Abstract
The lipid droplet-associated fat specific protein 27 (FSP27) suppresses lipolysis and thereby enhances triglyceride accumulation in adipocytes. We and others have recently found FSP27 to be a remarkably short-lived protein (half-life, 15 min) due to its rapid ubiquitination and proteasomal degradation. Thus, we tested the hypothesis that lipolytic agents such as tumor necrosis factor-α (TNF-α) and isoproterenol modulate FSP27 levels to regulate FFA release. Consistent with this concept, we showed that the lipolytic actions of TNF-α, interleukin-1β (IL-1β), and IFN-γ are accompanied by marked decreases in FSP27 expression and lipid droplet size in mouse adipocytes. Similar depletion of FSP27 using short interfering RNA (siRNA) mimicked the lipolysis-enhancing effect of TNF-α, while maintaining stable FSP27 levels using expression of hemagglutinin epitope-tagged FSP27 blocked TNF-α-mediated lipolysis. In contrast, we show the robust lipolytic action of isoproterenol is paradoxically associated with increases in FSP27 levels and a delayed degradation rate corresponding to decreased ubiquitination. This catecholamine-mediated increase in FSP27 abundance, probably a feedback mechanism for restraining excessive lipolysis by catecholamines, is mimicked by forskolin or 8-bromo-cAMP treatment and is prevented by the protein kinase A (PKA) inhibitor KT5720 or by PKA depletion using siRNA. Taken together, these data identify the regulation of FSP27 as an important intermediate in the mechanism of lipolysis in adipocytes in response to TNF-α and isoproterenol.
Keywords: catecholamine, cytokine, FSP27, lipid droplets, protein stability, ubiquitination
Storing excess energy for future use during starvation is critical for the survival of mammals. Much of this energy is stored in the form of triacylglycerol (TAG) within lipid droplets, which are present most abundantly in adipocytes and which, in turn, accumulate in depots such as subcutaneous and visceral adipose tissue in mice and humans. TAG in lipid droplets is mobilized during starvation by lipase-catalyzed hydrolysis (lipolysis) to release energy in the forms of glycerol and free fatty acids, providing fuel to other cell types such as muscle and liver. Previous work investigating formation of lipid droplets and regulation of lipolysis has elucidated the importance of lipid droplet-associated proteins for these processes (1, 2). Based on shared sequence homology, one set of lipid droplet proteins is grouped as the perilipin-adipophilin-tail interacting protein 47 (PAT/TIP47) family of proteins (3). PAT-related proteins are functionally conserved from mammals to lower organisms such as Drosophila and Dictyostelium spp (4). In Drosophila, two PAT domain proteins are encoded by the Lsdp1 and Lsd2 genes. Drosophila loss-of-function Lsd2 mutants are lean, whereas Lsd2 overexpression causes obesity (5). In mammals, PAT proteins can be divided into exchangeable TAG-associated PAT proteins (EPATs) or constitutively TAG-associated PAT proteins (CPATs). EPATs include the TIP47/perilipin-3 (PLIN3), S3-12/PLIN4, and oxidative tissues-enriched PAT protein (OXPAT)/myocardial lipid droplet protein (MLDP)/PLIN5 families. These proteins are stably expressed in cytosol and bind nascent TAG droplets. CPATs include the perilipin/PLIN1 and adipophilin/ADRP/PLIN2 families, which are bound to neutral lipid droplets and are rapidly degraded when dissociated from the lipid droplets (3, 6). Thus, expression of the different sets of lipid droplet proteins contributes to the finely tuned regulation of TAG deposition in lipid droplets.
Lipolysis of TAG into glycerol and fatty acids is activated by various physiological stimuli such as β-adrenergic agonists during fasting and tumor necrosis factor-α (TNF-α) secreted by macrophages that infiltrate adipose tissues in obesity. Stimulation of β-adrenergic receptors by the catecholamines epinephrine, norepinephrine, and isoproterenol activates adenylyl cyclase, increasing intracellular cAMP levels and activating cAMP-dependent protein kinase A (PKA). This protein kinase catalyzes phosphorylation of hormone-sensitive lipase (HSL) as well as perilipin, which can then interact and enhance lipolysis (7–12). On the other hand, TNF-α decreases perilipin expression, which may contribute to increased lipolysis, albeit with a longer time course, and to a lesser extent than the effect of catecholamines. Additionally, preventing the depletion of perilipin by TNF-α using expression of perilipin via an adenovirus vector has been shown to protect against TNF-α-mediated lipolysis (13). In addition, adipocytes from perilipin null mice display a higher basal lipolytic rate (14, 15). These mice exhibit an attenuated lipolytic response to β-adrenergic stimulation, reinforcing the importance of perilipin in the mechanism of lipase action to enhance TAG hydrolysis.
Recently, our laboratory identified mouse FSP27 as a highly expressed adipocyte protein that associates with lipid droplets (16, 17) and has significant homology to domains in perilipin that are thought to be responsible for triglyceride shielding from lipases and for lipid droplet targeting and anchoring (18). These findings have been confirmed and extended to show that FSP27 expression is associated with increased lipid droplet size and triglyceride accumulation in adipose and nonadipose cell types (16, 19–21). FSP27 is a member of the cell death-inducing DFF45-like effector (CIDE) family of proteins that have a common CIDE N domain at the N-terminus and a CIDE C domain at the C-terminus (22). Thus, human FSP27 is also denoted CIDEC, and a homozygous nonsense mutation in the CIDEC gene has been reported to be associated with lipodystrophy and insulin-resistant diabetes in a human patient (23). FSP27 was first identified as an adipocyte-specific gene product (24) and was later shown to have homology to the 45-kDa subunit of DNA fragmentation factor (22). It is highly expressed in white adipose tissue in mice as well as in humans and has been shown to be upregulated during 3T3-L1 adipogenesis (20). Despite having CIDE domains, FSP27 upregulation during adipogenesis does not lead to apoptosis (20). Proteomic analysis of lipid droplet-associated proteins in adipocyte homogenates has also revealed FSP27 to be in the lipid droplet fraction (25). Studies using both cultured cells and intact mice have confirmed that FSP27 depletion decreases lipid droplet size in adipocytes (16, 21), suggesting that it is necessary for formation or maintenance of the large unilocular lipid droplets characteristic of adipocytes of white fat in mice and humans.
Because short interfering RNA (siRNA)-mediated depletion of FSP27 causes increased lipolysis of lipid droplet TAGs in adipocytes and its overexpression enhances TAG deposition in cells, our aim in the present study was to examine the potential role of FSP27 in the physiological regulation of lipolysis by TNF-α and catecholamines. Our studies have shown that the FSP27 protein exhibits rapid turnover, with a half-life of less than 15 min. It is ubiquitinated and degraded via the proteasome, as are CIDEA and other lipid droplet proteins such as adipocyte triglyceride lipase (ATGL) (26, 27). These results are consistent with those in a paper published during preparation of the manuscript (28). We also report that TNF-α, interleukin-1β (IL-1β), or IFN-γ treatment of mouse adipocytes causes rapid FSP27 depletion followed by decreased lipid droplet size and enhanced lipolysis. Conversely, sustained FSP27 expression in adipocytes from a viral expression vector protects against the action of TNF-α on lipid droplet size and lipolysis. In contrast, isoproterenol surprisingly increased FSP27 protein levels by decreasing FSP27 ubiquitination and degradation, apparently as part of a feedback mechanism to restrain high lipolytic rates. Our study highlights the importance of the FSP27 lipid droplet protein as a key target of TNF-α and β-adrenergic agonists in the mechanisms whereby these agents modulate lipolysis in adipocytes.
MATERIALS AND METHODS
Materials
Affinity purified rabbit polyclonal FSP27 antibody was generated in the laboratory and also was kindly provided by Drs. Kasuga and Nishino, Kobe University Graduate School of Medicine, Kobe, Japan. Polyclonal perilipin antibody was purchased from Fitzgerald (product no. 20R-PP004). Polyclonal green fluorescent protein (GFP) antibody (product no. 2555), monoclonal hemagglutinin (HA) antibody (product no. 2367), and monoclonal ATGL antibody (product no. 2439) were purchased from Cell Signaling. Monoclonal ubiquitin antibody P4D1 (no. sc-8017) was purchased from Santa Cruz Biotechnology, and Vti1a antibody was from BD Transduction Laboratory (product no. 611220). Recombinant murine TNF-α (product no. 654245), cycloheximide (product no. 2397650), and MG132 (product no. 474790) were obtained from Calbiochem. Recombinant murine IL-6 (product no. I9646), recombinant murine IL-1β (product no. I5271), recombinant murine IFN-γ (product no. I4777), isoproterenol (product no. 15627-56), biotin (product no. B4639), and pantothenic acid (product no. P6292) were purchased from Sigma. 8-Bromo-cAMP (product no. CN-115), forskolin (product no. CN-100), and KT-5720 (product no. EI-199) were from Enzo Life Sciences; leupeptin and aprotinin were from Roche; and NH4Cl was from EMD.
siRNA
All siRNAs were purchased from Dharmacon (Chicago, IL). The siRNA sequences were 5′-CAGUCGCGUUUGCGACUGG-3′ for Scr; 5′-CAACUAAGAAGAUCGAUGUUU-3′ for FSP27; 5′-GCACAUUUAUCCCGGUGUAUU-3′ for ATGL; 5′-GUGGUUUGCCACGACUGACUU-3′ for PKAca; and 5′-AGAGUUUCUAGCCAAAGCCUU-3′ for PKAcb.
Cell culture and siRNA transfection in 3T3-L1 adipocytes
3T3-L1 fibroblasts were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS, 50 µg/ml penicillin, and 50 µg/ml streptomycin (complete DMEM). After cells reached quiescence, they were differentiated into adipocytes as previously described (29). Adipocytes that were differentiated for 4 days were transfected with siRNA duplexes using a Bio-Rad electroporator, also described previously (29, 30).
Mouse primary adipocyte and preadipocyte isolation and culture
Axillary fat pads were removed from 8-week-old C57Bl/6J male mice. After homogenization, tissue was incubated in 2 mg/ml collagenase in 0.1 M HEPES, 0.12 M NaCl, 50 mM KCl, 5 mM d-glucose, 1.5% BSA, 1 mM CaCl2 for 30 min at 37°C. Samples were then filtered through a 100 µm strainer. Following a 10 min centrifugation at 1,000 rpm, adipocytes were collected as the floating fraction and washed three times in PBS before TNF-α treatment. The stromal--vascular fraction (SVF) was resuspended in 10 ml of red blood cell lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) for 10 min, filtered through a 40 µm mesh, and centrifuged for 10 min at 1,000 rpm. The cell pellet was then resuspended in growing medium (DMEM-F12 medium containing 10% FBS, 50 µg/ml penicillin, 50 µg/ml streptomycin) and seeded in 6-well plates until confluence. Two days after confluency, differentiation was induced using growing medium supplemented with 17 µM pantothenic acid, 33 µM biotin, 0.25 µM dexamethasone, 500 µM isobutylmethylxanthine (IBMX), and 85 nM insulin for 48 h. On day 2, medium was changed, and cells were incubated in growing medium supplemented with 17 µM pantothenic acid, 33 µM biotin, and 85 nM insulin for 6 more days. Cells were treated, once differentiated, on day 8.
