Abstract
L-4F, an apolipoprotein A-I (apoA-I) mimetic peptide (also known as APL180), was administered daily by either intravenous (IV) infusion for 7 days or by subcutaneous (SC) injection for 28 days in patients with coronary heart disease in two distinct clinical studies. L-4F was well tolerated at all doses tested. Despite achieving plasma levels (mean maximal plasma concentration of 2,907 ng/ml and 395 ng/ml, following IV infusion and SC injection, respectively), that were effective in previously published animal models, treatment with L-4F, as assessed by biomarkers of HDL function such as HDL-inflammatory index (HII), and paraoxonase activity, did not improve. Paradoxically, there was a 49% increase in high-sensitivity C-reactive protein (hs-CRP) levels after seven IV infusions of 30 mg L-4F (P < 0.05; compared with placebo) and a trend for hs-CRP increase in subjects receiving 30 mg SC injection for 28 days. In a subsequent, ex vivo study, addition of L-4F at concentrations of 150, 375, or 1,000 ng/ml to plasma from subjects prior to L-4F treatment resulted in significant dose-dependent HII improvement. In conclusion, in vivo L-4F treatment, delivered by either SC injection or IV infusion, did not improve HDL functional biomarkers despite achieving plasma levels that improved identical biomarkers ex vivo and in animal models.
Keywords: apolipoprotein, atherosclerosis, high density lipoprotein, C-reactive protein, coronary heart disease, diabetes
Epidemiologic data support that elevated levels of HDL cholesterol are associated with an improvement in cardiovascular risk (1). HDL particles provide atherosclerosis protection by way of their role in reverse cholesterol transport and potentially their direct anti-inflammatory, antioxidant, and antithrombotic properties. However, there is an increasing body of literature that not all HDL particles are equal in their cardioprotective effects and that certain properties of the HDL particle itself confer either pro- or anti-inflammatory effects (2). Thus, HDL function, rather than the absolute level of HDL-cholesterol, may be a more accurate indicator for risk of developing atherosclerosis and of manifesting its clinical sequelae (2). This hypothesis has led to investigation of HDL as both a biomarker for cardiovascular risk and a therapeutic target to be functionally manipulated (3). Increasing the plasma concentrations of the main apolipoprotein in HDL, apolipoprotein A-I (apoA-I), through intravenous (IV) administration has been considered an attractive approach. However, treatment with recombinant HDL or even apoA-I is difficult because the commercial production of apoA-I (a 243-amino acid protein) is not trivial and requires complexing with lipid prior to IV administration. Therefore, smaller peptides that “mimic” or augment apoA-I activity have been explored as novel therapeutics (4).
The apoA-I mimetic peptides D-4F and L-4F (APL180) have shown promise in a number of animal models (4) and in early human trials with D-4F (5). The mechanism of action of these peptides has been reported to be due to their extraordinary ability to bind oxidized and oxidizable lipids with four to six orders of magnitude higher affinity, as compared with native apoA-I (6). After oral administration in mice, these mimetic peptides have been reported to improve the ability of HDL to inhibit LDL-induced monocyte chemoattractant protein-1 (MCP-1) production by human aortic endothelial cells as measured by monocyte migration in vitro and quantified as the HDL-inflammatory index (HII) (6–12).
The maximal plasma concentration (Cmax) of 4F (L or D) in the mouse studies were on the order of 100 to 300 ng/ml of peptide after oral administration (8, 9). In ex vivo studies with human plasma, addition of 250 to 1,000 ng/ml of peptide resulted in a decreased HII (13, 14). In a previous clinical study with D-4F, administration of a single oral dose of 300 mg or 500 mg achieved low Cmax plasma levels (approximately 10 ng/ml) due to the poor bioavailability of the oral formulation. Despite this fact, this oral formulation of D-4F improved the HII in patients with coronary heart disease (CHD) or a CHD equivalent compared with placebo, whereas lower oral doses did not have an effect (5).
Oxidized lipids bind with similar high affinity to D-4F and L-4F (6). D-4F is synthesized from d-amino acids which are poorly degraded in mammals, as discussed previously (15). This led to prolonged tissue retention times of D-4F, particularly in liver and kidney, in preliminary studies in animals (data not shown). In contrast, L-4F is synthesized from l-amino acids and was rapidly degraded in mammalian tissues (data not shown). Despite these differences, the effects of D-4F and L-4F on biomarkers and lesion area were similar when administered by subcutaneous (SC) injection in cholesterol-fed rabbits (12). Therefore, human studies with L-4F were initiated to probe the HDL anti-inflammatory effects of apoA-I mimetics. The studies described herein are the first clinical studies of L-4F administered to humans by either IV infusion (ClinicalTrials.gov number, NCT00568594) or by SC injection (ClinicalTrials.gov number, NCT00907998).
For these initial short studies in CHD patients, in addition to safety and pharmacokinetics, biomarkers of HDL function and systemic inflammation were assessed. The following pharmacodynamic (PD) biomarker endpoints were measured: HII using a cell-based assay, paraoxonase (PON), high-sensitivity C-reactive protein (hs-CRP), and interleukin-6 (IL-6) levels. We report here that despite achieving plasma levels of L-4F comparable to or well above those achieved in mouse models (8, 9) and in the early human studies with D-4F (5), no significant change in HII or PON were observed. Unexpectedly, there was a trend toward increases in hs-CRP and IL-6 levels.
MATERIALS AND METHODS
Peptides
The peptide L-4F (Ac-D-W-F-K-A-F-Y-D-K-V-A-E-K-F-K-E-A-F-NH2) was synthesized from l-amino acids by solid-phase synthesis as described (9). For in vitro studies, a control peptide, scrambled L-4F, with the same l-amino acids as in L-4F but in a sequence that does not promote α-helical formation (Ac-D-W-F-A-K-D-Y-F-K-K-A-F-V-E-E-F-A-K-NH2) was synthesized by solid-phase synthesis as described previously (8). For both the IV and SC studies, the peptides were provided to the clinical trial sites as a lyophilized powder in a sterile trehalose-phosphate buffer. Prior to use, the powder was reconstituted with sterile water for injection (SWFI) to produce vials containing 0.2 or 3 mg/ml (IV study) and 10 or 30 mg/ml (SC study). Placebo consisted of lyophilized trehalose-phospate buffer that was reconstituted with SWFI. Multiple vials (APL180 and/or placebo) were used to produce the required dose for the IV study.
Patient populations and study designs
Results presented herein are from two separate studies, an IV infusion study (APL180A2201; Fig. 1A) and an SC injection study (APL180A2210B; Fig. 1B). The IV infusion study initially included eight subjects at each dose level treated with IV infusions daily for 7 days to establish safety/tolerability. Additional patients were enrolled in the 30 mg dose group to increase the number of subjects and allow for more-robust statistical analyses. The SC study involved a single SC injection daily for 28 days. For both the IV and SC studies, males and females (post-menopausal or surgically sterile) between the ages of 18 and 75 years with stable CHD or a CHD equivalent and on a stable dose of a statin (>8 weeks) were eligible. CHD and equivalents were defined by the National Cholesterol Education Program Adult Treatment Panel III criteria (16), and CHD patients were required to be event- and procedure-free (e.g., no documented myocardial infarction (MI), unstable angina, coronary revascularization) for at least 6 months prior to inclusion. CHD equivalent patients included those with a history of symptomatic carotid artery disease (e.g., transient ischemic attack or stroke of carotid origin); patients with peripheral artery disease; diabetes mellitus (excluded if HbA1c ≥9%); 20% 10 year risk of CHD (Framingham point score ≥16 for men and ≥23 for women); or patients with other clinical forms of atherosclerotic disease, including >50% stenosis on angiography or ultrasound or other forms of clinical atherosclerotic disease (e.g., renal artery disease). All subjects provided informed written consent before participating in any study procedures. The study was approved by the Independent Ethics Committee and/or Institutional Review Board at each study center and local health authorities. The studies were conducted in accordance with good clinical practice and the ethical principles of the Declaration of Helsinki. Both studies were registered in accord with the International Committee of Medical Journal Editors (http://prsinfo.clinicaltrials.gov/icmje.html).
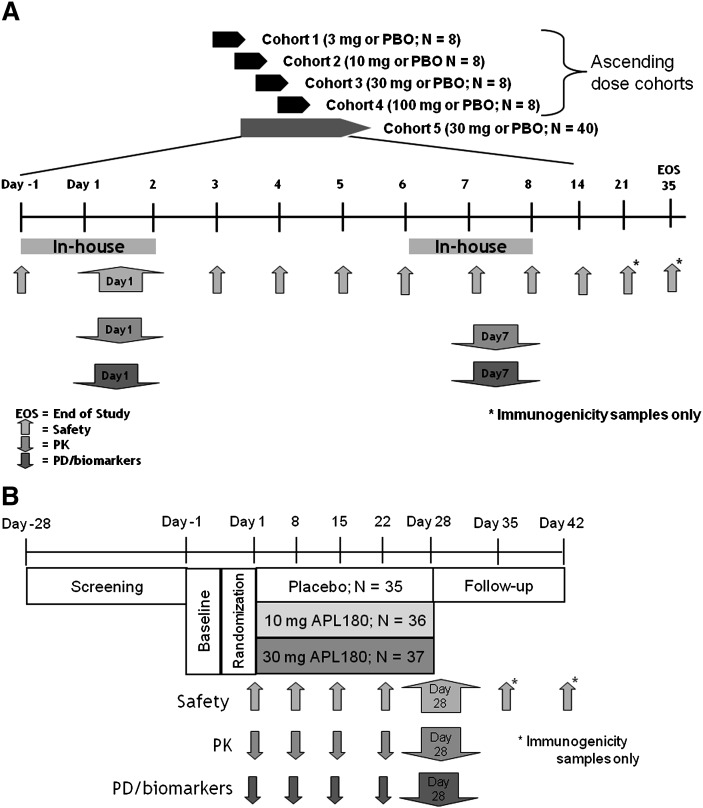
Fig. 1.
