Abstract
LIS1 and NDEL1 are known to be essential for the activity of cytoplasmic dynein in living cells. We previously reported that LIS1 and NDEL1 directly regulated the motility of cytoplasmic dynein in an in vitro motility assay. LIS1 suppressed dynein motility and inhibited the translocation of microtubules (MTs), while NDEL1 dissociated dynein from MTs and restored dynein motility following suppression by LIS1. However, the molecular mechanisms and detailed interactions of dynein, LIS1, and NDEL1 remain unknown. In this study, we dissected the regulatory effects of LIS1 and NDEL1 on dynein motility using full-length or truncated recombinant fragments of LIS1 or NDEL1. The C-terminal fragment of NDEL1 dissociated dynein from MTs, whereas its N-terminal fragment restored dynein motility following suppression by LIS1, demonstrating that the two functions of NDEL1 localize to different parts of the NDEL1 molecule, and that restoration from LIS1 suppression is caused by the binding of NDEL1 to LIS1, rather than to dynein. The truncated monomeric form of LIS1 had little effect on dynein motility, but an artificial dimer of truncated LIS1 suppressed dynein motility, which was restored by the N-terminal fragment of NDEL1. This suggests that LIS1 dimerization is essential for its regulatory function. These results shed light on the molecular interactions between dynein, LIS1, and NDEL1, and the mechanisms of cytoplasmic dynein regulation.
Keywords: Cell Motility, Dynein, Molecular Motors, Neurodegeneration, Protein-Protein Interactions, LIS1, NDEL1
Introduction
Cytoplasmic dynein is a multi-subunit protein complex that moves along microtubules. It is involved in various cellular and subcellular activities, such as cell migration, vesicle transport, and organelle positioning (1, 2). Dynein interacts with several accessory proteins to accomplish this wide range of activities throughout the cell cycle (3). The best-characterized of these accessory proteins is dynactin, a multiprotein complex that is almost universally associated with dynein-dependent functions (4). Platelet-activating factor acetylhydrolase, isoform 1b, subunit 1 (PAFAH1B1; commonly known as lissencephaly 1, LIS1)3 and its binding partner nuclear distribution gene E homolog (A. nidulans)-like 1 (NDEL1) also contribute to many dynein functions involving nuclear and spindle positioning and centrosomal movement (3).
LIS1 was first identified as a protein associated with the smooth brain disease, lissencephaly (5, 6). Homozygous loss of Lis1 or Ndel1 is lethal in mice (7, 8). The C terminus of LIS1 binds to cytoplasmic dynein (9), whereas the N terminus contains a LisH homodimerization domain (10). Between these domains is a coiled-coil region that imparts flexibility to the LIS1 dimer (11), suggesting that LIS1 can alter its conformation between an “open state,” in which the coiled-coil regions form a random helix, and a “closed state,” in which the coiled-coil regions form a superhelix (12). NDEL1 contains an N-terminal coiled-coil domain that interacts with LIS1, and an unstructured C terminus that directly binds to dynein (13, 14). LIS1 and NDEL1 are thought to associate with cytoplasmic dynein as a heterotetramer (11). Emerging evidence suggests that LIS1 and NDEL1 contribute to many dynein-related activities in neurons; reduced expression of LIS1 and NDEL1 in neural precursor cells increased average centrosome-nucleus spacing, and LIS1 interference caused inhibition of centrosomal migration (15) and somal translocation (16). Reduction of LIS1 and NDEL1 in a mitotic cell line impaired prophase nuclear envelope invagination (17). However, although many observations suggest that LIS1 and NDEL1 regulate dynein at the cellular level, the underlying mechanism remains elusive. A previous study demonstrated that LIS1 and NDEL1 regulated dynein motility in an in vitro gliding assay (18). The binding of LIS1 to dynein impaired the translocation of microtubules (MTs) bound to a dynein-coated glass surface (suppression), whereas the binding of NDEL1 dissociated MTs from the dynein surface (dissociation). However, the binding of both LIS1 and NDEL1 restored dynein motility (restoration). In this study, we used fragments of LIS1 and NDEL1 to identify the functional regions, and investigated the molecular interactions among cytoplasmic dynein, LIS1, and NDEL1. The results contribute to our understanding of the mechanisms of cytoplasmic dynein regulation by LIS1 and NDEL1.
