Abstract
In vivo single-voxel magnetic resonance spectroscopy (MRS) at 4.7T and ex vivo high-resolution proton magnetic resonance spectroscopy (HR-NMR) at 500 MHz were used to study the composition of adipose tissues in Zucker obese and Zucker lean rats. Lipid composition was characterized by unsaturation and polyunsaturation indexes and mean chain lengths. In vitro experiments were conducted in known mixtures of triglycerides and oils in order to validate the method. To avoid inaccuracies due to partial peak overlapping in MRS, peak quantification was performed after fitting of spectral peaks by using the QUEST algorithm. The intensity of different spectral lines was also corrected for T2 relaxation. Albeit with different sensitivity and accuracy, both techniques revealed that white adipose tissue is characterized by lower unsaturation and polyunsaturation indexes in obese rats compared with controls. HR-NMR revealed similar differences in brown adipose tissue. The present findings confirm the hypothesis that obese and lean Zucker rats have different adipose tissue composition.
Keywords: oil, fat, obese Zucker, unsaturation
Recent biochemical studies have emphasized the roles of saturated and unsaturated fatty acids in obesity and diabetes (1–7). For example, relatively high levels of saturated fatty acids and low levels of PUFAs are found in individuals with insulin resistance and metabolic syndrome (8).
Recent studies have suggested a close relationship between a number of widespread disorders and fat properties (1–7), highlighting the need for efficient means of characterizing lipid composition in vivo (1–7). Parameters such as mean unsaturation, mean polyunsaturation, and mean chain length can provide useful information about diet, fat distribution, and metabolism (9–14). The above-mentioned parameters of lipid deposits can be obtained in vitro using high resolution proton magnetic resonance spectroscopy (HR-NMR) (15–20). The disadvantages associated with in vitro techniques are well known: invasiveness and time-consuming extraction protocols (21). The main advantage is high resolution, which allows discrimination between olefinic proton peaks and glycerol methilenic peaks.
To the best of our knowledge, ours was one of the first groups to suggest that in vivo magnetic resonance spectroscopy (MRS) can provide information about fat composition in living animals (22). In that paper, chemical shift imaging was used to obtain parametric maps of PUFA distribution in adipose tissue of living rats. Recently, other groups have proposed in vivo single-voxel localized spectroscopy for the characterization of lipid tissue in animals (23) and humans (24) at 7 T. Single-voxel techniques take advantage of the possibility to select a relatively small volume of interest (of the order of 1–2 mm3 in animals) that allows to obtain good voxel-based shimming and consequently, well-resolved resonances (23).
However, with optimized single-voxel techniques in vivo spectra are also affected by relatively high line-width with partially overlapping peaks. Reliable extraction of lipid parameters from in vivo spectra requires suitable methods for spectral analysis and processing (23, 24). Lipid parameters are calculated from the relationships between the areas of different peaks in NMR spectra (8, 23, 24). Strobel et al. (23) have demonstrated that reliable calculation of peak areas requires fitting of spectral lines and correction of peak intensity by the T2 value. In the present paper, the method published by Strobel et al. was translated at 4.7 T and applied to the investigation in vivo of adipose tissue composition in Zucker rats.
In vivo studies were conducted in parallel with ex vivo HR-NMR, assumed to be a “gold standard” for the determination of lipid parameters. The choice of adopting HR-NMR as a gold standard is supported by a number of studies in oils and tissue extracts (see, for example, Refs. 15–17, 19).
Experiments were first conducted by HR-NMR in mixtures of triglycerides (TGs) of known composition in order to validate the relationships used for the calculation of lipid parameters. In order to correct for systematic differences between the indexes computed with HR-NMR and with in vivo MRS, five phantom oils (one animal and four vegetable) were measured by both instruments and the lipidic indexes obtained were used to calculate the linear regression; the values of HR-NMR were assumed to be the gold standard. Finally, in vivo MRS and ex vivo HR-NMR were applied to adipose tissues of Zucker rats, a widely used experimental model of obesity (25, 26). The Zucker obese rats present abnormalities similar to those seen in human metabolic syndrome and are widely accepted as an experimental model of insulin resistance. The lipid profile of obese subjects has been extensively investigated (27–29) in order to find a connection between the nature of the disorder and related metabolic problems. Therefore, in vivo and ex vivo spectra of adipose tissue of both obese and control rats were scanned in vivo using localized MRS and later, after extraction, using in vitro HR-NMR. Both white adipose tissue (WAT) and brown adipose tissue (BAT) were investigated, but only the former could be properly characterized using MRS, due to the low quality of in vivo spectra in the interscapular region arising from the respiratory motion. Both WAT and BAT showed different lipid profiles in obese and control rats. Specifically, unsaturation and polyunsaturation indexes of control rats were higher than those of obese rats. We speculate that the high degree of saturation of fat might explain the well-documented tendency of obese subjects to present cardiovascular and related disorders.
MATERIALS AND METHODS
Chemicals
Vegetable (peanut, sunflower, olive, soy) and animal (cod liver) oils were of commercial origin. Chemicals were purchased from Sigma-Aldrich (Milano, Italy). NMR tubes (5-mm outer diameter) were from Wilmad (New Jersey).
Animals
Male adult Zucker obese (fa/fa) rats (n = 8) and Zucker lean rats (n = 8) were obtained from Harlan, UK. Animals had free access to water and standard rat feed (Teklad Global 18% Protein Rodent Diet, Harlan, UK). According to the manufacturer, the fatty acids composition of the feed was: linoleic acid (31.35 g/kg), linolenic acid (2.76 g/kg), oleic acid (12.59 g/kg), palmitic acid (7.64 g/kg), and stearic acid (1.5 g/kg). Other fatty acids were present in the lower percentages (<0.26 g/kg).
