Abstract
Apolipoprotein A-V (apoA-V) is a potent regulator of intravascular triglyceride (TG) metabolism, yet its plasma concentration is very low compared with that of other apolipoproteins. To examine the basis for its low plasma concentration, the secretion efficiency of apoA-V was measured in stably transfected McA-RH7777 rat hepatoma cells. Pulse-chase experiments revealed that only ∼20% of newly synthesized apoA-V is secreted into culture medium within 3 h postsynthesis and that ∼65% undergoes presecretory turnover; similar results were obtained with transfected nonhepatic Chinese hamster ovary cells. ApoA-V secreted by McA-RH7777 cells was not associated with cell surface heparin-competable binding sites. When stably transfected McA-RH7777 cells were treated with oleic acid, the resulting increase in TG synthesis caused a reduction in apoA-V secretion, a reciprocal increase in cell-associated apoA-V, and movement of apoA-V onto cytosolic lipid droplets. In a stably transfected doxycycline-inducible McA-RH7777 cell line, apoA-V expression inhibited TG secretion by ∼50%, increased cellular TG, and reduced Z-average VLDL1 particle diameter from 81 to 67 nm; however, no impact on apoB secretion was observed. These data demonstrate that apoA-V inefficiently traffics within the secretory pathway, that its intracellular itinerary can be regulated by changes in cellular TG accumulation, and that apoA-V synthesis can modulate VLDL TG mobilization and secretion.
Keywords: apolipoprotein B, lipid trafficking, lipoprotein assembly
Apolipoprotein A-V (apoA-V), a member of the exchangeable apolipoprotein family synthesized predominantly in the liver, is a potent regulator of intravascular triglyceride (TG) metabolism (1). When it is overexpressed in transgenic mice, apoA-V reduces plasma TG levels by 65%, whereas inactivation of the apoA-V gene increases plasma TG by 4-fold (2). The preponderance of current literature suggests that apoA-V affects plasma TG turnover by stimulating LPL-mediated lipolysis of TG-rich lipoproteins, either directly or indirectly (3–7). ApoA-V has also been found to serve as a ligand for LDL receptor family members and other potential lipoprotein receptors and may thus contribute to the clearance of TG-rich lipoproteins and their remnants (8–11). However, recent studies have revealed that the effects of apoA-V on plasma TG concentration are complex and variable. In humans, several loss-of-function and null apoA-V alleles are associated with both reduced plasma apoA-V levels and elevated plasma TG (12, 13), yet other studies have found both positive and negative associations between plasma apoA-V and TG concentrations (7, 14, 15). Moreover, recent studies in mice have found a positive correlation between plasma apoA-V and TG concentrations (16, 17).
Despite its apparent impact on intravascular TG-rich lipoprotein lipolysis and clearance, a peculiar characteristic of apoA-V is that its plasma concentration is in the range of 100–200 µg/l, which is ∼10,000-fold lower than apoA-I and ∼1,000-fold lower than apoA-IV and corresponds to ∼1 molecule of apoA-V for every 1,000 VLDL particles (18, 19). This presents a conundrum as to how an apolipoprotein circulating at such low levels could exert such a potent effect on plasma TG metabolism and concentration. Although it is certainly possible that apoA-V could function in plasma at extreme substoichiometric concentrations relative to that of TG-rich lipoproteins, it has also been suggested that apoA-V might function within the hepatocyte to directly modulate hepatic TG metabolism and secretion (19, 20). Indeed, the apoA-V gene was first identified based on its marked upregulation in rats following partial hepatectomy (21), suggesting that it could play a role in the conservation of intracellular lipids needed for liver regeneration. While an effect of apoA-V on TG production has not been observed in all studies (4, 5, 22), Schaap et al. (3) documented reduced hepatic TG production following adenovirus-mediated expression of human apoA-V in mouse liver. Most recently, the discovery that apoA-V may reside on cytosolic lipid droplets (23, 24) further supports the concept that apoA-V responds to and perhaps modulates aspects of intracellular hepatic TG metabolism.
In the current study, the secretory trafficking of apoA-V was examined in both hepatic and nonhepatic cells under basal conditions and during oleic acid-stimulated TG synthesis. Results of these studies suggest that the low plasma concentrations of apoA-V may be due, in part, to its inherently inefficient exocytic trafficking and that TG accumulation within hepatoma cells further antagonizes apoA-V secretion. Interestingly, these studies also revealed that in a stably transfected, inducible cell line, apoA-V gene expression reduces TG secretion, suggesting an extravascular mechanism by which apoA-V could modulate TG metabolism and plasma TG levels.
EXPERIMENTAL PROCEDURES
Cell culture
McA-RH7777 cells were grown in DMEM containing 4.5 g/l glucose and 10% FBS. Chinese hamster ovary K1 (CHO-K1) cells were maintained in DMEM-Ham's F-12 solution containing 10% FBS. All medium solutions were supplemented with 100 units/ml penicillin and 100 µg/ml streptomycin. Cells were grown in 100 mm dishes at 37°C in an atmosphere containing 5% Co2.
