Abstract
A simple, high-yielding preparation of monolysocardiolipin (MLCL) by phospholipase A2 hydrolysis of cardiolipin (CL) in methanol on a semi-preparative scale is described. In methanol, phospholipase A2 preferentially hydrolyzes CL to MLCL. This selectivity results in ∼80% yield of MLCL. The synthesized MLCL and dilysocardiolipin were characterized by NMR and ESI-MS/MS. Only the sn-2 position of CL was hydrolyzed by phospholipase A2 in methanol.
Keywords: cardiolipin, phospholipase, mass spectrometry, dilysocardiolipin
Cardiolipin (CL) is a pseudosymmetric phospholipid that is localized in the mitochondrial inner membrane. CL works in concert with various mitochondrial enzymes (1, 2) and is suggested to act as a signaling molecule in apoptosis and mitophagy (3). CL oxidation or depletion can cause mitochondrial functional and structural damage (4–6).
Removal of one acyl chain results in generation of monolysocardiolipin (MLCL), which is an important metabolite of CL (7, 8). MLCL has been used as an intermediate in the synthesis of spin-labeled CL to study the interaction of CL with mitochondrial enzymes (9–11). Because a role for MLCL has been suggested in apoptosis, this molecule has been used to study its interaction with various enzymes involved in lipid remodeling and apoptosis (12–15). Recently, MLCL was recognized as a biomarker for Barth syndrome (16, 17) and accurate measurement of MLCL depends on a ready source of well-characterized MLCL.
MLCL usually is prepared by hydrolysis of CL with phospholipase A2 (PLA2), which hydrolyzes acyl chains only at the sn-2 position. Because CL has two sn-2 acyl chains, the reaction preferentially generates dilysocardiolipin (DLCL) by hydrolyzing both sn-2 chains (9). Therefore, the yield of MLCL depends both on the reaction conditions and stopping the reaction when MLCL is at a maximum. Recently, Boldyrev et al. (18) reported preparing MLCL with a significantly improved yield (39%), but the reaction procedure is complicated.
A previous report mentioned using ethanol as solvent to make MLCL (19). Herein, we extend that report, describing a simple, high-yielding preparation of MLCL using methanol as the reaction solvent. DLCL also was prepared stoichiometrically in methanol. The synthesized MLCL and DLCL were fully characterized using ESI-MS/MS and NMR.
EXPERIMENTAL PROCEDURES
Material
CL [bovine heart, disodium salt; the composition of this CL has been analyzed previously (20)] and PLA2 (porcine pancreas in ammonium sulfate suspension) were purchased from Sigma-Aldrich (St. Louis, MO), Whatman TLC plates (silica gel, 60Å, 250µm) from Fisher Scientific (Pittsburgh, PA), and silica gel (60, 230-400 mesh) from EMD chemicals (Gibbstown, NJ).
Instrumentation
The 1H-NMR spectra of CL, MLCL, and DLCL were recorded on Varian 400 MHz NMR in a 2:1 solution of CDCl3:CD3OD. Mass spectral data were acquired on a Thermo Finnigan LCQ Deca spectrometer. The MS and MS/MS spectra were obtained by flow injection analysis in the negative ion mode with a collision energy of 40 eV.
Synthesis of monolysocardiolipin
PLA2 (10 μl, ∼26 unit) was added to a solution of 3.5 mg of CL and 200 μl of CaCl2 (0.5 mM in H2O) in 1 ml methanol. The cloudy reaction mixture was stirred at room temperature and the reaction monitored by TLC as described below. After 14 h of stirring, the reaction mixture was filtered through cotton and the filtrate was evaporated to dryness under a N2 stream. The residue was dissolved in a solution of MeOH:CHCl3, 1:1 and applied to a silica gel column (2.5 × 20 cm) eluted with a solution of MeOH:CHCl3:H2O (6:2:0.2). MLCL was obtained as a white solid with ∼80% yield by weight.
