Abstract
Glutamate-induced excito-neurotoxicity likely contributes to non-cell autonomous neuronal death in neurodegenerative diseases. Microglial clearance of dying neurons and associated debris is essential to maintain healthy neural networks in the central nervous system. In fact, the functions of microglia are regulated by various signaling molecules that are produced as neurons degenerate. Here, we show that the soluble CX3C chemokine fractalkine (sFKN), which is secreted from neurons that have been damaged by glutamate, promotes microglial phagocytosis of neuronal debris through release of milk fat globule-EGF factor 8, a mediator of apoptotic cell clearance. In addition, sFKN induces the expression of the antioxidant enzyme heme oxygenase-1 (HO-1) in microglia in the absence of neurotoxic molecule production, including NO, TNF, and glutamate. sFKN treatment of primary neuron-microglia co-cultures significantly attenuated glutamate-induced neuronal cell death. Using several specific MAPK inhibitors, we found that sFKN-induced heme oxygenase-1 expression was primarily mediated by activation of JNK and nuclear factor erythroid 2-related factor 2. These results suggest that sFKN secreted from glutamate-damaged neurons provides both phagocytotic and neuroprotective signals.
Keywords: Antioxidant, Chemokines, Glutamate, MAP Kinases (MAPKs), Phagocytosis, HO-1, Fractalkine, Microglia
Introduction
Glutamate toxicity is a major cause of neuronal cell death in various neurologic disorders, including ischemia, inflammation, epilepsy, and neurodegenerative diseases. Microglia, macrophage-like resident immune cells in the central nervous system, accumulate in the lesions observed in such neurodegenerative disorders as Alzheimer disease (AD)2 and Parkinson disease, where this cell type is thought to have both neurotoxic and neuroprotective properties (1). When activated by LPS, neurotoxic microglia release large amounts of glutamate through gap junction hemichannels (2, 3), resulting in neuronal damage. On the other hand, neuroprotective microglia release neurotrophic factors and anti-inflammatory cytokines, and remove unwanted debris via phagocytosis. We have shown that microglia activated by the Toll-like receptor 9 ligand CpG attenuate oligomeric amyloid β (Aβ) neurotoxicity by producing the antioxidant enzyme heme oxygenase-1 (HO-1) and phagocytosing Aβ (4). HO-1 expression is up-regulated by various stressors, resulting in antioxidant effects to counteract neurodegenerative disease pathophysiology (5, 6). Furthermore, the antioxidative effects of HO-1 are derived from induction of various anti-inflammatory responses and other cytoprotective processes (7).
Microglial phagocytosis maintains neural networks by clearing neurotoxic molecules, such as Aβ and cellular debris. Microglia express cell surface receptors that regulate phagocytosis, including phosphatidylserine (PS) receptor (8), triggering receptor expressed on myeloid cells 2 (TREM2) (9), the scavenger receptor CD36 (10), and the purine receptor P2Y6 (11). Microglia also produce an opsonin, milk fat globule-EGF factor 8 (MFG-E8), which mediates signaling between microglia and apoptotic cells expressing such cell surface molecules as PS (12). MFG-E8 may also be involved in Aβ phagocytosis, and its expression is reportedly reduced in AD (13). These observations suggest that microglia may have both neurotoxic and neuroprotective roles under physiologic or pathologic conditions.
Recently, several lines of evidence have suggested that damaged neurons are not merely passive targets of microglia, but rather regulate microglial activity through cytokines, nucleotides, and chemokines (14). Degenerating neurons also produce signaling molecules that regulate microglia-mediated phagocytosis and neuroprotection. Some of this signaling may be controlled by chemokines and chemokine receptors, which are widely expressed throughout the central nervous system (15). We hypothesized that the CX3C chemokine fractalkine (FKN; CX3CL1), which has been detected as both soluble and membrane-anchored forms, plays a pivotal role in signaling between degenerating neurons and microglia, because FKN and its receptor CX3CR1 are highly expressed in brain tissue (16), particularly in neurons and microglia (17–19). We previously demonstrated that FKN functions as an intrinsic inhibitor of microglial neurotoxicity (20). In addition, FKN is neuroprotective in rat hippocampal neuronal cultures (21). Finally, FKN has been shown to enhance the clearance of apoptotic cells by macrophages (22).
In the present study, we show that soluble FKN (sFKN) is released from glutamate-exposed neurons, resulting in MFG-E8-induced enhancement of microglial phagocytosis of neuronal debris. Moreover, this chemokine induces HO-1 expression, which suppresses glutamate neurotoxicity to promote neuronal survival.
EXPERIMENTAL PROCEDURES
Reagents
l-Glutamate, goat IgG, and LPS were purchased from Sigma (St. Louis, MO). FKN (the chemokine domain) and anti-mouse FKN-neutralizing antibody were obtained from R&D Systems (Minneapolis, MN). Anti-mouse MFG-E8 antibody and JNK peptide inhibitor (L-JNKI) were purchased from MBL (Nagoya, Japan). Hamster IgG was obtained from Jackson ImmunoResearch. The MAPK inhibitors PD98059 (MEK1 inhibitor), U0126 (MEK1/2 inhibitor), SB203580 (p38 inhibitor), and SP600125 (JNK inhibitor) were purchased from Calbiochem (Gibbstown, NJ). Stannus mesoporphyrin (SnMP), a specific inhibitor of HO-1, was obtained from Frontier Scientific (Logan, UT), and dissolved in an arginine-containing solution as previously described (23, 24).
Cell Culture
The protocols for animal experiments were approved by the Animal Experiment Committee of Nagoya University.
Primary neuronal cultures were prepared from the cortices of C57BL/6 mice embryos at embryonic day 17 (E17) as described previously (25). Briefly, cortical fragments were dissociated into single cells in dissociation solution (Sumitomo Bakelite, Akita, Japan), and resuspended in nerve culture medium (Sumitomo Bakelite). Neurons were seeded onto 12-mm polyethyleneimine-coated glass coverslips (Asahi Techno Glass Corp., Chiba, Japan) at a density of 5.0 × 104 cells/well in 24-well plates and incubated at 37 °C in a humidified atmosphere containing 5% CO2. The purity of the cultures was more than 95% as determined by NeuN-specific immunostaining.
Microglia were isolated from primary mixed glial cell cultures prepared from newborn C57BL/6 mice at 14 days in vitro (DIV) using the “shaking off” method, which has been described previously (26). The purity of the cultures was 97–100% as determined by immunostaining for the Fc receptor. Cultures were maintained in DMEM supplemented with 10% fetal calf serum, 5 μg/ml of bovine insulin, and 0.2% glucose. Microglia were seeded at a density of 7.0 × 104 or 1.0 × 105 cells/well in 96- or 48-well plates.
