Abstract
TMEM16A (ANO1) functions as a calcium-activated chloride channel (CaCC). We developed pharmacological tools to investigate the contribution of TMEM16A to CaCC conductance in human airway and intestinal epithelial cells. A screen of ∼110,000 compounds revealed four novel chemical classes of small molecule TMEM16A inhibitors that fully blocked TMEM16A chloride current with an IC50 < 10 μm, without interfering with calcium signaling. Following structure-activity analysis, the most potent inhibitor, an aminophenylthiazole (T16Ainh-A01), had an IC50 of ∼1 μm. Two distinct types of inhibitors were identified. Some compounds, such as tannic acid and the arylaminothiophene CaCCinh-A01, fully inhibited CaCC current in human bronchial and intestinal cells. Other compounds, including T16Ainh-A01 and digallic acid, inhibited total CaCC current in these cells poorly, but blocked mainly an initial, agonist-stimulated transient chloride current. TMEM16A RNAi knockdown also inhibited mainly the transient chloride current. In contrast to the airway and intestinal cells, all TMEM16A inhibitors fully blocked CaCC current in salivary gland cells. We conclude that TMEM16A carries nearly all CaCC current in salivary gland epithelium, but is a minor contributor to total CaCC current in airway and intestinal epithelia. The small molecule inhibitors identified here permit pharmacological dissection of TMEM16A/CaCC function and are potential development candidates for drug therapy of hypertension, pain, diarrhea, and excessive mucus production.
Keywords: Anion Transport, Calcium, Chloride Channels, Fluorescence, High Throughput Screening
Introduction
Calcium-activated chloride channels (CaCCs)2 are ubiquitously expressed in epithelial and nonepithelial cells, where they are involved in epithelial fluid secretion, sensory signal transduction, smooth muscle contraction, oocyte fertilization, and other functions (1–3). CaCCs are potential drug targets for hypertension, asthma, secretory diarrheas, and pain (3).
Three groups reported that the TMEM16A (anoctamin-1, ANO1) gene encodes a CaCC (4–6), showing calcium-activated Cl− currents following heterologous expression. TMEM16A is expressed broadly in mammalian tissues, including tracheal, intestinal, and glandular epithelia, smooth muscle cells, and interstitial cells of Cajal in the gastrointestinal tract (4, 7–9). TMEM16A is also expressed in various tumors, where it has been proposed to play a role in tumor cell proliferation (4, 10). TMEM16A knock-out mice die soon after birth because of tracheomalacia (11). CaCC current measurements in these mice suggested a major role of TMEM16A in epithelial chloride secretion in the airways (12) and salivary gland (4, 13). However, the contribution of TMEM16A to CaCC conductance in adult tissues is not known because of the neonatal lethality of knock-out mice, the lack of potent and selective inhibitors, and the presence of multiple TMEM16 isoforms, some of which, including TMEM16B, also have CaCC activity (6, 14). Knowledge of the contribution of TMEM16A to CaCC activity is important in human disease pathogenesis and for development of new therapies, such as CaCC activators for cystic fibrosis (CF), and CaCC inhibitors for hypertension, asthma, and non-CFTR-dependent secretory diarrheas.
Here, we developed small molecule pharmacological tools to investigate TMEM16A involvement in CaCC conductance in various human epithelial cell cultures. TMEM16A inhibitors were identified by high throughput screening using a cell-based plate reader assay involving measurement of calcium agonist-induced iodide influx in FRT cells co-expressing human TMEM16A and the fluorescent iodide-sensing protein YFP-H148Q/I152L/F46L. Analysis of inhibitor effects on CaCC currents in human epithelial cell cultures indicated, contrary to expectation, only a minor role of TMEM16A to total CaCC current in airway and intestinal epithelia.
EXPERIMENTAL PROCEDURES
Materials and Solutions
Amiloride, ATP, UTP, ionomycin, and other chemicals, unless otherwise indicated, were purchased from Sigma. CFTRinh-172 and CaCCinh-A01 were synthesized as described (15, 16). T16Ainh-A01 was purchased from Asinex (San Diego, CA). The compound collections used for screening included: ∼100,000 synthetic small molecules from ChemDiv (San Diego, CA) and Asinex, and ∼7500 purified natural products from Analyticon (Potsdam, Germany), Timtek (Newark, NJ), and Biomol (Plymouth Meeting, PA). Compounds were maintained as dimethyl sulfoxide stock solutions. Structure-activity analysis was done on analogs purchased from ChemDiv and Asinex. The HCO3−-buffered solution contained 120 mm NaCl, 5 mm KCl, 1 mm MgCl2, 1 mm CaCl2, 10 mm d-glucose, 5 mm HEPES, and 25 mm NaHCO3 (pH 7.4). In the half-Cl− solution 65 mm NaCl in the HCO3−-buffered solution was replaced by sodium gluconate.
Cell Culture and RNAi Knockdown
FRT cells were stably transfected with human TMEM16A (TMEM16A(abc), cDNA provided by Dr. Luis Galietta, Gaslini Institute, Genoa, Italy) and the halide sensor YFP-H148Q/I152L/F46L. Cells were plated in 96-well black-walled microplates (Corning Inc., Corning, NY) at a density of 20,000 cells/well in Coon's modified F-12 medium supplemented with 5% fetal calf serum, 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. T84 and Calu-3 cells were cultured in DMEM/Ham's F-12 (1:1) medium containing 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Primary cultures of homozygous ΔF508-CFTR-expressing human bronchial epithelial cells were obtained and grown as described (17). The human submandibular cell line A253 (ATCC HTB 41) was cultured in complete McCoy's 5A medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin.
siRNA against TMEM16A was purchased from Dharmacon (Lafayette, CO; SMART pools), and negative control siRNA was from Invitrogen. siRNA transfection with Lipofectamine 2000 (Invitrogen) was performed according to the manufacturer's protocol. Briefly, cells were washed three to five times with PBS, and 100 nm TMEM16A siRNA was added with Lipofectamine 2000. Six hours after transfection, the solution was replaced with culture medium. The cells were transfected with siRNA a second time at 24 h. Short circuit current was measured 2 days after the second transfection.
