Abstract
We have recently identified a neuroprotective role for omega-3 polyunsaturated fatty acids (n-3 PUFAs) in a toxin-induced mouse model of Parkinson's disease (PD). Combined with epidemiological data, these observations suggest that low n-3 PUFA intake is a modifiable environmental risk factor for PD. In order to strengthen these preclinical findings as prerequisite to clinical trials, we further investigated the neuroprotective role of n-3 PUFAs in Fat-1 mice, a transgenic model expressing an n-3 fatty acid desaturase converting n-6 PUFAs into n-3 PUFAs. Here, we report that the expression of the fat-1 transgene increased cortical n-3:n-6 PUFA ratio (+28%), but to a lesser extent than dietary supplementation (92%). Such a limited endogenous production of n-3 PUFAs in the Fat-1 mouse was insufficient to confer neuroprotection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity as assessed by dopamine levels, tyrosine hydroxylase (TH)-positive neurons and fibers, as well as nigral Nurr1 and dopamine transporter (DAT) mRNA expression. Nevertheless, higher cortical docosahexaenoic acid (DHA) concentrations were positively correlated with markers of nigral dopaminergic neurons such as the number of TH-positive cells, in addition to Nurr1 and DAT mRNA levels. These associations are consistent with the protective role of DHA in a mouse model of PD. Taken together, these data suggest that dietary intake of a preformed DHA supplement is more effective in reaching the brain and achieving neuroprotection in an animal model of PD.
Keywords: docosahexaenoic acid, dopamine, fatty acids, dopaminergic neurons, catecholamines, brain lipids
Parkinson's disease (PD) is the second most prevalent neurodegenerative disorder after Alzheimer's (1). PD patients suffer from several motor (resting tremors, bradykinesia, muscular rigidity, and gait disturbance) and nonmotor symptoms resulting from a massive loss of dopaminergic (DAergic) neurons in the substantia nigra pars compacta (SNpc), which leads to a reduction in striatal dopamine (DA) content. This disease remains incurable, and only symptomatic treatments are available to patients (1–3). We recently identified a remarkable neuroprotective effect of omega-3 polyunsaturated fatty acids (n-3 PUFAs) using a mouse model of PD (4). Indeed, consumption of a n-3 PUFA-enriched diet for 10 months led to higher levels of docosahexaenoic acid (DHA: 22:6 n-3) in the brain, which protected from the detrimental effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a neurotoxin modeling PD nigrostriatal denervation (4). Increased expression of the brain-derived neurotrophic factor (BDNF) was established as an important factor underlying the neuroprotective action of DHA (5). These results, in combination with epidemiological correlative analyses (6), suggest that n-3 PUFA deficiency may be a modifiable environmental risk factor for PD (7).
PUFAs can be subdivided into n-3 and n-6 subclasses, which are mainly obtained in vegetable and animal sources. For example, cold water fatty fish, such as salmon and tuna, are among the richest sources of long chain n-3 PUFAs, including eicosapentaenoic acid (EPA: 20:5 n-3) and DHA (8, 9). N-3 and n-6 PUFAs have been considered essential fatty acids ever since the consequences of their deficiencies on brain development and functions were demonstrated (9–11). The importance of n-3 PUFAs in maintaining a general health status is widely accepted, as many studies have associated a high n-3 PUFA intake with beneficial effects on various conditions such as cardiovascular diseases, depression, and Alzheimer's disease [see reviews (12–14)]. In mammals, the conversion of n-6 into n-3 PUFAs is not possible due to the lack of a specific enzyme that adds a double bond between the third and fourth carbon from the methyl terminal. Therefore, the supply of n-3 PUFAs in mammals depends entirely on dietary intake. Invertebrate species, such as the roundworm Caenorhabditis elegans, harbor a fat-1 gene, which encodes the enzyme n-3 PUFA desaturase that catalyzes the n-6→n-3 PUFA conversion (15, 16). Kang et al. (15) incorporated this gene, with slight modifications, into the transgenic Fat-1 mouse. This resulted in a murine model exhibiting increased levels of n-3 PUFAs with diminished n-6 PUFA concentrations within different organs, including the brain (15). The Fat-1 mouse was investigated in various disease settings and beneficial effects of the transgene were reported on liver neoplasia (17) and atherosclerotic lesions (18) as well as in the prevention of cerebral seizures induced by pentylenetetrazol (19). In addition, increased spatial learning performances, enhanced dendritic spine density, and neurogenesis were observed in this model (20).
To provide a stronger rationale for clinical trials and to formulate public health recommendations, further preclinical data are needed to ascertain the mechanisms of action of n-3 PUFAs in PD animal models. To eliminate confounding dietary factors, we investigated the neuroprotective effects of endogenous n-3 PUFAs produced by the fat-1 transgene in an animal model of PD-like DAergic denervation. For this purpose, we exposed Fat-1 mice to MPTP, a neurotoxin that replicates several features of PD.
MATERIALS AND METHODS
Fat-1 transgenic mice, genotyping, and diet
Heterozygous Fat-1 mice and nontransgenic littermates (NonTg) were bred on the same C57BL/6 genetic background and all mice were genotyped. Ear punches were incubated with 10 mM NaOH and 0.1 mM EDTA for 2 h at 95°C and submitted to a 2-step PCR with Titanium Taq (Clontech, Mountain View, CA) and specific forward (5′-CGGTTTCTGCGATGGATCCCAC-3′) and reverse (5′-CCGGTGAAAACGCAGAAGTTGTTG-3′) primers. Amplification of a 631-bp band confirmed the fat-1 genotype. Mice were reproduced and maintained throughout their lifespan, from weaning to euthanasia, on a diet low in n-3 PUFAs and enriched in n-6 PUFAs (Table 1). Purified diet formulation was precisely determined in collaboration with Research Diets Inc. (New Brunswick, NJ) in order to maintain batch-to-batch consistency and to eliminate the presence of contaminants such as phytoestrogens or pesticides commonly found in laboratory chow. Levels of fatty acids in the diet were quantified using gas chromatography (see section Lipid extraction and gas chromatography and Table 1).
TABLE 1.
Diet composition
| Content | High n-6/Low n-3 PUFA diet |
|---|---|
| Proteins (% w/w) | 20.3 |
| Carbohydrates (% w/w) | 66 |
| Calories per diet weight (kcal/g) | 3.9 |
| Ingredients (g/kg) | |
| Casein | 200 |
| DL-Methionine | 3 |
| Corn starch | 150 |
| Sucrose | 500 |
| Cellulose, BW200 | 50 |
| Corn oil | 25 |
| Safflower | 25 |
| Choline bitartrate | 2 |
| Cholesterol, USP | 0.5 |
| Fatty acid content as determined by gas chromatography (g/kg of diet) | |
| n-3 PUFAs | 0.36 |
| Linolenic acid | 0.36 |
| Eicosapentaenoic acid | 0 |
| Docosahexaenoic acid | 0 |
| n-6 PUFAs | 36.25 |
| Linoleic acid | 36.04 |
| Arachidonic acid | 0 |
| n-6/n-3 PUFA ratio | 101.79 |
n-3 PUFA, omega-3 polyunsaturated fatty acid; n-6 PUFA, omega-6 polyunsaturated fatty acid.