For TLC analysis, about 1 µl of reaction mixture was removed at each time point, applied to the TLC plate, and developed with a solution of MeOH:CHCl3:H2O (6:2:0.2). The spots on TLC plates were visualized by I2 vapor and the intensity of each spot was quantified using ImageJ software (ImageJ 1.42q, http://rsb.info.nih.gov/ij/). Peak areas corresponding to MLCL and DLCL were multiplied by 4/3 and 2, respectively, to normalize the peak areas to that of CL. The relative amounts of CL, MLCL, and DLCL were calculated by dividing the peak area for each component by the total peak area of CL, MLCL, and DLCL in each lane.
DLCL was prepared by treating 3.5 mg of CL with PLA2 (40 μl, ∼104 unit) and 400 μl of CaCl2 (0.5 mM in H2O) in 1 ml methanol for 2 h.
RESULTS
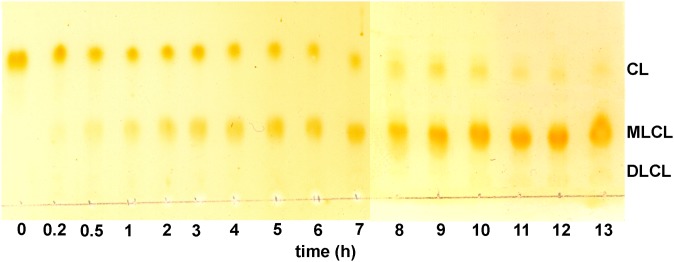
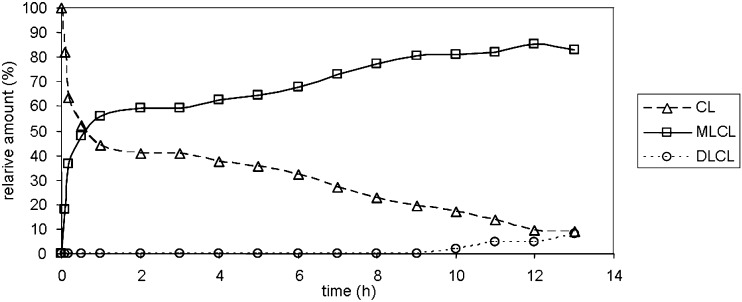
The TLC of the reaction mixture is shown in Fig. 1. Three spots are identified as CL, MLCL, and DLCL, and their relative retention factor (Rf) values are well matched with their expected polarities. The absence of spots below DLCL suggests that trilysocardiolipin is not generated. The relative amounts of MLCL, DLCL and CL analyzed during the reaction are shown in Fig. 2. At early reaction times (before 1 h) when CL is the major component, MLCL increases rapidly with a corresponding decrease in CL. After 1 h when MLCL is the major component, MLCL increases slowly with a slow decrease in CL. However, there is still no detectable DLCL, clearly showing a low hydrolytic rate of MLCL. DLCL starts increasing slowly only after 9 h when MLCL accounts for more than 70% of the reaction mixture. The maximum yield of MLCL under these reaction conditions is between 10 and 14 h of reaction with about 80% yield, according to TLC analysis.
Fig. 1.
The scanned image of a TLC of the reaction mixture as a function of reaction time. The reaction mixture was spotted on TLC at the indicated times and visualized by I2 vapor. The spots were identified as CL, MLCL, and DLCL.
Fig. 2.
The change in relative amounts of CL, MLCL and DLCL during reaction. TLC image was quantified using NIH Image J software. The density of spots that correspond to MLCL and CLCL was multiplied ×3/4 and ×2, respectively.
As expected, an increased amount of PLA2 accelerates the hydrolysis reaction. When the same amount of CL in 3.5 ml methanol is treated with 40 µL (104 unit) PLA2, CL is almost completely hydrolyzed in 2 h to DLCL.
