Abstract
Palmitic acid (PA) upregulates oxidized LDL receptor-1 (LOX-1), a scavenger receptor responsible for uptake of oxidized LDL (oxLDL), and enhances oxLDL uptake in macrophages. However, the precise underlying mechanism remains to be elucidated. PA is known to induce endoplasmic reticulum (ER) stress in various cell types. Therefore, we investigated whether ER stress is involved in PA-induced LOX-1 upregulation. PA induced ER stress, as determined by phosphorylation of PERK, eIF2α, and JNK, as well as induction of CHOP in macrophage-like THP-1 cells. Inhibitors [4-phenylbutyric acid (PBA), sodium tauroursodeoxycholate (TUDCA), and salubrinal] and small interfering RNA (siRNA) for the ER stress response decreased PA-induced LOX-1 upregulation. Thapsigargin, an ER stress inducer, upregulated LOX-1, which was decreased by PBA and TUDCA. We next examined whether unsaturated FAs could counteract the effect of PA. Both oleic acid (OA) and linoleic acid (LA) suppressed PA-induced LOX-1. Activation of the ER stress response observed in the PA-treated cells was markedly attenuated when the cells were cotreated with OA or LA. In addition, OA and LA suppressed thapsigargin-induced LOX-1 upregulation with reduced activation of ER stress markers. Our results indicate that activation of ER stress is involved in PA-induced LOX-1 upregulation in macrophages, and that OA and LA inhibit LOX-1 induction through suppression of ER stress.
Keywords: palmitic acid, endoplasmic reticulum, fatty acid, low density lipoprotein receptor
The elevated levels of nonesterified FAs (NEFAs) observed in obesity and type 2 diabetes have been suggested to be risk factors for cardiovascular disease (1). Although the mechanisms by which NEFA contributes to the pathogenesis and/or progression of atherosclerosis are still not completely understood, a number of studies have shown deleterious effects of NEFA, such as impairment of endothelium-dependent vasodilation (2) and activation of proinflammatory responses in the vascular wall (3). In addition, our previous study demonstrated that palmitic acid (PA) upregulates lectin-like oxidized LDL receptor-1 (LOX-1), a scavenger receptor responsible for uptake of oxidized LDL (oxLDL), and promotes uptake of oxLDL in macrophages (4). Accumulation of oxLDL in the intima and subsequent uptake of oxLDL by macrophages to make foam cells are thought to be crucial steps in the initiation and progression of atherosclerosis. Therefore, our results suggest that PA may facilitate the course of atherosclerosis by promoting foam cell formation with enhanced LOX-1 expression and uptake of oxLDL. However, the precise mechanism underlying PA-induced LOX-1 upregulation remains to be elucidated.
The endoplasmic reticulum (ER) is known to integrate cellular responses to stress, in addition to its functions in protein synthesis, folding, and transportation (5). When excessive amounts of unfolded and misfolded proteins are accumulated in the ER lumen, ER stress is induced, and the elevated ER stress is associated with several human diseases (6, 7). Under conditions that induce ER stress, the unfolded protein response (UPR) is activated to reduce the level of new protein synthesis, increase folding capacity, and degrade terminally misfolded proteins (6). When ER stress occurs, three ER transmembrane sensors, protein kinase-like ER kinase (PERK), inositol-requiring kinase/endonuclease-1 (IRE-1), and activating transcription factor-6 (ATF-6), are activated to initiate adaptive responses (8). PERK is a serine kinase that phosphorylates eukaryotic translation initiation factor 2α (eIF2α) to attenuate general protein synthesis. IRE-1 interacts with the adaptor protein TNF receptor-associated factor-2, leading to an interaction with a mitogen-activated protein kinase kinase kinase, ASK-1, which subsequently phosphorylates c-JUN N-terminal kinase (JNK). C/EBP homologous protein (CHOP) is a proapototic bZIP transcriptional factor that is mainly regulated by ATF-4- and ATF-6-dependent pathways upon ER stress. Prolonged ER stress can lead to apoptosis (9). Chronic conditions associated with diabetes and obesity, such as low-grade inflammation, hyperglycemia, and hyperlipidemia have recently been shown to induce ER stress (10). ER stress is activated in macrophages from atherosclerotic lesions in mice and humans (11–15), and reduction of ER stress in macrophages alleviates atherosclerosis in mice (14, 16), suggesting that macrophage ER stress may contribute to the pathogenesis of atherosclerosis, especially linking macrophage apoptosis to plaque vulnerability. In addition to the role of ER stress in the induction of apoptosis, there is evidence showing that ER stress increases expression of the scavenger receptors CD36 and SR-A in macrophages (12, 17), suggesting that ER stress promotes foam cell formation.
Recent studies have shown that PA induces ER stress in macrophages (14) as well as pancreatic β-cells (9) and liver cells (18). Therefore, to elucidate the underlying mechanisms, we first examined whether ER stress is involved in PA-induced upregulation of LOX-1. Moreover, it has been reported that unsaturated FAs such as oleic acid (OA) and linoleic acid (LA) have protective effects against the lipotoxic effects of the saturated FA, PA (19, 20). Therefore, we examined whether unsaturated FAs could inhibit the induction of LOX-1, and how ER stress is modified by unsaturated FAs. Here we have demonstrated that pharmacological manipulation and siRNA treatment to reduce ER stress response mitigates PA-induced LOX-1 upregulation, and that the unsaturated FAs OA and LA counteract the effects of PA through inhibition of ER stress.
MATERIALS AND METHODS
Materials
Sodium tauroursodeoxycholate (TUDCA), 4-phenylbutyric acid (PBA), and NEFA (PA, OA, and LA) were purchased from Sigma-Aldrich (St. Louis, MO). Stock solutions (5 mM) of NEFA were made by mixing 0.1 M NEFA and 5% BSA. Thapsigargin, salubrinal, and SP600125 were purchased from Calbiochem (San Diego, CA). Antibodies against PERK, eIF2, phospho-eIF2, and CHOP were purchased from Cell Signaling Technology (Beverly, MA). Antibodies against LOX-1, β-actin, and phospho-PERK were obtained from R&D Systems (Minneapolis, MN), Sigma-Aldrich, and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. DiI-oxLDL was purchased from Biomedical Technologies (Stoughton, MA).
Cell culture
The human monocytic leukemia cell line THP-1 and the murine macrophage cell line Raw264.7 were obtained from the American Type Culture Collection (Manassas, VA). THP-1 cells were maintained in RPMI-1640 medium (Invitrogen; Carlsbad, CA) supplemented with 10% FBS and 1% penicillin/streptomycin (P/S) at 37°C under 5% CO2. Differentiation of THP-1 cells was induced by treatment with 100 nM PMA (Sigma) in RPMI-1640 supplemented with 10% FBS and 1% P/S for 48 h. After 48 h of differentiation, the cells were used for the experiments.