MPTP treatment and experimental design
Fat-1(+) and NonTg mice were assigned to the following groups: vehicle/saline-treated (NonTg: n=15 or Fat-1: n=16) or MPTP-treated (NonTg: n=18 or Fat-1: n=19). Mice were housed in groups of 3 to 4 per cage under standard conditions throughout the experiments with free access to food and water and handled under the same conditions by one investigator. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Laval University Animal Welfare Committee. The MPTP treatment was performed at 6 months of age and consisted of 7 ip injections of a MPTP•HCl solution (20 mg free base/kg) (Sigma, St. Louis, MO), freshly dissolved in 0.9% saline. MPTP was administered twice on the first 2 days of the experimental protocol at 12 h intervals, and once a day on the 3 following days, as previously described (4, 21). Remaining animals received 0.9% saline ip following the regime used for MPTP. This particular MPTP procedure was selected to produce a moderate DAergic denervation (4, 21), and for the purpose of comparison with previous data collected with n-3 PUFA-enriched diets (4).
Tissue preparation for postmortem analyses
Animals were euthanized two weeks after the last injection under deep anesthesia with ketamine and xylazine mixture and perfused via intracardiac infusion with 1× PBS. Brains were collected, the frontal cortex was dissected for fatty acid analyses, and the two hemispheres were separated. The left hemisection was snap-frozen in 2-methyl-butane and then stored at –80°C for cryostat coronal brain sections of 12 μm and 20 μm for HPLC analyses. The right hemisection was separated at the level of bregma -1.70 mm, the caudal section was postfixed in 4% paraformaldehyde pH 7.4 and cut onto a freezing microtome (coronal brain section of 25 μm). The remaining rostral section was dissected to isolate the striatum for Western immunoblotting experiments.
Lipid extraction and gas chromatography
Approximately 20 mg of frozen frontal cortex tissue from each mouse was used for fatty acid extractions and analyses. The frontal cortex was selected because it is minimally affected by MPTP treatment (4) and allowed us to isolate the effect of the transgene on brain fatty acid profiles. Weighed brain tissues were homogenized successively with 0.9% NaCl, butylhydroxytoluene-methanol (Sigma, St. Louis, MO), and chloroform (J.T. Baker, Phillipsburg, NJ) with 22:3n-3 methyl ester internal standard (Nu-Chek Prep, Elysian, MN) at a concentration of 500 μg/g of tissue. After centrifugation at 2400 g for 7 min, the lower layer was collected (22). This procedure was repeated twice and the two extracts were pooled and brought to dryness under a stream of N2. Lipid extracts were transmethylated with methanol:benzene (4:1) and acetyl chloride at 98°C for 90 min. After cooling down, 6% K2CO3 was added. A 15 min centrifugation at 514 g allowed phase separation and the upper layer was collected in a gas chromatography autosampler vial and capped under N2. Fatty acid methyl esters were quantified using a model 6890 series gas chromatograph (Agilent Technologies, Palo Alto, CA) using a FAST-GC method. Five microliters of each sample were injected at a 25:1 split ratio. Tissue fatty acid methyl ester peak identification was performed by comparison to the peak retention times of a 28-component methyl ester reference standard (GLC-462; Nu-Chek Prep) (23).
Immunohistochemical evaluation of TH-positive neurons
Paraformaldehyde postfixed sections were processed using standard immunohistochemical procedures as previously described (4, 21). Briefly, sections were incubated overnight at 4°C with rabbit anti-tyrosine hydroxylase (TH) (1:5000; Pel-Freez, Rogers, AR) in 0.1% Triton X-100, and 5% normal goat serum in PBS (137 mM NaCl, 2.7 mM KCl, 10 mM sodium phosphate dibasic, 2 mM potassium phosphate monobasic, pH 7.4). The overnight incubation was followed by 1 h incubation at room temperature in a PBS solution containing 0.1% Triton X-100, 5% normal goat serum, and biotinylated goat anti-mouse IgG (Vector Laboratories, Burlington, ON, Canada; 1:1500). An avidin-biotin peroxidase complex (Vector Laboratories) combined with a 3,3′-diaminobenzidine tetrahydrochloride (Sigma) immunoreaction was used to visualize bound antibodies. Following reaction of 3,3′-diaminobenzidine tetrahydrochloride with TH, sections were counterstained with cresyl violet (Sigma), dehydrated, and coverslipped.
In situ hybridization
Nurr1 and DA transporter (DAT) probes were produced, synthesized, and labeled as previously described (4, 21). Coronal brain sections were mounted onto Snowcoat X-tra slides (Surgipath, Winnipeg, MB, Canada) and air-dried overnight at room temperature. Brain sections were prepared for overnight hybridization as reported (4, 21). The [35S]UTP-radiolabeled complementary RNA probe was added to a hybridization mix (1× Denhart's solution, 10% dextran sulfate, 50% deionized formamide, and 35S coupled 2 × 106 cpm/μl probe) and heated at 80°C for 5 min. Each slide was covered with 100 μl of the hybridization solution and coverslipped. The hybridization was carried out overnight on a slide warmer at 58°C. After hybridization, slides were rinsed in successive baths of standard salt sodium citrate and RNase A solution before being dehydrated in increasing concentrations of ethanol. Tissue sections were then exposed to Biomax MR autoradiography films (Kodak, New Haven, CT) for 5 d for Nurr1 and 5 h for DAT (4, 24).
Quantification of TH-immunoreactive neurons
The loss of TH-positive neurons was determined by unbiased stereological counts of TH-positive cells under bright-field illumination, as reported (4). Every fifth section through the SNpc was analyzed using the Stereo Investigator software (MicroBrightfield, Colchester, VT) integrated with an E800 Nikon microscope (Nikon Canada Inc., Mississauga, ON, Canada). After delineation of the SNpc at low magnification (4× objective), a point grid was overlaid onto each section. Immunostained cells were counted using the optical fractionator method at higher magnification (20× objective). The counting variables were as follows: distance between counting frames (150 μm × 150 μm), counting frame size (100 μm), and guard zone thickness (2 μm). Cells were counted only if they did not intersect forbidden lines. The optical fractionator method (25) was used to count TH-positive and TH-negative (cresyl violet-positive only) cellular profiles. Stereological counts were performed blindly by two independent investigators.
Densitometric measurements of Nurr1 and DAT mRNA levels in the SNpc
Levels of autoradiographic labeling for Nurr1 and DAT in the SNpc were quantified by computerized densitometry, as previously shown (4, 21). Optical densities of the autoradiograms were translated into μCi/g of tissue using 14C radioactivity standards (ARC 146-14C standards, American Radiolabeled Chemical Inc., St. Louis, MO). The average labeling for each area was calculated from three adjacent brain sections (same levels) of the same mouse (stereotaxic coordinates, A-P levels, −2.92 mm to −3.52 mm) (26). Background intensities measured in the white layer of the superior colliculus, which lacks detectable Nurr1 and DAT, were subtracted from every measurement (26).
Catecholamine quantification
DA, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) were measured by HPLC with electrochemical detection, according to a slightly modified version of the previously published protocol (4). Extracts of anterior striata (stereotaxic coordinates, A-P levels, +1.34 mm to +0.94 mm) were collected from mouse brains, and 200 μl of perchloric acid (0.1 N; Mallinckrodt Baker, Phillipsburg, NJ) were added to generate a supernatant. Fifty microliters of supernatant from striatal tissues were directly injected into the HPLC consisting of a Waters 717 plus Autosampler Automatic Injector, a Waters 1525 Binary Pump equipped with an Atlantis dC18 (3 μm) column, a Waters 2465 Electrochemical Detector, and a glassy carbon electrode (Waters, Lachine, QC, Canada). The electrochemical detector was set at 10 nA. The mobile phase consisted of 47.8 mM NaH2PO4, 0.9 mM sodium octyl sulfate (Mallinckrodt Baker), 0.4 mM EDTA, 2 mM NaCl, and 8% MeOH (Mallinckrodt Baker) at pH 2.9 and was delivered at 0.8 ml/min. Peaks were identified using the Breeze software (Waters). HPLC quantifications were normalized to protein concentrations. Protein measurements were determined with a bicinchoninic acid protein assay kit using BSA as standard (Pierce, Rockford, IL) according to the manufacturer's protocol (4).