In vivo MRS were acquired for all animals and a reduced number of them (n = 5 obese and n = 4 lean) were used for Folch's extraction protocol (21) and HR-NMR measurements. All studies were conducted in accordance with the guidelines of the Committee for Animal Research at the University of Verona.
HR-NMR and MRS experiments
Triglycerides (TG) solutions were prepared by mixing different volumes of pure glyceryl tripalmitate (16:0), glyceryl trioleate (18:0), glyceryl trilinoleate (18:2), glyceryl trilinolenate (18:3), and deuterated chloroform to reach a final volume of 600 μl. Nine phantom solutions were prepared by mixing the above-mentioned TGs according the following ratios: 100:0:0:0, 50:50:0:0, 0:100:0:0, 0:50:50:0, 0:0:100:0, 0:0:50:50, 0:0:0:100, 50:0:0:50, 33:34:33:0, 0:0:20:80. From these ratios, the theoretical unsaturation and polyunsaturation indexes were calculated and compared with the measured HR-NMR values.
Oil standard samples for HR-NMR were prepared by diluting 20 μl of oil in 600 μl of deuterated chloroform (CDCl3) and placed in 5 mm NMR tubes. Plain oil was used for MRS. Oil samples were prepared simultaneously from the same bottle for both MRS and HR-NMR. Animal fat was extracted from rat WAT and BAT using Folch's standard method (21). After extraction, HR-NMR samples were prepared by diluting a few μl of extracted fat in 600 μl of CDCl3.
MRS experiments were carried out using a 4.7T Biospec System (Bruker, Germany) equipped with a birdcage coil (72 mm internal diameter) and a flat surface coil (15 mm in diameter). Spectra were obtained using a point resolved spectroscopy sequence with repetition time/echo time (TR/TE) = 4000/22 ms, number of collected spectra = 256 (17 min of acquisition time), bandwidth = 20.03 ppm, voxel size = 1–2 mm3. No water suppression was applied. For TR = 4000ms, no correction of T1 was necessary (23). To perform T2-correction, both for oil phantoms and animal adipose tissue, spectra were acquired at different TEs (from 6 to 50 ms, TR = 2500 ms) according to Strobel et al. (23). Animals were preanesthetized with 5% of isofluorane in a mixture of O2 and air and then kept anesthetized with 1–1.5% of isofluorane. The surface coil was carefully positioned over the inguinal part of the rat where WAT deposit is situated. Oil standard samples were laid on the surface coil and inserted in the center of the birdcage transmitter coil. Temperature was approximately 22°C.
HR-NMR spectra were acquired using a Bruker DRX spectrometer operating at 500.13 MHz for 1H nuclei, with a 5 mm TXI probe. HR-NMR spectra were acquired at 298 K, with a repetition time of 2.64 s, spectral width 5 kHz, flip angle of 45°, total number of transients of 128 and 16K acquisition data points. No line broadening was applied in data processing and spectra were zero filled to 32K. Chloroform signal (δ = 7.27 ppm) was used as chemical shift reference, except for samples containing TMS standard (δ = 0.00 ppm). The software Topspin 1.3 (Bruker, Germany) was used to acquire and process the data; baseline corrections, phase adjustments, and calculation of integrals were manually performed using the tools implemented in this software.
Data analysis
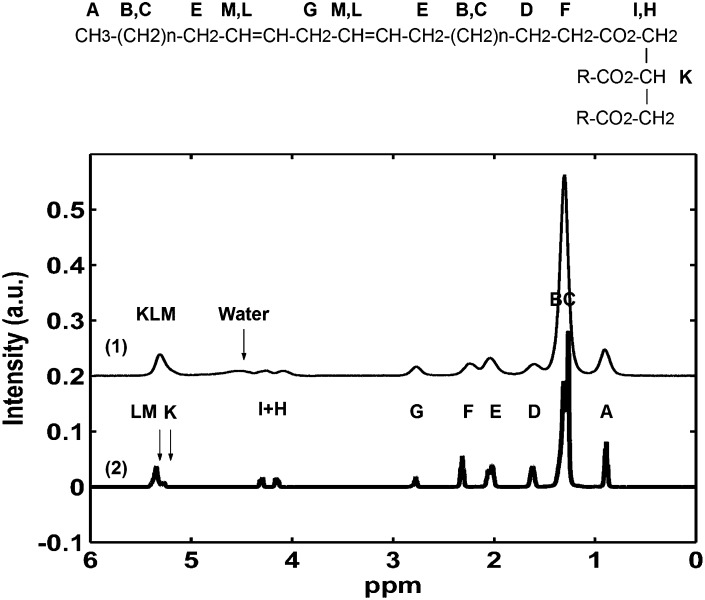
Peaks of proton spectra were assigned to chemical groups of tryglicerides according to the nomenclature reported in Fig. 1. In accordance with references (30) and (23), the following formulas were used to calculate the lipid indexes, starting from the peak areas [letters refer to the area under the curve (AUC) corresponding to each peak]:
| (Eq. 1) |
| (Eq. 2) |
| (Eq. 3) |
Fig. 1.
Representative MRS (1) and HR-NMR spectra (2) of WAT acquired respectively in vivo and ex vivo, after extraction. Peaks are marked using the same nomenclature as the lipid molecular formula.
Because the peak K overlaps with the LM group in MRS, LM is obtained by (Eq. 4); (which was experimentally validated using AUCs of corresponding peaks in HR-NMR).
| (Eq. 5) |
| (Eq. 6) |
where ft represents the triunsaturated fatty acids component, which was assumed negligible, ft=0, according to Strobel et. al (23).