Neuron-microglia co-cultures were prepared by adding 1.0 × 105 microglia in 100 μl of neuronal medium to neuronal cultures (5.0 × 104 neuronal cells) on DIV 14 in 24-well plates. The cultures were maintained in nerve culture medium.
Measurement of Soluble FKN Levels
Secreted soluble FKN from mouse primary microglia and cortical neurons was measured using an ELISA kit (R&D Systems) according to the manufacturer's instructions. Neurons were treated with l-glutamate (1 or 10 μm) for 6–48 h at 37 °C. Supernatants were then collected and assessed for FKN levels.
RT-PCR
Total RNA was extracted from microglia and neurons using an RNeasy Mini Kit (Qiagen, Tokyo, Japan). A first-strand cDNA library was obtained using SuperScript II (Invitrogen, Tokyo) and oligo(dT)12–18 (Invitrogen) as the first-strand primer. Negative control reactions were performed using the same system after heat denaturation of reverse transcriptase. RT-PCRs to amplify of transcripts encoding mouse FKN, CX3CR1, iNOS, TNF-α, MFG-E8, HO-1, and GAPDH were performed using 0.1 μg of first-strand cDNA, Blend Taq polymerase (Toyobo Co., Osaka, Japan), and oligonucleotide primers (Table 1).
TABLE 1.
PCR primers and expected sizes of PCR products
| Gene | Sequence (5′ to 3′) | Expected size |
|---|---|---|
| bp | ||
| CX3CL1 | ||
| Sense | 5′-TATCAGCTAAACCAGGAGTC | 652 |
| Antisense | 5′-CTGGGTTTATCTCCTCAGAC | |
| CX3CR1 | ||
| Sense | 5′-ACTGACATCTACCTCCTGAACC | 620 |
| Antisense | 5′-CTCAGGTCCCTCTTCATGTC | |
| iNOS | ||
| Sense | 5′-CCCTTCCGAAGTTTCTGGCAGCAGC | 497 |
| Antisense | 5′-GGCTGTCAGAGCCTCGTGGCTTTGG | |
| TNFα | ||
| Sense | 5′-TTCTGTCTACTGAACTTCGGGGTGATCGGTCC | 692 |
| Antisense | 5′-GTATGAGATAGCAAATCGGCTGACGTGTGGG | |
| MFG-E8 | ||
| Sense | 5′-CACTGTGAAACCGAGACCAACTACTAC | 548 |
| Antisense | 5′-GCTTTATGTACTGTGCCTCCAGAGTC | |
| HO-1 | ||
| Sense | 5′-CTATGTAAAGCGTCTCCA | 343 |
| Antisense | 5′-GTCTTTGTGTTCCTCTGTC | |
| GAPDH | ||
| Sense | 5′-ACTCACGGCAAATTCAACG | 817 |
| Antisense | 5′-CCCTGTTGCTGTAGCCGTA | |
Western Blotting
Cells from the BV-2 mouse microglial cell line were treated with FKN, and cell lysates were boiled after the addition of sample buffer (1 m Tris-HCl, 20% SDS, and 2.5% glycerol). Cytoplasmic and nuclear fractions from the cells were separated using NE-PERTM Nuclear and Cytoplasmic extraction reagents (Pierce). Fifty micrograms of total protein were separated on a 5–20% Tris glycine SDS-polyacrylamide gel and blotted onto Hybond-P PVDF membranes (GE Healthcare, Buckinghamshire, UK). Membranes were blocked with 5% skim milk in Tris-buffered saline containing 0.05% Tween 20 for 1 h at room temperature. The antibodies to detect phosphorylated and total MAPK and Hsp90 (Cell Signaling, Danvers, MA), histone H1 antibody (Abcam, Cambridge, UK), or nuclear factor erythroid 2-related factor 2 (Nrf2; Santa Cruz Biotechnology, Santa Cruz, CA) were applied at the concentrations recommended by the manufacturers. Secondary antibodies were horseradish peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG (GE Healthcare) and were used at a dilution of 1:1000. SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific) was used according to the manufacturer's instructions. The intensities of the bands were calculated using CS Analyzer 1.0 (Atto Corporation, Tokyo).
Evaluation of Microglial Phagocytosis
Primary mouse cortical neurons in 24-well plates were labeled on DIV 14 with 1 μm CM-DiI (Molecular Probes (Invitrogen)), and treated with 1 or 10 μm glutamate overnight at 37 °C. Less than 30% of the neurons survived, whereas almost 100% of the neurons in the vehicle-treated samples survived. Surviving neurons were identified based on cytoskeletal morphology as previously described (25). After changing the culture medium, microglia were added to these neuronal cultures (1:2 ratio for neurons to microglia) with or without FKN for 24 h. Cells were subsequently fixed in 4% paraformaldehyde. Microglia were stained with Cy5-conjugated rat anti-mouse CD11b monoclonal antibody prior to fixation. In separate experiments, microglia were co-cultured with DiI-stained damaged neurons, FKN, and anti-MFG-E8 antibody for 24 h. Phagocytic uptake of neuronal debris by microglia was estimated based on the detection of DiI-stained neuronal debris (red) in CD11b-positive microglia (green); the phagocytosis index was calculated as the percentage of red staining that overlapped with green staining (shown in yellow) among all of the microglia.
Measurement of HO-1, TNF-α, NO, and Glutamate Levels
Supernatants from microglia were assessed using ELISA kits for TNF-α (BD Pharmingen, Tokyo). Cell extracts from microglia in extraction buffer (1% Nonidet P-40 in PBS) were assayed for HO-1 with an ELISA kit (Takara Bio, Mie, Japan). The level of NO was determined using a Griess reaction, as reported previously (27). To measure glutamate levels, a colorimetric assay kit (Yamasa Corporation, Tokyo) was used as described previously (28).
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde for 15 min at room temperature, blocked, and permeabilized with 0.05% Triton X-100. Neurons were stained with mouse polyclonal anti-MAP-2 antibody (1:1000; Chemicon, Temecula, CA) and secondary antibody conjugated to Alexa 488 (1:1000; Invitrogen). Microglia were stained with Cy5-conjugated rat anti-mouse CD11b monoclonal antibody (1:300, BD Pharmingen) prior to fixation. Images were analyzed using a deconvolution fluorescence microscope system (BZ-8000; Keyence Corporation, Osaka).
Surviving neurons were identified based on their cytoskeletons as described previously (25). Viable neurons were stained strongly with anti-MAP-2 antibody, whereas damaged neurons showed weaker staining. MAP-2-positive neurons were counted in representative areas in each well. During five independent trials, more than 200 neurons were evaluated in each well by a scorer who was blind to the experimental conditions. The number of viable neurons in untreated cultures was set as 100%.
