Abstract
Occupational exposure to chromium (Cr) compounds has been shown to cause serious toxic and carcinogenic effects. The skin is an important target for the compounds in industrially exposed Cr workers. c-Jun NH2-terminal kinase (JNK) regulates cell proliferation, apoptosis, and differentiation. This protein's effects on cellular response depend upon the cell type and stimuli. The mechanisms by which hexavalent chromium (Cr(VI)) leads to apoptosis in the skin are unclear at present. The aim of this study is to examine whether JNK regulates apoptosis in Cr(VI)-exposed mouse JB6 epidermal cells. The present study showed that Cr(VI) induced apoptotic cell death through JNK activation. The blockage of JNK by small interference RNA (si-RNA) transfection suppressed Cr(VI)-induced apoptotic cell death with the concomitant downregulation of antiapoptotic Bcl-2 family proteins, mitochondrial membrane depolarization (Δψm), caspase activation, and poly (ADP-ribose) polymerase cleavage. However, inhibition of c-Jun expression by si-RNA transfection enhanced cytotoxicity, which corresponded to increasing apoptosis and Δψm. This phenomenon is associated with p53 activation caused by increasing reactive oxygen species (ROS) levels because of the downregulation of superoxide dismutase expression in si-c-Jun–transfected cells. Taken together, Cr(VI) induces apoptosis via JNK-mediated signaling, whereas c-Jun activation acts as an inhibitor of apoptotic signaling. Additionally, ROS generated by Cr(VI) is a pivotal regulator of JNK.
Keywords: apoptosis, Cr(VI), JNK, c-Jun, ROS
Chromium (Cr) compounds are widely used in industry and have serious toxic and carcinogenic effects on animals and humans (Cohen et al., 1993; Costa, 1997). Hexavalent chromium (Cr(VI)) enters the body by inhalation, ingestion, or absorption through the skin. For occupational exposure, the airways and skin are the primary routes of uptake (Costa, 1997; Shelnutt et al., 2007). Upon entering the cell, Cr(VI) is reduced by cellular reductants to its lower oxidation states, pentavalent chromium and tetravalent Cr (Shi et al., 1999). These intermediate states of Cr are reactive and can produce reactive oxygen species (ROS) (Shi and Dalal, 1989, 1990c), which cause DNA strand breaks, base modification, lipid peroxidation, and transcription factor activation (Ding and Shi, 2002; Hodges et al., 2001; Shi et al., 1999; Stohs et al., 2000; Xu et al., 1992; Ye et al., 1995). Occupational exposure to Cr(VI) is a well-established cause of skin damage such as skin ulcers, irritant contact dermatitis, and delayed hypersensitivity reactions (Hansen et al., 2006; OSHA, 2006; Shelnutt et al., 2007). The mildest skin reactions consist of erythema, edema, papules, and vesicles (OSHA, 2006). Cr(VI) also causes allergenic skin eczema, atopic dermatitis (Hansen et al., 2006), and dermal toxicity (Joseph et al., 2008; Shelnutt et al., 2007).
Cellular events such as cell proliferation, apoptosis, and differentiation are tightly regulated by mitogen-activated protein kinases (MAPK) including c-Jun NH2-terminal kinase (JNK), extracellular signal regulated kinase (ERK), and p38 kinase (Liu et al., 2002; Tobiume et al., 2001). ROS are critical mediators of cellular signaling, which regulate many cellular processes where MAPK signaling is closely involved (Clerk et al., 1998; Liu et al., 2002; Son et al., 2009, 2010b; Wang et al., 1998). In particular, JNK-mediated signaling is important in modulating apoptosis signaling in response to oxidative stress (Chang and Karin, 2001; Wang et al., 1998). JNK acts as an upstream effecter of transcription factor activator protein-1 (AP-1) (Derijard et al., 1994; Kyriakis et al., 1994; Ventura et al., 2003). AP-1 is a multimeric protein consisting of Jun (c-Jun, JunB, and JunD) and Fos (c-Fos, FosB, Fra1, and Fra2) subunits. AP-1 binds to the TRE (TPA DNA response element) and regulates many kinds of early response gene expression (Munoz et al., 1996). AP-1 also mediates either cell survival or death, which are caused through varying combinations of Jun subproteins (Leppa and Bohmann, 1999). Importantly, c-Jun is the major phosphorylation target of JNK (Davis, 1995).
We recently showed that Cr(VI) induced mitochondrial-mediated and caspase-dependent apoptosis in epidermal cells through activation of p53, which was mainly mediated by ROS generated by the chemical (Son et al., 2010a). This supports the suggestion that ROS could play a crucial role in Cr(VI)-mediated cell death. There is also considerable evidence emphasizing the involvement of MAPK-mediated signaling in Cr(VI)-induced apoptosis. Actually, Cr(VI) activates JNK, ERK, and/or p38 in rat H4 hepatoma, murine embryonic stem cells, and human bronchial epithelial or lung carcinoma cell line (CL) 3 cells (Chen et al., 2009; Chuang et al., 2000; Kim and Yurkow, 1996; Samet et al., 1998). These findings and our previous data indicate that among MAPK, JNK has important roles in Cr(VI)-treated cells because JNK is activated sensitively in the presence of ROS (Ge et al., 2008; Shen and Liu, 2006; Son et al., 2009, 2010b). It is of note that almost all the previous studies have used high concentrations of Cr(VI) over short exposure times, as opposed to the chronic exposure to low doses of Cr(VI) which occurs occupationally. Moreover, the cellular mechanisms by which JNK mediates apoptosis in Cr(VI)-treated cells remain unclear. The present study has further examined the possible role of JNK in regulating cellular responses to low concentrations of Cr(VI) in skin epidermal JB6 cells. Here, we demonstrate the critical roles of JNK-mediated signaling in facilitating ROS-mediated apoptosis in Cr(VI)-exposed JB6 cells.
MATERIALS AND METHODS
Chemicals and laboratory wares.
Unless specified otherwise, all chemicals and laboratory wares were purchased from Sigma Chemical Co. (St Louis, MO) and Falcon Labware (Bectone-Dickinson, Franklin Lakes, NJ). Eagle's minimal essential medium (EMEM), fetal bovine serum (FBS), gentamicin, and L-glutamine were purchased from Gibco Co. (Gibco BRL, NY). Dihydroethidium was obtained from Molecular Probes (Eugene, OR).
Cell culture and treatment.
The JB6 mouse epidermal cell line, Cl 41 (American Type Culture Collection, CRL-2010), and cells stably transfected with the AP-1-luciferase reporter plasmid (JB6/AP-1 cells) were cultured in EMEM containing 5% FBS, 2mM L-glutamine, and 50 μg of gentamicin per milliliter. The cells were grown at 37°C in a 5% CO2 atmosphere. The monolayer cells were trypsinized, and 1 million cells per milliliter were resuspended in either 3 ml or 200 μl of the media and spread onto either 60-mm culture dish or 96-well plates, respectively. After the cells had reached 80% confluence, the culture medium was replaced with fresh medium containing 0.5% FBS and various concentrations of Na2Cr2O7 (0–10μM).
Plasmids and transfection.
The SOD1-Myc-DDK– and SOD2-enhanced green fluorescent protein–tagged plasmids were purchased from Origene (Rockville, MD) and Addgene (Cambridge, MA), respectively. Transfection was performed using the Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacture's protocol. Briefly, JB6 cells were seeded in six-well culture plates and transfected with 4 μg of plasmids at approximately 50% confluency. Overexpressed cellular levels of the proteins specific for Cu/Zn superoxide dismutase (SOD) (SOD1) or MnSOD (SOD2) were checked by immunoblotting, and the cells were maintained using G418 for stable cell lines.
Measurement of cell viability.