Study design schematics for intravenous (IV) and subcutaneous (SC) studies. A: Overview of APL1802201, a randomized, double-blinded, placebo-controlled, ascending multiple-dose study of L-4F given as daily IV infusions for 7 consecutive days. B: Overview of APL1802210B, a parallel, randomized, double-blind, placebo-controlled, multiple-dose study of L-4F given as daily SC injections for 28 days. PBO, placebo.
As shown in Fig. 1A, the IV study was a randomized, double-blinded, placebo-controlled study with ascending multiple doses of L-4F given as daily IV infusions for seven consecutive days. Initially for this study, each ascending dose cohort consisted of eight patients randomized to receive L-4F (3, 10, 30, or 100 mg) or placebo (6:2) (APL180A2201), and then a larger cohort of 40 patients (randomized 1:1 to receive 30 mg L-4F or placebo) were added. Eligible patients reported to the site for baseline assessments 24 h prior to their dose (day −1) and remained domiciled until day 2. On day 1, following an overnight fast, patients received an IV dose of L-4F or placebo. The dose was administered as a 2 h IV infusion, where 47% of the dose was given at a constant rate for the first 15 min, then the rate of infusion was decreased and the remaining 53% was administered over the next 1 h and 45 min. This dosing regime was chosen such that the exposures obtained would approximate those expected from a SC injection. Following dosing on day 1, patients continued to fast for an additional 4 h. Safety, pharmacokinetic (PK), and PD assessments were conducted predose and continued throughout the first 24 h. Patients received their second daily IV dose at the clinic, were closely monitored for at least 1 h post dose, and then were released from the clinic. Patients returned to the site each morning for their daily IV infusions. Patients were readmitted to the site on day 6 prior to supper and stayed domiciled until day 8. Following an overnight fast, the procedures on day 1 were repeated on day 7. The patients were released from the site 24 h after their seventh dose, then returned 7, 14, and 28 days later (study days 14, 21, and 35) to undergo immunogenicity and safety assessments.
As shown in Fig. 1B, the SC study (APL180A2210B) was a parallel, randomized, double-blind, placebo-controlled, multiple-dose (daily SC injections for 28 days) design. A total of 104 patients were randomized to three distinct dose arms (10 or 30 mg L-4F, or placebo) in a 1:1:1 ratio. Eligible patients reported to the site for baseline assessments on day −1 and remained domiciled until day 2. On day 1, following an overnight fast, the first injection was administered. The doses were given in the abdomen, and the injection sites were rotated daily between the four quadrants (upper and lower right, upper and lower left). Safety, PK, and PD assessments were conducted predose and continued throughout the first 24 h. Patients received their second dose at the clinic on the morning of day 2, were closely monitored for at least 4 h, and then were released from the clinic. The daily SC doses were administered by a trained nurse on an out-patient basis until day 27, at which time the nurse assessed local tolerance of SC injections, other adverse events (AEs) and changes to comedication. On days 8, 15, and 22, additional safety, PK, and PD assessments were made. On day 28, patients reported to the site after an overnight fast and continued to fast for 4 h postdose. PK/PD samples were collected predose and for 24 h postdose. The patients were released from the site 24 h after their 28th dose, then returned 7 and 14 days later (days 35 and 42) to undergo immunogenicity and safety assessments.
In both studies, the choice of a placebo control over a scrambled peptide control was chosen due to the time and resources required to qualify the scrambled peptide for use in humans.
Safety assessments
All AEs and serious adverse events (SAEs) were recorded during the study period. The treating physician assessed all AEs for severity and determined the likelihood of a possible relationship to the study drug. In addition, blood hematologic and chemistry profiles and urinalysis, vital signs, body weight, physical condition, and 12-lead electrocardiogram (ECG) were monitored regularly throughout the studies. In the SC study, injection site reactions (ISRs) were monitored daily for severity of pain, redness, induration, swelling, hemorrhage, and itching, and it was left to the discretion of the investigator whether to report the ISR as an AE.
Pharmacokinetics
Blood samples for pharmacokinetic assessment were obtained at regular intervals up to 24 h postdose on day 1 and days 7/28 (IV/SC, respectively). In the SC study, weekly samples (days 8, 15, and 22) were also obtained to determine trough levels. In brief, blood samples (2 ml) were collected in sodium-heparin tubes, plasma was extracted, and samples were frozen at –70°C until analysis. Plasma levels of intact L-4F were determined using a validated LC-MS/MS methodology with a lower limit of quantitation of 2.5 ng/ml. For analysis, protein was precipitated from the plasma sample using acetonitrile and centrifugation in a 96-well format on a TomTec Quadra 96 System. Solid-phase extraction was performed on the supernatant using a Waters Oasis HLB µElution plate and using an elution mixture of methanol-water-acetic acid. Samples were then injected onto an Ace C8 5 µm column, and eluted with a gradient of acetonitrle in formic acid. MS was carried out on an API3000 Applied Biosystem using TurboIonspray® with positive-ion mode.
The steady-state pharmacokinetic parameters, including peak plasma concentrations (Cmax), area under the plasma concentration-time curve during dosing interval of 24 h (AUCtau), time to reach peak concentrations (Tmax), total clearance following IV dosing (Cl), terminal half-life (T1/2), and average daily concentration (Cavg) were estimated using noncompartmental methods (WinNonlin, version 5.2).
Biomarker assessments
Blood samples for biomarker assessment were obtained predose and at 0.25 h, 2 h (end of infusion), 4, 8, 12, and 24 h postdose on day 1 and day 7 for the IV study. During the SC study, samples were collected predose on days 1, 2, 8, 15, and 28, as well as at 2, 4, and 8 h postdose on day 28. Biomarker measurements included the HII, which was determined using previously described methods (10, 12). To assess the reproducibility of the cell-based assay used for determining the HII, a single plasma sample from one subject was divided into four aliquots, and each was processed on a different fast-protein liquid chromatography (FPLC) system to obtain the HDL-containing fractions; these fractions were then tested on four different plates containing the same human aortic endothelial cells exposed to the same standard control LDL on the same day. MCP-1 was then assayed by bioassay using the same normal human peripheral blood monocytes from a single normal donor but using different chambers on the same day. The results are shown in Table 1 and indicate a coefficient of variation of 21%.
TABLE 1.
Determining the reproducibility of the HII
| Assay | HII Value | |
|---|---|---|
| Intra-assay variability | #1 | 1.04 |
| #2 | 1.15 | |
| #3 | 1.13 | |
| #4 | 1.61 | |
| Mean ± SD (CV) | 1.23 ± 0.26 (21%) | |
| Inter-day variation | ||
| Day 1 | 1.34 ± 0.36 | |
| Day 2 | 1.36 ± 0.44 | |
CV, coefficient of variation; HII, HDL-inflammatory index. To determine the intra-assay reproducibility of the HII, plasma from one subject was divided into four aliquots and each was processed on a different fast-protein liquid chromatography (FPLC) system to obtain the HDL-containing fractions. These fractions were tested on four different plates containing the human aortic endothelial cells from the same preparation and exposed to the same standard control LDL on the same day. Monocyte chemoattractant protein-1 was then assayed by monitoring membrane migration of the same normal human monocytes but using different chambers on the same day. To determine the inter-day reproducibility of the HII, four subjects from the multi-dose IV study were randomly selected, and their plasma samples were divided into two aliquots that were processed on different days and assayed using different human aortic endothelial cells and different monocytes.