Real-time PCR
Total RNA was isolated and treated with DNase using a Qiagen RNeasy mini kit (Qiagen; Valencia, CA) according to the manufacturer's instructions. RNA was reverse transcribed using a high-capacity cDNA reverse transcription kit (Applied Biosystems; Foster City, CA). Quantitative gene expression analysis was performed by real-time PCR on an AB 7500 fast real-time PCR system (Applied Biosystems). PCR was performed using TaqMan gene expression assays of LOX-1 (Hs00234028_m1 and Mm00454586_m1). The 18S rRNA (Hs99999901_m1) was amplified in the same reaction to act as a reference.
IRE-1, PERK, and ATF-6 siRNA
THP-1 cells were transfected after 48 h differentiation with 10 nM Stealth Select RNAi (Invitrogen) directed against IRE-1 (HSS140847, HSS176615), PERK (HSS190343, HSS190344), or ATF-6 (HSS117915, HSS177036) using Lipofectamine RNAiMAX transfection reagent (Invitrogen) for 48 h. Subsequently, cells were treated with 200 μM PA for 8 h, and RNA was harvested. Stealth RNAi Negative Control Duplex (Low GC) was used as a negative control.
Western blotting
Cells were washed with ice-cold PBS then lysed in cold cell lysis buffer containing 25 mM Tris-HCl (pH 8.0), 10 mM EGTA, 10 mM EDTA, 1% NP-40, 100 mM NaF, 10 mM Na4P2O7, 10 mM Na3VO4, 1 mM PMSF, 2 ng/ml aprotinin, and 10 mM β-glycerophosphate. Protein concentration was measured using a BCA protein assay reagent kit (Pierce Chemical; Rockford, IL). The cell lysates were then subjected to SDS-PAGE followed by Western blotting. Antigen-antibody complexes were detected using an appropriate HRP-labeled secondary antibody with the ECL detection system (Amersham Biosciences; Piscataway, NJ) according to the manufacturer's protocol. The resulting bands were analyzed densitometrically using ImageQuant software (Molecular Dynamics; Sunnyvale, CA).
oxLDL uptake
Cells were incubated with 200 μM PA for 24 h in DMEM supplemented with 2% FBS. In addition, 25 μg/ml DiI-oxLDL was then added to the media and incubated for a further 4 h at 37°C. The cells were washed with ice-cold PBS and lysed in 2 mM SDS. Fluorescence in the lysate was determined with excitation and emission wavelengths of 530 and 590 nm, respectively. The results were normalized to total cell protein concentration.
Preparation of peritoneal macrophages
Total peritoneal cells were harvested by washing the peritoneal cavity of adult male C57BL/6J mice with ice cold PBS. Collected peritoneal cells were centrifuged and suspended in RPMI-1640 containing 10% FBS and 1% P/S (complete medium). Cells were plated in 24-well tissue culture plates at a density 1.5 × 106 cells/well, and then cultured at 37°C under 5% CO2 for 2 h to allow macrophages to adhere to culture dishes. To purify macrophages, nonadherent cells were removed by three washings with RPMI-1640. The cells were cultured at 37°C under 5% CO2 for 7 h and used for the studies. All animal procedures used in this study were conducted according to the guidelines of the Animal Ethical Committee of Kyorin Pharmaceutical Co., Ltd.
Statistical analysis
To determine the significance of differences among multiple groups, data were analyzed by one-way ANOVA followed by Dunnett's test. P < 0.05 was considered statistically significant.
RESULTS
Alleviation of ER stress mitigates PA-induced LOX-1 upregulation in macrophages
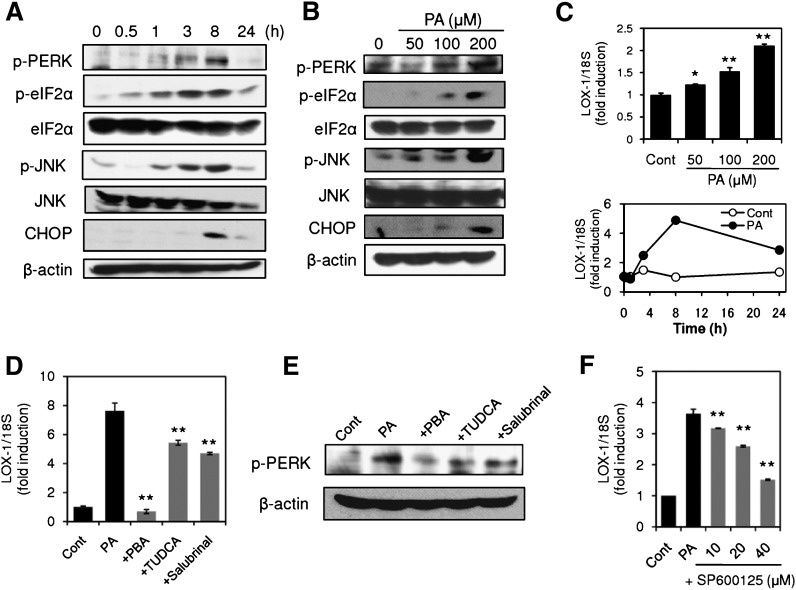
To elucidate the contribution of ER stress to PA-induced LOX-1 upregulation, we first examined whether PA (50–200 μM) induces ER stress in macrophage-like THP-1 cells. Under these experimental conditions, no obvious cytotoxicity was observed when cell viability was determined by the XTT assay; however, significant reduction in cell viability was detected at higher concentrations (500 μM). As shown in Fig. 1A, B, treatment of the cells with PA induced ER stress in a time-dependent and concentration-dependent manner, as determined by phosphorylation of PERK, eIF2α, and JNK as well as induction of CHOP. These ER stress markers were evident as early as 1 h after PA treatment and reached the maximum level at 8 h. On the other hand, upregulation of LOX-1 was induced by PA in a concentration-dependent manner (50–200 μM); 200 μM PA-induced LOX-1 induction was evident as early as 3 h, and the maximum induction was observed 8 h after PA treatment (Fig. 1C). Comparing the time course of ER stress activation with LOX-1 induction, ER stress markers became evident at an earlier time point than LOX-1 induction. Because we confirmed activation of ER stress response preceding LOX-1 induction, we next examined whether alleviation of ER stress could mitigate PA-induced LOX-1 upregulation. As indicated in Fig. 1D, the two chemical chaperones, PBA and TUDCA, small compounds that facilitate proper protein folding and stability, significantly inhibited PA-induced LOX-1 upregulation. In addition, the reported UPR signal inhibitor, salubrinal, also significantly reduced the induction of LOX-1. Consistent with the inhibitory effects on LOX-1 upregulation, treatment of the cells with PBA, TUDCA, or salubrinal reduced levels of PERK phosphorylation in the PA-treated cells (Fig. 1E). These results indicated that alleviation of ER stress could mitigate PA-induced LOX-1 upregulation. In addition, we investigated the effect of the JNK inhibitor SP600125 on PA-induced LOX-1 induction, because the AP-1 site, which is activated by JNK, is found within the LOX-1 promoter region. As shown in Fig. 1F, SP600125 prevented PA-induced LOX-1 in a concentration-dependent manner.