Sample preparation and Western immunoblots
Samples were homogenized in 8 vols of lysis buffer (150 mM NaCl, 10 mM NaH2PO4, 1% (v/v) Triton X-100, 0.5% SDS, and 0.5% sodium deoxycholate) containing a cocktail of protease inhibitors (Roche, Mississauga, ON, Canada) and phosphatase inhibitors (1 mM tetrasodium pyrophosphate and 50 mM sodium fluoride). Samples were sonicated (3 × 10 s) and centrifuged at 100,000 g for 20 min at 4°C. The supernatant was collected and stored at −80°C. The protein concentration in each fraction was determined with a bicinchoninic acid protein assay kit. Ten micrograms of total protein per sample were added to Laemmli loading buffer and heated to 95°C for 5 min. Samples were then loaded and subjected to SDS-polyacrylamide (8%) gel electrophoresis. Proteins were electroblotted onto 0.45 μm Immobilon PVDF membranes (Millipore, Billerica, MA) and blocked in 5% nonfat dry milk and 1% BSA in 1× PBS for 1 h. Membranes were immunoblotted with primary antibodies, rabbit anti-TH (Pel-Freez; 1:5,000), mouse anti-glial fibrillary acidic protein (GFAP) (Sigma; 1:10,000), mouse anti-actin (ABM Inc, Richmond, BC, Canada; 1:10,000), mouse anti-synaptophysin (Chemicon-Millipore; 1:10,000), mouse anti-PSD-95 (Neuromab, Davis, CA; 1:5,000) and with appropriate secondary antibodies, goat anti-rabbit or anti-mouse (Jackson Immunoresearch, West Grove, PA; 1:100,000) followed by the addition of chemiluminescence reagents (KPL, Mandel Scientific, Guelph, ON, Canada). Band intensities were quantified using a KODAK Image Station 4000 Digital Imaging System (Molecular Imaging Software version 4.0.5f7, Eastman Kodak, New Haven, CT).
Immunofluorescence
Post-fixed sections were incubated overnight at 4°C with 0.4% Triton X-100 and rabbit anti-GFAP (Waco Pure Chemical Industries, Richmond, VA; 1:1000). A 2.5 h incubation was then carried out at room temperature in a PBS solution containing goat Alexa 488-conjugated anti-rabbit antibody (1:500; Invitrogen/Molecular Probes, Eugene, OR). Following three washes in PBS, sections were placed in a solution containing 4′,6-diamidino-2-phenylindole (DAPI) (0.022%) for 7 min at room temperature and washed again twice before being mounted on slides, coverslipped using anti-fading mounting medium Fluoromount-G (SouthernBiotech, Birmingham, AL), and sealed with nail polish.
Statistical analyses and image preparation of postmortem material
Statistical analyses were performed using the JMP software version 6.0.2 (SAS Institute Inc., Cary, IL) and Prism 4 (GraphPad software, CA). Student's t-tests were used to analyze fatty acid profiles. One-way ANOVAs were performed for all the other experimentations and log adjustments were used to normalize variances when needed. Newman-Keuls multiple comparison tests were performed for posthoc analyses. Photomicrographs were taken with a Microfire 1.0 camera (Optronics, Goleta, CA) connected to an E800 Nikon microscope (Nikon Inc., Québec, QC, Canada) using the Picture Frame imaging software (Microbrightfield) while immunofluorescence specimens were examined using an epifluorescence microscope (Olympus Provis AX70, Melville, NY) and photographs were taken using a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI). All images were prepared for illustration in Adobe Photoshop 7.0.
RESULTS
The fat-1 transgene expression moderately increases the n-3:n-6 PUFA ratio
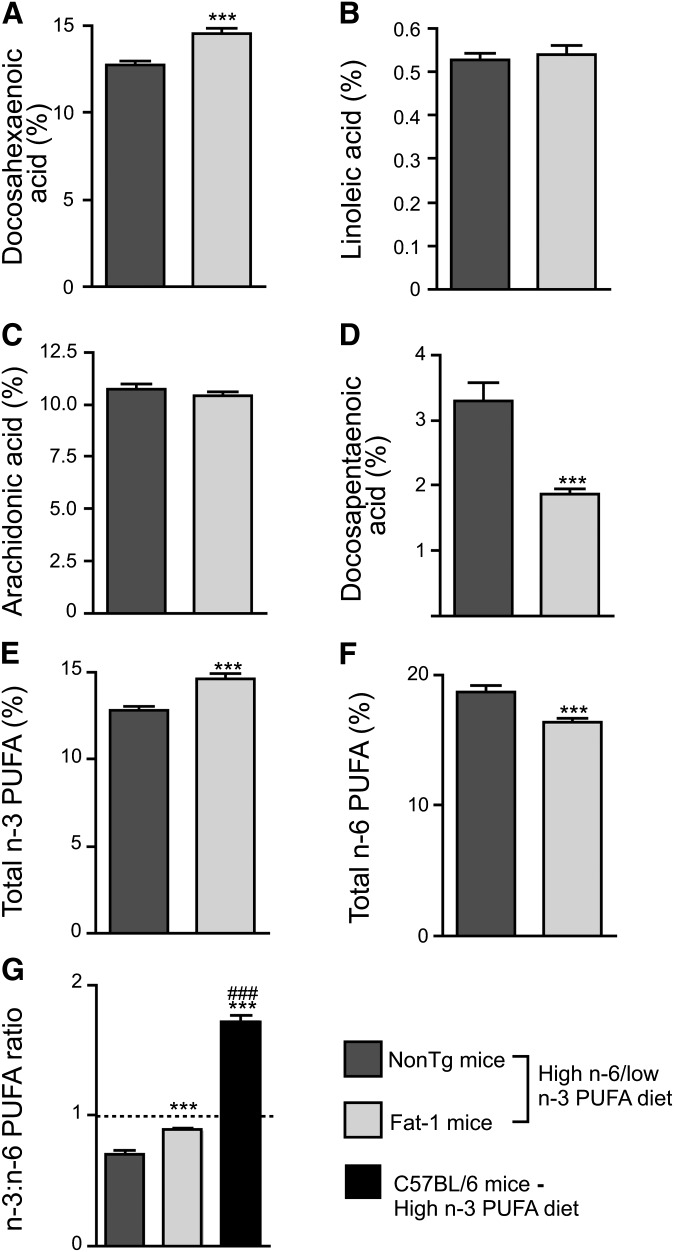
Due to the action of the fat-1 transgene, DHA levels (Fig. 1A; p < 0.001) and total n-3 PUFA concentrations (Fig. 1E; 12.79% vs. 14.62% in Fat-1 mice, p < 0.001) were increased whereas total n-6 PUFAs were decreased in the cortex of 6-month-old Fat-1 mice (Fig. 1F; 18.66% vs. 16.38% in Fat-1 mice, p < 0.001), compared with NonTg mice. In addition, expression of fat-1 decreased the n-6 docosapentaenoic acid content (Fig. 1D; p < 0.001), a biomarker of n-3 PUFA deprivation elevated in NonTg mice exposed to low n-3 PUFA intake. Finally, a 28% increase of the cortical n-3:n-6 PUFA ratio in Fat-1 mice was also detected, reaching almost 1:1 (Fig. 1G; p < 0.001). However, the effect of the fat-1 transgene on the brain PUFA profile was surprisingly limited in comparison with the effect of a 10 month n-3 PUFA supplementation as previously observed (4). Indeed, the fat-1 transgene increased the n-3:n-6 PUFA ratio by a modest 28% relative to NonTg mice fed the high n-6 PUFA diet, whereas a 10-month exposure to an n-3 PUFA-enriched diet for 10 months increased the n-3:n-6 PUFA ratio by as much as 92% (Fig. 1G; p < 0.001).