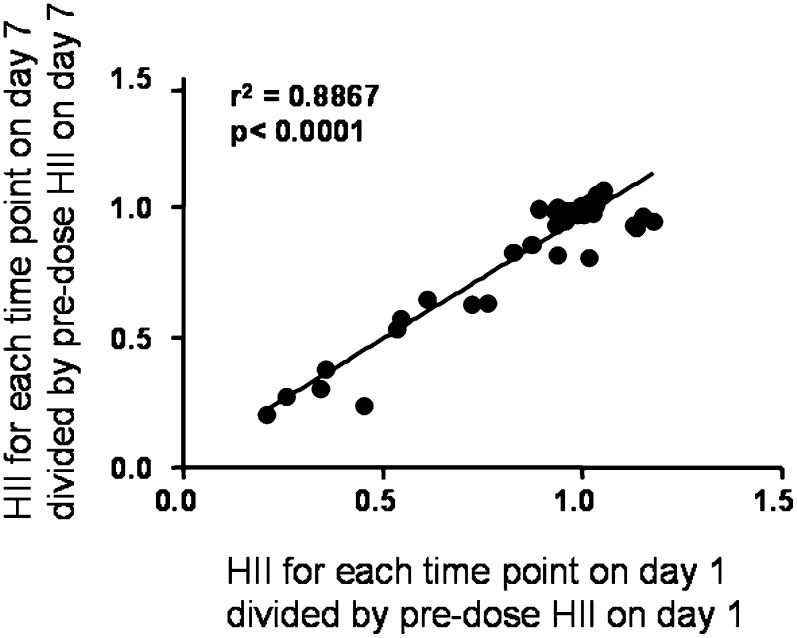
To further support the reproducibility of the cell-based assay used for determining the HII, four subjects from the IV study were randomly selected, and their plasma samples were divided into two aliquots that were then processed on different days and assayed using different human aortic endothelial cells and different monocytes. The mean ± SD of the HII values obtained on the two different assay days are shown in Table 1 and indicate a mean difference of 1.5%. A third approach to characterize the reproducibility of the HII was as follows: the HII values obtained at each time point on the first and seventh days following infusion (time points assayed after infusion were 15 min, and 2, 4, 8, 12, and 24 h) for the four subjects described in Table 1 were divided by the predose HII value on the first and seventh day, respectively, and plotted against each other. The results are shown in Fig. 2 and confirmed the reproducibility of the method using samples obtained on different days.
Fig. 2.
Reproducibility of the HDL-inflammatory index (HII) for samples obtained on different days. The HII values obtained at each time point on the first and seventh days following infusion (time points assayed after infusion were 15 min, 2 h, 4 h, 8 h, 12 h, and 24 h) for the four subjects described in Table 2 were divided by the predose HII value on the first and seventh days, respectively, and plotted against each other. The regression line is shown as a solid black line. The slope of the line = 0.9376; y intercept = 0.0272; x intercept = −0.02901.
Other biomarkers assessed in these studies were PON activity, hs-CRP, and IL-6. PON activity was assessed, in sodium-heparin plasma using paraoxon as a substrate, as previously described (7). Serum hs-CRP and IL-6 levels were assessed using the ImmunDiagnostik CRP ELISA Kit No. K9710s, and R and D systems Quantikine® HS Human IL-6 Immunoassay, No. SS600B, respectively. Lipoprotein and apolipoprotein analyses were performed by Northwest Research Laboratories (Seattle, WA). Lipoprotein analysis utilized their betaquantification method (density gradient ultracentrifugation). ApoA-I and apoA-II were analyzed using a nephelometric method (Ab/Ag complex) on a Behring Nephelometer II autoanalyzer. Immunogenicity toward L-4F was monitored using a direct ELISA method. Patient samples were diluted 1:20 and then incubated on L-4F-coated microtitre plates for 3 h at room temperature. The detecting antibody [a 1:8,000 dilution of a goat anti-monkey IgG-HRP, γ specific (No SAB1303) from Open Biosystems; which also binds human IgG] was added to the plates and incubated for 1 h at room temperature. BM blue PO-Substrate, No. 1484281 (Roche), was used for detection. A lower limit of quantitation of 0.5 μg/ml was determined using human IgG as a control.
Ex vivo studies
Prior to the completion of the SC study but after the completion of all of the IV studies, the ability of L-4F to improve HII after addition of the peptide ex vivo to plasma samples from a subset of subjects was determined using methods previously described (13, 14).
Statistical methods
An ANCOVA with classification by treatment and baseline as the covariate was performed on change from baseline in biomarker data at each time point for the IV study and SC study separately. The null hypothesis H0: Δ = 0 versus the alternative hypothesis HA: Δ ≠ 0, where Δ denotes the mean difference between an L-4F dose and placebo treatment, was tested at the 5% significance level. Additionally, average change from baseline on study days where multiple measurements were taken over time was analyzed using the same approach described above. With the exception of HII data, all biomarker data were log-transformed prior to statistical analysis.
RESULTS
Baseline demographics, medical history, and baseline biomarkers for subjects enrolled in the IV study (Table 2) were similar between the two largest cohorts (30 mg L-4F and placebo; N = 26 and 28, respectively). Some imbalances were noted among the remaining cohorts of this study (e.g., no females enrolled in the 100 mg cohort) due to the small number of subjects enrolled (N = 6). The baseline demographics and medical history for subjects enrolled in the SC study were similar (Table 3). Some imbalances were noted between baseline biomarker values (e.g., more black patients enrolled in the 10 mg cohort).
TABLE 2.
Baseline demography of IV study (APL810A2201) by dose group
| APL180 | |||||
|---|---|---|---|---|---|
| Demographic Variables | 3 mgN = 6 | 10 mgN = 6 | 30 mgN = 26 | 100 mgN = 6 | PlaceboN = 28 |
| Age (mean ± SD), years | 55.8 ± 6.49 | 60.0 ± 4.65 | 59.7 ± 7.47 | 64.2 ± 4.71 | 60.7 ± 7.57 |
| Sex, male (n, %) | 4 (66.7%) | 4 (66.7%) | 19 (73.1%) | 6 (100%) | 19 (67.9%) |
| Sex, female (n, %) | 2 (33.3%) | 2 (33.3%) | 7 (26.9%) | 0 | 9 (32.1%) |
| Race (n, %) | |||||
| Caucasian | 5 (83.3%) | 6 (100%) | 21 (80.8%) | 5 (83.3%) | 22 (78.6%) |
| Black | 1 (16.7%) | 0 | 3 (11.5%) | 0 | 5 (17.9%) |
| Asian | 0 | 0 | 0 | 1 (16.7%) | 0 |
| Other | 0 | 0 | 2 (7.7%) | 0 | 1 (3.6%) |
| Height (mean ± SD), cm | 173.33 ± 11.553 | 173.83 ± 4.119 | 171.54 ± 9.318 | 171.83 ± 8.232 | 172.21 ± 9.681 |
| Weight (mean ± SD), kg | 88.97 ± 10.191 | 90.17 ± 10.926 | 87.52 ± 14.724 | 81.62 ± 20.786 | 89.10 ± 17.896 |
| BMI (mean ± SD), kg/m2 | 29.64 ± 2.485 | 29.96 ± 4.441 | 29.63 ± 3.567 | 27.30 ± 4.525 | 29.82 ± 3.870 |
| Cardiovascular disease (n, %) | 1 (17%) | 1 (17%) | 9 (35%) | 4 (67%) | 11 (39%) |
| Diabetes (n, %) | 4 (67%) | 5 (83%) | 13 (50%) | 5 (50%) | 16 (57%) |
| Hypertension (n, %) | 5 (83%) | 2 (33%) | 16 (62%) | 4 (67%) | 20 (71%) |
| Hyperlipidemia (n, %) | 4 (67%) | 6 (100%) | 13 (50%) | 3 (50%) | 12 (43%) |
| HDL-C (mean ± SD), mg/dl | 47.83 ± 17.43 | 40.33 ± 8.94 | 43.69 ± 12.72 | 48.5 ± 13.78 | 44.61 ± 10.24 |
| ApoA-I (mean ± SD), mg/dl | 147.00 ± 34.32 | 109.17 ± 26.67 | 126.77 ± 20.58 | 142.33 ± 23.65 | 127.68 ± 23.29 |
| HII (mean, range) | 1.64 (1.37–1.88) | 1.86 (1.19–2.49) | 1.67 (1.23–2.28) | 1.57 (1.30–1.75) | 1.67 (1.13–2.30) |
| PON (geometric mean, range), U/ml | 95.0 (55.8–229.4) | 92.9 (59.9–156.2) | 97.3 (35.1–276.0) | 79.8 (35.1–184.0) | 112.6 (44.4–338.0) |
| hs-CRP (geometric mean, range), mg/L | 3.2 (1.1–16.8) | 2.4 (0.8–21.0) | 2.0 (0.1–47.8) | 1.8 (0.3–10.0) | 2.1 (0.3–16.9) |
| IL-6 (geometric mean, range), pg/ml | 2.45 (1.75–2.92 | 2.48 (1.10–12.52) | 1.98 (0.63–6.00) | 2.01 (1.78–2.30) | 1.83 (0.58–6.39) |
apoA-I, apolipoprotein A-I; BMI, body mass index; hs-CRP, high-sensitivity C-reactive protein; HDL-C, HDL-cholesterol; IL-6, interleukin-6; IV, intravenous; PON, paraoxonase.
TABLE 3.
