Abstract
Glyceroneogenesis, a metabolic pathway that participates during lipolysis in the recycling of free fatty acids to triglycerides into adipocytes, contributes to the lipid-buffering function of adipose tissue. We investigated whether glyceroneogenesis could be affected by human immunodeficiency virus (HIV) protease inhibitors (PIs) responsible or not for dyslipidemia in HIV-infected patients. We treated explants obtained from subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) depots from lean individuals. We observed that the dyslipidemic PIs nelfinavir, lopinavir and ritonavir, but not the lipid-neutral PI atazanavir, increased lipolysis and decreased glyceroneogenesis, leading to an increased release of fatty acids from SAT but not from VAT. At the same time, dyslipidemic PIs decreased the amount of perilipin and increased interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) secretion in SAT but not in VAT. Parthenolide, an inhibitor of the NFκB pathway, counteracted PI-induced increased inflammation and decreased glyceroneogenesis. IL-6 (100 ng) inhibited the activity of phosphoenolpyruvate carboxykinase, the key enzyme of glyceroneogenesis, in SAT but not in VAT. Our data show that dyslipidemic but not lipid-neutral PIs decreased glyceroneogenesis as a consequence of PI-induced increased inflammation in SAT that could have an affect on adipocytes and/or macrophages. These results add a new link between fat inflammation and increased fatty acids release and suggest a greater sensitivity of SAT than VAT to PI-induced inflammation.
Keywords: human immunodeficiency virus, interleukin-6, lipolysis, phosphoenolpyruvate carboxykinase, protease inhibitors, tumor necrosis factor-α
Adipose tissue exerts two important functions involved in the regulation of lipid metabolism and insulin sensitivity: 1) storage of FFA as triglycerides (TG) into adipocytes and their disposal by lipolysis, and 2) secretion of adipokines and cytokines that could promote either insulin sensitivity or resistance in target tissues. Type 2 diabetes has been shown to be associated with disturbances in glucose and lipid metabolism, with modifications in systemic levels of adipokines, cytokines, and FFAs, partly as a consequence of adipose tissue dysfunction and inflammation. In particular, dysregulation of FFA metabolism would be an essential cause of metabolic anomalies because a defect in their storage into adipocytes could lead to their ectopic depot in the liver, muscles, heart, and pancreas, where they play an important role in dyslipidemia, insulin resistance, and altered glucose tolerance (1). Recently, the antiretroviral drugs given to patients to control human immunodeficiency virus (HIV) infection were recognized as responsible for metabolic alterations and abnormal adipose tissue distribution, together with modifications in adipokines, cytokines, and FFAs, and with ectopic depots of lipids in nonfat tissues, arguing for mechanisms common to those reported in diabetes (2, 3).
We recently highlighted, in human adipose tissue, the importance of the metabolic pathway, glyceroneogenesis (GNG), which is able to limit FFA release to blood under physiological fasting situations and which is a new target of thiazolidinedione action (4–6). FFA re-esterification via GNG was first described by Ballard et al. (7) and Reshef et al. (8) and then was functionally identified as an important pathway for lipid homeostasis (reviewed in ref. 9). GNG is an abbreviated version of gluconeogenesis that provides glycerol-3-phosphate, synthesized mainly from pyruvate and lactate inside adipocytes to recycle into TG, the FFA excessively produced by lipolysis during fasting. White adipose tissue does not oxidize fatty acids for energy to any appreciable extent; it exhibits a negligible level of glycerol kinase activity and does not contain sufficient glycogen to supply the amount of glucose required to account for the glycerol-3-phosphate needed to re-esterify fatty acids to TG (10). Thus, the hydrolysis of 1 mol of TG (lipolysis) leads to 1 mol of glycerol and 3 mol of FFA, giving a theoretical FFA/glycerol ratio of 3; but, the simultaneous activation of GNG, which decreases FFA release without affecting that of glycerol, gives a FFA/glycerol ratio less than 3. We previously demonstrated, in human adipose tissue, that inhibiting cytosolic phosphoenolpyruvate carboxykinase (PEPCK-C) activity by mercaptopicolinate restored the FFA/glycerol ratio to 3 and, thus, confirmed that the decrease of the FFA/glycerol ratio to less than 3 is an index of GNG efficiency (4). GNG moderates FFA delivery during lipolysis situation and is thought to adjust the amounts of FFA released to meet the body's exact needs. Specific suppression of the PCK1 gene, which encodes its key enzyme (PEPCK-C) in adipose tissue resulted in mice with increased FFA release due to decreased GNG (11). Very recently, GNG was described as the predominant pathway for TG synthesis in rat adipose tissue, not only during fasting but also under high-glucose diet conditions (12). Our studies of the performance of GNG in human subcutaneous adipose tissue (SAT) revealed that GNG was inversely related to body mass index (BMI), suggesting the possibility it is involved in the increased systemic FFA level observed in overweight subjects (4).
Increased levels of FFA and proinflammatory cytokines have been reported in some HIV-infected patients under antiretroviral treatment (reviewed in reference 13). Increased FFA level has been linked to treatments that include protease inhibitors (PIs) and has also been associated with the occurrence of metabolic alterations, dyslipidemia, and insulin resistance (14–16). Even if the ability of individual molecules to induce these alterations varies according to the molecule, PIs were collectively found to increase total and LDL cholesterol and also TG (17–19). However, among PIs, some of them affect mainly lipid metabolism (e.g., lopinavir [LPV], ritonavir [RTV], and, to a lesser extent, nelfinavir [NFV]), while others, such as atazanavir (ATV), appear to be lipid-friendly (17, 18, 20–22). Additionally, some PIs were shown to directly affect adipose tissue lipid metabolism, even in the absence of an abnormal fat distribution. Indeed, studies performed in healthy volunteers revealed the ability of LPV/RTV to increase FFA levels after 5 days or 4 weeks, independently of an effect on insulin sensitivity or altered body fat (23, 24). Otherwise, in HIV-infected patients under PI therapy, stopping the PI resulted in decreased FFA levels without body fat modifications (15). Fat samples obtained from HIV-infected patients treated with a combination of antiretroviral drugs showed an increased rate of lipolysis (25, 26). However, the ability of different PIs to directly alter human adipose tissue lipid metabolism under fasting conditions has not been evaluated.
Due to the occurrence of lipoatrophy in a number of HIV-infected patients, resulting from thymidine analog reverse-transcriptase inhibitor (NRTI) toxicity (27), a number of studies have evaluated subcutaneous adipose tissue samples obtained from HIV-infected patients. A local inflammation was generally observed, with macrophage infiltration, increased expression of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), together with decreased expression of the main adipogenic genes (2, 13, 25, 27–33). This was associated with an increased level of systemic proinflammatory cytokines, such as IL-6 and TNF-α (2, 25, 32, 33), and of FFA (14, 16). The relative roles of the different antiretroviral drugs in that setting are difficult to decipher as these patients are generally taking a combination of NRTIs and PIs. We previously observed that stopping thymidine analog NRTIs in HIV-infected patients with or without lipoatrophy reduced fat inflammation (31). However, the specific role of each of the different classes of antiretroviral drugs is difficult to analyze (33). Therefore, we investigated whether some PIs were able to directly affect inflammation in human fat samples.
In the present study, we investigated whether a treatment with different PIs could lead to an alteration of GNG, lipolysis, and the inflammatory profile in human adipose tissue from lean subjects in as much as HIV-infected patients in France have a mean BMI of 22–23 kg/m2 (34). We evaluated four PIs associated to different extents with lipid alterations in HIV-infected patients for their effects on the lipolytic rate, FFA/TG cycling via GNG, and induction of inflammation in human adipose tissue explants. Because most systemic FFA comes from SAT metabolism in lean humans (35), we also investigated at the same time, and for the first time, samples of SAT and VAT from the same individual to test whether they were similarly affected.
Our data show that NFV, RTV, and LPV, but not the lipid-neutral PI ATV, led to increased FFA delivery from human SAT but not from VAT, as a consequence of both increased basal lipolysis and decreased GNG in SAT. As perilipin inhibits basal hydrolysis by adipocyte lipases of neutral lipids stored in the lipid droplet, we evaluated its amount and observed a decrease in parallel to the increase in basal lipolysis in response to NFV, RTV, and LPV. We also searched for a link between the depot-specific effects of PIs on GNG and the simultaneous secretion of some proinflammatory cytokines. We found that IL-6 and TNF-α secretion and gene expression were related to GNG inhibition induced by NFV, which was used as a representative of the dyslipidemic PIs. Indeed, the inhibitor of NFκB activation, parthenolide, corrected the NFV-induced adverse effects in SAT: it prevented the production of cytokines and restored FFA/TG cycling via GNG. Our data suggest that the inflammatory state of SAT induced by some PIs contributes to inhibition of the GNG pathway, leading to an increased FFA release. Considering the major contribution of SAT, versus that of VAT, for the control of FFA homeostasis, these data also highlight the importance of GNG in this process and suggest a greater susceptibility of SAT than VAT to PI-induced inflammation.
MATERIALS AND METHODS
Materials
Nelfinavir, lopinavir, ritonavir, and atazanavir were purchased from Santa Cruz Biotechnology, Santa Cruz, CA.
Culture of adipose tissue explants for monitoring lipolytic rate and fatty acid re-esterification
In the first set of experiments (described in legends to Figs. 1–4), samples of abdominal SAT and omental VAT were obtained from 16 healthy, young premenopausal women (age, 37.1 ± 7.0 years old) with BMI from 18.7 to 26.5 kg/m2 (average BMI = 22.8 ± 3.2 kg/m2) undergoing elective surgery at Cochin Hospital (Dr. C. Chapron) for benign noninfectious diseases: either for uterine myoma ablation or for exploratory laparotomy for endometriosis. Subjects were otherwise healthy and did not receive any medication or hormonal supplementation except for contraceptive pills. In the second set of experiments, SAT samples were obtained from 3 lean males undergoing surgery for benign biliary tract affection at St.-Antoine Hospital (Dr. O. Scatton). All subjects gave written informed consent, and all procedures were approved by the ethics committee of Cochin Hospital. Samples, removed at the beginning of the surgical procedure were immediately transferred to the laboratory in DMEM containing 10% fetal bovine serum and 12.5 mM glucose. The tissue was carefully dissected to remove visible blood vessels and connective tissue and then cut into small pieces of 20–35 mg wet weight, distributed in 12-well culture plates with about 400 mg/well, and incubated, in 2.5 ml of DMEM containing 10% fetal bovine serum and 12.5 mM glucose, biotin, pantothenate, and antibiotics as described previously (4) in a humidified atmosphere of 10% Co2 at 37°C. Medium was changed every day, and glucose concentration was decreased to 5 mM and supplemented with 10 µM of one of the following PI molecules: NFV, RTV, LPV, or ATV for 72 h. On day 3, overnight media samples were conserved for the determination of cytokines, and samples were incubated in glucose- and serum-free DMEM containing 3% (w:v) low-fat BSA for 4 h, then in 1 ml of Krebs-Ringer bicarbonate buffer containing 3% (w:v) fatty acid-free BSA and [14C1]pyruvate (1 µCi/ml) as precursor of glycerol-3-phosphate. The isotopic dilution of [14C1]pyruvate was about 1:130. After 1 h, the incubation medium was discarded for the estimation of lipolytic FFA and glycerol. Simultaneously, the corresponding tissue fragments were frozen in liquid nitrogen before lipid extraction according to the simplified method of Bligh and Dyer (36). The subsequent incorporation of [14C1]pyruvate into the lipid moiety was estimated by counting the radioactivity associated with this fraction and was used to appreciate the level of FFA that had been re-esterified during the 1 h lipolytic process. For each tissue sample, some explants were selected at day 3 for the determination of PEPCK-C activity and TG content.