Fig. 1.
Fat-1 transgenic mice display significant changes in fatty acid profiles. As assessed in the frontal cortex by gas chromatography, DHA (A) and total n-3 PUFAs (E) were increased in Fat-1 mice compared with NonTg mice. Despite the absence of differences in linoleic (B) and arachidonic acids (C), n-6 docosapentaenoic acid (D) and total n-6 PUFAs (F) were significantly decreased in Fat-1 mice. These alterations led to an increase in the brain n-3:n-6 PUFA ratio reaching nearly 1:1, but the fat-1 transgene was comparatively less potent than dietary DHA supplementation in increasing the brain n-3:n-6 PUFA ratio [see previous published data (4)] (G). Data are expressed as a percentage of total fatty acids. *** P < 0.001 versus NonTg mice and ### P < 0.001 versus NonTg and Fat-1 mice.
The fat-1 transgene does not impede MPTP striatal neurotoxic effects
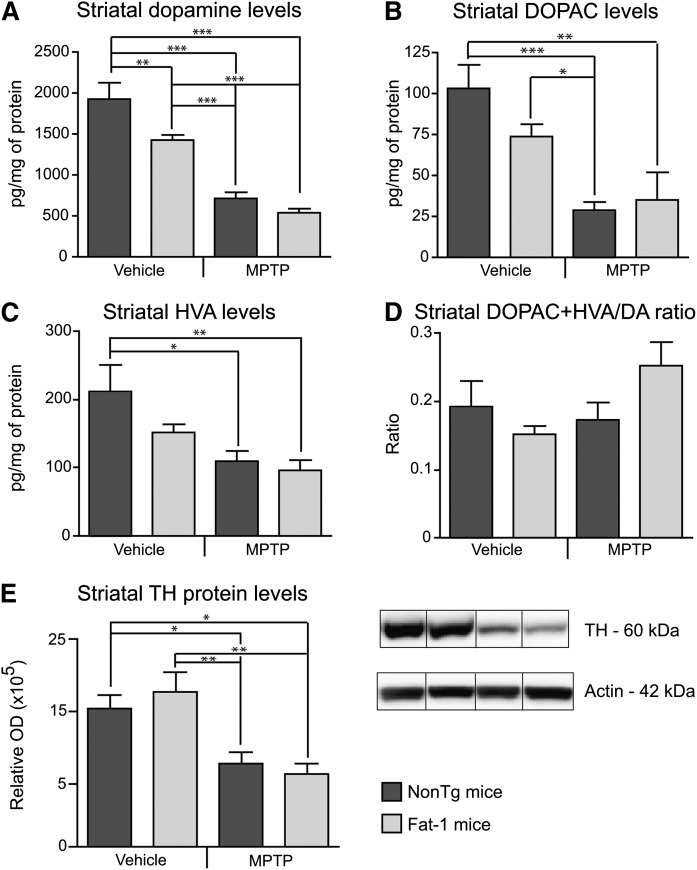
As previously reported with the specific MPTP administration regime used in this study (4, 21), the neurotoxin significantly decreased striatal DA content. Reductions of 63% and 72% in striatal DA levels were noted in MPTP-treated NonTg (p < 0.001) and Fat-1 mice (p < 0.001), respectively. A spontaneous decrease of DA was also observed in saline-treated Fat-1 mice (Fig. 2A; p < 0.01). DOPAC (Fig. 2B) and HVA (Fig. 2C), two metabolites of DA, were also decreased by the MPTP treatment in both groups. The (DOPAC+HVA)/DA ratio did not undergo significant changes (Fig. 2D). Levels of striatal TH-immunoreactive fibers were also decreased following the MPTP lesion, as compared with saline-treated mice in either the NonTg (Fig. 2E; p < 0.05) or Fat-1 groups (Fig. 2E; p < 0.01), as assessed by Western analysis. The expression of the fat-1 transgene per se did not protect from MPTP-induced DA and fiber loss, as both Fat-1 and NonTg MPTP-treated mice depicted consistent levels of these makers (Fig. 2). Synaptic markers, synaptophysin as well as the synaptosomal-associated protein (SNAP-25) and the postsynaptic density marker (PSD-95) were also analyzed by immunoblotting. Neither MPTP nor the fat-1 transgene measurably affected the expression of these pre- and post-synaptic proteins (data not shown).
Fig. 2.
The fat-1 transgene does not prevent the MPTP-induced decreases in dopamine. Levels of DA (A) and its metabolites DOPAC (B) and HVA (C) were quantified by HPLC coupled to electrochemical detection. Decreases in DA, DOPAC, and HVA were observed following MPTP, independently of the transgene. Similar observations were established by Western analyses as striatal TH-immunoreactive fibers were decreased in MPTP-treated NonTg and Fat-1 mice (E). Values are expressed as mean ± SEM. * P < 0.05; ** P < 0.01; *** P < 0.001.