Baseline demography of SC study (APL810A2210B) by dose group
| APL180 | |||
|---|---|---|---|
| Demographic variables | 10 mgN = 36 | 30 mgN = 37 | PlaceboN = 35 |
| Age (mean ± SD), years | 59.2 ± 7.35 | 60.8 ± 8.11 | 61.3 ± 7.53 |
| Sex, male (n, %) | 26 (72.2%) | 27 (73.0%) | 25 (71.4%) |
| Sex, female (n, %) | 10 (27.8%) | 10 (27.0%) | 10 (28.6%) |
| Race (n, %) | |||
| Caucasian | 31 (86.1%) | 35 (94.6%) | 32 (91.4%) |
| Black | 5 (13.9%) | 1 (2.7%) | 1 (2.9%) |
| Asian | 0 | 1 (2.7%) | 1 (2.9%) |
| Pacific Islander | 0 | 0 | 1 (2.9%) |
| Height (mean ± SD), cm | 89.4 ± 14.7 | 86.3 ± 16.0 | 89.1 ± 13.7 |
| Weight (mean ± SD), kg | 172.0 ± 8.7 | 173.3 ± 10.2 | 171.9 ± 10.3 |
| BMI (mean ± SD), kg/m | 30.1 ± 3.5 | 28.6 ± 3.4 | 30.0 ± 2.8 |
| Cardiovascular disease (n, %) | 18 (50%) | 15 (41%) | 16 (46%) |
| Diabetes (n, %) | 29 (81%) | 27 (83%) | 26 (74%) |
| Hypertension (n, %) | 28 (78%) | 25 (68%) | 27 (77%) |
| Hyperlipidemia (n, %) | 33 (92%) | 36 (97%) | 35 (100%) |
| HDL-C (mean ± SD), mg/dl | 39.28 ± 10.94 | 42.85 ± 8.41 | 46.55 ± 13.87 |
| ApoA-I (mean ± SD), mg/dl | 114.31 ± 26.28 | 124.97 ± 21.01 | 129.10 ± 24.10 |
| HII (mean, range) | 1.71 (1.2–2.5) | 1.55 (1.0–2.4) | 1.67 (1.0–2.1) |
| PON (geometric mean, range), U/ml | 189.3 (30.8–492.1) | 142.0 (27.1–395.3) | 173.0 (31.3–559.8) |
| hs-CRP (geometric mean, range), mg/l | 4.7 (0.3–32.7) | 2.2 (0.1–10.9) | 3.1 (0.1–16.6) |
| IL-6 (geometric mean, range), pg/ml | 2.66 (0.9–9.3) | 2.27 (0.9–5.8) | 2.14 (0.7–6.4) |
SC, subcutaneous.
Safety and tolerability
L-4F was well tolerated when administered IV for seven daily doses over the 3–100 mg dose range. Of the 72 patients enrolled in the IV study, 70 patients completed the study. There were no deaths reported and no discontinuations for AEs; two patients withdrew the consent and were discontinued. One subject who completed the study suffered an SAE (vertebro-basilar cerebrovascular accident) 3 weeks after completion of dosing (7 daily doses of 100 mg L-4F). This event was deemed to be unrelated to the study drug by the investigator. Most of the AEs reported in the study were mild and were similar in frequency between L-4F- and placebo-treated subjects (Table 4). A total of 23/44 (52%) L-4F-treated subjects reported at least one AE compared with 17/28 (61%) of placebo-treated subjects. In L-4F-treated subjects, the most frequent AEs reported were general disorders and administration site conditions (24%, compared with 14% in placebo), nervous system disorders (20%, compared with 25% in placebo) and musculoskeletal and connective tissue disorders (18%, compared with 29% in placebo). With the exception of more-frequent ISRs in subjects treated with 100 mg L-4F (67%) (Table 4; see supplementary Table I), AE frequency was similar across all dose groups.
TABLE 4.
Summary of adverse events by body system in IV and SC studies
| IV Study (APL180A2201) | SC Study (APL180A2210B) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Body System | L-4F3 mg(N = 6) | L-4F10 mg(N = 6) | L-4F30 mg(N = 26) | L-4F100 mg(N = 6) | All L-4Fsubjects(N = 44) | Placebo(N = 28) | L-4F10 mg(N = 36) | L-4F30 mg(N = 37) | All L-4Fsubjects(N = 73) | Placebo(N = 35) |
| Any body System | 3 (50.0) | 2 (33.3) | 13 (50.0) | 5 (83.3) | 23 (52.3) | 17 (60.7) | 24 (66.7) | 29 (78.4) | 53 (72.6) | 17 (48.6) |
| Blood and lymphatic system disorders | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.8) | 0 | 1 (1.4) | 0 |
| Cardiac disorders | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.8) | 1 (2.7) | 2 (2.7) | 1 (2.9) |
| Ear and labyrinth disorders | 1 (16.7) | 0 | 0 | 0 | 1 (2.3) | 0 | 1 (2.8) | 0 | 1 (1.4) | 0 |
| Eye disorders | 0 | 0 | 1 (3.8) | 0 | 1 (2.3) | 0 | 0 | 1 (2.7) | 1 (1.4) | 0 |
| Gastrointestinal disorders | 0 | 0 | 4 (15.4) | 2 (33.3) | 6 (13.6) | 3 (10.7) | 7 (19.4) | 5 (13.5) | 12 (16.4) | 4 (11.4) |
| General disorders and administration site conditions* | 2 (33.3) | 0 | 4 (15.4) | 4 (66.7) | 10 (22.7) | 4 (14.3) | 14 (38.9) | 17 (45.9) | 31 (42.5) | 6 (17.1) |
| Infections and infestations | 1 (16.7) | 0 | 2 (7.7) | 0 | 3 (6.8) | 0 | 6 (16.7) | 4 (10.8) | 10 (13.7) | 2 (5.7) |
| Injury, poisoning, and procedural complications | 0 | 0 | 1 (3.8) | 2 (33.3) | 3 (6.8) | 4 (14.3) | 0 | 3 (8.1) | 3 (4.1) | 2 (5.7) |
| Investigations (abnormal lab values) | 1 (16.7) | 0 | 0 | 0 | 1 (2.3) | 0 | 1 (2.8) | 1 (2.7) | 2 (2.7) | 5 (14.3) |
| Metabolism and nutrition disorders | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.8) | 0 | 1 (1.4) | 1 (2.9) |
| Musculoskeletal and connective tissue disorders | 1 (16.7) | 2 (33.3) | 3 (11.5) | 2 (33.3) | 8 (18.2) | 8 (28.6) | 4 (11.1) | 4 (10.8) | 8 (11.0) | 2 (5.7) |
| Nervous system disorders | 2 (33.3) | 1 (16.7) | 5 (19.2) | 1 (16.7) | 9 (20.5) | 7 (25.0) | 3 (8.3) | 5 (13.5) | 8 (11.0) | 4 (11.4) |
| Psychiatric disorders | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.9) |
| Renal and urinary disorders | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (8.1) | 3 (4.1) | 1 (2.9) |
| Respiratory, thoracic and mediastinal disorders | 0 | 0 | 0 | 0 | 0 | 0 | 3 (8.3) | 1 (2.7) | 4 (5.5) | 0 |
| Skin and subcutaneous tissue disorders | 0 | 1 (16.7) | 1 (3.8) | 2 (33.3) | 4 (9.1) | 3 (10.7) | 5 (13.9) | 5 (13.5) | 10 (13.7) | 3 (8.6) |
| Vascular disorders | 1 (16.7) | 1 (16.7) | 2 (7.7) | 0 | 4 (9.1) | 0 | 3 (8.3) | 2 (5.4) | 5 (6.8) | 0 |
*In the IV study, the most common infusion site reaction was pain at the site of infusion (9% in APL180 treated compared with 7% in placebo. In the SC study, the most common injection site reactions among APL180 treated subjects were itching (71%) and redness (70%), while in placebo, hemorrhage (23%), and itching (20%) were most commonly reported.
L-4F was well tolerated when administered SC for 28 daily doses (10 and 30 mg). Of the 108 subjects enrolled in the SC study, 104 completed the study. There were no deaths reported. One subject who received 10 mg L-4F was discontinued for an SAE (abdominal pain), and three subjects withdrew consent (all L-4F-treated). Two SAEs were reported during the study. One subject who received 10 mg L-4F was admitted to the hospital for severe abdominal pain, and one placebo-treated subject experienced symptomatic sinus tachycardia 3 days after receiving the final dose of study medication. Neither SAE was suspected by the investigator to be related to the study medication. A total of 53/73 (73%) L-4F-treated subjects reported at least one AE, compared with 17/35 (49%) of placebo-treated subjects. In L-4F-treated subjects, the most frequently reported AEs were general disorders and administration site conditions (42%, compared with 17% in placebo) and gastrointestinal disorders (16%, compared with 11% in placebo). ISRs increased in frequency and severity score with increasing dose (Table 4; see supplementary Table II). ISRs were reported by 97%, 81%, and 46% of 30 mg, 10 mg, and placebo-treated subjects, respectively, whereas moderate-to-severe ISRs were reported by 43%/14% (moderate/severe), 27%/3%, and 6%/0% of 30 mg, 10 mg, and placebo-treated subjects, respectively. Two patients receiving 30 mg daily SC withdrew consent after experiencing ISRs but did not cite the ISRs as the reason for withdrawal. Among the remaining participants, there was no discontinuation for ISRs in the 108 participants in the SC study.