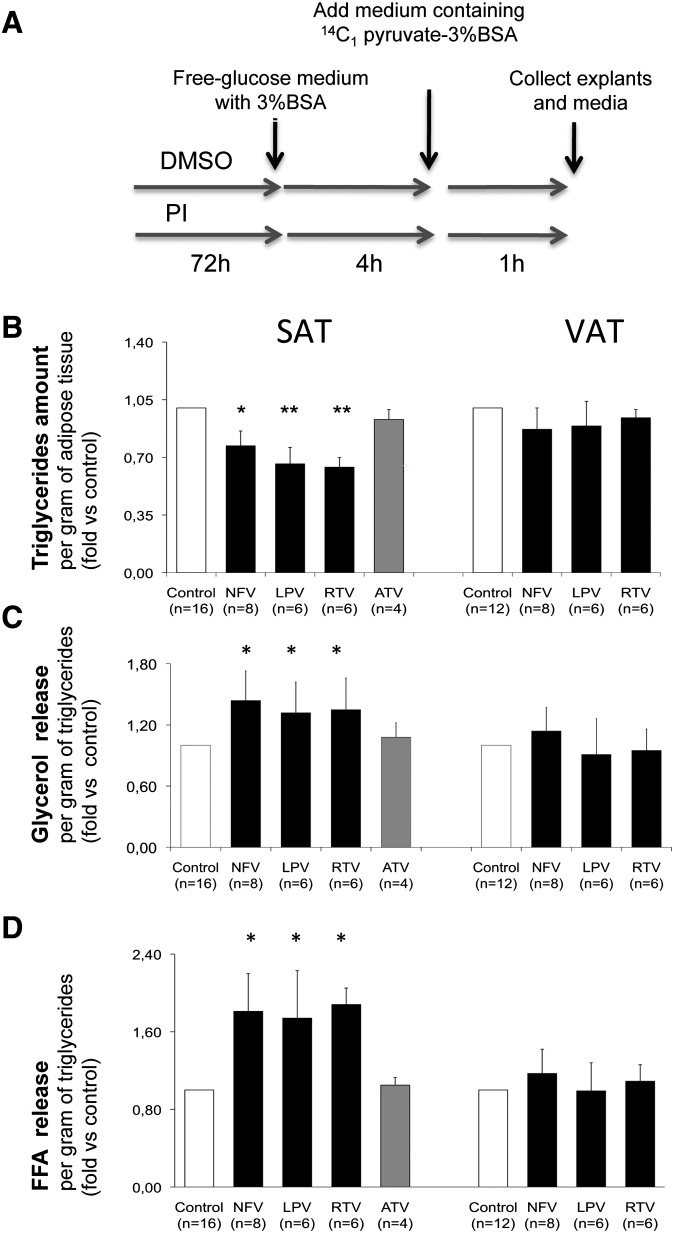
Fig. 1.
Effect of PIs on TG content and glycerol and FFA release in subcutaneous and visceral human fat explants. SAT and VAT fat pads were pretreated from day 1 to 3 with either ethanol (control) or NFV, 10 µM; LPV, 10 µM; RTV, 10 µM; and ATV, 10 µM as described in Material and Methods and according to the scheme presented in A. The number of subjects are shown in brackets, and all experiments were performed in duplicate. Bars represents means ± SD relative to respective controls; *, P < 0.05; and **, P < 0.01. B: Variation of TG content expressed per gram of tissue relative to respective controls values. C: Glycerol concentration was measured in the culture medium after a 1 h incubation in order to evaluate the lipolytic rate in response to antiretroviral agents in fat explants. Results are expressed per gram of TG and are relative to their respective control values. D: FFA release was simultaneously evaluated in the medium after 1 h of basal lipolysis in the presence of 2.5 mM pyruvate. Results are expressed per gram of TG and are relative to their respective control values.
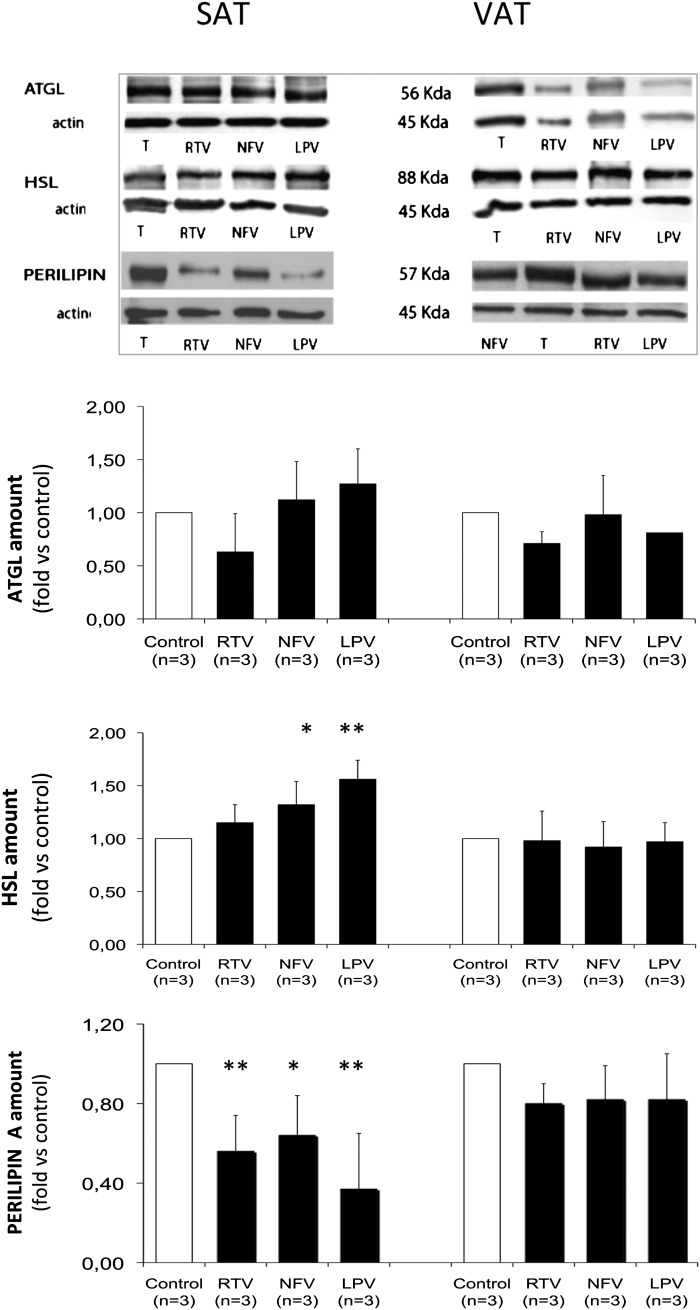
Fig. 2.
Effects of PIs on ATGL and HSL lipases and on perilipin protein levels in subcutaneous and visceral human fat pads. SAT and VAT fat pads were pretreated as shown in Fig. 1A. Upper panels: representative Western blots presented for each protein. Blotting was performed on whole fat explants after removing fat coat and fragments. Lower panels: Corresponding quantifications were performed versus β-actin amount and expressed in fold changes versus control. Data are means ± SD from three individuals; *, P < 0.05; and **, P < 0.01 versus control.
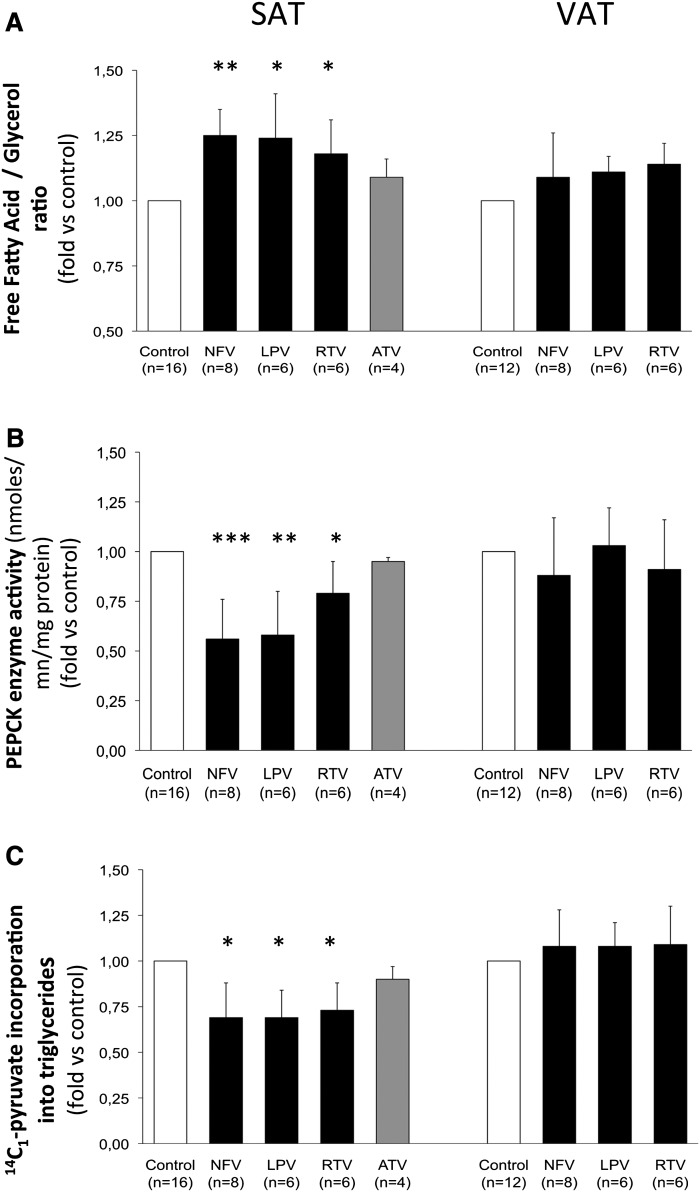
Fig. 3.
GNG-dependent re-esterification of FFA liberated during lipolysis in subcutaneous and visceral human fat pads. SAT and VAT fat pads were pretreated from day 1 to 3 with either ethanol (control) or NFV, 10 µM; LPV, 10 µM; RTV, 10 µM; and ATV, 10 µM, as described in Material and Methods and according to the scheme in Fig. 1A. The number of subjects are in brackets, and all experiments were performed in duplicate. Results are expressed as means ± SD and are relative to the level of their respective control, *, P < 0.05; **, P < 0.01; and ***, P < 0.001 versus control A. FFA/glycerol ratio was calculated from the determination of both parameters in the incubation medium of each fat explant. The GNG-dependent fatty acid re-esterification process decreases the FFA/glycerol ratio because it affects only one parameter, the FFA concentration. B: PEPCK-C activity was evaluated in human SAT and VAT cytosolic fractions in response to the different treatments. C: Evaluation of the GNG-dependent re-esterification of FFA liberated during lipolysis in fat pads incubated for 1 h in KRPB-BSA containing 2.5 mM pyruvate and 1 µCi 14C1-radiolabeled pyruvate. Lipids were then extracted, and radioactivity was measured as described in Materials and Methods.