Generation of adenovirus and infection of adipocytes with adenovirus
The coding sequence of mouse FSP27 with an HA-epitope tag on the N-terminal end was cloned into the pAdTrack adenoviral vector at Sal1/BglII restriction enzyme sites in the multiple cloning region. The construct was confirmed by sequencing. The construct was then linearized by Pme1 digestion and transformed into BJ5813 electrocompetent cells (Stratagene) to integrate the construct by homologous recombination into an adenoviral backbone, as described for the Adeasy system for viral vector generation (31). Empty vector with GFP was used as control. Expression levels of HA-tagged FSP27 and GFP were standardized with titration and confirmed with Western blot analysis using FSP27, HA, and GFP antibodies. For infection of adipocytes, control or FSP27 adenovirus was directly added for 24 h to adipocytes on the fourth day after initiation of differentiation. At the end of 24 h, the medium was changed to fresh complete DMEM, and cells were cultured for another 48 h before being harvested. All experiments were designed such that the cells were exposed to virus 72 h prior to harvesting.
TNF-α, IL-1β, IFN-γ, IL-6, and isoproterenol treatments and assay to measure glycerol release
To treat adipocytes with TNF-α (3T3-L1 and primary adipocytes), IL-6, IL-1β, IFN-γ, or isoproterenol (3T3-L1) adipocytes were washed once with PBS to remove the phenol red. Then, 10 to 20 ng/ml TNF-α, or 1, 10, 20, or 100 ng/ml IL-1β, or 50–100 ng/ml IFN-γ, or 50–100 ng/ml IL-6, or 10 µM isoproterenol was added to phenol red-free high-glucose DMEM supplemented with 1% sodium pyruvate, 1% l-glutamine, 0.1% biotin, 10% FBS, 1% penicillin, and 1% streptomycin for the indicated times. For serum starvation, cells were washed once with PBS, and phenol red-free high-glucose DMEM supplemented with 1% sodium pyruvate, 1% l-glutamine, and 0.1% biotin, and 2% BSA was added, and then the mixture was incubated overnight, and the cells were treated with TNF-α. After TNF-α, IL-6, IL-1β, IFN-γ, or isoproterenol treatment, the medium was collected and assayed for glycerol content by using a commercial colorimetric assay kit from Sigma as per manufacturer's protocol.
FSP27 stability assay
Mature 3T3-L1 adipocytes were treated with 5 µg/ml leupeptin, 5 µg/ml aprotinin, 10 µM MG132, or 2.5 mM NH4Cl for 2 h, and protein was harvested with SDS lysis buffer and analyzed using Western blotting. For cycloheximide chase studies, mature adipocytes were washed with PBS and treated with 5 µg/ml cycloheximide for 15, 30, and 60 min, and then protein was harvested with SDS lysis buffer and analyzed by Western blotting.
Detection of FSP27 ubiquitination
Mature 3T3-L1 adipocytes were treated with 10 µM MG132 alone or in combination with 10 ng/ml TNF-α or 10 µM isoproterenol for 3 h. Cells were washed twice with ice cold PBS and harvested with radioimmunoprecipitation assay buffer containing 0.5% SDS. The lysate was passed through a syringe and precleared with protein A beads for 1 h. FSP27 was immunoprecipitated with either nonimmune rabbit IgG or rabbit FSP27 antibody from the precleared lysate diluted with lysis buffer to a final SDS concentration of 0.1%. Immunoprecipitated protein was analyzed by Western blotting and probed with ubiquitin and FSP27 antibody.
Oil Red O staining for lipid droplets
Adipocytes grown on coverslips were washed three times with cold PBS, fixed in 4% paraformaldehyde for 10 min, and permeabilized and blocked with 0.05% Triton X-100 and 5% FBS in PBS for 30 min. Oil Red O (0.4%) in isopropyl alcohol solution was mixed with distilled water in a 60:40 ratio and filtered through 0.45 µm filters. This freshly prepared Oil Red O solution was added to permeabilized cells for 30 min. These cells were then washed three times with ice cold PBS and mounted using Prolong Gold plus 4',6-diamidino-2-phenylindole (DAPI) mounting medium (Invitrogen), and lipid droplets were imaged by confocal microscopy with a Nikon 60× planapochromat objective. All images were taken with identical exposures in any given experiment.
Confocal microscopy
Images were taken using a Zeiss Axiophot microscope with Hamamatsu digital camera and processed with MetaMorph version 6.1 imaging software. Quantitation of the lipid droplet size was done using Axiovision digital version 4.1 imaging software by selecting individual lipid droplets or groups of lipid droplets, where appropriate.
RNA isolation and quantitative reverse transcription-PCR
Total RNA from 3T3-L1 adipocytes were isolated using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. cDNA was synthesized from 1 µg of total RNA using an iScript cDNA synthesis kit (Bio-Rad). Quantitative real-time reverse transcription (RT)-PCR was done using a MyIQ real-time PCR system with iQ SYBR Green mixture (Bio-Rad) and specific primers to amplify the genes. Primer sequences are available upon request.
Statistical analysis
Results are expressed as means ± SEM, and the significance of the change was assessed using Student's t-test or one-way ANOVA test.
RESULTS
FSP27 has a short half-life and is degraded by the proteasome after ubiquitination
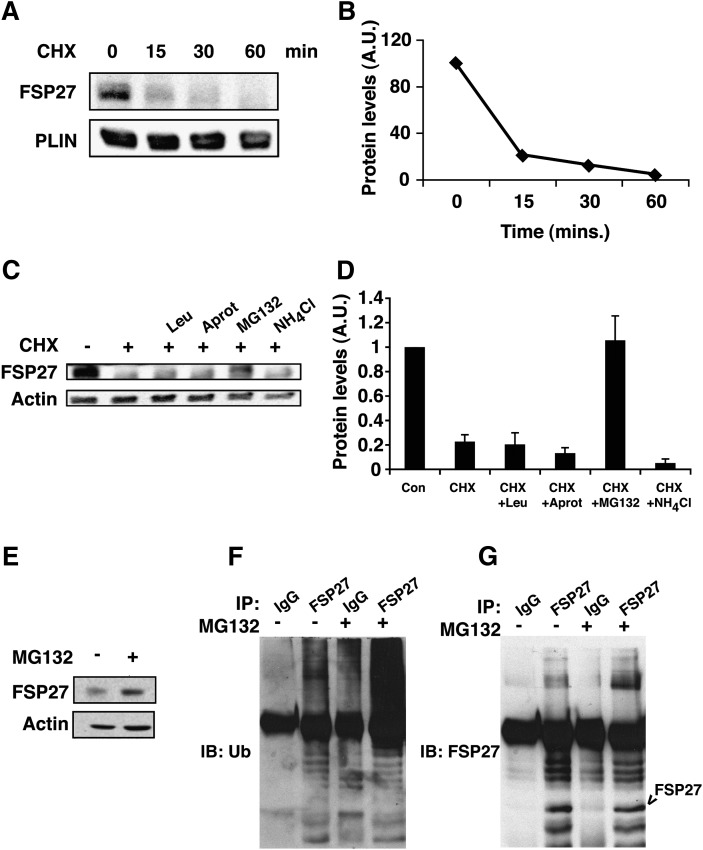
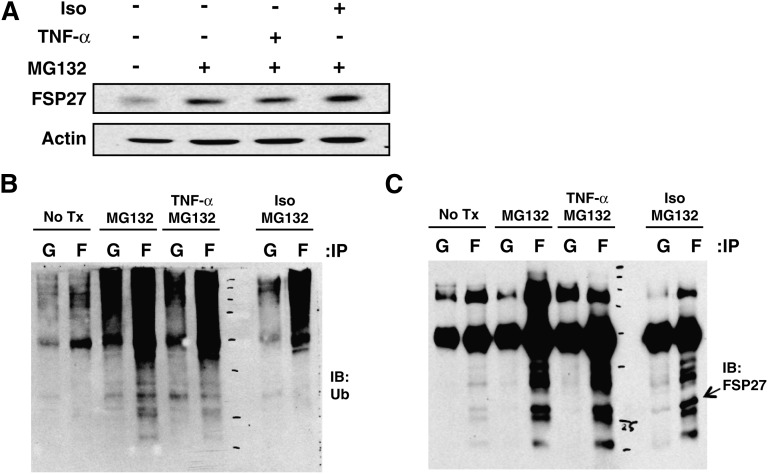
Previous studies have shown that FSP27 depletion increases lipolysis in adipocytes, while FSP27 expression promotes enlarged lipid droplet formation in COS cells and preadipocytes (16, 21). These data suggest that the changes in cytosolic FSP27 levels could modulate lipid metabolism or storage and could be a mechanism by which various stimuli modulate lipolysis. Therefore, in order to study the role of FSP27 in regulation of lipolysis in response to various lipolytic stimuli, we studied the regulation of FSP27 protein levels. First we determined the stability of FSP27 by treating mature adipocytes with 5µg/ml cycloheximide to block new protein synthesis and measured intact FSP27 protein. We found that FSP27 is an unusually short-lived protein and has a half-life of less than 15 min (Fig. 1A, B). Addition of the proteasome inhibitor, MG132, inhibited the degradation of FSP27 (Fig. 1C–E). However, neither the general peptidase inhibitors aprotinin and leupeptin nor the lysosome inhibitor NH4Cl was able to prevent degradation of FSP27 (Fig. 1C, D). Upon blockade of proteasomal degradation with MG132, multiple bands detectable with anti-ubiquitin antibody and FSP27 antibody could be detected specifically in FSP27 immunoprecipitates (Fig. 1 F, G). These results suggest that the FSP27 protein is rapidly destroyed by ubiquitination and proteasomal degradation, raising the possibility that FSP27 levels could be quickly modulated as a mechanism for regulation of lipolysis. These data are consistent with those in a paper published as we were finalizing our manuscript, which also showed FSP27 has a short half-life and is ubiquitinated and degraded via the proteasome (28).
Fig. 1.
FSP27 has a short half-life and is degraded by the proteasome after ubiquitination. A: Representative Western blots of adipocyte lysates following 15, 30, and 60 min treatments with 5 µg/ml cycloheximide (CHX) compared with untreated (0) cells show rapid degradation of FSP27. B: Densitometric measurement of FSP27 protein levels after cycloheximide treatment (as shown in panel A). C: Representative Western blots of FSP27 show protection of FSP27 from degradation in lysates from cells treated with the proteasome inhibitor MG132 but not by peptidase inhibitor leupeptin (Leu) or aprotinin (Aprot) or lysosomal inhibitor NH4Cl. D: Densitometric measurements showing protection of FSP27 by MG132. E: FSP27 immunoblot (IB) of total lysates of 3T3-L1 adipocytes with or without MG132. F: Lysates from control (−) or MG132-treated (+) cells were immunoprecipitated (IP) with anti-FSP27 antibody (FSP27) or nonimmune rabbit IgG (IgG), and immunoprecipitates were probed with anti-ubiquitin (Ub) monoclonal antibody. G: Immunoprecipitates (as shown in panel F) probed with anti-FSP27 antibody. Data shown are representative of four or more separate experiments.