3-(4,5-Dimethylthiazol-2yl-)-2,5-diphenyl tetrazolium bromide (MTT) was used to evaluate viability of cells. After transfection with small interference RNA (si-RNA), JB6 cells were treated with or without 10μM of Na2Cr2O7 for 48 h. Thereafter, 5 μl of an MTT solution (5 mg/ml in PBS as stock solution) was added into each well of 96-well plates and incubated for 4 h at 37°C. MTT-reducing activity of cells was measured by treating them with acidic isopropanol prior to reading at 560 nm with Spectra Max Plus384 (Molecular Devices, Sunnyvale, CA).
FITC-Annexin V/propidium iodide staining.
JB6 cells were washed twice with PBS before being suspended in 1× binding buffer (10mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.4, 140mM NaOH, and 2.5mM CaCl2). Five microliters of fluorescein isothiocyanate (FITC)-labeled Annexin V were mixed with 100 μl of cell suspension containing 1 × 105 cells, and the cells were incubated at room temperature for 10 min. Afterward, 2 μl of propidium iodide (PI) solution (10 μg/ml) was added into the cells followed by additional 10 min incubation on ice. The scatter parameters of the cells (20,000 cells per experiment) were analyzed using a Fluorescence-Activated Cell Sorter Calibur system. Four cell populations were identified according to the following interpretation: viable population in the lower left quadrant (low PI and FITC signals), early apoptotic population in the lower right quadrant (low PI and high FITC signals), necrotic population in the upper left quadrant (high PI and low FITC signals), and late apoptotic or necrotic population in the upper right quadrant (high PI and high FITC signals).
Measurement of mitochondrial membrane potential.
The mitochondrial membrane potential (ΔΨm) was monitored using 5,5′,6,6′-tetra-chloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1), a lipophilic cationic fluorescence dye. JC-1 is capable of selectively entering mitochondria, where it forms monomers, and emits green fluorescence (FL-1) when ΔΨm is relatively low. At a high ΔΨm, JC-1 aggregates and gives red fluorescence (FL-2) (Cossarizza et al., 1993). Thus, the red and green fluorescence of JC-1 reflect the change of ΔΨm of the mitochondrial membrane. Briefly, cells (0.5 × 106 cells per well) were seeded into 60-mm culture dishes and transfected with si-RNA and the JB6 cells were cultured in MEM supplemented with or without 10μM of Na2Cr2O7 for 48 h. Cells were trypsinized, washed in ice-cold PBS, and incubated with 2μM JC-1 at 37°C for 20 min. Finally, cells were washed twice with PBS and analyzed by flow cytometry.
Western blot analyses.
Cell lysates were made in lysis buffer (50mM Tris-Cl, pH 7.4, 1mM EDTA, 150mM NaCl, 1% Nonidet P-40, 0.25% Na-deoxycholate, and 1 μg/ml of aprotinin, leupeptin, and pepstatin). Equal amounts of protein (20 micrograms per sample) were separated by the NuPAGE Bis-Tris electrophoresis system (Invitrogen) and blotted onto nitrocellulose membrane (Whatman, Dassel, Germany). Blots were probed with primary antibodies for either 2 h at room temperature or overnight at 4°C before incubation with horseradish peroxidase–conjugated anti-IgG in a blocking buffer for 1 h. Polyclonal antibody specific for SOD1 (SC-11407) and monoclonal antibodies specific for β-actin (SC-47778) and p-c-Jun NH2-terminal kinase (p-JNK) (SC-6252) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Bcl-2 (2876), Bcl-xL (2762), Bid (2003), p-p53 (Ser 15) (9284), caspase-8 (4927), c-Jun (9162), p-c-Jun (9261), JNK (9252), cleaved caspase-3 (Asp 175) (9661), and glutathion peroxidase1 (GPX1) (3206) polyclonal antibodies were purchased from Cell Signaling (Beverly, MA). The monoclonal antibodies specific to p53 (OP03) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (A00839-40) and polyclonal antibody specific to Cu/ZnSOD (07403) were purchased from Oncogene, Genscript, and Millipore, respectively. The poly (ADP-ribose) polymerase (PARP) (SA-249) and catalase (CAT) (NB100–79910) antibodies were purchased from Biomol (Plymouth Meeting, PA) and Novus (Littleton, CO), respectively. Secondary antibodies and enhanced chemiluminescence substrate were from Pierce (Rockford, IL). The blots were exposed to Hyperfilm (Amersham Pharmacia Biotech), and bands were quantified with ImageJ densitometry software (National Institutes of Health, Bethesda, MD).
Measurement of cellular superoxide anion () production.
Dihydroethidium was used for staining , which are produced by intact cells (Qian et al., 2003; Zamzami et al., 1995). Briefly, cells (0.2 × 106 cells per well) were seeded into six-well culture plates and transfected with si-RNA and the JB6 cells were cultured in the presence or absence of 10μM of Na2Cr2O7 for 24 h. Finally, the cells were exposed to dihydroethidium at a final concentration of 2μM for 30 min and processed for flow cytometric analysis.
Electron spin resonance assay.
All electron spin resonance (ESR) measurements were conducted using a Bruker EMX spectrometer (Bruker Instruments, Billerica, MA) and a flat cell assembly, as described previously (Son et al., 2010a). A spin trap, 5,5-dimethyl-1-pyrroline-1-oxide, was charcoal purified and distilled to remove all ESR detectable impurities before use. Hyperfine couplings were measured (to 0.1 G) directly from magnetic field separation using potassium tetraperoxochromate and 1,1-diphenyl-2-picrylhydrazyl as reference standards. The Acquisit program was used for data acquisitions and analyses (Bruker Instruments). Reactants were mixed in test tubes to a total final volume of 0.5 ml. The reaction mixture was then transferred to a flat cell for ESR measurement. Experiments were performed at room temperature and under ambient air.
si-RNA transfection.
Silencer predesigned si-RNA for mouse JNK1 (si-RNA ID: 188653), JNK2 (si-RNA ID: 73119), p53 (si-RNA ID: 187425), c-Jun (si-RNA ID: 155944), negative control (AM4611), and positive control GAPDH (si-RNA ID: 4390849) were obtained from Ambion (Austin, TX). JB6 cells were seeded in 96- or six-well culture plates and transfected at approximately 50% confluency with the si-RNA duplexes using Lipofectamine RNAi MAX (Invitrogen) according to the manufacturer's instructions. Medium was changed after 6 h to minimize cytotoxicity. Cellular levels of the proteins specific for the si-RNA transfection were checked by immunoblotting, and all experiments were performed 24 h after transfection.
Assay of AP-1 activity.
Stably transfected JB6 cells with AP-1-luciferase reporter plasmid were kindly provided from Dr Min Ding (National Institute for Occupational Safety and Health, Morgantown, WV). When JB6/AP-1 cells reached 80–90% confluence, the cells were incubated in a low serum (0.5% FBS) containing EMEM for 24 h to minimize basal AP-1 activity. The cells were exposed to various concentrations of Na2Cr2O7 (0–10μM) for 24 h. The cells were also exposed to Na2Cr2O7 (10μM) for various times (0 h–24 h). The luciferase activity specific to AP-1 was then measured by using a luciferase assay kit (Promega, Madison, WI) as described elsewhere (Ding et al., 1999). The results were expressed as relative AP-1 activity compared with control.
Statistical analysis.
All the data have been expressed as mean ± SE. One-way ANOVA using SPSS version 10.0 software was used for multiple comparisons. A value of p < 0.05 was considered statistically significant.
