Abstract
A major immunological response during neuroinflammation is the activation of microglia, which subsequently release proinflammatory mediators such as prostaglandin E2 (PGE2). Besides its proinflammatory properties, cyclooxygenase-2 (COX-2)-derived PGE2 has been shown to exhibit anti-inflammatory effects on innate immune responses. Here, we investigated the role of microsomal PGE2 synthase-1 (mPGES-1), which is functionally coupled to COX-2, in immune responses using a model of lipopolysaccharide (LPS)-induced spinal neuroinflammation. Interestingly, we found that activation of E-prostanoid (EP)2 and EP4 receptors, but not EP1, EP3, PGI2 receptor (IP), thromboxane A2 receptor (TP), PGD2 receptor (DP), and PGF2 receptor (FP), efficiently blocked LPS-induced tumor necrosis factor α (TNFα) synthesis and COX-2 and mPGES-1 induction as well as prostaglandin synthesis in spinal cultures. In vivo, spinal EP2 receptors were up-regulated in microglia in response to intrathecally injected LPS. Accordingly, LPS priming reduced spinal synthesis of TNFα, interleukin 1β (IL-1β), and prostaglandins in response to a second intrathecal LPS injection. Importantly, this reduction was only seen in wild-type but not in mPGES-1-deficient mice. Furthermore, intrathecal application of EP2 and EP4 agonists as well as genetic deletion of EP2 significantly reduced spinal TNFα and IL-1β synthesis in mPGES-1 knock-out mice after LPS priming. These data suggest that initial inflammation prepares the spinal cord for a negative feedback regulation by mPGES-1-derived PGE2 followed by EP2 activation, which limits the synthesis of inflammatory mediators during chronic inflammation. Thus, our data suggest a role of mPGES-1-derived PGE2 in resolution of neuroinflammation.
Keywords: Cyclic AMP (cAMP), Cyclooxygenase (COX) Pathway, Inflammation, Innate Immunity, Prostaglandins, Tumor Necrosis Factor (TNF), Spinal Cord, mPGES-1, Microglia
Introduction
Neurodegenerative disorders, including Alzheimer and Parkinson disease, multiple sclerosis, and spinal cord or peripheral nerve injury, are associated with neuroinflammation (1, 2). Its initiation, maintenance and resolution are regulated by various cell types, including resident microglia, astroglia, and oligodendrocytes as well as invading blood leukocytes. Lipopolysaccharide (LPS) has traditionally been used to simulate innate immune responses in the central nervous system (CNS) by activating toll-like receptor-4 of microglia (3). Upon activation, microglia release inflammatory mediators such as cytokines, chemokines, free radicals, nitric oxide, or prostaglandins (4). One of the earliest events during LPS-induced neuroinflammation is the synthesis and release of the proinflammatory cytokine TNFα by microglia, which reaches maximum concentrations 2–8 h after the initial inflammatory stimulus (5). In effector cells, TNFα induces the expression of multiple proteins that further enhance the inflammatory response, including cyclooxygenase-2 (COX-2) and the functionally coupled microsomal PGE2 synthase-1 (mPGES-1)2 (6, 7). After 24 h, TNFα levels decrease to base-line levels whereas the activation of glia persists for several days (8). The mechanisms controlling the precise time course of the early innate immune responses depend on positive and negative feedback regulation loops and are not fully understood.
PGE2, which acts via E-prostanoid (EP) receptors, fulfills contrasting roles as regulator of inflammatory responses (9). Its well known proinflammatory properties are the reason for the clinical use of the anti-inflammatory acting COX inhibitors (10–12). In this regard, enhanced PGE2 synthesis in the CNS can damage and/or sensitize neurons, resulting in lesions or enhanced pain transmission (13–15). However, PGE2 also has anti-inflammatory properties. It mediates bradykinin-induced neuroprotection and blocks LPS- and ATP-induced cytokine synthesis in cultured microglia or in neuron-glia cocultures (16, 17). The anti-inflammatory and neuroprotective effects of PGE2 are suggested to be mediated via microglial EP2 and EP4 receptors. However, several studies have shown that EP2 or EP4 receptors are not expressed in microglia of naïve or injured animals in vivo (15). Accordingly, although COX-2 inhibitors elevate TNFα synthesis after a single intraperitoneal LPS injection, they do not have an effect on TNFα synthesis after a single intracerebroventricular LPS injection, indicating that PG-driven negative feedback controls TNFα production in the periphery but not in the CNS (18).
Here, we used the well described model of chronic neuroinflammation by repetitive spinal LPS injection. In this model, where EP2 expression was up-regulated in microglia, we investigated the pro- and anti-inflammatory properties of PGE2 in vivo as well as the roles of COX-2 and mPGES-1 during termination of innate immune responses. We found that PGE2 attenuates LPS-induced TNFα synthesis in the spinal cord via EP2 receptors accompanied by a negative feedback inhibition on COX-2 expression. This effect was markedly reduced in mPGES-1-deficient mice which do not exhibit elevated PGE2 levels in response to LPS.
EXPERIMENTAL PROCEDURES
Animals
Crl:CFW(Sw) mPGES-1-deficient mice were kindly provided by Sanofi Aventis (Bridgewater, CT) and have been described previously (19). EP2 knock-out mice have been described previously (20). Transgenic mice were compared with strain-, age-, and sex-matched controls. For generation of spinal cultures, pregnant Sprague-Dawley rats were purchased from Janvier (Le Genest, St. Isle, France). In all experiments the ethics guidelines for investigations in conscious animals were obeyed, and the procedures were approved by the local Ethics Committee.
Reagents
EP receptor-specific ligands (EP1 agonist ONO-DI-004, EP2 agonist ONO-AE1-259, EP3 agonist ONO-AE-248, and EP4 agonist ONO-AE1-329) were kindly provided by Dr. Maruyama (ONO Pharmaceuticals, Sakurei, Japan). Cicaprost (dissolved in PBS) was a gift from Schering (Berlin, Germany). Generation and use of EP2- and EP4-specific antibodies have been described in detail previously (21). For immunohistochemistry antibodies against neuronal nuclei (Millipore), polyclonal IBA1 (Waco, Richmond, VA), glial fibrillary acidic protein (Sigma), and 7-amino actinomycin D (BD Pharmingen) were used.
Spinal Cord Cell Culture
Mixed spinal cord cell cultures were prepared as described previously with some changes (6, 19). Briefly, whole spinal cords were prepared from Sprague-Dawley rats at embryonic day 16–17. After dissociation, cells were plated onto poly-l-lysine (Sigma)-coated 3.5-cm dishes and cultivated for 6 days in neurobasal medium (Invitrogen) containing B-27 supplement (Invitrogen) and 0.01 μg/ml murine nerve growth factor (NGF 7S) (Invitrogen). No mitosis inhibitors were added during cultivation. Stimulations were performed in neurobasal medium containing B-27 supplement. These mixed spinal cultures contained large proportions of neurons, astrocytes, and microglia.