TNF-α and other inflammatory cytokines decrease FSP27 levels in 3T3-L1 adipocytes
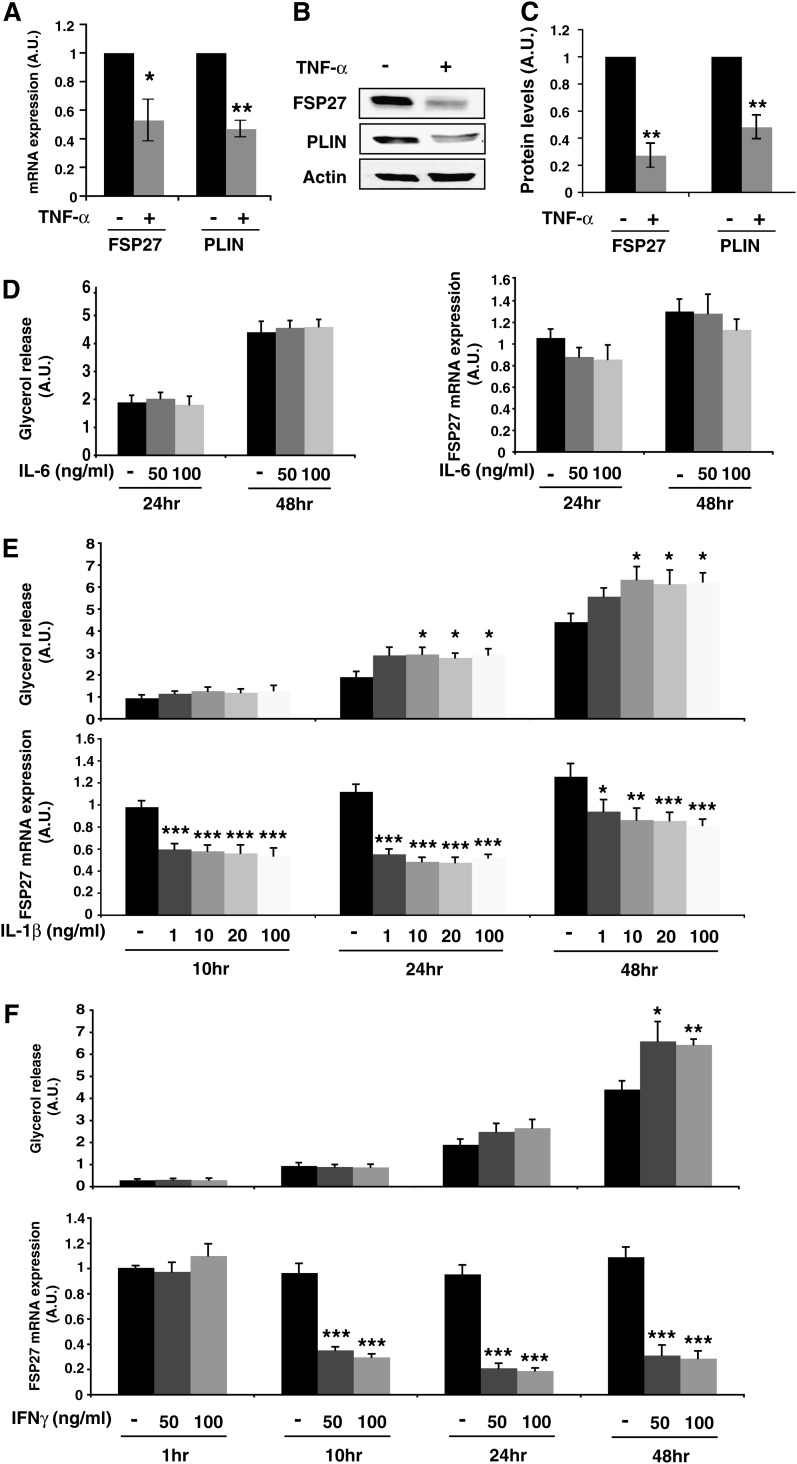
TNF-α is an inflammatory cytokine that has profound effects on adipocytes, including stimulation of lipolysis and downregulation of numerous adipocyte-expressed genes. Because FSP27 depletion causes increased lipolysis in adipocytes, we sought to determine whether TNF-α alters FSP27 levels when adipocytes are treated with TNF-α at concentrations that stimulate lipolysis. The lowest concentration and shortest duration of TNF-α treatment that stimulated lipolysis optimally were established by treating fully differentiated 3T3-L1 adipocytes with various concentrations of TNF-α for different time periods and measuring rates of glycerol release (data not shown). The strongest effects on lipolysis were obtained when cultured adipocytes were treated with 10 ng/ml TNF-α for 16 h. Under these conditions, approximately 1.5-fold increase in glycerol release from adipocytes was observed in response to the cytokine. After mature adipocytes were treated with TNF-α, FSP27 and perilipin mRNA were analyzed using quantitative real-time PCR. Treating adipocytes with TNF-α significantly decreased the mRNA transcripts of both FSP27 and perilipin (Fig. 2A). Downregulation of FSP27 mRNA after TNF-α treatment was also observed previously with Northern blotting (20), and downregulation of perilipin by the cytokine is consistent with results from a previous study (13). The effects of TNF-α on the protein levels of FSP27 and perilipin were also analyzed using Western blotting, which showed a significant decrease in FSP27 and perilipin levels after TNF-α treatment (Fig. 2B, C). Therefore, TNF-α treatment of adipocytes at a dose that stimulates lipolysis dramatically downregulates FSP27 both at the mRNA and the protein levels.
Fig. 2.
TNF-α as well as IL-1β and IFN-γ but not IL-6 decrease FSP27 levels and increase glycerol release in 3T3-L1 adipocytes. A–C: Cells treated with 10 ng/ml TNF-α for 16 h. A: Quantitative RT-PCR of Fsp27 and perilipin mRNA levels; B) representative immunoblot of FSP27, perilipin, and actin (loading control); C: densitometric quantitation of immunoblots of FSP27 and perilipin protein levels. A–C, Data are expressed as means ± SEM for six different experiments; P values were calculated using Student's t-test; *, P < 0.05; **, P < 0.0005. A.U., arbitrary units. D: 3T3-L1 adipocytes were treated with IL-6 at the indicated concentrations and times. D, left panel, glycerol release; D, right panel, quantitative RT-PCR of FSP27 mRNA levels. E, F: Cells treated with IL1-β or IFN-γ, respectively, at the indicated concentrations. and times are shown. Upper panels, glycerol release; lower panels, quantitative RT-PCR of FSP27 mRNA levels. D–F, Data are expressed as means ± SEM from three to five experiments performed in duplicate. P values were calculated using Student's t-test relative to the time-matched untreated condition; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
As other inflammatory mediators such as IL-1β and IFN-γ have been shown to induce lipolysis in adipocytes (32–35), we wondered if FSP27 downregulation was specific to TNF-α-mediated lipolysis or if it was also regulated by other cytokines. To answer this question, we treated differentiated 3T3-L1 adipocytes with IL-6, IL-1β, or IFN-γ. As described previously (36), IL-6 treatment (50–100 ng/ml) for 24 to 48 h did not induce any increase in glycerol release and did not affect the FSP27 mRNA or protein level (Fig. 2D and Supplementary Fig. S1A). However, in accordance with previous studies (33, 34), IL-1β (Fig. 2E and Supplementary Fig. S1B) induced a time-dependent increase in glycerol release detectable after 24 h treatment with doses as low as 10 ng/ml. Interestingly, FSP27 levels were decreased by ∼40% after 10 h of treatment, and the effect was maintained over time. These results suggest that the mechanism by which IL-1β induces lipolysis could involve FSP27 regulation, as we describe here for TNF-α-mediated lipolysis. In accordance with previous studies that showed induction of lipolysis following IFN-γ treatment (32, 33, 35), IFN-γ induced an increase in glycerol release at 24 h treatment that reached significant levels at 48 h, whereas it decreased FSP27 expression by ∼70–80% by 10 h of treatment (Fig. 2F and Supplementary Fig. S1C). Thus, modulation of FSP27 levels is associated with increased lipolysis induced by other proinflammatory signals known to profoundly affect adipose tissue biology.
TNF-α decreases FSP27 levels in primary adipocytes
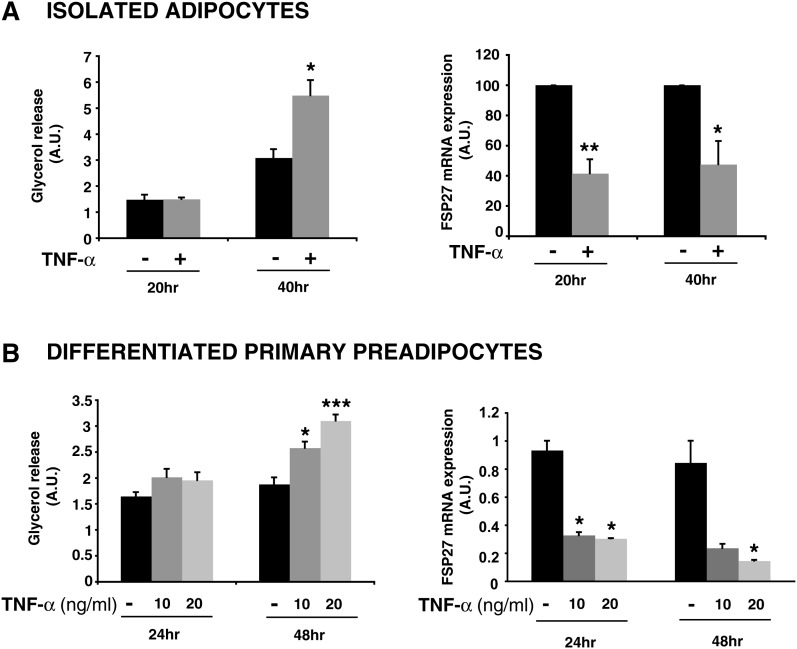
In order to determine if the effect of TNF-α on FSP27 levels was relevant in primary adipocytes, we separated the adipocyte fraction from the SVF from subcutaneous adipose tissue of C57Bl/6J mice. As shown in Fig. 3A, treatment of the isolated adipocytes with 10 ng/ml TNF-α for 20 to 40 h ex vivo resulted in a significant ∼1.5-fold increase in glycerol release at the later time point. Interestingly, TNF-α treatment decreases FSP27 mRNA expression by ∼60% by 20 h. To confirm these data, we differentiated the preadipocytes from the SVF into adipocytes in vitro and treated the mature adipocytes with 10 to 20 ng/ml TNF-α for 24 to 48 h. We also observed a 70% to 80% decrease in FSP27 mRNA levels in response to either dose of TNF-α at as soon as 24 h of treatment, whereas glycerol release was increased significantly by 48 h (Fig. 3B). Thus, in primary and in vitro-differentiated adipocytes as well as in cultured cells, depletion of FSP27 could be part of the mechanism by which TNF-α increases lipolysis.
Fig. 3.