Statistical Analysis
Statistically significant differences between the experimental groups were determined by one-way ANOVA followed by Dunnett or Tukey tests for multiple comparisons. Statistical analysis was performed using the software program Prism 4 for Windows (GraphPad Software, San Diego, CA). p values less than 0.05 were considered to be significant.
RESULTS
Neurons Exposed to Glutamate Release sFKN, Promoting Microglial Clearance of Neuronal Debris
The expression of FKN and its receptor CX3CR1 was examined in neurons and microglia. FKN mRNA was expressed in neurons and CX3CR1 mRNA was mainly expressed in microglia (Fig. 1A). sFKN protein was detected in the supernatant from unstimulated neuronal cultures, but not from microglial cultures, indicating that sFKN was secreted from neurons rather than microglia (Fig. 1B). In a previous report, we confirmed the expression of fractalkine in microglia. However, the expression was found to be less by using a more specific primer in RT-PCR and an ELISA kit in this study.
FIGURE 1.
Excitotoxically damaged neurons release sFKN to promote microglial clearance of neuronal debris. A, expression of FKN and CX3CR1 mRNA in microglia (Mi) and neurons (Neu) was assessed using RT-PCRs. GAPDH expression was used as a control. B, sFKN in the culture medium of microglia and neuronal cultures was measured using ELISAs. C, neurons were treated with glutamate at the indicated concentrations. sFKN concentrations in the neuronal culture supernatants were measured. Results show the mean ± S.E. (n = 3). Glutamate treatment significantly induced sFKN expression in 6 h compared with untreated control samples. *, p < 0.05; **, p < 0.01 (one-way ANOVA with Dunnett's post hoc test). D, microglial phagocytosis assay. Primary mouse cortical neurons were labeled with CM-DiI, and treated with or without glutamate (Glu) for 24 h. The culture medium was changed and microglia were added to the culture with or without sFKN for 24 h. A few DiI-incorporated microgila were detected in co-cultures of untreated neurons (NT) and microglia (a). sFKN (10 nm) did not increase the number of phagocytosed cells (b). Neurons that were pretreated with 10 μm glutamate (pre-Glu neurons) showed an increase in microglial phagocytosis (c); 10 nm sFKN increase the number of DiI-incorporated microglia (d). The arrows in the fluorescence micrograph denote microglial phagocytosis of DiI-stained neuronal debris. Scale bar = 20 μm. E, quantification of the phagocytosis index, which is defined as the percentage of total microglia staining (green) that overlaps with DiI staining (red) (relevant areas are shown in yellow). The columns indicate the mean ± S.E. obtained from three independent experiments, each of which included analysis of 10 randomly selected fields. Significant differences compared with untreated samples (*) or samples without sFKN treatment (#) are noted. ***, p < 0.001; ###, p < 0.001; n.s., not significant (one-way ANOVA with Tukey's post hoc test). F–H, microglia treated with the indicated concentrations of sFKN did not induce the production of glutamate (G), nitrite and iNOS (H), or TNF-α (I) as measured using the assay kit or ELISAs for protein levels, and mRNA expression levels of iNOS (H) or TNF-α (I) as measured using RT-PCR.
A previous study in rats showed that cleavage of FKN from neuronal membranes was enhanced by excitotoxic stimuli (29). We therefore examined whether glutamate enhanced sFKN release from mouse primary cortical neurons. As shown in Fig. 1C, both 1 and 10 μm glutamate enhanced sFKN release from these neurons, with the maximum effect observed 6 h after administration of 10 μm glutamate.
We then assessed whether the release of sFKN from neurons damaged by glutamate affected microglial clearance of neuronal debris. We developed a novel microglial phagocytosis assay using primary mouse cortical neurons; microglial phagocytosis has been previously assessed by uptake of fluorescent beads (30, 31). Mouse primary cortical neurons were labeled with fluorescent DiI, and treated with 10 μm glutamate for 24 h to induce damage. Microglia were then added and the cells were cultured for an additional 24 h. Phagocytic uptake of DiI-labeled neuronal debris was examined. Microglia did not markedly phagocytose labeled healthy neurons (Fig. 1D, panel a, and E), an observation that was not affected by treatment with 10 nm sFKN (Fig. 1D, panel b, and E). On the other hand, microglia engulfed neurons that were pretreated with 10 μm glutamate (pre-Glu) (Fig. 1D, panel c, and E). This process was enhanced by treatment with 10 nm sFKN (Fig. 1D, panel d, and E).
We also confirmed that sFKN-treated microglia engulfed pre-Glu neurons by immunostaining for the phagocytic marker Rab7, which signals phagosome maturation. Neuronal debris that was engulfed after pretreatment with 10 μm glutamate colocalized with Rab-7 in the cytoplasm of sFKN-treated microglia (supplemental Fig. S1).
We also assessed the effects of sFKN on microglial activation. sFKN did not induce the production of such neurotoxic molecules as glutamate, NO, and TNFα in microglia (Fig. 1, F–H).
sFKN Up-regulates Microglial Phagocytosis of Excitotoxically Damaged Neurons via MFG-E8 Production
We examined how sFKN enhanced microglial phagocytosis of excitotoxically damaged neurons. sFKN up-regulated the mRNA and protein expression of the PS receptor MFG-E8 in microglia in a dose-dependent manner (Fig. 2A).
FIGURE 2.
sFKN-induced microglial phagocytosis of excitotoxically damaged neurons is mediated through expression of MFG-E8. A, microglia were treated with the indicated concentrations of sFKN for 24 h, and MFG-E8 mRNA and protein expression levels were measured using ELISAs and RT-PCRs, respectively. The columns indicate the mean ± S.E. (n = 3). Treatment with 10 nm sFKN significantly increased MFG-E8 protein levels compared with untreated (NT) samples. *, p < 0.05 (one-way ANOVA with Dunnett's post hoc test). B, microglial phagocytosis of primary cortical neurons exposed to glutamate was examined by addition of anti-MFG-E8 antibody or isotype-matched hamster IgG control: (a) without sFKN, (b) 100 nm sFKN, (c) 100 nm sFKN and 10 μg/ml anti-MFG-E8 antibody, and (d) 100 nm sFKN and 20 μg/ml anti-MFG-E8 antibody, (e) 100 nm sFKN and 20 μg/ml of hamster IgG control. The arrows denote phagocytosis of neuronal debris (red) by microglia (green). Scale bar = 20 μm. C, phagocytosis index. The columns indicate mean ± S.E. from three independent experiments. In each experiment, 10 randomly selected fields were analyzed. sFKN significantly increased phagocytosis of neural debris, which was dose-dependently suppressed by anti-MFG-E8 antibody. ***, p < 0.001 compared with cultures without antibody (one-way ANOVA with Tukey's post hoc test).