Transfection and selection of stable clones
McA-RH7777 and CHO-K1 cells in 100 mm dishes were transfected at ∼30% confluence with 16 µg of apoA-V expression plasmid (20) and 2 µg of pSV2-neo (25), using FuGENE 6 (Roche Molecular Biochemicals). Twenty-four hours posttransfection, cells were subjected to selection with DMEM-10% FBS supplemented with 750 µg/ml G418 (Cellgro). Selection medium was replaced every 48 h for 10 days. Individual clones were isolated, expanded, and maintained in 250 µg/ml G418. To generate inducible cell clones, apoA-V was inserted into plasmid pTRE2hyg (Clontech), which was cotransfected into McA-RH7777 cells at a 1:1 ratio (20 µg of total DNA) with plasmid pTet-On (Clontech). Twenty-four hours posttransfection, cells were subjected to selection with DMEM-10% FBS supplemented with 750 µg/ml G418 and 200 µg/ml hygromycin. Selection medium was replaced every 48 h for ∼14 days. Individual clones were selected and maintained in a solution of 250 µg/ml G418 and 100 µg/ml hygromycin. To induce apoA-V expression, cells were incubated for the indicated times with 1 µg/ml doxycycline (Dox; BD Biosciences). Individual clones were analyzed for inducible expression by immunoblot analysis. Two clones (no. 1 and 6) were characterized, and one clone was used for experiments described in Results.
Metabolic radiolabeling and analysis
Cells in 100 mm dishes were labeled for the indicated times with 100 µCi/ml of [35S]Met-Cys (EasyTag Express protein labeling mixture; Perkin-Elmer) in Met- and Cys-deficient DMEM, followed in some cases by chase using complete DMEM containing 2.5 mM Met and 1 mM Cys. Unless otherwise indicated, all pulse and chase media quantities contained 10% FBS. Following each labeling period, cells were placed on ice, and cells and media were harvested, subjected to immunoprecipitation with anti-human apoA-V serum (Supplementary Fig. S1) or anti-human apoB (Academy Bio-medical, Houston, TX), and analyzed by SDS-PAGE (26). Band intensities were quantified using a Fujifilm BAS5000 phosphorimaging unit. In some experiments, cells were incubated with increasing concentrations of porcine heparin (product no. H-7005; Sigma ). For labeling of lipids, 10 µCi/ml [3H]oleate (Perkin-Elmer) was added to cells for the times indicated. After monolayers were washed, cells were lysed in solution containing 1% Triton X-100, 150 mM NaCl, 25 mM Tris (pH 7.4),1 mM PMSF, 1 µg/ml leupeptin, and 1 µg/ml pepstatin. Both the clarified cell lysate and the media samples were supplemented with lipid markers and extracted with chloroform-methanol, as described previously (27, 28). Lipids were fractioned by thin layer chromatography in a neutral solvent (heptane-ether-acetic acid [90:30:1]) tank (29). The TG-containing fraction was visualized by incubation in iodine vapor, cut from the plate, and quantified by liquid scintillation counting.
Immunofluorescence microscopy
Stably transfected McA-RH7777 cells were plated on poly-l-lysine-coated coverslips and 24 h later fixed in 3.7% formaldehyde in PBS for 20 min, followed by incubation for 1 h in PBS containing 10 mM glycine, 0.1% saponin, and 3% BSA. Cells were then incubated for 1 h in primary antibody diluted in PBS containing 0.1% saponin and 1% BSA. A dilution of 1:300 was used for rabbit anti-human apoA-V serum, and 1:50 dilution was used for mouse anti-human ADRP (Fitzgerald Industries International, Inc.). Cells were then incubated with rhodamine-conjugated goat anti-rabbit IgG and FITC-conjugated goat anti-mouse IgG, (1:20) (Jackson ImmunoResearch) for 1 h in PBS containing 0.1% saponin and 1% BSA. Cells were postfixed with 3.7% formaldehyde in PBS, mounted with ProLong Gold antifade reagent (Invitrogen), and viewed with a Zeiss Axioplan 2 microscope with a 63× oil objective.
Isolation of lipid droplets
McA-RH7777 cells were cultured with and without 0.8 mM oleate complexed to 1.5% BSA in DMEM-10% FBS. After 24 h, cells were homogenized, and lipid droplets were isolated by sucrose gradient centrifugation, as described (26). Twelve 1 ml fractions collected from the top of the gradient by using an Autodensiflow gradient fractionator (Labconco) were subjected to TCA precipitation, SDS-PAGE, and immunoblot analysis using anti-human apoA-V serum. TG content of the lipid droplet fraction was determined by enzymatic assay (Wako).
VLDL particle diameter distribution
The hydrodynamic diameters of VLDL particles produced by transfected McA-RH7777 cells were measured using a Zetasizer Nano-S® model ZEN1600 dynamic laser light-scattering instrument (Malvern Instruments) at 633 nm. Following density gradient ultracentrifugation, as described previously (30, 31), gradients were fractionated into twelve 1 ml fractions from the top, using an Auto Densi-Flow gradient fractionator. Fractions of VLDL1 (Svedberg units of flotation [Sf] >100) and VLDL2 (Sf 20–100) were transferred to a quartz cuvette, and light scatter readings were performed at 20°C. Gradient samples were subsequently subjected to immunoprecipitation with anti-apoB antibodies and analyzed by SDS-PAGE.
RESULTS
Analysis of apoA-V secretion kinetics in hepatic and nonhepatic cells
Previous studies have documented inefficient secretion of apoA-V from transiently transfected COS cells undergoing continuous metabolic radiolabeling with [35S]Met-Cys (20). However, as endogenous apoA-V expression is predominantly limited to hepatocytes, we compared the secretory behavior of apoA-V in stably transfected rat hepatoma cells (McA-RH7777) with that in CHO cells. To examine secretion quantitatively, pulse-chase analyses were performed. After a 10 min pulse with [35S]Met-Cys and a 120 min chase, only 20% of newly synthesized apoA-V was secreted from McA-RH7777 cells (Fig. 1A) and 38% from CHO cells (Fig. 1B). In addition to limited recovery of apoA-V from medium, little cell-associated apoA-V remained after the 120 min chase (∼10%). Hence, in addition to its limited secretion, the majority of newly synthesized apoA-V appeared to undergo rapid presecretory turnover.