RESULTS
Cr(VI) Leads to Activation of JNK in Skin Epidermal Cells
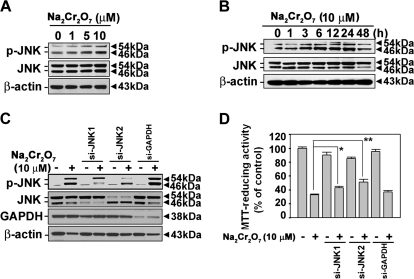
A 24-h treatment of cells with Cr(VI) resulted in increased levels of phosphorylated JNK in a dose-dependent manner (Fig. 1A). This JNK activation was decreased after 48 h incubation when the cells treated with 10μM of Na2Cr2O7 (Fig. 1B). For further verification of JNK's involvement, we transfected the cells with si-RNAs specific to JNK1 or JNK2, by which the expression of both proteins was suppressed (Fig. 1C). The Cr(VI)-induced decrease in cell viability was attenuated significantly in the cells transfected with either si-JNK1 (p < 0.05) or si-JNK2 (p < 0.01) (Fig. 1D). Treatment with 10μM Na2Cr2O7 for 48 h in cells transfected with si-JNK1 and si-JNK2 increased the MTT-reducing activity to 43 and 51%, respectively, compared with the 10μM Na2Cr2O7-treated cells (33%). These results indicated that JNK-mediated signaling is involved in Cr(VI)-induced cell death in JB6 cells.
FIG. 1.
JNK signaling is involved in Cr(VI)-induced cell death in JB6 cells. (A) JB6 cells were incubated with Na2Cr2O7 at various concentrations (0–10μM) for 24 h. (B) Cells were exposed to 10μM Na2Cr2O7 for various times (0–48 h). Protein lysates were analyzed by a NuPAGE Bis-Tris electrophoresis system, and the levels of phosphorylated JNK and total JNK were detected by Western blot analysis. JB6 cells were transfected with si-RNAs specific to JNK1 or JNK2. After 24 h incubation, they were exposed to 10μM Na2Cr2O7. Finally, the protein levels were analyzed by Western blot at 24 h (C), and MTT assay was performed at 48 h (D). β-Actin and GAPDH were used as control protein and control transfection, respectively. The results are shown as the mean ± SE of three separate experiments. *p < 0.05 and **p < 0.01 versus the Na2Cr2O7 treatment alone (ANOVA, Scheffe's test).
JNK Activation Is Closely Involved in Cr(VI)-Induced Apoptosis and Mitochondria Stress
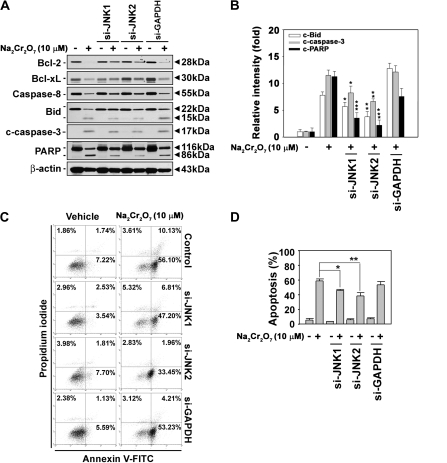
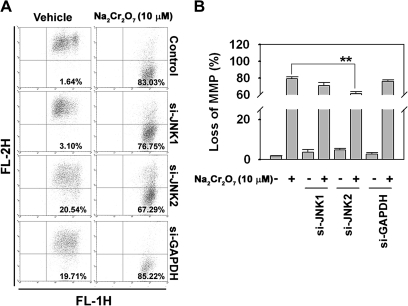
In order to clarify the involvement of JNK in Cr(VI)-induced cytotoxicity, various apoptosis detection assays were subsequently performed. Cr(VI) treatment resulted in the induction of cleaved caspase-3 and the decrease of cellular Bcl-2 and Bcl-xL levels, as expected. In addition, decrease of procaspase-8 and increase of cleavage of Bid and PARP proteins were observed in the cells. However, these changes in Cr(VI)-exposed cells were blocked by transfecting them with JNK-specific si-RNAs (Figs. 2A and 2B). Quantification of band intensity data showed that Cr(VI)-induced increases in cleaved Bid, caspase-3, and PARP were clearly reduced by either si-JNK1 or si-JNK2 transfection. Cr(VI) treatment increased early and late apoptotic population in JB6 cells. Approximately 3% of the cell population expressing high PI and low FITC signals, indicative of necrotic cells, was observed when the cells were exposed to 10μM Na2Cr2O7. Under the same conditions, more than 60% of cell populations were apoptotic (Figs. 2C and 2D). The blockage of JNK expression by its specific si-RNA transfection attenuated the Cr(VI)-induced increase of Annexin V–positively stained cells. The protective effect of si-JNK1 and si-JNK2 transfection upon Cr(VI)-induced apoptosis was 15% (p < 0.05) and 21% (p < 0.01), respectively, which suggested the involvement of JNK-mediated signaling in Cr(VI)-mediated apoptosis. Cr(VI)-mediated reduction of ΔΨm was also inhibited by si-JNK transfection (Fig. 3), where the cells transfected with si-JNK2 recovered the content of ΔΨm in a significant level. This result indicated that JNK2 signaling more closely regulated mitochondrial stress than JNK1 in Cr(VI)-treated JB6 cells.
FIG. 2.
JNK-mediated signaling plays a critical role in Cr(VI)-induced apoptosis in skin epidermal cells. JB6 cells were transfected with si-JNKs for 24 h. They were exposed to 10μM Na2Cr2O7 for an additional 48 h and then processed by Western blot analysis (A) and Annexin V/PI staining (C). (B) The intensity of bands expressed as the mean ± SE relative to the control of triplicate experiments after normalizing the bands to β-actin. (D) The percentage of apoptotic populations was summed from early apoptotic cells (Annexin V+/PI−) and late apoptotic cells (Annexin V+/PI+). si-GAPDH and β-actin were used as control si-RNA and control protein, respectively. The results are shown as the mean ± SE of three separate experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 versus the Cr(VI) treatment alone (ANOVA, Scheffe's test).
FIG. 3.
Cr(VI) induced mitochondrial membrane potential (ΔΨm) depolarization through JNK signaling pathway. (A) JB6 cells were treated with Cr(VI) for 48 h following transfection with si-JNKs. The cells were then incubated with 2μM JC-1 dye for 15 min, and the intensities of FL-1 and FL-2 fluorescence were measured by flow cytometry. (B) The percentage of depolarized populations was calculated from three independent experiments. **p < 0.01 versus the Cr(VI) treatment alone (ANOVA, Scheffe's test).