EXPERIMENTAL PROCEDURES
Cloning, Expression, and Purification of Proteins
DNA encoding the N-terminal fragment of human NDEL1 (NN; amino acids 44–183), conjugated to a hexahistidine tag, was amplified by PCR and ligated into the NdeI/EcoR I-digested pET-17b expression vector (Novagen). DNA encoding the C-terminal fragment of human NDEL1 (NC; amino acids 156–345) was amplified by PCR and ligated into NdeI/PstI-digested pCold-ProS2 vector (Takara Bio). This construct has an N-terminal polyhistidine tag followed by a ProS2 tag protein to promote solubility. The plasmids were transformed into the BL21-CodonPlus (DE3) RIL (Stratagene) host strain. Cells were grown at 37 °C to an absorbance of 0.6 at 600 nm. The temperature was reduced to 20 °C (15 °C for NC), and 0.1 mm (1 mm for NC) isopropyl-1-thio-β-d-galactopyranoside was added. After 3 h (20 h for NC) of induction, cells were harvested by centrifugation and resuspended in lysis buffer (50 mm Tris-HCl, 150 mm NaCl, and 20 mm imidazole, pH 7.0) in the presence of a protease inhibitor mixture (Roche Applied Science), and lysed with a sonicator followed by centrifugation. The soluble protein in the supernatant was purified by Ni-IMAC resin (Bio-Rad) and eluted with elution buffer (50 mm Tris-HCl, 150 mm NaCl, and 300 mm imidazole, pH 7.0). The protein solutions were desalted using a NAP-5 column (GE Healthcare). The ProS2 tag was removed from the NC fragment by HRV3C protease (Novagen) digestion. Because the NC solution contained degradation products, we analyzed the NC concentration by SDS-PAGE together with the Bradford method.
GST-LIS1 and GST-NDEL1 were purified as described previously (18). GST-tagged, N-terminal-deleted LIS1 (GST-ΔN-LIS1) was generated using a Bac-to-Bac baculo-system (Invitrogen) with SF-9 or High Five insect cells (BD Biosciences). In brief, cDNA encoding amino acids 57–410 of human LIS1 protein conjugated to a GST tag was cloned into the EcoRI site of pFASTBACHTa (Invitrogen), followed by transfection into DH10BAC cells (Invitrogen). Purified bacmid DNA was transfected into SF-9 cells for amplification of infectious baculovirus using LipoTAXI (Stratagene). After amplification, baculovirus was inoculated into High Five insect cells. Recombinant proteins were purified using GST-Sepharose (GE Healthcare), and the GST tag of GST-ΔN-LIS1 was removed by thrombin digestion (Sigma).
Preparation of Cytoplasmic Dynein and Tubulin
Cytoplasmic dynein was prepared from porcine brain as described previously (19, 20). Anion-exchange column (UnoQ-1; Bio-Rad)-purified cytoplasmic dynein peaks were pooled in HPLC buffer (35 mm Tris-HCl, 5 mm MgSO4, 1 mm EGTA, 0.5 mm EDTA, 1 mm DTT, 10 μm ATP, pH 7.2) supplemented with 24% sucrose. Tubulin was purified from porcine brain (21). Proteins were quantified as described (22), using BSA as a standard protein.
In Vitro Motility Assay
MT gliding movement was observed under a dark-field microscope (23) in buffer A (50 mm potassium acetate, 10 mm PIPES-KOH, 2 mm MgSO4, 1 mm EGTA, 1 mm DTT), supplemented with 1 mm ATP and 40 μm paclitaxel. The velocities of more than 50 MTs translocated for at least 20 s were measured under each set of conditions. Immobile MTs were not considered for velocity measurements.
MT Binding Assay
Dynein was mixed with NN, NC, or NDEL1 in buffer A at a 1:10 molar ratio of dynein:NN/NC/NDEL1. The mixed solutions were added to 10 mm ATP and 40 μm paclitaxel, and then centrifuged at 53,000 rpm for 10 min at 25 °C, using a 100.2 rotor in an Optima TLX ultracentrifuge (Beckman Coulter). The supernatants and pellets were subjected to SDS-PAGE. The images of the gels were digitized, and the bands were quantified. The mean values were obtained from three experiments.
Sucrose Density Gradient Centrifugation and Gel Filtration Analysis
The sedimentation coefficients (S) of NDEL1 fragments, GST-ΔN-LIS1, and ΔN-LIS1 in buffer B (12 mm PIPES-KOH, 2 mm MgCl2, 1 mm EGTA, 1 mm DTT, pH 6.8) were determined by 10–40% (w/v) sucrose gradient centrifugation at 40,000 rpm for 20 h at 2 °C in a P50S2 rotor (Hitachi). Standard proteins were β-amylase (S = 8.9), alcohol dehydrogenase (S = 7.4), BSA (S = 4.3), carbonic anhydrase (S = 3.2), and cytochrome c (S = 1.9). Gel filtration chromatography (TSKgel G3000SWXL; Tosoh) was performed in buffer B supplemented with 300 mm potassium acetate to prevent nonspecific adsorption. Stokes' radii were determined from the elution volumes using standard marker proteins. Molecular weights were calculated from the sedimentation coefficients and Stokes' radii, as previously described (23).