Fig. 1.
Kinetics of apoA-V secretion. McA-RH7777 (McA) (A) and CHO-K1 (B) cells, stably transfected with human apoA-V, were pulse radiolabeled with [35S]Met-Cys for 10 min and chased for the times indicated. After each chase time, apoA-V in cells and medium fractions was recovered by immunoprecipitation and analyzed by SDS-PAGE and phosphorimaging analysis. The percent secretion is calculated as the amount of radiolabeled apoA-V present at each chase time point as a percentage of apoA-V synthesized during the 10 min pulse (0 min chase).
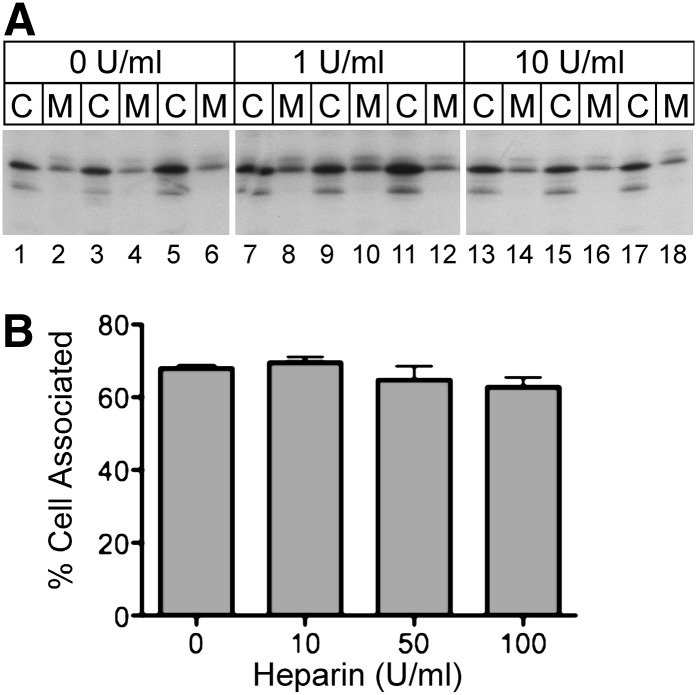
As apoA-V can associate with heparin sulfate proteoglycans (6, 32), the limited recovery of apoA-V from medium fractions could result from binding of newly secreted apoA-V to the cell surface. To determine if newly secreted apoA-V associated with heparin-competable binding sites in McA-RH7777 cells, transfected cells were radiolabeled with [35S]Met-Cys in the presence and absence of heparin, and the distribution of cell-associated and medium apoA-V was examined. As shown in Fig. 2A, the relative distribution of apoA-V in cell and medium fractions was unaffected by the presence of heparin at concentrations up to 10 U/ml. To determine if higher concentrations of heparin might affect cell association, the experiment was repeated using concentrations up to 100 U/ml; however, as shown in Fig. 2B, no significant change in cell-associated apoA-V was detected. These results suggest that the low apoA-V secretion efficiency shown in Fig. 1 is not due to cell surface proteoglycan-mediated sequestration. To explore whether apoA-V bound to the surface of McA-RH7777 cells via some other mechanism, staining of live cells with anti-apoA-V antibody was performed. No cell staining was observed unless cells were first permeabilized with saponin (Supplementary Fig. S2).
Fig. 2.
Effect of heparin on apoA-V cell association. A: Stably transfected McA-RH7777 cells were radiolabeled with [35S]Met-Cys for 2 h, followed by addition of the indicated concentration of porcine heparin and further incubation for 6 h. Cell lysates (C) and medium (M) samples were immunoprecipitated with anti-human apoA-V serum and subjected to SDS-PAGE and fluorography. B: The experimental results shown in panel A were repeated in triplicate, using the indicated concentrations of porcine heparin. Radioactive band intensities were quantified by phosphorimaging analysis and expressed as the percentage of total radiolabeled apoA-V associated with the cell pellet (means ± SEM; N = 3).
Effect of oleate on apoA-V secretion efficiency and subcellular localization
ApoA-V synthesis is upregulated in hepatic regeneration (21), a condition associated with hepatic TG accumulation (33–36). Hence, we explored whether the intracellular trafficking of apoA-V was affected by alterations in cellular TG synthesis and accumulation. Stably transfected McA-RH7777 cells were incubated with and without oleate before and during pulse-chase analyses. Inclusion of oleate caused a marked (∼46%) reduction in the secretion of apoA-V from stably transfected McA-RH7777 cells (Fig. 3A, B) and a corresponding (∼36%) increase in cell-associated apoA-V (Fig. 3C). The oleate-induced alteration in secretion occurred exclusively at the level of trafficking and subcellular localization, as oleate had no effect on the high percentage of apoA-V that underwent presecretory turnover during the 120 min chase (Fig. 3D).
Fig. 3.
Oleate-induced TG synthesis reduces apoA-V secretion efficiency. A: Stably transfected McA-RH7777 cells were pretreated without oleate (−OA) and with oleate (+OA) (0.8 mM oleate complexed to 1.5% BSA) for 2 h and subjected to pulse-chase analysis, as described in the legend to Fig. 1, in the continued absence and presence of OA. B: Duplicate dishes of cells incubated without and with OA were subjected to pulse radiolabeling for 10 min, followed by a 120 min chase. The percentages of initial (0 min chase) cell-associated radiolabeled apoA-V recovered from medium (B) and cell fractions (C) after the 120 min chase are shown. D: Percentage of initial radiolabeled apoA-V lost during the 120 min chase. For panels B–D, error bars show data ranges.