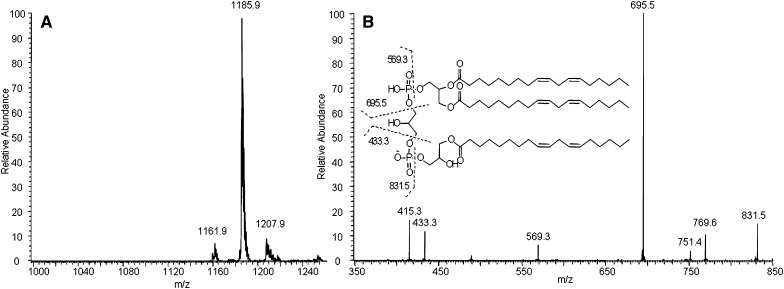
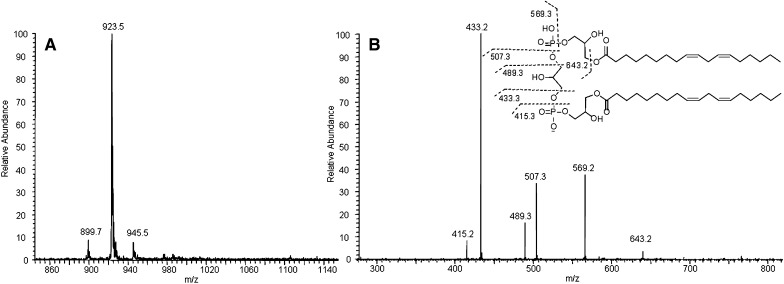
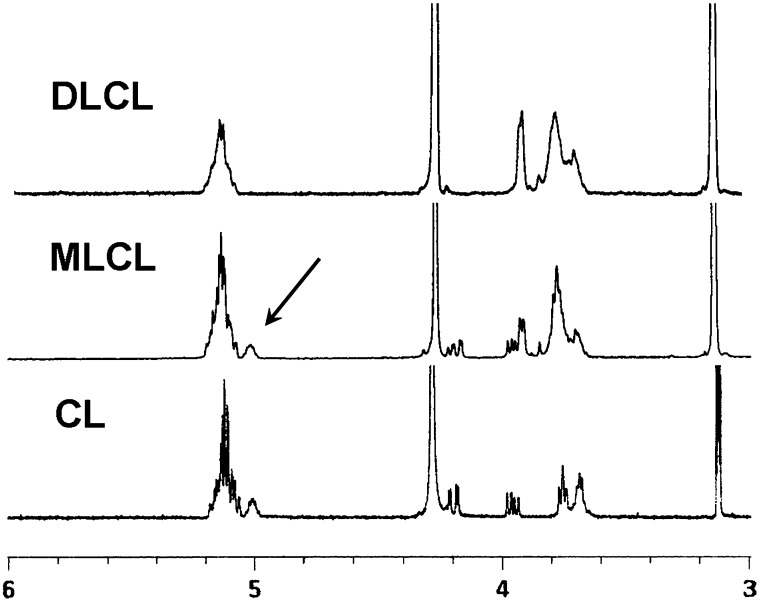
The structure of MLCL and DLCL was characterized by ESI-MS/MS and NMR. The observed peak at m/z 1186 by MS corresponds to the molecular weight of MLCL (Fig. 3A). MS/MS of the precursor ion (m/z 1186) is shown in Fig. 3B. Peaks at m/z 831.5 and 695.5 correspond to the diacylglycerol phosphatidylglycerol phosphatidate and diacylglycerol phosphatidate, and the peaks at m/z 569.3 and 433.3 correspond to their hydrolyzed counterparts. The MS and MS/MS spectra of DLCL are shown in Fig. 4. The absence of fragment ions at m/z 831.5 and 695.5 indicates that only one acyl chain was hydrolyzed from each phosphatidylglycerol phosphadiate moiety (Fig. 4B). MLCL and DLCL were further analyzed by 1H-NMR and the spectra compared with previously reported results (Fig. 5) (21). The peak at 5.0 ppm corresponds to the protons at the sn-2 position and shifts upfield with hydrolysis of the acyl chain. The extent of the upfield shift depends on the position of the hydrolysis. Hydrolysis at the sn-2 position shifts by about 10 ppm, whereas hydrolysis at the sn-1 position shifts by about 2 ppm (22). The observed peak at 5.0 ppm and the absence of a peak at 4.8 ppm of MLCL and absence of any peak at about 5 ppm of DLCL prove that the position of hydrolysis is only at the sn-2 position. Although studies have shown that the acyl chains can migrate to an empty hydroxyl group (23), our current characterization methods cannot detect the occurrence of acyl chain migration during hydrolysis. The combination of MS/MS and NMR spectra unequivocally characterizes the structure of MLCL and DLCL.