The total unsaturation value, ui, was calculated by considering the ratio between the AUC of the olefinic protons, LM, and the terminal methyl protons, A. The fu index provides an alternative way to express unsaturation: the degree of unsaturation is measured using the ratio between the allylic protons, E, and the acetalic protons, F. Similarly, the degree of polyunsaturation can be calculated using two indexes, pi and fd. The pi index is obtained by dividing AUC of peak G for the AUCs of methyl protons, A, whereas fd uses the values of acetalyc F peaks instead of A. The numerical coefficients in the above-reported relationships account for the number of protons which contribute to each resonance (21). It should be noted that equation 4 is valid if the samples do not contain significant amounts of mono- and di-glyceride, that is to say that the equations 7 and 8 are satisfied:
| (Eq. 7) |
| (Eq. 8) |
The above condition was experimentally verified by HR-NMR spectra both in oils and animal tissue extracts.
Finally, the theoretical values of the previously indexes of pure TGs mixtures (see Materials and Methods, HR-NMR and MRS experiments) were manually calculated starting from the molecular formulas and from the medium number of double bonds for each chain.
Analysis of MRS data
After acquisition, MRS spectra were transferred to a PC for analysis. The values of AUC were obtained by fitting different peaks in the spectra. Several algorithms, both in time domain and frequency domain, were tested including AMARES (31), HLSVD (32), QUEST (33) included in jMRUI software (34), simple integration, and LCModel (35). QUEST was selected because the lipidic indexes obtained after QUEST analysis of spectral peaks showed the best agreement with HR-NMR data and the smallest computed SD in each group of animals with respect to the other algorithms. For QUEST quantification, a database formed by 11 Lorentzian peaks at different ppm values was created using the jMRUI simulation tool. The frequency of each peak was fixed to mimic the corresponding lipid peak, A, BC, D, E, F, G, I, H, K, and LM. Moreover, the default weighting function of QUEST was applied in order to correct baseline distortions. For rat spectra, an additional Lorentzian was added to fit the water peak at 4.7 ppm.
T2-correction of AUCs was performed according to Strobel et al. (23) by fitting the mono-exponential model function to experimental data:
| (Eq. 9) |
where MTE is the amplitude of a peak at echo time TE, and M0 is its initial amplitude.
Statistics
The Mann-Whitney U-test was used to assess the statistical significance of differences between obese rats and controls. MATLAB was used for the computation of R2 both for comparing HR-NMR values with theoretical ones on TG phantoms, and for calibrating MRS values with HR-NMR in phantom oils measurements. In the first case, theoretical values were assumed to be the regressors and the HR-NMR ones the endogenous variables; in the second, the HR-NMR values were assumed to be the regressors (because HR-NMR measurements were used as the gold standard as previously noted) and the in vivo values were assumed as endogenous variables.
RESULTS
TGs and Oil phantoms
Experiments were initially conducted by HR-NMR in TGs mixtures of known composition in order to validate the relationships used to extract the lipidic indexes. Results are reported in Table 1, where the experimental values for mean unsaturation and polyunsaturation are compared with the theoretical values. Table 1 shows that ui values are well correlated (R2 = 0.9982) to the theoretical values of the unsaturation independently from the polyunsaturation. On the contrary, the fu index fails to express the true unsaturation at polyunsaturation values greater than zero. Both pi and fd are linearly correlated to the theoretical value of the polyunsaturation (R2 = 0.9980 and R2 = 0.9992, respectively) but values calculated according to pi are closer to the theoretical values (slope closer to one, plots not shown). The ui and pi parameters were consequently used to express unsaturation and polyunsaturation of lipids in oil phantoms and animals.
TABLE 1.
Values of unsaturation and polyunsaturation for different TGs mixtures
| Sample number | Unsaturation(Prepared) | Polyunsaturation(Prepared) | uia(experimental) | pia(experimental) | fua(experimental) | fda(experimental) |
|---|---|---|---|---|---|---|
| #1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| #2 | 0.50 | 0.00 | 0.50 | 0.00 | 0.47 | 0.00 |
| #3 | 1.00 | 0.00 | 1.01 | 0.00 | 0.99 | 0.00 |
| #4 | 1.50 | 0.50 | 1.49 | 0.50 | 0.99 | 0.46 |
| #5 | 2.00 | 1.00 | 2.03 | 1.07 | 0.97 | 0.96 |
| #6 | 2.50 | 1.50 | 2.37 | 1.49 | 0.95 | 1.34 |
| #7 | 3.00 | 2.00 | 2.93 | 2.15 | 0.90 | 1.83 |
| #8 | 1.01 | 0.33 | 0.95 | 0.32 | 0.63 | 0.29 |
| #9 | 2.80 | 1.80 | 2.70 | 1.86 | 0.93 | 1.63 |
Values are calculated by the known volumes of TGs used to prepare the mixtures or experimentally determined by using the indexes ui, pi, fu, fd.
The experimental error was estimated to be in the range 1–2%.