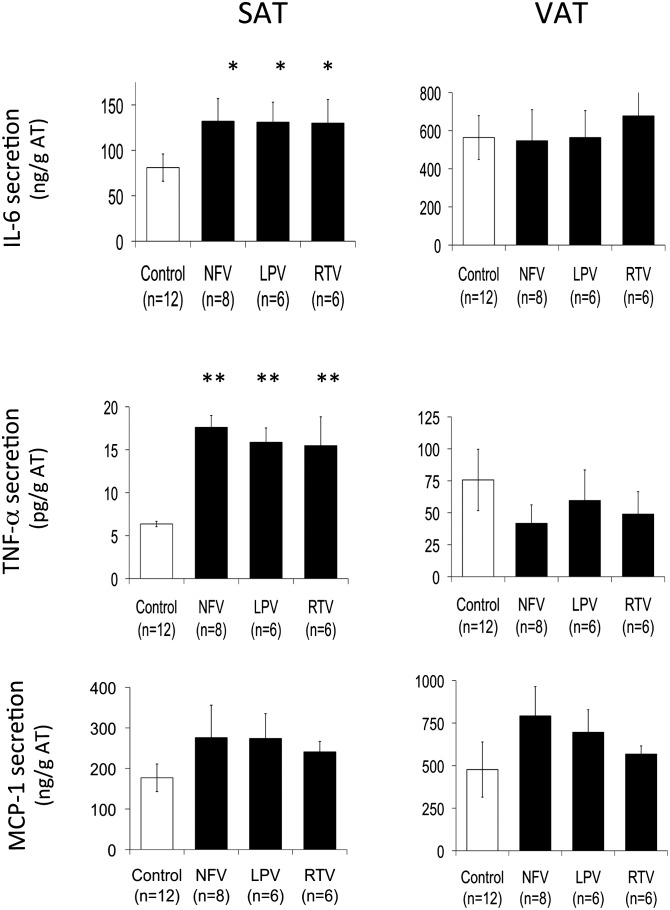
Fig. 4.
Effect of PIs on proinflammatory cytokines and chemokine secretion from human subcutaneous and visceral fat pads. SAT and VAT fat pads were pretreated from day 1 to 3 with either ethanol (control) or NFV, 10 µM; LPV, 10 µM; RTV, 10 µM; and ATV, 10 µM, as described in Material and Methods and according to the scheme in Fig. 1A. IL-6, TNF-α, and MCP1 secretion were measured in the last 18 h incubation medium. The number of subjects are in brackets, and results are expressed in nanograms or pg/g of tissue ± SD for each reproduced in duplicate; *, P < 0.05; and **, P < 0.01 versus control.
Culture of adipose tissue explants for gene expression analysis (single-dose NFV protocol)
SAT and VAT were obtained from 9 other women (average age = 38.6 ± 6.9 years old) with BMI from 20.5 to 24.2 kg/m2 (average BMI = 22.3 ± 1.5 kg/m2). Explants were prepared as described above but treated with a single NFV dose (10 μM) either during 14 h or 28 h (from day 1 to day 2), or at day 2 during 6 h incubation in DMEM, glucose 5 mM, and 0.5% FFA-free BSA. Media quantities and explants were used for cytokines determination and RNA isolation or PEPCK-C enzyme activity measurement. In addition, in the second set of experiments, the effects of ATV and NFV were evaluated in parallel on cytokine and PEPCK expression.
Determination of PEPCK-C-specific activity and biochemical analyses
PEPCK-C was estimated in a postmitochondrial fraction, prepared from adipose tissue previously homogenized in 10 mM Tris-HCl buffer, pH 7.4, containing 1 mM dithiothreitol. PEPCK-C activity was determined as described by Duff and Snell (37) by the acute radioactive method of Chang and Lane (38), with saturating substrate concentration and 1:30 isotope dilution of [14C]NaHCO3; the reaction being performed at 30 min at 37°C. Protein content was determined using a BCA protein assay kit from Sigma-Aldrich (Tafkirchen, Germany). Concentrations of glycerol and FFA released in the Krebs-Ringer bicarbonate buffer were monitored using free glycerol reagent (Sigma-Aldrich, Tafkirchen, Germany) and an acyl-CoA oxidase kit from Roche Applied Science (Meylan, France), respectively. The TG content of adipose tissue explants was determined with a kit (Sigma-Aldrich, Tafkirchen, Germany).
Levels of adipokines, cytokines, and chemokines secreted into the culture medium
Medium was collected at the time indicated in the figures legend and frozen in liquid nitrogen. Then, multiple cytokine/chemokines analysis kits (human adipocyte LINCOplex kit; HADCYT-61-K; Linco®) were used to quantify concentrations of IL-6, TNF-α, and monocyte chemoattractant protein 1 (MCP1) in the medium, Millipore multiscreen 96-well filtration plates (furnished) were used for the assays (Millipore, Billerica, MA). All samples were tested at least twice with a Bioplex 200 system (Bio-Rad, Marnes la Coquette, France). All analyses were performed according to the manufacturer's protocol. Dilutions of 1:50 and 1:500 were needed for quantification of MCP1 and IL-6 in SAT or VAT, respectively. TNF-α secretion did not require any dilution.
RNA and quantitative RT-PCR
RNA extraction was performed using an RNeasy Lipid kit (Qiagen, Courtaboeuf, France), and the extract's respective quality was assessed by gel electrophoresis. First-strand cDNA was synthesized using a High Capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA), and quantitative RT-PCR measurements using SYBR Green were carried out with a Roche thermocycler. mRNA variations were estimated relative to those of 18S rRNA. The primer sequence for hPCK1 Sense was 5′-GCTCTGAGGAGGAGAATGG-3′; 5′-TGCTCTTGGGTGACGATAAC-3′ for hPCK1 Antisense; 5′-CAAATTCGGTACATCCTCGAC-3′ for hIL6 Sense; 5′-TTTCACCAGGCAAGTCTCCT-3′ for hIL6 Antisense; 5′-AATAGGCTGTTCCCATGTAGC-3′ for hTNF-α Sense; 5′-AGAGGCTCAGCAATGAGTGA-3′ for hTNF-α Antisense; 5′-GAGCGAAAGCATTTGCCAAG-3′ for h18S Sense; and 5′-GGCATCGTTTAGGTCGGAA-3′ for h18S Antisense.
Western blotting
Adipose tissue was homogenized in Laemmli buffer (tissue lyzer; Qiagen, Courtaboeuf, France). Extracts were centrifuged first at 10,000 rpm, at 4°C, for 8 min to remove the fat coat, and then at 14,000 rpm, at 4°C, for 10 min to remove insoluble material; then supernatants were diluted by ultrasounds and immediately separated on an SDS-7.5% polyacrylamide gel and electroblotted. The nitrocellulose blot was incubated with antibodies against hormone-sensitive lipase (HSL) (Cell Signaling Technology, Boston, MA), adipose triacylglycerol lipase (ATGL) (Santa Cruz Biotechnology), and perilipin (Progen, GmbH, Heidelberg, Germany), nonspecific protein binding to the nitrocellulose being reduced by preincubation in blocking buffer (0.2% BSA, 10 mM Tris, 150 mM NaCl, and 0.02% Tween-20). After blots were incubated with a relevant second antibody, immunoreactive bands were visualized using enhanced chemiluminescence (catalog no. RPN22090L/AE) Western blot protocol (Amersham, GE Healthcare Europe, Saclay, France). Band intensities were quantitated by a Chimigenius (Amersham Bioscience, Pantin, France) and expressed versus corresponding β actin amount.
Statistical analysis
Data were analyzed by paired t-test. Significant differences were assumed for P values <0.05.
RESULTS
Comparison of the fatty acid/triglyceride cycling activities between human SAT and VAT
FFA/TG cycling represents the balance between lipolysis of adipose tissue TG and the simultaneous FFA re-esterification that occurs via GNG (39). We therefore measured both glycerol output (basal lipolytic index) and FFA output in the medium of fat pads from human SAT and VAT and then calculated the FFA/glycerol ratio as an index of GNG efficiency (Table 1). The lipolytic index was always higher in VAT than in SAT for a given subject, even if the mean values were not significantly different because of great interindividual variations. The absolute FFA output per gram of VAT was 2-fold higher than from SAT (P < 0.05). We confirmed that in SAT from lean women under basal conditions (4), the FFA amount actually released represented about one-third of what can be expected from the lipolytic index (as indicated by a FFA/glycerol ratio of 1.05 ± 0.46, instead of the expected value of 3), while the FFA output from VAT was about half of what can be expected (FFA/glycerol ratio of 1.57 ± 0.48).
Table 1.
Comparison of the lipolytic rate and FFA re-esterification via glyceroneogenesis between human subcutaneous (SAT) and visceral (VAT) adipose tissue.
| Human Fat Depot (n=12) | SAT | VAT |
| Glycerol output (G) in μmoles/g of AT/1h = lipolytic index | 0.44 ± 0.11 | 0.66 ± 0.23 |
| Fatty acid output (FFA) in μmoles/g of AT/1h | 0.46 ± 0.19 | 1.04 ± 0.34 |
| Free fatty acid versus glycerol output (FFA/G) in μmoles/g of AT/1h (without GNG, should be 3) | 1.05 ± 0.46 | 1.57 ± 0.48 |
| [14C1|-pyruvate incorporation into triglycerides in nmoles/g of AT/1h = FFA re-esterification via GNG | 5.60 ± 3.29 | 8.11 ± 2.86 |
| PEPCK-C specific activity in nmoles/mn/mg protein | 0.67 ± 0.21 | 1.35 ± 0.64 |
Glycerol and FFA release were measured in the culture medium and FFA re-esterification via glyceroneogenesis was appreciated by measuring the [14C1|-pyruvate incorporation into triglycerides and the PEPCK-C enzyme activity in subcutaneous and visceral fat explants from 12 lean women. The results are expressed as mean ± SEM. *(p < 0.05) between SAT and VAT mean values.
We also directly assessed the GNG pathway by simultaneously measuring the incorporation of [14C1]pyruvate into TG and PEPCK-C-specific activity in both fat depots. The rates of GNG-dependent FFA re-esterification and PEPCK-C-specific activity tended to be higher in VAT than in SAT (Table 1).
Effect of PIs on the lipolytic rate (glycerol and FFA output) of human SAT and VAT
In the first set of experiments, human adipose tissue explants from two fat depots were pretreated for 72 h with NFV, RTV, or LPV and then were glucose-depleted according to the scheme described in the legend to Fig. 1A. In the second set of experiments, human SAT explants were treated with NFV and ATV. We decided to maintain maximum concentrations (Cmax) (10 μm) over 3 days, which is different from the in vivo situation. Indeed, we wanted to sum up, in a short period of time, the adverse effects of drugs observed in patients after a longer-term treatment. We have used these Cmax concentrations in our previous studies. We have previously addressed the point related to PI concentrations in culture medium by evaluation of the level of bound NFV under culture conditions and observed that the unbound concentration of NFV was 1% of the total concentration (40). This value is close to those reported for sera concentrations of patients treated with NFV and those treated with LPV and RTV.