Shu et al. (23, 24) demonstrated that an apoA-V-green fluorescent protein fusion protein and native apoA-V could associate with cytosolic lipid droplets in oleate-treated McA-RH7777 cells. Presumably, this localization arises from the retrotranslocation (or other mode of trafficking) of apoA-V from the endoplasmic reticulum (ER) lumen into the cytosol, whereupon it associates with lipid droplets (37). We therefore hypothesized that the reduced secretion of apoA-V observed with oleate treatment was caused by a corresponding increase in delivery of apoA-V to lipid droplets. To explore this possibility, stably transfected McA-RH7777 cells were incubated with and without oleate for 24 h. The addition of oleate promoted increased synthesis and accumulation of neutral lipids, as shown by an increased intensity of Nile red staining (Fig. 4A). Indirect immunofluorescence microscopy demonstrated that oleate treatment was accompanied by increased apoA-V binding to lipid droplet structures (Fig. 4B), which were identified based on colocalization with the lipid droplet binding protein, ADRP (38). Oleate-induced relocalization of apoA-V onto lipid droplets was further confirmed biochemically by isolating lipid droplets via cell homogenization and sucrose density centrifugation (39). Relative to that of controls, oleate treatment resulted in a ∼16-fold increase in the TG contained in the lipid droplet fraction (5.42 μg versus 88.18 µg of TG in control versus in oleate-treated cells, respectively) (Fig. 4C, fraction 1). When gradient fractions were subjected to SDS-PAGE and immunoblot analysis, the apoA-V content in fraction 1 was also seen to increase by ∼10-fold in oleate-treated versus control cells. These data indicate that the movement of apoA-V onto lipid droplets may directly compete with the exocytic trafficking of apoA-V.
Fig. 4.
Effect of oleate-induced TG accumulation on apoA-V subcellular localization. McA-RH7777 cells stably transfected with human apoA-V were incubated with (+) or without (−) oleate (OA), as described in the legend to Fig. 3, in the presence of 10% FBS for 16 h. A: Cells were stained with Nile red, as described (53) and examined by fluorescence microscopy. B: Cells were fixed and stained with anti- apoA-V and anti-ADRP antibodies as described in Experimental Procedures. Areas of colocalization of apoA-V (rhodamine, red) and ADRP (FITC, green) are detected in the overlay (yellow). C: Cells were homogenized and subjected to sucrose gradient centrifugation (39). Twelve 1 ml fractions were collected and subjected to precipitation with TCA, SDS-PAGE, and immunoblot analysis using anti-human apoA-V serum. Fraction 1 (top) contains the cellular lipid droplet fraction (39).
Effect of apoA-V expression on apoB and triglyceride secretion
Based on its low concentration in plasma, the possibility that apoA-V modulates intracellular hepatic TG metabolism has been proposed (3, 19, 20). Hence, we explored whether the expression of apoA-V could affect TG secretion in McA-RH7777 hepatoma cells. The McA-RH7777 cells stably transfected with apoA-V shown in Figs. 1–4 responded to oleate by increasing TG secretion and apoB mass by ∼2-fold (data not shown). However, to rule out possible phenotypic variability associated with clonally selected cell lines, we generated McA-RH7777 cell lines that expressed apoA-V under the control of a Dox-inducible promoter. As shown in Fig. 5A, these cells displayed undetectable basal apoA-V expression and a robust induction when cells were incubated with Dox. To examine the consequences of apoA-V expression on TG secretion, cells were labeled with [3H]oleate in the absence and presence of Dox for 24 h. Induction of apoA-V expression resulted in a ∼50% increase in cellular TG content (Fig. 5B) and a roughly corresponding decrease in TG secretion (Fig. 5C). To assess the impact of apoA-V on apoB, control and induced cells were subjected to radiolabel pulse-chase analysis with [35S]Met-Cys (Fig. 5D). As shown in Fig. 5D, E, apoA-V expression appeared to have no impact on the secretion or the intracellular stability of apoB.
Fig. 5.
Effect of apoA-V on apoB and TG secretion. A: McA-RH7777 cells, which express human apoA-V under the control of a Dox-inducible promoter, were incubated without (−) or with (+) 1 µg/ml Dox for 48 h. Cells were labeled with [35S]Met-Cys for 4 h, also without and with Dox, and cell lysates were subjected to immunoprecipitation with anti-apoA-V serum, SDS-PAGE, and phosphorimaging analysis. B, C: Cells were incubated in medium containing 20% FBS and 0.4 mM oleate complexed to 0.75% BSA, without (−) or with (+) 1 µg/ml Dox for 24 h, followed by radiolabeling with [3H]oleate (10 µCi/ml) in the same medium for 24 h. Cells (B) and medium (C) fractions were extracted with chloroform-methanol, and the lipid extracts were fractionated by TLC. TG bands were quantified by liquid scintillation counting. D: Cells cultured for 48 h, as described in legends to panels B and C, in the absence or presence of Dox, were pulse radiolabeled with [35S]Met-Cys for 30 min and chased with unlabeled medium for 0 or 2 h, as indicated. ApoB in cells and medium samples was immunoprecipitated and subjected to SDS-PAGE and phosphorimaging analysis. E: Percentage of newly synthesized apoB (0 h chase) recovered from medium after a 2 h chase. Control (without Dox [−]) efficiency was set to 100% (values shown in panels B, C, and E are means ± SEM; N = 3). All data were analyzed using a paired Student t-test (*, P < 0.05).
