
- CRE
- cAMP response element
- PP2
- 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine
- ATM/ATR
- ataxia-telangiectasia mutated/ATM and Rad3-related.
REFERENCES
- 1. Lang F., Busch G. L., Ritter M., Völkl H., Waldegger S., Gulbins E., Häussinger D. (1998) Physiol. Rev. 78, 247–306 [DOI] [PubMed] [Google Scholar]
- 2. Yu S. P., Choi D. W. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 9360–9362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deutsch C., Slater L., Goldstein P. (1982) Biochim. Biophys. Acta 721, 262–267 [DOI] [PubMed] [Google Scholar]
- 4. Westfall P. J., Patterson J. C., Chen R. E., Thorner J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 12212–12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burg M. B., Ferraris J. D., Dmitrieva N. I. (2007) Physiol. Rev. 87, 1441–1474 [DOI] [PubMed] [Google Scholar]
- 6. Sheikh-Hamad D., Gustin M. C. (2004) Am. J. Physiol. Renal Physiol 287, F1102–F1110 [DOI] [PubMed] [Google Scholar]
- 7. Lunn J. A., Jacamo R., Rozengurt E. (2007) J. Biol. Chem. 282, 10370–10379 [DOI] [PubMed] [Google Scholar]
- 8. Corrales R. M., Luo L., Chang E. Y., Pflugfelder S. C. (2008) Cornea 27, 574–579 [DOI] [PubMed] [Google Scholar]
- 9. Dai W. (2005) Oncogene 24, 214–216 [DOI] [PubMed] [Google Scholar]
- 10. Donohue P. J., Alberts G. F., Guo Y., Winkles J. A. (1995) J. Biol. Chem. 270, 10351–10357 [DOI] [PubMed] [Google Scholar]
- 11. Hamanaka R., Smith M. R., O'Connor P. M., Maloid S., Mihalic K., Spivak J. L., Longo D. L., Ferris D. K. (1995) J. Biol. Chem. 270, 21086–21091 [DOI] [PubMed] [Google Scholar]
- 12. Li B., Ouyang B., Pan H., Reissmann P. T., Slamon D. J., Arceci R., Lu L., Dai W. (1996) J. Biol. Chem. 271, 19402–19408 [DOI] [PubMed] [Google Scholar]
- 13. Conn C. W., Hennigan R. F., Dai W., Sanchez Y., Stambrook P. J. (2000) Cancer Res. 60, 6826–6831 [PubMed] [Google Scholar]
- 14. Wang Q., Xie S., Chen J., Fukasawa K., Naik U., Traganos F., Darzynkiewicz Z., Jhanwar-Uniyal M., Dai W. (2002) Mol. Cell. Biol. 22, 3450–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ouyang B., Pan H., Lu L., Li J., Stambrook P., Li B., Dai W. (1997) J. Biol. Chem. 272, 28646–28651 [DOI] [PubMed] [Google Scholar]
- 16. Wang L., Dai W., Lu L. (2007) J. Biol. Chem. 282, 32121–32127 [DOI] [PubMed] [Google Scholar]
- 17. Wang L., Gao J., Dai W., Lu L. (2008) J. Biol. Chem. 283, 25928–25935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karin M., Liu Z., Zandi E. (1997) Curr. Opin. Cell Biol. 9, 240–246 [DOI] [PubMed] [Google Scholar]
- 19. Shaulian E., Karin M. (2001) Oncogene 20, 2390–2400 [DOI] [PubMed] [Google Scholar]
- 20. Liu H., Deng X., Shyu Y. J., Li J. J., Taparowsky E. J., Hu C. D. (2006) EMBO J. 25, 1058–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carrillo R. J., Dragan A. I., Privalov P. L. (2010) J. Mol. Biol. 396, 431–440 [DOI] [PubMed] [Google Scholar]
- 22. Hurst H. C. (1995) Protein Profile 2, 101–168 [PubMed] [Google Scholar]
- 23. Schumacher M. A., Goodman R. H., Brennan R. G. (2000) J. Biol. Chem. 275, 35242–35247 [DOI] [PubMed] [Google Scholar]
- 24. Galcheva-Gargova Z., Dérijard B., Wu I. H., Davis R. J. (1994) Science 265, 806–808 [DOI] [PubMed] [Google Scholar]
- 25. Kyriakis J. M., Banerjee P., Nikolakaki E., Dai T., Rubie E. A., Ahmad M. F., Avruch J., Woodgett J. R. (1994) Nature 369, 156–160 [DOI] [PubMed] [Google Scholar]
- 26. Rosette C., Karin M. (1996) Science 274, 1194–1197 [DOI] [PubMed] [Google Scholar]
- 27. Lee J. H., Kim M., Im Y. S., Choi W., Byeon S. H., Lee H. K. (2008) Invest. Ophthalmol. Vis. Sci. 49, 1827–1835 [DOI] [PubMed] [Google Scholar]
- 28. Lu L., Wang L., Shell B. (2003) Invest. Ophthalmol. Vis. Sci. 44, 5102–5109 [DOI] [PubMed] [Google Scholar]
- 29. Kimura K., Teranishi S., Yamauchi J., Nishida T. (2008) Invest. Ophthalmol. Vis. Sci. 49, 125–132 [DOI] [PubMed] [Google Scholar]
- 30. Saika S., Okada Y., Miyamoto T., Yamanaka O., Ohnishi Y., Ooshima A., Liu C. Y., Weng D., Kao W. W. (2004) Invest. Ophthalmol. Vis. Sci. 45, 100–109 [DOI] [PubMed] [Google Scholar]
- 31. Huang Z., Tunnacliffe A. (2004) J. Physiol. 558, 181–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Uhlik M. T., Abell A. N., Johnson N. L., Sun W., Cuevas B. D., Lobel-Rice K. E., Horne E. A., Dell'Acqua M. L., Johnson G. L. (2003) Nat. Cell Biol. 5, 1104–1110 [DOI] [PubMed] [Google Scholar]
- 33. Gupta S., Campbell D., Dérijard B., Davis R. J. (1995) Science 267, 389–393 [DOI] [PubMed] [Google Scholar]
- 34. Livingstone C., Patel G., Jones N. (1995) EMBO J. 14, 1785–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Dam H., Wilhelm D., Herr I., Steffen A., Herrlich P., Angel P. (1995) EMBO J. 14, 1798–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ouwens D. M., de Ruiter N. D., van der Zon G. C., Carter A. P., Schouten J., van der Burgt C., Kooistra K., Bos J. L., Maassen J. A., van Dam H. (2002) EMBO J. 21, 3782–3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee M., Kim J. Y., Anderson W. B. (2004) J. Biol. Chem. 279, 48692–48701 [DOI] [PubMed] [Google Scholar]
- 38. Nam J. S., Ino Y., Sakamoto M., Hirohashi S. (2002) Clin. Cancer Res. 8, 2430–2436 [PubMed] [Google Scholar]
- 39. Sarkaria J. N., Busby E. C., Tibbetts R. S., Roos P., Taya Y., Karnitz L. M., Abraham R. T. (1999) Cancer Res. 59, 4375–4382 [PubMed] [Google Scholar]
- 40. Cortez D. (2003) J. Biol. Chem. 278, 37139–37145 [DOI] [PubMed] [Google Scholar]
- 41. Wang L., Lu L. (2007) Invest. Ophthalmol. Vis. Sci. 48, 652–660 [DOI] [PMC free article] [PubMed] [Google Scholar]