Because some PIs have been shown to decrease the TG content of human mature subcutaneous adipocytes (41), likely by an inhibition of glucose transport via GLUT4 (42), we checked the TG content of fat explants after PI treatment. Figure 1B shows that NFV, RTV, and LPV decreased (by about 25% to 30%) the TG content of SAT but not VAT. Because adipose tissue lipolysis is known to be correlated with adipocyte size (43), we expressed the lipolytic rate of fat depots versus their respective TG content after PI treatment; data revealed that these three PIs increased lipolysis (by about 35%), as indicated by the release of glycerol, but only in SAT (Fig. 1C). In parallel, FFA release was also increased by these three PIs (Fig. 1D) but at a higher level (by about 80%) than expected from glycerol release. The effect of these PIs on acute lipolysis (after 1 h incubation with 1 μM isoproterenol) was also tested in some individuals, and the same depot-specific difference was observed (data not shown). To determine whether the effects of these three PIs was a class effect or whether they were related to their ability to induce dyslipidemia in HIV-infected patients, we then compared the effect of NFV with that of ATV, a lipid-friendly PI, in SAT. Results shown in Fig. 1B–D indicate that ATV did not affect TG content, glycerol, or FFA release in contrast to NFV.
To try to understand the different response to PI-induced lipolysis of the two fat depots, we analyzed the protein content of the two major lipases ATGL and HSL (Fig. 2). The amount of ATGL did not significantly differ between the two depots (possibly due to a high interindividual variation), while a treatment with PIs mildly increased the amount of HSL protein in SAT but not VAT. The proteolysis of perilipin has been proposed as the mechanism for enhanced basal lipolysis in response to NFV in murine adipocytes (44) and was recently found to be more robust in adipocyte cell lines derived from mice SAT than VAT (45). Therefore, we also evaluated the perilipin amount and, interestingly, observed that perilipin was decreased by NFV, RTV, and LPV in human SAT but not VAT (Fig. 2). The parallel and inverse variations in HSL and perilipin observed after a treatment with these PIs in SAT offers a rationale for increased lipolysis induced by these drugs.
Thus, as expected from in vivo studies, our data revealed that NFV, RTV, and LPV, but not ATV, were able to increase basal lipolysis in starved adipose tissue from lean subjects. In addition, we show for the first time that this effect is far more pronounced in SAT, perhaps as a result of a PI-induced decreased perilipin amount.
Moreover, the FFA release is increased by 81% ± 7% (Fig. 1D), which is double the %37 ± 6% expected from lipolysis (Fig. 1C). This could suggest that NFV, RTV, and LPV but not ATV induced in SAT an additional defect at the level of FFA re-esterification by GNG.
Effect of PIs on FFA re-esterification via GNG and PEPCK-C activity
To test the possible involvement of GNG in this process, we first calculated the FFA versus glycerol release amounts from SAT and VAT from each subject. NFV, RTV, and LPV but not ATV significantly increased the FFA/glycerol ratio in SAT; importantly, this PI-specific effect was not found in VAT (Fig. 3A). This suggested an impairment of GNG that was investigated by measuring the incorporation of [14C1]pyruvate into the glycerol-3-phosphate backbone of the main substrate of this pathway, into TG of both depots pretreated or not with PIs. The experiments were performed for 1 h during basal lipolysis, and the level of FFA being re-esterified with [14C1]pyruvate was evaluated. We actually found that NFV, RTV, and LPV but not ATV significantly decreased (by about 30%) the GNG-dependent FFA re-esterification process in SAT, without any significant effect of NFV, LPV, and RTV in VAT (Fig. 3C). We verified that these PIs did not modify the intracellular pool of pyruvate. These results strongly suggest that about half of the PI-induced FFA release from SAT was due to a decrease in their re-esterification capacities via GNG in this specific fat depot. We then investigated whether this defect of GNG was related to a direct effect on its key enzyme and found that NFV, RTV, and LPV but not ATV significantly decreased PEPCK-C activity (by 25% to 45%) only in SAT (Fig. 3B).
Our data show, for the first time, that i) the dyslipidemic PIs NFV, RTV, and LPV alter FFA re-esterification via GNG in human adipose tissue, whereas lipid-friendly ATV did not, and ii) that this effect takes place specifically in SAT via a significant decrease of the GNG key enzyme activity and efficiency, thus contributing to an excessive release of FFA from SAT and not from VAT in the situation of basal lipolysis.
Effects of PIs on adipose tissue inflammation
We then decided to determine if this PI effect on GNG affecting SAT was related to a depot-specific inflammatory process by evaluating the ability of these PIs to modify the secretion of proinflammatory cytokines. First, a comparison of the secretion profiles of the two fat depots confirmed data previously reported in the literature (46), i.e., MCP1 and IL-6 are secreted in 2.5- and 6-fold higher amounts, respectively, by VAT than SAT (Fig. 4). As to TNF-α secretion, we found, as expected, a very low level of secretion (10,000 times less than IL-6). Moreover, VAT from lean individuals released 10-fold more TNF-α than SAT (Fig. 4).
We then analyzed the effect of PIs on cytokine secretion by the two depots. IL-6 secretion from SAT was enhanced by treatment with NFV, LPV, and RTV (1.6×) but not from VAT (Fig. 4, upper panels). Accordingly, TNF-α secretion was 2.5-fold increased by the PI-treatment in SAT, whereas it was not significantly changed in VAT. In contrast, these PIs tended to increase MCP1 secretion in both depots (Fig. 4, lower panels). Our results suggest that SAT exhibited a lower inflammation state in the basal situation than VAT and was more responsive than VAT to a treatment with PIs as regards the secretion of proinflammatory cytokines IL-6 and TNF-α.
NFV but not ATV adversely affects IL-6 and PEPCK-C gene expression in SAT
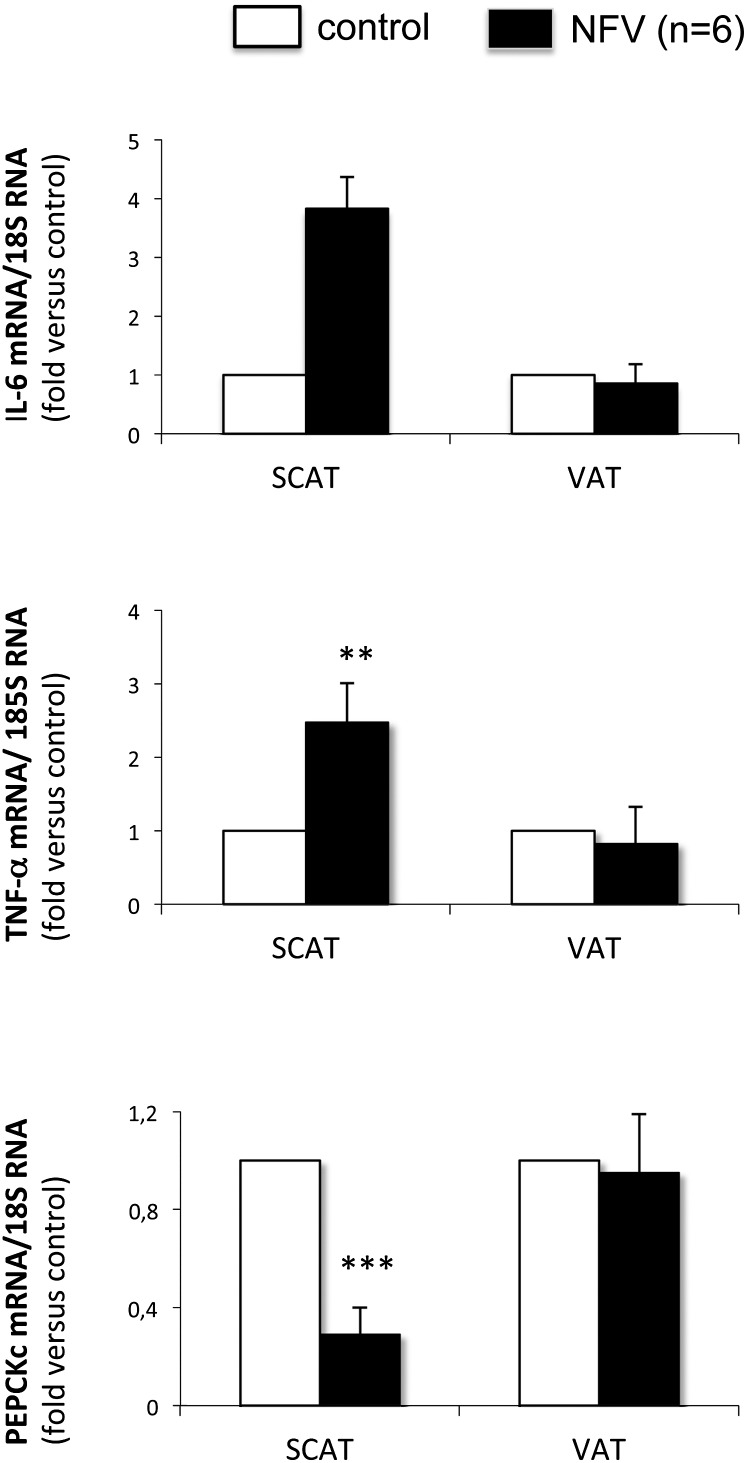
We further checked the effect of NFV, taken as a representative of the dyslipidemic PIs, on IL-6 and TNF-α gene expressions in human SAT and VAT under short-term conditions. Time-course studies revealed that the maximal effect was obtained after 6 h but persisted up to 14 h. Our data confirm that the levels of IL-6 and TNF-α mRNA were specifically increased (by 3.8- and 2.5-fold, respectively) in SAT in response to a treatment with NFV, while this treatment did not alter their mRNA levels in VAT (Fig. 5). The analysis, in parallel, of the PEPCK-C gene expression showed that its mRNA amount was markedly affected in SAT (70% decrease at 14 h after a treatment with NFV) but not VAT (Fig. 5, lower panel). Importantly, we did not observe any significant effect of ATV on amounts of IL-6 and PEPCK-C mRNA (data not shown). Thus, our data revealed that NFV concordantly increased IL-6 and TNF-α expression and decreased that of PEPCK-C in SAT but not in VAT, while ATV did not exert these adverse effects in SAT.
Fig. 5.
Effect of NFV on IL-6, TNF-α, and PEPCK-C gene expression in human subcutaneous and visceral adipose tissue. Fat pads were treated for 14 h in DMEM, glucose 5 mM, 0.5% low-fat BSA. mRNA concentrations were analyzed by quantitative RT-PCR, normalized to 18S rRNA, and expressed relative to their respective controls. Amounts of IL-6, TNF-α, and PEPCK-C mRNA were about 64, 4, and 2 higher in VAT than in SAT. Data are means ± SD from six individuals. **, P < 0.01; and ***, P < 0.001 versus control.