Nigral dopaminergic markers correlate with brain DHA levels
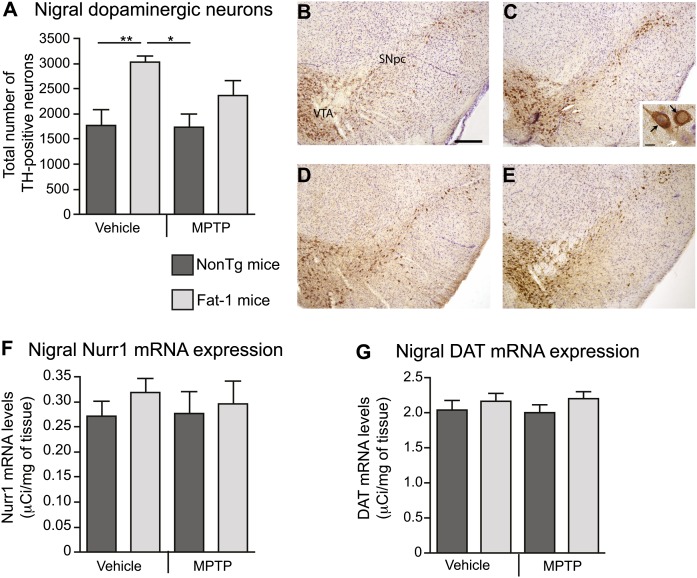
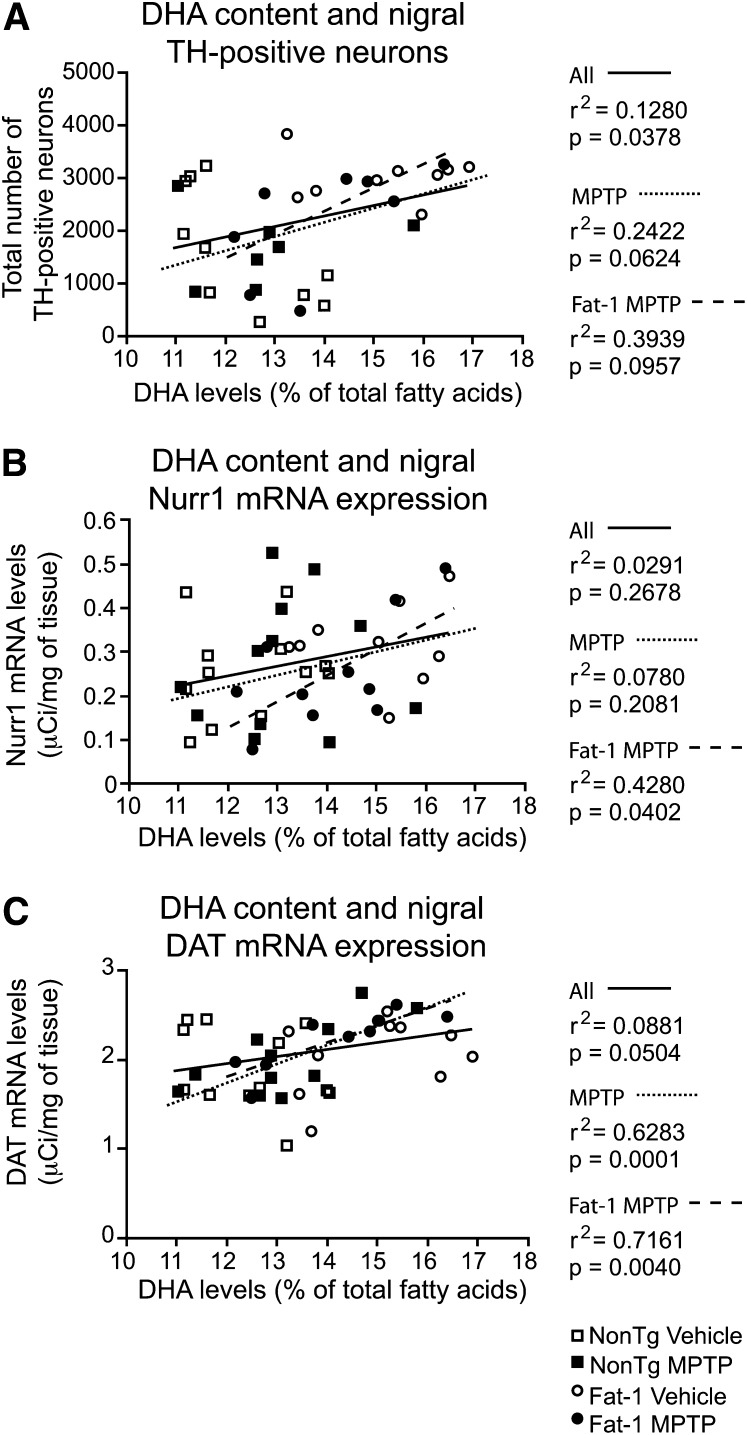
Although the fat-1 transgene did not exert a neuroprotective effect, as assessed with nigral markers of the DAergic system (Fig. 3), significant correlations were detected with brain DHA levels. The MPTP protocol used here typically induces between 30% and 40% neuronal degeneration in the SNpc (4, 21, 27, 28), which is accompanied by a 60% striatal DA loss (4, 28). As depicted on the histogram (Fig. 3A) and visualized on the photomicrographs (Fig. 3B–E), the MPTP insult did not decrease the number of TH-immunostained neurons in treated NonTg mice as compared with saline-treated NonTg mice. Consistent with the TH immunohistochemistry data, no effect of the MPTP injection was detected on nigral Nurr1 mRNA levels, an important nuclear receptor for DAergic regulation and survival (29) (Fig. 3F). Similar observations were noted for DAT mRNA levels, essential for the reuptake of DA into the presynaptic compartment of DAergic synapses (Fig. 3G). Nevertheless, a significant association between TH-positive neurons and DHA levels was noted (Fig. 4A; r2 = 0.13, p = 0.038). A correlation was also established between Nurr1 mRNA levels and brain DHA levels (Fig. 4B; r2 = 0.43, p = 0.040) in the MPTP-treated Fat-1 mice group. A similar significant relationship was detected between DAT mRNA levels and brain DHA levels (Fig. 4C; r2 = 0.63, p = 0.0001) in MPTP-treated mice independently of transgene expression, as well as in MPTP-treated Fat-1 mice (Fig. 4C; r2 = 0.72, p = 0.004).
Fig. 3.
MPTP administration does not affect the number of nigral dopaminergic cells in Fat-1 mice and control littermates. Stereological counts of TH-positive cells in the SNpc did not reveal significant changes between NonTg mice treated with either saline (B) or MPTP (A, D). An increased number of TH-positive neurons was observed in saline-treated Fat-1 mice (C) compared with NonTg mice either treated with saline or MPTP. In the inset (C), the white arrow points to a cresyl-positive neuron, whereas black arrows identify TH-positive neurons. Measurements of optical densities following in situ hybridization for Nurr1 (F) and DAT (G) mRNA levels did not reveal any differences between groups. Values are expressed as mean ± SEM. * P < 0.05; scale bar in B = 100 μm applies to C, D, and E, and in C (inset) = 10 μm.
Fig. 4.
TH-positive neurons, as well as Nurr1 and DAT mRNA expression, correlate with cortical DHA levels. A correlation between the total number of nigral TH-positive neurons and DHA (% of total fatty acids) was observed (A). Significant associations were also noted for Nurr1 (B) and DAT (C) mRNA expression in Fat-1 MPTP-treated mice. Each animal is depicted by a symbol associated to a specific group: □, NonTg Vehicle; ■, NonTg MPTP; ○, Fat-1 Vehicle; •, Fat-1 MPTP. Correlations were performed with either all animals (solid line), MPTP-treated mice (dotted line) or Fat-1 MPTP-treated mice (dashed line).
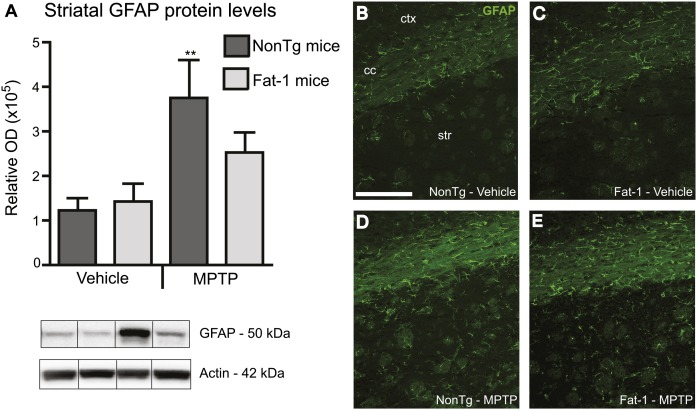
Fat-1 transgene attenuates striatal astrogliosis
N-3 PUFAs are natural anti-neuroinflammatory agents [see reviews (30–32)]; thus, inflammatory cell markers were evaluated in the striatum. Western analyses revealed a significant increase in GFAP immunoreactive protein levels following MPTP exposure (Fig. 5). This increased astrogliosis was attenuated in Fat-1 mice because no significant difference was observed between MPTP-treated Fat-1 mice and saline-treated groups (Fig. 5A). Western blot results were further corroborated with visual assessment of immunofluorescence, where an increase in the number of GFAP-immunoreactive cells was observed in NonTg mice treated with MPTP in comparison to saline-treated groups and Fat-1 mice (Fig. 5B–E). Ionized calcium binding adaptor molecule-1 (Iba-1), which specifically labels macrophages and microglia, was also analyzed by immunoblotting but no difference was noted between groups (data not shown).