Fig. 1.
Alleviation of endoplasmic reticulum (ER) stress mitigates palmitic acid (PA)-induced oxidized LDL receptor-1 (LOX-1) upregulation in THP-1 cells. A: Cells were treated with 200 μM PA, and ER stress markers were analyzed by Western blot. eIF2α, eukaryotic translation initiation factor 2α; JNK, c-JUN N-terminal kinase; CHOP, C/EBP homologous protein. B: Western blot analysis of ER stress markers in cells stimulated with PA for 6 h at the indicated concentrations. C: (upper) LOX-1 gene expression in cells stimulated with PA for 24 h at the indicated concentrations. LOX-1 expression was quantified by real-time PCR and normalized relative to 18S rRNA. Data are expressed as means ± SE of three independent experiments. * P < 0.05, ** P < 0.01 versus Cont (lower). Time course of changes in PA (200 μM)-induced LOX-1 upregulation. Data are averaged values from two experiments. D, E: Effects of 4-phenylbutyric acid (PBA), sodium tauroursodeoxycholate (TUDCA), and salubrinal on PA-induced LOX-1 upregulation. THP-1 cells were stimulated with 200 μM PA in the presence or absence of 4-phenylbutyric acid (PBA) (20 mM), TUDCA (2 mM), and salubrinal (40 μM). LOX-1 expression (D) and phosphorylation of protein kinase-like ER kinase (PERK) (E) were analyzed by real-time PCR and Western blot, respectively. Data in D are expressed as means ± SE of five independent experiments. ** P < 0.01 versus PA. F: Concentration-dependent inhibition of PA-induced LOX-1 induction by SP600125. Data are expressed as means ± SE of three independent experiments. ** P < 0.01 versus PA.
PA-induced LOX-1 upregulation is inhibited by salubrinal and SP600125 in primary mouse macrophages
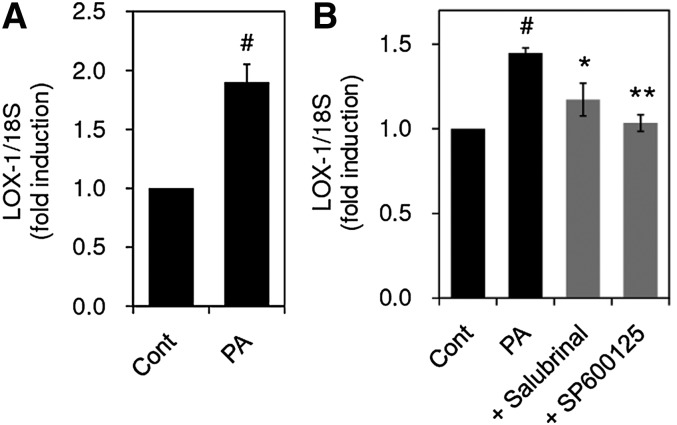
To confirm whether PA upregulates LOX-1 in primary mouse macrophages obtained from the peritoneal cavity, as in the case of THP-1 cells, macrophages were treated with 200 μM PA, and the expression level of LOX-1 was determined. As shown in Fig. 2A, 200 μM PA enhanced LOX-1 expression in primary macrophages. We then examined the effects of salubrinal and SP600125 on PA-induced LOX-1 upregulation. Concomitant treatment of the cells with either salubrinal or SP600125 significantly abrogated PA-induced LOX-1 induction (Fig. 2B).
Fig. 2.
Salubrinal and SP600125 inhibit PA-induced LOX-1 upregulation in primary mouse macrophages. A: Primary macrophages obtained from mouse peritoneal cavity were stimulated with 200 μM PA for 6 h, and LOX-1 expression was quantified by real-time PCR and normalized relative to 18S rRNA. B: Primary macrophage cells were stimulated with 200 μM PA in the presence or absence of salubrinal (40 μM) or SP600125 (40 μM). After 6 h incubation, cells were harvested and LOX-1 expression was quantified. Data are expressed as means ± SE (n = 3). * P < 0.05, ** P < 0.01 versus PA, # P < 0.05 versus Cont.
IRE-1 and PERK are involved in PA-induced LOX-1 upregulation
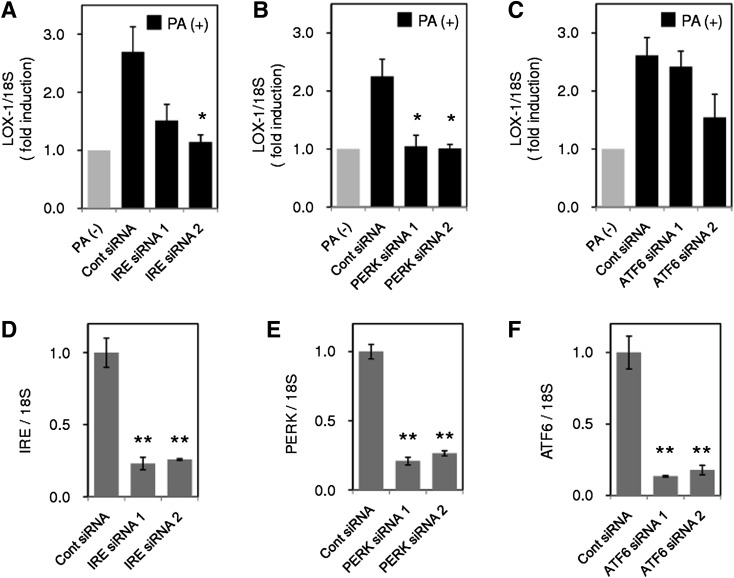
Because studies using inhibitors for ER stress suggested the contribution of ER stress to LOX-1 upregulation, we next investigated the effects of gene silencing of three major UPR pathways (IRE-1, PERK, and ATF6) on PA-induced LOX-1 upregulation. For this purpose, we used two different siRNA constructs to knock down each gene expression of IRE-1, PERK, and ATF-6. As shown in Fig. 3A, IRE-1 gene silencing by IRE siRNA 1 and IRE siRNA 2 reduced PA-induced LOX-1 by 69.8% and 91.6% (P < 0.05), respectively. When PERK gene expression was suppressed by either siRNA, almost complete inhibition of LOX-1 induction was observed (96.3% and 99.4%, P < 0.05; Fig. 3B). Activation of the IRE-1 and PERK pathways by PA stimulation was supported by phosphorylation of JNK and PERK, respectively (Fig. 1A). In contrast to IRE-1 and PERK, siRNAs directed against ATF-6 failed to lower PA-induced LOX-1 significantly (Fig. 3C). All siRNAs used in this study showed efficient inhibition of each target gene expression (Fig. 3D–F). These results suggest that the IRE-1 and PERK pathways mainly contribute to PA-induced LOX-1 upregulation.