Inhibition of the NFκB inflammatory pathway prevented NFV-induced inflammation and restored PEPCK-C expression and enzyme activity in human SAT
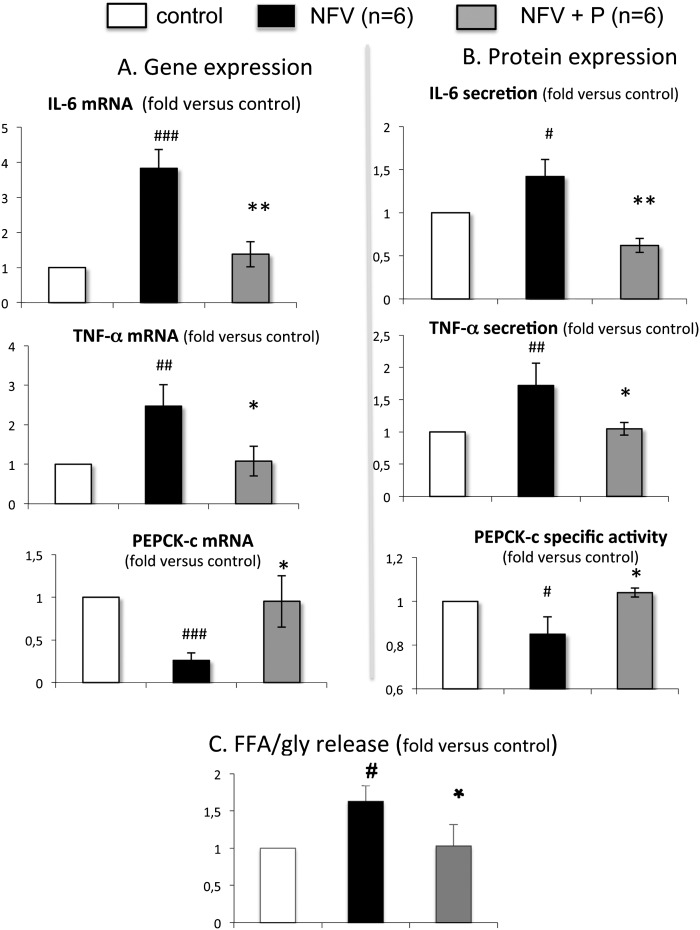
To get further insight into the NFV-adverse effects on human SAT and to investigate whether the NFV opposite effects on IL-6, TNF-α, and PEPCK-C gene expression were linked, we used parthenolide, an anti-inflammatory drug able to specifically inhibit the NFκB pathway and tested its ability to prevent NFV-induced effects. To that end, we treated SAT explants with parthenolide 1 h before NFV addition; then, after 6 h or 14 h incubation, IL-6, TNF-α, or PEPCK-C mRNA was quantified. Our data clearly demonstrated that parthenolide was able to significantly prevent the NFV-dependent increase in IL-6 and TNF-α mRNA levels in SAT, while the level of PEPCK-C mRNA was restored to the control level (Fig. 6A).
Fig. 6.
Effect of the inhibitor of NFκB activation, parthenolide, on NFV-induced inflammation and NFV-altered GNG in human subcutaneous adipose tissue. Parthenolide (P), 30 μM, was added 1 h before NFV, 10 µM. mRNA concentrations were analyzed by quantitative RT-PCR, normalized to 18S rRNA, and expressed relative to their respective control values. Cytokine secretion and PEPCK-C enzyme activity levels were measured as in Materials and Methods. Data are means ± SD from six individuals. #, P < 0.05; ##, P < 0.01; ###, P < 0.001 NFV versus control and *, P < 0.05; and **, P < 0.01 NFV+P versus NFV. A: Analysis of the mRNA amounts (duration of the treatment was 6 h for IL-6 and TNF-α and 18 h for PEPCK-C gene expression analysis). B: Measure of secretion of cytokines in culture medium and PEPCK-C enzyme activity in fat pads after 28 h. C: Measure of glycerol and FFA in the culture medium after a 2 h period of isoproterenol-induced lipolysis.
We also checked whether the NFV-induced variation of gene expression was associated with a variation in the level of the cytokine secretion in the medium or of PEPCK-C enzyme activity. To that end, we performed similar experiments but with a longer incubation time (28 h) and measured cytokine, glycerol, and FFA secretion in the culture medium and PEPCK-C enzyme activity in the explants. Results shown in Fig. 6B indicate that parthenolide prevented NFV-induced IL-6 and TNF-α secretion and simultaneously rescued PEPCK-C enzyme activity in SAT. In parallel, the NFV-induced increase of FFA/glycerol ratio (1.63×, P < 0.05 versus control) was prevented when parthenolide was added together with NFV (1.03×, P < 0.05 versus NFV) (Fig. 6C). Thus, our data indicate that NFV was able to activate the NFκB inflammatory pathway, resulting in increased IL-6 and TNF-α gene expression and secretion and in decreased GNG. Such modifications were identified in SAT but not VAT (data not shown).
IL-6 might mediate NFV effect, resulting in repression of PEPCK-C activity in SAT
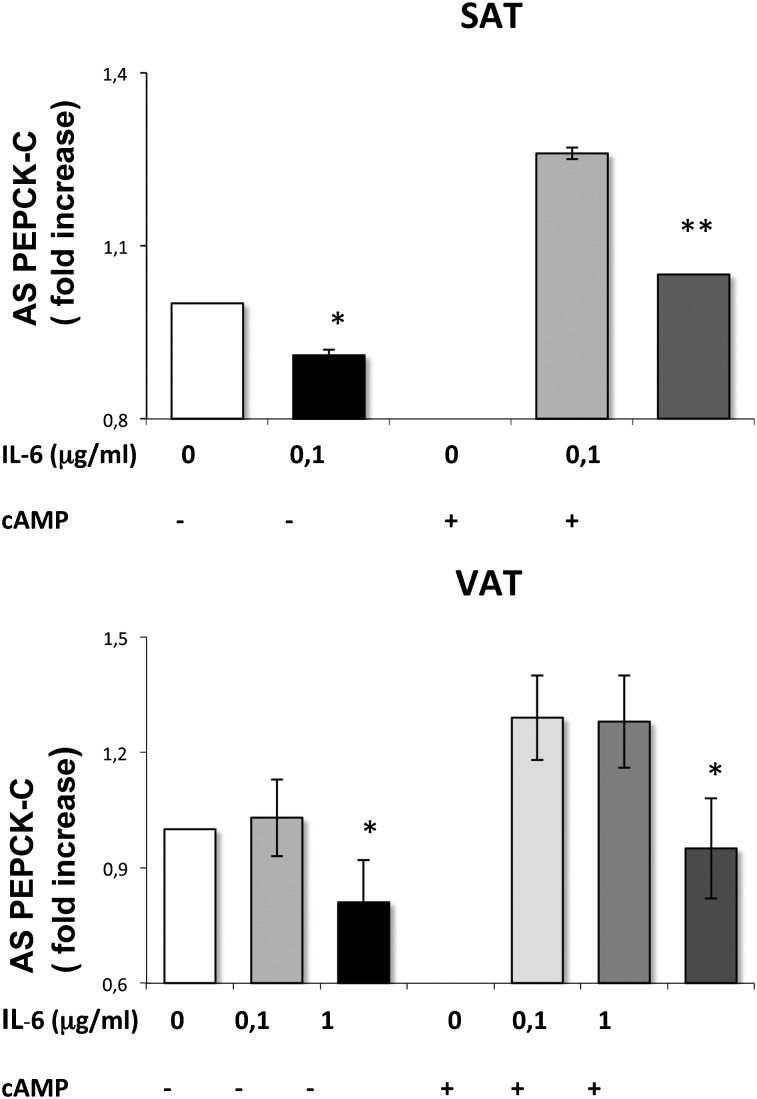
Since the inhibitory effect of NFV on PEPCK-C was prevented when the NFκB pathway was inhibited, we wondered whether the amount of IL-6 secreted by adipose tissue in response to NFV could mediate its effect on PEPCK-C activity in human SAT. We did not check the effect of TNF-α due to the very low expression (pg/ml) in human adipose tissue (cf. Fig. 4); dose amounts previously reported to exert an effect in vitro were on the order of nanograms (47). We incubated SAT and VAT explants with purified human IL-6 for 24 h in the presence or absence of cAMP (0.5 mM), since it has been previously shown that NFκB activation inhibited PEPCK-C transcription induced by cAMP in the liver (48). Our data show that the addition of 0.1μg of IL-6/ml in the culture medium significantly inhibited PEPCK-C enzyme activity in SAT but not VAT (Fig. 7), the inhibition being more marked in the presence of cAMP (−17%) than in its absence (−10%). Of importance, the dose of IL-6 (0.1μg/ml) added to the culture medium was chosen to double the average IL-6 secretion from untreated SAT in 24 h and to mimic the IL-6 amount secreted after NFV treatment (which increased by about 1.6-fold in SAT). In contrast, such a dose of IL-6 is lower than the average IL-6 secretion of untreated VAT (0.570 μg/ml). Thus, we investigated the effect of a higher dose of IL-6 (1 μg/ml) on VAT (which doubled the average IL-6 secretion from VAT). We found that the higher dose of IL-6 was able to significantly decrease PEPCK-C activity both in the absence and presence of cAMP in VAT (Fig. 7). Therefore, our results show that IL-6 is able to decrease PEPCK-C activity in both fat depots but with different sensitivities. These data suggest that the NFV-induced IL-6 production in SAT could be involved in the observed inhibition of PEPCK-C in this fat depot.
Fig. 7.
Effect of purified human IL-6 on basal and cAMP-induced PEPCK-C enzyme activity in subcutaneous and visceral adipose tissue. Fat pads were treated with 0.1 and/or 1μg/ml IL-6 over 24 h in the presence (or not) of D-butyril cAMP (0.5 mM), which was added 1 h after IL-6 and then homogenized for measurement of PEPCK-C enzyme activity. Each value represents the mean of duplicate determinations from three different explants and were expressed in fold changes versus their respective control values. *, P < 0.05; and **, P < 0.01 versus control.
DISCUSSION
The main finding of this work is the observation, for the first time in human fat, of depot-specific alteration in FFA release induced by some PIs with known in vivo side-effects on lipid metabolism but not by a lipid-neutral PI. When tested ex vivo on adipose tissue explants from lean subjects, the PIs NFV, LPV, and RTV enhanced FFA release from human SAT, but not the VAT depot, by simultaneously increasing lipolysis and decreasing FFA re-esterification via GNG, while ATV was devoid of any effect in that setting. These depot-specific disorders were found to be parallel to the depot-specific decrease of perilipin content in the case of lipolysis and to be linked to a higher inflammatory response in the case of GNG. Since most systemic FFA come from the subcutaneous fat depot in lean humans (35), such new data highlight the functional importance of GNG, particularly in SAT, for maintaining FFA homeostasis, and confirm the different metabolic behaviors of the two major fat depots in human.
The presence of an increased glycerol turnover has been previously reported in fasted HIV-infected patients in PI therapy, leading to increased circulating levels of FFA (15, 16). A role for PI in that setting was suggested by van der Walk et al. (15), as stopping PI allowed a reduced level of lipolysis. Those patients were generally diagnosed with lipodystrophy. However, stopping PI in those patients reduced lipolysis without modifications in fat distribution (15). In addition, in healthy volunteers, the association of LPV-RTV was able to increase FFA levels after a short-time treatment without altered fat distribution (23, 24). Therefore, the effect of PIs on adipose tissue appears to be independent of the presence or absence of lipodystrophy. In vitro studies performed with cultured adipocytes revealed the ability of some PIs to increase FFA release, arguing for increased lipolysis (49, 50). Here, we propose, for the first time, that the increased lipolysis induced by some PIs likely concerns human SAT.