Cr(VI) Activates AP-1 in Skin Epidermal Cells
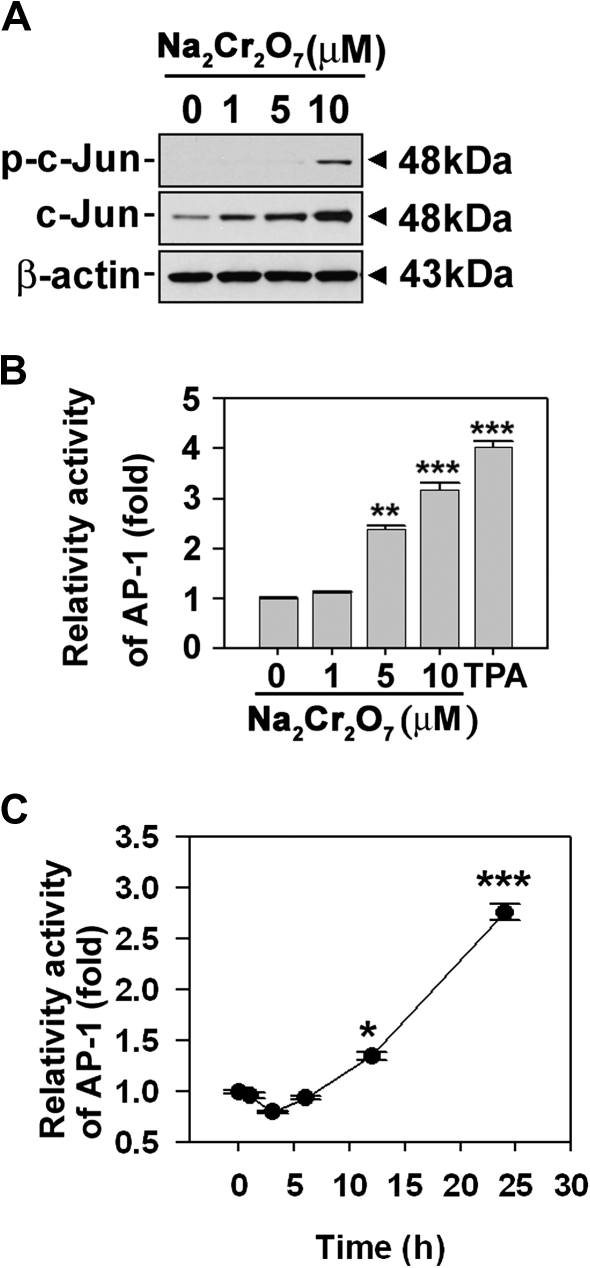
Because the transcription factor AP-1 and its subfamily c-Jun are well-known downstream mediators of JNK, we examined whether Cr(VI) is able to stimulate c-Jun phosphorylation and AP-1 activation using JB6 cells transfected with an AP-1-luciferase reporter plasmid (Li et al., 1997). Cellular levels of c-Jun and p-c-Jun were augmented by treating the cells with Cr(VI) in a dose-dependent manner (Fig. 4A). Cr(VI) treatment also dramatically increased the luciferase activity specific for AP-1 in a dose- (Fig. 4B) and time-dependent manner (Fig. 4C), which suggested the involvement of AP-1–mediated signaling in cellular responses to Cr(VI).
FIG. 4.
Cr(VI) increased AP-1 activity in skin epidermal cells. (A) JB6 cells were incubated with Na2Cr2O7 at the various concentrations (0–10μM) for 24 h. The levels of phosphorylated c-Jun and total c-Jun were detected by Western blot analysis. β-Actin was used as a control protein. (B) JB6 cells transfected with AP-1 promoter gene were exposed to the indicated concentrations (0–10μM) of Na2Cr2O7 for 24 h. (C) Cells were exposed to 10μM Na2Cr2O7 for various times (0–24 h). The AP-1 activity by luciferase assay, presented as relative AP-1 induction compared with the untreated control cells. 12-O-tetradecanoylphorbol-13-acetate (20 ng/ml) was used as positive control for AP-1 activity. *p < 0.05, **p < 0.01, and ***p < 0.001 versus the unexposed control values (ANOVA, Scheffe's test).
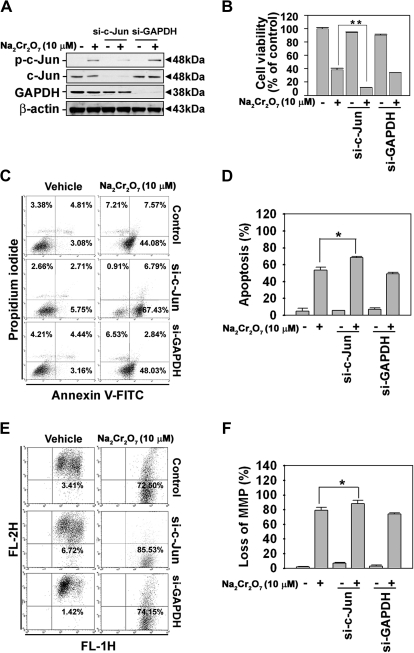
c-Jun Mediates Survival Signal in Cr(VI)-Exposed Skin Epidermal Cells
We subsequently examined the role of c-Jun in Cr(VI)-exposed cells after si-c-Jun transfection. Figure 5 shows that the inhibition of c-Jun expression by si-RNA transfection facilitated the Cr(VI)-induced toxicity in the skin epidermal cells. In fact, the decrease in cell viability and the increase in Annexin V–positive cells, which had led in the Cr(VI)-treated cells, were accelerated significantly in the cells transfected with si-c-Jun. For example, when Cr(VI)-exposed cells were transfected with si-c-Jun, cell viability decreased to 11% compared with only Cr(VI)-treated cells (38%) (Fig. 5B). Apoptosis assay results also showed that the percentage of apoptotic cells, 53%, increased to 68% in si-c-Jun–transfected cells (Figs. 5C and 5D). Cr(VI)-induced decreases in ΔΨm accelerated in si-c-Jun–transfected cells when the cells were flow cytometrically analyzed after staining with JC-1 (Figs. 5E and 5F). These results suggested that c-Jun activation might have a protective effect against Cr(VI)-derived toxicity in skin epidermal cells.
FIG. 5.
Suppression of c-Jun expression by si-RNA transfection accelerated apoptotic cell death in Cr(VI)-exposed JB6 cells. JB6 cells were transfected with si-RNA specific to c-Jun for 24 h, after which they were exposed to 10μM Na2Cr2O7 for additional 48 h and then processed for MTT assays (B), Annexin V/PI staining (C), or mitochondrial membrane potential (ΔΨm) (E) measurement. The knockdown in c-Jun levels was confirmed by Western blot analysis after 24 h Cr(VI) treatment (A). The percentages of apoptotic populations (D) and mitochondrial membrane–depolarized populations (F) were calculated from three independent experiments. si-GAPDH and β-actin were used as control si-RNA and control protein, respectively. *p < 0.05 and **p < 0.01 versus the Cr(VI) treatment alone (ANOVA, Scheffe's test).