Fig. 3.
PA-induced LOX-1 is suppressed by siRNA directed against inositol-requiring kinase/endonuclease-1 (IRE-1) and PERK. THP-1 cells were transfected with siRNA for 48 h directed against IRE-1 (A), PERK (B), and activating transcription factor-6 (ATF-6) (C), and LOX-1 expression was analyzed by real-time PCR after PA stimulation (200 μM, 8 h), and normalized relative to 18S rRNA. Expression of the target gene of each siRNA, IRE-1 (D), PERK (E), and ATF-6 (F), was also quantified. Data are expressed as means ± SE of three independent experiments. ** P < 0.01, * P < 0.05 versus Cont siRNA.
ER stress caused by thapsigargin treatment upregulates LOX-1 expression
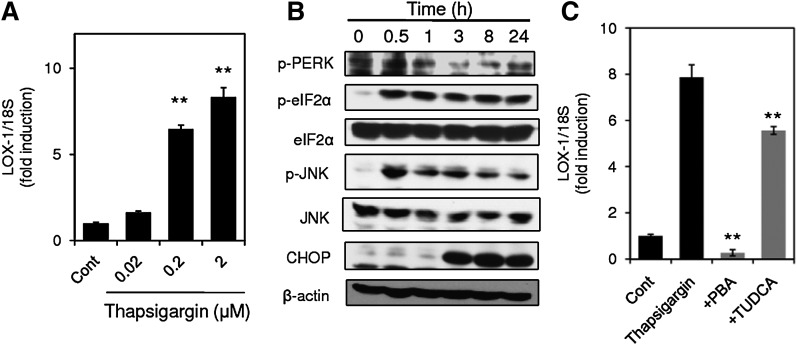
Because studies using inhibitors and siRNAs for ER stress response suggested the contribution of ER stress response to LOX-1 upregulation, we investigated whether a well-known ER stress inducer, thapsigargin, upregulates LOX-1 expression. Treatment of the cells with thapsigargin upregulated LOX-1 expression in a concentration-dependent manner 24 h after treatment (Fig. 4A), accompanying phosphorylation of PERK, eIF2α, and JNK, as well as induction of CHOP (Fig. 4B). These observations suggested that ER stress caused by thapsigargin treatment may bring about upregulation of LOX-1 expression. To confirm this possibility, the effects of inhibition of ER stress on thapsigargin-induced LOX-1 upregulation were examined. As shown in Fig. 4C, both PBA and TUDCA significantly suppressed thapsigargin-induced LOX-1 upregulation.
Fig. 4.
ER stress caused by thapsigargin upregulates LOX-1 expression. A: LOX-1 expression in THP-1 cells stimulated with thapsigargin for 24 h at the indicated concentrations was analyzed by real-time PCR. Data are expressed as means ± SE of three independent experiments. ** P < 0.01 versus Cont. B: THP-1 cells were incubated with 2 μM thapsigargin for the indicated periods, and cell lysates were subjected to Western blot analysis for phospho-PERK and β-actin. C: Effects of PBA and TUDCA on thapsigargin-induced LOX-1 expression. THP-1 cells were stimulated with 2 μM thapsigargin in the presence or absence of PBA and TUDCA for 24 h. LOX-1 expression was quantified by real-time PCR and normalized relative to 18S rRNA. Data are expressed as means ± SE of three independent experiments. ** P < 0.01 versus thapsigargin.
OA and LA inhibit PA-induced LOX-1 upregulation and enhanced uptake of oxLDL
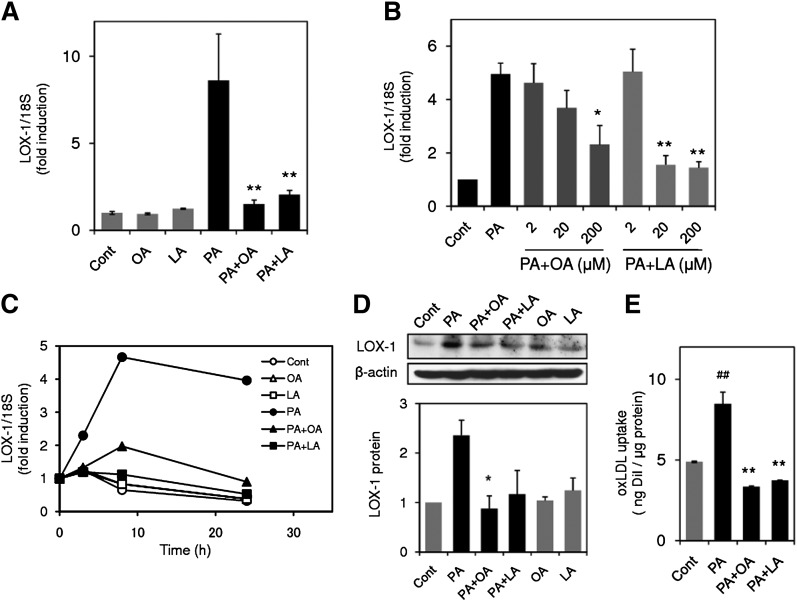
Unsaturated FAs such as OA and LA are known to show a protective effect on lipotoxic effects induced by PA (19, 20); however, the effects of these unsaturated FAs on PA-induced LOX-1 upregulation have not yet been explored. As shown in Fig. 5A, upregulation of LOX-1 expression was significantly prevented by concomitant application of either 200 μM OA or 200 μM LA to the PA (200 μM)-treated cells. The inhibitory effects of OA and LA were concentration dependent (2–200 μM; Fig. 5B) and could be observed throughout the experimental period (4 h–24 h, Fig. 5C). Prevention of LOX-1 induction by these unsaturated FAs was also confirmed by Western blot at the protein level (Fig. 5D). In accordance with upregulation of LOX-1 gene expression and protein level, PA promoted uptake of oxLDL, and both LA and OA prevented this enhancement of oxLDL induced by PA treatment (Fig. 5E).
Fig. 5.