We next assessed whether anti-MFG-E8 neutralizing antibody prevented sFKN-induced microglial phagocytosis. Unstimulated microglia did not appear to phagocytose pre-Glu neurons (Fig. 2B, panel a), whereas 100 nm sFKN markedly enhanced microglial phagocytosis (Fig. 2B, panel b). Although anti-MFG-E8 neutralizing antibody at a concentration of 10 μg/ml had no effect on phagocytosis (Fig. 2B, panel c), 20 μg/ml of anti-MFG-E8 neutralizing antibody completely blocked sFKN-induced microglial uptake of DiI-labeled neuronal debris (Fig. 2B, panel d). However, 20 μg/ml of hamster IgG, isotype-matched control of anti-MFG-E8 antibody, had no effect on phagocytosis of microglia (Fig. 2B, panel e). Quantitative analysis confirmed these findings (Fig. 2C). These results indicate that sFKN enhances microglial phagocytic uptake of excitotoxically damaged neuronal cells via MFG-E8 expression.
sFKN-treated Microglia Exhibit Neuro-protective Activity against Glutamate-induced Excitotoxicity
Phagocytic clearance of neuronal debris in response to sFKN may contribute to the stability of neuronal networks. We next examined whether sFKN directly promotes neuronal survival in neuronal cultures (Fig. 3A, panels a–e) or neuron-microglia co-cultures (Fig. 3A, panels f–j). Unstimulated neurons stained with anti-MAP-2 antibody had no detectable morphologic abnormalities and possessed intact cell bodies and dendrites; microglia stained with anti-CD11b antibody also appeared normal (Fig. 3A, panels a and f, and B). Glutamate (10 μm) induced neuronal cell damage in both types of cultures, with the survival rates decreasing to 32.1 and 47.1%, respectively (Fig. 3, A, panels b and g, and B). Treatment with 100 nm sFKN rescued ∼70% of neurons from excitotoxic death in the co-cultures, but not in the neuronal cultures (Fig. 3, A, panels c and h, and B). Addition of anti-FKN antibody significantly attenuated neuronal survival in the co-cultures (22.6%) (Fig. 3, A, panel i, and B). However, addition of goat IgG, an isotype-matched Ig control for anti-FKN antibody, had no significant effect on the survival rate (Fig. 3A, panels e and j, and B). We confirmed that sFKN by itself did not affect neuronal survival even after long exposures, whereas 10 nm sFKN was also neuroprotective in neuron-microglia co-cultures (Fig. 3B and supplemental Fig. S2, A and B). Additionally, the neuroprotective activity of sFKN was evaluated by Annexin V and PI staining. Glutamate treatment remarkably increased the number of Annexin V and PI positive-staining neurons, whereas sFKN treatment significantly decreased the number of Annexin V and PI positive-staining neurons in the co-cultures (supplemental Fig. S2, C–E).
FIGURE 3.
sFKN exerts neuroprotective effects in the presence of microglia. A, untreated neuronal cultures (NT; a) and neuron-microglia co-cultures (f; 1:2 neurons to microglia). Glutamate (Glu) induced neuronal loss in both neuronal (b) and neuron-microglia co-cultures (g). Addition of 100 nm sFKN did not significantly increase the survival rate (c), whereas the same treatment significantly increased survival in the presence of microglia (h). Anti-FKN antibody reduced survival of neurons in the neuron-microglia co-cultures (i), but this antibody had no significant effect in neuronal cultures (d). Addition of goat IgG (isotype-matched control for anti-FKN antibody) had no effect on the survival rate (e and j). Neurons were stained with anti-MAP-2 antibody (green), and microglia were stained with a Cy5-conjugated anti-CD11b antibody (red). Scale bar = 50 μm. B, neuronal survival was estimated as the percentage of intact neurons in the sample relative to the untreated sample. The columns indicate the mean ± S.E. from three independent experiments. In each experiment, 10 randomly selected fields were analyzed. *, indicates significant differences compared with Glu treatment (*, p < 0.05; ***, p < 0.001; n.s., not significant).
The Neuroprotection of sFKN-treated Microglia Is Mediated by Enhanced Phagocytosis and the Clearance of Damaged Neurons via MFG-E8
Furthermore, we examined whether the enhanced phagocytosis and the clearance of damaged neurons by FKN-treated microglia would promote survival of neurons. Glutamate-induced neuronal cell damage (Fig. 4, A, panels a and b, and B) was inhibited by 100 nm sFKN in neuron-microglia co-cultures (Fig. 4, A, panel c, and B). Addition of 20 μg/ml of anti-MFG-E8 neutralizing antibody significantly inhibited neuroprotection by sFKN (Fig. 4, A, panels d, and B). On the other hand, hamster IgG, isotype-matched control of this antibody, had no effect on sFKN-induced neuroprotection (Fig. 4, A, panel e, and B). Both anti-MFG-E8 antibody alone and IgG control alone did not affect the neuronal survival rate (Fig. 4, A, panel f, and B).
FIGURE 4.
Inhibition of endogenous MFG-E8 expression abrogates sFKN-induced neuroprotection. A, untreated neuron-microglia co-cultures (NT; a). The cultures were pretreated with (c–e) or without (b and f) sFKN for 3 h in the presence of 20 μg/ml of anti-MFG-E8 antibody (d and f) or 20 μg/ml of isotype-matched hamster IgG control (e). The cultures were then treated with glutamate for 24 h (b–e). Neurons were stained with anti-MAP-2 antibody (green), and microglia were stained with Cy5-conjugated anti-CD11b antibody (red). Scale bar = 50 μm. B, the neuronal survival rate in the presence of sFKN and anti-MFG-E8 antibody or control IgG was estimated. Columns indicate the mean ± S.E. from three independent experiments, each of which included analysis of 10 randomly selected fields. * indicates significant differences compared with sFKN treatment (***, p < 0.001).
sFKN Neuroprotection Is Mediated by Microglial Production of HO-1
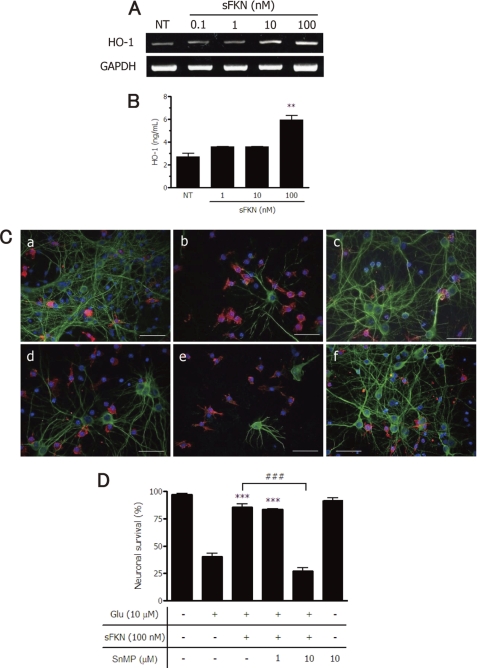
Interestingly, we found that sFKN dose dependently increased HO-1 mRNA and protein levels (Fig. 5, A and B). Glutamate-induced neuronal cell damage (Fig. 5C, panel b), which was compared with intact cultures (Fig. 5C, panel a), was almost completely inhibited by 100 nm sFKN in neuron-microglia co-cultures (Fig. 5C, panel c). This neuroprotective effect of sFKN was abolished by treatment with 10 μm SnMP, a HO-1 inhibitor (Fig. 5C, panel e); a lower concentration of SnMP did not exert this effect (Fig. 5C, panel d). 10 μm SnMP alone was not toxic for neurons in the presence of microglia (Fig. 5C, panel f). Quantitative analysis confirmed the effects of sFKN and the HO-1 inhibitor (Fig. 5D).