Induction of Spinal Neuroinflammation
Neuroinflammation was induced by an intrathecal injection of LPS. Mice were lightly anesthetized with isoflurane (Forene; Abbott), and a small incision (5-mm) was made in the skin over the sacral spinal column. 5 μl of 2 μg/μl LPS in ACSF solution or ACSF alone (in control animals) was injected as described by Hylden and Wilcox (22). Successful injection was signaled by a flinch of the animal's tail. After injection the skin was closed with a Michel clip. Priming was achieved by an intrathecal LPS injection 24 h before the second LPS injection.
Cytokine Determination in Cell Culture Medium and Tissue Homogenate
TNFα and IL-1β were measured by enzyme immunoassay (EIA) kits from R&D Systems (Minneapolis, MN) according to the manufacturer's protocol. Cell culture supernatant was used directly. Snap-frozen spinal cord (L4–6) tissue was thawed in 100 μl of phosphate-buffered saline (PBS) and homogenized with a potter. Then, 300 μl of PBS was added, and tissue was homogenized further by sonification. After centrifugation (10,000 × g), protein concentrations were determined in the supernatant, and 50 μg of protein/in 50 μl of PBS was used for the EIA.
cAMP Accumulation
cAMP levels were determined as described previously using the cAMP ELISA from R&D Systems (23). Cells were preincubated for 5 min with 100 μm isobutylmethylxanthine and then treated for 15 min as indicated.
Western Blot Analysis
Spinal cord cells were directly homogenized in hot (95 °C) Laemmli buffer. Then, cell lysates containing 30 μg of protein were separated on a 15% SDS-polyacrylamide gel. mPGES-1 was detected with a polyclonal antibody (1:1000) from AgriSera (Vännes, Sweden), COX-2, and EP2 with antibodies from Cayman (Ann Arbor, MI). An antibody against extracellular signal regulated kinase-2 (ERK-2) (Santa Cruz Biotechnology, Santa Cruz, CA) was used to detect for equal loading.
EP2 and EP4 receptors and COX-2 from spinal cord tissue were detected in membrane fractions. L4–6 spinal segments were homogenized in PBS with a potter and by sonification. Then the homogenate was centrifuged for 10 min at 10,000 × g and for 1 h at 170,000 × g. The pellet of the last centrifugation was resuspended in 50 μl of PBS containing 0.25 m sucrose and was used for Western blotting. Equal loading of membrane fraction was controlled with an antibody against β-actin (1:5000) from Santa Cruz Biotechnology.
RT-PCR
2 μg of total RNA from rat spinal cord was annealed with oligo(dT) primer and reverse-transcribed using Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI). A VIC-TAMARA-labeled probe for 18 S rRNA quantification was from Applied Biosystems. Primers for amplification of EP2 were 5′-CCTGCCGCTGCTCAACTACG-3′ and 5′-GTCTCCTCTGCCATCGAAGTCCTC-3′ and for EP4 5′-CTGAACAGCCCGGTGACCATTCC-3′ and 5′-GCCGGCCAGCCGCTTGTCCAC-3′. Real-time PCR was performed using the TaqMan system (Applied Biosystems) and the Absolute Blue quantitative PCR SYBR Green ROX mix (Thermo Fisher Scientific) according to the manufacturer's instructions. Relative expression of EP2 and EP4 mRNA was determined using the comparative cycle threshold method, normalizing relative values to the average expression of 18 S rRNA.
Immunohistochemistry
Spinal (L4–5) tissues were prepared from mice that were perfused intracardially with 0.9% saline followed by 4% paraformaldehyde/PBS (pH 7.4). After a 24-h incubation in 30% sucrose/PBS, the tissue was cryostat-sectioned at 10 μm and stored at 4 °C until use. EP2 (1:2000) and EP4 receptors (1:1000) were detected with polyclonal rabbit antibodies (21), using the streptavidin-Cy3 detection system (Vector Laboratories, Burlingame, CA) according to the manufacturer's protocol. Here, signals were amplified by incubation with a biotinylated anti-streptavidin antibody followed by an additional incubation with streptavidin-Cy3 conjugate. For costaining we used DAPI (Applied Biochem) and CD11b:FITC (Serotec, Oxford, UK). For multiepitope ligand cartography spinal cord cells were grown on poly-l-lysine-coated coverslips as described above and fixed with 4% paraformaldehyde in PBS for 20 min at room temperature, permeabilized with 0.1% Triton X-100, and blocked with 5% goat serum in PBS. For the multiepitope ligand cartography analysis, antibodies were labeled with FITC as described (19, 24–26).
Determination of Prostanoids by Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
LC-MS/MS analysis of PGF2α, PGE2, PGD2, TXB2, and 6-keto-PGF1α from spinal tissue, and tissue culture supernatant was performed as described previously (27, 28).
Briefly, lumbar spinal cords were homogenized in 400 μl of PBS. 100 μl of homogenate containing 1 μg/μl protein was mixed with 50 μl of water, 20 μl of methanol, and 20 μl of internal standard solution (25 ng/ml [2H4]PGE2, [2H4]PGD2 and [2H4]TXB2, and 10 ng/ml [2H4]PGF2α and [2H4]6-keto-PGF1α in methanol) and extracted twice with 800 μl of ethyl acetate. The combined organic phases were removed at a temperature of 45 °C under a gentle stream of nitrogen. The residues were reconstituted with 50 μl of acetonitrile/water/formic acid (20:80:0.0025, v/v, pH 4.0) and injected into the LC-MS/MS system. For detailed instrumentation and condition see online supplemental methods of Linke et al. (28).
For the detection of eicosanoids in cell culture supernatants, 500 μl of cell medium was mixed with 500 μl of 35 mm H3PO4, 20 μl of methanol, and 20 μl of internal standard solutions. Prostaglandins were extracted with solid phase extraction as described previously (28).
Statistics
For comparison between groups, data were subjected to two-tailed Student's t test except where mentioned otherwise.
RESULTS
Activation of EP2 and EP4 Receptors Inhibits LPS-induced TNFα Release in Primary Spinal Cord Cells
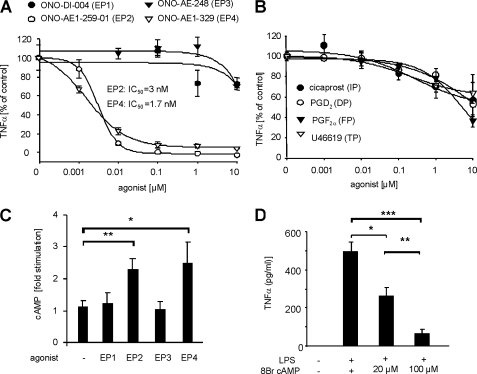
We generated primary cultures from spinal cords of E17 embryonic rats in the absence of mitosis inhibitors to allow the survival of neurons and the growth of glia cells. Treatment of spinal cord cultures with 1 μg/ml LPS increased the TNFα concentration in the cell culture medium from undetectable levels to 507 ± 25 pg/ml (n = 6). EP2 and EP4 receptor agonists almost completely inhibited LPS-induced TNFα synthesis with IC50 values of 3 nm and 1.7 nm, respectively (Fig. 1A). In contrast, agonists of other prostanoid receptors, such as EP1, EP3 (Fig. 1A), DP, FP, IP and TP (Fig. 1B) exhibited only marginal effects on TNFα synthesis at concentrations that were >1000-fold higher than their respective KD values. EP2 and EP4 are known to activate cAMP synthesis through the stimulatory G protein Gs (29). Accordingly, agonists for EP2 and EP4 significantly increased the cAMP concentration in the spinal cord cells at a concentration of 0.1 μm (Fig. 1C), and the stable cAMP analog 8-bromo-cAMP was sufficient to mimic the effect of the EP2 and EP4 agonists on TNFα synthesis (Fig. 1D).