TNF-α also decreases FSP27 levels and increases glycerol release in adipocytes isolated from mouse adipose tissue. A: Primary adipocytes were isolated from subcutaneous adipose tissue of C57Bl/6J mice and treated with 10 ng/ml TNF-α for 20 or 40 h. A, left panel: glycerol released into medium; right panel: quantitative RT-PCR of FSP27 mRNA level (shown as a percentage of the time-matched untreated sample). Data are expressed as means ± SEM from two different experiments performed in duplicate. A.U., arbitrary units. B: Primary preadipocytes from the SVF were induced to differentiate as described in Materials and Methods. Mature adipocytes were then treated with 10–20 ng/ml TNF-α for 24 or 48 h. B, left panel: glycerol release; B, right panel: quantitative RT-PCR. Data are expressed as means ± SEM from one experiment performed three to five times. P values were calculated using Student's t-test relative to the time-matched untreated condition; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FSP27 depletion enhances TNF-α-stimulated lipolysis
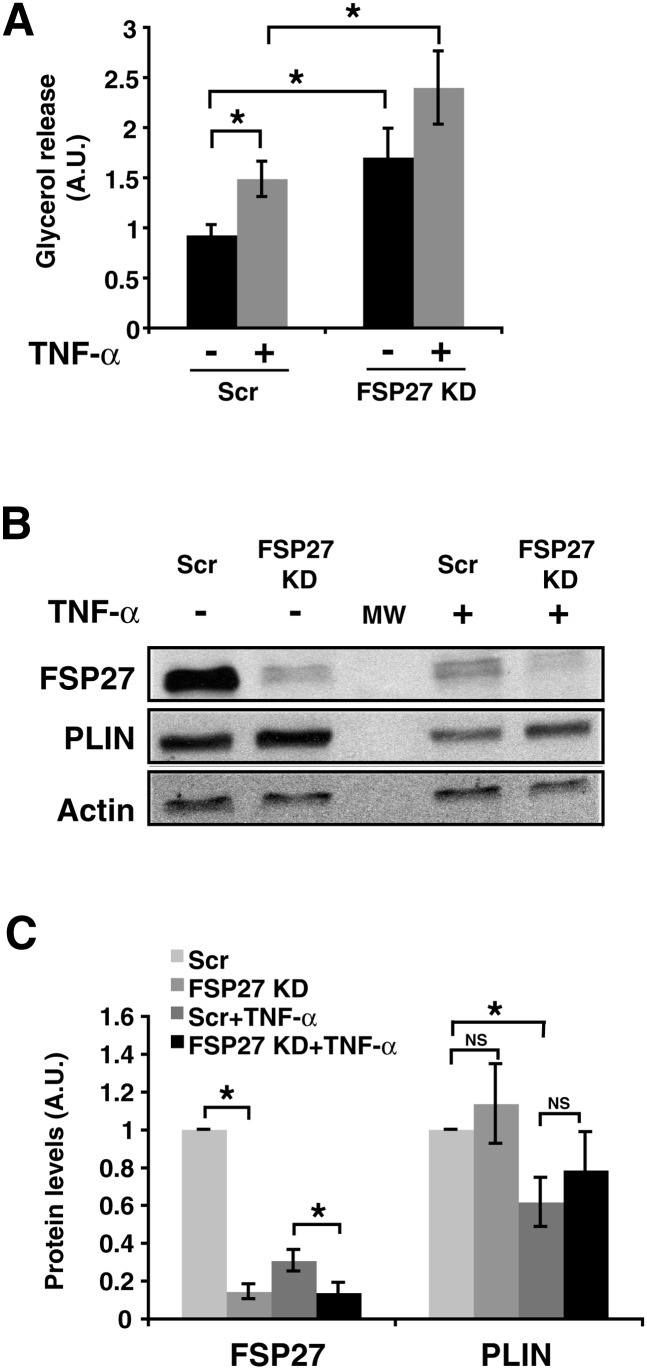
To determine whether FSP27 protein downregulation by TNF-α plays a role in increased lipolysis, we first measured lipolysis in adipocytes after siRNA-mediated depletion of FSP27 with or without TNF-α treatment (Fig. 4). Cultured adipocytes were transfected with either scrambled (Scr) or FSP27 siRNA, and glycerol release was measured in basal and TNF-α-treated cells. Treating cells with TNF-α and silencing of FSP27 expression both caused an increase of ∼50% in glycerol release, while the combination of these treatments resulted in a 2–2.5-fold increase in lipolysis (Fig. 4A). Both FSP27 silencing and TNF-α treatment caused similarly dramatic decreases in FSP27 protein levels (Fig. 4 B, C). Under these conditions, TNF-α caused a smaller but still significant decrease in perilipin levels, as previously reported (13). However, FSP27 depletion by gene silencing did not significantly change perilipin levels, although we detected increased glycerol release, suggesting that perilipin is unable to compensate for decreased FSP27 levels in preventing lipolysis under these conditions. Thus, depletion of FSP27 correlates with increased glycerol release, implicating control of FSP27 as a potential mechanism of regulation of lipolytic rate.
Fig. 4.
TNF-α treatment of 3T3-L1 adipocytes increases lipolysis that is enhanced by further depletion of FSP27 using siRNA. A: Glycerol released in medium was measured in 3T3-L1 adipocytes electroporated with scrambled (Scr) or FSP27 siRNA and treated with 10 ng/ml TNF-α for 16 h. A.U., arbitrary units. B: Western blot analysis using FSP27, perilipin (PLIN), and actin antibodies showing FSP27 and PLIN protein levels in control and under TNF-α-treated conditions. MW, molecular weight. C: Densitometric analysis of FSP27 and PLIN protein levels in siRNA or TNF-α-treated cells. Data are expressed as means ± SEM for six different experiments. KD, knock-down. P values were calculated using ANOVA; *, P < 0.05; **, P < 0.0005.
TNF-α-mediated decrease in FSP27 is associated with reduced lipid droplet size and increased glycerol release
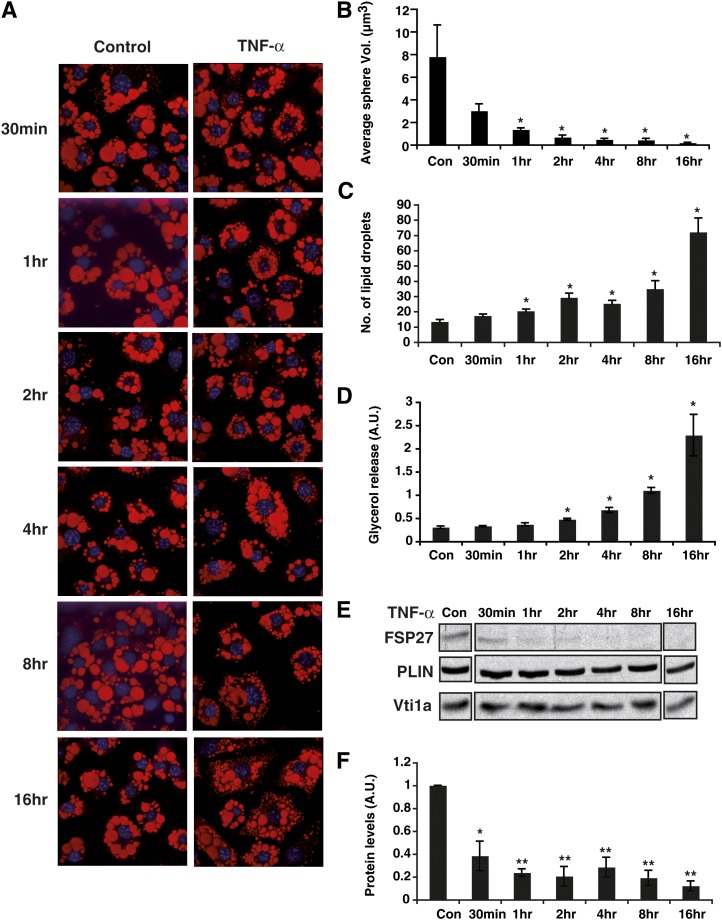
To further address potential regulation of lipolysis by FSP27, we examined temporal relationships among TNF-α-mediated changes in decreased FSP27 protein levels, lipid droplet morphology, and adipocyte lipolytic rates. Mature 3T3-L1 adipocytes were treated with 10 ng/ml TNF-α for various times between 30 min and 16 h, and media samples were collected and assayed for glycerol release. There was a significant increase in lipolysis starting at 2 h after addition of TNF-α to the adipocytes, while the highest increase in lipolytic rate occurred at 16 h (Fig. 5D). Whereas FSP27 protein levels started to decrease as early as 30 min after addition of TNF-α, the protein was almost completely undetectable after 2 h of treatment (Fig. 5E, F). The sizes of lipid droplets within adipocytes were already reduced after only 30 min of TNF-α treatment, and increased numbers of lipid droplets could also be observed at this time (Fig. 5A–C). These changes became statistically significant after 1 h of TNF-α treatment. Thus, the rapid TNF-α-mediated decrease in FSP27 protein was temporally associated with the formation of numerous smaller lipid droplets, consistent with the idea that FSP27 downregulation facilitates morphological changes of lipid droplets. Increased glycerol release follows these events, suggesting that the change in lipid droplet morphology that results from loss of FSP27 in turn permits subsequent events that increase lipolytic rate.
Fig. 5.
TNF-α-mediated decrease in FSP27 is associated with reduced lipid droplet size and increased glycerol release. Mature 3T3-L1 adipocytes were treated with TNF-α for 30 min, 1 h, 2 h, 4 h, 8 h, and 16 h. A: Confocal microscope images showing lipid droplets stained with Oil Red O (red) and nuclei stained with DAPI (blue). Images are representative of 90 random fields imaged from three different experiments. B: Quantification of lipid droplet volume using MetaMorph imaging software. Data are means ± SEM of 10 cells for each condition from different experiments. Con, control. C: Quantification of numbers of lipid droplets per cell for each time point. D: Glycerol released into medium after 10 ng/ml TNF-α treatment for 30 min, 1 h, 2 h, 4 h, 8 h, and 16 h. E: Western blot analysis showing FSP27 levels after 10 ng/ml TNF-α treatment for 30 min, 1 h, 2 h, 4 h, 8 h, and 16 h. F: Densitometry showing decrease of FSP27 protein levels after TNF-α treatment. Data are expressed as means ± SEM from three different experiments. P values were calculated using ANOVA;*, P < 0.05; **, P < 0.005.
Expression of adenoviral FSP27 protects against TNF-α-mediated lipolysis and reduced lipid droplet size
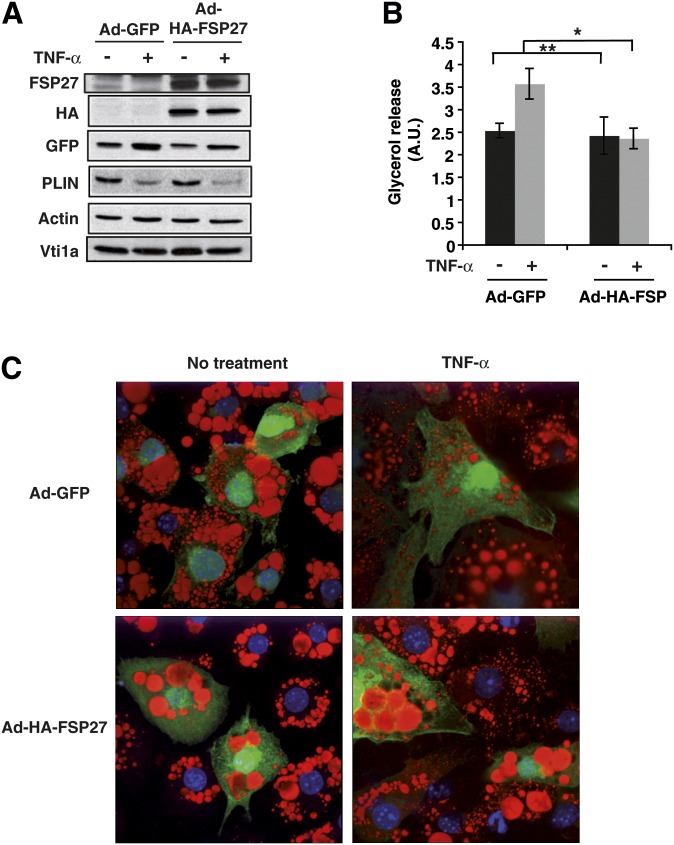
To test the hypothesis that the action of TNF-α on adipocyte lipolysis relies on its ability to deplete FSP27 protein levels, we developed a strategy to maintain FSP27 levels in adipocytes in the presence of TNF-α. To achieve this, we generated an HA-tagged FSP27 (HA-FSP27) construct incorporated into an adenovirus expression system that also elicited GFP expression from a different promoter, thus maintaining FSP27 levels when the endogenous FSP27 expression was reduced by TNF-α. Adenovirus expressing GFP alone was used to infect control cells. When these reagents were used, recombinant adenovirus-directed expression of HA-FSP27 remained essentially constant, while TNF-α treatment depleted endogenous FSP27 in control vector-infected cells, as expected (Fig. 6A). Under these conditions, the expected decrease in perilipin protein level in response to the cytokine was also observed. Despite this, adenovirus-mediated expression of HA-FSP27 in cultured adipocytes blocked the increase in lipolysis caused by TNF-α (Fig. 6B).