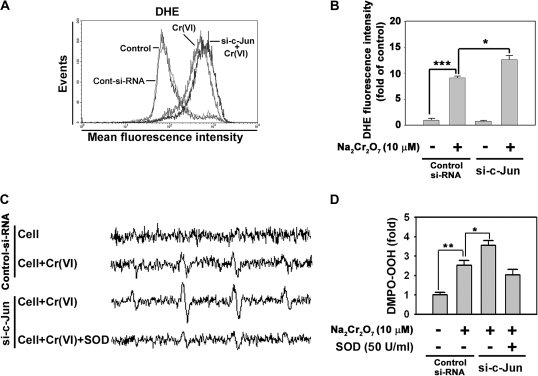
c-Jun Regulates Intracellular Level in Cr(VI)-Exposed Cells
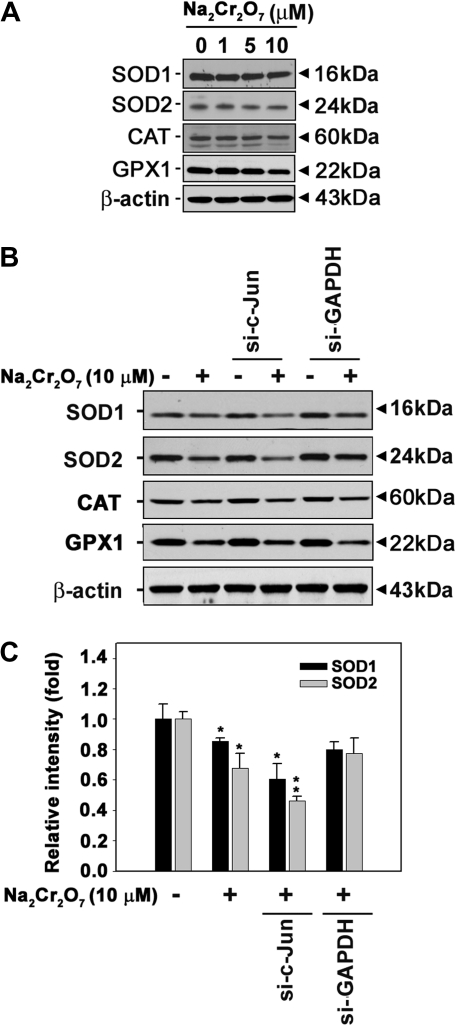
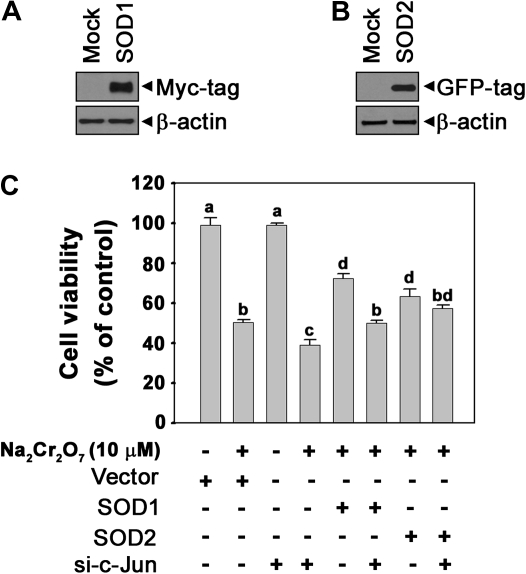
In order to understand the exact role of c-Jun in Cr(VI)-mediated cell death, we determined intracellular ROS levels using flow cytometry. Fluorescence intensity of dihydroethidium, which is specific for , was increased in si-c-Jun–transfected cells as compared with the cells exposed to Cr(VI) alone (Figs. 6A and 6B). This result was supported by ESR measurements. These spin adducts showed an increased intensity cells transfected with si-c-Jun relative to the cells treated with Cr(VI) alone (Figs. 6C and 6D). In particular, the addition of SOD (50 U/ml) reduced the signal intensity of the spectrum, thereby suggesting that the increase in spin adduct intensity in the si-c-Jun–transfected cells was because of the generation of . These results suggested that alteration of levels is dependent upon c-Jun in Cr(VI)-exposed cells. Western blot analysis confirmed the involvement of in Cr(VI)-mediated toxicity in skin epidermal cells. Antioxidant enzymes such as CAT, SOD1, SOD2, and GPX1 were slightly decreased in Cr(VI)-exposed cells (Fig. 7A). However, the Cr(VI)-mediated downregulation of SOD1 and SOD2 was transiently augmented by si-c-Jun transfection (Figs. 7B and 7C). These results suggested that c-Jun regulates SOD expression in Cr(VI)-exposed cells. It is possible that c-Jun survival signals in response to Cr(VI) are through the upregulation of cellular SOD expression. To further understand this phenomenon, we overexpressed SOD in the cells (Fig. 8). Overexpression of SOD1 or SOD2 caused Cr(VI)-induced cell death to decrease significantly (p < 0.05). This result indicated that expression of SOD regulated cell death in Cr(VI)-exposed JB6 cells. Moreover, both overexpressed SOD1 and SOD2 significantly attenuated si-c-Jun–mediated increases in cell death in Cr(VI)-exposed cells. This result further supported the hypothesis that c-Jun mediated cell survival signaling through regulation of SOD expression in Cr(VI)-exposed JB6 cells.
FIG. 6.
Transfection with si-c-Jun increases intracellular levels in Cr(VI)-exposed cells. (A) JB6 cells were transfected with si-c-Jun for 24 h, were exposed to 10μM Na2Cr2O7 for additional 24 h, and then processed for flow cytometry after treatment with 5μM DHE for 30 min. (B) The fluorescence intensity of DHE was expressed as the mean ± SE relative to the treatment control. (C) After transfection with si-c-Jun for 24 h, ESR spectra were recorded after the addition of 10μM Na2Cr2O7 and 100mM DMPO to transfected cells (1 × 106) with or without SOD (50 U/ml). (D) The signal intensity of DMPO-OOH was expressed as the mean ± SE relative to the treatment control. *p < 0.05, **p < 0.01, and ***p < 0.001 versus the unexposed control si-RNA or the Cr(VI)-exposed control si-RNA (ANOVA, Scheffe's test). The ESR spectrometer settings were as follows: frequency, 9.8 GHz; power, 39.91 mW; modulation frequency, 100 kHz; receiver gain, 5.02 × 105; time constant, 40.96 ms; modulation amplitude, 1.00 G, scan time, 60 s; and magnetic field, 3451 ± 100 G. All spectra shown are an accumulation of 16 scans. DHE, dihydroethidium; DMPO, 5,5-dimethyl-1-pyrroline 1-oxide.
FIG. 7.
Transfection with si-c-Jun attenuates SOD expression in Cr(VI)-exposed cells. (A) JB6 cells were incubated with Na2Cr2O7 at various concentrations (0–10μM) for 24 h, and then the protein levels were detected by Western blot analysis. (B) Cells were transfected with si-c-Jun for 24 h, were exposed to 10μM Na2Cr2O7 for additional 24 h, and then analyzed by Western blot. (C) The cellular protein levels of SOD1 or SOD2 were calculated using densitometry software and are expressed as the mean ± SE relative to the control after normalizing the bands to β-actin. Representative data were shown from triplicate experiments. *p < 0.05 and **p < 0.01versus the unexposed control. si-GAPDH and β-actin were used as control si-RNA and control protein, respectively.
FIG. 8.
SOD overexpression attenuates c-Jun knockdown–mediated increasing cell death in Cr(VI)-exposed cells. SOD1-Myc-DDK– and SOD2-EGFP–tagged plasmids were transfected into the JB6 cells. Overexpressed cellular levels of the proteins specific for SOD1 (A) or SOD2 (B) were confirmed by Western blotting. (C) SOD1 or SOD2 transformed JB6 cells were transfected with si-c-Jun for 24 h and incubated with Na2Cr2O7 (10μM) for additional 48 h, after which an MTT assay was performed. The different superscripts represent significant differences (p < 0.05) between the groups according to Duncan's multiple range tests. β-actin and pCMV6 were used as a control protein and control vector, respectively.
c-Jun Acts Upstream Effecter of p53 in Cr(VI)-Exposed Skin Epidermal Cells
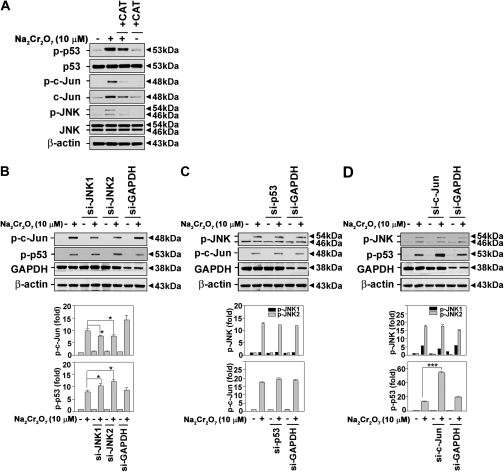
We previously demonstrated that Cr(VI) caused apoptotic cell death in JB6 cells, where ROS generation and p53 upregulation play important roles (Son et al., 2010a). We examined the effects of Cr(VI) on the phosphorylation of JNK and c-Jun in the presence of CAT. We also evaluated whether there are the correlation between JNK-mediated signaling and p53 expression. Treating the cells with CAT (500 U/ml) diminished the Cr(VI)-mediated increases in p-c-Jun, p-JNKs, and p-p53 levels (Fig. 9A). These findings suggested that Cr(VI)-generated ROS participated in the activation of JNK/c-Jun–mediated signaling. The inhibition of JNK by transfection with specific si-RNA attenuated Cr(VI)-mediated induction of p-c-Jun levels, but there was increase in the p-p53 level (Fig. 9B). When the cells were transfected with si-p53, the levels of p-JNK and p-c-Jun were not affected regardless of the presence of Cr(VI) (Fig. 9C). si-c-Jun transfection did not change the phosphorylation state of JNK in Cr(VI)-exposed cells, although it did lead to a dramatic increase in p-p53 levels (p < 0.001) (Fig. 9D). These results suggested that the activation of c-Jun may play a role in cell survival by suppressing p53 expression as well as by regulating SOD expression in Cr(VI)-exposed cells.