FIGURE 1.
Anti-inflammatory properties of PGE2 are mediated through EP2 and EP4 in primary spinal cord cultures. A, effects of different EP receptor agonists on LPS-induced TNFα release from spinal cultures. Cells were incubated with 1 μg/μl LPS and the respective agonist concentrations for 3 h. TNFα concentrations were determined in the culture supernatants by EIA. Data are shown as mean ± S.E. (error bars) of 3–6 experiments. B, same as D except that agonists for IP, DP, FP, and TP receptors were used. Data are shown as mean ± S.E. of 3–6 experiments. C, EP agonist-stimulated cAMP synthesis in primary spinal cord cultures. Cells were preincubated with 0.1 μm isobutylmethylxanthine for 5 min and then stimulated with 0.1 μm ONO-DI-004 (EP1) ONO-AE1-259-01 (EP2), ONO-AE-248 (EP3), or ONO-AE1-329 (EP4) for 15 min. Data are shown as mean ± S.E. of at least 6 experiments. Student's t test: *, p ≤ 0.05; **, p ≤ 0.01. D, TNFα release from LPS-stimulated primary spinal cord cultures. Embryonic spinal cord cultures were incubated with 1 μg/ml LPS and the respective concentration of 8-bromo-cAMP (8Br cAMP) for 3 h. Data shown represent the mean ± S.E. (n = 3). Student's t test: *, p ≤ 0.01; **, p ≤ 0.005; ***, p ≤ 0.001.
PGE2 Is a Negative Feedback Regulator during Spinal LPS-induced Immune Responses
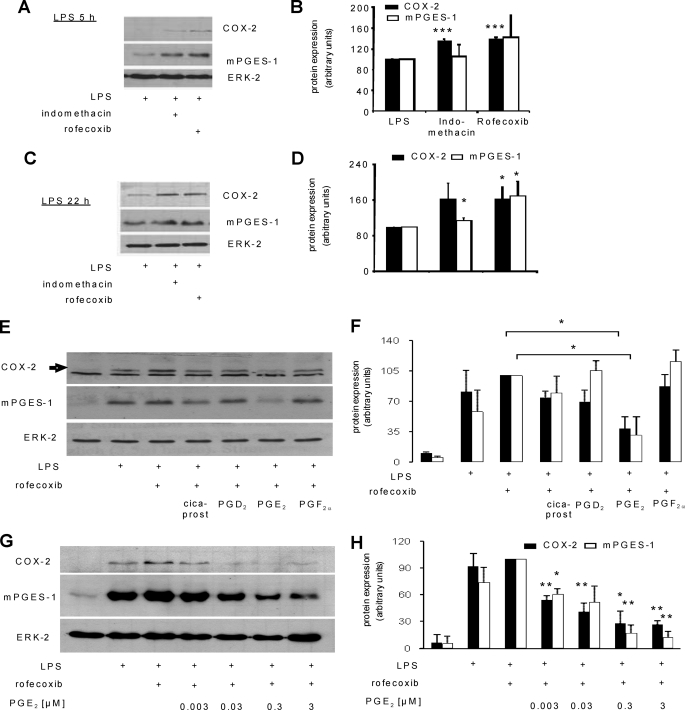
TNFα induces spinal COX-2 and mPGES-1 expression as well as prostanoid synthesis in vivo and in vitro in primary spinal cord cultures (6, 7). Because activation of EP2 and EP4 receptors efficiently blocks LPS-induced TNFα synthesis in primary spinal cord cultures, we hypothesized that PGE2 may function as a negative feedback regulator to the enzymes necessary for its own production. Therefore, we incubated primary spinal cord cultures for various time periods with LPS and blocked endogenous prostanoid synthesis using either the unselective COX-1/2 inhibitor indomethacin or the selective COX-2 inhibitor rofecoxib. Both substances increased LPS-induced COX-2 expression significantly (Fig. 2, A and B). There was a tendency toward increased mPGES-1 expression after 5 h which reached significance only at late time points (Fig. 2, C and D). These findings suggest that COX-2-derived prostanoids inhibit LPS-induced expression of both genes. To identify prostanoids inhibiting LPS-induced COX-2 and mPGES-1 expression, we stimulated primary spinal cord cultures with LPS, inhibited endogenous prostanoid synthesis with rofecoxib, and added different prostanoids to activate their respective receptors. Due to the instability of prostacyclin, cicaprost was used as an IP receptor agonist (30). Interestingly, PGE2 was the only prostanoid that was able to block LPS-induced COX-2 and mPGES-1 expression significantly (Fig. 2, E and F). Notably, the inhibitory effect of PGE2 occurred at low nanomolar concentrations (Fig. 2, G and H).
FIGURE 2.
PGE2 is a negative feedback regulator during LPS-induced immune responses in primary spinal cord cultures. A–D, Western blot analyses of COX-2 (72 kDa) and mPGES-1 (16 kDa) protein levels in lysates of spinal cord cells after incubation with 1 μg/ml LPS, 3 μm indomethacin, or 3 μm rofecoxib for 5 (A and B) or 22 h (C and D). ERK-2 (42 kDa) was used to control for equal loading. B and C, densitometric analyses of 3–4 independent experiments. Student's t test: *, p ≤ 0.05; ***, p ≤ 0.001. E and F, same as in A except spinal cord cells have been incubated with 1 μg/ml LPS, 3 μm rofecoxib, and 1 μm prostanoids for 21 h. F, densitometric analysis of three independent experiments. Student's t test: *, p ≤ 0.05. G and H, same as in A except that increasing PGE2 concentrations were used. H, densitometric analysis of 3 independent experiments. Student's t test: *, p ≤ 0.05; **, p ≤ 0.01.