Fig. 6.
Adenoviral expression of FSP27 protects against TNF-α-mediated lipolysis and lipid droplet size diminution. 3T3-L1 adipocytes were infected with control virus (Ad-GFP) or HA-FSP27-expressing virus (Ad-HA-FSP27) on the fourth day of differentiation. On the fifth day, cells were serum starved overnight, treated with 10 ng/ml TNF-α for 24 h, and analyzed for protein, glycerol release, and lipid droplet morphology. A: Western blot analysis using FSP27, HA, GFP, perilipin (PLIN), actin, and Vti1a antibodies show maintenance of HA-tagged FSP27 protein levels even after treatment of TNF-α. Actin is the loading control for HA, GFP, and PLIN, and Vti1a is the loading control for FSP27. B: Glycerol released into the medium in 24 h with or without TNF-α treatment. A. U., arbitrary units. C: Confocal microscopy image showing lipid droplets stained with Oil Red O (red), nuclei stained with DAPI (blue), and GFP expression (green) in 3T3-L1 adipocytes expressing Ad-GFP or Ad-HA-FSP27 in the presence or absence of TNF-α. Data are expressed as means ± SEM for five individual experiments. P values were calculated using ANOVA; *, P < 0.05; **, P < 0.0005.
TNF-α treatment, isoproterenol, and FSP27 depletion in cultured adipocytes have been shown to cause a time-dependent change in the morphology of lipid droplets such that they are smaller in size and increased in number (3, 16). Therefore, we wanted to test whether high FSP27 expression could prevent this change in lipid droplet morphology upon TNF-α treatment. Control or HA-FSP27 adenovirus was added to differentiated adipocytes seeded on coverslips, and cells were treated with 10 ng/ml TNF-α. Cells were then stained with Oil Red O to visualize lipid droplets. Treatment of the cultured adipocytes with TNF-α for 16 h caused formation of lipid droplets that were smaller and more abundant than in control cells, as expected. However, TNF-α treatment of adipocytes expressing HA-FSP27 maintained larger, fewer lipid droplets than cells expressing GFP alone or uninfected adipocytes in the same field (Fig. 6C). These results demonstrate that sustained FSP27 expression can block the effects of TNF-α on both lipid droplet morphology and lipolysis, even though perilipin levels are diminished. This suggests that depletion of FSP27 is part of the mechanism by which TNF-α increases lipolysis in adipocytes.
Isoproterenol delays degradation of FSP27 in 3T3-L1 adipocytes
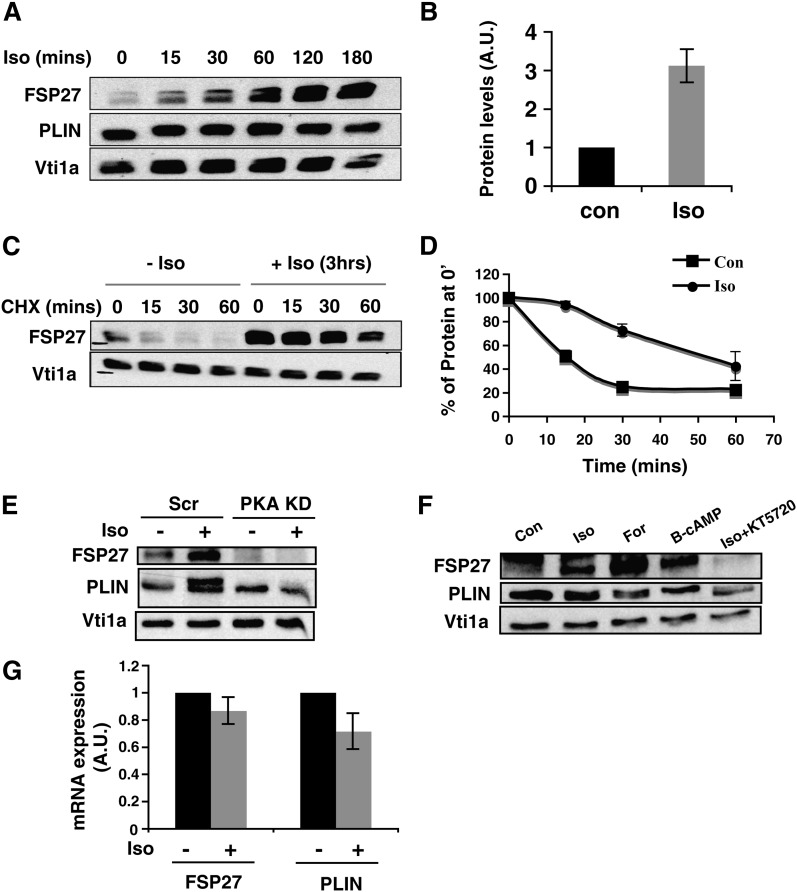
We also tested the role of FSP27 in β-adrenergic receptor-stimulated lipolysis, which is a normal physiological pathway of starvation-induced lipolysis in adipocytes. First, we tested the effect of the β-adrenergic receptor agonist, isoproterenol, on FSP27 levels. Surprisingly, treatment of mature adipocytes with 10 µM isoproterenol for 3 h increased FSP27 protein levels in a time-dependent manner without significant effects on mRNA levels (Fig. 7A, B, G).Since FSP27 is a short-lived protein, the effects of isoproterenol treatment on degradation of FSP27 in adipocytes was determined by treating cells with 10 µM isoproterenol for 3 h and then treating them with cycloheximide to block new protein synthesis. We observed delayed degradation of FSP27 after isoproterenol treatment, increasing its half-life from about 15 min to more than 1 h (Fig. 7C, D). Because β-adrenergic receptor stimulation increases cAMP levels and activates PKA, we next tested whether isoproterenol-mediated stabilization of FSP27 occurs through this same canonical PKA pathway. Depletion of PKA by using siRNA decreased the basal FSP27 level and inhibited upregulation of FSP27 after isoproterenol treatment (Fig. 7E). Effectiveness of the PKA knockdown was evident from the lack of perilipin phosphorylation (detected as a shifted band in isoproterenol-treated cells) upon PKA silencing (Fig. 7E). FSP27 upregulation after isoproterenol treatment was inhibited also by the PKA inhibitor KT5720, while the effect of isoproterenol on FSP27 was mimicked by either the adenylate cyclase activator forskolin or 8-bromo-cAMP, a soluble cAMP analog, in combination with IBMX, a phosphodiesterase inhibitor (Fig. 7F). Therefore, β-adrenergic stimulation signals via increased intracellular cAMP levels and PKA activation to upregulate FSP27 protein by delaying its degradation.
Fig. 7.
Isoproterenol delays degradation of FSP27 to increase protein level in 3T3-L1 adipocytes. A: Lysates from adipocytes treated with 10 µM isoproterenol (Iso) for 15, 30, 60, 120, or 180 min were immunoblotted for FSP27, perilipin (PLIN), or Vti1a (loading control). B: Densitometry of immunoblot shows increased FSP27 protein level after 180 min of 10 µM isoproterenol treatment of mature adipocytes. A.U., arbitrary units; Con, control. C: Mature adipocytes treated with 10 µM isoproterenol, pulsed with 5 µg/ml cycloheximide (CHX), and chased for 15, 30, and 60 min show delayed degradation of FSP27 in the presence of isoproterenol compared with that of control (0). D: Densitometry of FSP27 level in the presence or absence of 10 µM isoproterenol shows delayed FSP27 degradation in the presence of isoproterenol. E: Adipocytes transfected with either Scr or PKA siRNA and treated with or without 10 µM isoproterenol. The effect of isoproterenol on FSP27 is abrogated when PKA is knocked down (KD). The absence of perilipin phosphorylation despite isoproterenol treatment in adipocytes treated with PKA siRNA is used as the positive control. F: Mature adipocytes were treated with 10 µM isoproterenol, 5 µM forskolin (For), 1 mM 8-bromo-cAMP (B-cAMP) plus IBMX, or 80 µM KT5720 plus 10 µM isoproterenol. The effect of isoproterenol on FSP27 is mimicked by forskolin and 8-bromo-cAMP and inhibited by KT5720. G: Mature adipocytes treated with 10 µM isoproterenol were harvested for RNA and analyzed for FSP27 mRNA. It shows no change in FSP27 transcript with isoproterenol treatment. Data are expressed as means ± SEM for six individual experiments.
Expression of FSP27 protects against isoproterenol-mediated diminution of lipid droplet size but not against lipolysis
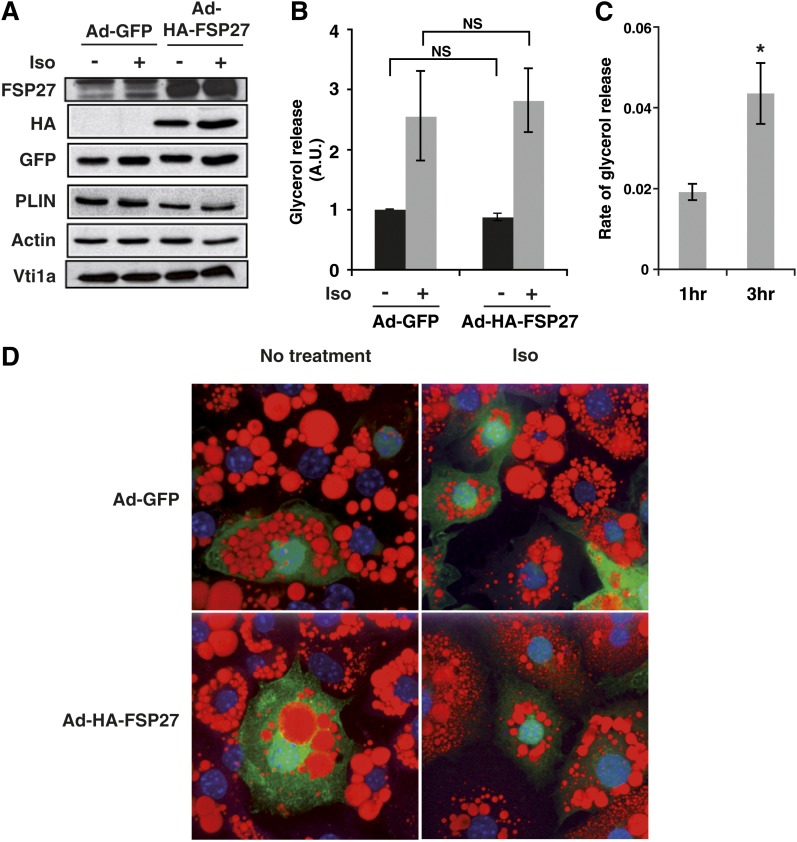
Based on the results described above, the lipolytic action of isoproterenol paradoxically increases the abundance of FSP27 protein, which normally acts to inhibit lipolysis under basal conditions. We hypothesize that isoproterenol upregulates FSP27 as a feedback mechanism to restrain excessive lipolysis induced by potent β-adrenergic stimuli. Therefore, we next tested whether expression of HA-FSP27 could attenuate the action of isoproterenol on lipolysis. The 3T3-L1 adipocytes were infected with control adenovirus or HA-FSP27 adenovirus for 72 h, as described previously, and treated with 10 µM isoproterenol for 3 h. Using adenovirus as vector, we were able to express FSP27 protein in excess of the isoproterenol-mediated increase in endogenous FSP27 (Fig. 8A). When adipocytes expressing HA-FSP27 were treated with 10 µM isoproterenol for 3 h, the sharp increase in lipolysis was still observed (Fig. 8B). As isoproterenol upregulates FSP27, it is possible that the protective effect of FSP27 on lipolysis is already saturated, and thus the adenovirus-expressed FSP27 does not have an additional protective effect. Thus, we tested whether there are any differences in the rates of lipolysis mediated by catecholamines in the absence of FSP27. As isoproterenol treatment increases FSP27 protein within 3 h, we reasoned that experimentally reducing isoproterenol's ability to upregulate FSP27 using siRNA-mediated gene silencing might result in increased rates of lipolysis within that time frame. Thus, FSP27 siRNA or Scr siRNA was transfected into adipocytes on the fourth day after differentiation. Then, 72 h after siRNA transfection, adipocytes were treated with 10 µM isoproterenol for 3 h, and the rates of glycerol release in the medium was measured at the end of 1 h and 3 h. Results show that the difference in rates of glycerol release in response to isoproterenol in adipocytes treated with FSP27 compared with Scr siRNA was significantly higher at 3 h than at 1 h (Fig. 8C). This suggests that increased FSP27 protein in response to isoproterenol is an intrinsic mechanism that serves to dampen its potent lipolytic effect, as we observe increased lipolysis by isoproterenol when FSP27 cannot be upregulated.