FIG. 9.
Signal transduction pathways involved in Cr(VI)-induced apoptosis in skin epidermal cells. (A) Cells were treated with CAT (500 U/ml) in the presence or absence of Na2Cr2O7 (10μM) for 24 h. Cells were transfected with si-RNA specific to JNK1, JNK2 (B), p53(C), and c-Jun (D) for 24 h, after which they were exposed to 10μM Na2Cr2O7 for an additional 24 h, and protein levels were detected by immunoblot analysis. si-GAPDH and β-actin were used as control si-RNA and control protein, respectively. The intensity of bands was expressed as the mean ± SE relative to the control of triplicate experiments after normalizing the bands to β-actin. *p < 0.05 and ***p < 0.001 versus the Cr(VI) treatment alone (ANOVA, Scheffe's test).
DISCUSSION
We have previously reported that Cr(VI) induced apoptosis through ROS-mediated p53 activation in JB6 cells (Son et al., 2010a). However, the p53 signaling pathway did not fully explain the mechanisms underlying Cr(VI)-induced apoptosis. The purpose of the present study is to determine whether there are other mediators, which participate in the induction of apoptosis in Cr(VI)-exposed cells. In this study, we have investigated specific mechanisms associated with JNK activation in response to ROS induced by Cr(VI) in JB6 cells. JNK exerts dual roles. It facilitates Cr(VI)-mediated apoptosis through the augmentation of caspase activation and mitochondrial stress. It also activates antiapoptotic c-Jun signaling in Cr(VI)-exposed cells, which leads to diminished p53 activation through upregulation of SOD expression.
Previous studies have demonstrated that Cr(VI) induced cell death by apoptosis (Bagchi et al., 2001; Pritchard et al., 2001; Singh et al., 1998). In parallel, our current findings show that Cr(VI) induces phosphorylation of JNKs on skin epidermal cells in a dose- and time-dependent manner, which causes cell death through apoptotic mechanisms. Cr(VI)-induced cytotoxicity and apoptosis were decreased by si-JNK transfection (Figs. 1 and 2). This is because the increases in activated forms of cleaved caspase-3, Bid, and PARP by Cr(VI) were attenuated in si-JNK transfection (Figs. 2A and 2B). Moreover, mitochondrial membrane depolarization by Cr(VI) was significantly inhibited by si-JNK transfection (Fig. 3). However, JNK-mediated signaling did not play a major role in Cr(VI)-exposed cell death. This is because knockdown of JNKs with si-RNA prevented only 20–30% of Cr(VI)-induced apoptosis or loss of ΔΨm. Our previous study demonstrated that p53 signaling was also involved of Cr(VI)-induced apoptosis in JB6 cells (Son et al., 2010a). Mitochondria play an important role in apoptosis induced by caspase-dependent or independent pathways, with Bcl-2 family proteins being critical for this induction. Our data show that decreases in Bcl-2 and Bcl-xL levels are attenuated by either si-JNK1 or si-JNK2 transfection (Fig. 2A). This indicates that activation of JNK by Cr(VI) causes downregulation of Bcl-2 and Bcl-xL proteins. Bcl-2 and Bcl-xL are the major antiapoptotic proteins integrated in the mitochondrial membrane. They prevent apoptosis through either heterodimerization with proapoptotic proteins or their direct pore-forming effects on the outer membrane of mitochondria (Gross et al., 1999; Harris and Thompson, 2000; Nechushtan et al., 2001).
Although there is considerable evidence that c-Jun activation leads to apoptosis, c-Jun has diverse functions in stress response and apoptosis. For example, c-Jun is a positive regulator of cell growth in cultured fibroblast and hepatoblasts (Eferl et al., 1999; Hilberg et al., 1993; Johnson et al., 1993). Moreover, the antiapoptotic function of c-Jun has been demonstrated in UV-induced cell death (Wisdom et al., 1999) and is consistent with increased apoptosis in cultured hepatoblasts and erythroblasts lacking c-Jun (Eferl et al., 1999). Therefore, the effect of c-Jun activation depends on the cell context and stimuli. In this study, we found that c-Jun acted in a prosurvival manner in Cr(VI)-exposed JB6 cells. Figure 5 shows that knocking down c-Jun expression by si-RNA transfection significantly enhances cytotoxicity and apoptosis in Cr(VI)-exposed cells. Moreover, decreases in ΔΨm are augmented by si-c-Jun transfection. These effects seem to be caused by c-Jun inhibition of p53 activity, as evidenced by the dramatic increase in phosphorylated p53 levels following si-c-Jun transfection (Fig. 9D). These findings demonstrate that c-Jun acts as an antiapoptotic factor rather than an effecter of apoptosis in Cr(VI)-exposed cells. c-Jun acts as a negative regulator of p53 expression by directly interacting with the p53 promoter (Schreiber et al., 1999). However, our promoter assay shows that p53 luciferase activity is not significantly changed by si-c-Jun transfection (data not shown). This means that c-Jun regulates p53 activation indirectly. One possible mechanism by which c-Jun suppresses p53 is that c-Jun regulates SOD expression. ESR and fluorescence analyses have revealed that intracellular production of by Cr(VI) increases in si-c-Jun–transfected cells when compared with cells treated with Cr(VI) alone (Fig. 6). Our previous studies, as well as those by other groups, have shown that Cr(VI) leads to the generation of ROS such as ·OH, , and H2O2 (Bagchi et al., 2001; Ding and Shi, 2002; Son et al., 2010a; Wang et al., 2000). Cr(VI) directly generates ROS during its reduction and subsequent reaction with cellular small molecules, such as glutathione and H2O2 (Shi and Dalal, 1988, 1990a,b). Moreover, cells stimulated by Cr(VI) also generate ROS through nicotinamide adenine dinucleotide phosphate oxidase activation (Shi and Dalal, 1989, 1990d; Wang et al., 2000). Otherwise, cells contain several ROS scavenging enzymes, such as CAT, GPX, and SOD. SOD is one of the primary antioxidant enzymes. In eukaryotic cells, there are two main forms of intracellular SOD: manganese-containing SOD(MnSOD) and copper/zinc-containing SOD(Cu/ZnSOD). MnSOD (SOD2) is located in the mitochondria matrix, whereas Cu/ZnSOD (SOD1) is found primarily in the cytoplasm. SOD regulates cell growth by changing the level of intracellular ROS (Liu et al., 2002). In the present study, the expression of SOD1 and SOD2 are decreased by si-c-Jun transfection in Cr(VI)-exposed cells when compared with the cells treated with Cr(VI) alone (Fig. 7). However, the expression of CAT or GPX1 is not changed by si-c-Jun transfection. When cells overexpressed SOD1 or SOD2, Cr(VI)-induced cell death was attenuated (Fig. 8). This result shows that SOD reduces ROS production and prevents cell death in Cr(VI)-exposed JB6 cells. Moreover, the increase in cell death by c-Jun knockdown with si-RNA was prevented by both SOD1 and SOD2 overexpression. These results demonstrate that c-Jun regulates an intracellular balance in ROS levels through SOD expression in Cr(VI)-exposed JB6 cells. Although, overexpression of both SOD1 and SOD2 is effective, SOD2-overexpressed cells show more a pronounced protective effect than SOD1-overexpressed cells. This suggests that c-Jun more closely regulates SOD2 than SOD1 in Cr(VI)-exposed JB6 cells. Furthermore, the knockdown of c-Jun with si-RNA transfection increases (Fig. 6); this increase in ROS levels accelerates activation of p53 signaling (Fig. 9D). In addition, CAT downregulates Cr(VI)-induced activation of p53, JNK, and c-Jun (Fig. 9A). These results indicate that intracellular ROS generated by Cr(VI) is closely involved in Cr(VI)-induced apoptosis. Presumably, the signaling pathways of JNK, c-Jun, and p53 are simultaneously activated by Cr(VI) in JB6 cells.