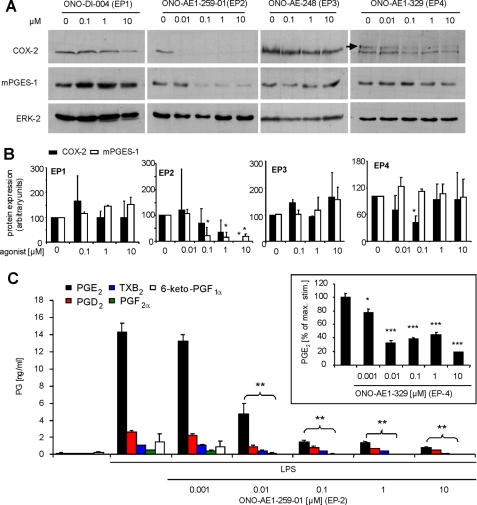
To identify the EP receptor subtypes that mediated this negative feedback regulation by PGE2, we repeated the experiments using selective EP receptor agonists instead of PGE2 and found that EP2 and EP4 agonists inhibited LPS-induced COX-2 expression at low nanomolar concentrations. Similarly, EP2 agonists inhibited mPGES-1 expression (Fig. 3A) whereas both EP1 and EP3 agonists did not reduce COX-2 or mPGES-1 expression. Effects of the EP1 agonist at very high concentrations (10 μm) might be attributed to loss of receptor selectivity (Ki = 150 nm for EP1) (Fig. 3, A and B) (31).
FIGURE 3.
EP2 and EP4 mediate the negative feedback regulation by PGE2. A and B, spinal cord cells were stimulated with 1 μg/ml LPS, 3 μm rofecoxib, and the respective EP receptor agonist for 21 h. Protein levels were determined by Western blotting. B, densitometric analysis of at least 2 independent experiments. Student's t test: *, p ≤ 0.05. C, prostanoid concentrations in supernatants of spinal cord cultures that have been stimulated with 1 μg/ml LPS and the respective ONO-AE1–259-01 (EP2 agonist) concentrations. Inset, prostanoid concentrations in cultures treated with the indicated ONO-AE1-329 (EP4 agonist) concentrations. Data are shown as the average of at least 3 independent experiments. Student's t test: *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.005.
Next, we investigated whether or not EP2 and EP4 activation indeed causes a negative feedback inhibition which blocks the LPS-stimulated prostaglandin release. Therefore, we incubated primary spinal cord cultures with LPS and EP2 and EP4 agonists and determined prostanoid concentrations by LC-MS/MS. The EP2 agonist dose-dependently reduced the release of PGE2, PGD2, TXB2 (a stable metabolite of TXA2), PGF2α, and 6-keto-PGF1α (a stable metabolite of prostacyclin) (Fig. 3C). Similar results were obtained for inhibition of LPS-induced PGE2 synthesis using the EP4 agonist (Fig. 3C, inset). Taken together, these data suggest that COX-2-derived PGE2 limits its own synthesis via EP2/EP4 activation.
Influence of Altered Prostanoid Synthesis in mPGES-1-deficient Mice on Cytokine Synthesis during Acute LPS-induced Neuroinflammation
So far the in vitro data suggest that PGE2, but not other prostanoids, induces a negative feedback regulation via COX-2. Therefore, we investigated whether or not impaired endogenous PGE2 synthesis in mPGES-1-deficient mice correlates with increased innate immune responses and COX-2 activity also in vivo during LPS-induced neuroinflammation.
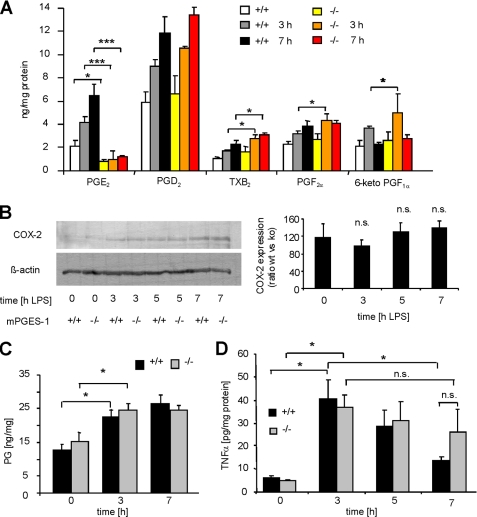
First, we determined the contribution of mPGES-1 to spinal prostanoid synthesis in response to a single intrathecal LPS injection. Prostanoid concentrations were measured in homogenates of spinal tissue from L4–5 segments in naïve animals as well as 3 h and 7 h after LPS injection by LC-MS/MS analysis. mPGES-1-knock-out mice exhibited significantly lower basal PGE2 levels and, in contrast to wild-type mice, no LPS-induced PGE2 synthesis was observed (Fig. 4A). In accordance with a previously described redirection of the prostanoid synthesis in mPGES-1-deficient mice (19, 32), spinal cords of mPGES-1-knock-out mice exhibited higher TXB2, PGF2α and 6-keto-PGF1α concentrations 3 and 7 h after stimulation with LPS. COX-2 protein levels in the spinal cord were increased 3–7 h after LPS injection (Fig. 4B). In mPGES-1-deficient mice, this up-regulation was not further increased compared with wild-type animals, indicating that initial immune responses that are induced by a single LPS injection are not affected by mPGES-1 deletion. Because mPGES-1 deficiency should not influence the quantitative metabolism of PGH2, we calculated the sum of prostanoids and used this value as a measure for COX-2 activity. In accordance to our findings for COX-2 expression, mPGES-1-deficient mice did not differ in their total prostanoid levels compared with wild-type mice 3–7 h after a single LPS injection (Fig. 4C).
FIGURE 4.
Cytokine synthesis and COX-2 expression after one LPS injection (acute neuroinflammation) in mPGES-1-deficient mice. A, determination of prostanoid levels in tissue homogenates of L4–5 spinal segments of wild-type and mPGES-1-knock-out mice by LC-MS/MS. Tissue was prepared at the indicated time points after an intrathecal injection of 10 μg of LPS. Data shown represent the mean ± S.E. from 8 animals per genotype and time point. Student's t test: *, p ≤ 0.05. B, COX-2 protein levels in membrane fractions of tissue homogenates from A. For each condition lysates from all eight animals were pooled. Left, densitometric analysis of 4–6 independent experiments. C, sum of PGE2, PGD2, PGF2α, 6-keto-PGF1α, and TXB2 concentrations calculated for the same tissue homogenates as in A. D, determination of TNFα by EIA in the same tissue homogenates as in A. Student's t test: *, p ≤ 0.05; ***, p ≤ 0.001; n.s., not significant.
Finally, we compared the time course of spinal TNFα synthesis after a single intrathecal LPS injection. Both wild-type and mPGES-1 knock-out mice showed substantially increased TNFα levels in spinal cord homogenates 3 h after LPS injection. 7 h after LPS injection, TNFα levels were still elevated but started to decline (Fig. 4D). This decline was significant in wild-type but not in mPGES-1-deficient mice, giving a first hint that termination of TNFα synthesis might depend on mPGES-1-derived PGE2 production.
LPS Priming Induces EP2 and EP4 Expression in Spinal Microglia and/or Invading Macrophages
Immunocytochemical analysis showed that EP2 receptors were predominantly expressed in amoeboid-shaped microglia (as shown by IBA staining), indicating that EP2 receptors are expressed on activated microglia. To a lesser extent, EP2 was also expressed in neurons (as shown by neuronal nuclei staining) (supplemental Figs. 1 and 2).