Fig. 8.
Expression of adenoviral FSP27 protects against isoproterenol-mediated lipid droplet size diminution but not lipolysis. The 3T3-L1 adipocytes were infected with control virus (Ad-GFP) or HA-FSP27-expressing virus (Ad-HA-FSP27) on the fourth day of differentiation. On the fifth day, cells were serum starved overnight, treated with 10 µM isoproterenol (Iso) for 3 h, and analyzed for protein, glycerol release, and lipid droplet morphology. A: Western blot analysis using FSP27, HA, GFP, PLIN, Actin, and Vti1a antibodies. Actin is the loading control for HA, GFP, and PLIN, and Vti1a is the loading control for FSP27. B: Glycerol released into the medium in 3 h with or without isoproterenol. C: Difference in the rate of glycerol released [calculated using the equation (FSP27 KD + Iso) – (Scr + iso), where KD is knockdown] in response to isoproterenol from 3T3-L1 adipocytes treated with Scr or FSP27 siRNA shows increased rate of lipolysis at 3 h compared with 1 h. D: Confocal microscopy image shows lipid droplets stained with Oil Red O (red), nuclei stained with DAPI (blue), and GFP (green) expression in 3T3-L1 adipocytes expressing Ad-GFP or Ad-HA-FSP27 in the presence or absence of isoproterenol. Data are expressed as means ± SEM for three individual experiments. P values were calculated using ANOVA for data shown in panel B and Student's t-test for data shown in panel C; *, P < 0.05.
Our previous results show that lipolysis and lipid droplet dispersion are distinct processes. Thus, we also tested the effect of FSP27 on isoproterenol-mediated lipid droplet dispersion. Our results show that HA-FSP27 expression from adenovirus was able to protect against the isoproterenol-mediated decrease in lipid droplet size. Isoproterenol caused decreased lipid droplet size in cells expressing GFP alone and in uninfected cells, while cells expressing FSP27 showed persistently larger lipid droplets, even in the presence of isoproterenol (Fig. 8D). Taken together, these data indicate that lipolysis and change in lipid droplet morphology in response to isoproterenol are two distinct processes, supporting previous observations (10, 37) and that FSP27 has a role in the formation of larger lipid droplets, independent of the lipolysis.
Ubiquitination of FSP27 in response to TNF-α and isoproterenol
As isoproterenol upregulated FSP27 protein by delaying its degradation, we hypothesized that isoproterenol would decrease FSP27 ubiquitination. Ubiquitination of FSP27 might also be regulated in response to TNF-α, which decreases FSP27 levels. Mature adipocytes were treated with the proteasome inhibitor, MG132, alone or in combination with isoproterenol or TNF-α for 3 h, and FSP27 and ubiquitinated proteins were detected in FSP27 immunoprecipitates. Analysis of FSP27 by using ubiquitin antibody showed decreased ubiquitination of FSP27 in response to isoproterenol (Fig. 9 A, B, C). Conversely, ubiquitination of FSP27 in response to TNF-α was similar to that with MG132-only treatment. Thus, the observed stabilization of FSP27 protein in response to isoproterenol treatment correlates with reduced ubiquitin conjugation to FSP27.
Fig. 9.
Isoproterenol (Iso) alters ubiquitination of FSP27 to modulate FSP27 in 3T3-L1 adipocytes. Mature adipocytes were treated with MG132 alone or with TNF-α or isoproterenol, FSP27- immunoprecipitated using FSP27 antibody, and probed with ubiquitin and FSP27 antibody. A: Western blot showing total lysate of adipocytes treated with MG132 alone or with TNF-α or isoproterenol. B: FSP27 immunoprecipitated (IP) with FSP27 antibody and probed with ubiquitin (Ub) antibody. C: FSP27 immunoprecipitated with FSP27 antibody and probed with FSP27 antibody. Data are representative of four separate experiments. Lanes G, IgG; lanes F, FSP27.
ATGL silencing using siRNA and treatment with TNF-α and isoproterenol
Mouse adipocytes contain multiple lipases including HSL and ATGL that participate in mediating basal and stimulated lipolysis (11, 38–41). Consistent with the concept that these lipases are required for lipolysis, the sizes of lipid droplets were reported to be larger in ATGL- or HSL-depleted adipocytes (11, 42, 43). Thus, we examined whether the lipase ATGL is required to mediate the enhanced lipolysis caused by FSP27 depletion and TNF-α treatment. We used siRNA to deplete the lipase ATGL in 3T3-L1 adipocytes and assayed glycerol release. As shown in Supplementary Fig. S2, ATGL depletion by siRNA prevented stimulation of lipolysis by TNF-α treatment or by FSP27 silencing. Isoproterenol was also unable to enhance lipolysis in the absence of ATGL (data not shown) as expected and reported previously (11, 38, 39, 41). These results strongly suggest that TNF-α and FSP27 regulate adipocyte lipolysis through the canonical lipases regulated by catecholamines, which includes TAG hydrolysis catalyzed by the lipase ATGL.
DISCUSSION
The present work and another recent study (28) show that FSP27 has a very short half-life and that its rapid turnover is due to ubiquitination and degradation via the proteasome (Fig. 1). These characteristics are similar to those of other lipid droplet proteins such as ADRP and another CIDE protein, CIDEA (26, 27). Recent parallel studies have also shown that three lysine residues in the C-terminal region of FSP27, K224, K226, and K236, are critical for ubiquitination and therefore for the stability of FSP27 protein (28). Overexpression of the FSP27 mutant lacking these three lysines was shown to stabilize exogenous and endogenous FSP27 and increase lipid droplet storage in adipocytes. Our results advance this finding by demonstrating that acute regulation of FSP27 levels is one of the mechanisms by which external stimuli such as TNF-α and other inflammatory cytokines modulate lipolysis (Figs. 2, 3, 4, 6).
The cytokine TNF-α has been reported to affect rates of lipolysis in adipocytes at multiple levels, including enhancing cAMP-dependent signaling pathways as well as altering amounts and activities of triglyceride lipases and other lipid droplet-associated proteins (1, 44–46). Here we show that TNF-α causes rapid and dramatic depletion of the lipid droplet protein FSP27. This depletion is associated with the decreased volume of lipid droplets and increased lipolysis seen in response to the cytokine (Fig. 5). Remarkably, restoration of FSP27 protein levels by adenovirus-mediated overexpression in TNF-α-treated cells is sufficient to block both the droplet diminution and the increased lipolysis induced by TNF-α treatment (Fig. 6). This result suggests that downregulation of FSP27 is an essential and early step in the sequence of TNF-α-triggered events that lead to increased triglyceride turnover in adipocytes. Interestingly, this regulation of FSP27 does not seem to be specific to TNF-α, as IL-1β and IFN-γ, cytokines that have been shown to induce adipocyte lipolysis, also decrease FSP27 levels before the glycerol levels increase in the medium (Fig. 2 D–F and Supplementary ).
Data presented here show nearly complete inhibition of FSP27 protein expression by TNF-α treatment in cultured adipocytes. We also observed a significant decrease in FSP27 mRNA after TNF-α treatment (Fig. 2A), consistent with results previously reported (20). Thus, the decrease in FSP27 protein level could be primarily due to the decrease in FSP27 transcript levels, although we have not ruled out other mechanisms that affect the protein itself. These results in 3T3-L1 adipocytes seem to also be relevant in primary cells, as we obtained the same results in adipocytes prepared from mouse fat tissue (Fig. 3). A previous study has shown that TNF-α decreases the expression of a transcription factor, C/EBPα, corresponding to decreased activity of a FSP27 promoter-CAT reporter construct in cultured adipocytes. Thus, downregulation of FSP27 transcripts by TNF-α could be partly due to a decrease in C/EBPα levels (20, 24, 47). It has also been shown that transcription of FSP27 is PPARγ-dependent in hepatocytes (48) and adipocytes (18, 49) and that the effect of TNF-α on FSP27 transcription could be due to the previously observed decrease in PPARγ protein levels in TNF-α-treated adipocytes (50, 51). TNF-α has also been reported to regulate lipolysis in 3T3-L1 adipocytes through mitogen-activated protein kinases (52); however, inhibition of these kinases was not able to block the depletion of FSP27 transcripts (20). The detailed molecular mechanisms whereby TNF-α downregulates FSP27 mRNA and protein levels is an important topic for future investigations.
Based on the previous work cited above, the depletion of FSP27 by TNF-α action could be one mechanism acting in parallel with other mechanisms by which TNF-α stimulates lipolysis in adipocytes. For example, we observed that when FSP27 is depleted using siRNA and the cells are then treated with TNF-α, the increase in glycerol release is greater than with FSP27 depletion or TNF-α treatment alone (Fig. 4A). However, this also correlates with lower levels of FSP27 protein after FSP27 silencing with siRNA plus TNF-α treatment compared with that of either treatment alone (Fig. 4B, C). Importantly, there was no significant change in perilipin expression under these conditions. These data not only support the role of FSP27 in TNF-α-stimulated lipolysis but also indicate that total lipolytic rates correlate with the level of FSP27 present in adipocytes. The key role of FSP27 in TNF-α-stimulated lipolysis is further supported by the ability of FSP27 to block TNF-α-stimulated lipolysis in 3T3-L1 adipocytes when there is sustained FSP27 expression using adenovirus infection (Fig. 6B). Consistent with the protection against lipolysis, the phenotype of lipid droplets with FSP27 expression was large and few in number, as opposed to smaller, numerous droplets that occurred in response to TNF-α treatment in control cells (Fig. 6C). Again, under these conditions, the effects of sustained expression of FSP27 on TNF-α-mediated lipolysis and lipid droplet phenotypic change occurred despite depletion of perilipin (Fig. 6A, B), suggesting that the role of FSP27 is independent of perilipin protein levels. Therefore, our data imply that FSP27 depletion is an important mechanism for TNF-α action on lipolysis under the conditions of our experiments.