With the exception of a few minor and transient abnormal clinical laboratory parameters, no other notable changes in blood chemistry, hematology, urinalysis, ECGs, or vital signs were observed, compared with placebo in either study. No evidence of immunogenicity against the peptide was obtained after 28 days of SC treatment (data not shown).
Pharmacokinetics
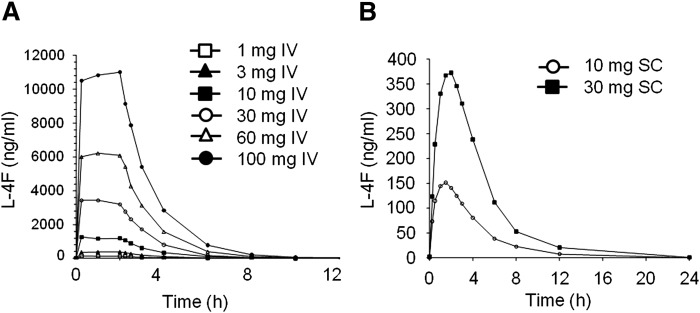
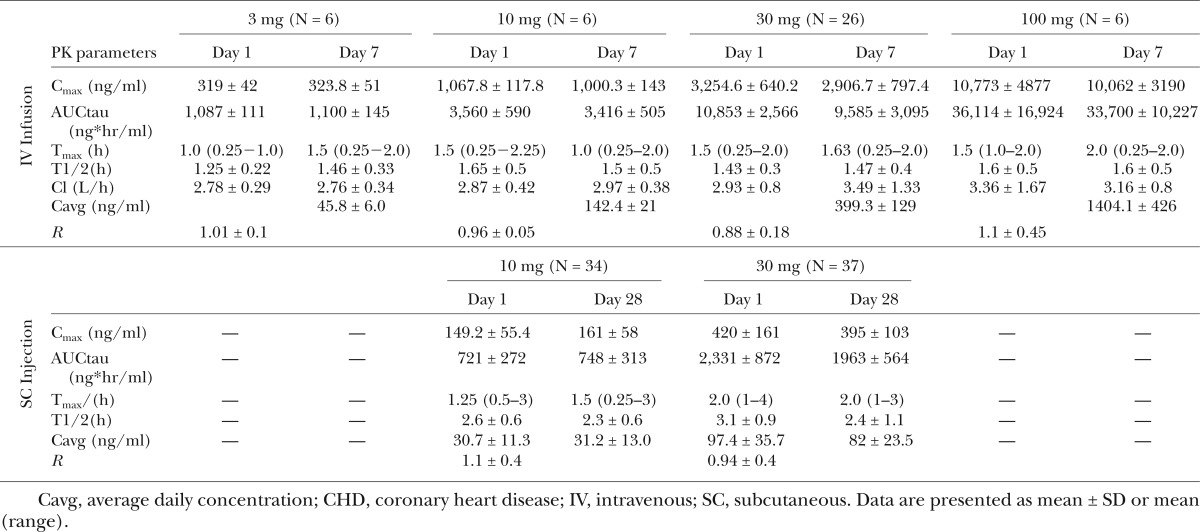
Following IV infusion of L-4F, both Cmax and AUC increased in a dose-proportional manner over the 3–100 mg dose range (Fig. 3A). Cmax, AUC, and Cavg levels ranged from 300–10,000 ng/ml, 1,100–22,700 ng*hr/ml, and 45.8–1,404 ng/ml, respectively. All PK parameters were similar on day 1 and day 7, and no accumulation of L-4F was observed following multiple IV dosing. L-4F is eliminated with a terminal half-life of approximately 1.5 h (Table 5), supporting the lack of accumulation with multiple dosing.
Fig. 3.
Pharmacokinetics of L-4F following IV and SC administration. The plasma levels of L-4F increased proportional to dose during the 2 h IV infusion of 3–100 mg (A) and following the SC injection of 10 or 30 mg (B). The values shown are the average for the subjects described in Tables 2 and 3.
TABLE 5.
Multiple ascending dose administration via IV and SC routes of administration in CHD patients
Following SC injection, L-4F was absorbed, rapidly reaching peak concentrations with in 1.5–2.0 h. Both Cmax and AUC increased in a dose-proportional manner between the 10 and 30 mg dose (Fig. 3B). Cmax, AUC, and Cavg levels ranged from ∼150–420 ng/ml, 700–2,500 ng/hr/ml, and 30–97 ng/ml, respectively. All PK parameters were similar on day 1 and day 28, and no accumulation of L-4F was observed following multiple SC dosing. L-4F was eliminated with a terminal half-life of ∼2.5–3.0 h (Table 5) supporting lack of accumulation with multiple dosing.
Biomarkers
Plasma lipid and lipoprotein levels.
There were no notable changes in plasma total cholesterol, LDL-cholesterol, HDL-cholesterol, or triglyceride values between subjects treated with placebo or L-4F in either study (data not shown). There was a trend toward lower levels of apoA-I (∼8% compared with 3% for placebo) and apoA-II (∼9% compared with 4% for placebo) following single and multiple IV doses of 30 mg APL180. These differences reached statistical significance compared with placebo at 2 h postdose on both day 1 and day 7 and 24 h postdose on day 7 (data not shown).
HII
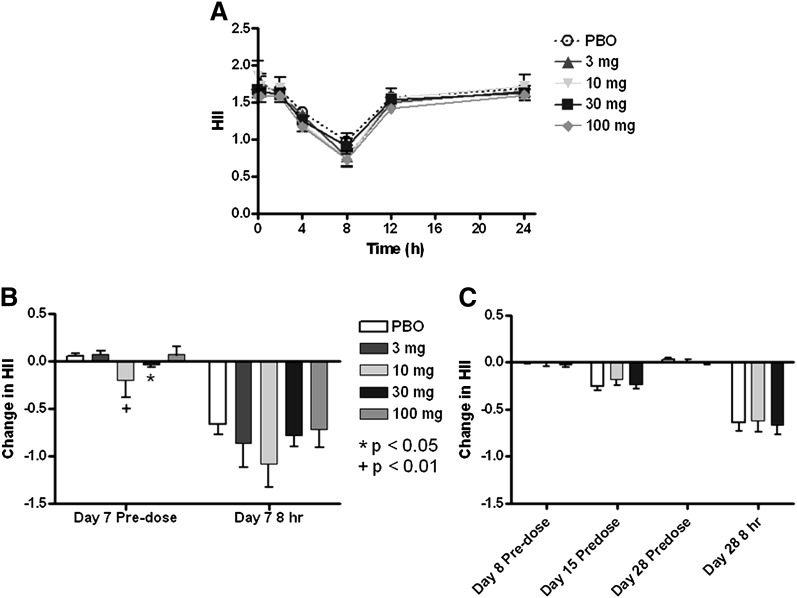
In these studies, each time point was compared with its placebo control. A single IV infusion of L-4F did not significantly improve HII compared with placebo (Fig. 4A), despite achieving plasma levels of L-4F that reached >10,000 ng/ml. At trough, following six consecutive daily IV doses of 10 or 30 mg L-4F, the predose level of HII was significantly reduced compared with placebo (Fig. 4B; P = 0.004 and 0.037, respectively) and tended to be lower than placebo throughout the day, following dose 7, although it was not statistically different from placebo at 8 h postdose. During daily SC treatment with L-4F at 10 mg/day or 30 mg/day, there was no significant reduction in HII predose (trough) on day 8, 15, or 28, compared with placebo (Fig. 4C). In addition, at 8 h postdose on day 28, HII was not reduced, compared with placebo, despite achieving plasma levels of approximately 150 ng/ml and approximately 400 ng/ml for the 10 mg/day and 30 mg/day doses, respectively (Table 5).
Fig. 4.
HII following IV and SC administration of L-4F. HII decreased over 8 h following a single IV infusion of L-4F and placebo (A). Although a few statistically significant decreases compared with placebo were observed in the HII following six or more IV doses (B), the reductions were minimal (≤0.1), and no dose response was evident. No decrease in HII relative to day 1 predose was observed following multiple SC injections of L-4F (C). The data shown are mean ± SEM. PBO, placebo.
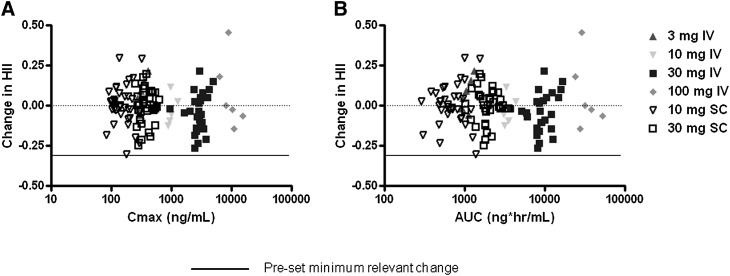
Although there were a few statistically significant decreases observed in the HII, the reductions were minimal (≤0.1), compared with the expected minimum change of 0.3, based on previously reported results with D-4F (5), and there was no dose–response or exposure–response (Cmax or AUC) relationship discernible (Fig. 5). This lack of decrease in HII was despite achieving Cmax levels over the range of 300–10,000 ng/ml.
Fig. 5.