Co-sedimentation Assay
To investigate the interactions between dynein and the NDEL1 fragments, GST-ΔN-LIS1, or ΔN-LIS1, dynein was mixed with NN, NC, GST-ΔN-LIS1, or ΔN-LIS1 in buffer B supplemented with 1 mm ATP. The molar ratio of dynein to NN, NC, or ΔN-LIS1 in the mixture was 1:10 (1:30 for GST-ΔN-LIS1). The mixed solutions were layered on 10–40% (w/v) sucrose gradients and centrifuged at 33,000 rpm for 18 h at 2 °C in a P50S2 rotor (Hitachi). After centrifugation, the gradients were divided into fractions of equal volume and analyzed by SDS-PAGE. The gels were stained with silver.
RESULTS
The C-terminal Region of NDEL1 Facilitates the Dissociation of Dynein from MTs
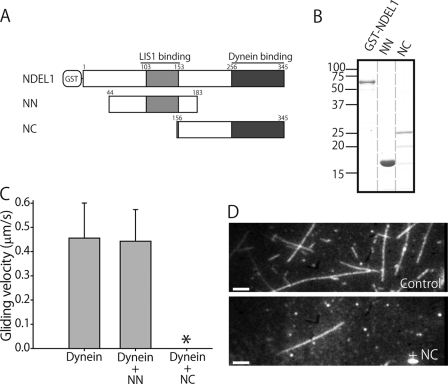
We initially focused on the role of NDEL1 in dynein regulation. NDEL1 associates with LIS1 through its N-terminal coiled-coil domain (14), and with dynein through its C-terminal domain (9). To examine whether these two domains were separately and independently responsible for the two NDEL1 functions, we dissected NDEL1 into an N-terminal fragment, NN (amino acids 44–183), containing a LIS1 binding region, and a C-terminal fragment, NC (amino acids 156–345), containing a dynein binding region (Fig. 1A). NN was expressed stably in Escherichia coli, but NC was not stable, and the protein solution contained degradation products (Fig. 1B). We tried to eliminate these degradation products using gel filtration chromatography, but they eluted at the same time as intact NC. The degradation products could arise if NC were largely unstructured, as predicted (13, 14). Sucrose density gradient centrifugation and gel filtration analysis indicated that NN was a dimer while NC was a monomer (Table 1). We further examined the effects of these fragments on dynein activity.
FIGURE 1.
Effects of NDEL1 fragments on dynein function. A, schematic diagram of NDEL1 and its fragments. B, NDEL1, NN, and NC electrophoresed on a 10% polyacrylamide gel. C, MT gliding velocities of dynein in the absence (Dynein, n = 58) or presence of NDEL1 fragments (NN, n = 55). * denotes few MTs binding to the dynein-coated surface. The error bars indicate standard deviations. D, dark-field images of bound MTs in the absence (Dynein) and presence of the NC fragment (Dynein + NC). Bars, 5 μm.
TABLE 1.
Oligomeric states of NDEL1 fragments and truncated LIS1
The Stokes' radius, rStokes, was calculated from the gel filtration data. The sedimentation coefficient, s20,w, was estimated by sucrose density gradient centrifugation and calculated at 20 °C in water. The estimated Mr was calculated from rStokes and s20,w. The polypeptide Mr was calculated from the primary sequence.