Screening Procedures
High throughput screening was done using an automated screening platform (Beckman) equipped with FluoStar fluorescence plate readers (BMG Labtechnologies, Durham, NC) as described (15). Each well of a 96-well plate was washed three times with PBS (200 μl/wash), leaving 50 μl of PBS. Test compounds (0.5 μl) were added to each well at 25 μm final concentration. After 10 min, 96-well plates were transferred to a plate reader for fluorescence assay. Each well was assayed individually for TMEM16A-mediated I− influx by recording fluorescence continuously (400 ms/point) for 2 s (base line), then 50 μl of a 140 mm I− solution containing 200 μm ATP was added. The initial rate of I− influx was computed from fluorescence data by nonlinear regression.
Short Circuit Current
Snapwell inserts containing TMEM16A-expressing FRT cells, T84 cells, or human bronchial epithelial cells were mounted in Ussing chambers (Physiologic Instruments, San Diego, CA). Amiloride, CFTRinh-172, UTP, ATP, and TMEM16A inhibitors were added to the apical solution, and an equal volume of vehicle was added at the same time to the basolateral solution. Symmetrical HCO3−-buffered solutions were used for T84 cells and human bronchial epithelial cells. For FRT cells, the hemichambers were filled with the half-Cl− solution (apical) and the HCO3−-buffered solution (basolateral). Cells were bathed for a 10-min stabilization period and aerated with 95% O2/5% CO2 at 37 °C or room temperature. In some experiments, for measurement of apical chloride conductance, the basolateral membrane was permeabilized with nystatin (360 μg/ml), and a chloride gradient was applied in which the basolateral membrane was bathed with the HCO3−-buffered solution, and in the apical solution 120 mm NaCl was replaced by sodium gluconate. Short circuit current was measured using an EVC4000 Multi-Channel V/I Clamp (World Precision Instruments, Sarasota, FL) and recorded using PowerLab/8sp (AD Instruments, Castle Hill, Australia).
Patch Clamp
Whole cell recordings were made at room temperature on TMEM16A-expressing FRT cells and human submandibular A253 cells. The pipette solution contained 130 mm CsCl, 0.5 mm EGTA, 1 mm MgCl2, 1 mm Tris-ATP, and 10 mm HEPES (pH 7.2). The bath solution contained 140 mm N-methyl-d-glucamine-Cl, 1 mm CaCl2, 1 mm MgCl2, 10 mm glucose, and 10 mm HEPES (pH 7.4). Pipettes were pulled from borosilicate glass and had resistances of 3–5 megohms after fire polishing. Seal resistances were between 3 and 10 gigohms. After establishing the whole cell configuration, TMEM16A was activated by 100 μm ATP or by 275 nm free calcium in the pipette solution (1 mm CaCl2 added to pipette solution). Whole cell currents were elicited by applying hyperpolarizing and depolarizing voltage pulses from a holding potential of 0 mV to potentials between −100 mV and +100 mV in steps of 20 mV. Recordings were made at room temperature using an Axopatch-200B (Axon Instruments). Currents were digitized with a Digidata 1440A converter (Axon Instruments), filtered at 5 kHz, and sampled at 1 kHz.
Cytoplasmic Calcium Measurements
FRT cells in 96-well black-walled microplates were loaded with Fluo-4 NW at 48 h after plating per the manufacturer's protocol (Invitrogen). Fluo-4 fluorescence was measured with a FLUOstar Optima fluorescence plate reader (BMG Labtechnologies) equipped with syringe pumps and custom excitation/emission filters (485/538 nm).
Immunoblot Analysis
FRT, A253, T84, and ΔF508-CFTR-expressing human bronchial epithelial cells were lysed with cell lysis buffer (50 mm Tris-HCl (pH 7.4), 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mm NaCl, 1 mm EDTA, 1 mm Na3VO4, and protease inhibitor mixture (Roche Applied Science)). Cell debris was removed by centrifugation, and proteins in the supernatant were resolved by SDS-PAGE and immunoblotted using standard procedures (transfer to polyvinylidene difluoride membrane, 1-h blocking in 5% nonfat dry milk, primary TMEM16A antibody (1:1000 dilution, ab16293; Abcam Inc., Cambridge, MA) and secondary antibody (1:3000 dilution) incubations, and enhanced chemiluminescence detection).
RESULTS
Identification of Small Molecule TMEM16A Inhibitors
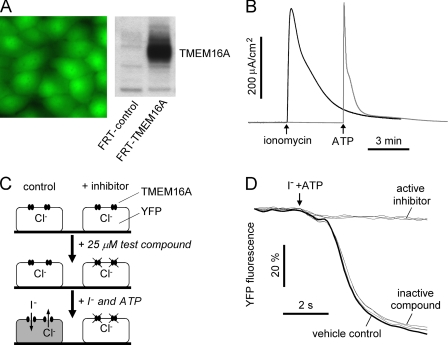
High throughput screening was done using FRT cells that were stably transfected with human TMEM16A and the iodide-sensitive fluorescent protein YFP-H148Q/I152L/F46L. FRT cells were chosen because of their low basal halide transport, rapid growth on uncoated plastic, high and stable expression of transfected cDNAs, and formation a tight epithelium (for short circuit current analysis), as well as our prior success in chloride channel drug discovery using these cells (15, 18). Fig. 1A shows cytoplasmic YFP fluorescence in the transfected cells and immunoblot verification of TMEM16A protein expression. Fig. 1B shows robust CaCC current in the TMEM16A-expressing cells in response to the calcium agonists ATP and ionomycin. Agonist-stimulated current was absent in nontransfected FRT cells (data not shown).