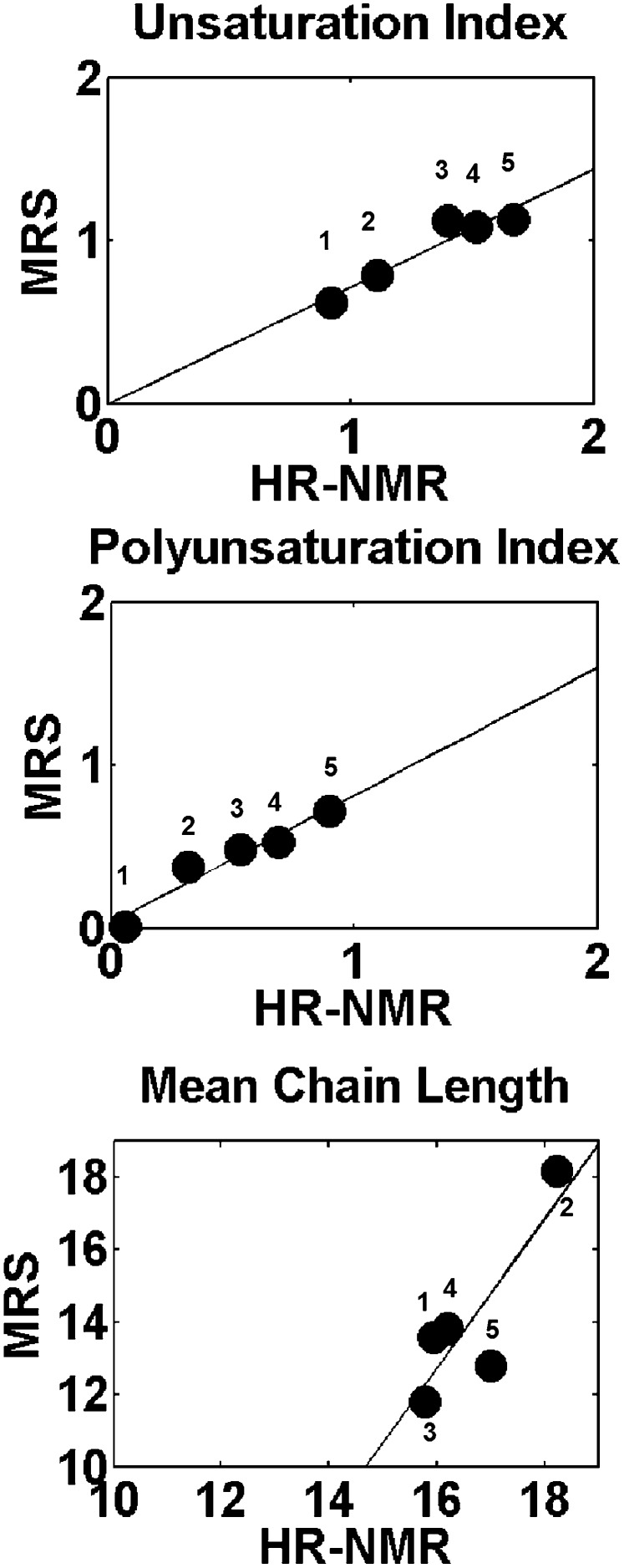
In order to investigate the correlation between lipidic indexes extracted from HR-NMR and MRS spectra, oil phantoms were measured by the two techniques. Five oils were included in the analysis: peanut, sunflower, olive, soy, and cod liver. The ui, pi, and mcl parameters were computed from their spectra (both HR-NMR and MRS). Results are shown in Fig. 2. A good correlation was observed between the values of ui and pi parameters calculated from HR-NMR and MRS spectra. The correlation is less satisfactory for the parameter mcl, most likely because its determination from MRS is affected by large inaccuracy due to error propagation.
Fig. 2.
Correlation between lipid indexes extracted from MRS and HR-NMR data for different oil phantoms: for the unsaturation index R2 =0.95, for the polyunsaturation index R2 =0.97, and for the mean chain length R2 =0.85. Labels 1, 2, 3, 4, and 5 refer to peanut, sunflower, olive, soy, and cod liver oils.
Animal lipid analysis
The lipid parameters ui, pi, and mcl obtained in WAT of obese and lean rats are reported in Fig. 3–5. In each figure, the first histogram is relative to the determination by HR-NMR, whereas the second and third histograms are relative to the determination by MRS. In the third histogram, the coefficients obtained from the oil calibration were used to correct the indexes calculated in vivo from MRS. Hereafter, this procedure will be referred to as the “HR-NMR-based calibration”.
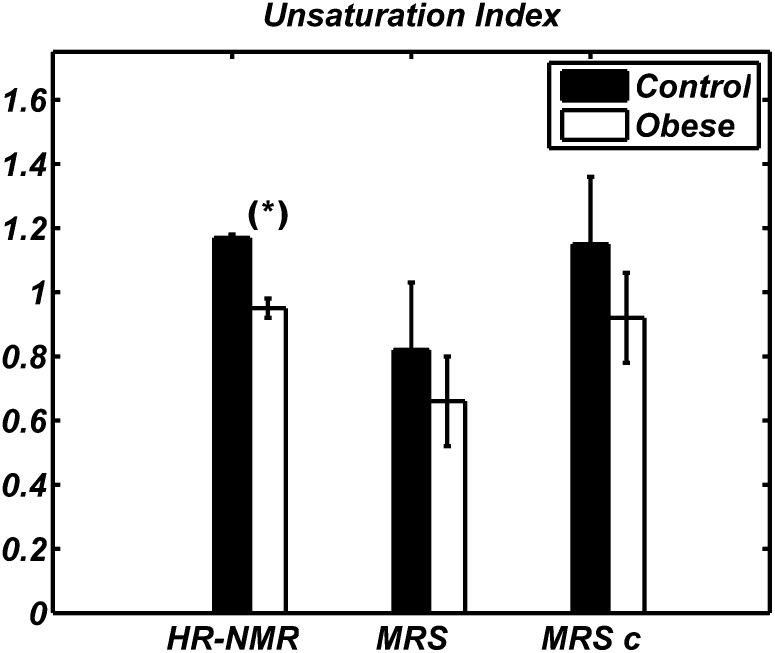
Fig. 3.