In murine adipocytes, PI-induced increased lipolysis was not linked to PKA activation, to modulation of cAMP content, or to an alteration of the mitogen-activated protein kinase kinase pathway (50). It was assigned to a decrease in perilipin (44) that was found to be even more robust in murine adipocyte cell lines derived from SAT than from VAT (45). We confirm here that the perilipin amount was also more decreased by some PIs in human SAT than in VAT, which could explain, at least in part, the PI-induced lipolysis in this fat depot.
Lipolysis is governed by ATGL and HSL, which act sequentially, even in basal lipolysis, and represent 95% of the TG hydrolase activity in adipocytes (51). We report here a moderate PI-induced increase in HSL amount, restricted to SAT, while there were no significant changes in ATGL amounts in human SAT and VAT. In contrast, two recent reports of the effects of RTV and NFV on a murine adipocyte cell line showed that HSL content was not modified by these two PIs (52, 53), while ATGL content was increased by RTV (53). These discrepancies could be due to species differences, use of cell lines versus explants, and also to the length of the RTV treatment, performed over the entire differentiation process in adipocytes.
A study performed in a murine adipocyte cell line revealed a disproportion between FFA and glycerol releases from NFV-treated adipocytes (52), suggesting an additional alteration of FFA re-esterification via GNG. We show here, for the first time, that the FFA/TG cycle mediated by GNG was inhibited by three PIs in human SAT but not VAT. A recent report performed with a murine adipocyte cell line also described such RTV-induced decrease of pyruvate use for FA re-esterification into TG (53). In parallel, these authors observed a RTV-induced increased glycerol utilization for TG backbone but suggested, referring to our previous work (4), that this process could be of minor importance in human SAT, where glycerol phosphate formation by glycerol kinase accounts for only 14% of the total FFA re-esterified. We confirm this hypothesis (the negligible importance of glycerol kinase for glycerol-phosphate formation in response to PIs) because we were not able to find any significant PI-induced variation of glycerol use for TG backbone in human adipose tissues (results not shown).
Thus, our data suggest that NFV, RTV, and LPV exerted on human SAT a combination of increased lipolysis and decreased FFA re-esterification, potentially exacerbating FFA delivery into the circulation under fasting conditions. Furthermore, the alteration of lipolysis and GNG only concerned SAT and not VAT, suggesting a specific deleterious impact of these PIs on lipid metabolism from the peripheral fat depot. Only one study previously investigated the effect of PIs on the lipid-buffering function of human adipose tissue. This work compared the effects of some PIs on human SAT and VAT samples from the same subjects but was performed under feeding conditions. The authors found a specific alteration of the capacity of FFA storage into SAT (54). This could be due to the ability of some PIs to inhibit GLUT4 transporter and therefore glucose entrance and resulting lipogenesis (55). In this regard, we also observed that the PI-induced decrease of TG content was restricted to SAT under our experimental conditions. Combined with our results obtained under fasting conditions, these data suggest that some PIs are able to induce disturbances in the total lipid-buffering capacity of human SAT under both fasting and feeding conditions. The in vivo relevance of this hypothesis is reinforced by our observation that a lipid-friendly PI, ATV, did not alter these functions in human SAT. Thus, our data suggest that some PIs could alter SAT lipid metabolism and increase systemic FFA level, as observed in some HIV-infected patients taking antiretroviral therapies, therefore leading to lipotoxicity and metabolic complications as systemic FFA come mainly from SAT in lean humans (35).
However, our ex vivo results could differ from the in vivo situation, and, probably, the simple model tested here may not be as complex as the real in vivo situation. HIV patients generally receive a combination of antiretroviral drugs, which could exert potential additive side-effects. Moreover, lipid alterations observed in HIV-infected patients could result from the effect of PIs on different tissues. Our study has several limitations. We have studied adipose tissue from non-HIV-infected subjects. Therefore, we have not addressed a possible role for HIV infection. The drugs were used at a single concentration (corresponding to their Cmax in the patients) and added separately, whereas they are given in combination in HIV-infected patients. RTV is now given as a boosting molecule, and it would be important to evaluate its effect on GNG at lower concentrations. Finally, our biopsy samples are mostly from female subjects. Even if lipid alterations from PI effects are frequent in HIV-infected women (18), it is possible that some alterations at the level of adipose tissue are different in males. Therefore, we compared the effect of NFV in three SAT explants issued from lean men. We found that NFV exerted similar side-effects on FFA release via altered lipolysis and GNG similar to samples issued from women (results not shown).
Importantly, the use of these drugs as tools to better understand adipocyte biology allowed us to discover that decreased FFA re-esterification via GNG was associated with increased IL-6 and TNF-α production, both alterations being reverted when the NFκB pathway was inhibited. Therefore, our data suggest that NFV, LPV, and RTV were able to activate some proinflammatory pathways in human SAT only and raised the intriguing question why VAT was less sensitive to PI-induced alteration than SAT. This may be related not only to the basal metabolic differences between adipocytes from the two localizations (56) but also to the in situ presence of a different number of macrophages exhibiting various phenotypes (M1, proinflammatory or M2). The direct deleterious effects of some PIs on adipose tissue cells have been demonstrated in vitro by studies performed with either adipocyte or macrophage, two cell types associated in adipose tissue. Studies using murine adipocyte cell models revealed the toxicity of some PIs on adipocyte differentiation and metabolism (40, 57). In human adipocytes derived from SAT (41, 58) and in human macrophages (41), some PIs increased secretion of chemokines and proinflammatory cytokines and induced an oxidative stress in the two cell types and decreased adiponectin and leptin release from adipocytes (41). A unique recent study compared the effect of NFV on immortalized rat preadipocyte cell lines isolated from intra-abdominal (epididymal) or subcutaneous (inguinal) fat depots (45). In that study, the authors actually observed a higher sensitivity of adipocytes from SAT to NFV action, despite similar intracellular concentrations of NFV. It has also been reported that VAT adipocytes would have a greater number of mitochondria and better detoxification enzyme equipment, leading to a decreased sensitivity to the oxidative stress produced by some PIs (59).
Comparison of our results obtained from human SAT explants with a preserved tissue architecture, and which contain the different adipose cell types, with those observed in human adipocytes isolated from SAT (41) could help to decipher the respective role of adipocytes versus other cell types. The tested PIs identically provoked a decrease of leptin (results not shown) and an increase of IL-6 secretion in SAT as well as in adipocytes isolated from human SAT (41). However, as we also observed NFV-induced increased TNF-α gene expression in SAT that was not observed in isolated adipocytes (41), this suggests that this PI-induced inflammatory response takes place mainly in macrophages. Importantly, 50% to 90% of the IL-6 secreted by SAT is produced by macrophages (46, 60). This suggests that the adipose tissue macrophages could be an important target of PI action and that, possibly, the different sensitivity levels to PI we observed between SAT and VAT could come from differences in their resident macrophages, which would vary in number and phenotypes according to the fat depot as previously shown (60–62). Accordingly, it was shown that human SAT was more sensitive to minor variations of TNF-α concentration than VAT (63), perhaps in relation to a significantly higher expression of TNF-R1 (64).
By exploring the mechanism of the selective PEPCK-C inhibition by PIs in SAT, we found that it was related to the activation of the NFκB pathway, since both the increases of IL-6 and TNF-α and the decrease of PEPCK-C gene expression were corrected by parthenolide, an inhibitor of IKKβ phosphorylation. IL-6, TNF-α, and more generally LPS-induced inflammation have been shown to downregulate cAMP-induced PEPCK-C gene transcription by activating the NFκB pathway in hepatocytes (65, 66). Thus, we tested the direct effect of purified IL-6 on SAT and VAT explants (TNF-α was not evaluated because of its very low production). We observed that IL-6 was able to inhibit both basal and cAMP-induced PEPCK-C activity at the concentration of 0.1 μg/ml in human SAT, while a higher dose (1 μg/ml) was required in VAT. Therefore, our results show that IL-6 is able to decrease PEPCK-C activity in both fat depots but only when the amount added doubles their respective basal secretion. These data suggest that NFV-induced IL-6 production in SAT could be involved in the observed inhibition of PEPCK-C in this fat depot, whereas the absence of an effect on PEPCK-C in VAT is likely in relation to the absence of NFV-induced IL-6 production. Data also suggest that PEPCK-C in VAT is less responsive to IL-6, probably due to its higher basal release.
The in vivo relevance of our findings that IL-6 is a negative modulator of GNG, which could have repercussions on the size of adipose tissue, is reinforced by the phenotype of IL-6−/− mice, which develop mature-onset obesity with an increase of all fat pads (but with a higher increase in the subcutaneous fat depot) (67). Similarly, TNF-α−/− mice are obese and have lower levels of systemic FFA (68). In humans, under noninflammatory conditions, IL-6 is secreted to a significant extent by adipose tissue (69) and could be a physiologically negative regulator of PCK1 and, thus, of GNG. IL-6 plasma concentration is increased in HIV-infected patients in accordance with its increased expression in SAT (29) and, in obese subjects, proportionately to the fat mass (70). This could be related to adipose tissue inflammation in both situations. As increased IL-6 plasma levels are implicated in hyperlipidemia (71, 72), we hypothesize that these alterations could be at least in part related to the inhibition of FFA re-esterification via GNG in both situations.
Finally, our data argue for the functional importance of GNG in human FFA homeostasis. Nye et al. (9) suggested that the lipolytic process could induce the release of more FFA than is essential for immediate energy needs and that the simultaneous re-esterification of some of these FFA could participate in fine-tuning the whole process. Our data suggest that inflammatory processes could mediate inhibition of PEPCK-C expression in adipose tissue, particularly in SAT. Obesity is associated with a low-grade inflammation of adipose tissue (73), as observed in adipose tissue from HIV-infected patients. Android obesity and PI-treated HIV-infected patients often present the similarity of an increased VAT. In this regard, given the known association between visceral fat accumulation and metabolic alterations, it is tempting to hypothesize that VAT could perhaps became hypertrophied in some pathological situations by taking up the excess of FFA released from SAT.
Acknowledgments
The authors thank the surgery teams of Drs. Chapron, Soubrane, and Scatton for providing tissue samples and the patients for their kind agreement. The authors also thank M. Caron-Debarle and M. Auclair for constant and kind help in the laboratory, Nadège Brunel, IFR 65 plateforme microdosages, for cytokine determination, and Dr. J. A. Boutin for support and help with the manuscript.
Footnotes
Abbreviations:
- ATGL
- adipose triacylglycerol lipase
- Cmax
- maximum concentrations
- HIV
- human immunodeficiency virus
- HSL
- hormone-sensitive lipase
- MCP1
- monocyte chemoattractant protein 1
- PEPCK-C
- cytosolic phosphoenolpyruvate carboxykinase
- PI
- protease inhibitor
- SAT
- subcutaneous adipose tissue
- VAT
- visceral adipose tissue
This work was supported by INSERM, Agence Nationale pour la recherche sur le Sida et les hépatites virales (ANRS), SIDACTION, and la Fondation de France. S. L. received a fellowship from ANRS, C. V. from DRASS, and S. K. from MESR.