The role of FSP27 in lipolysis mediated by β-adrenergic receptor activation is different than its role in TNF-α-mediated lipolysis. The β-adrenergic receptor activation surprisingly increases FSP27 levels in a time-dependent manner (Fig. 7A, B), which contrasts with the inhibitory effects of TNF-α described above. The increase in FSP27 is due to decreased ubiquitination and degradation (Fig. 9B, C). We also show that β-adrenergic receptor activation signals through the canonical PKA pathway and increases intracellular cAMP, as the addition of forskolin and soluble 8-bromo-cAMP mimics the effect, whereas PKA inhibition by KT5720 or PKA depletion using siRNA abrogates the effect (Fig. 7E, F). A recent study shows that oleic acid treatment of adipocytes also increases FSP27 levels in a time-dependent manner and that FSP27 stabilization after isoproterenol treatment is dependent upon TAG synthesis (28). Oleic acid also increases expression of the lipid droplet protein ADRP via the transcription factor AP1 and the PPAR response element (53). The increase in FSP27 after oleic acid treatment may involve similar mechanisms because the FSP27 promoter also has a PPAR response element. Therefore, the mechanism that upregulates FSP27 after oleic acid treatment could be different from the β-adrenergic pathway, which has no effect on transcription.
Nonetheless, the increase in FSP27 in response to a catecholamine lipolytic stimulus is counterintuitive, and adenovirus-mediated expression of FSP27 does not reduce the robust lipolytic effect of isoproterenol (Fig. 8B). However, it is possible that the adenovirus-mediated expression of FSP27 has no further effect against isoproterenol-mediated lipolysis because the function of the isoproterenol-enhanced FSP27 protein is already at the maximum level. On the other hand, when FSP27 is depleted using siRNA and the adipocytes are treated with isoproterenol, there is an increased rate of lipolysis at 3 h, correlating with the difference in cellular FSP27 levels between Scr and FSP27 siRNA-treated adipocytes (Fig. 8C). A previous study showed impaired isoproterenol-mediated lipolysis when FSP27 was depleted in 3T3-L1 adipocytes (54); however, data were presented as the ratio of NEFA released under isoproterenol-stimulated condition to NEFA released under basal conditions, and the decrease in the ratio seen under FSP27-silenced conditions could be due to increased basal NEFA release as a result of FSP27 silencing. We observed similar increases in glycerol release after isoproterenol treatment in FSP27-silenced cells and control cells. Furthermore, our results show depletion of FSP27 is not a necessary step for isoproterenol-mediated lipolysis; however, FSP27 depletion using siRNA increases the rate of lipolysis in response to isoproterenol. Thus, we hypothesize that upregulation of FSP27 protein is a feedback mechanism to restrain excessive lipolysis after strong lipolytic stimuli such as catecholamines.
Our data also suggest that lipolysis and lipid droplet size diminution in response to lipolytic agents are different processes. The decrease in lipid droplet size is measurable prior to the detectable release of glycerol in adipocytes treated with TNF-α (Fig. 5A–D). In addition, adenoviral FSP27 expression protects against both lipid droplet size decrease and lipolysis in response to TNF-α. Furthermore, FSP27 appears to be a point of divergence between TNF-α and β-adrenergic agonist-stimulated lipolysis since overexpression of FSP27 does not block increased lipolysis induced by isoproterenol treatment of adipocytes. However, adenovirus-directed expression of FSP27 is able to protect against isoproterenol-mediated lipid droplet reduction. Thus, our study establishes lipolysis and lipid droplet morphology changes as distinct processes.
Although FSP27 shares structural and functional similarities with perilipin, there is evidence for divergent and nonredundant roles for these two lipid droplet proteins. Structurally, FSP27 was noted to contain domains homologous to TAG shielding and targeting domains in perilipin (18). Phenotypes of mice null for both proteins include resistance to diet-induced obesity (14, 15, 21); however, perilipin null mice are glucose-intolerant, whereas FSP27 null mice have normal glucose tolerance (55). These data suggest divergence in the functions of the two proteins in whole-body metabolism. Both perilipin and FSP27 can be downregulated by TNF-α; however, under our experimental conditions, siRNA-mediated knockdown of FSP27 led to increased lipolysis despite no significant change in perilipin level (Fig. 4A–C). In addition, sustained expression of FSP27 using adenovirus to overcome TNF-α effects on FSP27 was able to protect against the lipolytic effect of TNF-α despite the depletion of perilipin. Taken together, these results show that FSP27, although similar to perilipin, is an important lipid droplet protein with an independent function that is not compensated by other lipid droplet proteins such as perilipin.
In addition to effects of lipid droplet proteins like FSP27 and perilipin, lipolysis ultimately depends on the enzymatic activity of lipases such as HSL and ATGL. Lipid droplet TAG is hydrolyzed by ATGL into diacylglycerol, which, in turn, is acted upon by HSL to release free fatty acids and glycerol (41, 56). It has been shown that HSL is required for catecholamine-stimulated lipolysis (43, 57). The localization of HSL to lipid droplets could be perilipin phosphorylation-independent in the basal state and perilipin phosphorylation-dependent under the stimulated condition (58). However, the lipolytic effect of TNF-α has been considered to be due to its effect on various proteins and transcription factors that mediate changes in expression of multiple targets, and the requirement of specific lipases for its action has not been reported. Here we show that ATGL is required for TNF-α and isoproterenol to stimulate lipolysis (Supplementary Fig. S2). Furthermore, in the absence of the lipase ATGL, depletion of FSP27 does not increase lipolysis. Thus, both FSP27 and TNF-α require ATGL to mediate their effects on lipolysis in adipocytes, similar to the actions of catecholamines. Important issues for future experiments are characterization of the molecular mode by which FSP27 mediates its effect on lipolysis and whether its mechanism of action involves direct or indirect engagement of the ATGL and HSL lipases.
Note added in proof
Yoshikazu Tamori, one of the co-authors on the paper, was inadvertently left out of the manuscript accepted for publication. All other authors and the Journal's Editor-in-Chief approved the addition after the article was in the proof stage. Dr. Tamori will appear as an author in all forms of the article except the originally-accepted Paper In Press.
Supplementary Material
Acknowledgments
The authors thank Drs. Kasuga and Nishino, Kobe University Graduate School of Medicine, Kobe, Japan, for providing affinity purified rabbit polyclonal FSP27 antibody. We also thank Dr. Yong-Xu Wang, University of Massachusetts Medical School, Worcester, MA, for helping to make the adenovirus vector for FSP27 expression, and Dr. Paul Furcinitti for use of confocal microscopy facilities. We also thank Dr. Joseph Virbasius for help in preparation of the manuscript.
Footnotes
Abbreviations:
- A.U.
- arbitrary unit
- ATGL
- adipose triacylglycerol lipase
- C/EBPα
- CCAAT/enhancer binding protein α
- CIDEC
- cell death-inducing DNA fragmentation factor like effector-α C
- DAPI
- 4',6-diamidino-2-phenylindole
- FSP27
- fat specific protein 27
- GFP
- green fluorescent protein
- HA
- hemagglutinin
- HSL
- hormone-sensitive lipase
- IBMX
- isobutylmethylxanthine
- IL
- interleukin
- OXPAT/MLDP
- oxidative tissues-enriched PAT protein/myocardial lipid droplet protein
- PAT
- perilipin-adipophilin-TIP47
- PKA
- protein kinase A
- PLIN
- perilipin
- PPARγ
- peroxisome proliferator-activated receptor γ
- SVF
- stromal–vascular fraction
- TAG
- triacylglycerol
- TIP47
- tail interacting protein 47
- TNF-α
- tumor necrosis factor-α
This work was supported by National Institutes of Health Grants DK-30898 and DK-60837 (to M. P. Czech), American Diabetes Association Grant: 7-08-RA-57, USDA, Agricultural Research Service under Contract no. 58-1950-7-707 and NIH Grant DK0822574 (to A. S. Greenberg). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other funding agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures.
REFERENCES
- 1.Wang S., Soni K. G., Semache M., Casavant S., Fortier M., Pan L., Mitchell G. A. 2008. Lipolysis and the integrated physiology of lipid energy metabolism. Mol. Genet. Metab. 95: 117–126. [DOI] [PubMed] [Google Scholar]
- 2.Ducharme N. A., Bickel P. E. 2008. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 149: 942–949. [DOI] [PubMed] [Google Scholar]
- 3.Wolins N. E., Brasaemle D. L., Bickel P. E. 2006. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 580: 5484–5491. [DOI] [PubMed] [Google Scholar]
- 4.Miura S., Gan J. W., Brzostowski J., Parisi M. J., Schultz C. J., Londos C., Oliver B., Kimmel A. R. 2002. Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J. Biol. Chem. 277: 32253–32257. [DOI] [PubMed] [Google Scholar]
- 5.Gronke S., Beller M., Fellert S., Ramakrishnan H., Jackle H., Kuhnlein R. P. 2003. Control of fat storage by a Drosophila PAT domain protein. Curr. Biol. 13: 603–606. [DOI] [PubMed] [Google Scholar]
- 6.Kimmel A. R., Brasaemle D. L., McAndrews-Hill M., Sztalryd C., Londos C. 2010. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J. Lipid Res. 51: 468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brasaemle D. L., Levin D. M., Adler-Wailes D. C., Londos C. 2000. The lipolytic stimulation of 3T3–L1 adipocytes promotes the translocation of hormone-sensitive lipase to the surfaces of lipid storage droplets. Biochim. Biophys. Acta. 1483: 251–262. [DOI] [PubMed] [Google Scholar]
- 8.Carmen G. Y., Victor S. M. 2006. Signalling mechanisms regulating lipolysis. Cell. Signal. 18: 401–408. [DOI] [PubMed] [Google Scholar]
- 9.Granneman J. G., Moore H. P., Granneman R. L., Greenberg A. S., Obin M. S., Zhu Z. 2007. Analysis of lipolytic protein trafficking and interactions in adipocytes. J. Biol. Chem. 282: 5726–5735. [DOI] [PubMed] [Google Scholar]
- 10.Marcinkiewicz A., Gauthier D., Garcia A., Brasaemle D. L. 2006. The phosphorylation of serine 492 of perilipin a directs lipid droplet fragmentation and dispersion. J. Biol. Chem. 281: 11901–11909. [DOI] [PubMed] [Google Scholar]
- 11.Miyoshi H., Perfield J. W., II, Obin M. S., Greenberg A. S. 2008. Adipose triglyceride lipase regulates basal lipolysis and lipid droplet size in adipocytes. J. Cell. Biochem. 105: 1430–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore H. P., Silver R. B., Mottillo E. P., Bernlohr D. A., Granneman J. G. 2005. Perilipin targets a novel pool of lipid droplets for lipolytic attack by hormone-sensitive lipase. J. Biol. Chem. 280: 43109–43120. [DOI] [PubMed] [Google Scholar]
- 13.Souza S. C., de Vargas L. M., Yamamoto M. T., Lien P., Franciosa M. D., Moss L. G., Greenberg A. S. 1998. Overexpression of perilipin A and B blocks the ability of tumor necrosis factor alpha to increase lipolysis in 3T3–L1 adipocytes. J. Biol. Chem. 273: 24665–24669. [DOI] [PubMed] [Google Scholar]