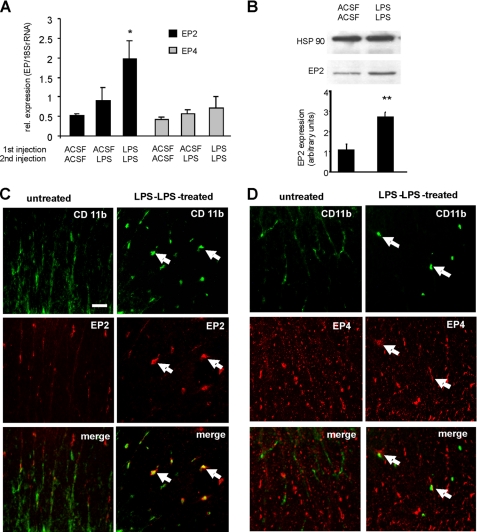
Because EP2 is not detected in ramified spinal microglia in vivo (15), we hypothesized that microglia activation and subsequent EP2 and EP4 receptor up-regulation after LPS pretreatment (priming) might be a precondition for the negative feedback regulation of PGE2 in the spinal cord. To test this hypothesis, we compared spinal EP2 and EP4 expression after a single or repeated intrathecal LPS injection. Wild-type and mPGES-1-deficient mice, respectively, received by intrathecal application 27 h and 3 h before spinal cord preparation either twice a vehicle injection (ACSF) or a vehicle and a LPS injection, or two LPS injections. A significant up-regulation of EP2 mRNA, but not of EP4 mRNA, was seen using real-time RT-PCR in mice receiving two LPS injections (Fig. 5A). The up-regulation of EP2 was confirmed by Western blot analysis (Fig. 5B). Next, we analyzed EP2 and EP4 receptor expression in spinal sections of naïve and LPS-primed mice. CD11b costaining allowed the identification of microglia and/or macrophages, whose invasion into the spinal cord can also be induced by LPS (33). Upon LPS priming, amoeboid-shaped cells with strong CD11b staining appeared in the spinal cord. Costaining with antibodies against EP2 and EP4 revealed that both receptors are expressed in the amoeboid CD11b-positive cells (Fig. 5, B and C). However, costaining of CD11b-positive cells with EP4 was only rarely seen (Fig. 5C). Thus, the up-regulation of EP2 and its more prominent expression in CD11b-positive cells indicate that EP2 mediates the anti-inflammatory properties of PGE2 after repeated LPS stimulation.
FIGURE 5.
LPS priming induces EP2 and EP4 expression in spinal microglia and/or invading macrophages. A, mRNA levels of EP2 and EP4 were determined by real-time RT-PCR in L4–5 spinal segments of wild-type mice receiving 27-h and 3-h prior preparation of either ACSF twice or ACSF and LPS twice or LPS twice. Protein homogenates of six animals were pooled. B, same as A except that EP2 expression is shown by Western blot analysis. In the lower panel the densitometric analysis of expression in 3–4 mice is shown. Student's t test: **, p ≤ 0.01. C and D, immunohistochemical analysis of spinal cord tissue sections from naïve and LPS-primed mice. EP2 (B) and EP4 (C) expression shown in red were costained with CD11b for microglia and/or macrophages (green). Shown are representative regions from spinal cord white matter. Scale bars, 20 μm.
Reduction of Immune Responses after LPS Priming Is mPGES-1-dependent
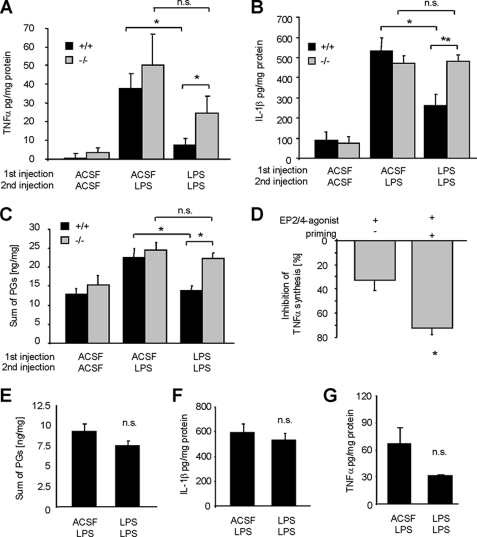
Next, we investigated immune responses in wild-type and mPGES-1-deficient mice after increasing EP2 and EP4 expression in the spinal cord by LPS priming. Here, we compared TNFα, IL-1β, and prostanoid synthesis 3 h after LPS injection in mice of both genotypes which received LPS or vehicle 24 h earlier. We found that TNFα, IL-1β, and prostanoid concentrations (sum of PGE2, PGD2, TXB2, PGF2α and 6-keto-PGF1α) were significantly lower after the second LPS administration in LPS-primed wild-type mice compared with mice that were not primed (Fig. 6, A–C). Most importantly, in accordance with our hypothesis that PGE2 inhibits LPS-induced innate immune responses, TNFα, IL-1β, prostanoid concentrations, and COX-2 expression were not significantly decreased in LPS-primed mPGES-1-deficient mice (Fig. 6, A–C, and supplemental Figs. 3 and 4).
FIGURE 6.
LPS priming reduces immune responses in a mPGES-1- and EP2/4-dependent manner. A, determination of TNFα concentrations by EIA from tissue homogenates of L4–5 spinal segments from mPGES-knock-out or wild-type mice receiving 27-h and 3-h prior preparation of either ACSF twice or ACSF and LPS twice or LPS twice. n.s., not significant. B, same as in A except IL-1β levels were determined. C, same as in A and B, except that prostanoids were determined. Data are shown as the sum of all prosatanoids (PGE2, PGD2, PGF2α, 6-keto-PGF1α, and TXB2) detected in the tissue samples. D, 100 μm EP2 and EP4 agonists were injected intrathecally together with LPS in naïve or LPS-primed mPGES-knock-out mice. After 2 h, L4–5 spinal segments were dissected, and TNFα levels were determined by EIA. Data are shown in percent inhibition compared with mPGES-1-knock-out mice which received no agonists. All data are shown as the mean ± S.E. (error bars) from 6–8 animals per genotype and stimulation condition. Student's t test: *, p ≤ 0.05. E–G, EP2-knock-out mice were injected intrathecally. together with LPS in naïve or LPS-primed mPGES-knock-out mice. After 2 h L4–5 spinal segments were dissected, and prostanoids (E), IL-1β (F), or TNFα (G) levels were determined by EIA. All data are shown as the mean ± S.E. from 8 animals per condition.
To show that the altered response to LPS in mPGES-1-deficient mice is due to the lack of PGE2, we applied EP2 and EP4 agonists together with LPS to mPGES-1-deficient mice. Indeed, EP2 and EP4 agonists reduced spinal TNFα concentrations in mPGES-1-deficient mice in primed and unprimed animals (Fig. 6D). However, the reduction of TNFα concentrations was significantly more pronounced in LPS-primed mPGES-1 knock-out mice. Interestingly, in EP2 knock-out mice, no significant decrease in the synthesis of prostanoids, IL-1β, or TNFα was observed (Fig. 6, E–G), suggesting that in vivo EP2 is the receptor mediating the anti-inflammatory response of PGE2 in our model of neuroinflammation. In summary, our data suggest that LPS priming renders the spinal cord competent for negative feedback regulation via mPGES-1-derived PGE2 by up-regulating EP2 receptors.