Fig. 5.
Attenuation of the MPTP-mediated astrocytic response in Fat-1 mice. MPTP administration induced an increase in GFAP protein levels in the striatum of NonTg mice, but not in Fat-1 mice, as quantified by Western analysis (A). This observation was further confirmed by immunofluorescence (B–E) where a greater number of GFAP-positive cells was observed in the striatum. Cells displayed an activated inflammatory profile in NonTg MPTP-treated (D) as compared with saline-treated (B, C) and Fat-1 MPTP-treated mice (E). GFAP, glial fibrillary acidic protein; cc, corpus callosum; str, striatum; ctx, cortex; OD, optical density. ** P < 0.01 versus vehicle groups; scale bar in B = 200 μm applies to C, D, and E.
DISCUSSION
The objectives of this study were 2-fold: 1) determining the capacity of the fat-1 transgene to convert n-6 into n-3 PUFAs in the brain as well as 2) investigating the neuroprotective action of endogenously produced n-3 PUFAs against MPTP neurotoxicity. We first confirmed that Fat-1 mice have higher cortical n-3 PUFA levels. However, the efficacy of the n-3 fatty acid desaturase was found to be inconsistent between Fat-1 mice, as the brain n-3:n-6 PUFA ratio ranged from 0.72 to 1.0 within the same group. Of note, mice were carefully genotyped twice in order to distinguish transgenic mice from NonTg littermates, an approach also used by other research groups (19, 33, 34), whereas other authors have relied exclusively on phenotyping the animal with the fatty acid profiles (20). As reported by our group (4, 35) and more recently by several other research teams (34, 36), dietary DHA supplementation readily increases n-3:n-6 PUFA ratio over 1.5. This effect is much more significant than with fat-1 transgene as reported previously (33, 34) and substantiated by our data. Most studies using 8- to 12-week-old Fat-1 mice have reported brain fatty acid profiles similar to levels observed here for 6-month-old Fat-1 mice, thus excluding age as a discriminating factor (15, 33, 34). Importantly, previous studies investigating Fat-1 mice used diets enriched in n-6 PUFAs and low in n-3 PUFAs in order to detect more specifically the transgene effects (15, 20).
The MPTP regime and administration route selected here consistently produce a moderate nigral neuronal degeneration ranging from 30 to 40% of nigral TH-positive cells (4, 21, 27, 28) and a 60–70% reduction in striatal DA (4, 28). However, in Fat-1 mice as well as in their NonTg littermates, no significant loss of DAergic cells was detected in the SNpc, despite the use of three different reliable nigral markers. This surprising observation may be explained by a strain-specific effect of the MPTP. In fact, Fat-1 mice were initially produced on a C57BL/6 × C3H mixed genetic background and remaining C3H genes could have interfered with the MPTP regime. MPTP neurotoxicity has been shown to depend on the mouse strain, with pure C57BL/6 ranking among the most sensitive to MPTP toxicity (37).
Despite a weaker effect on nigral components, MPTP injections led to massive loss of DA levels as well as striatal TH-positive fibers, confirming the DAergic denervation. However, the fat-1 transgene did not exert beneficial effects on striatal components evaluated here. This contrasts with our previous observation of a neuroprotective effect of long-term intake of a diet enriched in n-3 PUFAs (4). As stated above, the fact that the fat-1 transgene barely raised the n-3:n-6 PUFA ratio to nearly 1.0, as compared with the 1.5 ratio observed with dietary intake of DHA, is the most likely explanation for the inability of the fat-1 transgene to prevent the effect of MPTP. Nevertheless, the increase in DHA levels induced either by the fat-1 transgene or by the n-3 PUFA-enriched diet was positively correlated with nigral components evaluated here such as TH-positive neurons, as well as Nurr1 and DAT mRNA levels. These associations strongly support the contention that DHA exerts beneficial effects in the MPTP mouse model of PD.
Among the unexpected effects observed here for the fat-1 transgene are the spontaneous decrease in striatal DA concentrations and the increase in nigral TH-positive neurons found in saline-treated Fat-1 mice. However, these observations are in agreement with previous studies demonstrating that n-3 PUFA-deficient rats show increased striatal and prefrontal cortical DA levels (38, 39) along with a reduced number of DAergic neurons in the SNpc and in the ventral tegmental area (40). This result reinforces the assertion that n-3 PUFAs have a strong impact on the DAergic system per se, as proposed by several authors [see review (41)].
N-3 PUFAs orchestrate a wide spectrum of cellular activities via their direct incorporation into the cell membrane phospholipids or following their intracellular release. Anti-apoptotic (35, 42), anti-oxidant (35, 43, 44), and anti-inflammatory (45–47) effects of n-3 PUFAs have been discussed. In addition, n-3 PUFAs stand as natural potential modulators of neurotrophic factors such as BDNF (5, 43, 48). With respect to the potential anti-apoptotic properties of n-3 PUFAs, a protein microarray analysis conducted in Fat-1 mice revealed a pattern of expression involved in the enhancement of neuronal survival and function associated with increased hippocampal expression of nitric oxide synthase, the tumor suppressor phosphatase and tensin homolog, and mitogen-activated protein kinase-2 (MAPK-2) (49). The anti-inflammatory properties of n-3 PUFAs can be explained by their inhibition of arachidonic acid metabolism through enzymatic competition for cyclooxygenases (COXs). By decreasing the concentration of arachidonic acid and, consequently, arachidonic acid-derived pro-inflammatory eicosanoids, the accumulation of n-3 PUFAs in the brain favors DHA-derived mediators, which are less pro-inflammatory (30, 31). Recently, several authors reported the anti-inflammatory properties of mediators derived from EPA and DHA and which belong to the resolvin and protectin families (50–52). These EPA- and DHA-derived mediators were shown to decrease leukocyte infiltration, COX-2 expression, and the transcription factor NF-κB (50, 53). Clues that the fat-1 transgene has an anti-inflammatory activity were also given by reports that brain COX-2 levels are reduced in 12-week-old Fat-1 mice (33). This dampening of the inflammatory response was also associated with a beneficial effect as observed in Fat-1 modeling pancreatitis and inflamed colon (54, 55). Finally, the decrease in GFAP levels observed here in Fat-1 mice following MPTP lesion supports the hypothesis of an anti-inflammatory role for the fat-1 transgene despite the absence of a clear neuroprotective effect.
Taken together, our results confirm the capacity of the fat-1 transgene to produce n-3 PUFAs, thereby increasing the brain n-3:n-6 PUFA ratio. However, the increase in brain DHA provided by fat-1 was insufficient to induce a frank neuroprotective effect against MPTP neurotoxicity, in comparison to the effects reached with DHA dietary supplementation. Nevertheless, the strong correlations between nigral constituents and DHA levels found in the present study reinforce the hypothesis that n-3 PUFAs are beneficial against MPTP-induced denervation. They also support the implication of n-3 PUFAs in reducing inflammatory processes. Overall, the present data combined with our previous work strongly suggest that dietary intervention with preformed DHA constitutes a potent method to achieve neuroprotective levels in the brain, particularly in the context of PD.
Acknowledgments
The authors would like to thank Dr Richard Poulin for his valuable editorial comments.