REFERENCES
- 1.Savage D. B., Petersen K. F., Shulman G. I. 2007. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol. Rev. 87: 504–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sevastianova K., Sutinen J., Kannisto K., Hamsten A., Ristola M., Yvi-järvinen H. 2008. Adipose tissue inflammation and liver fat in patients with highly active antiretroviral therapy-associated lipodystrophy. Am. J. Physiol. Endocrinol. Metab. 295: E85–E91. [DOI] [PubMed] [Google Scholar]
- 3.Gan S. K., Samaras K., Thompson C. H., Kraegen E. W., Carr A., Cooper D. A., Chisholm D. J. 2002. Altered myocellular and abdominal fat partioning predict disturbance in insulin action in HIV protease inhibitor-related lipodystrophy. Diabetes. 51: 3163–3169. [DOI] [PubMed] [Google Scholar]
- 4.Leroyer S. N., Tordjman J., Chauvet G., Quette J., Chapron C., Forest C., Antoine B. 2006. Rosiglitazone controls fatty acid cycling in human adipose tissue by means of glyceroneogenesis and glycerol phosphorylation. J. Biol. Chem. 281: 13141–13149. [DOI] [PubMed] [Google Scholar]
- 5.Tordjman J., Chauvet G., Quette J., Beale E. G., Forest C., Antoine B. 2003. Thiazolidinediones block fatty acid release by inducing glyceroneogenesis in fat cells. J. Biol. Chem. 278: 18785–18790. [DOI] [PubMed] [Google Scholar]
- 6.Tordjman J., Leroyer S., Chauvet G., Quette J., Chauvet C., Tomkiewicz C., Chapron C., Barouki R., Forest C., Aggerbeck M., et al. 2007. Cytosolic aspartate aminotransferase, a new partner in adipocyte glyceroneogenesis and an atypical target of thiazolidinedione. J. Biol. Chem. 282: 23591–23602. [DOI] [PubMed] [Google Scholar]
- 7.Ballard F. J., Hanson R. W., Leveille G. A. 1967. Phosphoenolpyruvate carboxykinase and the synthesis of glyceride-glycerol from pyruvate in adipose tissue. J. Biol. Chem. 242: 2746–2750. [PubMed] [Google Scholar]
- 8.Reshef L., Hanson R. W., Ballard F. J. 1970. A possible physiological role for glyceroneogenesis in rat adipose tissue. J. Biol. Chem. 245: 5979–5984. [PubMed] [Google Scholar]
- 9.Nye C., Kim J., Kalhan S. C., Hanson R. W. 2008. Reassessing triglyceride synthesis in adipose tissue. Trends Endocrinol. Metab. 19: 356–361. [DOI] [PubMed] [Google Scholar]
- 10.Hanson R. W., Reshef L. 2003. Glyceroneogenesis revisited. Biochimie. 85: 1199–1205. [DOI] [PubMed] [Google Scholar]
- 11.Olswang Y., Cohen H., Papo O., Cassuto H., Croniger C. M., Hakimi P., Tilghman S. M., Hanson R. W., Reshef L. 2002. A mutation in the peroxisome proliferator-activated receptor gamma-binding site in the gene for the cytosolic form of phosphoenolpyruvate carboxykinase reduces adipose tissue size and fat content in mice. Proc. Natl. Acad. Sci. U S A. 99: 625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nye C. K., Hanson R. W., Kalhan S. C. 2008. Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat. J. Biol. Chem. 283: 27565–27574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villaroya F., Domingo P., Giralt M. 2010. Drug-induced lipotoxicity: lipodystrophy associated with HIV-1 infection and antiretroviral treatment. Biochim. Biophys. Acta. 1801: 392–399. [DOI] [PubMed] [Google Scholar]
- 14.Hadigan C., Borgonha S., Rabe J., Young V., Grinspoon S. 2002. Increased rates of lipolysis among human immunodeficiency virus-infected men receiving highly active antiretroviral therapy. Metabolism. 51: 1143–1147. [DOI] [PubMed] [Google Scholar]
- 15.van der Valk M., Allick G., Weverling G. J., Romijn J. A., Ackermans M. T., Lange J. M., van Eck-Smit B. L., van Kuijk C., Endert E., Sauerwein H. P., et al. 2004. Markedly diminished lipolysis and partial restoration of glucose metabolism, without changes in fat distribution after extended discontinuation of protease inhibitors in severe lipodystrophic human immunodeficient virus-1-infected patients. J. Clin. Endocrinol. Metab. 89: 3554–3560. [DOI] [PubMed] [Google Scholar]
- 16.Woerle H. J., Mariuz P. R., Meyer C., Reichman R. C., Popa E. M., Dostou J. M., Welle S. L., Gerich J. E. 2003. Mechanisms for the deterioration in glucose tolerance associated with HIV protease inhibitor regimens. Diabetes. 52: 918–925. [DOI] [PubMed] [Google Scholar]
- 17.Fontas E., van Leth F., Sabin C. A., Friis-Møller N., Rickenbach M., d'Arminio Monforte A., Kirk O., Dupon M., Morfeldt L., Mateu S., et al. 2004. Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: are different antiretroviral drugs associated with different lipid profiles? J. Infect. Dis. 189: 1056–1074. [DOI] [PubMed] [Google Scholar]
- 18.Anastos K., Lu D., Shi Q., Tien P. C., Kaplan R. C., Hessol N. A., Cole S., Vigen C., Cohen M., Young M., et al. 2007. Association of serum lipid levels with HIV serostatus, specific antiretroviral agents, and treatment regimens. J. Acquir. Immune Defic. Syndr. 45: 34–42. [DOI] [PubMed] [Google Scholar]
- 19.Mallon P. W. 2007. Antiretroviral therapy-induced lipid altération: in-vitro, animal and human studies. Curr. Opin. HIV AIDS. 4: 282–292. [DOI] [PubMed] [Google Scholar]
- 20.Fisac C., Virgili N., Ferrer E., Barbera M. J., Fumero E., Vilarasau C., Podzamczer D. A. 2003. Comparison of the effects of nevirapine and nelfinavir on metabolism and body habitus in antiretroviral-naive human immunodeficiency virus-infected patients: a randomized controlled study. J. Clin. Endocrinol. Metab. 88: 5186–5192. [DOI] [PubMed] [Google Scholar]
- 21.Wood R., Phanuphak P., Cahn P., Pokrovsky V., Rozenbaum W., Pantaleo G., Sension M., Murphy R., Mancini M., Kelleher T., et al. 2004. Long-term efficacy and safety of atazanavir with stavudine and lamivudine in patients previously treated with nelfinavir or atazanavir. J. Acquir. Immune Defic. Syndr. 36: 684–692. [DOI] [PubMed] [Google Scholar]
- 22.Carey D., Amin J., Boyd M., Petoumenos K., Emery K. 2010. Lipid profiles in HIV-infected adults receiving atazanavir and atazanavir/ritonavir: systematic review and meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 65: 1878–88.. [DOI] [PubMed] [Google Scholar]
- 23.Noor M. A., Parker R. A., O'Mara E., Grasela D. M., Currie A., Hodder S. L., Fiedorek F. T., Haas D. W. 2004. The effects of HIV protease inhibitors atazanavir and lopinavir/ritonavir on insulin sensitivity in HIV-seronegative healthy adults. AIDS. 18: 2137–2144. [DOI] [PubMed] [Google Scholar]
- 24.Lee G.A., Seneviratne T., Noor M. A., Lo J. C., Schwarz J. M., Aweeka F. T., Mulligan K., Schambelan M., Grunfeld C. 2004. HIV proteases inhibitors increase adiponectin levels in HIV-negative men. J. Acquir. Immune Defic. Syndr. 36: 645–647. [DOI] [PubMed] [Google Scholar]
- 25.Johnson J. A., Albu J. B., Engelson E. S., Fried S. K., Inada Y., Ionescu G., Kotler D. P. 2003. Increased systemic and adipose tissue cytokines in patients with HIV-associated lipodystrophy. Am. J. Physiol. Endocrinol. Metab. 286: E261–E271. [DOI] [PubMed] [Google Scholar]
- 26.Kotler D. P., Ionescu G., Johnson J. A., Inada Y., Engelson E. S., Albu J. B. 2003. Studies of adipose tissue metabolism in human immunodeficiency virus-associated lipodystrophy. Clin. Infect. Dis. 37: S47–S51. [DOI] [PubMed] [Google Scholar]
- 27.Caron-Debarle M., Lagathu C., Boccara F., Vigouroux C., Capeau J. 2010. HIV-associated lipodystrophy: from fat injury to premature aging. Trends Mol. Med. 16: 218–229. [DOI] [PubMed] [Google Scholar]
- 28.Bastard J.-P., Caron M., Vidal H., Jan V., Auclair M., Vigouroux C., Luboinski J., Laville M., Maachi M., Girard P.-M., et al. 2002. The association between altered expression of adipogenic factor SREBP1 in lipoatrophic adipose tissue from HIV-1-infected patients and abnormal adipocyte differentiation and insulin resistance. Lancet. 359: 1026–1031. [DOI] [PubMed] [Google Scholar]
- 29.Jan V., Cervera P., Maachi M., Baudrimont M., Kim M., Vidal H., Girard P. M., Levan P., Rozenbaum W., Lombes A., et al. 2004. Altered fat differentiation and adipocytokine expression are inter-related and linked to morphological changes and insulin resistance in HIV-1-infected lipodystrophic patients. Antivir. Ther. 9: 555–564. [PubMed] [Google Scholar]
- 30.Nolan D., Hammond E., James I., McKinnon E., Mallal S. 2003. Contribution of nucleoside-analogue reverse transcriptase inhibitor therapy to lipoatrophy from the population to the cellular level. Antivir. Ther. 8: 617–626. [PubMed] [Google Scholar]
- 31.Kim M., Leclercq P., Lanoy E., Cervera P., Antuna-Puente B., Maachi M., Dorofeev E., Slama L., Valantin M. A., Costagliola D., et al. 2007. A 6-month interruption of antiretroviral therapy improves adipose tissue function in HIV-infected patients: the ANRS EP29 Lipostop Study. Antivir. Ther. 12: 1273–1283. [PubMed] [Google Scholar]
- 32.Vigouroux C., Maachi M., Nguyen T-H., Coussieu C., Gharakhanian S., Funahashi T., Matsuzawa Y., Shimomura I., Rozenbaum W., Capeau J., et al. 2003. Serum adipocytokines are related to lipodystrophy and metabolic disorders in HIV-infected men under antiretroviral therapy. AIDS. 17: 1503–1511. [DOI] [PubMed] [Google Scholar]
- 33.Hammond E., Nolan D. 2007. Adipose tissue inflammation and alterd adipokine and cytokine production in antirétroviral therapy-associated lipodystrophy. Curr. Opin. HIV AIDS. 4: 274–281. [DOI] [PubMed] [Google Scholar]
- 34.Savès M., Raffi F., Capeau J., Rozenbaum W., Ragnaud J. M., Perronne C., Basdevant A., Leport C., Chêne G. 2002. Factors related to lipodystrophy and metabolic alterations in patients with human immunodefiency virus infection receiving highly active antiretroviral therapy. Clin. Infect. Dis. 34: 1396–1405. [DOI] [PubMed] [Google Scholar]