FIGURE 1.
Identification of small molecule inhibitors of human TMEM16A. A, TMEM16A-transfected cells showing green cytoplasmic YFP-H148Q/I152L/F46L fluorescence (left) and TMEM16A protein by immunoblotting (right). B, short circuit current analysis of TMEM16A-expressing FRT cells. Representative current traces showing ionomycin- and ATP-stimulated TMEM16A chloride current are shown. C, screening protocol. FRT cells stably expressing TMEM16A and the YFP halide sensor were incubated for 10 min with test compound. Fluorescence was monitored in response to the addition of iodide and ATP. D, fluorescence measured in single wells of 96-well plates, showing vehicle control and examples of active and inactive compounds.
As diagrammed in Fig. 1C, screening to identify TMEM16A inhibitors was done in 96-well plates in which test compounds (at 25 μm) were added 10 min before the assay to a physiological chloride-containing solution bathing the cells. The assay involved addition of an iodide solution containing ATP. Iodide influx was measured in individual wells from the initial time course of decreasing YFP fluorescence. Examples of inactive and active compounds are shown in Fig. 1D, along with negative (dimethyl sulfoxide vehicle) control. Active compounds reduced iodide influx, as seen by a reduced rate of decreasing fluorescence.
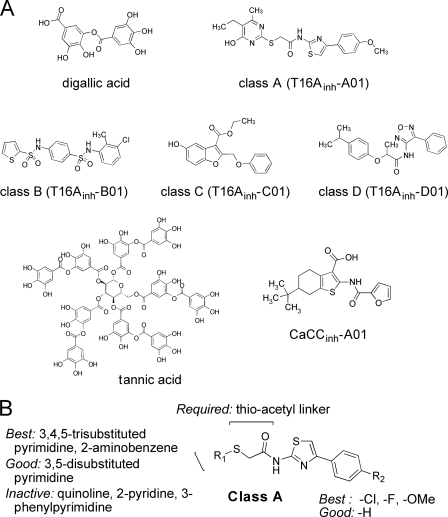
Screening of ∼110,000 drug-like small molecules and natural products yielded 13 compounds that inhibited TMEM16A-mediated iodide influx by >50% at 10 μm. The compounds fell into six distinct chemical classes, one of which (CaCCinh-A01) was identified previously in a phenotype screen for CaCC inhibitors done using human intestinal HT-29 cells (16). Another compound, digallic acid, is related chemically to tannic acid, which we identified previously as a CaCC inhibitor (19). Following verification by measurements of short circuit current and cytoplasmic calcium (see below) and structure-activity studies, chemical structures of the most potent compound of each class are shown in Fig. 2A. Except for digallic acid and CaCCinh-A01, the structures of the new inhibitor classes (named T16Ainh-Xxx, where X refers to classes A, B, C, or D, and xx is the compound identifying number) are unrelated chemically to previously reported CaCC inhibitors or to known CFTR inhibitors including CFTRinh-172, GlyH-101, and PPQ (structures not shown).
FIGURE 2.
Chemical structures of TMEM16A inhibitors. A, structures shown of the most potent TMEM16A inhibitor of each of four classes (T16Ainh-X01, where X = A, B, C, or D), along with structure of digallic acid and the previously identified CaCC inhibitors CaCCinh-A01 (16) and tannic acid (19). B, summary of SAR analysis for class A TMEM16A inhibitors. Inhibition data for most potent class A compounds are summarized in Table 1. Functional data and SAR analysis for class B–D compounds provided in supplementary Figs. S1–S3, respectively.
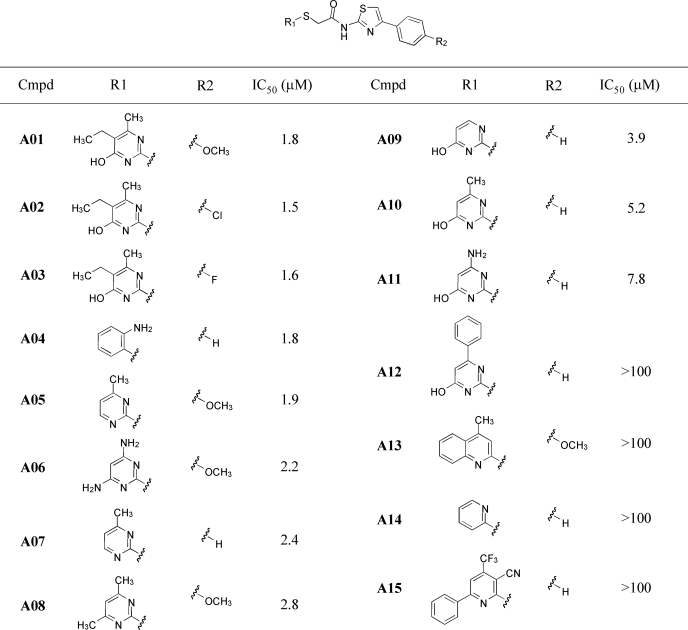
Analysis of structure-activity relationships (SARs) was done by screening of more than 800 chemical analogs of class A, B, C, and D compounds identified in the primary screen. Activity data for the most active TMEM16A inhibitors of class A are summarized in Table 1. Activity data and a summary of SAR analysis for class B, C, and D compounds are provided in supplemental Figs. S1–S3, respectively. Fig. 2B summarizes the SAR analysis of class A compounds, which consist of a 2-amino,4-phenythiazole core coupled to a second heterocycle (R1) via a thio-acetyl linker. For the second heterocycle, pyrimidine and 2-aminobenzene (T16Ainh-A04) gave the most potent inhibition. Other heterocycles such as quinoline (T16Ainh-A13) and 2-pyridine (T16Ainh-A14, A15) were inactive. Substitution on the pyrimidine ring reduced inhibition potency. 3,4,5-Trisubstituted analogs (T16Ainh-A01, A02, A03) were among the most potent inhibitors, with IC50 of 1.5–1.8 μm. A bulky group such as phenyl at the 3-position reduced inhibition (T16Ainh-A12, IC50 >100 μm), although smaller substituents including amine, hydroxy, and alkyl groups were tolerated. Substitutions (R2) on the phenyl ring of the thiazole with electron-withdrawing (chloride, fluoride) and donating groups (methoxy) had minimal effect on inhibition potency.