Fig. 3.
MS (A) and MS/MS (B) spectra of MLCL and proposed structures of fragment ions.
Fig. 4.
MS (A) and MS/MS (B) spectra of DLCL and proposed structures of fragment ions.
Fig. 5.
1H-NMR spectra of CL, MLCL, and DLCL. The presence of only one peak corresponding to CH-O-CO group of MLCL as indicated by the arrow shows that MLCL has been hydrolyzed at the sn-2 position.
DISCUSSION
PLA2 hydrolysis of CL in methanol is a very effective method to prepare MLCL. Reaction in methanol results in a high yield of MLCL and the reaction and work-up process are very simple. Moreover, after reaching the maximum, the amount of MLCL decreased only slowly. This slow decline is a great advantage, because the end product over time will be converted to undesirable side products.
Acknowledgments
The authors thank Dr. Bernard Tandler and the Hoppel Laboratory “Writing with Style” group for editorial assistance.
Footnotes
Abbreviations:
- CL
- cardiolipin
- DLCL
- dilysocardiolipin
- MLCL
- monolysocardiolipin
- PLA2
- phospholipase A2
This work was supported in part by the National Institutes of Health/National Institute on Aging grant (P01 AG015885). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Hoffmann B., Stockl A., Schlame M., Beyer K., Klingenberg M. 1994. The reconstituted ADP/ATP carrier activity has an absolute requirement for cardiolipin as shown in cysteine mutants. J. Biol. Chem. 269: 1940–1944. [PubMed] [Google Scholar]
- 2.Petrosillo G., Casanova G., Matera M., Ruggiero F. M., Paradies G. 2006. Interaction of peroxidized cardiolipin with rat-heart mitochondrial membranes: induction of permeability transition and cytochrome c release. FEBS Lett. 580: 6311–6316. [DOI] [PubMed] [Google Scholar]
- 3.Kagan V. E., Bayir H. A., Belikova N. A., Kapralov O., Tyurina Y. Y., Tyurin V. A., Jiang J., Stoyanovsky D. A., Wipf P., Kochanek P. M., et al. 2009. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic. Biol. Med. 46: 1439–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J., Rodriguez M. E., Oleinick N. L., Anderson V. E. 2010. Photo-oxidation of cardiolipin and cytochrome c with bilayer-embedded Pc 4. Free Radic. Biol. Med. 49: 718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J., Fujioka H., Oleinick N. L., Anderson V. E. 2010. Photosensitization of intact heart mitochondria by the phthalocyanine Pc 4: correlation of structural and functional deficits with cytochrome c release. Free Radic. Biol. Med. 49: 726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Q., Lesnefsky E. J. 2006. Depletion of cardiolipin and cytochrome c during ischemia increases hydrogen peroxide production from the electron transport chain. Free Radic. Biol. Med. 40: 976–982. [DOI] [PubMed] [Google Scholar]
- 7.Esposti M. D., Cristea I. M., Gaskell S. J., Nakao Y., Dive C. 2003. Proapoptotic Bid binds to monolysocardiolipin, a new molecular connection between mitochondrial membranes and cell death. Cell Death Differ. 10: 1300–1309. [DOI] [PubMed] [Google Scholar]
- 8.Danos M., Taylor W. A., Hatch G. M. 2008. Mitochondrial monolysocardiolipin acyltransferase is elevated in the surviving population of H9c2 cardiac myoblast cells exposed to 2-deoxyglucose-induced apoptosis. Biochem. Cell Biol. 86: 11–20. [DOI] [PubMed] [Google Scholar]