The finding that apoA-V impacts TG but not apoB secretion suggests an effect on particle size but not particle number. To assess apoB particle characteristics in the presence and absence of apoA-V expression, stably transfected McA-RH7777 cells treated with and without Dox were metabolically radiolabeled with [35S]Met-Cys for 4 h, and medium samples were subjected to cumulative rate density gradient ultracentrifugation (30, 31). No apparent change in apoB density distribution was observed in response to apoA-V expression (Fig. 6A); however, when the VLDL1 fraction (Sf, >100) was analyzed by dynamic laser light scattering, the Dox-treated cells displayed a 26 nm reduction in peak VLDL1 particle size diameter, from 66 to 42 nm, and a 14 nm reduction in Z-average diameter, from 81 to 67 nm (Fig. 6B); apoA-V had no impact on the VLDL2 (Sf, 20–100) peak diameter of ∼31 nm, as expected. These data indicate that the reduced TG secretion observed upon induction of apoA-V expression is due primarily to attenuation of second-step particle maturation, essential for the formation of TG-rich VLDL.
Fig. 6.
ApoA-V expression impacts lipoprotein particle size distribution. Inducible McA-RH7777 cells were incubated in the absence (−) or presence (+) of Dox for 48 h and radiolabeled as described in the legend to Fig. 5A. A: Medium samples were harvested and subjected to cumulative rate flotation ultracentrifugation, as described previously (30, 31). Size distributions of particles contained in the VLDL1 (B) and VLDL2 (C) fractions were determined using dynamic laser light scattering analysis.
DISCUSSION
One of the signature characteristics of apoA-V is that its plasma concentration is extremely low compared with its homologous relatives in the exchangeable apolipoprotein family, apoA-I and apoA-IV, which circulate at levels that are 10,000- and 1,000-fold higher, respectively (1, 14, 15, 18, 19). Although little is known about the catabolic fate of plasma apoA-V, our observation that only a small fraction of newly synthesized apoA-V is secreted from McA-RH7777 hepatoma cells into medium suggests that inefficient secretion of apoA-V from the liver into the plasma compartment may, in part, contribute to its low plasma concentration. Although Shu et al. (32) recently estimated that the mass of plasma apoA-V in human apoA-V transgenic mice was 4-fold greater than the amount present in the entire liver, this relationship does not necessarily reflect secretion efficiency, as many factors, including presecretory turnover and plasma residence time, can affect this ratio.
The basis for the inefficient secretion of apoA-V is unknown. It is possible that apoA-V lacks effective anterograde transport properties critical for exiting from the ER or that its hydrophobicity and insolubility in the absence of lipid (20, 40) affect its folding and transport competence. In either case, prolonged residence time in the ER may promote retrograde translocation of apoA-V into the cytosol (41). Although many such dislocated proteins are targeted for turnover, the affinity of apoA-V for lipid droplets may protect a population from degradation, particularly as cytosolic lipid droplet formation may provide an escape route from the ER to the cytosol for some ER-localized proteins (37). It is therefore likely that within the cell, apoA-V must continually associate with lipids to maintain its solubility, and thus, its trafficking may be particularly sensitive to lipid fluxes from the ER membrane, whether into the ER lumen or into the cytosol on the surface of lipid droplets (42).
The present data also provide evidence of a linkage between intracellular apoA-V trafficking and TG metabolism. Treatment of stably transfected McA-RH7777 hepatoma cells with oleate, which increased intracellular TG accumulation, caused a dramatic inhibition of apoA-V secretion and a reciprocal increase in intracellular apoA-V. Fluorescence confocal microscopy established that this was due to the association of apoA-V with the surface of cytosolic lipid droplets, as observed previously (23, 24, 32). Hence, it appears that hepatic TG synthesis or accumulation may drive a dynamic competition between apoA-V secretion and lipid droplet association.
The consequence of apoA-V expression and localization was explored using a regulatable apoA-V expression system. When transfected apoA-V expression was induced with Dox, neither the secretion nor the density distribution of apoB in McA-RH7777 cells was altered dramatically. This finding agrees with observations of Shu et al. (23), who also noted no change in apoB secretion or density gradient distribution in Hep3B hepatoma cells stably transfected with human apoA-V. However, in the current study, apoA-V expression was associated with a ∼50% reduction in TG secretion and a corresponding increase in cellular TG content. To explore the basis for this observation, particle size analysis was performed. While the VLDL2 fraction displayed no significant size change in Dox-treated cells, the VLDL1 peak particle diameter was reduced by ∼26 nm upon Dox-mediated induction of apoA-V expression (Fig. 6). Assuming a VLDL1:VLDL2 ratio of 1:3 (Fig. 6A), this change in particle diameter corresponds to a ∼40% reduction in VLDL volume, a value in reasonable agreement with the ∼50% reduction in radiolabeled TG secretion shown in Fig. 5C. Hence, the reduction in TG content in medium from apoA-V-expressing McA-RH7777 cells appears to result from a reduction in VLDL particle size distribution but not in particle number. These data are consistent with findings of Schaap at al. (3), who found that adenovirus-mediated expression of human apoA-V inhibited VLDL-TG secretion, also without affecting particle number. While other studies have failed to establish a link between apoA-V expression and VLDL secretion in transgenic mice (5), it is worth noting that upon induction of apoA-V in vivo during liver regeneration (21), hepatic TG synthesis and accumulation are upregulated without an accompanying increase in VLDL secretion (43).
Considering these observations, we speculate that depending on the relative rates of TG and VLDL synthesis, apoA-V either enters the secretory pathway, possibly on the surface of nascent VLDL, and becomes subject to presecretory degradation or buds from the cytoplasmic side of the ER membrane on the surface of lipid droplets (27). Thus, under specific metabolic conditions, apoA-V could modulate VLDL assembly by facilitating the diversion and sequestration of a pool of metabolically active TG within the ER membrane toward lipid droplet formation and away from apoB lipidation. As ablation of apoA-V expression does not appear to increase hepatic TG secretion (44), this suggests that apoA-V may exert mainly an inhibitory effect on VLDL-TG secretion, which becomes robust only as hepatic TG synthesis increases.