DISCUSSION
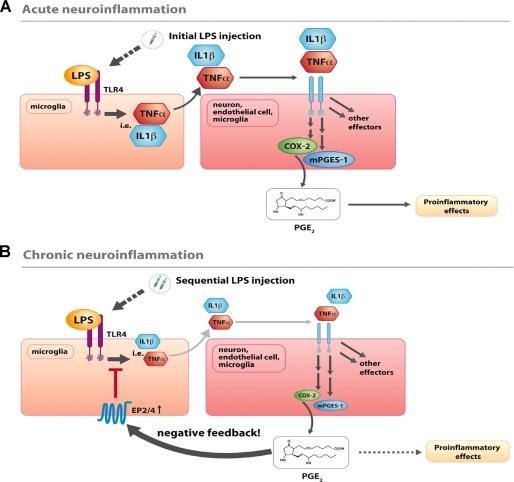
COX-2-derived PGE2 is a known regulator of neuroinflammatory processes in several neurological disorders such as multiple sclerosis, traumatic brain injury, Alzheimer and Parkinson disease as well as spinal cord injury (15, 34). In experimental models, conditions of neuroinflammation, including increased PGE2 synthesis, can be induced by LPS injection into the CNS (3). Here, we describe that PGE2 limits cytokine and prostaglandin synthesis mainly through EP2 activation in a model of LPS-induced neuroinflammation and that mPGES-1 is a critical enzyme in this negative feedback regulation (Fig. 7).
FIGURE 7.
Schematic summary of the results showing the consequences of mPGES-1 mediated PGE2 synthesis for LPS-induced neuroinflammation. A, single intrathecal LPS injection, which simulates acute neuroinflammation, activates microglial toll-like receptor-4, causing a marked increase of cytokine release (i.e. TNFα and IL-1β). Via activation of their receptors they induce in microglia, neurons, or endothelial cells an up-regulation of COX-2 and mPGES-1 resulting in a selective synthesis of PGE2. Through neuronal EP2, PGE2 induces deleterious effects including neurodegeneration, excitotoxicity, or sensitization. B, if LPS was injected sequentially (24 h and 3 h before preparation), a situation of chronic neuroinflammation is mimicked. As a consequence, EP2 and EP4 are up-regulated in microglia enabling PGE2 to act via an additional pathway in microglia with a function as anti-inflammatory negative feedback regulator. As a proximate cause of this pathway, innate immune responses including cytokine and prostanoid synthesis induced by a second LPS injection are attenuated, promoting the resolution of neuroinflammation.
Previously, PGE2 was shown to block innate immune responses from microglia in vitro via EP2 and EP4 (16, 17, 35). Here, we found that EP2- and EP4-expressing spinal microglia had an amoeboid morphology that is characteristic for activated microglia. In vivo, EP2 has a predominantly neuronal localization in the murine spinal cord, whereas ramified spinal microglia does not express EP2 (14, 15, 36, 37). However, EP2 and EP4 expression in microglia and astrocytes, respectively, was induced in the superoxide dismutase model for lateral sclerosis or in the ischemic brain, suggesting that the expression of microglial EP2 is limited to activated microglia (15, 38, 39).
Because only activated microglia expressed EP2 and EP4, we activated microglia by intrathecal application of LPS 24 h before administering a second LPS injection. In this model increased CD11b immunoreactivity was detected in amoeboid-shaped cells in the spinal cord, which is indicative for an activation of resident microglia or immigration of macrophages into the spinal cord. Most importantly, after LPS priming, the CD11b-positive cells expressed EP2 and EP4. However, only EP2 is up-regulated after the first LPS stimulation, and genetic deletion of EP2 was sufficient to prevent the anti-inflammatory effects of PGE2 suggesting that in our in vivo model mostly EP2 mediates the anti-inflammatory effects of PGE2. The appearance of EP2 suggests that an initial inflammation (priming) in the spinal cord is necessary to enable the inhibitory effect of PGE2 on cytokine synthesis and to initiate a negative feedback loop. Accordingly, LPS-primed mice produced less TNFα, IL-1β, and prostanoids compared with mice that were not primed. These data are supported by the findings that the synthesis of proinflammatory cytokines as well as antigen-presenting cell functions were diminished after systemic pretreatment with LPS (8).
Surprisingly, in mPGES-1-deficient mice, the attenuation of immune responses after LPS priming was completely absent, suggesting that PGE2 is a component of priming-induced resolution of inflammation. Furthermore, we found that proximate to PGE2 signaling in microglia COX-2 expression was also blocked. A selective shift of COX-2-mediated prostanoid synthesis by mPGES-1 to PGE2 might therefore be a key step in the termination of cytokine and prostaglandin production. Previously it was postulated that COX-2-activity in the CNS has no effect on LPS-induced cytokine synthesis (18). However, in that study the TNFα synthesis was determined in response to a single LPS application. In accordance to this finding, we also observed no differences between wild-type and mPGES-1-deficient mice after a single LPS application. The negative feedback by PGE2 was only observed when LPS was injected twice. This dependence of microglial activation suggests a physiological function of the negative feedback loop mainly during chronic neuroinflammation. Hence, a single LPS injection was not sufficient to increase EP2 or EP4 expression within the next 3 h in the spinal cord. Consequently, the time course of spinal TNFα concentrations also showed no significant differences between wild-type and mPGES-1-deficient mice after a single intrathecal LPS injection. The period of TNFα synthesis after a single LPS injection seems to be too short to induce microglial EP2 expression and, therefore, to permit the negative feedback inhibition of PGE2.
Several cAMP-elevating agents can inhibit or at least reduce TNFα synthesis (40, 41). Because EP2, EP4, IP, DP, and certain splice variants of EP3 increase cAMP generation (42), all of these receptors are theoretically able to attenuate TNFα-mediated immune responses by PGE2. However, we found that in primary spinal cord cultures only activation of EP2 and EP4 was able to block TNFα synthesis, whereas the activation of other prostanoid receptors had no effect on the cytokine synthesis. These data were supported by the fact that mPGES-1-deficient mice lacked the negative feedback regulation. We and others showed that mPGES-1 deficiency causes a redirection of eicosanoid synthesis from PGE2 to other prostanoids causing, for example, a functional compensation for the loss of PGE2 in zymosan-induced hyperalgesia (19, 32). Due to the availability of the COX-2 product PGH2, the tissue-specific expression of other terminal prostaglandin synthases decides the fate of PGH2 and directs the specific prostaglandin production. However, in this study no functional compensation by other prostanoids was observed, showing that also in vivo only PGE2 was able to induce negative feedback regulation and to inhibit cytokine synthesis or COX-2 and mPGES-1 expression. The observed PGE2-specific anti-inflammatory effect underlines the importance of the selective isomerization of COX-2-derived PGH2 by mPGES-1 in the regulation of neuroinflammatory processes.