Footnotes
Abbreviations:
- BDNF
- brain-derived neurotrophic factor
- COX
- cyclooxygenase
- DA
- dopamine, DAergic, dopaminergic
- DAT
- dopamine transporter
- DHA
- docosahexaenoic acid
- DOPAC
- 3,4-dihydroxyphenylacetic acid
- EPA
- eicosapentaenoic acid
- GFAP
- glial fibrillary acidic protein
- HVA
- homovanillic acid
- MPTP
- 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NonTg
- nontransgenic
- SNpc
- substantia nigra pars compacta
- TH
- tyrosine hydroxylase
This study was supported by Parkinson Society Canada, and by the Institute of Nutrition, Metabolism and Diabetes (INMD) of the Canadian Institutes of Health Research (CIHR) and the Canada Foundation for Innovation (F. Cicchetti and F. Calon). M. Bousquet was supported by a Vanier Canada Graduate Scholarship from the CIHR. The work of F. Calon was supported by a New Investigator Award from the Clinical Research Initiative and the CIHR Institute of Aging (CAN-76833). The work of F. Cicchetti was supported by a New Investigator Award from the CIHR.
REFERENCES
- 1.Lees A. J., Hardy J., Revesz T. 2009. Parkinson's disease. Lancet. 373: 2055–2066. [DOI] [PubMed] [Google Scholar]
- 2.Schapira A. H. 2009. Neurobiology and treatment of Parkinson's disease. Trends Pharmacol. Sci. 30: 41–47. [DOI] [PubMed] [Google Scholar]
- 3.Schapira A. H., Bezard E., Brotchie J., Calon F., Collingridge G. L., Ferger B., Hengerer B., Hirsch E., Jenner P., Le Novere N., et al. 2006. Novel pharmacological targets for the treatment of Parkinson's disease. Nat. Rev. Drug Discov. 5: 845–854. [DOI] [PubMed] [Google Scholar]
- 4.Bousquet M., Saint-Pierre M., Julien C., Salem N., Jr, Cicchetti F., Calon F. 2008. Beneficial effects of dietary omega-3 polyunsaturated fatty acid on toxin-induced neuronal degeneration in an animal model of Parkinson's disease. FASEB J. 22: 1213–1225. [DOI] [PubMed] [Google Scholar]
- 5.Bousquet M., Gibrat C., Saint-Pierre M., Julien C., Calon F., Cicchetti F. 2009. Modulation of brain-derived neurotrophic factor as a potential neuroprotective mechanism of action of omega-3 fatty acids in a parkinsonian animal model. Prog. Neuropsychopharmacol. Biol. Psychiatry. 33: 1401–1408. [DOI] [PubMed] [Google Scholar]
- 6.de Lau L. M., Bornebroek M., Witteman J. C., Hofman A., Koudstaal P. J., Breteler M. M. 2005. Dietary fatty acids and the risk of Parkinson disease: the Rotterdam study. Neurology. 64: 2040–2045. [DOI] [PubMed] [Google Scholar]
- 7.Calon F., Cicchetti F. 2008. Can we prevent Parkinson's disease with n-3 polyunsaturated fatty acids? Future Lipidol. 3: 133–137. [Google Scholar]
- 8.Racine R. A., Deckelbaum R. J. 2007. Sources of the very-long-chain unsaturated omega-3 fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Curr. Opin. Clin. Nutr. Metab. Care. 10: 123–128. [DOI] [PubMed] [Google Scholar]
- 9.Salem N., Jr, Hullin F., Yoffe A. M., Karanian J. W., Kim H. Y. 1989. Fatty acid and phospholipid species composition of rat tissues after a fish oil diet. Adv. Prostaglandin Thromboxane Leukot. Res. 19: 618–622. [PubMed] [Google Scholar]
- 10.Crawford M. A., Bazinet R. P., Sinclair A. J. 2009. Fat intake and CNS functioning: ageing and disease. Ann. Nutr. Metab. 55: 202–228. [DOI] [PubMed] [Google Scholar]
- 11.Fiennes R. N., Sinclair A. J., Crawford M. A. 1973. Essential fatty acid studies in primates linolenic acid requirements of capuchins. J. Med. Primatol. 2: 155–169. [DOI] [PubMed] [Google Scholar]
- 12.Hansen S. N., Harris W. S. 2007. New evidence for the cardiovascular benefits of long chain omega-3 fatty acids. Curr. Atheroscler. Rep. 9: 434–440. [DOI] [PubMed] [Google Scholar]
- 13.Calon F., Cole G. 2007. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: evidence from animal studies. Prostaglandins Leukot. Essent. Fatty Acids. 77: 287–293. [DOI] [PubMed] [Google Scholar]
- 14.Stahl L. A., Begg D. P., Weisinger R. S., Sinclair A. J. 2008. The role of omega-3 fatty acids in mood disorders. Curr. Opin. Investig. Drugs. 9: 57–64. [PubMed] [Google Scholar]
- 15.Kang J. X., Wang J., Wu L., Kang Z. B. 2004. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 427: 504. [DOI] [PubMed] [Google Scholar]
- 16.Kang Z. B., Ge Y., Chen Z., Cluette-Brown J., Laposata M., Leaf A., Kang J. X. 2001. Adenoviral gene transfer of Caenorhabditis elegans n-3 fatty acid desaturase optimizes fatty acid composition in mammalian cells. Proc. Natl. Acad. Sci. USA. 98: 4050–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffitts J., Saunders D., Tesiram Y. A., Reid G. E., Salih A., Liu S., Lydic T. A., Busik J. V., Kang J. X., Towner R. A. 2010. Non-mammalian fat-1 gene prevents neoplasia when introduced to a mouse hepatocarcinogenesis model Omega-3 fatty acids prevent liver neoplasia. Biochim. Biophys. Acta. 1801: 1133–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan J. B., Huang L. L., Rong R., Tan R., Wang J., Kang J. X. 2010. Endogenously decreasing tissue n-6/n-3 fatty acid ratio reduces atherosclerotic lesions in apolipoprotein E-deficient mice by inhibiting systemic and vascular inflammation. Arterioscler. Thromb. Vasc. Biol. 30: 2487–2494.. [DOI] [PubMed] [Google Scholar]
- 19.Taha A. Y., Huot P. S., Reza-Lopez S., Prayitno N. R., Kang J. X., Burnham W. M., Ma D. W. 2008. Seizure resistance in fat-1 transgenic mice endogenously synthesizing high levels of omega-3 polyunsaturated fatty acids. J. Neurochem. 105: 380–388. [DOI] [PubMed] [Google Scholar]
- 20.He C., Qu X., Cui L., Wang J., Kang J. X. 2009. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc. Natl. Acad. Sci. USA. 106: 11370–11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibrat C., Saint-Pierre M., Bousquet M., Levesque D., Rouillard C., Cicchetti F. 2009. Differences between subacute and chronic MPTP mice models: investigation of dopaminergic neuronal degeneration and alpha-synuclein inclusions. J. Neurochem. 109: 1469–1482. [DOI] [PubMed] [Google Scholar]
- 22.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 23.Masood A., Stark K. D., Salem N., Jr 2005. A simplified and efficient method for the analysis of fatty acid methyl esters suitable for large clinical studies. J. Lipid Res. 46: 2299–2305. [DOI] [PubMed] [Google Scholar]
- 24.Gibrat C., Bousquet M., Saint-Pierre M., Levesque D., Calon F., Rouillard C., Cicchetti F. 2010. Cystamine prevents MPTP-induced toxicity in young adult mice via the up-regulation of the brain-derived neurotrophic factor. Prog. Neuropsychopharmacol. Biol. Psychiatry. 34: 193–203. [DOI] [PubMed] [Google Scholar]