Observations from animal and human studies provide further evidence of the linkage between apoA-V gene expression and hepatic TG synthesis, storage, and secretion. Shu et al. (32) observed that both the hepatic TG content and the apoA-V lipid droplet association were increased in human apoA-V transgenic mice, whereas inactivation of the mouse apoA-V gene had little effect. Although the impact on plasma TG was not examined in that study, Pamir et al. (45) found that when human apoA-V transgenic mice were fed a high-fat and high-sucrose diet, fasting plasma TG levels fell instead of increasing, suggesting that apoA-V gene expression had inhibited diet-induced hepatic VLDL-TG secretion. Werner et al. (46) observed that hepatic steatosis induced by essential fatty acid deficiency is accompanied by increased apoA-V gene expression; yet, Huang et al. (47) found that plasma apoA-V levels were 45% lower in obese, insulin-resistant, dyslipidemic subjects, suggesting that hepatic steatosis, which is a concomitant condition of the metabolic syndrome, reduced hepatic apoA-V secretion. Presently, the mechanism of linkage between apoA-V and TG metabolism is not well understood, but it is relevant that the apoA-V gene contains two E-box elements that can bind SREBP1c (48), a nuclear factor that plays a central role in regulating hepatic TG synthesis (49, 50), and that the human apoA-V promoter contains a PPARα response element (51, 52) and, thus, is upregulated by PPARα agonists, which are also potent modulators of hepatic TG metabolism (50).
In summary, our data from a hepatic cell line model establishes that apoA-V is inefficiently secreted, that stimulation of TG synthesis significantly inhibits apoA-V secretion and redirects the trafficking of apoA-V to the surface of cytosolic lipid droplets, and that upregulation of apoA-V gene expression reduces VLDL-TG with little apparent effect on apoB secretion itself. These data suggest that in addition to its well established function in regulating plasma TG levels by catalyzing the peripheral lipolysis and clearance of TG-rich lipoproteins, apoA-V may also play a critical role in modulating hepatic lipoprotein secretion and TG storage. ApoA-V may thus stand at the crossroads between hepatic lipid export and storage and may be an important factor in determining human susceptibility to hepatic steatosis, lipotoxicity, and insulin sensitivity.
Supplementary Material
Footnotes
Abbreviations:
- apo
- apolipoprotein
- CHO
- Chinese hamster ovary
- Dox
- doxycycline
- ER
- endoplasmic reticulum
- Sf
- Svedberg flotation
- TG
- triglyceride
This work was supported by National Institutes of Health Grants HL-49373 (G.S.S.) and HL-30897 (R.B.W.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures.
REFERENCES
- 1.Forte T. M., Shu X., Ryan R. O. 2009. The ins (cell) and outs (plasma) of apolipoprotein A-V. J. Lipid Res. 50: S150–S155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pennacchio L. A., Olivier M., Hubacek J. A., Cohen J. C., Cox D. R., Fruchart J. C., Krauss R. M., Rubin E. M. 2001. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 294: 169–173. [DOI] [PubMed] [Google Scholar]
- 3.Schaap F. G., Rensen P. C. N., Voshol P. J., Vrins C., Van der Vliet H. N., Chamuleau R., Havekes L. M., Groen A. K., Van Dijk K. W. 2004. ApoAV reduces plasma triglycerides by inhibiting very low density lipoprotein-triglyceride (VLDL-TG) production and stimulating lipoprotein lipase-mediated VLDL-TG hydrolysis. J. Biol. Chem. 279: 27941–27947. [DOI] [PubMed] [Google Scholar]
- 4.Fruchart-Najib J., Baugé E., Niculescu L. S., Pham T., Thomas B., Rommens C., Majd Z., Brewer B., Pennacchio L. A., Fruchart J. C. 2004. Mechanism of triglyceride lowering in mice expressing human apolipoprotein A5. Biochem. Biophys. Res. Commun. 319: 397–404. [DOI] [PubMed] [Google Scholar]
- 5.Merkel M., Loeffler B., Kluger M., Fabig N., Geppert G., Pennacchio L. A., Laatsch A., Heeren J. 2005. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J. Biol. Chem. 280: 21553–21560. [DOI] [PubMed] [Google Scholar]
- 6.Lookene A., Beckstead J. A., Nilsson S., Olivecrona G., Ryan R. O. 2005. Apolipoprotein A-V-heparin Interactions: implications for plasma lipoprotein metabolism. J. Biol. Chem. 280: 25383–25387. [DOI] [PubMed] [Google Scholar]
- 7.Talmud P. J. 2007. Rare APOA5 mutations–Clinical consequences, metabolic and functional effects: An ENID review. Atherosclerosis. 194: 287–292. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson S. K., Lookene A., Beckstead J. A., Gliemann J., Ryan R. O., Olivecrona G. 2007. Apolipoprotein A-V interaction with members of the low density lipoprotein receptor gene family. Biochemistry. 46: 3896–3904. [DOI] [PubMed] [Google Scholar]