| Constructs | rStokes | S20,w | Estimated Mr | Polypeptide Mr | Ratio | Oligomeric state |
|---|---|---|---|---|---|---|
| nm | ×10−13s | |||||
| NN | 4.2 | 1.9 | 32,900 | 16,500 | 2.0 | Dimer |
| NC | 3.0 | 1.5 | 18,600 | 22,100 | 0.84 | Monomer |
| GST- Δ N-LIS1 | 5.1 | 5.8 | 122,000 | 65,300 | 1.9 | Dimer |
| Δ N-LIS1 | 3.3 | 3.4 | 45,700 | 40,000 | 1.1 | Monomer |
The results of co-sedimentation assays and GST pull-down assays demonstrated that NN interacted with LIS1 but not with dynein, while NC interacted with dynein but not with LIS1 (supplemental Fig. S1), whereas full-length NDEL1 interacted with both dynein and LIS1, as shown by Yamada et al. (18). In an in vitro gliding assay, the addition of dynein alone caused MTs to translocate on the dynein-coated surface at an average velocity of 0.46 ± 0.15 μm/s (mean ± S.D.) in the presence of 1 mm ATP (supplemental Movie S1). In the presence of NN, MTs translocated at an average velocity of 0.44 ± 0.13 μm/s, similarly to the control (Fig. 1C). In the presence of NC, however, MTs hardly bound to the dynein surface (Fig. 1D), and the few MTs that were bound to the dynein surface were easily dissociated (supplemental Movie S2). Interestingly, in the absence of ATP, the number of MTs bound to the dynein-coated surface was comparable to that in the control, despite the presence of NC. However, when a buffer containing 1 mm ATP was introduced to the chamber, MTs started to dissociate from the surface. The dissociation was thus nucleotide dependent, and a similar phenomenon was observed with full-length NDEL1. These results demonstrate that the C-terminal region is sufficient to facilitate the dissociation of dynein from MTs in the presence of ATP.
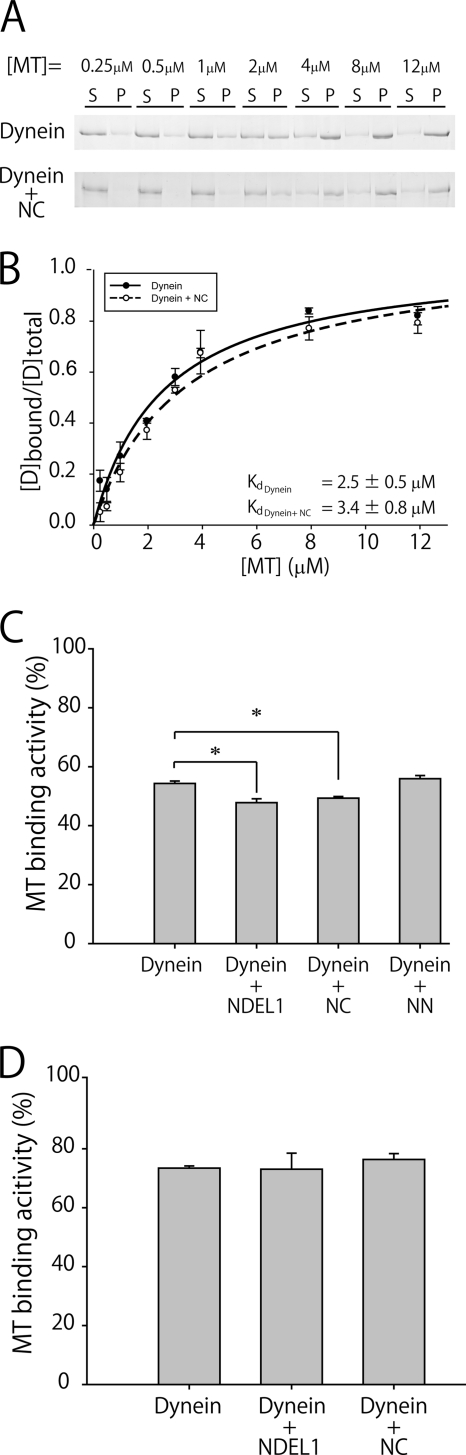
To confirm these results, we performed an MT co-pelleting assay. Without NC, cytoplasmic dynein bound to MTs and the dissociation constant (Kd) was 2.5 ± 0.5 μm. In the presence of NC, on the other hand, the dissociation constant increased to 3.4 ± 0.7 μm (Fig. 2, A and B). In addition, we investigated the effect of the N-terminal fragment of NDEL1, NN, and full-length NDEL1 in the presence of 0.3 μm MTs. The percentage of dynein bound to MTs in the presence of NDEL1 was 47.9 ± 0.6% (mean ± S.D.; Fig. 2C), which was comparable to the NC condition (49.6 ± 0.6%), but significantly less than the control (54.3 ± 0.8%; p < 0.005 by Student's t test). As expected from the in vitro gliding assay, NN had little effect on MT binding activity. In the absence of ATP, neither the NC fragment nor full-length NDEL1 altered the binding affinity of dynein to MTs (Fig. 2D), and the binding affinities were higher than those in the presence of ATP, demonstrating a similar tendency to that identified by the in vitro gliding assay. However, the differences in dissociation constants and the amounts of dynein bound to MTs were lower than expected from the in vitro gliding assay observations that MTs bound to the dynein surface were rarely observed in the presence of NC/NDEL1. This apparent weak effect of NC/NDEL1 probably reflects the geometrical differences between the two experiments. A previous study has shown that cytoplasmic dynein has the ability to crosslink MTs into bundles, indicating that it can interact with MTs through its tail region in addition to its stalk head, which is the main binding site of dynein to MTs (24). In MT co-pelleting assays, dynein can bind to MTs through its tail region, because dynein is free in the solution. In an in vitro gliding assay, on the other hand, dynein is considered to be fixed to the glass surface mainly through its tail region, and NC effectively weakens the interaction between the stalk and MTs. This experimental difference may generate the apparent weak effect of the C-terminal fragment of NDEL1 in the MT co-pelleting assay compared with the in vitro gliding assay. Nonetheless, these results demonstrate that the NC fragment was sufficient to weaken the MT-dynein interaction, and because the NC fragment was a monomer, dimerization of NDEL1 was shown to be not essential for the dissociation of MTs.