- 25.Glaser J. R., Glaser E. M. 2000. Stereology, morphometry, and mapping: the whole is greater than the sum of its parts. J. Chem. Neuroanat. 20: 115–126. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G., Franklin K. J. 2001. The mouse brain in stereotaxic coordinates. Academic Press, San Diego: 296. [Google Scholar]
- 27.Tremblay M. E., Saint-Pierre M., Bourhis E., Levesque D., Rouillard C., Cicchetti F. 2006. Neuroprotective effects of cystamine in aged parkinsonian mice. Neurobiol. Aging. 27: 862–870. [DOI] [PubMed] [Google Scholar]
- 28.Sun L., Xu S., Zhou M., Wang C., Wu Y., Chan P. 2010. Effects of cysteamine on MPTP-induced dopaminergic neurodegeneration in mice. Brain Res. 1335: 74–82. [DOI] [PubMed] [Google Scholar]
- 29.Saucedo-Cardenas O., Quintana-Hau J. D., Le W. D., Smidt M. P., Cox J. J., De Mayo F., Burbach J. P., Conneely O. M. 1998. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc. Natl. Acad. Sci. USA. 95: 4013–4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orr S. K., Bazinet R. P. 2008. The emerging role of docosahexaenoic acid in neuroinflammation. Curr. Opin. Investig. Drugs. 9: 735–743. [PubMed] [Google Scholar]
- 31.Calder P. C. 2006. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids. 75: 197–202. [DOI] [PubMed] [Google Scholar]
- 32.Laye S. 2010. Polyunsaturated fatty acids, neuroinflammation and well being. Prostaglandins Leukot. Essent. Fatty Acids. 82: 295–303. [DOI] [PubMed] [Google Scholar]
- 33.Boudrault C., Bazinet R. P., Kang J. X., Ma D. W. 2010. Cyclooxygenase-2 and n-6 PUFA are lower and DHA is higher in the cortex of fat-1 mice. Neurochem. Int. 56: 585–589. [DOI] [PubMed] [Google Scholar]
- 34.Orr S. K., Tong J. Y., Kang J. X., Ma D. W., Bazinet R. P. 2010. The fat-1 mouse has brain docosahexaenoic acid levels achievable through fish oil feeding. Neurochem. Res. 35: 811–819. [DOI] [PubMed] [Google Scholar]
- 35.Calon F., Lim G. P., Yang F., Morihara T., Teter B., Ubeda O., Rostaing P., Triller A., Salem N., Jr, Ashe K. H., et al. 2004. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 43: 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez S. E., Berg B. M., Moore K. A., He B., Counts S. E., Fritz J. J., Hu Y. S., Lazarov O., Lah J. J., Mufson E. J. 2010. DHA diet reduces AD pathology in young APPswe/PS1 Delta E9 transgenic mice: possible gender effects. J. Neurosci. Res. 88: 1026–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonsalla P. K., Heikkila R. E. 1986. The influence of dose and dosing interval on MPTP-induced dopaminergic neurotoxicity in mice. Eur. J. Pharmacol. 129: 339–345. [DOI] [PubMed] [Google Scholar]
- 38.Zimmer L., Durand G., Guilloteau D., Chalon S. 1999. n-3 polyunsaturated fatty acid deficiency and dopamine metabolism in the rat frontal cortex. Lipids. 34(Suppl): S251. [DOI] [PubMed] [Google Scholar]
- 39.Delion S., Chalon S., Guilloteau D., Besnard J. C., Durand G. 1996. alpha-Linolenic acid dietary deficiency alters age-related changes of dopaminergic and serotoninergic neurotransmission in the rat frontal cortex. J. Neurochem. 66: 1582–1591. [DOI] [PubMed] [Google Scholar]
- 40.Ahmad S. O., Park J. H., Radel J. D., Levant B. 2008. Reduced numbers of dopamine neurons in the substantia nigra pars compacta and ventral tegmental area of rats fed an n-3 polyunsaturated fatty acid-deficient diet: a stereological study. Neurosci. Lett. 438: 303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chalon S. 2006. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot. Essent. Fatty Acids. 75: 259–269. [DOI] [PubMed] [Google Scholar]
- 42.Calon F., Lim G. P., Morihara T., Yang F., Ubeda O., Salem N., Jr, Frautschy S. A., Cole G. M. 2005. Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer's disease. Eur. J. Neurosci. 22: 617–626. [DOI] [PubMed] [Google Scholar]
- 43.Wu A., Ying Z., Gomez-Pinilla F. 2004. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma. 21: 1457–1467. [DOI] [PubMed] [Google Scholar]
- 44.Yavin E., Brand A., Green P. 2002. Docosahexaenoic acid abundance in the brain: a biodevice to combat oxidative stress. Nutr. Neurosci. 5: 149–157. [DOI] [PubMed] [Google Scholar]
- 45.Calder P. C. 2005. Polyunsaturated fatty acids and inflammation. Biochem. Soc. Trans. 33: 423–427. [DOI] [PubMed] [Google Scholar]
- 46.De Smedt-Peyrusse V., Sargueil F., Moranis A., Harizi H., Mongrand S., Laye S. 2008. Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J. Neurochem. 105: 296–307. [DOI] [PubMed] [Google Scholar]
- 47.Simopoulos A. P. 2002. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 21: 495–505. [DOI] [PubMed] [Google Scholar]
- 48.Rapoport S. I., Rao J. S., Igarashi M. 2007. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot. Essent. Fatty Acids. 77: 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Menesi D., Kitajka K., Molnar E., Kis Z., Belleger J., Narce M., Kang J. X., Puskas L. G., Das U. N. 2009. Gene and protein expression profiling of the fat-1 mouse brain. Prostaglandins Leukot. Essent. Fatty Acids. 80: 33–42. [DOI] [PubMed] [Google Scholar]
- 50.Hong S., Gronert K., Devchand P. R., Moussignac R. L., Serhan C. N. 2003. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J. Biol. Chem. 278: 14677–14687. [DOI] [PubMed] [Google Scholar]
- 51.Serhan C. N., Gotlinger K., Hong S., Arita M. 2004. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat. 73: 155–172. [DOI] [PubMed] [Google Scholar]
- 52.Bazan N. G. 2005. Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 15: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marcheselli V. L., Hong S., Lukiw W. J., Tian X. H., Gronert K., Musto A., Hardy M., Gimenez J. M., Chiang N., Serhan C. N., et al. 2003. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J. Biol. Chem. 278: 43807–43817. [DOI] [PubMed] [Google Scholar]
- 54.Weylandt K. H., Nadolny A., Kahlke L., Kohnke T., Schmocker C., Wang J., Lauwers G. Y., Glickman J. N., Kang J. X. 2008. Reduction of inflammation and chronic tissue damage by omega-3 fatty acids in fat-1 transgenic mice with pancreatitis. Biochim. Biophys. Acta. 1782: 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gravaghi C., La Perle K. M., Ogrodwski P., Kang J. X., Quimby F., Lipkin M., Lamprecht S. A. 2010. Cox-2 expression, PGE(2) and cytokines production are inhibited by endogenously synthesized n-3 PUFAs in inflamed colon of fat-1 mice. J. Nutr. Biochem. Epub ahead of print (doi:10.1016/j.jnutbio.2010.03.003). [DOI] [PubMed] [Google Scholar]