- 9.Dichlberger A., Cogburn L. A., Nimpf J., Schneider W. J. 2007. Avian apolipoprotein A-V binds to LDL receptor gene family members. J. Lipid Res. 48: 1451–1456. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson S. K., Christensen S., Raarup M. K., Ryan R. O., Nielsen M. S., Olivecrona G. 2008. Endocytosis of apolipoprotein A-V by members of the low density lipoprotein receptor and the Vps10p domain receptor families. J. Biol. Chem. 283: 25920–25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beigneux A. P., Davies B. S., Gin P., Weinstein M. M., Farber E., Qiao X., Peale F., Bunting S., Walzem R. L., Wong J. S., et al. 2007. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 5: 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Priore Oliva C., Pisciotta L., Volti G. L., Sambataro M. P., Cantafora A., Bellocchio A., Catapano A., Tarugi P., Bertolini S., Calandra S. 2005. Inherited apolipoprotein A-V deficiency in severe hypertriglyceridemia. Arterioscler. Thromb. Vasc. Biol. 25: 411–417. [DOI] [PubMed] [Google Scholar]
- 13.Marçais C., Verges B., Charrière S., Pruneta V., Merlin M., Billon S., Perrot L., Drai J., Sassolas A., Pennacchio L. A., et al. 2005. Apoa5 Q139X truncation predisposes to late-onset hyperchylomicronemia due to lipoprotein lipase impairment. J. Clin. Invest. 115: 2862–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakel H., Nowak M., Helleboid-Chapman A., Fruchart-Najib J., Fruchart J-C. 2006. Is apolipoprotein A5 a novel regulator of triglyceride-rich lipoproteins? Ann. Med. 38: 2–10. [DOI] [PubMed] [Google Scholar]
- 15.Kluger M., Heeren J., Merkel M. 2008. Apoprotein A-V: An important regulator of triglyceride metabolism. J. Inherit. Metab. Dis. 31: 281–288. [DOI] [PubMed] [Google Scholar]
- 16.Nelbach L., Shu X., Konrad R. J., Ryan R. O., Forte T. M. 2008. Effect of apolipoprotein A-V on plasma triglyceride, lipoprotein size, and composition in genetically engineered mice. J. Lipid Res. 49: 572–580. [DOI] [PubMed] [Google Scholar]
- 17.Vaessen S. F. C., Dallinga-Thie G. M., Ross C. J. D., Splint L. J., Castellani L. W., Rensen P. C. N., Hayden M. R., Schaap F. G., Kuivenhoven J. A. 2009. Plasma apolipoprotein AV levels in mice are positively associated with plasma triglyceride levels. J. Lipid Res. 50: 880–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assmann G. 1982. Lipid metabolism and atherosclerosis. FK Schattauer Verlag GmbH, Stuttgart, Germany. [Google Scholar]
- 19.Olofsson S. O. 2005. ApoA-V—The regulation of a regulator of plasma triglycerides. Arterioscler. Thromb. Vasc. Biol. 25: 1097–1099. [DOI] [PubMed] [Google Scholar]
- 20.Weinberg R. B., Cook V. R., Beckstead J. A., Martin D. D., Gallagher J. W., Shelness G. S., Ryan R. O. 2003. Structure and interfacial properties of human apolipoprotein A-V. J. Biol. Chem. 278: 34438–34444. [DOI] [PubMed] [Google Scholar]
- 21.van der Vliet H. N., Sammels M. G., Leegwater A. C., Levels J. H., Reitsma P. H., Boers W., Chamuleau R. A. 2001. Apolipoprotein A-V: a novel apolipoprotein associated with an early phase of liver regeneration. J. Biol. Chem. 276: 44512–44520. [DOI] [PubMed] [Google Scholar]
- 22.Qu S., Perdomo G., Su D., D'Souza F. M., Shachter N. S., Dong H. H. 2007. Effects of apoA-V on HDL and VLDL metabolism in APOC3 transgenic mice. J. Lipid Res. 48: 1476–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shu X., Chan J., Ryan R. O., Forte T. M. 2007. Apolipoprotein A-V association with intracellular lipid droplets. J. Lipid Res. 48: 1445–1450. [DOI] [PubMed] [Google Scholar]
- 24.Shu X., Ryan R. O., Forte T. M. 2008. Intracellular lipid droplet targeting by apolipoprotein A-V requires the carboxyl-terminal segment. J. Lipid Res. 49: 1670–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Southern P. J., Berg P. 1982. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J. Mol. Appl. Genet. 1: 327–341. [PubMed] [Google Scholar]
- 26.Shelness G. S., Thornburg J. T. 1996. Role of intramolecular disulfide bond formation in the assembly and secretion of apolipoprotein B-100-containing lipoproteins. J. Lipid Res. 37: 408–419. [PubMed] [Google Scholar]
- 27.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 28.Sellers J. A., Hou L., Athar H., Hussain M. M., Shelness G. S. 2003. A Drosophila microsomal triglyceride transfer protein homolog promotes the assembly and secretion of human apolipoprotein B—implications for human and insect lipid transport and metabolism. J. Biol. Chem. 278: 20367–20373. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein J. L., Basu S. K., Brown M. S. 1983. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 98: 241–260. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Tran K., Yao Z. 1999. The activity of microsomal triglyceride transfer protein is essential for accumulation of triglyceride within the microsomes in McA-RH7777 cells. J. Biol. Chem. 274: 27793–27800. [DOI] [PubMed] [Google Scholar]