Unsaturation index values obtained by HR-NMR (left), MRS (middle), and MRS after HR-NMR-based calibration (right, indicated as MRSc) in WAT of Zucker obese (white) and lean (black) animals. Data are shown as mean ± SD for the two groups of animals. HR-NMR values were statistically lower in obese than in control rats (* P < 0.05); this trend was also observed using MRS, but the difference was not statistically significant because of the large standard deviation affecting the in vivo data.
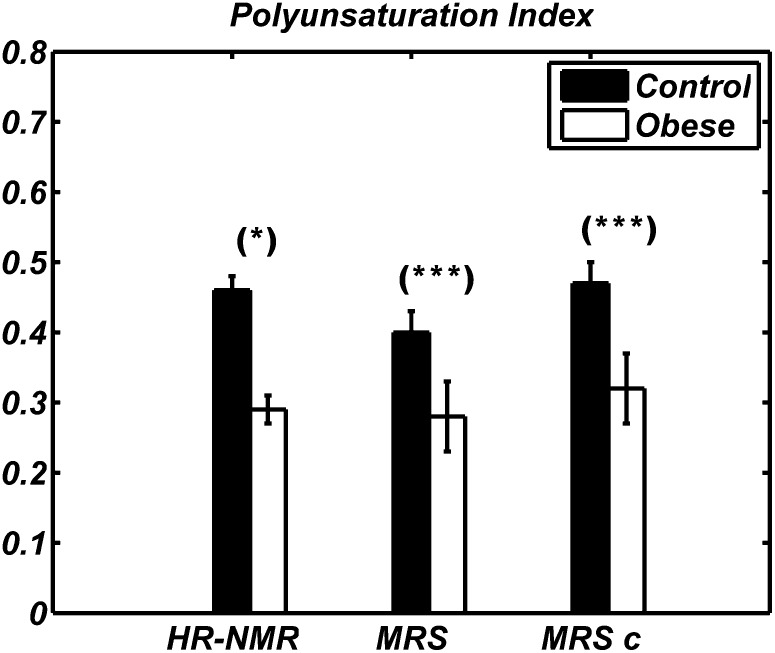
Fig. 4.
Polyunsaturation index values obtained by HR-NMR (left), MRS (middle), and MRS after HR-NMR-based calibration (right, MRSc) in WAT of Zucker obese (white) and lean (black) animals. Data are shown as mean ± SD for the two groups of animals. The difference seen by MRS between obese and control rats is statistically significant (* P < 0.05, *** P < 0.001).
Fig. 5.
Mean chain length values obtained by HR-NMR (left), MRS (middle), and MRS after calibration (right, MRSc) in WAT of Zucker obese (white) and lean (black) animals. Data are shown as mean ± SD for the two groups of animals. No significant differences were observed.
Figure 3 shows clearly a smaller ui value in Zucker obese rats compared with lean ones, as determined by HR-NMR of fat extracts. Although qualitatively in agreement with the HR-NMR results, the mean ui values measured by MRS did not show a statistically significant difference between the groups. The pi value measured by HR-NMR (Fig. 4) was also much lower in obese compared with lean animals and this difference was also detectable with statistical significance (P < 0.001) in MRS data. Analogously, HR-NMR detected clear differences in both fu and fd (data not shown), but statistically significant differences were found in vivo only for fd (P < 0.001). Results for mcl from HR-NMR and MRS, the latter with and without HR-NMR-based calibration, are shown in Fig. 5; neither technique reported a difference in mean chain length between control and obese animals.
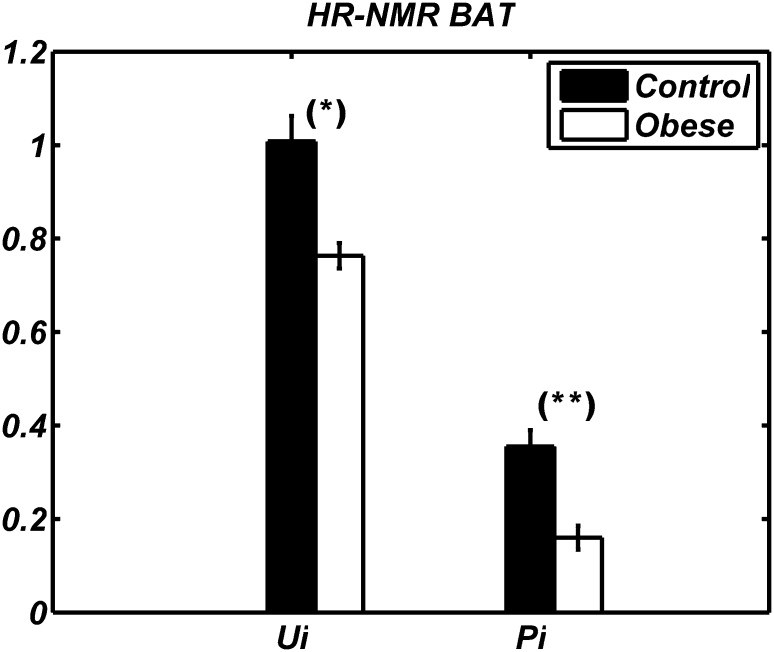
Figure 6 shows lipidic indexes obtained by HR-NMR in BAT. A strong decrease in both ui and pi (24.8% and 55.6%, respectively) was observed in BAT of obese rats compared with controls. In addition, we detected differences between BAT and WAT; in both groups, WAT had higher unsaturation and polyunsaturation indexes than BAT. Indeed, the ui value of WAT was 14% and 19% higher than BAT in control and obese animals, respectively. Similarly, the pi value of WAT was 45% and 22% higher than BAT in control and obese animals, respectively.
Fig. 6.
The ui and pi values (respectively right and left bars) of BAT of Zucker obese (white) and lean (black) animals obtained by HR-NMR. Data are shown as mean ± SD for the two groups of animals. The difference between obese and control rats is statistically significant (* P < 0.05, ** P < 0.01).