- 35.Koutsari C., Jensen M. D. 2006. Thematic review series: patient-oriented research. Free fatty acid metabolism in human obesity. J. Lipid Res. 47: 1643–1650. [DOI] [PubMed] [Google Scholar]
- 36.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 37.Duff D. A., Snell K. 1982. Limitations of commonly used spectrophotometric assay methods for phosphoenolypyruvate carboxykinase activity in crude extracts of muscle. Biochem. J. 206: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang H. C., Lane M. D. 1966. The enzymatic carboxylation of phosphoenolpyruvate. II. Purification and properties of liver mitochondrial phosphoenolpyruvate carboxykinase. J. Biol. Chem. 241: 2413–2420. [PubMed] [Google Scholar]
- 39.Beale E. G., Hammer R. E., Antoine B., Forest C. 2004. Disregulated glyceroneogenesis: PCK1 as a candidate diabetes and obesity gene. Trends Endocrinol. Metab. 15: 129–135. [DOI] [PubMed] [Google Scholar]
- 40.Caron M., Auclair M., Sterlingot H., Kornprobst M., Capeau J. 2003. Some HIV protease inhibitors alter lamin A/C maturation and stability, SREBP-1 nuclear localization and adipocyte differentiation. AIDS. 17: 2437–2444. [DOI] [PubMed] [Google Scholar]
- 41.Lagathu C., Eustace B., Prot M., Frantz D., Gu Y., Bastard J.-P., Maachi M., Azoulay S., Briggs M., Caron M., et al. 2007. Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir. Ther. 12: 489–500. [PubMed] [Google Scholar]
- 42.Murata H., Hruz P. W., Mueckler M. 2002. Indinavir inhibits the glucose transporter isoform Glut4 at physiologic concentrations. AIDS. 16: 859–863. [DOI] [PubMed] [Google Scholar]
- 43.Johnson J. A., Fried S. K., Pi-Sunyer F. X., Albu J. B. 2001. Impaired insulin action in subcutaneous adipocytes from women with visceral obesity. Am. J. Physiol. Endocrinol. Metab. 280: E40–E49. [DOI] [PubMed] [Google Scholar]
- 44.Kovsan J., Ben-Romano R., Souza S. C., Greenberg A. S., Rudich A. 2007. Regulation of adipocyte lipolysis by degradation of the perilipin protein: nelfinavir enhances lysosome-mediated perilipin proteolysis. J. Biol. Chem. 282: 21704–21711. [DOI] [PubMed] [Google Scholar]
- 45.Kovsan J., Osnis A., Maissel A., Mazor L., Tarnovscki T., Hollander L., Ovadia S., Meier B., Klei J., Bashan N., et al. 2009. Depot-specific adipocyte cell lines reveal differential drug-induced responses of white adipocytes–relevance for partial lipodystrophy. Am. J. Physiol. Endocrinol. Metab. 296: E315–E322. [DOI] [PubMed] [Google Scholar]
- 46.Fain J. N., Madan A. K., Hiler M. L., Cheema P., Bahouth S. W. 2004. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 145: 2273–2282. [DOI] [PubMed] [Google Scholar]
- 47.Kim J. Y., Tillison K., Lee J.-H., Rearick D. A., Smas C. M. 2006. The adipose tissue triglyceride lipase ATGL/PNPLA2 is down regulated by insulin and TNFα in 3T3–L1 adipocytes and is a target for transactivation by PPARγ. Am. J. Physiol. Endocrinol. Metab. 291: E115–E127. [DOI] [PubMed] [Google Scholar]
- 48.Waltner-Law M., Daniels M. C., Sutherland C., Granner D. K. 2000. NF-kappa B inhibits glucocorticoid and cAMP-mediated expression of the phosphoenolpyruvate carboxykinase gene. J. Biol. Chem. 275: 31847–31856. [DOI] [PubMed] [Google Scholar]
- 49.Lenhard J. M., Furfine E. S., Jain R. G., Ittoop O., Orband-Miller L. A., Blanchard S. G., Paulik M. A., Weiel J. E. 2000. HIV protease inhibitors block adipogenesis and increase lipolysis in vitro? Antiviral Res. 47: 121–129. [DOI] [PubMed] [Google Scholar]
- 50.Adler-Wailes D. C., Liu H., Ahmad F., Feng N., Londos C., Manganiello V., Yanovski J. A. 2005. Effects of the human immunodeficiency virus-protease inhibitor, ritonavir, on basal and catecholamine-stimulated lipolysis. J. Clin. Endocrinol. Metab. 90: 3251–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schweiger M., Schreiber R., Haermmerle G., Lass A., Fledelius C., Jacobsen P., Tornquist H., Zechner R., Zimmermann R. 2006. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J. Biol. Chem. 281: 40236–40241. [DOI] [PubMed] [Google Scholar]
- 52.Rudich A., Vanounou S., Riesenberg K., Porat M., Tirosh A., Harman-Boehm I., Greenberg A. S., Schlaeffer F., Bashan N. 2001. The HIV protease inhibitor nelfinavir induces insulin resistance and increases basal lipolysis in 3T3–L1 adipocytes. Diabetes. 50: 1425–1431. [DOI] [PubMed] [Google Scholar]
- 53.Adler-Wailes D. C., Guiney E. L., Wolins N. E., Yanovski J. A. 2010. Long-term ritonavir exposure increases fatty acid and glycerol recycling in 3T3–L1 adipocytes as compensatory mechanisms for increased triacylglycerol hydrolysis. Endocrinology. 151: 2097–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cianflone K., Zakarian R., Stanculescu C., Germinario R. 2006. Protease inhibitor effects on triglyceride synthesis and adipokine secretion in human omental and subcutaneous adipose tissue. Antivir. Ther. 11: 681–691. [PubMed] [Google Scholar]
- 55.Hruz P. W., Murata H., Oiu H., Mueckler M. 2002. Indinavir induces acute and reversible peripheral insulin resistance in rats. Diabetes. 51: 937–942. [DOI] [PubMed] [Google Scholar]
- 56.Tchkonia T., Lenburg M., Thomou T., Giorgadze N., Frampton G., Pirtskhalava T., Cartwright A., Cartwright M., Flanagan J., Karagiannides I., et al. 2007. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am. J. Physiol. Endocrinol. Metab. 292: E298–E307. [DOI] [PubMed] [Google Scholar]
- 57.Lagathu C., Bastard J. P., Auclair M., Maachi M., Kornprobst M., Capeau J., Caron M. 2004. Antiretroviral drugs with adverse effects on adipocyte lipid metabolism and survival alter the expression and secretion of proinflammatory cytokines and adiponectin in vitro. Antivir. Ther. 9: 911–920. [PubMed] [Google Scholar]
- 58.Vernochet C., Azoulay S., Duval D., Guedj R., Cottrez F., Vidal H., Ailhaud G., Dani C. 2005. Human immunodeficiency virus protease inhibitors accumulate into cultured human adipocytes and alter expression of adipocytokines. J. Biol. Chem. 280: 2238–2243. [DOI] [PubMed] [Google Scholar]
- 59.Deveaud C., Beauvoit B., Hagry S., Galinier A., Carrière A., Salin B., Schaeffer J., Caspar-Bauguil S., Fernandez Y., Gordien J. B., et al. 2005. Site specific alterations of adipose tissue mitochondria in 3′-azido-3′-deoxythymidine (AZT)-treated rats: an early stage in lipodystrophy? Biochem. Pharmacol. 70: 90–101. [DOI] [PubMed] [Google Scholar]
- 60.Dolinkova M., Dostalova I., Lacinova Z., Michalsky D., Haluzikova D., Mraz M., Kasalicky M., Haluzik M. 2008. The endocrine profile of subcutaneous and visceral adipose tissue of obese patients. Mol. Cell. Endocrinol. 291: 63–70. [DOI] [PubMed] [Google Scholar]
- 61.Harman-Boehm I., Blüher M., Redel H., Sion-Vardy N., Ovadia S., Avinoach E., Shai I., Klöting N., Stumvoll M., Bashan N., et al. 2007. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J. Clin. Endocrinol. Metab. 92: 2240–2247. [DOI] [PubMed] [Google Scholar]
- 62.Aron-Wisnewsky J., Tordjman J., Poitou C., Darakhshan F., Hugol D., Basdevant A., Aissat A., Guerre-Milllo M., Clement K. 2009. Human adipose tissue macrophages: M1 and M2 cell surface markers in subcutaneous and omental depots and after weight loss. J. Clin. Endocrinol. Metab. 94: 4619–4623. [DOI] [PubMed] [Google Scholar]
- 63.Grohmann M., Sabin M., Holly J., Shield J., Crowne E., Stewart C. 2005. Characterization of differentiated subcutaneous and visceral adipose tissue from children: the influences of TNF-alpha and IGF-I. J. Lipid Res. 46: 93–103. [DOI] [PubMed] [Google Scholar]
- 64.Hube F.M., Birgel M., Lee Y.M., Hauner H. 1999. Expression pattern of tumour necrosis factor receptors in subcutaneous and omental human adipose tissue: role of obesity and non-insulin-dependent diabetes mellitus. Eur. J. Clin. Invest. 29: 672–678. [DOI] [PubMed] [Google Scholar]
- 65.Hill M., Mc Callum R. 1991. Altered transcriptional regulation of phosphoenolpyruvate carboxykinase in rats following endotoxin treatment. J. Clin. Invest. 88: 811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hill M., Mc Callum R. 1992. Identification of tumor necrosis factor as a transcriptional regulator of the phopshoenolpyruvate carboxykinase gene following endotoxin treatment of mice. Infect. Immun. 60: 4040–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wallenius V., Walleniys K., Ahren B., Rudling M., Carlsten H., Dickson S., Ohlsson C., Jansson J-O. 2002. Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 8: 75–79. [DOI] [PubMed] [Google Scholar]
- 68.Uysal K. T., Wiesbrock S. M., Marino M. W., Hotamisligli G. S. 1992. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 389: 610–614. [DOI] [PubMed] [Google Scholar]
- 69.Mohamed-Ali V., Flower L., Sethi J., Hotamisligil G., Gray R., Humphries S. E., York D. A., Pinkney J. 2001. beta-Adrenergic regulation of IL-6 release from adipose tissue: in vivo and in vitro studies. J. Clin. Endocrinol. Metab. 86: 5864–5869. [DOI] [PubMed] [Google Scholar]
- 70.Vgontzas N. A., Papanicolaou D. A., Bixler E. O., Kales A., Tyson K., NatureChrousos G. P. 1997. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J. Clin. Endocrinol. Metab. 82: 1313–1316. [DOI] [PubMed] [Google Scholar]
- 71.Rotter V., Nagaev I. 2003. Interleukin-6 (IL-6) induces insulin resistance in 3T3–L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J. Biol. Chem. 278: 45777–45784. [DOI] [PubMed] [Google Scholar]
- 72.Kolb H., Mandrup-Poulsen T. 2005. An immune origin of type 2 diabetes? Diabetologia. 48: 1038–1050. [DOI] [PubMed] [Google Scholar]
- 73.Cancello R., Clement K. 2006. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG. 113: 1141–1147. [DOI] [PubMed] [Google Scholar]