FIGURE 2.
Effects of NDEL1 fragments and full-length NDEL1 on dynein-MT interactions. A, SDS-PAGE gels showing the binding of cytoplasmic dynein to MTs in the presence/absence of NC with different MT concentrations. S, supernatant; P, pellet. B, binding curves obtained from MT co-pelleting assays repeated three times. In the control condition, the dissociation constant, Kd, was 2.5 ± 0.5 μm. In the presence of the C-terminal fragment of NDEL1, the Kd was 3.4 ± 0.7 μm. C and D, amount of dynein bound to MTs in the presence (C) or absence (D) of ATP. The error bars indicate standard deviations. The p value was calculated using Student's t test (*, p < 0.005).
The N-terminal Region of NDEL1 Releases the Suppression of Dynein Motility by LIS1
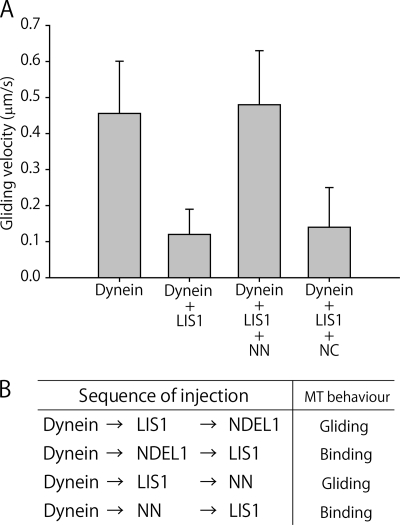
As mentioned above, the NN fragment had no direct effect on dynein function. However, NN contains a LIS1-binding domain, and was therefore expected to influence the suppression of dynein motility by LIS1. To address this issue, we examined the effect of the NDEL1 fragments on the suppression of dynein motility by performing an in vitro gliding assay in the presence of LIS1. When dynein was mixed with LIS1, the gliding velocity of MTs decreased to 0.12 ± 0.07 μm/s (Fig. 3A and supplemental Movie S3) and many immobile MTs were observed. Our previous study demonstrated that this impairment of MT translocation could be attributed to the direct blocking of dynein motility by LIS1, rather than by the tethering of MTs to the glass surface by LIS1 (18). When dynein was incubated with both LIS1 and NN, MTs translocated at a velocity 0.48 ± 0.15 μm/s (n = 60). This restoration of motility was similar to that caused by full-length NDEL1 (18). In contrast, the addition of NC had little effect on the suppression. These results suggest that the N-terminal region of NDEL1 was sufficient to restore dynein motility after LIS1-induced suppression. The co-sedimentation assay and GST pull-down assay showed that NN binds to LIS1, but not to dynein (supplemental Fig. S1), so the results of the motility assay also suggest that the binding of NN to LIS1 could restore dynein motility. To examine this idea, we modified the in vitro gliding assay to introduce the regulatory proteins sequentially. In the standard assay, dynein and LIS1 and/or NDEL1 were mixed and then introduced to the observation chambers simultaneously, whereas in the modified assay, dynein was introduced first, followed sequentially by the other proteins. If the protein introduced after dynein did not associate with dynein, the protein would be washed away. When NDEL1/NN was introduced prior to LIS1, MT motility was not restored and MTs bound to the surface (Fig. 3B). However, when LIS1 was introduced prior to NDEL1/NN, MTs showed gliding movements, demonstrating restoration of LIS1 suppression. Restoration could therefore be explained by direct binding of the NN fragment to LIS1.
FIGURE 3.