DISCUSSION
In vivo localized proton magnetic resonance spectroscopy has been proposed as a noninvasive tool to measure lipid composition in adipose tissues of animals and humans (22–24). In the present study, localized in vivo and high resolution ex vivo magnetic resonance spectroscopy were used to investigate the composition of adipose tissues in Zucker obese and lean rats.
Preliminary experiments were performed in TGs mixtures of known composition by HR-NMR in order to establish the validity of the relationships used to calculate polyunsaturation and unsaturation indexes. Both ui and fu (equations 1 and 5) (30, 23) are reported in the literature as indexes of the total mean lipid unsaturation, that is to say, the mean number of double bonds for each chain of triglyceride. The ui index is correlated to the integral of the vinylic protons (LM), whereas fu exploits the allylic ones (E), i.e., the protons in α-position to the double bonds. In the latter case, a high polyunsaturation, and therefore the presence of diallylic G peaks, affects the relationship between the number of double bonds and the value of the AUC of E. Hence, fu provides an index of unsaturation smaller than ui, where the difference arises from the total mean polyunsaturation of lipids, as demonstrated by our data acquired in TGs mixtures of known composition. Similarly, pi and fd values (equations 2 and 6) (30, 23) are reported in the literature as measurements of the mean number of conjugated double bonds for lipid chain; both indexes are calculated by the diallylic G peak that is proportional to the degree of polyunsaturation and, consequently, give similar final results if mono- and diglycerides are present in low concentrations. The results we have obtained in TGs of known composition point out that pi values are slightly closer to the theoretical ones.
Qualitative differences between WAT of Zucker obese and lean animals were apparent from HR-NMR and also from MRS, although in MRS the difference in ui was not statistically significant. The accuracy of the MRS quantification method is crucial for the standard deviation of the mean values and, consequently, for statistical significance of the results. As a general consideration, the polyunsaturation expressed either by the pi or by fd indexes can be well estimated from MRS data because the G peak (2.75 ppm) is distant from other peaks. In contrast, the determination of ui is performed by considering the peak A (0.9 ppm), which may partially overlap with the huge peak BC (at 1.3 ppm), and LMK, which is formed by two indistinguishable signals, LM and K. Consequently, the quantification of LM from MRS data can be difficult and this may contribute to an increased inter-subjects standard deviation. Similarly, the fu index is calculated based on partially overlapping peaks such as E and F. It is noteworthy that inaccuracies due to partial overlapping of peaks will be less important at higher fields thanks to the larger frequency separation of NMR peaks.
From the biological point of view, results obtained in the present study showed that adipose tissue in Zucker obese rats is qualitatively different from that of Zucker lean rats. Specifically, lower values for ui and pi were observed in WAT and BAT from obese rats compared with control animals. A similar result for pi was already found by our group in WAT of ob/ob mice (13). The biological meaning of this finding remains to be clarified. The ui and pi parameters may be related to the degree of activation of lipolysis, i.e., the degree of activity of adipose tissue. In the present study, obese rats showed lower ui and pi than lean rats, which indicates that adipose tissue in obese animals is more active than in lean ones. This may be related to the well-documented metabolic characteristics of this animal model, such as hyperinsulinemia, hyperlipidemia, and hyperadiponectinemia (26, 36). Previous studies have confirmed high metabolic activity in Zucker fa/fa rats (37). A different interpretation for these results could be a selective accumulation of saturated fats or a defect in accumulating unsaturated fats. A defect in accumulating unsaturated fats might be supported by a deficit in gene expression of specific transporters such as CD 36 (38). It should be noted that a decrease in unsaturation with the increase of the body mass index has been also found in skeletal human muscle (8). Such a decrease was interpreted focusing on the desaturase enzymes, termed Δ9, Δ6, Δ5, and Δ4, and positing increased activity of the first two and decreased activity of Δ5 (39). Moreover, the observed differences between WAT and BAT are in line with previously reported findings in the rat showing a higher proportion of unsaturation and polyunsaturation of fatty acids in the lipid deposits of BAT in comparison with WAT (30).
Without the need of water suppression pulses and thanks to the inherently high signal, MRS acquisitions on fatty tissues can be easily implemented in clinical examinations (24) and, especially at high magnetic field, could provide useful information on the fat deposits composition. Clinical data could contribute to clarify the relationship between the properties of fat and some widespread disorders such as for example obesity, insulin resistance, and diabetes.
CONCLUSIONS
In vivo single-voxel MRS at 4.7 T and ex vivo HR-NMR at 500 MHz were used to study the composition of adipose tissues in Zucker obese and Zucker lean rats. Albeit with different sensitivity and accuracy, both techniques revealed that WAT is characterized by different unsaturation and polyunsaturation indexes in obese rats compared with controls, confirming the hypothesis of lipid metabolism abnormality in Zucker rats. HR-NMR showed similar differences in BAT. It remains to be investigated whether similar differences exist in human fat deposits and their potential diagnostic usefulness.
Acknowledgments
The authors thank Dr. Flavia Merigo for the helpful discussion and scientific support.
Footnotes
Abbreviations:
- AUC
- area under the curve
- BAT
- brown adipose tissue
- HR-NMR
- high resolution proton magnetic resonance spectroscopy
- MRS
- magnetic resonance spectroscopy
- mcl
- mean chain length
- TE
- echo time
- TR
- repetition time
- ui
- unsaturation index
- pi
- polyunsaturation index
- TG
- triglycerides
- WAT
- white adipose tissue
E. Mosconi is a PhD student supported by VenetoNanotech, Italy; D. M. Sima is a postdoctoral fellow of the Fund for Scientific Research Flanders. This research has been supported by Research Council KUL: GOA MaNet, CoE EF/05/006 Optimization in Engineering (OPTEC); Belgian Federal Science Policy Office: IUAP P6/04 (DYSCO, Dynamical systems, control and optimization', 2007-2011); EU: FAST (FP6-MC-RTN-035801). The scientific responsibility is assumed by its authors.