Oleic acid (OA) and linoleic acid (LA) inhibit PA-induced LOX-1 upregulation. A: LOX-1 gene expression in THP-1 cells stimulated with 200 μM PA in the presence or absence of OA (200 μM) and LA (200 μM) for 24 h. LOX-1 expression was quantified by real-time PCR and normalized relative to 18S rRNA. Data are expressed as means ± SE from four independent experiments. ** P < 0.01 versus PA. B: Concentration-dependent inhibition of PA-induced LOX-1 upregulation by OA and LA. LOX-1 expression in THP-1 cells stimulated with 200 μM PA in the presence or absence of OA and LA at the indicated concentration for 24 h. Data are expressed as means ± SE of three independent experiments. ** P < 0.01, * P < 0.05 versus PA. C: Time course of changes in LOX-1 expression in THP-1 cells stimulated with 200 μM PA with or without OA (200 μM) and LA (200 μM). Data are average values from two independent experiments. D: (top) Representative Western blot for LOX-1 from three independent experiments, which showed the same trends as in cells treated with 200 μM PA for 24 h. (bottom) Bar graphs show the results of densitometric analysis of Western blots for LOX-1 protein. Data are expressed as means ± SE from three independent experiments. E: PA promotes uptake of oxidized LDL (oxLDL), and OA or LA prevents enhanced uptake of oxLDL. Cells were incubated with 200 μM PA in the presence or absence of 200 μM OA or LA for 24 h, followed by incubation with DiI-labeled oxLDL for 4 h. After washing and lysis, fluorescence of DiI in the lysate was measured at 530/590 nm. Data are expressed as means ± SE of three independent experiments. ## P < 0.01 versus control group (Cont), ** P < 0.01 versus PA.
OA and LA inhibit PA-induced activation of ER stress markers
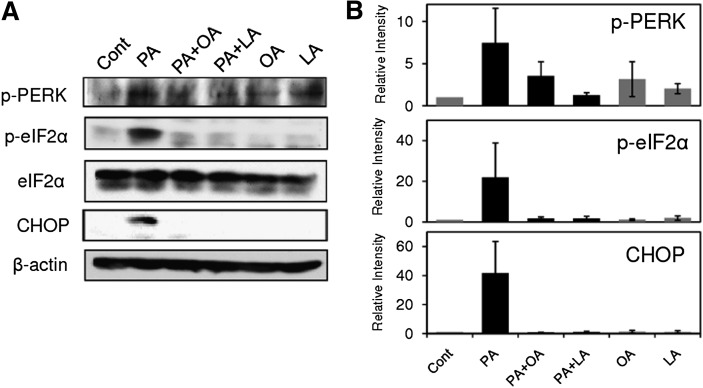
Our results indicating the involvement of ER stress in PA-induced LOX-1 upregulation prompted us to investigate whether OA and LA could inhibit activation of ER stress markers caused by PA treatment. As shown in Fig. 6A, treatment with 200 μM PA induced phosphorylation of PERK and eIF2α at 8 h, and these increments in phosphorylation levels were markedly inhibited by concomitant treatment with either OA (200 μM) or LA (200 μM). In addition, OA and LA almost completely prevented PA-induced CHOP induction (Fig. 6A). Densitometric analysis showed that there were obvious trends for OA and LA to reduce ER stress markers, although the data did not reach statistical significance (Fig. 6B).
Fig. 6.
OA and LA inhibit PA-induced activation of ER stress markers. A: THP-1 cells were incubated with 200 μM PA in the presence or absence of OA (200 μM) and LA (200 μM) for 8 h, and cell lysates were subjected to Western blot analysis for ER stress markers. Data show representative Western blots from three independent experiments that showed the same trends. B: Bar graphs show densitometric analysis of Western blots. Data are expressed as means ± SE from three independent experiments.
OA and LA prevent thapsigargin-induced ER stress and LOX-1 upregulation
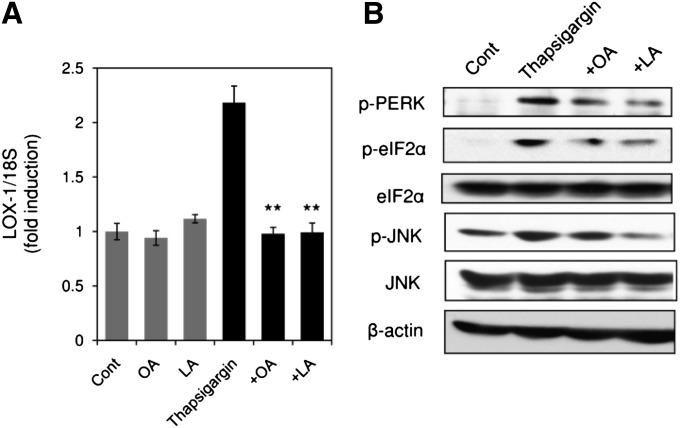
To define whether OA and LA can inhibit LOX-1 upregulation by alleviation of ER stress, the effects of OA and LA on ER stress-induced upregulation of LOX-1 were examined. As shown in Fig. 7A, OA (200 μM) and LA (200 μM) completely inhibited upregulation of LOX-1 expression induced by 2 μM thapsigargin. To confirm inhibition of ER stress response by OA and LA, the effects of OA and LA on activation of ER stress markers induced by thapsigargin treatment were examined. Thapsigargin (2 μM) induced phosphorylation of PERK, eIF2α, and JNK 24 h after the treatment in THP-1 cells, and the activation of these ER stress markers was alleviated by concomitant application of OA (200 μM) or LA (200 μM) to the thapsigargin-treated cells (Fig. 7B). Our data showed that OA and LA prevented upregulation of LOX-1 and ER stress response induced by thapsigargin, another ER stress inducer, indicating that OA and LA exert inhibitory effects on PA-induced LOX-1 upregulation through the mechanisms associated with alleviation of ER stress.
Fig. 7.
OA and LA alleviate ER stress response caused by thapsigargin. A: Effects of OA (200 μM) and LA (200 μM) on 2 μM thapsigargin-induced LOX-1 upregulation. LOX-1 expression 24 h after thapsigargin treatment was quantified by real-time PCR and normalized relative to 18S rRNA. Data are expressed as means ± SE of four independent experiments. * P < 0.05 versus thapsigargin. B: ER stress markers in THP-1 cells stimulated with thapsigargin in the presence or absence of OA and LA for 3 h were analyzed by Western blot.