FIGURE 5.
sFKN exerts neuroprotective effects via expression of HO-1 in microglia. Microglia were treated with the indicated concentrations of sFKN for 24 h, and HO-1 mRNA (A) and protein (B) expression levels were measured using RT-PCRs and ELISAs, respectively. The columns indicate the mean ± S.E. (n = 3). **, p < 0.01 compared with untreated control (NT) samples. C, effects of an HO-1 inhibitor on the neuroprotective function of sFKN. Untreated neuron-microglia co-cultures (NT; a). The cultures without pretreatment (b and f) or pretreated with sFKN (c–e) for 3 h in the presence of the HO-1 inhibitor SnMP at 1 μm (c) or 10 μm (d and f). The cultures were treated with glutamate for 24 h. Neurons were stained with anti-MAP-2 antibody (green), and microglia were stained with Cy5-conjugated anti-CD11b antibody (red). Scale bar, 50 μm. D, the neuronal survival rate in the presence of sFKN and SnMP was estimated. Columns indicate the mean ± S.E. from three independent experiments, each of which included analysis of 10 randomly selected fields. ***, p < 0.001 compared with neuronal survival in the presence of only glutamate. ###, p < 0.001 compared with cultures without SnMP.
sFKN Exerts Neuroprotective Effects via Activation of ERK and JNK MAPK Signaling
FKN is reportedly involved in the activation of various intracellular signaling pathways, including ERK1/2, JNK, and p38 MAPKs, and PI3K (19, 32, 33). We explored which MAPK signaling pathways contributed to the neuroprotective effects of FKN. BV-2 cells were treated with 100 nm sFKN for various time periods, revealing time-dependent phosphorylation of ERK1/2 (p42/44 MAPK) and JNK, but not p38 MAPK (Fig. 6A). Moreover, the activation level of these MAPKs by 10 nm sFKN treatment was lower than that by 100 nm sFKN (supplemental Fig. S3A). The ERK activation level was similar to results obtained following LPS treatment. On the other hand, glutamate treatment had no effect on the activation of these MAPKs (supplemental Fig. S3B).
FIGURE 6.
sFKN exerts neuroprotective effects via ERK and JNK MAPK signaling. A, protein extracts from BV-2 cells were analyzed by immunoblotting with antibodies specific for phosphorylated and total MAPKs (ERK1/2, JNK, and p38). Cells were treated with 100 nm sFKN for the indicated time periods or 1 μg/ml of LPS for 60 min. *, p < 0.05; **, p < 0.01 compared with untreated control (NT) samples. B, neuron-microglia co-cultures were pretreated with (a–d) or without (e and f) 100 nm sFKN for 3 h in the presence of MAPK inhibitors (MEK1/2 (b and e), 1 μm U0126; JNK (c and f), 10 μm JNK peptide inhibitor L-JNKI; p38 (d), 10 μm SB203580). The cultures were then treated with 10 μm glutamate for 24 h. Staining for neurons (MAP2; green) and microglia (CD11b; red) was then performed. Scale bar, 50 μm. C, neuronal survival rate against glutamate excitotoxicity in the presence of 100 nm sFKN and each MAPK inhibitor (MEK1/2, 1 μm U0126; MEK1, 10 μm PD98059 (PD); JNK, 10 μm L-JNKI and 10 μm SP600125 (SP); p38, 10 μm SB203580 (SB)). The columns indicate the mean ± S.E. from three independent experiments, each of which included analysis of 10 randomly selected fields. ***, p < 0.001 compared with the cultures without MAPK inhibitors.
The neuroprotective effects of 100 nm sFKN (Fig. 6B, panel a) was almost completely canceled by treatment with the MEK1/2 inhibitor U0126 (Fig. 6B, panel b) or the JNK peptide inhibitor L-JNKI (Fig. 6B, panel c), whereas the p38 inhibitor SB203580 had little effect (Fig. 6B, panel d). Thus, U0126 or L-JNKI treatment alone had no effect on the survival of neurons (Fig. 6B, panels e and f), and other MAPK inhibitors were not toxic for neuron-microglia co-cultures (Fig. 6C). U0126 inhibits both MEK1 and MEK2, and thereby completely suppresses the activation of ERK, a kinase downstream of MEK. On the other hand, the ERK inhibitor PD98059 inhibits only MEK1. JNK inhibitors primarily act at two sites: targeting the JNK peptide (e.g. L-JNKI) or JNK kinase (e.g. SP600125). We found that these inhibitors of MEK (PD98059) or JNK (SP600125) also blocked sFKN-induced neuroprotection (Fig. 6C).
sFKN Induces Microglial HO-1 Production via JNK-Nrf2 Signaling
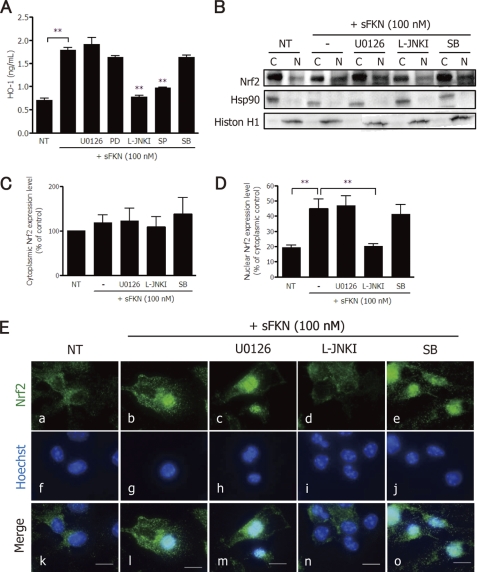
We examined whether MAPK signaling pathways are involved in HO-1 production by microglia. Inhibitors of JNK MAPK (JNK peptide, L-JNKI; JNK kinase, SP600125) significantly suppressed sFKN-induced HO-1 expression (Fig. 7A). Inhibitors of ERK MAPK (MEK1/2, U0126; MEK1, PD98059) or p38 MAPK (SB203580) did not suppress HO-1 expression (Fig. 7A). Similarly, the PI3K inhibitor wortmannin had no effect on sFKN-induced HO-1 expression (supplemental Fig. S4).