- 31.Chung S., Gebre A. K., Seo J., Shelness G. S., Parks J. S. 2010. A novel role for ABCA1-generated large pre-β migrating nascent HDL in the regulation of hepatic VLDL triglyceride secretion. J. Lipid Res. 51: 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shu X., Nelbach L., Ryan R. O., Forte T. M. 2010. Apolipoprotein A-V associates with intrahepatic lipid droplets and influences triglyceride accumulation. Biochim. Biophys. Acta. 1801: 605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shteyer E., Liao Y., Muglia L. J., Hruz P. W., Rudnick D. A. 2004. Disruption of hepatic adipogenesis is associated with impaired liver regeneration in mice. Hepatology. 40: 1322–1332. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez M. A., Albor C., Ingelmo-Torres M., Nixon S. J., Ferguson C., Kurzchalia T., Tebar F., Enrich C., Parton R. G., Pol A. 2006. Caveolin-1 is essential for liver regeneration. Science. 313: 1628–1632. [DOI] [PubMed] [Google Scholar]
- 35.Brasaemle D. L. 2006. A metabolic push to proliferate. Science. 313: 1581–1582. [DOI] [PubMed] [Google Scholar]
- 36.Farrell G. C. 2004. Probing Prometheus: fat fueling the fire? Hepatology. 40: 1252–1255. [DOI] [PubMed] [Google Scholar]
- 37.Ploegh H. L. 2007. A lipid-based model for the creation of an escape hatch from the endoplasmic reticulum. Nature. 448: 435–438. [DOI] [PubMed] [Google Scholar]
- 38.Brasaemle D. L., Barber T., Wolins N., Serrero G., Blanchette-Mackie E., Londos C. 1997. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J. Lipid Res. 38: 2249–2263. [PubMed] [Google Scholar]
- 39.Brasaemle D. L., Wolins N. E. 2006. Isolation of lipid droplets from cells by density gradient centrifugation. Current Protocols in Cell Biology. Bonifacino J. S., Dasso M., Harford J. B., Lippincott-Schwartz J., Yamada K. M., John Wiley & Sons Inc., NY: 3.15.11–13.15.12.. [Google Scholar]
- 40.Wong-Mauldin K., Raussens V., Forte T. M., Ryan R. O. 2009. Apolipoprotein A-V N-terminal domain lipid interaction properties in vitro explain the hypertriglyceridemic phenotype associated with natural truncation mutants. J. Biol. Chem. 284: 33369–33376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagola K., Mehnert M., Jarosch E., Sommer T. 2010. Protein dislocation from the ER. Biochim. Biophys. Acta. In press. [DOI] [PubMed] [Google Scholar]
- 42.Martin S., Parton R. G. 2006. Lipid droplets: a unified view of a dynamic organelle. Nat. Rev. Mol. Cell Biol. 7: 373–378. [DOI] [PubMed] [Google Scholar]
- 43.Tijburg L. B., Nyathi C. B., Meijer G. W., Geelen M. J. 1991. Biosynthesis and secretion of triacylglycerol in rat liver after partial hepatectomy. Biochem. J. 277: 723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grosskopf I., Baroukh N., Lee S. J., Kamari Y., Harats D., Rubin E. M., Pennacchio L. A., Cooper A. D. 2005. Apolipoprotein A-V deficiency results in marked hypertriglyceridemia attributable to decreased lipolysis of triglyceride-rich lipoproteins and removal of their remnants. Arterioscler. Thromb. Vasc. Biol. 25: 2573–2579. [DOI] [PubMed] [Google Scholar]
- 45.Pamir N., McMillen T. S., Li Y-I., Lai C-M., Wong H., LeBoeuf R. C. 2009. Overexpression of apolipoprotein A5 in mice is not protective against body weight gain and aberrant glucose homeostasis. Metabolism. 58: 560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Werner A., Havinga R., Bos T., Bloks V. W., Kuipers F., Verkade H. J. 2005. Essential fatty acid deficiency in mice is associated with hepatic steatosis and secretion of large VLDL particles. Am. J. Physiol. Gastrointest. Liver Physiol. 288: G1150–G1158. [DOI] [PubMed] [Google Scholar]
- 47.Huang X-s., Zhao S-P., Hu M., Bai L., Zhang Q., Zhao W. 2010. Decreased apolipoprotein A5 is implicated in insulin resistance-related hypertriglyceridemia in obesity. Atherosclerosis. 210: 563–568. [DOI] [PubMed] [Google Scholar]
- 48.Jakel H., Nowak M., Moitrot M., Dehondt H., Hum D. W., Pennacchio L. A., Fruchart-Najib J., Fruchart J. C. 2004. The liver X receptor ligand T0901317 down-regulates APOA5 gene expression through activation of SREBP-1c. J. Biol. Chem. 279: 45462–45469. [DOI] [PubMed] [Google Scholar]
- 49.Browning J. D., Horton J. D. 2004. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 114: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldberg I. J., Ginsberg H. N. 2006. Ins and outs modulating hepatic triglyceride and development of nonalcoholic fatty liver disease. Gastroenterology. 130: 1343–1346. [DOI] [PubMed] [Google Scholar]
- 51.Prieur X., Schaap F. G., Coste H., Rodríguez J. C. 2005. Hepatocyte nuclear factor-4alpha regulates the human apolipoprotein AV gene: identification of a novel response element and involvement in the control by peroxisome proliferator-activated receptor-gamma coactivator-1alpha, AMP-activated protein kinase, and mitogen-activated protein kinase pathway. Mol. Endocrinol. 19: 3107–3125. [DOI] [PubMed] [Google Scholar]
- 52.Prieur X., Lesnik P., Moreau M., RodrÌguez J. C., Doucet C., Chapman M. J., Huby T. 2009. Differential regulation of the human versus the mouse apolipoprotein AV gene by PPARalpha: Implications for the study of pharmaceutical modifiers of hypertriglyceridemia in mice. Biochim. Biophys. Acta. 1791: 764–771. [DOI] [PubMed] [Google Scholar]
- 53.Listenberger L. L., Han X., Lewis S. E., Cases S., Farese R. V., Jr., Ory D. S., Schaffer J. E. 2003. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA. 100: 3077–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.