TABLE 1.
TMEM16A inhibition by class A compounds
Structure-activity relationship of class A inhibitors is shown. IC50 was determined from fluorescence plate reader assay.
Characterization of TMEM16A Inhibitors
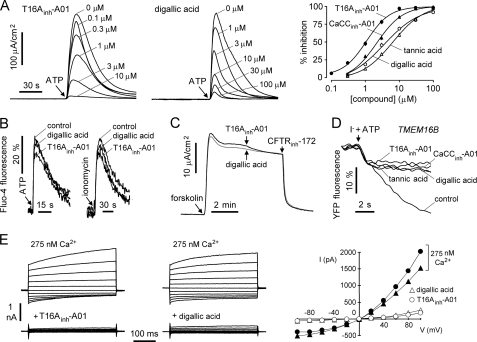
Inhibitors were characterized by electrophysiological and intracellular calcium measurements. Fig. 3A shows a short circuit current in TMEM16A-expressing FRT cells in which the basolateral membrane was permeabilized with amphotericin B, and a transepithelial chloride gradient was applied, such that the observed current is a direct, quantitative measure of apical membrane TMEM16A chloride conductance. Test compounds were added 5 min prior to TMEM16A activation by 100 μm ATP. Compounds T16Ainh-A01 and digallic acid fully inhibited an ATP-induced short circuit current. Concentration-inhibition data for four inhibitors, which will be used further below, are shown in Fig. 3A (right), with fitted IC50 values: T16Ainh-A01 (1.1 μm), digallic acid (3.6 μm), CaCCinh-A01 (2.1 μm), and tannic acid (6.4 μm).
FIGURE 3.
Characterization of small molecule TMEM16A inhibitors. A, left, short circuit (apical membrane) current measured in TMEM16A-expressing FRT cells in the presence of a transepithelial chloride gradient (see “Experimental Procedures”). Inhibitors were added 5 min prior to TMEM16A activation by 100 μm ATP. A, right, summary of dose-response data (mean ± S.E., n = 4). B, cytoplasmic calcium measured by Fluo-4 fluorescence. Cells were pretreated for 5 min with 10 μm T16Ainh-A01 or 100 μm digallic acid (or vehicle control), with 100 μm ATP or 1 μm ionomycin added as a calcium agonist as indicated. C, CFTR activated by 10 μm forskolin in primary cultured human bronchial epithelial cells and 10 μm T16Ainh-A01 (black line) or 100 μm digallic acid (gray line) added to apical bath as indicated. D, CaCC current measured in TMEM16B-transfected FRT cells expressing YFP-H148Q/I152L/F46L, showing inhibition by 10 μm T16Ainh-A01, 100 μm digallic acid, 30 μm CaCCinh-A01, and 100 μm tannic acid. E, whole cell TMEM16A current recorded at a holding potential at 0 mV and pulsing to voltages between ±100 mV (in steps of 20 mV) in the absence and presence of 10 μm T16Ainh-A01 (left) or 100 μm digallic acid (center). TMEM16A was stimulated by 275 nm free calcium in pipette solution. E, right, current/voltage (I/V) plot of mean currents at the middle of each voltage pulse.
Fig. 3B shows Fluo-4 fluorescence measurement of ATP and ionomycin-stimulated cytoplasmic calcium elevation. Cytoplasmic calcium was not altered by 10 μm T16Ainh-A01 or 100 μm digallic acid, as shown, or by the other TMEM16A inhibitors in Fig. 2A (data not shown). 10 μm T16Ainh-A01 and 100 μm digallic acid had little effect on CFTR Cl− conductance (inhibited by <10%; Fig. 3C). Fig. 3D shows that T16Ainh-A01, digallic acid, CaCCinh-A01, and tannic acid each inhibited the TMEM16 isoform TMEM16B, which has been reported to have CaCC activity (6, 14). Whole cell patch clamp analysis was done to determine inhibition mechanisms of T16Ainh-A01 and digallic acid (Fig. 3E). 10 μm T16Ainh-A01 and 100 μm digallic acid inhibited nearly completely TMEM16A chloride current (induced by 275 nm free calcium in the pipette) at all voltages, indicating a voltage-independent block mechanism.
TMEM16A Inhibitors Block Calcium-activated Chloride Conductance in Salivary Gland Cells
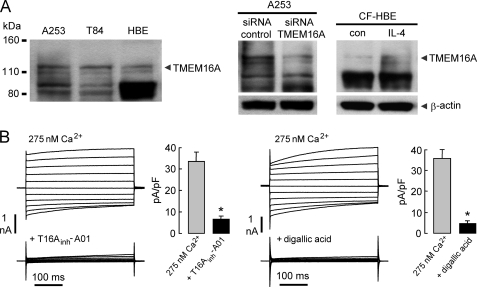
We studied three types of human epithelia-derived cell cultures to determine the contribution of TMEM16A to calcium-stimulated chloride currents: A253 cells (salivary gland-derived), T84 cells (colon-derived), and human bronchial epithelial cells (primary cultures of bronchial epithelium). Fig. 4A shows immunoblot analysis of TMEM16A protein in each cell type, in TMEM16A siRNA-treated A253 cells, and in interlukin-4 (IL-4)-treated human bronchial epithelial cells. Although several nonspecific bands were seen using available antibodies, bands were seen at the correct molecular size of TMEM16A, which were reduced by siRNA knockdown and increased by IL-4 treatment. Fig. 4B shows whole cell patch clamp recordings of A253 cells in the presence of 10 μm CFTRinh-172 in the bath solution to inhibit CFTR. Characteristic outwardly rectifying CaCC currents were seen. 10 μm T16Ainh-A01 and 100 μm digallic acid strongly inhibited chloride current (induced by 275 nm free calcium in the pipette).