FIGURE 7.
sFKN induces microglial HO-1 expression via JNK-Nrf2 signaling. A, after microglia were treated with sFKN and the indicated MAPK inhibitors for 24 h, HO-1 protein expression levels were measured using ELISAs. **, p < 0.01 compared with sFKN-treated samples. B, BV-2 cells were treated with various MAPK inhibitors (MEK1/2, 1 μm U0126; JNK, 10 μm L-JNKI; or p38, 10 μm SB203580 (SB)) in the presence or absence of 100 nm sFKN. Protein extracts from cytoplasmic (c) or nuclear (n) fractions were analyzed by immunoblotting with the antibodies specific for Nrf2, Hsp90, and histone H1. Hsp90 expression was used as a cytoplasmic protein control. Histon H1 was used as nuclear protein expression control. C, relative amount of Nrf2 per cytoplasmic protein Hsp90 in BV-2 cells. The columns show the mean ± S.E. from three independent experiments. D, relative amount of Nrf2 per nuclear protein histone H1 in BV-2 cells. The columns show the mean ± S.E. from three independent experiments. * indicates significant differences compared with sFKN-treated nuclear extracts (**, p < 0.01). E, immunofluorescence images of Nrf2 (green; a–e) and nuclei (Hoechst blue; f–j) in microglia treated with 100 nm sFKN in the presence of various MAPK inhibitors (U0126 (c, h, and m), L-JNKI (d, i, and n), SB (e, j, and o)). The merged images (k–o) show decreased levels of nuclear Nrf2 in response to L-JNKI (n). Scale bar, 10 μm.
Nrf2 has been implicated in the induction of HO-1 expression and reportedly plays a pivotal role in cellular survival (34). Indeed, HO-1 expression is regulated by nuclear translocation of Nrf2 (35). We therefore examined whether sFKN induces Nrf2 translocation from the cytoplasm to the nucleus via activation of MAPK pathways. Using Western blot analysis, nuclear expression of Nrf2 was increased by 100 nm sFKN (Fig. 7B). Among the various MAPK inhibitors, only the JNK inhibitor L-JNKI inhibited Nrf2 translocation (Fig. 7, B and C). Cytoplasmic Nrf2 expression was not affected significantly by these inhibitors (Fig. 7, B and D). Immunofluorescence staining of Nrf2 in microglia confirmed these results. 100 nm sFKN promoted nuclear translocation of Nrf2 (Fig. 7E, panels a, b, f, g, k, and l). Among the various MAPK inhibitors, only the JNK inhibitor L-JNKI disrupted Nrf2 translocation (Fig. 7E, panels c–e, h–j, and m–o). We confirmed that Nrf2 was translocated to the nucleus by sFKN in the neuron-microglia co-cultures treated with glutamate (supplemental Fig. S5).
DISCUSSION
Appropriate clearance of neuronal debris by microglia is essential for the maintenance of healthy neuronal networks (36). Various signals from damaged neurons, including nucleotides and chemokines, must therefore recruit and activate microglia (11, 14). Microglia respond to FKN through CX3CR1. In a previous study (20), we found that FKN serves a neuroprotective function against activated microglia-induced neurotoxicity. A lack of CX3CR1 reportedly results in progressive neuronal cell death in an animal model of neurodegenerative disease (37). Despite a controversial report showing that knocking out CX3CR1 prevents neuronal loss in a mouse model of AD (38), many others have offered evidence supporting the neuroprotective roles of FKN. Plasma sFKN level are lower in patients with AD (39). Thus, FKN that is released from damaged neurons may signal microglia for support, although the precise mechanisms have not yet been clarified. Interestingly, FKN cleavage is reportedly enhanced in neuropathic pain (40) and cerebral ischemia (41), and in response to apoptosis inducers (8) and glutamate (21, 29).
In this study, we found that sFKN is released from mouse cortical neurons when they are excitotoxically damaged by glutamate. sFKN then enhances phagocytic uptake of neuronal debris by microglia. Microglial phagocytosis has been generally assessed by uptake of fluorescently labeled beads. In some reports, the SH-SY5Y and Neuro2a neuroblastoma cell lines and the BV-2 microglial cell line have been used in phagocytosis assays (8, 42). It is preferred, however, to use damaged neurons to assess phagocytosis of neuronal debris by microglia. Here, we developed a novel approach to assess microglial phagocytic uptake using DiI-labeled neurons. The reliability of the assay was validated by colocalization of DiI-labeled debris and the phagocytic marker Rab-7. In this assay, microglial phagocytosis of neuronal debris was markedly enhanced by 10 nm sFKN. This sFKN concentration is similar to that released from neurons after exposure to 10 μm glutamate (Fig. 1C).
We then explored phagocytosis-related factors that were expressed in microglia in response to sFKN. sFKN enhanced expression of the PS receptor MFG-E8 in microglia, which is involved in clearing apoptotic cells (8). We also found that MFG-E8 expression was up-regulated under excitotoxic conditions, such as those mediated by high levels of glutamate. Moreover, expression levels of the other PS receptor T cell immunoglobulin mucin domain 4, TREM2, and CD36 also dose dependently increased in response to sFKN treatment (data not shown). These molecules may also promote phagocytic activity in microglia. Thus, sFKN released from damaged neurons may provide an “eat me” signal through several different pathways. On the other hand, sFKN-treated microglia exhibited dose-dependent neuroprotective effects against glutamate-induced neurotoxicity, suggesting that sFKN functions as a “help me” signal from damaged neurons. We found that the enhanced phagocytosis and the clearance of damaged neurons by FKN-treated microglia promote survival of neurons via MFG-E8. The neuroprotection of 10 nm sFKN can be due to microglial phagocytic activity via MFG-E8.
FKN is reported to activate various intracellular signaling pathways, including ERK1/2 (21, 32, 33), JNK (32), p38 (32, 43), and PI3K (19, 33, 44). Among these pathways, PI3K and p38 MAPK signaling mediates the toxic activities of microglia through production of certain proinflammatory cytokines. We examined signaling pathways that may have been involved in sFKN-induced microglial neuroprotection using the microglial cell line BV-2; with the aid of specific MAPK inhibitors, we demonstrated that sFKN acts through ERK and JNK MAPK, but not through p38 MAPK. Moreover, we showed that JNK MAPK signaling drives the expression of the antioxidant enzyme HO-1 via Nrf2 nuclear translocation. Nrf2 is an important transcription factor for the expression of genes encoding phase II detoxifying and antioxidant enzymes (45). The subcellular localization of Nrf2 is a key regulator of HO-1 expression in response to various stress stimuli (34, 46). Nuclear translocation and phosphorylation of Nrf2 are induced by ERK1/2, p38, and PI3K (34). Our study showed that sFKN enhances the nuclear translocation of Nrf2 via activation of JNK MAPK in microglia. HO-1, a member of the heat shock protein family, is a lysosomal enzyme that oxidatively cleaves heme to produce biliverdin, carbon monoxide, and iron. This enzyme also helps to reduce oxidative stress-induced factors, such as reactive oxygen species. Numerous studies have demonstrated that up-regulation of HO-1 expression in the central nervous system may be beneficial to counteract neuroinflammation and neurodegenerative diseases (7).