Scatter plot of exposure on change in HII. Change in HII from predose day 1 to predose day 7 (IV) or day 28 (SC) for each subject (y axis) was plotted as a function of L-4F exposure achieved (x axis) as measured by maximal plasma concentration (Cmax) (A) or area under the curve (AUC) (B). No dose–response or exposure–response relationship was observed.
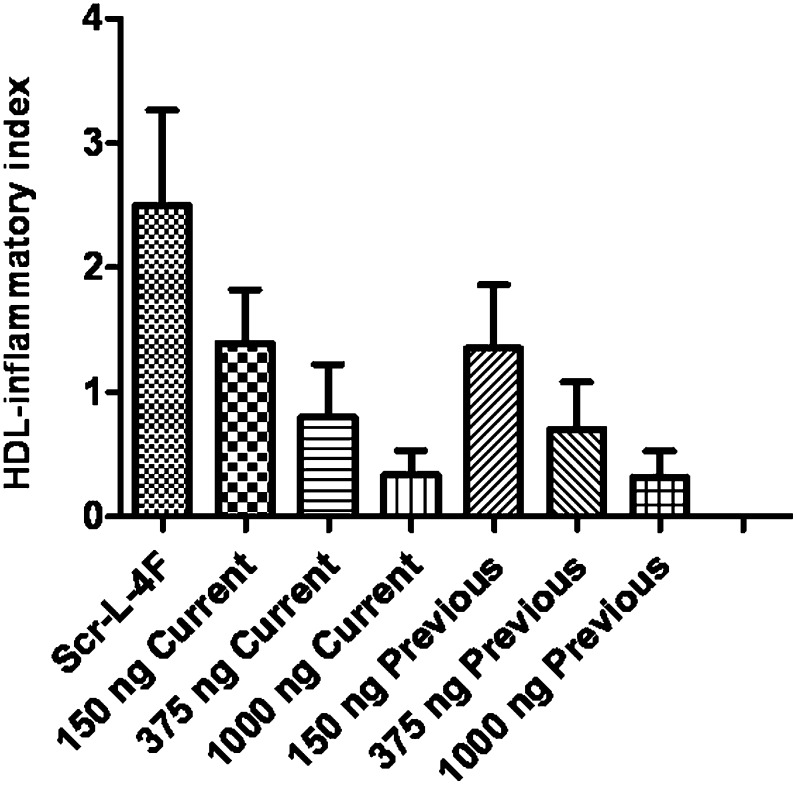
We previously reported that addition of 250 to 1,000 ng/ml of peptide in vitro to human plasma, followed by isolation of the HDL, resulted in significant improvement in HII (13, 14). After the results of the IV studies were available (but before the outcome of the SC study was known) the potency of the current batch of L-4F peptide was compared with a previously studied batch (13, 14) using in vitro experiments. When the peptides were added ex vivo to patient plasma, the results (Fig. 6) indicate that i) there was no difference in this assay between the two sources of peptides, and ii) there was a dose-dependent improvement in HII after addition in vitro of either peptide to plasma from randomly chosen subjects in the SC study at peptide concentrations that were ineffective in vivo.
Fig. 6.
Ex vivo incubation of L-4F to plasma from CHD subjects. Predose plasma samples from day 2 of the SC study were randomly chosen from 20 subjects in the study. Each plasma sample was incubated in vitro for 15 min at 37°C with 1,000 ng/ml of scrambled L-4F (Scr-L-4F) or 150 ng/ml, 375 ng/ml or 1,000 ng/ml of the L-4F peptide that was used in the clinical studies described here (Current) or L-4F peptide that had been used in previously published preclinical studies (13, 14; Previous). The plasma samples were then separated by fast-protein liquid chromatography (FPLC), and the HDL fractions were added to cultures of human aortic endothelial cells to determine the HII, as described in Materials and Methods. The data shown are mean ± SD. There was no difference in HII between the two sources of peptides. A similar dose-dependent improvement (decrease) in HII was observed after ex vivo addition of both peptides.
PON activity
There was no significant change in PON activity compared with placebo following single or multiple doses administered via either IV or SC (data not shown).
hs-CRP
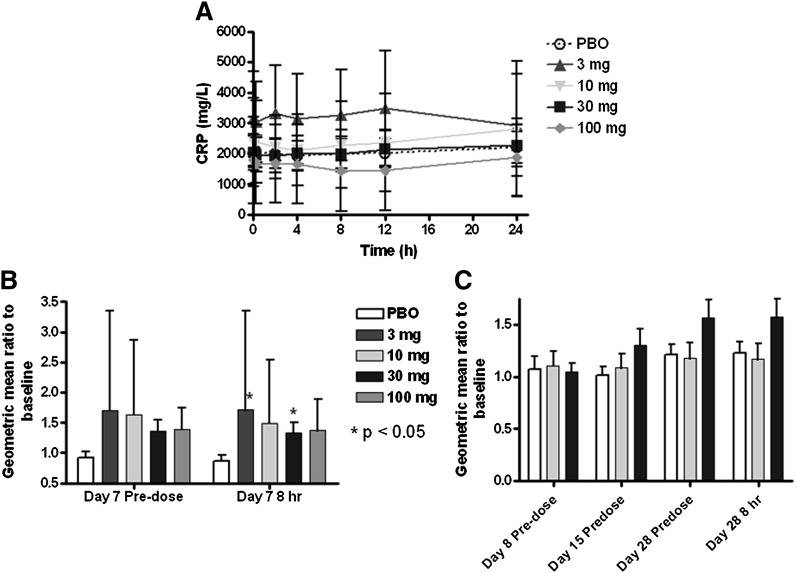
A single IV infusion of L-4F did not alter hs-CRP levels (Fig. 7A). Unexpectedly, after six daily IV infusions of L-4F, a trend for increase in hs-CRP was observed for all doses, although these did not reach statistical significance (P < 0.1, but >0.05; Fig. 7B). The increase was maintained following the seventh dose, with 8 h and 24 h postdose on day 7 being statistically higher for the 30 mg group, compared with placebo (ratio 1.34 and 1.51, P = 0.047 and 0.038, respectively). Despite the small number of subjects in the other L-4F dose groups, a similar trend for an increase in hs-CRP on day 7 was observed for each dose group (Fig. 7B and data not shown), although only the 3 mg (8 h postdose) reached statistical significance, compared with placebo. A similar but nonsignificant trend toward an increase in hs-CRP levels was observed after administration of 30 mg daily SC of L-4F starting on day 15 (Fig. 7C). The 10 mg of L-4F given SC for 28 days had no effect on hs-CRP levels.
Fig. 7.
High-sensitivity C-reactive protein (hs-CRP) following IV and SC administration of L-4F. A single IV infusion of L-4F did not alter hs-CRP levels (A). A trend for increase in hs-CRP was observed for all doses at trough on day 7 (B), although these did not reach statistical significance (P < 0.1, but > 0.05). The increases were statistically higher compared with placebo for the IV 3 and 30 mg groups compared with placebo at 8 h postdose on day 7 (B). A similar, but nonsignificant trend toward an increase in hs-CRP levels was observed after daily SC administration of 30 mg (but not 10 mg) of L-4F (C). Geometric mean ± SEM are given in A, and geometric mean ratio ± SEM are given in B and C. PBO, placebo.
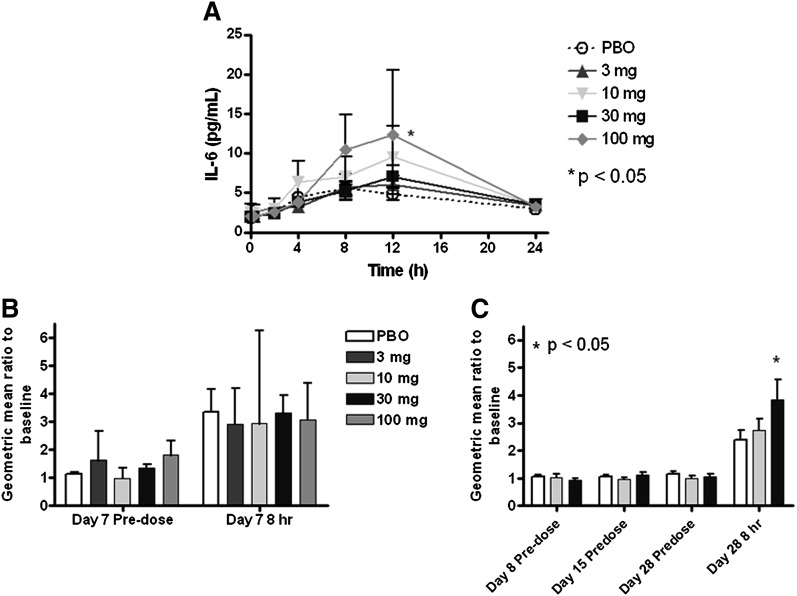
IL-6 levels
An increase in IL-6 during the day was observed following single and multiple IV doses of L-4F and placebo (Fig. 8A, and data not shown), but only reached significance for the 100 mg dose at 12 h postdose on day 1 (Fig. 8A) and the 3 mg dose at 2 h postdose on day 7 (data not shown). There was a trend for the increases to be larger in the L-4F-treatment groups, but the trend did not increase with dose.