Activities of NDEL1 fragments in restoring dynein motility following LIS1 suppression. A, MT gliding velocities of dynein in the presence of LIS1 and NDEL1 fragments. B, modified in vitro gliding assays. Dynein was introduced into the chamber first, followed sequentially by the other proteins. Buffer A with 1 mm ATP and 40 μm taxol was added between each protein introduction to remove excess, unbound proteins. Binding means that MTs were bound to the dynein-coated surface and did not move.
Dimerization of LIS1 Is Essential for the Suppression of Dynein Motility
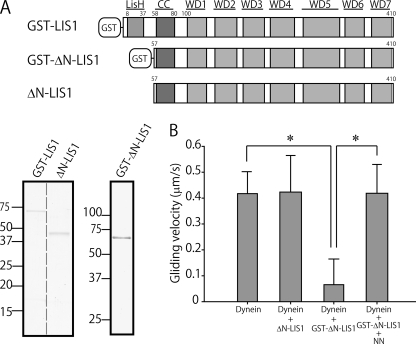
Homodimerization of LIS1 has been suggested to be important for its function (16). We therefore examined the effects of LIS1 dimerization on its regulatory functions. The N-terminal LisH motif in LIS1 (amino acids 8–37) and a single residue downstream of the LisH-motif, Trp-55, have been identified as being mainly responsible for its dimerization, while the coiled-coil region (amino acids 58–81) does not contribute to the dimerization (10–12). We truncated LIS1 by deleting the LisH-motif and expressing it as a fusion protein of GST (GST-ΔN-LIS1, amino acids 57–410; Fig. 4A). GST forms a dimer, and was removed by proteolysis to obtain a monomeric form of LIS1. Sucrose density gradient centrifugation and gel filtration analysis revealed that GST-ΔN-LIS1 was a dimerized protein, while ΔN-LIS1 was a monomeric protein (Table 1). The results of the co-sedimentation assay confirmed that both GST-ΔN-LIS1 and ΔN-LIS1 bound to dynein (supplemental Fig. S2). ΔN-LIS1 had little effect on dynein motility in an in vitro motility assay, confirming the importance of LIS1 dimerization for its regulatory function (supplemental Movie S4). The gliding velocity of MTs was similar to that of the control (Fig. 4B). The artificial dimer GST-ΔN-LIS1, however, significantly decreased the gliding velocity of MTs (p < 0.001; supplemental Movie S5). In addition, NN restored dynein motility after suppression by the artificial dimer. These results indicate that dimerization of LIS1 is important for its regulatory function, and that the LisH motif is not essential for the suppression and restoration of dynein motility by NN.
FIGURE 4.
Dimerization of LIS1 is necessary for its function. A, schematic diagram of GST-LIS1, GST-ΔN-LIS1, and ΔN-LIS1, and SDS-PAGE gel image. B, MT gliding velocities of dynein in the presence of 1 mm ATP. The error bars indicate standard deviations. The numbers of MTs measured were 55 (Dynein), 68 (Dynein + ΔN-LIS1), 66 (Dynein + GST-ΔN-LIS1), and 61 (Dynein + GST-ΔN-LIS1 + NN). The p value was calculated using Student's t test (*, p < 0.001).
DISCUSSION
Our previous study revealed that LIS1 suppressed the translocation of MTs in an in vitro gliding assay and that NDEL1 restored dynein motility (18). In the current study, we dissected NDEL1 into two fragments and showed that the C-terminal, dynein binding fragment (NC) was sufficient to cause the dissociation of MTs from a dynein-coated surface, while the N-terminal, LIS1-binding fragment (NN) was sufficient to restore dynein motility. We demonstrated that the two NDEL1 fragments were separately and independently responsible for NDEL1's two main functions. Using a modified in vitro gliding assay, we showed that the direct binding of NN to LIS1, rather than to dynein, restored dynein motility following suppression by LIS1.
The results also demonstrated that LIS1 dimerization is important for its regulatory function, but that the LisH motif, which is responsible for the dimerization of the native protein, is not essential. Artificially dimerized LIS1 (GST-ΔN-LIS1) was able to regulate dynein motility in an in vitro gliding assay, suggesting that cooperation between the two C-terminal regions of LIS1 might be necessary and sufficient for LIS1 function. A Lis1 mutant mouse lacking the LisH motif displayed aberrant layering in the developing cortex, and defective neuronal migration (25). Our findings provide evidence that the dimerization of LIS1 is essential for its regulation of dynein.