FIGURE 4.
TMEM16A inhibitors block CaCC chloride current in human salivary gland epithelial cells. A, left, immunoblot of TMEM16A protein in A253 salivary gland cells, T84 intestinal cells, and primary cultured human bronchial epithelial cells. Right, TMEM16A immunoblot at 24 h after incubation with 10 ng/ml IL-4 in human bronchial epithelial cells. Center, TMEM16A immunoblot at 48 h after the two transfections with nontargeting control siRNA and siRNA against TMEM16A in A253 cells. Representatives of three sets of studies are shown. B, whole cell patch clamp recordings of A253 cells. 10 μm T16Ainh-A01 (left) and 100 μm digallic acid (right) inhibition of TMEM16A chloride current induced by 275 nm free calcium in the pipette solution is shown. The bar graphs summarize current density data measured at +80 mV (mean ± S.E., n = 4).
TMEM16A Inhibitors Block Calcium-activated Chloride Conductance Poorly in Intestinal and Airway Epithelial Cells
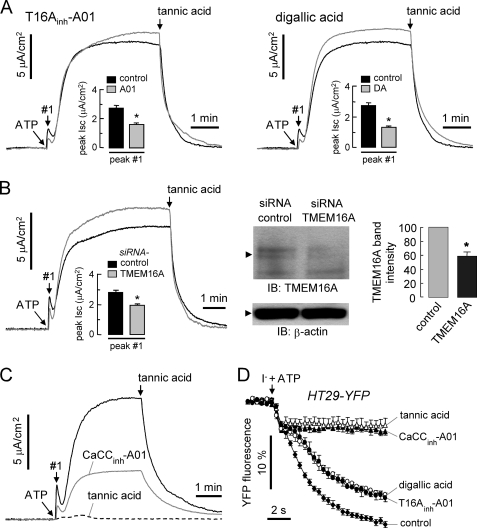
Short circuit current measurements in T84 cells showed strong CaCC current following calcium elevation by 100 μm ATP (Fig. 5A). As above, in these studies CFTRinh-172 was present to ensure that chloride current is CaCC-dependent rather than CFTR-dependent because calcium elevation in some cell types can activate CFTR through calcium-sensitive adenylyl cyclases (17). We found that 10 μm T16Ainh-A01 and 100 μm digallic acid had little effect on total CaCC current, but selectively blocked the early, transient current elevation following ATP addition (Fig. 5A). In contrast, 30 μm CaCCinh-A01 and 100 μm tannic acid strongly inhibited CaCC current following ATP stimulation (Fig. 5C). These findings suggest that TMEM16A accounts for only a small fraction of total CaCC current in T84 cells. To confirm this interpretation, siRNA knockdown was done. TMEM16A knockdown by siRNA in T84 cells reduced TMEM16A protein expression by 42 ± 5% (Fig. 5B). Short circuit current measurements showed an effect of TMEM16A knockdown qualitatively similar to that seen with T16Ainh-A01 and digallic acid. Knockdown reduced the early, transient current elevation following ATP addition, but had little effect on total CaCC current. Inhibitor effects were also studied in a different human intestinal cell line, HT-29. Fig. 5D shows that 10 μm T16Ainh-A01 and 100 μm digallic acid had little effect on CaCC activity, whereas 30 μm CaCCinh-A01 and 100 μm tannic acid strongly inhibited CaCC in YFP-expressing HT29 cells, supporting the interpretation of data in T84 cells.
FIGURE 5.
TMEM16A inhibitors block the initial transient CaCC current in human intestinal epithelial cells following agonist stimulation. A, short circuit current measured in T84 cells. At 3 min after pretreatment with 10 μm T16Ainh-A01 (left) or 100 μm digallic acid (right), CaCC was activated by 100 μm ATP. Bar graphs summarize ATP-induced initial peak (#1, mean ± S.E., n = 7; *, p < 0.05). Remaining UTP-induced current was completely blocked by 100 μm tannic acid as indicated. CFTR was inhibited by pretreatment of 20 μm CFTRinh-172. B, left, short circuit current in T84 cells measured at 48 h after the two transfections with nontargeting control siRNA and siRNA against TMEM16A. Bar graph summarizes ATP-induced peak current (mean ± S.E., n = 4; *, p < 0.05). Right, immunoblot (IB) of TMEM16A in T84 cells transfected with control and TMEM16A siRNA (mean ± S.E., n = 3; *, p < 0.05). C, representative short circuit current data for pretreatment with 30 μm CaCCinh-A01 or 100 μm tannic acid. D, effect of 100 μm tannic acid, 30 μm CaCCinh-A01, 100 μm digallic acid, and 10 μm T16Ainh-A01 on CaCC activity in YFP-expressing HT29 cells (mean ± S.E., n = 4).