The various MAPK pathways operate independently of each other (47). In this study, only JNK MAPK was involved in HO-1 expression through Nrf2 nuclear translocation. ERK signaling was also involved in the neuroprotective effects of sFKN. ERK contributes to cellular survival, proliferation, and differentiation through phosphorylation of a number of transcription factors, including cAMP response element-binding protein, activating transcription factor 1, and Myc (48). Further studies are needed to clarify the neuroprotective mechanism of the ERK pathway. Nevertheless, the neuroprotective effects of sFKN may be mediated by both the JNK-Nrf2-HO-1 and ERK signaling pathways.
Our data are consistent with the scheme shown in Fig. 8. Mild to moderate neuronal damage induced by glutamate enhances sFKN release from cellular membranes, and sFKN induces the expression of MFG-E8, JNK-Nrf2-HO-1, and ERK in microglia through CX3CR1. MFG-E8 enhances the phagocytic uptake of neuronal debris and thereby promotes neuronal survival, and JNK-Nrf2-HO-1 exerts antioxidant effects. Expression of MFG-E8, which is involved in Aβ phagocytosis by macrophages, is reduced in AD brains (13).
FIGURE 8.
Model of the role of sFKN in microglial phagocytosis and neuroprotection. sFKN, which is secreted from neurons that are damaged by glutamate, promotes microglial phagocytosis of neuronal debris through the release of MFG-E8. sFKN also induces the expression of the antioxidant enzyme HO-1 in microglia via Nrf2 recruitment and activation of the JNK MAPK signaling pathway. The neuroprotective effects of sFKN are also mediated in part by activation of ERK MAPK, although the downstream signaling pathway has not yet been elucidated. Therefore, sFKN may be an intrinsic neuroprotectant for damaged yet surviving neurons.
Furthermore, Nrf2 gene therapy has been shown to improve memory in the mouse model of AD (49). Therefore, FKN may prevent some of the pathogenic processes that underlie AD. Our study also suggests that the FKN-CX3CR1 signaling axis is crucial for healthy neuronal networks, and may provide a pharmacotherapeutic target in the treatment of neurodegenerative diseases.
Supplementary Material
Acknowledgment
We appreciate the helpful suggestions from Professor M. Takekawa (Dept. of Cell Signaling and Molecular Medicine, RIEM, Nagoya University).
This work was supported by the Program for Promotion of Fundamental Studies in Health Sciences from the National Institute of Biomedical Innovation (NIBIO), a Grant-in-aid for the global COE program from the Ministry of Education, Culture, Sports, Science and Technology of Japan, grants from the Ministry of Health, Labor and Welfare of Japan, and the New Energy and Industrial Technology Development Organization (NEDO).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
- AD
- Alzheimer disease
- DIV
- days in vitro
- FKN
- fractalkine
- HO-1
- heme oxygenase-1
- Aβ
- amyloid β
- PS
- phosphatidylserine
- TREM2
- triggering receptor expressed on myeloid cells
- MFG-E8
- milk fat globule-EGF factor 8
- sFKN
- soluble fractalkine
- PI
- propidium iodide
- SnMP
- stannus mesoporphyrin
- Nrf2
- nuclear factor erythroid 2-related factor 2
- pre-Glu
- pretreated with 10 μm glutamate
- ANOVA
- analysis of variance.
REFERENCES
- 1. Farfara D., Lifshitz V., Frenkel D. (2008) J. Cell Mol. Med. 12, 762–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Takeuchi H., Jin S., Wang J., Zhang G., Kawanokuchi J., Kuno R., Sonobe Y., Mizuno T., Suzumura A. (2006) J. Biol. Chem. 281, 21362–21368 [DOI] [PubMed] [Google Scholar]
- 3. Takeuchi H. (2010) Clin. Exp. Neuroimmun. 1, 12–21 [Google Scholar]
- 4. Doi Y., Mizuno T., Maki Y., Jin S., Mizoguchi H., Ikeyama M., Doi M., Michikawa M., Takeuchi H., Suzumura A. (2009) Am. J. Pathol. 175, 2121–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elbirt K. K., Bonkovsky H. L. (1999) Proc. Assoc. Am. Physicians 111, 438–447 [PubMed] [Google Scholar]
- 6. Pappolla M. A., Chyan Y. J., Omar R. A., Hsiao K., Perry G., Smith M. A., Bozner P. (1998) Am. J. Pathol. 152, 871–877 [PMC free article] [PubMed] [Google Scholar]
- 7. Syapin P. J. (2008) Br. J. Pharmacol. 155, 623–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fuller A. D., Van Eldik L. J. (2008) J. Neuroimmune. Pharmacol. 3, 246–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takahashi K., Rochford C. D., Neumann H. (2005) J. Exp. Med. 201, 647–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stolzing A., Grune T. (2004) FASEB J. 18, 743–745 [DOI] [PubMed] [Google Scholar]
- 11. Koizumi S., Shigemoto-Mogami Y., Nasu-Tada K., Shinozaki Y., Ohsawa K., Tsuda M., Joshi B. V., Jacobson K. A., Kohsaka S., Inoue K. (2007) Nature 446, 1091–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanayama R., Tanaka M., Miwa K., Shinohara A., Iwamatsu A., Nagata S. (2002) Nature 417, 182–187 [DOI] [PubMed] [Google Scholar]
- 13. Boddaert J., Kinugawa K., Lambert J. C., Boukhtouche F., Zoll J., Merval R., Blanc-Brude O., Mann D., Berr C., Vilar J., Garabedian B., Journiac N., Charue D., Silvestre J. S., Duyckaerts C., Amouyel P., Mariani J., Tedgui A., Mallat Z. (2007) Am. J. Pathol. 170, 921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Biber K., Neumann H., Inoue K., Boddeke H. W. (2007) Trends Neurosci. 30, 596–602 [DOI] [PubMed] [Google Scholar]
- 15. Tran P. B., Miller R. J. (2003) Nat. Rev. Neurosci. 4, 444–455 [DOI] [PubMed] [Google Scholar]