Fig. 8.
Interleukin-6 (IL-6) following IV and SC administration of L-4F. A postdose increase in IL-6 was observed following single IV doses of both L-4F and placebo (A), but only reached statistical significance for the 100 mg dose at 12 h postdose. No change in IL-6 compared with placebo was observed at any dose level prior to (predose) or 8 h after the seventh IV dose (B). There were no predose increases in IL-6 levels with either 10 or 30 mg L-4F at any time during the SC study compared with placebo (C). However, after dosing on day 28 in the SC study, there was a significant increase in IL-6 levels 8 h after the 30 mg dose of L-4F, compared with placebo. Geometric mean ± SEM are given in A, and geometric mean ratio ± SEM are given in B and C. PBO, placebo.
There were no predose increases in IL-6 levels at any time during the SC study, compared with placebo with either the 10 or 30 mg L-4F dose (Fig. 8C). However, after dosing on day 28 in the SC study there was a significant increase in IL-6 levels 8 h after the 30 mg dose of L-4F compared with placebo (P = 0.021).
DISCUSSION
L-4F was tested for safety/tolerability and evidence of favorable effects on HDL properties and systemic inflammatory PD biomarkers in two studies in patients with CHD or CHD equivalents. Seven daily IV infusion and 28 daily SC injections of L-4F were found to be well tolerated in this patient population. There were no drug-related SAEs, and with the exception of ISRs, which were more frequent with L-4F SC treatment, AE frequency was similar to placebo. No notable changes in vital signs or laboratory parameters were observed. Dose-proportional increase in L-4F exposure was observed with both IV and SC administration. L-4F was eliminated rapidly, with a terminal half-life of ∼1.5 h and 2.5 h when administered via IV infusion and SC injection, respectively. Given the short half-life, no accumulation of L-4F was observed following multiple-dose administration via IV or SC routes. No improvement in biomarkers of HDL anti-inflammatory functions (HII and PON) was detected after a single or multiple doses. A trend toward an increase in inflammatory markers (hs-CRP and IL-6) was noted following multiple doses of L-4F.
The two studies here confirm previous studies (5, 10) indicating that patients with CHD or CHD equivalents have elevated HII values even when on a stable dose of a statin, compared with HII values that have been previously reported in healthy normal volunteers (10, 14).
Despite achieving plasma levels of L-4F in vivo that greatly exceeded those achieved in animal models (8, 9) and humans administered D-4F (5), there was no notable improvement in HII compared with placebo in these studies. Although there was a small change in the HII after six daily IV infusions of 30 mg L-4F, which achieved mean plasma Cmax levels of 2,907 ng/ml (Fig. 4), no trend in exposure/response was observed (Fig. 5). In the studies reported here, there was a time-dependent decrease in HII in the placebo group at 4 and 8 h after the start of the IV infusion (Fig. 4A, B) and 8 h after SC administration of peptide on day 28 (Fig. 4C). In the previous study of oral D-4F (5), there was also a decrease in HII after placebo administration. In the studies of Bloedon et al. (5), administration of an oral dose of D-4F of 30 or 100 mg did not reduce HII more than placebo, but administering a dose of 300 mg or 500 mg did (5). The protocol in the studies reported here and those previously reported for D-4F (5) required food and medicines to be withheld until 2 h after administration of either the placebo or the peptide. The patients in the studies reported here and those previously reported for D-4F (5) were all required to take a statin, which has previously been shown to reduce HII (10). Additionally, almost all also took other medications, including aspirin, angiotensin-converting enzyme (ACE) inhibitors, and β blockers. Thus, the “placebo” patients in these studies probably took medications during the morning of the study that are known (statins) or are likely to reduce HII (e.g., ACE inhibitors). In contrast to the failure of L-4F to produce a significant reduction in HII in vivo compared with placebo, adding L-4F to the plasma of these subjects ex vivo, at concentrations similar to those achieved in vivo (i.e., 150 ng/ml, 375 ng/ml, or 1,000 ng/ml) dose dependently improved HII utilizing identical protocols to determine HII (Fig. 6).
The HII assay is a cell-based assay, where an HII of <1.0 is considered to represent an anti-inflammatory effect (inhibition of LDL-induced, MCP-1-mediated monocyte migration), whereas an HII >1.0 is considered pro-inflammatory (increased migration) (10). When administered with a statin, both the D-4F and L-4F peptides cause atherosclerotic lesion regression in mouse models (9, 11). In cholesterol-fed rabbits, administration by SC injection of either D-4F or L-4F resulted in significant lesion inhibition that was correlated with the HII values. Although the HII was significantly correlated with lesion area and serum amyloid-A (SAA) levels, it did not correlate with HDL-cholesterol levels in cholesterol-fed rabbits (12). This suggested that in this particular animal model, the anti-inflammatory properties of HDL were physiologically distinct from the plasma cholesterol levels. In this rabbit study (12), there was no difference in efficacy between D-4F and L-4F when administered by SC injection.
One could speculate that since the mechanism of action of L-4F appears to be related to its remarkable ability to bind oxidized lipids (6) compared with apoA-I, the apparent differences observed between in vivo and ex vivo activities may be explained by the concentration of such lipids in each situation. In the case of the ex vivo experiment, the number of oxidized lipids present on HDL is fixed, with a limited supply in the plasma to replenish those sequestered by L-4F. In this case, 150 ng/ml of L-4F is sufficient to remove/sequester most of the oxidized lipids that affect HII from the plasma samples. However, in vivo, L-4F may quickly be saturated with oxidized lipids, and in the physiologic state, the sequestered plasma lipids can easily and rapidly be replenished from tissue stores of oxidized lipids. This theory is similar to that proposed by Tsimikas et al. (17) to explain the increase in number of oxidized phospholipids (OxPL) per apoB (OxPL/apoB) and the increase in circulating lipoprotein [a], previously shown to bind OxPL, following 16 weeks of treatment with atorvastatin 80 mg/day. This rapid efflux of pro-inflammatory oxidized lipids from the tissues could also be a potential explanation for the increase in hs-CRP. Future studies should determine the saturation levels of oxidized lipids for the 4F peptide and determine whether adding the peptide saturated with these lipids to cells such as hepatocytes or macrophages in vitro will stimulate the production of IL-6. Such studies could lend strong support for the argument regarding the saturation of the peptide with lipid in vivo in the studies reported here. Although the possibilities described above focus on “oxidized lipids,” it is also possible that in vivo, something other than an oxidized lipid may have interacted with the peptide noncovalently and inactivated the peptide's anti-inflammatory action. Such a noncovalent interaction would not affect measurement of the plasma levels of the peptide but could affect the activity.
Another possibility for explaining the failure to favorably alter HII in the studies reported here compared with previous studies (5, 7–9, 11) is the fact that the previous studies used D-4F, which is resistant to degradation by mammalian tissues. However, the determination of plasma concentrations of L-4F in the current studies was by LC-MS/MS, indicating that the l-peptide used in these studies was indeed intact at the time of plasma sampling.
The total dose administered and the modes of administration are other differences between the current and the previous study in humans (5). Although the current studies clearly reached plasma concentrations higher than those achieved with D-4F (Cmax = 149 to 10,000 ng/ml compared with ∼10 ng/ml, respectively), the actual dose given was considerably lower in the current studies and the mode of administration distinctly different (IV or SC doses of 3 to 100 mg L-4F compared with oral doses of 300 and 500 mg D-4F). One could postulate that in vivo, in humans, it is not the plasma level that is physiologically relevant but rather the dose that is in contact with the tissues. Perhaps, the oral doses that produced low plasma levels of peptide (5) were successful not because of the plasma levels achieved, but because of high doses of peptide that interacted with the epithelium of the alimentary tract and removed oxidized lipids by this contact. An additional possibility is that perhaps oral administration of peptide removes oxidized lipids in the intestine during the process of enterohepatic recirculation of bile. These hypotheses will need to be tested in future studies.
Despite initial studies in both animals and humans that have demonstrated that 4F is anti-inflammatory, the two studies reported here suggest evidence of a pro-inflammatory effect of L-4F. Why did the administration of an apoA-I mimetic peptide in the current studies significantly increase hs-CRP levels in the multiple-dose IV study (Fig. 7B)? Endotoxin contamination of the peptide that was administered in these studies can be ruled out for multiple reasons: 1) the peptide was synthesized under Good Manufacturing Practices, subjected to vigorous testing in accordance with health authority regulations, and was found to be free of endotoxin; 2) there was no increase in hs-CRP after a single IV infusion (Fig. 7A), and there was no further increase post infusion on day 7 in the multiple-dose IV study; 3) the increase in hs-CRP did not increase with an increasing dose of L-4F (Fig. 7B); and 4) finally, there was no difference in MCP-1 production by human endothelial cell cultures after ex vivo exposure to the peptide used in the current studies (Fig. 6) and the peptide used in previous studies, in which SAA levels in rabbits were decreased by L-4F administration (12). Thus, the explanation for the increase in hs-CRP values after multiple doses of L-4F compared with placebo in the IV study (Fig. 7B) is probably not that it is due to endotoxin.