DISCUSSION
It has been suggested that elevated levels of NEFA are associated with increased risk of cardiovascular disease (1). However, the precise mechanism by which NEFA promotes cardiovascular disease has not been clarified. Previously, we found that PA enhanced LOX-1 expression and promoted uptake of oxLDL in macrophages (4). To elucidate the underlying mechanism of these effects, we examined the contribution of the ER stress response to PA-induced LOX-1 upregulation in the present study. Our results demonstrated that the ER stress response contributes to the upregulation of LOX-1. This was supported by a number of observations. First, treatment of macrophage cells with PA resulted in activation of ER stress markers and phosphorylation of PERK, eIF2a, and JNK. Second, activation of these ER stress markers preceded LOX-1 upregulation. Third, the concentration of PA (100 and 200 μM) that caused activation of ER stress markers also induced upregulation of LOX-1. Fourth, pharmacological manipulation to reduce the ER stress response, such as treatment with PBA, TUDCA, and salubrinal, alleviated PA-induced LOX-1 upregulation. Fifth, siRNA directed against IRE-1 and PERK, important molecules in ER stress response pathways, alleviated PA-induced LOX-1. Sixth, a well-known ER stress inducer, thapsigargin, also increased the level of LOX-1 expression. These results suggest that PA causes upregulation of LOX-1 via activation of the ER stress response. To our knowledge, this is the first study to show a relationship between ER stress response and regulation of LOX-1 expression. However, ER stress may not be the only mechanism underlying PA-induced LOX-1 upregulation, because PA could induce LOX-1 at 50 μM, whereas ER stress markers appeared only at 100 μM. The concentration of PA used in the current study can be considered within the pathophysiological range because it is reported that postprandial plasma concentration of NEFA reaches about 1 mM (21) in type 2 diabetes, and PA is the most abundant NEFA (∼28%) in plasma (22). In addition to regulation of LOX-1, there have been several reports that ER stress response enhances the levels of expression of the scavenger receptors CD36 (17) and SR-A (12) in macrophages. These studies show that tunicamycin and cholesterol loading enhance expression of CD-36 and SR-A via ER stress, respectively. In our previous study, PA upregulated only LOX-1, but not other scavenger receptors such as CD36, SR-AI, SR-BI, and CD68 (4). The difference in scavenger receptor induced by ER stress among the studies may be explained by the difference in the ER stress inducer used in these studies. These observations, together with our findings, suggest that uptake of oxLDL by macrophages, leading to foam cell formation, may be promoted by the ER stress response through upregulation of scavenger receptors.
Recent studies have shown that elevation of ER stress markers in macrophages infiltrating atherosclerotic lesions can be detected in both humans and mice (15, 23). It was also demonstrated that markers of the ER stress response are notably elevated in macrophages from both early and advanced atherosclerotic lesions in a murine model of atherosclerosis (23). There is a striking correlation between ER stress markers and plaque vulnerability (15). More recently, treatment with the chemical chaperone PBA to alleviate ER stress was shown to result in a dose-dependent reduction in macrophage ER stress and apoptosis in atherosclerotic lesions in apolipoprotein E knockout mice (14). Consistent with these results, our findings of ER stress-mediated LOX-1 upregulation in macrophages suggested that the ER stress response may contribute to the progression of atherosclerotic lesions by enhancing foam cell formation. In addition to its role in oxLDL uptake, LOX-1 has been demonstrated to modulate apoptosis in cardiomyocytes (24). LOX-1 mediates oxLDL-induced apoptosis in endothelial cells (25). In our study, neither increase in caspase-3 activity nor cell toxicity was observed upon stimulation with 200 μM PA (data not shown), whereas upregulation of LOX-1 occurred. A higher concentration (500 μM) of PA caused significant reduction of cell viability (data not shown). These observations indicate that PA induces upregulation of LOX-1 at a lower concentration than apoptosis induction. Hence, it is possible that accumulated oxLDL in atherosclerotic lesions could facilitate macrophage apoptosis via upregulated LOX-1. This hypothesis is supported by the observation that LOX-1 expression in atherosclerotic plaques is positively correlated with plaque instability in an animal atherosclerosis model (26). Accordingly, the macrophage ER stress response-induced upregulation of LOX-1 may also be related to plaque vulnerability by facilitating macrophage apoptosis in addition to its promotional effect on foam cell formation.
Among the major ER stress response pathways, PERK-eIF2α-ATF-4, ATF-6, IRE-1-XBP-1, and IRE-1-JNK, only the AP-1 site, which is activated by JNK, can be found within the LOX-1 promoter region. In addition, the LOX-1 promoter does not include the CHOP-C/EBP motif. In our study, PA-induced LOX-1 induction was concentration-dependently inhibited by SP600125 (Fig. 1F), suggesting an important role of JNK in PA-induced LOX-1 upregulation. It is reported that activation of JNK occurs downstream of the PERK pathway (27, 28), as well as the IRE-1 pathway. Consistent with these observations, knockdown of PERK and IRE-1 using siRNA significantly reduced PA-induced LOX-1 induction (Fig. 3A, B). Therefore, PERK- and IRE-1-dependent activation of JNK may play an important role in PA-induced LOX-1. Involvement of AP-1 site activation is indicated in high-glucose-induced LOX-1 (29), in accordance with our results. In addition to the involvement of the JNK-AP-1 pathway, we previously demonstrated that inhibition of p38 decreased the extent of PA-induced LOX-1 induction (4). In liver cells, mitochondrial dysfunction activates the ER stress response accompanying p38 activation, and alleviation of ER stress using a chemical chaperone reduces p38 activation (30). It has been shown that activation of p38 is necessary for CHOP induction in cholesterol-loaded macrophages (31). These observations suggest a link between the ER stress response and activation of mitogen-activated protein (MAP) kinases. In our study, the level of PERK phosphorylation was partially decreased by addition of the inhibitor of p38, SB203580, to PA-treated cells, whereas treatment with PBA or TUDCA failed to mitigate activation of p38 (data not shown). Taken together, it is plausible that signaling pathways involving ER stress and activation of MAP kinases play an important role in PA-induced LOX-1 upregulation.
Another important finding in the present study was that PA-induced upregulation of LOX-1 could be inhibited by the unsaturated acids OA and LA. Several previous studies have indicated protective effects of OA and LA on PA-induced lipotoxicity. Kadotani et al. (19) showed that OA and LA antagonize PA-induced COX-2 expression with suppression of PA-evoked intracellular signals, including p38, JNK, ERK1/2, and PKCθ in C2C12 myotubes. Staiger et al. (20) demonstrated that OA and LA inhibit PA-induced apoptosis in human coronary artery endothelial cells. In accordance with these studies, we also demonstrated antagonistic effects of OA and LA against PA-induced LOX-1 upregulation. With regard to the underlying mechanisms, several observations indicated the involvement of suppression of ER stress. PA caused LOX-1 upregulation with activation of ER stress markers, and alleviation of ER stress by use of chemical chaperones mitigated the LOX-1 upregulation. The LOX-1 upregulation was inhibited by OA and LA with concomitant reduction of ER stress markers. OA and LA also inhibited thapsigargin-induced LOX-1 upregulation as well as activation of ER stress markers. Taken together, these observations suggested that OA and LA prevent LOX-1 upregulation through alleviation of ER stress. In line with our data, OA has been shown to diminish PA-induced ER stress in H4IIE and primary liver cells (18) and macrophages (14). In preadipocytes, OA and LA counteract PA-induced apoptosis by reducing ER stress (32). However, it is unclear how those unsaturated FAs exert protective effects against ER stress. ER stress can be alleviated by activation of peroxisome proliferator-activated receptor α (PPARα) and -γ (33, 34). OA and LA are known to be ligands for both PPARα and -γ, although they show stronger binding to PPARα (35). Therefore, reduction of ER stress conferred by OA and LA might be mediated by PPARα or -γ activation. Further studies are required to elucidate the contributions of PPARα- and -γ-associated mechanisms to the effects of OA and LA.