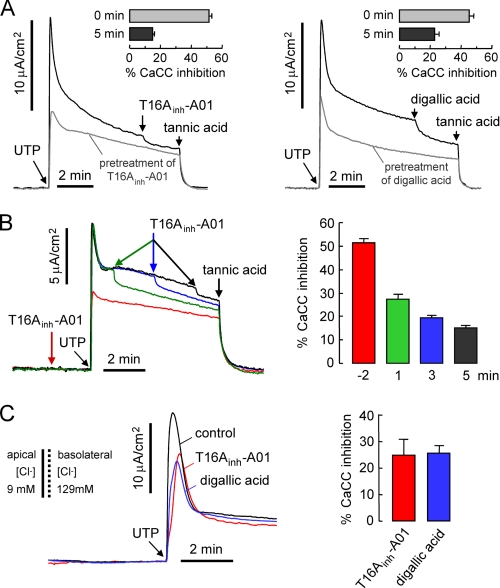
Experiments were also done in primary cultures of human bronchial epithelial cells from CF subjects, which lack functional CFTR and thus avoid the potentially confounding issue of calcium-induced CFTR activation. Fig. 6A shows large CaCC currents in these cells following UTP stimulation. As found in the intestinal cells, T16Ainh-A01 and digallic acid inhibited initial peak current by ∼50%, whereas these compounds had relatively little effect on the CaCC current measured at 5 min after UTP stimulation. Similar results were found for non-CF bronchial epithelial cell cultures studied in the presence of CFTRinh-172 (data not shown). These data support the conclusion that TMEM16A is responsible mainly for the early, transient CaCC chloride current following agonist stimulation in airway and intestinal epithelial cells. Fig. 6B shows UTP-stimulated CaCC current in which T16Ainh-A01 was added before versus at three different times after UTP. The reduced T16Ainh-A01 inhibition with time after UTP addition supports the interpretation that TMEM16A contributes mainly to the early, transient CaCC current. Finally, to confirm the effect of T16Ainh-A01 and digallic acid on apical CaCC current, we measured apical membrane chloride conductance in CF bronchial epithelial cells following basolateral membrane permeabilization by 360 μg/ml nystatin and in the presence of a basolateral-to-apical chloride gradient. 10 μm T16Ainh-A01 and 100 μm digallic acid each reduced UTP-stimulated peak current by ∼25% (Fig. 6C).
FIGURE 6.
TMEM16A inhibitors poorly block CaCC chloride current in human bronchial epithelial cells. A, short circuit current measured in primary cultures of CF human bronchial epithelial cells. CaCC was activated by 100 μm UTP after 3-min pretreatment with 10 μm T16Ainh-A01 (left) or 100 μm digallic acid (right). Bar graphs summarize inhibition of UTP-induced initial peak current (0 min) and current at 5 min (mean ± S.E., n = 7–9). Remaining UTP-induced current was completely blocked by 100 μm tannic acid as indicated. Epithelial sodium channel was inhibited by 10 μm amiloride. B, short circuit current showing UTP-induced CaCC current in CF human bronchial epithelial cells with 10 μm T16Ainh-A01 added at four different time points (−2 min, 1 min, 3 min, 5 min) as indicated. Bar graph summarizes inhibition of CaCC at each time point (mean ± S.E., n = 7–9). C, apical chloride conductance measured after basolateral membrane permeabilization by nystatin (360 μg/ml) and with indicated apical and basolateral solution [Cl−]. Cells were pretreated with 10 μm T16Ainh-A01 (red curve) or 100 μm digallic acid (blue curve), and CaCC was activated by 100 μm UTP. Bar graph summarizes inhibition of UTP-induced initial peak current (mean ± S.E. (error bars), n = 3).
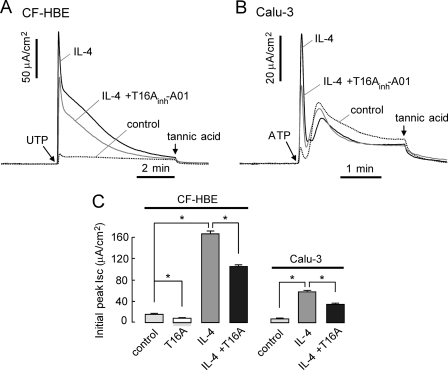
Last, experiments were done in cells treated with IL-4, which strongly increases TMEM16A expression in human airway epithelium (5). As mentioned above, Fig. 4A confirmed a marked increase in TMEM16A expression after 24-h incubation with IL-4 in primary cultures of CF human bronchial epithelium. In CF human bronchial epithelial cells and Calu-3 cells, IL-4 treatment produced ∼10-fold increases in UTP-induced initial peak current (Fig. 7). T16Ainh-A01-sensitive current increased by ∼8-fold in IL-4-treated CF human bronchial epithelial cells compared with untreated cells (Fig. 7C). These results support the interpretation that TMEM16A is a minor contributor to total CaCC current in unstimulated airway epithelial cells.
FIGURE 7.
IL-4 induced CaCC up-regulation in CF human bronchial epithelial cells. A, short circuit current. IL-4 was applied at 10 ng/ml for 24 h. CaCC was activated by 100 μm UTP after 3-min pretreatment with 10 μm T16Ainh-A01. CF-HBE, CF human bronchial epithelial cells. B, short circuit current measured in Calu-3 cells. IL-4 was applied at 10 ng/ml for 24 h. CaCC was activated by 100 μm ATP after 3-min pretreatment with 10 μm T16Ainh-A01. CFTR was inhibited by pretreatment of 10 μm CFTRinh-172. C, bar graph summarizes inhibition of UTP-induced initial peak current (mean ± S.E. (error bars), n = 3–5; *, p < 0.05).
DISCUSSION
The discovery of TMEM16A as a CaCC is an important advance, as the molecular identity of CaCCs has been unclear, with conflicting data reported for various CaCC candidates including bestropins, ClCs and tweety (reviewed in refs. 20, 21). TMEM16A is ubiquitously expressed in epithelia (8, 22) and has been proposed as a major CaCC in epithelial fluid transport. One goal of our study here was to develop small molecule tools to quantify the contribution of TMEM16A to CaCC in major epithelial tissues where it is expressed, including the airways, intestine, and salivary gland. These tissues are of particular importance, because, if TMEM16A is the major CaCC in these epithelia, then TMEM16A modulators may be of utility in the therapy of CF, certain secretory diarrheas, and salivary gland hypofunction. Our approach was to identify TMEM16A inhibitors by high throughput screening using a target-based fluorescence assay of iodide influx in TMEM16A-transfected cells. Following characterization and optimization by structure-activity analysis, the inhibitors were tested for their efficacy in inhibiting CaCC conductance in human airway, intestinal, and salivary gland cells. We previously reported a phenotype-based screen to identify small molecule CaCC inhibitors in the human intestinal epithelial cell line, HT-29 (16), as well as a target-based screen that identified wine and tea gallotannins as CaCC inhibitors (19).