- 14.Tansey J. T., Sztalryd C., Gruia-Gray J., Roush D. L., Zee J. V., Gavrilova O., Reitman M. L., Deng C. X., Li C., Kimmel A. R., et al. 2001. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc. Natl. Acad. Sci. U S A. 98: 6494–6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Botas J., Anderson J. B., Tessier D., Lapillonne A., Chang B. H., Quast M. J., Gorenstein D., Chen K. H., Chan L. 2000. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat. Genet. 26: 474–479. [DOI] [PubMed] [Google Scholar]
- 16.Puri V., Konda S., Ranjit S., Aouadi M., Chawla A., Chouinard M., Chakladar A., Czech M. P. 2007. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J. Biol. Chem. 282: 34213–34218. [DOI] [PubMed] [Google Scholar]
- 17.Puri V., Virbasius J. V., Guilherme A., Czech M. P. 2008. RNAi screens reveal novel metabolic regulators: RIP140, MAP4k4 and the lipid droplet associated fat specific protein (FSP) 27. Acta Physiol. ( Oxf. ). 192: 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puri V., Ranjit S., Konda S., Nicoloro S. M., Straubhaar J., Chawla A., Chouinard M., Lin C., Burkart A., Corvera S., et al. 2008. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc. Natl. Acad. Sci. U S A. 105: 7833–7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller P., Petrie J. T., De Rose P., Gerin I., Wright W. S., Chiang S. H., Nielsen A. R., Fischer C. P., Pedersen B. K., MacDougald O. A. 2008. Fat-specific protein 27 regulates storage of triacylglycerol. J. Biol. Chem. 283: 14355–14365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J. Y., Liu K., Zhou S., Tillison K., Wu Y., Smas C. M. 2008. Assessment of fat-specific protein 27 in the adipocyte lineage suggests a dual role for FSP27 in adipocyte metabolism and cell death. Am. J. Physiol. Endocrinol. Metab. 294: E654–E667. [DOI] [PubMed] [Google Scholar]
- 21.Nishino N., Tamori Y., Tateya S., Kawaguchi T., Shibakusa T., Mizunoya W., Inoue K., Kitazawa R., Kitazawa S., Matsuki Y., et al. 2008. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J. Clin. Invest. 118: 2808–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inohara N., Koseki T., Chen S., Wu X., Nunez G. 1998. CIDE, a novel family of cell death activators with homology to the 45 kDa subunit of the DNA fragmentation factor. EMBO J. 17: 2526–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubio-Cabezas O., Puri V., Murano I., Saudek V., Semple R. K., Dash S., Hyden C. S., Bottomley W., Vigouroux C., Magre J., et al. 2009. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med. 1: 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danesch U., Hoeck W., Ringold G. M. 1992. Cloning and transcriptional regulation of a novel adipocyte-specific gene, FSP27. CAAT-enhancer-binding protein (C/EBP) and C/EBP-like proteins interact with sequences required for differentiation-dependent expression. J. Biol. Chem. 267: 7185–7193. [PubMed] [Google Scholar]
- 25.Brasaemle D. L., Dolios G., Shapiro L., Wang R. 2004. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3–L1 adipocytes. J. Biol. Chem. 279: 46835–46842. [DOI] [PubMed] [Google Scholar]
- 26.Xu G., Sztalryd C., Lu X., Tansey J. T., Gan J., Dorward H., Kimmel A. R., Londos C. 2005. Post-translational regulation of adipose differentiation-related protein by the ubiquitin/proteasome pathway. J. Biol. Chem. 280: 42841–42847. [DOI] [PubMed] [Google Scholar]
- 27.Chan S. C., Lin S. C., Li P. 2007. Regulation of Cidea protein stability by the ubiquitin-mediated proteasomal degradation pathway. Biochem. J. 408: 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nian Z., Sun Z., Yu L., Toh S. Y., Sang J., Li P. 2010. Fat-specific protein 27 undergoes ubiquitin-dependent degradation regulated by triacylglycerol synthesis and lipid droplet formation. J. Biol. Chem. 285: 9604–9615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang Z. Y., Zhou Q. L., Coleman K. A., Chouinard M., Boese Q., Czech M. P. 2003. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc. Natl. Acad. Sci. U S A. 100: 7569–7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powelka A. M., Seth A., Virbasius J. V., Kiskinis E., Nicoloro S. M., Guilherme A., Tang X., Straubhaar J., Cherniack A. D., Parker M. G., et al. 2006. Suppression of oxidative metabolism and mitochondrial biogenesis by the transcriptional corepressor RIP140 in mouse adipocytes. J. Clin. Invest. 116: 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo J., Deng Z. L., Luo X., Tang N., Song W. X., Chen J., Sharff K. A., Luu H. H., Haydon R. C., Kinzler K. W., et al. 2007. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat. Protoc. 2: 1236–1247. [DOI] [PubMed] [Google Scholar]
- 32.Doerrler W., Feingold K. R., Grunfeld C. 1994. Cytokines induce catabolic effects in cultured adipocytes by multiple mechanisms. Cytokine. 6: 478–484. [DOI] [PubMed] [Google Scholar]
- 33.Feingold K. R., Doerrler W., Dinarello C. A., Fiers W., Grunfeld C. 1992. Stimulation of lipolysis in cultured fat cells by tumor necrosis factor, interleukin-1, and the interferons is blocked by inhibition of prostaglandin synthesis. Endocrinology. 130: 10–16. [DOI] [PubMed] [Google Scholar]
- 34.Lagathu C., Yvan-Charvet L., Bastard J. P., Maachi M., Quignard-Boulange A., Capeau J., Caron M. 2006. Long-term treatment with interleukin-1beta induces insulin resistance in murine and human adipocytes. Diabetologia. 49: 2162–2173. [DOI] [PubMed] [Google Scholar]
- 35.Patton J. S., Shepard H. M., Wilking H., Lewis G., Aggarwal B. B., Eessalu T. E., Gavin L. A., Grunfeld C. 1986. Interferons and tumor necrosis factors have similar catabolic effects on 3T3 L1 cells. Proc. Natl. Acad. Sci. U S A. 83: 8313–8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lagathu C., Bastard J. P., Auclair M., Maachi M., Capeau J., Caron M. 2003. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: prevention by rosiglitazone. Biochem. Biophys. Res. Commun. 311: 372–379. [DOI] [PubMed] [Google Scholar]
- 37.Miyoshi H., Perfield J. W., II, Souza S. C., Shen W. J., Zhang H. H., Stancheva Z. S., Kraemer F. B., Obin M. S., Greenberg A. S. 2007. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J. Biol. Chem. 282: 996–1002. [DOI] [PubMed] [Google Scholar]
- 38.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., et al. 2006. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 312: 734–737. [DOI] [PubMed] [Google Scholar]
- 39.Kershaw E. E., Hamm J. K., Verhagen L. A., Peroni O., Katic M., Flier J. S. 2006. Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes. 55: 148–157. [PMC free article] [PubMed] [Google Scholar]
- 40.Schweiger M., Schreiber R., Haemmerle G., Lass A., Fledelius C., Jacobsen P., Tornqvist H., Zechner R., Zimmermann R. 2006. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J. Biol. Chem. 281: 40236–40241. [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., et al. 2004. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 306: 1383–1386. [DOI] [PubMed] [Google Scholar]
- 42.Smirnova E., Goldberg E. B., Makarova K. S., Lin L., Brown W. J., Jackson C. L. 2006. ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep. 7: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S. P., Laurin N., Himms-Hagen J., Rudnicki M. A., Levy E., Robert M. F., Pan L., Oligny L., Mitchell G. A. 2001. The adipose tissue phenotype of hormone-sensitive lipase deficiency in mice. Obes. Res. 9: 119–128. [DOI] [PubMed] [Google Scholar]
- 44.Cawthorn W. P., Sethi J. K. 2008. TNF-alpha and adipocyte biology. FEBS Lett. 582: 117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahn Landstrom T., Mei J., Karlsson M., Manganiello V., Degerman E. 2000. Down-regulation of cyclic-nucleotide phosphodiesterase 3B in 3T3–L1 adipocytes induced by tumour necrosis factor alpha and cAMP. Biochem. J. 346: 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryden M., Arner P. 2007. Tumour necrosis factor-alpha in human adipose tissue–from signalling mechanisms to clinical implications. J. Intern. Med. 262: 431–438. [DOI] [PubMed] [Google Scholar]
- 47.Williams P. M., Chang D. J., Danesch U., Ringold G. M., Heller R. A. 1992. CCAAT/enhancer binding protein expression is rapidly extinguished in TA1 adipocyte cells treated with tumor necrosis factor. Mol. Endocrinol. 6: 1135–1141. [DOI] [PubMed] [Google Scholar]
- 48.Matsusue K., Kusakabe T., Noguchi T., Takiguchi S., Suzuki T., Yamano S., Gonzalez F. J. 2008. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab. 7: 302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim Y. J., Cho S. Y., Yun C. H., Moon Y. S., Lee T. R., Kim S. H. 2008. Transcriptional activation of Cidec by PPARgamma2 in adipocyte. Biochem. Biophys. Res. Commun. 377: 297–302. [DOI] [PubMed] [Google Scholar]
- 50.Ruan H., Hacohen N., Golub T. R., Van Parijs L., Lodish H. F. 2002. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3–L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes. 51: 1319–1336. [DOI] [PubMed] [Google Scholar]
- 51.Zhang B., Berger J., Hu E., Szalkowski D., White-Carrington S., Spiegelman B. M., Moller D. E. 1996. Negative regulation of peroxisome proliferator-activated receptor-gamma gene expression contributes to the antiadipogenic effects of tumor necrosis factor-alpha. Mol. Endocrinol. 10: 1457–1466. [DOI] [PubMed] [Google Scholar]
- 52.Souza S. C., Palmer H. J., Kang Y. H., Yamamoto M. T., Muliro K. V., Paulson K. E., Greenberg A. S. 2003. TNF-alpha induction of lipolysis is mediated through activation of the extracellular signal related kinase pathway in 3T3–L1 adipocytes. J. Cell. Biochem. 89: 1077–1086. [DOI] [PubMed] [Google Scholar]
- 53.Fan B., Ikuyama S., Gu J. Q., Wei P., Oyama J., Inoguchi T., Nishimura J. 2009. Oleic acid-induced ADRP expression requires both AP-1 and PPAR response elements, and is reduced by Pycnogenol through mRNA degradation in NMuLi liver cells. Am. J. Physiol. Endocrinol. Metab. 297: E112–E123. [DOI] [PubMed] [Google Scholar]
- 54.Magnusson B., Gummesson A., Glad C. A., Goedecke J. H., Jernas M., Lystig T. C., Carlsson B., Fagerberg B., Carlsson L. M., Svensson P. A. 2008. Cell death-inducing DFF45-like effector C is reduced by caloric restriction and regulates adipocyte lipid metabolism. Metabolism. 57: 1307–1313. [DOI] [PubMed] [Google Scholar]
- 55.Puri V., Czech M. P. 2008. Lipid droplets: FSP27 knockout enhances their sizzle. J. Clin. Invest. 118: 2693–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haemmerle G., Zimmermann R., Hayn M., Theussl C., Waeg G., Wagner E., Sattler W., Magin T. M., Wagner E. F., Zechner R. 2002. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J. Biol. Chem. 277: 4806–4815. [DOI] [PubMed] [Google Scholar]
- 57.Langin D., Dicker A., Tavernier G., Hoffstedt J., Mairal A., Ryden M., Arner E., Sicard A., Jenkins C. M., Viguerie N., et al. 2005. Adipocyte lipases and defect of lipolysis in human obesity. Diabetes. 54: 3190–3197. [DOI] [PubMed] [Google Scholar]
- 58.Miyoshi H., Souza S. C., Zhang H. H., Strissel K. J., Christoffolete M. A., Kovsan J., Rudich A., Kraemer F. B., Bianco A. C., Obin M. S., et al. 2006. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J. Biol. Chem. 281: 15837–15844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.