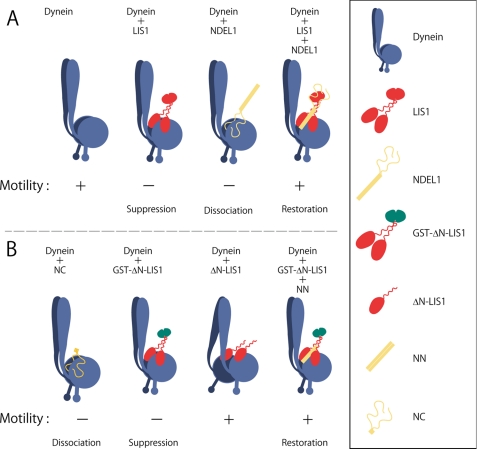
The schematic diagram shown in Fig. 5 represents our proposed mechanism of dynein motility regulation by full-length LIS1 and full-length NDEL1 (Fig. 5A), and by truncated LIS1 and NDEL1 fragments (Fig. 5B), based on the results of this study. In the absence of LIS1 and NDEL1, dynein translocates MTs in an in vitro gliding assay. The binding of LIS1 to dynein suppresses the motility and inhibits the translocation of MTs. Our preliminary study demonstrated, using a single-molecule assay, that the LIS1/dynein complex bound to MTs and did not move processively.4 This suggests that LIS1 regulates dynein motility directly, rather than by tethering MTs to the glass surface. The observation that truncated monomeric LIS1 did not affect dynein motility, while artificially dimerized LIS1 did suppress dynein motility, suggests that a single C-terminal region of LIS1 binds to one of two heavy chains of the dynein complex, and that the regulatory function of LIS1 requires the cooperativity of two C-terminal regions. In addition, the conformational flexibility of LIS1, which is mediated by the coiled-coil region (12), might play a role in the regulation of dynein motility.
FIGURE 5.
Schematic diagram of the proposed regulation of dynein motility. A, regulation by full-length LIS1 and full-length NDEL1. B, regulation by truncated LIS1 and NDEL1 fragments. The sizes of dynein, truncated LIS1 fragments, and NDEL1 fragments are not to scale.
The binding of the C-terminal region of NDEL1 causes the dissociation of MTs from dynein. The observation that MTs did not dissociate in the absence of ATP suggests that the dissociation could be attributed to the effects of NDEL1 on dynein motility, rather than to masking of the MT binding site of dynein by NDEL1. In addition, density gradient centrifugation and gel filtration analysis indicated that NC was a monomeric protein, suggesting that NDEL1 dimerization was not necessary to cause the dissociation of MTs.
The binding of both LIS1 and NN restores dynein motility. This restoration is mediated by direct binding of the N-terminal region of NDEL1 to LIS1, and does not require the LisH homodimerization domain. At present, however, the role of the C-terminal region of NDEL1 in the dynein/LIS1/NDEL1 complex (Fig. 5B, right) remains elusive. A previous study reported that the amount of NDEL1 bound to dynein was increased strongly in the presence of LIS1 (18), suggesting that the C terminus of NDEL1 may not interact with dynein in the dynein/LIS1/NDEL1 complex.
The in vitro gliding assay demonstrated no clear difference in motility between dynein alone and the dynein/LIS1/NDEL1 complex. McKenney et al. (26) recently applied an external load onto single molecules of dynein using optical tweezers, and reported that the complex of dynein and LIS1, with or without the NDEL1 homolog, NDE1, showed a force-persistent property, whereas dynein alone did not. The response of dynein molecules to external force is physiologically important, especially in the regulation of dynein motility within the cell, and thus deserves in vitro investigation.
In this study, we propose a molecular mechanism for the interactions among dynein, LIS1 and NDEL1, which contributes to our understanding of the mechanism of dynein motility regulation by LIS1 and NDEL1. However, further details of the mechanism responsible for the inhibition and restoration of motility, as well as the geometry and the structure of the complex, remain to be elucidated.
Supplementary Material

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2 and Movies S1–S5.
Y. Y. Toyoshima, unpublished data.
- LIS1
- lissencephaly 1
- NDEL1
- A. nidulans)-like 1
- MT
- microtubule.