Saturated FAs, including PA, have been suggested to have deleterious effects on pancreatic β cells (9), endothelial cells (2, 20), preadipocytes (32), and macrophages (3, 4). These adverse effects have been linked to the pathogenesis of metabolic diseases such as type 2 diabetes and atherosclerosis. In contrast, unsaturated FAs such as LA and OA have previously been shown to exert protective effects against the toxic effects of saturated FAs (19, 20). In the present study, we showed that OA and LA inhibited PA-induced LOX-1 upregulation by alleviation of ER stress. There are also several lines of evidence suggesting beneficial effects of unsaturated FA in clinical settings. Laaksonen et al. (36) reported that dietary LA intake may have substantial cardioprotective benefit. The inverse association of dietary LA intake with incident coronary heart disease was indicated in the Nurses' Health Study (37). These observations suggest that changes in the levels of unsaturated FAs may affect the risk of cardiovascular disease. The ratio of PA to LA can change substantially with diet and may be related to insulin sensitivity (38). The elevated ratio of saturated FA to unsaturated FA associated with increased stearoyl CoA desaturase activity was observed in the lipid composition of macrophages from FA binding protein-4 (aP2) knockout mice compared with those from wild-type controls (14). Taken together, these observations suggest that not only dietary intake but also altered regulation of intracellular lipid metabolism may affect the ratio of saturated to unsaturated FA in lipid composition, which, in turn, could affect the risk of metabolic diseases such as atherosclerosis and type 2 diabetes.
In conclusion, our results indicated that ER stress plays a critical role in PA-induced LOX-1 upregulation in macrophage cells. Because elevation of ER stress markers is observed in early and advanced atherosclerotic lesions (23) and there are correlations between ER stress markers and plaque vulnerability (15), the upregulation of macrophage LOX-1 caused by ER stress may contribute to the progression of atherosclerosis by enhancement of foam cell formation and plaque vulnerability. Our results also indicated that the unsaturated FAs OA and LA could prevent PA-induced LOX-1 induction through suppression of ER stress. These results suggest that changes in the ratio of saturated FA to unsaturated FA in lipid composition brought about by dietary intake or altered metabolic processes could modulate the course of atherosclerosis. Our findings may offer a new mechanism to explain in part the relationship between elevated NEFA and increased risk of cardiovascular disease.
Acknowledgments
The authors thank Akiko Umei for technical support.
Footnotes
Abbreviations:
- ATF-6
- activating transcription factor-6
- CHOP
- C/EBP homologous protein
- eIF2α
- eukaryotic translation initiation factor 2α
- ER
- endoplasmic reticulum
- IRE-1
- inositol-requiring kinase/endonuclease-1
- JNK
- c-JUN N-terminal kinase
- LA
- linoleic acid
- LOX-1
- oxidized LDL receptor-1
- NEFA
- nonesterified FA
- OA
- oleic acid
- oxLDL
- oxidized LDL
- PA
- palmitic acid
- PBA
- 4-phenylbutyric acid
- PERK
- protein kinase-like ER kinase
- P/S
- penicillin/streptomycin
- TUDCA
- sodium tauroursodeoxycholate
- UPR
- unfolded protein response
REFERENCES
- 1.Pilz S., Scharnagl H., Tiran B., Seelhorst U., Wellnitz B., Boehm B. O., Schaefer J. R., Marz W. 2006. Free fatty acids are independently associated with all-cause and cardiovascular mortality in subjects with coronary artery disease. J. Clin. Endocrinol. Metab. 91: 2542–2547. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg H. O., Tarshoby M., Monestel R., Hook G., Cronin J., Johnson A., Bayazeed B., Baron A. D. 1997. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J. Clin. Invest. 100: 1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi H., Kokoeva M. V., Inouye K., Tzameli I., Yin H., Flier J. S. 2006. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116: 3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishiyama J., Taguchi R., Yamamoto A., Murakami K. 2010. Palmitic acid enhances lectin-like oxidized LDL receptor (LOX-1) expression and promotes uptake of oxidized LDL in macrophage cells. Atherosclerosis. 209: 118–124. [DOI] [PubMed] [Google Scholar]
- 5.Ron D., Walter P. 2007. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8: 519–529. [DOI] [PubMed] [Google Scholar]
- 6.Harding H. P., Calfon M., Urano F., Novoa I., Ron D. 2002. Transcriptional and translational control in the mammalian unfolded protein response. Annu. Rev. Cell Dev. Biol. 18: 575–599. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman R. J., Scheuner D., Schroder M., Shen X., Lee K., Liu C. Y., Arnold S. M. 2002. The unfolded protein response in nutrient sensing and differentiation. Nat. Rev. Mol. Cell Biol. 3: 411–421. [DOI] [PubMed] [Google Scholar]
- 8.Minamino T., Kitakaze M. 2010. ER stress in cardiovascular disease. J. Mol. Cell. Cardiol. 48: 1105–1110. [DOI] [PubMed] [Google Scholar]
- 9.Karaskov E., Scott C., Zhang L., Teodoro T., Ravazzola M., Volchuk A. 2006. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology. 147: 3398–3407. [DOI] [PubMed] [Google Scholar]
- 10.Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Gorgun C., Glimcher L. H., Hotamisligil G. S. 2004. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 306: 457–461. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J., Werstuck G. H., Lhotak S., de Koning A. B., Sood S. K., Hossain G. S., Moller J., Ritskes-Hoitinga M., Falk E., Dayal S., et al. 2004. Association of multiple cellular stress pathways with accelerated atherosclerosis in hyperhomocysteinemic apolipoprotein E-deficient mice. Circulation. 110: 207–213. [DOI] [PubMed] [Google Scholar]
- 12.Han S., Liang C. P., DeVries-Seimon T., Ranalletta M., Welch C. L., Collins-Fletcher K., Accili D., Tabas I., Tall A. R. 2006. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 3: 257–266. [DOI] [PubMed] [Google Scholar]
- 13.Gargalovic P. S., Gharavi N. M., Clark M. J., Pagnon J., Yang W. P., He A., Truong A., Baruch-Oren T., Berliner J. A., Kirchgessner T. G., et al. 2006. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 26: 2490–2496. [DOI] [PubMed] [Google Scholar]