Because it is known that COX-2 and mPGES-1 expression in microglia, neurons, and endothelial cells is induced by proinflammatory cytokines, such as TNFα and IL-1β (6, 43–45), it was not surprising that activation of EP2 and EP4 receptors also prevented LPS-induced COX-2- and mPGES-1 expression as well as prostanoid synthesis. In accordance with the literature, our results suggest a dual contrasting role of PGE2 during neuroinflammation in the spinal cord. Early in inflammation, cytokines induce COX-2 expression in different cell types. This leads to an increased synthesis of various prostanoids causing deleterious effects on neurons (13, 14, 37, 46). Subsequently, expression of mPGES-1 shifts COX-2-derived prostanoid synthesis and PGE2 reaches maximum levels. However, during sustained inflammation, mimicked by a second LPS injection, microglial cells start to express EP2, which enables PGE2 to have an additional anti-inflammatory effect. PGE2 now blocks further cytokine synthesis, which is necessary for the induction of COX-2 expression. Taken together, our data may therefore identify mPGES-1 as an essential step for the control of innate immune responses during chronic inflammation in the spinal cord. They indicate that the same pathway that initially promotes inflammation later on contributes to its resolution, a process that physiologically is of high importance and may also have clinical consequences in pharmacotherapy with nonsteroidal anti-inflammatory drugs or selective mPGES-1 inhibitors, which are currently under development.
Previous studies on LPS preconditioning in the CNS focused mainly on its effect on hypoxic ischemia-induced neurodegeneration. It was shown that systemic LPS- or ischemia-induced preconditioning causes neuroprotection as a long term outcome through mechanisms involving several different pathways including COX-2, nitric-oxide synthase, and reactive oxygen species (47–49). Interestingly, also in the hypoxic slice culture model by Kim et al. (47) COX-2 activity was crucial for preconditioning-induced neuroprotection. Our study adds additional insights in the preconditioning effects by specifying the underlying downstream (of COX-2) mechanism to mPGES-1 activity and EP2 activation. It further demonstrates the existence of an anti-inflammatory preconditioning effect in the adult spinal cord after local LPS application. This indicates that during chronic neuroinflammation a direct pathogen action on resident microglia or invading macrophages is sufficient to dampen the ongoing innate immune responses. Whether this has a neuroprotective outcome needs to be investigated in further studies.
Despite the fact that we found anti-inflammatory properties, PGE2 has often been shown to promote neuronal damage during injury (13, 50). A reason for these differences may be that microglia-derived TNFα can be neuroprotective as described for ischemia (51). To date, it has not been established whether proinflammatory cytokines contribute to or limit neuronal injuries during neurological disorders, including ischemia, traumatic brain injury, multiple sclerosis, and Alzheimer or Parkinson disease (52). Therefore, it is difficult to predict how inhibition of mPGES-1 and COX-2, which may increase cytokine synthesis during chronic neuroinflammation, will affect the clinical outcome of these neurological disorders.
Supplementary Material
Acknowledgments
We thank Wiebke Becker and Marina Henke for excellent technical assistance.
This work was supported by Deutsche Forschungsgemeinschaft Grant GE695 and the LOEWE Lipid Signaling Forschungszentrum Frankfurt.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
- mPGES-1
- microsomal PGE2 synthase-1
- ACSF
- artificial cerebrospinal fluid
- EIA
- enzyme immunoassay
- EP
- E-prostanoid
- MS/MS
- tandem mass spectrometry
- PGE2
- prostaglandin E2
- TXB
- thromboxane A2 receptor
- IP
- PGI2 receptor
- DP
- PGD2 receptor
- TP
- thromboxane A2 receptor
- FP
- PGF2α receptor.
REFERENCES
- 1. Griffin R. S., Costigan M., Brenner G. J., Ma C. H., Scholz J., Moss A., Allchorne A. J., Stahl G. L., Woolf C. J. (2007) J. Neurosci. 27, 8699–8708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Skaper S. D. (2007) Ann. N.Y. Acad. Sci. 1122, 23–34 [DOI] [PubMed] [Google Scholar]
- 3. Lehnardt S., Massillon L., Follett P., Jensen F. E., Ratan R., Rosenberg P. A., Volpe J. J., Vartanian T. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 8514–8519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herber D. L., Maloney J. L., Roth L. M., Freeman M. J., Morgan D., Gordon M. N. (2006) Glia 53, 382–391 [DOI] [PubMed] [Google Scholar]
- 5. Shen A., Zhou D., Shen Q., Liu H. O., Sun L., Liu Y., Chen J., Yang J., Ji Y., Cheng C. (2009) Neurochem. Res. 34, 333–341 [DOI] [PubMed] [Google Scholar]
- 6. Brenneis C., Maier T. J., Schmidt R., Hofacker A., Zulauf L., Jakobsson P. J., Scholich K., Geisslinger G. (2006) FASEB J. 20, 1352–1360 [DOI] [PubMed] [Google Scholar]
- 7. Narita M., Shimamura M., Imai S., Kubota C., Yajima Y., Takagi T., Shiokawa M., Inoue T., Suzuki M., Suzuki T. (2008) Neuroscience 152, 477–486 [DOI] [PubMed] [Google Scholar]
- 8. Buenafe A. C., Bourdette D. N. (2007) J. Neuroimmunol. 182, 32–40 [DOI] [PubMed] [Google Scholar]
- 9. Hata A. N., Breyer R. M. (2004) Pharmacol. Ther. 103, 147–166 [DOI] [PubMed] [Google Scholar]
- 10. FitzGerald G. A., Patrono C. (2001) N. Engl. J. Med. 345, 433–442 [DOI] [PubMed] [Google Scholar]
- 11. Fitzgerald G. A. (2004) N. Engl. J. Med. 351, 1709–1711 [DOI] [PubMed] [Google Scholar]
- 12. Scholich K., Geisslinger G. (2006) Trends Pharmacol. Sci. 27, 399–401 [DOI] [PubMed] [Google Scholar]
- 13. Montine T. J., Milatovic D., Gupta R. C., Valyi-Nagy T., Morrow J. D., Breyer R. M. (2002) J. Neurochem. 83, 463–470 [DOI] [PubMed] [Google Scholar]