In summary, this study demonstrates the dual effects of JNK signaling in Cr(VI)-exposed cells. JNK induces apoptosis through the caspase-dependent and mitochondria-mediated pathways, where the decreases in Bcl-2/Bcl-xL level, activation of Bid through caspase-8 signaling, and mitochondrial stress lead to caspase activation and PARP cleavage. c-Jun signaling induced by JNK is a survival mechanism against Cr(VI)-induced apoptosis through upregulation of SOD expression in skin epidermal cells (Fig. 10). This study provides a further understanding of cell survival or death mechanisms in heavy metal–exposed skin epidermal cells.
FIG. 10.
The signaling pathways of JNK in Cr(VI)-exposed skin epidermal cells. Cr(VI) increases intracellular ROS level in JB6 cells. The ROS generated by Cr(VI) contribute to JNK activation. The activated JNK leads to mitochondrial dysfunction and apoptosis. c-Jun, which was activated by JNK, mediates survival signaling through inhibition of p53 activation because of upregulation of SOD expression in Cr(VI)-exposed JB6 cells.
FUNDING
National Institutes of Health (R01ES015518, 1R01CA119028, R01ES015375, 1R01CA116697); National Institute of Environmental Health Sciences training grant (T32ES07266).
Acknowledgments
We thank Dr Min Ding for stably transfected JB6 cells with AP-1-luciferase reporter plasmid.
References
- Bagchi D, Bagchi M, Stohs SJ. Chromium (VI)-induced oxidative stress, apoptotic cell death and modulation of p53 tumor suppressor gene. Mol. Cell. Biochem. 2001;222:149–158. [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chen L, Ovesen JL, Puga A, Xia Y. Distinct contributions of JNK and p38 to chromium cytotoxicity and inhibition of murine embryonic stem cell differentiation. Environ. Health Perspect. 2009;117:1124–1130. doi: 10.1289/ehp.0800157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SM, Liou GY, Yang JL. Activation of JNK, p38 and ERK mitogen-activated protein kinases by chromium(VI) is mediated through oxidative stress but does not affect cytotoxicity. Carcinogenesis. 2000;21:1491–1500. [PubMed] [Google Scholar]
- Clerk A, Fuller SJ, Michael A, Sugden PH. Stimulation of “stress-regulated” mitogen-activated protein kinases (stress-activated protein kinases/c-Jun N-terminal kinases and p38-mitogen-activated protein kinases) in perfused rat hearts by oxidative and other stresses. J. Biol. Chem. 1998;273:7228–7234. doi: 10.1074/jbc.273.13.7228. [DOI] [PubMed] [Google Scholar]
- Cohen MD, Kargacin B, Klein CB, Costa M. Mechanisms of chromium carcinogenicity and toxicity. Crit. Rev. Toxicol. 1993;23:255–281. doi: 10.3109/10408449309105012. [DOI] [PubMed] [Google Scholar]
- Cossarizza A, Baccarani-Contri M, Kalashnikova G, Franceschi C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolcarbocyanine iodide (JC-1) Biochem. Biophys. Res. Commun. 1993;197:40–45. doi: 10.1006/bbrc.1993.2438. [DOI] [PubMed] [Google Scholar]
- Costa M. Toxicity and carcinogenicity of Cr(VI) in animal models and humans. Crit. Rev. Toxicol. 1997;27:431–442. doi: 10.3109/10408449709078442. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Transcriptional regulation by MAP kinases. Mol. Reprod. Dev. 1995;42:459–467. doi: 10.1002/mrd.1080420414. [DOI] [PubMed] [Google Scholar]
- Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Ding M, Shi X. Molecular mechanisms of Cr(VI)-induced carcinogenesis. Mol. Cell. Biochem. 2002;234–235:293–300. [PubMed] [Google Scholar]
- Ding M, Shi X, Dong Z, Chen F, Lu Y, Castranova V, Vallyathan V. Freshly fractured crystalline silica induces activator protein-1 activation through ERKs and p38 MAPK. J. Biol. Chem. 1999;274:30611–30616. doi: 10.1074/jbc.274.43.30611. [DOI] [PubMed] [Google Scholar]
- Eferl R, Sibilia M, Hilberg F, Fuchsbichler A, Kufferath I, Guertl B, Zenz R, Wagner EF, Zatloukal K. Functions of c-Jun in liver and heart development. J. Cell Biol. 1999;145:1049–1061. doi: 10.1083/jcb.145.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Liu Z, Qi W, Shi X, Zhai Q. Chromium (VI) induces insulin resistance in 3T3-L1 adipocytes through elevated reactive oxygen species generation. Free Radic. Res. 2008;42:554–563. doi: 10.1080/10715760802155113. [DOI] [PubMed] [Google Scholar]
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Hansen MB, Menne T, Johansen JD. Cr(III) and Cr(VI) in leather and elicitation of eczema. Contact Dermatitis. 2006;54:278–282. doi: 10.1111/j.0105-1873.2006.00824.x. [DOI] [PubMed] [Google Scholar]
- Harris MH, Thompson CB. The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ. 2000;7:1182–1191. doi: 10.1038/sj.cdd.4400781. [DOI] [PubMed] [Google Scholar]
- Hilberg F, Aguzzi A, Howells N, Wagner EF. c-Jun is essential for normal mouse development and hepatogenesis. Nature. 1993;365:179–181. doi: 10.1038/365179a0. [DOI] [PubMed] [Google Scholar]
- Hodges NJ, Adam B, Lee AJ, Cross HJ, Chipman JK. Induction of DNA-strand breaks in human peripheral blood lymphocytes and A549 lung cells by sodium dichromate: association with 8-oxo-2-deoxyguanosine formation and inter-individual variability. Mutagenesis. 2001;16:467–474. doi: 10.1093/mutage/16.6.467. [DOI] [PubMed] [Google Scholar]
- Johnson RS, van Lingen B, Papaioannou VE, Spiegelman BM. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 1993;7:1309–1317. doi: 10.1101/gad.7.7b.1309. [DOI] [PubMed] [Google Scholar]
- Joseph P, He Q, Umbright C. Heme-oxygenase 1 gene expression is a marker for hexavalent chromium-induced stress and toxicity in human dermal fibroblasts. Toxicol. Sci. 2008;103:325–334. doi: 10.1093/toxsci/kfn048. [DOI] [PubMed] [Google Scholar]
- Kim G, Yurkow EJ. Chromium induces a persistent activation of mitogen-activated protein kinases by a redox-sensitive mechanism in H4 rat hepatoma cells. Cancer Res. 1996;56:2045–2051. [PubMed] [Google Scholar]
- Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Leppa S, Bohmann D. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene. 1999;18:6158–6162. doi: 10.1038/sj.onc.1203173. [DOI] [PubMed] [Google Scholar]
- Li JJ, Westergaard C, Ghosh P, Colburn NH. Inhibitors of both nuclear factor-kappaB and activator protein-1 activation block the neoplastic transformation response. Cancer Res. 1997;57:3569–3576. [PubMed] [Google Scholar]
- Liu SL, Lin X, Shi DY, Cheng J, Wu CQ, Zhang YD. Reactive oxygen species stimulated human hepatoma cell proliferation via cross-talk between PI3-K/PKB and JNK signaling pathways. Arch. Biochem. Biophys. 2002;406:173–182. doi: 10.1016/s0003-9861(02)00430-7. [DOI] [PubMed] [Google Scholar]