REFERENCES
- 1.Simonsen N., van 't Veer P., Strain J. J., Martin-Moreno J. M., Huttunen J. K., Fernández-Crehuet Navajas J., Martin B. C., Thamm M., Kardinaal A. F. M., Kok F. J., et al. 1998. Adipose tissue omega-3 and omega-6 fatty acid content and breast cancer in the EURAMIC study. Am. J. Epidemiol. 147: 342–352. [DOI] [PubMed] [Google Scholar]
- 2.Shannon J., King I. B., Moshofsky R., Lampe J. W., Gao D. L., Ray R. M., Thomas D. B. 2007. Erythrocyte fatty acids and breast cancer risk: a case-control study in Shangai, China. Am. J. Clin. Nutr. 85: 1090–1097. [DOI] [PubMed] [Google Scholar]
- 3.Manco M., Mingrone G., Greco A. V., Capristo E., Gniuli D., De Gaetano A., Gasbarrini G. 2000. Insulin resistance directly correlates with increased saturated fatty acids in skeletal muscle triglycerides. Metabolism. 49: 220–224. [DOI] [PubMed] [Google Scholar]
- 4.Goodpaster B. H., Wolf D. 2004. Skeletal muscle lipid accumulation in obesity, insulin resistance, and type 2 diabetes. Pediatr. Diabetes. 5: 219–226. [DOI] [PubMed] [Google Scholar]
- 5.Shoelson S. E., Herrero L., Naaz A. 2007. Obesity, inflammation, and insulin resistance. Gastroenterology. 132: 2169–2180. [DOI] [PubMed] [Google Scholar]
- 6.Dorfman S. E., Laurent D., Gounarides J. S., Li X., Mullarkey T. L., Rocheford E. C., Sari-Sarraf F., Hirsch E. A., Hughes T. E., Commerford S. R. 2009. Metabolic implications of dietary trans-fatty acids. Obesity (Silver Spring). 17: 1200–1207. [DOI] [PubMed] [Google Scholar]
- 7.Gerber M., Thiébaut A., Astorg P., Clavel-Chapelon F., Combe N. 2005. Dietary fat, fatty acid composition and risk of cancer. Eur. J. Lipid Sci. Technol. 107: 540–559. [Google Scholar]
- 8.Velan S. S., Said N., Durst C., Frisbee S., Frisbee J., Raylman R. R., Thomas M. A., Rajendran V. R., Spencer R. G., Alway S. E. 2008. Distinct patterns of fat metabolism in skeletal muscle of normal-weight, overweight and obese humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295: R1060–R1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malcom G. T., Bhattacharyya A. K., Velez-Duran M., Guzman M. A., Oalmann M. C., Strong J. P. 1989. Fatty acid composition of adipose tissue in humans: differences between subcutaneous sites. Am. J. Clin. Nutr. 50: 288–291. [DOI] [PubMed] [Google Scholar]
- 10.Ohno T., Ogawa K., Kuroshima A. 1992. Postnatal changes in fatty acids composition of brown adipose tissue. Int. J. Biometeorol. 36: 30–35. [DOI] [PubMed] [Google Scholar]
- 11.Sbarbati A., Guerrini U., Marzola P., Asperio R., Osculati F. 1997. Chemical shift imaging at 4.7 tesla of brown adipose tissue. J. Lipid Res. 38: 343–347. [PubMed] [Google Scholar]
- 12.Velan S. S., Durst C., Lemieux S. K., Raylman R. R., Sridhar R., Spencer R. G., Hobbs G. R., Thomas M. A. 2007. Investigation of muscle lipid metabolism by localized one- and two-dimensional MRS Techniques using a clinical 3T MRI/MRS scanner. J. Magn. Reson. Imaging. 25: 192–199. [DOI] [PubMed] [Google Scholar]
- 13.Calderan L., Marzola P., Nicolato E., Fabene P. F., Milanese C., Bernardi P., Giordano A., Cinti S., Sbarbati A. 2006. In vivo phenotyping of the ob/ob mouse by magnetic resonance imaging and 1H-magnetic resonance spectroscopy. Obesity (Silver Spring). 14: 405–414. (PubMed). [DOI] [PubMed] [Google Scholar]
- 14.Hwang J. H., Bluml S., Leaf A., Ross B. D. 2003. In vivo characterization of fatty acids in human adipose tissue using natural abundance 1H decoupled 13C MRS at 1.5T: clinical applications to dietary therapy. NMR Biomed. 16: 160–167. (PubMed). [DOI] [PubMed] [Google Scholar]
- 15.Jie M. S., Mustafa J. 1997. High-resolution nuclear magnetic resonance spectroscopy–applications to fatty acids and triacylglycerols. Lipids. 32: 1019–1034. [DOI] [PubMed] [Google Scholar]
- 16.Knothe G., Kenar J. A. 2004. Determination of the fatty acid profile by 1H-NMR spectroscopy. Eur. J. Lipid Sci. Technol. 106: 88–96. [Google Scholar]
- 17.Guillén M. D., Ruiz A. 2003. 1H nuclear magnetic resonance spectroscopy as a fast tool for determining the composition of acyl chains in acylglycerol mixtures. Eur. J. Lipid Sci. Technol. 105: 502–507. [Google Scholar]
- 18.Zancanaro C., Bolner A., Righetti C. 2001. NMR spectroscopic analysis of rat brain development: in vitro proton and carbon studies of whole tissue and its phospholipid fraction. Dev. Neurosci. 23: 107–112. [DOI] [PubMed] [Google Scholar]