- 16. Pan Y., Lloyd C., Zhou H., Dolich S., Deeds J., Gonzalo J. A., Vath J., Gosselin M., Ma J., Dussault B., Woolf E., Alperin G., Culpepper J., Gutierrez-Ramos J. C., Gearing D. (1997) Nature 387, 611–617 [DOI] [PubMed] [Google Scholar]
- 17. Harrison J. K., Jiang Y., Chen S., Xia Y., Maciejewski D., McNamara R. K., Streit W. J., Salafranca M. N., Adhikari S., Thompson D. A., Botti P., Bacon K. B., Feng L. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 10896–10901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishiyori A., Minami M., Ohtani Y., Takami S., Yamamoto J., Kawaguchi N., Kume T., Akaike A., Satoh M. (1998) FEBS Lett. 429, 167–172 [DOI] [PubMed] [Google Scholar]
- 19. Maciejewski-Lenoir D., Chen S., Feng L., Maki R., Bacon K. B. (1999) J. Immunol. 163, 1628–1635 [PubMed] [Google Scholar]
- 20. Mizuno T., Kawanokuchi J., Numata K., Suzumura A. (2003) Brain Res. 979, 65–70 [DOI] [PubMed] [Google Scholar]
- 21. Limatola C., Lauro C., Catalano M., Ciotti M. T., Bertollini C., Di Angelantonio S., Ragozzino D., Eusebi F. (2005) J. Neuroimmunol. 166, 19–28 [DOI] [PubMed] [Google Scholar]
- 22. Miksa M., Amin D., Wu R., Ravikumar T. S., Wang P. (2007) Mol. Med. 13, 553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yasui Y., Sasao E., Sakata M., Matsui N., Fukuishi N., Akagi R., Akagi M. (2007) Biol. Pharm. Bull. 30, 443–446 [DOI] [PubMed] [Google Scholar]
- 24. Li Q., Li J., Zhang L., Wang B., Xiong L. (2007) Life Sci. 80, 1087–1093 [DOI] [PubMed] [Google Scholar]
- 25. Mizuno T., Kurotani T., Komatsu Y., Kawanokuchi J., Kato H., Mitsuma N., Suzumura A. (2004) Neuropharmacology 46, 404–411 [DOI] [PubMed] [Google Scholar]
- 26. Suzumura A., Mezitis S. G., Gonatas N. K., Silberberg D. H. (1987) J. Neuroimmunol. 15, 263–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pollock J. S., Förstermann U., Mitchell J. A., Warner T. D., Schmidt H. H., Nakane M., Murad F. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 10480–10484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takeuchi H., Mizuno T., Zhang G., Wang J., Kawanokuchi J., Kuno R., Suzumura A. (2005) J. Biol. Chem. 280, 10444–10454 [DOI] [PubMed] [Google Scholar]
- 29. Chapman G. A., Moores K., Harrison D., Campbell C. A., Stewart B. R., Strijbos P. J. (2000) J. Neurosci. 20, 87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bocchini V., Rebel G., Massarelli R., Schuber F., Muller C. D. (1988) Int. J. Dev. Neurosci. 6, 525–534 [DOI] [PubMed] [Google Scholar]
- 31. Napoli I., Kierdorf K., Neumann H. (2009) Glia 57, 1660–1671 [DOI] [PubMed] [Google Scholar]
- 32. Cambien B., Pomeranz M., Schmid-Antomarchi H., Millet M. A., Breittmayer V., Rossi B., Schmid-Alliana A. (2001) Blood 97, 2031–2037 [DOI] [PubMed] [Google Scholar]
- 33. Meucci O., Fatatis A., Simen A. A., Miller R. J. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 8075–8080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Surh Y. J., Kundu J. K., Li M. H., Na H. K., Cha Y. N. (2009) Arch. Pharm. Res. 32, 1163–1176 [DOI] [PubMed] [Google Scholar]
- 35. Kensler T. W., Wakabayashi N., Biswal S. (2007) Annu. Rev. Pharmacol. Toxicol. 47, 89–116 [DOI] [PubMed] [Google Scholar]
- 36. Neumann H., Kotter M. R., Franklin R. J. (2009) Brain 132, 288–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cardona A. E., Pioro E. P., Sasse M. E., Kostenko V., Cardona S. M., Dijkstra I. M., Huang D., Kidd G., Dombrowski S., Dutta R., Lee J. C., Cook D. N., Jung S., Lira S. A., Littman D. R., Ransohoff R. M. (2006) Nat. Neurosci. 9, 917–924 [DOI] [PubMed] [Google Scholar]
- 38. Fuhrmann M., Bittner T., Jung C. K., Burgold S., Page R. M., Mitteregger G., Haass C., LaFerla F. M., Kretzschmar H., Herms J. (2010) Nat. Neurosci. 13, 411–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim T. S., Lim H. K., Lee J. Y., Kim D. J., Park S., Lee C., Lee C. U. (2008) Neurosci. Lett. 436, 196–200 [DOI] [PubMed] [Google Scholar]
- 40. Milligan E. D., Sloane E. M., Watkins L. R. (2008) J. Neuroimmunol. 198, 113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dénes A., Ferenczi S., Halász J., Környei Z., Kovács K. J. (2008) J. Cereb. Blood Flow Metab. 28, 1707–1721 [DOI] [PubMed] [Google Scholar]
- 42. Hsieh C. L., Koike M., Spusta S. C., Niemi E. C., Yenari M., Nakamura M. C., Seaman W. E. (2009) J. Neurochem. 109, 1144–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clark A. K., D'Aquisto F., Gentry C., Marchand F., McMahon S. B., Malcangio M. (2006) J. Neurochem. 99, 868–880 [DOI] [PubMed] [Google Scholar]
- 44. Lyons A., Lynch A. M., Downer E. J., Hanley R., O'Sullivan J. B., Smith A., Lynch M. A. (2009) J. Neurochem. 110, 1547–1556 [DOI] [PubMed] [Google Scholar]
- 45. Alam J., Cook J. L. (2007) Am. J. Respir. Cell Mol. Biol. 36, 166–174 [DOI] [PubMed] [Google Scholar]
- 46. Naidu S., Vijayan V., Santoso S., Kietzmann T., Immenschuh S. (2009) J. Immunol. 182, 7048–7057 [DOI] [PubMed] [Google Scholar]
- 47. Takekawa M., Tatebayashi K., Saito H. (2005) Mol. Cell 18, 295–306 [DOI] [PubMed] [Google Scholar]
- 48. Chang F., Steelman L. S., Lee J. T., Shelton J. G., Navolanic P. M., Blalock W. L., Franklin R. A., McCubrey J. A. (2003) Leukemia 17, 1263–1293 [DOI] [PubMed] [Google Scholar]
- 49. Kanninen K., Heikkinen R., Malm T., Rolova T., Kuhmonen S., Leinonen H., Ylä-Herttuala S., Tanila H., Levonen A. L., Koistinaho M., Koistinaho J. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 16505–16510 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.