The TMEM16A target-based screen yielded five classes of small molecule TMEM16A inhibitors with IC50 <10 μm and ∼100% inhibition at higher concentrations. Cytoplasmic calcium and patch clamp analysis suggested that these inhibitors target TMEM16A directly, rather than upstream processes such as agonist binding or calcium signaling. Analysis of chemical analogs of active compounds established structure-activity datasets that defined the structural determinants of TMEM16A inhibition. The most potent class A inhibitors contain an aminophenylthiazole moiety linked by a thio-acetyl moiety with a substituted pyrimidine. Various substitutions on the 3-, 4-, and 5-positions of the pyrimidine ring were tolerated, giving analogs with IC50 down to ∼1 μm for TMEM16A chloride current inhibition. Inhibitor classes A–D represent potential development candidates based on their broad SAR, favorable medicinal properties, and ease of synthetic chemistry; however, there is little predicted clinical utility of TMEM16A-selective inhibitors because their efficacy is limited to salivary gland and perhaps other tissues and some tumors.
An important finding in this study was that some compounds that strongly inhibited TMEM16A in TMEM16A-expressing FRT cells strongly inhibited CaCC current in salivary gland epithelial cells, but only partially and weakly in airway and intestinal cells. Other compounds inhibited CaCC current in all three epithelial cell types. The logical interpretation of these data is that TMEM16A carries most or all CaCC current in salivary gland cells, but only a small fraction of total CaCC current in airway and intestinal cells. The conclusion that TMEM16A is the principal CaCC in salivary gland epithelial cells is in agreement with recent transport measurements in submandibular glands from neonatal TMEM16A knock-out mice (13). The compounds that poorly inhibited CaCC current in airway and intestinal epithelial cells primarily blocked an early, transient chloride current following agonist stimulation. The involvement of TMEM16A in the early, transient chloride current was supported by RNAi knockdown experiments. Because the transient chloride current represents a small fraction of time-integrated, agonist-stimulated chloride current, we conclude that TMEM16A plays only a minor role in total CaCC current in airways and intestine.
IL-4 strongly increased TMEM16A expression (Fig. 4A) and calcium agonist-induced short circuit current in CF human bronchial epithelial cells and Calu-3 cells (Fig. 7), but not in T84 cells (data not shown). IL-4 treatment strongly increased the ATP-induced initial peak current, but did not inhibit the subsequent sustained current. T16Ainh-A01 reduced the initial peak current but not the sustained current. These results support the conclusion that TMEM16A comprises, in part, the initial CaCC current following agonist stimulation but is of minor importance in the time-integrated, total CaCC current in normal airway and intestinal epithelium.
Our findings have several implications for chloride transport in the airways and intestine. CFTR is considered to be the major chloride channel in human airways, whose deficiency in CF is associated with altered airway function leading to chronic infection and deterioration of lung function (23). Pharmacological activation of alternative chloride channels, such as CaCCs, is considered to have therapeutic values in CF, with two compounds currently in clinical trials that elevate cytoplasmic calcium (24, 25). Our data suggest that direct activators of TMEM16A are unlikely to be useful for CF therapy because of its minor contribution to total CaCC in airway epithelium. Indeed, we recently identified small molecule TMEM16A activators that strongly activate CaCC conductance in TMEM16A-transfected cells but have little effect in human bronchial epithelial cells. However, the activators are effective in IL-4-treated human bronchial epithelial cells, where the activator-induced current is blocked completely by T16Ainh-A01.3 Without clear identification of the major airway CaCC(s), a phenotype-based screen is needed to identify CaCC activators for potential CF therapy. In the intestine, there is evidence for involvement of enterocyte CaCCs in non-CFTR-dependent secretory diarrheas caused by certain drugs and microorganisms (26–29). As in airway epithelium, our findings here suggest that TMEM16A is a minor contributor to intestinal fluid secretion, mandating the need to identify the major enterocyte CaCC(s) and to evaluate potential antidiarrheal therapeutics, such as gallotannins (19), that are nonselective in their CaCC inhibition action.
The identity remains unknown of the CaCC(s) responsible for the majority of agonist-stimulated CaCC current in airway and intestinal epithelia. Our results rule out TMEM16B as an important contributor because the TMEM16A blockers strongly inhibited TMEM16B. Other TMEM16 isoforms may be responsible for CaCC currents, as isoforms TMEM16F, TMEM16H, and TMEM16J have been identified in airway epithelial and intestinal epithelia (12, 22, 30). However, little or no significant CaCC current has been found for these isoforms (30), at least in studies involving heterologous expression. It is interesting that some compounds, such as CaCCinh-A01 and tannic acid, act as nonselective CaCC inhibitors, suggesting common structural features of CaCCs in airway and intestinal epithelia.
In conclusion, we have identified small molecule pharmacological tools to dissect the physiological roles of TMEM16A. Functional studies using these compounds indicate that although TMEM16A is responsible for essentially all CaCC in salivary gland epithelium, it is a minor contributor to total, time-integrated CaCC current in airway and intestinal epithelia, mandating the need to establish the molecular identity of remaining CaCC(s). The nonselective CaCC inhibitors identified here and in our prior screening studies have potential utility for therapy of various human diseases associated with CaCC-dependent functions, including hypertension (smooth muscle contraction), pain (nococeptive neuron function), excessive airway mucus production, and certain tumors.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants HL73856, DK72517, DK86125, DK35124, EB00415, and EY13574. This work was also supported by Research Development Program and Drug Discovery grants from the Cystic Fibrosis Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
W. Namkung, P.-W. Phuan, and A. S. Verkman, unpublished data.
- CaCC
- calcium-activated chloride channel
- CF
- cystic fibrosis
- CFTR
- cystic fibrosis transmembrane conductance regulator
- SAR
- structure-activity relationship.