- 19.Wollenberg K. 1991. Quantitative triacylglycerol analysis of whole vegetable seeds by 1H and 13C magic angle sample spinning NMR spectroscopy. J. Am. Oil Chem. Soc. 68: 391–400. [Google Scholar]
- 20.Miyake Y., Yokomizo K., Matsuzaki N. 1998. Determination of unsaturated fatty acid composition by high-resolution nuclear magnetic resonance spectroscopy. J. Am. Oil Chem. Soc. 75: 1091–1094. [Google Scholar]
- 21.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipids from animal tissue. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 22.Lunati E., Farace P., Nicolato E., Righetti C., Marzola P., Sbarbati A., Osculati F. 2001. Polyunsaturated fatty acids mapping by 1H NMR-chemical shift imaging. Magn. Reson. Med. 46: 879–883. [DOI] [PubMed] [Google Scholar]
- 23.Strobel K., van den Hoff J., Pietzsch J. 2008. Localized proton magnetic resonance spectroscopy of lipids in adipose tissue at high spatial resolution in mice in vivo. J. Lipid Res. 49: 473–480. [DOI] [PubMed] [Google Scholar]
- 24.Ren J., Dimitrov I., Sherry A. D., Malloy C. R. 2008. Composition of adipose tissue and marrowfat in humans by 1H NMR at 7 Tesla. J. Lipid Res. 49: 2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bray G. A. 1977. The Zucker-fatty rat: a review. Fed. Proc. 36: 148–153. [PubMed] [Google Scholar]
- 26.Blond J. P., Henchiri C., Bézard J. 1989. Delta 6 and delta 5 desaturase activity in liver from obese zucker rats at different ages. Lipids. 24: 389–395. [DOI] [PubMed] [Google Scholar]
- 27.Perseghin G., Scifo P., De Cobelli F., Pagliato E., Battezzati A., Arcelloni C., Vanzulli A., Testolin G., Pozza G., Del Maschio A., et al. 1999. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans. Diabetes. 48: 1600–1606. [DOI] [PubMed] [Google Scholar]
- 28.Serkova N. J., Jackman M., Brown J. L., Liu T., Hirose R., Roberts J. P., Maher J. J., Niemann C. U. 2006. Metabolic profiling of livers and blood from obese Zucker rats. J. Hepatol. 44: 956–962. [DOI] [PubMed] [Google Scholar]
- 29.Brookes P. S., Buckingham J. A., Tenreiro A. M., Hulbert A. J., Brand M. D. 1998. The proton permeability of the inner membrane of liver mitochondria from ectothermic and endothermic vertebrates and from obese rats: correlations with standards metabolic rate and phospholipid fatty acid composition. Comp. Biochem. Physiol. 119B: 325–334. [DOI] [PubMed] [Google Scholar]
- 30.Zancanaro C., Nano R., Marchioro C., Sbarbati A., Boicelli A., Osculati F. 1994. Magnetic resonance spectroscopy investigations of brown adipose tissue and isolated brown adipocities. J. Lipid Res. 35: 2191–2199. (PubMed). [PubMed] [Google Scholar]
- 31.Vanhamme L., van den Boogaart A., Van Huffel S. 1997. Improved method for accurate and efficient quantification of mrs data with use of prior knowledge. J. Magn. Reson. 129: 35–43. [DOI] [PubMed] [Google Scholar]
- 32.Poullet J. B., Sima D. M., Van Huffel S. 2008. MRS signal quantification: a review of time- and frequency -domain methods. JMR. 195: 134–144. [DOI] [PubMed] [Google Scholar]
- 33.Ratiney H., Sdika M., Coenradie Y., Cavassila S., van Ormondt D., Graveron-Demilly D. 2005. Time-domain semi-parametric estimation based on a metabolite basis set. NMR Biomed. 18: 1–13.. [DOI] [PubMed] [Google Scholar]
- 34.Naressi A., Couturier C., Castang I., de Beer R., Graveron-Demilly D. 2001. Java -based graphical user interface for MRUI, a software package for quantitation of in vivo medical magnetic resonance spectroscopy signals. Comput. Biol. Med. 31: 269–286. [DOI] [PubMed] [Google Scholar]
- 35.Provencher S. W. 2001. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 14: 260–264. [DOI] [PubMed] [Google Scholar]
- 36.Oana F., Takeda H., Hayakawa K., Matsizawa A., Akahane S., Isaji M., Akahane M. 2005. Physiological difference between obese (fa/fa) Zucker rats and lean Zucker rats concerning adiponectin. Metabolism. 54: 995–1001. [DOI] [PubMed] [Google Scholar]
- 37.Guerre -Millo M., Guesnet P., Guichard C., Durand G., Lavau M. 1994. Alteration in membrane lipid order and composition in metabolically hyperactive fatty rat adipocytes. Lipids. 29: 205–209. [DOI] [PubMed] [Google Scholar]
- 38.Sbarbati A., Bertini M., Catassi C., Gagliardini R., Osculati F. 1998. Ultrastructural lesions in the small bowel of patients with cystic fibrosis. Pediatr. Res. 43: 234–239. [DOI] [PubMed] [Google Scholar]
- 39.Vessby B., Gustaffson I-B., Tenglabad S., Boberg M., Andersson A. 2002. Desaturation and elongation of fatty acids and insulin action. Ann. N. Y. Acad. Sci. 967: 183–195. [DOI] [PubMed] [Google Scholar]