The trend toward an increase in hs-CRP levels after 28 days of SC administration (Fig. 7C) and the significant increase in IL-6 levels 8 h after injection of 30 mg SC of L-4F on day 28 (Fig. 8C) may have been due to the high incidence of ISRs (97%) in this group (Table 4) but also may be due to the saturation of the peptide with pro-inflammatory lipids in vivo, which stimulated an acute-phase response. It should also be kept in mind that although the increase in hs-CRP in the multiple-dose IV study (Fig. 7B) occurred in all L-4F dose groups, it was not dose dependent. Thus, further studies will be required to determine if this finding is truly related to L-4F.
The implications for the future development of the 4F peptides from the studies reported here include the possibility that much higher doses of the peptide will be needed. If that is the case, because of the limitations on the concentration and volume of peptide that can be injected into humans subcutaneously, it is not likely that the 4F peptides will be administered to humans subcutaneously. If much higher doses of peptide will be required in vivo in humans, the cost of producing the peptide may limit the utility of these peptides as clinical therapeutic agents in diseases for which lower-cost treatments are currently effective. A recent publication from our laboratory (18) demonstrates that the 4F peptide binds lysophosphatidic acid (which induces migration and invasion of both mouse and human epithelial ovarian cancer cells) with extraordinary affinity (Kd = 0.000523 ± 0.0015 nM). It was demonstrated that administering L-4F orally (added to mouse chow at a dose of 100 mg/kg/day) to mice with a normal immune system that had been injected with mouse ovarian epithelial cancer cells significantly reduced both plasma levels of lysophosphatidic acid and tumor burden (18). Assuming that high doses of the 4F peptides will be required, assuming that the cost of production of such peptides will be high, and even assuming that hs-CRP levels would be elevated when the peptide was administered at high doses orally (which seems less likely), the use of these peptides in the treatment of diseases with an unmet clinical need such as ovarian cancer could still be attractive.
In summary, at the doses used in these studies, there was no improvement in selected markers of HDL anti-inflammatory function (HII, PON) or systemic inflammatory markers (hs-CRP, IL-6) following administration of L-4F to humans with CHD or CHD equivalents. The results of these clinical trials provide the basis for a number of hypotheses that will need to be tested in future studies. Among the issues that need to be addressed in future studies to determine if L-4F will be efficacious in cardiovascular disease is the determination as to whether L-4F will increase hs-CRP in humans when administered orally. Additionally, it will need to be determined whether the HII assay is a suitable biomarker for assessing peptide treatment and whether other functional assays such as cholesterol efflux may be better.
Acknowledgments
The authors are very grateful to the subjects who participated in these clinical trials.
Footnotes
Abbreviations:
- ACE
- angiotensin-converting enzyme
- AE
- adverse event
- apoA-I
- apolipoprotein A-I
- CHD
- coronary heart disease
- Cavg
- average daily concentration
- Cmax
- maximal plasma concentration
- ECG
- electrocardiogram
- FPLC
- fast-protein liquid chromatography
- HII
- HDL-inflammatory index
- hs-CRP
- high-sensitivity C-reactive protein
- ISR
- injection site reaction
- IV
- intravenous
- MCP-1
- monocyte chemoattractant protein-1
- OxPL
- oxidized phospholipids
- PD
- pharmacodynamic
- PK
- pharmacokinetic
- PON
- paraoxonase
- SAA
- serum amyloid-A
- SAE
- serious adverse event
- SC
- subcutaneous
- SWFI
- sterile water for injection
This work was supported in part by United States Public Health Service Grant HL-30568. A. M. Fogelman, S. T. Reddy, and M. Navab are principals in Bruin Pharma, and A. M. Fogelman is an officer in Bruin Pharma. C. E. Watson, N. Weissbach, L. Kjems, S. Ayalasomayajula, Y. Zhang, I. Chang, and A. Schecter are employees of Novartis Institutes for Biomedical Research
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two tables.
REFERENCES
- 1.Gordon T., Castelli W. P., Hjortland M. C., Kannel W. B., Dawber T. R. 1977. High density lipoprotein as a protective factor against coronary heart disease. Am. J. Med. 62: 707–714. [DOI] [PubMed] [Google Scholar]
- 2.Navab M., Reddy S. T., Van Lenten B. J., Anantharamaiah G. M., Fogelman A. M. 2009. The role of dysfunctional HDL in atherosclerosis. J. Lipid Res. 50 (Suppl.): 145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navab M., Anantharamaiah G. M., Reddy S. T., Van Lenten B. J., Fogelman A. M. 2009. HDL as a biomarker, potential therapeutic target and therapy. Diabetes. 58: 2711–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navab M., Shechter I., Anantharamaiah G. M., Reddy S. T., Van Lenten B. J., Fogelman A. M. 2010. Structure and function of HDL mimetics. Arterioscler. Thromb. Vasc. Biol. 30: 164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloedon L. T., Dunbar R., Duffy D., Pinell-Salles P., Norris R., DeGroot B. J., Movva R., Navab M., Fogelman A. M., Rader D. J. 2008. Safety, pharmacokinetics and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J. Lipid Res. 49: 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Lenten B. J., Wagner A. C., Jung C. L., Ruchala P., Waring A. J., Lehrer R. I., Watson A. D., Hama S., Navab M., Anantharamaiah G. M., et al. 2008. Anti-inflammatory apoA-I mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J. Lipid Res. 49: 2302–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navab M., Anantharamaiah G. M., Hama S., Garber D. W., Chaddha M., Hough G., Lallone R., Fogelman A. M. 2002. Oral administration of an apo A-I mimetic peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation. 105: 290–292. [DOI] [PubMed] [Google Scholar]
- 8.Navab M., Anantharamaiah G. M., Reddy S. T., Hama S., Hough G., Grijalva V. R., Wagner A. C., Frank J. S., Datta G., Garber D., et al. 2004. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation. 109: 3215–3220. [DOI] [PubMed] [Google Scholar]
- 9.Navab M., Ruchala P., Waring A.J., Lehrer R. I., Hama S., Hough G., Palgunachari M. N., Anantharamaiah G. M., Fogelman A. M. 2009. A novel method for oral delivery of apolipoprotein mimetic peptides synthesized from all L-amino acids. J. Lipid Res. 50: 1538–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ansell B. J., Navab M., Hama S., Kamranpour N., Fonarow G., Hough G., Rahmani S., Mottahedeh R., Dave R., Reddy S. T., et al. 2003. Inflammatory/anti-inflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 108: 2751–2756. [DOI] [PubMed] [Google Scholar]
- 11.Navab M., Anantharamaiah G. M., Hama S., Hough G., Reddy S. T., Frank J. S., Garber D. W., Handattu S., Fogelman A. M. 2005. D-4F and statins synergize to render HDL anti-inflammatory in mice and monkeys and cause lesion regression in old apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 25: 1426–1432. [DOI] [PubMed] [Google Scholar]
- 12.Van Lenten B. J., Wagner A. C., Navab M., Anantharamaiah G. M., Hama S., Reddy S. T., Fogelman A. M. 2007. Lipoprotein inflammatory properties and serum amyloid A levels but not cholesterol levels predict lesion area in cholesterol-fed rabbits. J. Lipid Res. 48: 2344–2353. [DOI] [PubMed] [Google Scholar]
- 13.Navab M., Anantharamaiah G. M., Reddy S. T., Hama S., Hough G., Grijalva V. R., Yu N., Ansell B. J., Datta G., Garber D. W., et al. 2005. Apolipoprotein A-I mimetic peptides. Arterioscler. Thromb. Vasc. Biol. 25: 1325–1331. [DOI] [PubMed] [Google Scholar]
- 14.Vaziri N. D., Moradi H., Pahl M. V., Fogelman A. M., Navab M. 2009. In vitro stimulation of HDL anti-inflammatory activity and inhibition of LDL pro-inflammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney Int. 76: 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garber D. W., Venkatachalapathi Y. V., Gupta K. B., Ibdah J., Phillips M. C., Hazelrig J. B., Segrest J. P., Anantharamaiah G. M. 1992. Turnover of synthetic class A amphipathic peptide analogues of exchangeable apolipoproteins in rats. Correlation with physical properties. Arterioscler. Thromb. 12: 886–894. [DOI] [PubMed] [Google Scholar]
- 16.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. 2001. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (adult treatment panel III). 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 17.Tsimikas S., Witztum J. L., Miller E. R., Sasiela W. J., Szarek M., Olsson A. G., Schwartz G. G. 2004. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation. 110: 1406–1412. [DOI] [PubMed] [Google Scholar]
- 18.Su F., Kozak K. R., Imaizumi S., Gao F., Amneus M. W., Grijalva V., Ng C., Wagner A., Hough G., Farias-Eisner G., et al. 2010. Apolipoprotein A-I (apoA-I) and apoA-I mimetic peptides inhibit tumor development in a mouse model of ovarian cancer. Proc. Natl. Acad. Sci. USA. 107: 19997–20002. [DOI] [PMC free article] [PubMed] [Google Scholar]