REFERENCES
- 1. Hartzell C., Putzier I., Arreola J. (2005) Annu. Rev. Physiol. 67, 719–758 [DOI] [PubMed] [Google Scholar]
- 2. Eggermont J. (2004) Proc. Am. Thorac. Soc. 1, 22–27 [DOI] [PubMed] [Google Scholar]
- 3. Verkman A. S., Galietta L. J. (2009) Nat. Rev. Drug Discov. 8, 153–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang Y. D., Cho H., Koo J. Y., Tak M. H., Cho Y., Shim W. S., Park S. P., Lee J., Lee B., Kim B. M., Raouf R., Shin Y. K., Oh U. (2008) Nature 455, 1210–1215 [DOI] [PubMed] [Google Scholar]
- 5. Caputo A., Caci E., Ferrera L., Pedemonte N., Barsanti C., Sondo E., Pfeffer U., Ravazzolo R., Zegarra-Moran O., Galietta L. J. (2008) Science 322, 590–594 [DOI] [PubMed] [Google Scholar]
- 6. Schroeder B. C., Cheng T., Jan Y. N., Jan L. Y. (2008) Cell 134, 1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hwang S. J., Blair P. J., Britton F. C., O'Driscoll K. E., Hennig G., Bayguinov Y. R., Rock J. R., Harfe B. D., Sanders K. M., Ward S. M. (2009) J. Physiol. 587, 4887–4904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang F., Rock J. R., Harfe B. D., Cheng T., Huang X., Jan Y. N., Jan L. Y. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 21413–21418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Manoury B., Tamuleviciute A., Tammaro P. (2010) J. Physiol. 588, 2305–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee C. H., Liang C. W., Espinosa I. (2010) Adv. Anat. Pathol. 17, 222–232 [DOI] [PubMed] [Google Scholar]
- 11. Rock J. R., Futtner C. R., Harfe B. D. (2008) Dev. Biol. 321, 141–149 [DOI] [PubMed] [Google Scholar]
- 12. Rock J. R., O'Neal W. K., Gabriel S. E., Randell S. H., Harfe B. D., Boucher R. C., Grubb B. R. (2009) J. Biol. Chem. 284, 14875–14880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Romanenko V. G., Catalán M. A., Brown D. A., Putzier I., Hartzell H. C., Marmorstein A. D., Gonzalez-Begne M., Rock J. R., Harfe B. D., Melvin J. E. (2010) J. Biol. Chem. 285, 12990–13001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stöhr H., Heisig J. B., Benz P. M., Schöberl S., Milenkovic V. M., Strauss O., Aartsen W. M., Wijnholds J., Weber B. H., Schulz H. L. (2009) J. Neurosci. 29, 6809–6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma T., Thiagarajah J. R., Yang H., Sonawane N. D., Folli C., Galietta L. J., Verkman A. S. (2002) J. Clin. Invest. 110, 1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De La Fuente R., Namkung W., Mills A., Verkman A. S. (2008) Mol. Pharmacol. 73, 758–768 [DOI] [PubMed] [Google Scholar]
- 17. Namkung W., Finkbeiner W. E., Verkman A. S. (2010) Mol. Biol. Cell 21, 2639–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tradtrantip L., Sonawane N. D., Namkung W., Verkman A. S. (2009) J. Med. Chem. 52, 6447–6455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Namkung W., Thiagarajah J. R., Phuan P. W., Verkman A. S. (2010) FASEB J. 24, 4178–4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galietta L. J. (2009) Biophys. J. 97, 3047–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flores C. A., Cid L. P., Sepúlveda F. V., Niemeyer M. I. (2009) Braz. J. Med. Biol. Res. 42, 993–1001 [DOI] [PubMed] [Google Scholar]
- 22. Kunzelmann K., Kongsuphol P., Aldehni F., Tian Y., Ousingsawat J., Warth R., Schreiber R. (2009) Cell Calcium 46, 233–241 [DOI] [PubMed] [Google Scholar]
- 23. Jacquot J., Tabary O., Le Rouzic P., Clement A. (2008) Int. J. Biochem. Cell Biol. 40, 1703–1715 [DOI] [PubMed] [Google Scholar]
- 24. Zeitlin P. L., Boyle M. P., Guggino W. B., Molina L. (2004) Chest 125, 143–149 [DOI] [PubMed] [Google Scholar]
- 25. Storey S., Wald G. (2008) Nat. Rev. Drug Discov. 7, 555–556 [DOI] [PubMed] [Google Scholar]
- 26. Takahashi A., Sato Y., Shiomi Y., Cantarelli V. V., Iida T., Lee M., Honda T. (2000) J. Med. Microbiol. 49, 801–810 [DOI] [PubMed] [Google Scholar]
- 27. Kahn M. E., Senderowicz A., Sausville E. A., Barrett K. E. (2001) Clin. Cancer Res. 7, 343–349 [PubMed] [Google Scholar]
- 28. Ramig R. F. (2004) J. Virol. 78, 10213–10220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rufo P. A., Lin P. W., Andrade A., Jiang L., Rameh L., Flexner C., Alper S. L., Lencer W. I. (2004) Am. J. Physiol. Cell Physiol. 286, C998–C1008 [DOI] [PubMed] [Google Scholar]
- 30. Schreiber R., Uliyakina I., Kongsuphol P., Warth R., Mirza M., Martins J. R., Kunzelmann K. (2010) J. Biol. Chem. 285, 7838–7845 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.