- Munoz C, Pascual-Salcedo D, Castellanos MC, Alfranca A, Aragones J, Vara A, Redondo JM, de Landazuri MO. Pyrrolidine dithiocarbamate inhibits the production of interleukin-6, interleukin-8, and granulocyte-macrophage colony-stimulating factor by human endothelial cells in response to inflammatory mediators: modulation of NF-kappa B and AP-1 transcription factors activity. Blood. 1996;88:3482–3490. [PubMed] [Google Scholar]
- Nechushtan A, Smith CL, Lamensdorf I, Yoon SH, Youle RJ. Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J. Cell Biol. 2001;153:1265–1276. doi: 10.1083/jcb.153.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSHA. Occupational exposure to hexavalent chromium. Final rule. Fed. Regist. 2006;71:10099–10385. [PubMed] [Google Scholar]
- Pritchard DE, Ceryak S, Ha L, Fornsaglio JL, Hartman SK, O'Brien TJ, Patierno SR. Mechanism of apoptosis and determination of cellular fate in chromium(VI)-exposed populations of telomerase-immortalized human fibroblasts. Cell Growth Differ. 2001;12:487–496. [PubMed] [Google Scholar]
- Qian Y, Luo J, Leonard SS, Harris GK, Millecchia L, Flynn DC, Shi X. Hydrogen peroxide formation and actin filament reorganization by Cdc42 are essential for ethanol-induced in vitro angiogenesis. J. Biol. Chem. 2003;278:16189–16197. doi: 10.1074/jbc.M207517200. [DOI] [PubMed] [Google Scholar]
- Samet JM, Graves LM, Quay J, Dailey LA, Devlin RB, Ghio AJ, Wu W, Bromberg PA, Reed W. Activation of MAPKs in human bronchial epithelial cells exposed to metals. Am. J. Physiol. 1998;275:L551–L558. doi: 10.1152/ajplung.1998.275.3.L551. [DOI] [PubMed] [Google Scholar]
- Schreiber M, Kolbus A, Piu F, Szabowski A, Mohle-Steinlein U, Tian J, Karin M, Angel P, Wagner EF. Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev. 1999;13:607–619. doi: 10.1101/gad.13.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelnutt SR, Goad P, Belsito DV. Dermatological toxicity of hexavalent chromium. Crit. Rev. Toxicol. 2007;37:375–387. doi: 10.1080/10408440701266582. [DOI] [PubMed] [Google Scholar]
- Shen HM, Liu ZG. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2006;40:928–939. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- Shi X, Chiu A, Chen CT, Halliwell B, Castranova V, Vallyathan V. Reduction of chromium(VI) and its relationship to carcinogenesis. J. Toxicol. Environ. Health B Crit. Rev. 1999;2:87–104. doi: 10.1080/109374099281241. [DOI] [PubMed] [Google Scholar]
- Shi XG, Dalal NS. On the hydroxyl radical formation in the reaction between hydrogen peroxide and biologically generated chromium(V) species. Arch. Biochem. Biophys. 1990a;277:342–350. doi: 10.1016/0003-9861(90)90589-q. [DOI] [PubMed] [Google Scholar]
- Shi XL, Dalal NS. On the mechanism of the chromate reduction by glutathione: ESR evidence for the glutathionyl radical and an isolable Cr(V) intermediate. Biochem. Biophys. Res. Commun. 1988;156:137–142. doi: 10.1016/s0006-291x(88)80815-5. [DOI] [PubMed] [Google Scholar]
- Shi XL, Dalal NS. Chromium (V) and hydroxyl radical formation during the glutathione reductase-catalyzed reduction of chromium (VI) Biochem. Biophys. Res. Commun. 1989;163:627–634. doi: 10.1016/0006-291x(89)92183-9. [DOI] [PubMed] [Google Scholar]
- Shi XL, Dalal NS. ESR spin trapping detection of hydroxyl radicals in the reactions of Cr(V) complexes with hydrogen peroxide. Free Radic. Res. Commun. 1990b;10:17–26. doi: 10.3109/10715769009145929. [DOI] [PubMed] [Google Scholar]
- Shi XL, Dalal NS. Evidence for a Fenton-type mechanism for the generation of •OH radicals in the reduction of Cr(VI) in cellular media. Arch. Biochem. Biophys. 1990c;281:90–95. doi: 10.1016/0003-9861(90)90417-w. [DOI] [PubMed] [Google Scholar]
- Shi XL, Dalal NS. NADPH-dependent flavoenzymes catalyze one electron reduction of metal ions and molecular oxygen and generate hydroxyl radicals. FEBS Lett. 1990d;276:189–191. doi: 10.1016/0014-5793(90)80539-u. [DOI] [PubMed] [Google Scholar]
- Singh J, Carlisle DL, Pritchard DE, Patierno SR. Chromium-induced genotoxicity and apoptosis: relationship to chromium carcinogenesis (review) Oncol. Rep. 1998;5:1307–1318. doi: 10.3892/or.5.6.1307. [DOI] [PubMed] [Google Scholar]
- Son YO, Hitron JA, Wang X, Chang Q, Pan J, Zhang Z, Liu J, Wang S, Lee JC, Shi X. Cr(VI) induces mitochondrial-mediated and caspase-dependent apoptosis through reactive oxygen species-mediated p53 activation in JB6 Cl41 cells. Toxicol. Appl. Pharmacol. 2010a;245:226–235. doi: 10.1016/j.taap.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YO, Jang YS, Shi X, Lee JC. Activation of JNK and c-Jun is involved in glucose oxidase-mediated cell death of human lymphoma cells. Mol. Cells. 2009;28:545–551. doi: 10.1007/s10059-009-0149-1. [DOI] [PubMed] [Google Scholar]
- Son YO, Lee JC, Hitron JA, Pan J, Zhang Z, Shi X. Cadmium induces intracellular Ca2+- and H2O2-dependent apoptosis through JNK- and p53-mediated pathways in skin epidermal cell line. Toxicol. Sci. 2010b;113:127–137. doi: 10.1093/toxsci/kfp259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohs SJ, Bagchi D, Hassoun E, Bagchi M. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J. Environ. Pathol. Toxicol. Oncol. 2000;19:201–213. [PubMed] [Google Scholar]
- Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura JJ, Kennedy NJ, Lamb JA, Flavell RA, Davis RJ. c-Jun NH(2)-terminal kinase is essential for the regulation of AP-1 by tumor necrosis factor. Mol. Cell. Biol. 2003;23:2871–2882. doi: 10.1128/MCB.23.8.2871-2882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Leonard SS, Ye J, Ding M, Shi X. The role of hydroxyl radical as a messenger in Cr(VI)-induced p53 activation. Am. J. Physiol. Cell Physiol. 2000;279:C868–C875. doi: 10.1152/ajpcell.2000.279.3.C868. [DOI] [PubMed] [Google Scholar]
- Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem. J. 1998;333(Pt 2):291–300. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom R, Johnson RS, Moore C. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J. 1999;18:188–197. doi: 10.1093/emboj/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wise JP, Patierno SR. DNA damage induced by carcinogenic lead chromate particles in cultured mammalian cells. Mutat. Res. 1992;280:129–136. doi: 10.1016/0165-1218(92)90008-n. [DOI] [PubMed] [Google Scholar]
- Ye J, Zhang X, Young HA, Mao Y, Shi X. Chromium(VI)-induced nuclear factor-kappa B activation in intact cells via free radical reactions. Carcinogenesis. 1995;16:2401–2405. doi: 10.1093/carcin/16.10.2401. [DOI] [PubMed] [Google Scholar]
- Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J. Exp. Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]