- 14. Reinold H., Ahmadi S., Depner U. B., Layh B., Heindl C., Hamza M., Pahl A., Brune K., Narumiya S., Müller U., Zeilhofer H. U. (2005) J. Clin. Invest. 115, 673–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao P., Waxman S. G., Hains B. C. (2007) J. Neurosci. 27, 2357–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caggiano A. O., Kraig R. P. (1999) J. Neurochem. 72, 565–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Noda M., Kariura Y., Pannasch U., Nishikawa K., Wang L., Seike T., Ifuku M., Kosai Y., Wang B., Nolte C., Aoki S., Kettenmann H., Wada K. (2007) J. Neurochem. 101, 397–410 [DOI] [PubMed] [Google Scholar]
- 18. Sacco S., Agnello D., Sottocorno M., Lozza G., Monopoli A., Villa P., Ghezzi P. (1998) J. Neurochem. 71, 2063–2070 [DOI] [PubMed] [Google Scholar]
- 19. Brenneis C., Coste O., Schmidt R., Angioni C., Popp L., Nusing R. M., Becker W., Scholich K., Geisslinger G. (2008) J. Cell Mol. Med. 12, 639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hizaki H., Segi E., Sugimoto Y., Hirose M., Saji T., Ushikubi F., Matsuoka T., Noda Y., Tanaka T., Yoshida N., Narumiya S., Ichikawa A. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 10501–10506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morath R., Klein T., Seyberth H. W., Nüsing R. M. (1999) J. Am. Soc. Nephrol. 10, 1851–1860 [DOI] [PubMed] [Google Scholar]
- 22. Hylden J. L., Wilcox G. L. (1980) Eur. J. Pharmacol. 67, 313–316 [DOI] [PubMed] [Google Scholar]
- 23. Pierre S. C., Häusler J., Birod K., Geisslinger G., Scholich K. (2004) EMBO J. 23, 3031–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coste O., Brenneis C., Linke B., Pierre S., Maeurer C., Becker W., Schmidt H., Gao W., Geisslinger G., Scholich K. (2008) J. Biol. Chem. 283, 32442–32451 [DOI] [PubMed] [Google Scholar]
- 25. Schubert W., Bonnekoh B., Pommer A. J., Philipsen L., Böckelmann R., Malykh Y., Gollnick H., Friedenberger M., Bode M., Dress A. W. (2006) Nat. Biotechnol. 24, 1270–1278 [DOI] [PubMed] [Google Scholar]
- 26. Pierre S., Maeurer C., Coste O., Becker W., Schmidtko A., Holland S., Wittpoth C., Geisslinger G., Scholich K. (2008) Mol. Cell. Proteomics 7, 2475–2485 [DOI] [PubMed] [Google Scholar]
- 27. Coste O., Pierre S., Marian C., Brenneis C., Angioni C., Schmidt H., Popp L., Geisslinger G., Scholich K. (2008) J. Cell. Mol. Med. 12, 995–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Linke B., Pierre S., Coste O., Angioni C., Becker W., Maier T. J., Steinhilber D., Wittpoth C., Geisslinger G., Scholich K. (2009) J. Proteome Res. 8, 4851–4859 [DOI] [PubMed] [Google Scholar]
- 29. Pierre S., Eschenhagen T., Geisslinger G., Scholich K. (2009) Nat. Rev. Drug Discov. 8, 321–335 [DOI] [PubMed] [Google Scholar]
- 30. Armstrong R. A., Lawrence R. A., Jones R. L., Wilson N. H., Collier A. (1989) Br. J. Pharmacol. 97, 657–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suzawa T., Miyaura C., Inada M., Maruyama T., Sugimoto Y., Ushikubi F., Ichikawa A., Narumiya S., Suda T. (2000) Endocrinology 141, 1554–1559 [DOI] [PubMed] [Google Scholar]
- 32. Trebino C. E., Eskra J. D., Wachtmann T. S., Perez J. R., Carty T. J., Audoly L. P. (2005) J. Biol. Chem. 280, 16579–16585 [DOI] [PubMed] [Google Scholar]
- 33. Ji K. A., Yang M. S., Jeong H. K., Min K. J., Kang S. H., Jou I., Joe E. H. (2007) Glia 55, 1577–1588 [DOI] [PubMed] [Google Scholar]
- 34. Cimino P. J., Keene C. D., Breyer R. M., Montine K. S., Montine T. J. (2008) Curr. Med. Chem. 15, 1863–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shie F. S., Montine K. S., Breyer R. M., Montine T. J. (2005) Glia 52, 70–77 [DOI] [PubMed] [Google Scholar]
- 36. Kawamura T., Yamauchi T., Koyama M., Maruyama T., Akira T., Nakamura N. (1997) Life Sci. 61, 2111–2116 [DOI] [PubMed] [Google Scholar]
- 37. Baba H., Kohno T., Moore K. A., Woolf C. J. (2001) J. Neurosci. 21, 1750–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liang X., Wang Q., Shi J., Lokteva L., Breyer R. M., Montine T. J., Andreasson K. (2008) Ann. Neurol. 64, 304–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Choi J. S., Kim H. Y., Chun M. H., Chung J. W., Lee M. Y. (2006) Cell Tissue Res. 324, 203–211 [DOI] [PubMed] [Google Scholar]
- 40. Taffet S. M., Singhel K. J., Overholtzer J. F., Shurtleff S. A. (1989) Cell. Immunol. 120, 291–300 [DOI] [PubMed] [Google Scholar]
- 41. Seldon P. M., Barnes P. J., Meja K., Giembycz M. A. (1995) Mol. Pharmacol. 48, 747–757 [PubMed] [Google Scholar]
- 42. Sugimoto Y., Negishi M., Hayashi Y., Namba T., Honda A., Watabe A., Hirata M., Narumiya S., Ichikawa A. (1993) J. Biol. Chem. 268, 2712–2718 [PubMed] [Google Scholar]
- 43. Engblom D., Saha S., Engström L., Westman M., Audoly L. P., Jakobsson P. J., Blomqvist A. (2003) Nat. Neurosci. 6, 1137–1138 [DOI] [PubMed] [Google Scholar]
- 44. Ikeda-Matsuo Y., Ikegaya Y., Matsuki N., Uematsu S., Akira S., Sasaki Y. (2005) J. Neurochem. 94, 1546–1558 [DOI] [PubMed] [Google Scholar]
- 45. Samad T. A., Moore K. A., Sapirstein A., Billet S., Allchorne A., Poole S., Bonventre J. V., Woolf C. J. (2001) Nature 410, 471–475 [DOI] [PubMed] [Google Scholar]
- 46. Zeilhofer H. U. (2005) Cell. Mol. Life Sci. 62, 2027–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim E., Raval A. P., Defazio R. A., Perez-Pinzon M. A. (2007) Neuroscience 145, 931–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lin H. Y., Huang C. C., Chang K. F. (2009) Pediatr. Res. 66, 254–259 [DOI] [PubMed] [Google Scholar]
- 49. Lin H. Y., Wu C. L., Huang C. C. (2010) Stroke 41, 1543–1551 [DOI] [PubMed] [Google Scholar]
- 50. Strauss K. I. (2008) Brain Behav. Immun. 22, 285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lambertsen K. L., Clausen B. H., Babcock A. A., Gregersen R., Fenger C., Nielsen H. H., Haugaard L. S., Wirenfeldt M., Nielsen M., Dagnaes-Hansen F., Bluethmann H., Faergeman N. J., Meldgaard M., Deierborg T., Finsen B. (2009) J. Neurosci. 29, 1319–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McCoy M. K., Tansey M. G. (2008) J. Neuroinflammation 5, 45 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.