- 14.Erbay E., Babaev V. R., Mayers J. R., Makowski L., Charles K. N., Snitow M. E., Fazio S., Wiest M. M., Watkins S. M., Linton M. F., et al. 2009. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15: 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myoishi M., Hao H., Minamino T., Watanabe K., Nishihira K., Hatakeyama K., Asada Y., Okada K., Ishibashi-Ueda H., Gabbiani G., et al. 2007. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 116: 1226–1233. [DOI] [PubMed] [Google Scholar]
- 16.Thorp E., Li G., Seimon T. A., Kuriakose G., Ron D., Tabas I. 2009. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 9: 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hua Y., Kandadi M. R., Zhu M., Ren J., Sreejayan N. 2010. Tauroursodeoxycholic acid attenuates lipid accumulation in endoplasmic reticulum-stressed macrophages. J. Cardiovasc. Pharmacol. 55: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei Y., Wang D., Gentile C. L., Pagliassotti M. J. 2009. Reduced endoplasmic reticulum luminal calcium links saturated fatty acid-mediated endoplasmic reticulum stress and cell death in liver cells. Mol. Cell. Biochem. 331: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadotani A., Tsuchiya Y., Hatakeyama H., Katagiri H., Kanzaki M. 2009. Different impacts of saturated and unsaturated free fatty acids on COX-2 expression in C(2)C(12) myotubes. Am. J. Physiol. Endocrinol. Metab. 297: E1291–E1303. [DOI] [PubMed] [Google Scholar]
- 20.Staiger K., Staiger H., Weigert C., Haas C., Haring H. U., Kellerer M. 2006. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-kappaB activation. Diabetes. 55: 3121–3126. [DOI] [PubMed] [Google Scholar]
- 21.van Wijk J. P., de Koning E. J., Castro Cabezas M., Rabelink T. J. 2005. Rosiglitazone improves postprandial triglyceride and free fatty acid metabolism in type 2 diabetes. Diabetes Care. 28: 844–849. [DOI] [PubMed] [Google Scholar]
- 22.Staiger H., Staiger K., Stefan N., Wahl H. G., Machicao F., Kellerer M., Haring H. U. 2004. Palmitate-induced interleukin-6 expression in human coronary artery endothelial cells. Diabetes. 53: 3209–3216. [DOI] [PubMed] [Google Scholar]
- 23.Zhou J., Lhotak S., Hilditch B. A., Austin R. C. 2005. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 111: 1814–1821. [DOI] [PubMed] [Google Scholar]
- 24.Spallarossa P., Fabbi P., Manca V., Garibaldi S., Ghigliotti G., Barisione C., Altieri P., Patrone F., Brunelli C., Barsotti A. 2005. Doxorubicin-induced expression of LOX-1 in H9c2 cardiac muscle cells and its role in apoptosis. Biochem. Biophys. Res. Commun. 335: 188–196. [DOI] [PubMed] [Google Scholar]
- 25.Chen X. P., Xun K. L., Wu Q., Zhang T. T., Shi J. S., Du G. H. 2007. Oxidized low density lipoprotein receptor-1 mediates oxidized low density lipoprotein-induced apoptosis in human umbilical vein endothelial cells: role of reactive oxygen species. Vascul. Pharmacol. 47: 1–9. [DOI] [PubMed] [Google Scholar]
- 26.Ishino S., Mukai T., Kume N., Asano D., Ogawa M., Kuge Y., Minami M., Kita T., Shiomi M., Saji H. 2007. Lectin-like oxidized LDL receptor-1 (LOX-1) expression is associated with atherosclerotic plaque instability–analysis in hypercholesterolemic rabbits. Atherosclerosis. 195: 48–56. [DOI] [PubMed] [Google Scholar]
- 27.Liang S. H., Zhang W., McGrath B. C., Zhang P., Cavener D. R. 2006. PERK (eIF2alpha kinase) is required to activate the stress-activated MAPKs and induce the expression of immediate-early genes upon disruption of ER calcium homoeostasis. Biochem. J. 393: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yacoub A., Liu R., Park M. A., Hamed H. A., Dash R., Schramm D. N., Sarkar D., Dimitriev I. P., Bell J. K., Grant S., et al. 2010. Cisplatin enhances protein kinase R-like endoplasmic reticulum kinase- and CD95-dependent melanoma differentiation-associated gene-7/interleukin-24-induced killing in ovarian carcinoma cells. Mol. Pharmacol. 77: 298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L., Sawamura T., Renier G. 2004. Glucose enhances human macrophage LOX-1 expression: role for LOX-1 in glucose-induced macrophage foam cell formation. Circ. Res. 94: 892–901. [DOI] [PubMed] [Google Scholar]
- 30.Lim J. H., Lee H. J., Ho Jung M., Song J. 2009. Coupling mitochondrial dysfunction to endoplasmic reticulum stress response: a molecular mechanism leading to hepatic insulin resistance. Cell. Signal. 21: 169–177. [DOI] [PubMed] [Google Scholar]
- 31.Devries-Seimon T., Li Y., Yao P. M., Stone E., Wang Y., Davis R. J., Flavell R., Tabas I. 2005. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J. Cell Biol. 171: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo W., Wong S., Xie W., Lei T., Luo Z. 2007. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3–L1 and rat primary preadipocytes. Am. J. Physiol. Endocrinol. Metab. 293: E576–E586. [DOI] [PubMed] [Google Scholar]
- 33.Evans-Molina C., Robbins R. D., Kono T., Tersey S. A., Vestermark G. L., Nunemaker C. S., Garmey J. C., Deering T. G., Keller S. R., Maier B., et al. 2009. Peroxisome proliferator-activated receptor gamma activation restores islet function in diabetic mice through reduction of endoplasmic reticulum stress and maintenance of euchromatin structure. Mol. Cell. Biol. 29: 2053–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman S. M., Qadri I., Janssen R. C., Friedman J. E. 2009. Fenofibrate and PBA prevent fatty acid-induced loss of adiponectin receptor and pAMPK in human hepatoma cells and in hepatitis C virus-induced steatosis. J. Lipid Res. 50: 2193–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murakami K., Ide T., Nakazawa T., Okazaki T., Mochizuki T., Kadowaki T. 2001. Fatty-acyl-CoA thioesters inhibit recruitment of steroid receptor co-activator 1 to alpha and gamma isoforms of peroxisome-proliferator-activated receptors by competing with agonists. Biochem. J. 353: 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laaksonen D. E., Nyyssonen K., Niskanen L., Rissanen T. H., Salonen J. T. 2005. Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch. Intern. Med. 165: 193–199. [DOI] [PubMed] [Google Scholar]
- 37.Hu F. B., Stampfer M. J., Manson J. E., Rimm E., Colditz G. A., Rosner B. A., Hennekens C. H., Willett W. C. 1997. Dietary fat intake and the risk of coronary heart disease in women. N. Engl. J. Med. 337: 1491–1499. [DOI] [PubMed] [Google Scholar]
- 38.Summers L. K., Fielding B. A., Bradshaw H. A., Ilic V., Beysen C., Clark M. L., Moore N. R., Frayn K. N. 2002. Substituting dietary saturated fat with polyunsaturated fat changes abdominal fat distribution and improves insulin sensitivity. Diabetologia. 45: 369–377. [DOI] [PubMed] [Google Scholar]