- 9.Cable M. B., Jacobus J., Powell G. L. 1978. Cardiolipin: a stereospecifically spin-labeled analogue and its specific enzymic hydrolysis. Proc. Natl. Acad. Sci. USA. 75: 1227–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell G. L., Knowles P. F., Marsh D. 1987. Spin-label studies on the specificity of interaction of cardiolipin with beef heart cytochrome oxidase. Biochemistry. 26: 8138–8145. [DOI] [PubMed] [Google Scholar]
- 11.Beyer K., Nuscher B. 1996. Specific cardiolipin binding interferes with labeling of sulfhydryl residues in the adenosine diphosphate/adenosine triphosphate carrier protein from beef heart mitochondria. Biochemistry. 35: 15784–15790. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y., Kelley R. I., Blanck T. J., Schlame M. 2003. Remodeling of cardiolipin by phospholipid transacylation. J. Biol. Chem. 278: 51380–51385. [DOI] [PubMed] [Google Scholar]
- 13.Reers M., Pfeiffer D. R. 1987. Inhibition of mitochondrial phospholipase A2 by mono- and dilysocardiolipin. Biochemistry. 26: 8038–8041. [DOI] [PubMed] [Google Scholar]
- 14.Liu J., Durrant D., Yang H. S., He Y., Whitby F. G., Myszka D. G., Lee R. M. 2005. The interaction between tBid and cardiolipin or monolysocardiolipin. Biochem. Biophys. Res. Commun. 330: 865–870. [DOI] [PubMed] [Google Scholar]
- 15.Tyurin V. A., Tyurina Y. Y., Osipov A. N., Belikova N. A., Basova L. V., Kapralov A. A., Bayir H., Kagan V. E. 2007. Interactions of cardiolipin and lyso-cardiolipins with cytochrome c and tBid: conflict or assistance in apoptosis. Cell Death Differ. 14: 872–875. [DOI] [PubMed] [Google Scholar]
- 16.Houtkooper R. H., Rodenburg R. J., Thiels C., van Lenthe H., Stet F., Poll-The B. T., Stone J. E., Steward C. G., Wanders R. J., Smeitink J., et al. 2009. Cardiolipin and monolysocardiolipin analysis in fibroblasts, lymphocytes, and tissues using high-performance liquid chromatography-mass spectrometry as a diagnostic test for Barth syndrome. Anal. Biochem. 387: 230–237. [DOI] [PubMed] [Google Scholar]
- 17.Kulik W., van Lenthe H., Stet F. S., Houtkooper R. H., Kemp H., Stone J. E., Steward C. G., Wanders R. J., Vaz F. M. 2008. Bloodspot assay using HPLC-tandem mass spectrometry for detection of Barth syndrome. Clin. Chem. 54: 371–378. [DOI] [PubMed] [Google Scholar]
- 18.Boldyrev I. A., Pavlova Iu B., Molotkovskii Iu G. 2009. Synthesis and characteristics of new fluorescent probes based on cardiolipin. Bioorg. Khim. 35: 239–244. [DOI] [PubMed] [Google Scholar]
- 19.Schlame M., Rustow B. 1990. Lysocardiolipin formation and reacylation in isolated rat liver mitochondria. Biochem. J. 272: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minkler P. E, Hoppel C. L. 2010. Separation and characterization of cardiolipin molecular species by reverse-phase ion pair high-performance liquid chromatography-mass spectrometry. J. Lipid Res. 51: 856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malhotra A., Xu Y., Ren M., Schlame M. 2009. Formation of molecular species of mitochondrial cardiolipin. 1. A novel transacylation mechanism to shuttle fatty acids between sn-1 and sn-2 positions of multiple phospholipid species. Biochim. Biophys. Acta. 1791: 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pluckthun A., Dennis E. A. 1982. Acyl and phosphoryl migration in lysophospholipids: importance in phospholipid synthesis and phospholipase specificity. Biochemistry. 21: 1743–1750. [DOI] [PubMed] [Google Scholar]
- 23.Abe M., Kitsuda S., Ohyama S., Koubori S., Murai M., Miyoshi H. 2010. Concise procedure for the synthesis of cardiolipins having different fatty acid combinations. Tetrahedron Lett. 51: 2071–2073. [Google Scholar]