REFERENCES
- 1. Vallee R. B., Williams J. C., Varma D., Barnhart L. E. (2004) J. Neurobiol. 58, 189–200 [DOI] [PubMed] [Google Scholar]
- 2. Vallee R. B., Seale G. E., Tsai J. W. (2009) Trends Cell Biol. 19, 347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kardon J. R., Vale R. D. (2009) Nat. Rev. Mol. Cell Biol. 10, 854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schroer T. A. (2004) Annu. Rev. Cell Dev. Biol. 20, 759–779 [DOI] [PubMed] [Google Scholar]
- 5. Reiner O., Carrozzo R., Shen Y., Wehnert M., Faustinella F., Dobyns W. B., Caskey C. T., Ledbetter D. H. (1993) Nature 364, 717–721 [DOI] [PubMed] [Google Scholar]
- 6. Wynshaw-Boris A. (2007) Clin. Genet. 72, 296–304 [DOI] [PubMed] [Google Scholar]
- 7. Hirotsune S., Fleck M. W., Gambello M. J., Bix G. J., Chen A., Clark G. D., Ledbetter D. H., McBain C. J., Wynshaw-Boris A. (1998) Nat. Genet. 19, 333–339 [DOI] [PubMed] [Google Scholar]
- 8. Sasaki S., Mori D., Toyo-oka K., Chen A., Garrett-Beal L., Muramatsu M., Miyagawa S., Hiraiwa N., Yoshiki A., Wynshaw-Boris A., Hirotsune S. (2005) Mol. Cell. Biol. 25, 7812–7827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sasaki S., Shionoya A., Ishida M., Gambello M. J., Yingling J., Wynshaw-Boris A., Hirotsune S. (2000) Neuron 28, 681–696 [DOI] [PubMed] [Google Scholar]
- 10. Gerlitz G., Darhin E., Giorgio G., Franco B., Reiner O. (2005) Cell Cycle 4, 1632–1640 [DOI] [PubMed] [Google Scholar]
- 11. Tarricone C., Perrina F., Monzani S., Massimiliano L., Kim M. H., Derewenda Z. S., Knapp S., Tsai L. H., Musacchio A. (2004) Neuron 44, 809–821 [DOI] [PubMed] [Google Scholar]
- 12. Mateja A., Cierpicki T., Paduch M., Derewenda Z. S., Otlewski J. (2006) J. Mol. Biol. 357, 621–631 [DOI] [PubMed] [Google Scholar]
- 13. Niethammer M., Smith D. S., Ayala R., Peng J., Ko J., Lee M. S., Morabito M., Tsai L. H. (2000) Neuron 28, 697–711 [DOI] [PubMed] [Google Scholar]
- 14. Derewenda U., Tarricone C., Choi W. C., Cooper D. R., Lukasik S., Perrina F., Tripathy A., Kim M. H., Cafiso D. S., Musacchio A., Derewenda Z. S. (2007) Structure 15, 1467–1481 [DOI] [PubMed] [Google Scholar]
- 15. Tsai J. W., Bremner K. H., Vallee R. B. (2007) Nat. Neurosci. 10, 970–979 [DOI] [PubMed] [Google Scholar]
- 16. Tsai J. W., Chen Y., Kriegstein A. R., Vallee R. B. (2005) J. Cell Biol. 170, 935–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hebbar S., Mesngon M. T., Guillotte A. M., Desai B., Ayala R., Smith D. S. (2008) J. Cell Biol. 182, 1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamada M., Toba S., Yoshida Y., Haratani K., Mori D., Yano Y., Mimori-Kiyosue Y., Nakamura T., Itoh K., Fushiki S., Setou M., Wynshaw-Boris A., Torisawa T., Toyoshima Y. Y., Hirotsune S. (2008) EMBO J. 27, 2471–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bingham J. B., King S. J., Schroer T. A. (1998) Methods Enzymol. 298, 171–184 [DOI] [PubMed] [Google Scholar]
- 20. Toba S., Toyoshima Y. Y. (2004) Cell Motil Cytoskeleton 58, 281–289 [DOI] [PubMed] [Google Scholar]
- 21. Sloboda R. D., Rosenbaum J. L. (1982) Methods Enzymol. 85, 409–416 [DOI] [PubMed] [Google Scholar]
- 22. Read S. M., Northcote D. H. (1981) Anal. Biochem. 116, 53–64 [DOI] [PubMed] [Google Scholar]
- 23. Furuta K., Edamatsu M., Maeda Y., Toyoshima Y. Y. (2008) J. Biol. Chem. 283, 36465–36473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amos L. A. (1989) J. Cell Sci. 93, 19–28 [DOI] [PubMed] [Google Scholar]
- 25. Cahana A., Escamez T., Nowakowski R. S., Hayes N. L., Giacobini M., von Holst A., Shmueli O., Sapir T., McConnell S. K., Wurst W., Martinez S., Reiner O. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 6429–6434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McKenney R. J., Vershinin M., Kunwar A., Vallee R. B., Gross S. P. (2010) Cell 141, 304–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.