Abstract
We have shown previously that chronic (32 weeks) exposure to occupationally relevant concentrations of the environmental pollutant trichloroethylene (TCE) induced autoimmune hepatitis (AIH) in autoimmune-prone MRL+/+ mice. In real-life, individuals are never exposed to only one chemical such as TCE. However, very little is known about the effects of chemical mixtures on the immune system. The current study examined whether coexposure to another known immunotoxicant, mercuric chloride (HgCl2), altered TCE-induced AIH. Female MRL+/+ mice were treated for only 8 weeks with TCE (9.9 or 186.9 mg/kg/day in drinking water) and/or HgCl2 (260 μg/kg/day, sc). Unlike mice exposed to either TCE or HgCl2 alone, mice exposed to both toxicants for 8 weeks developed significant liver pathology commensurate with early stages of AIH. Disease development in the coexposed mice was accompanied by a unique pattern of anti-liver and anti-brain antibodies that recognized, among others, a protein of approximately 90 kDa. Subsequent immunoblotting showed that sera from the coexposed mice contained antibodies specific for heat shock proteins, a chaperone protein targeted by antibodies in patients with AIH. Thus, although TCE can promote autoimmune disease following chronic exposure, a shorter exposure to a binary mixture of TCE and HgCl2 accelerated disease development. Coexposure to TCE and HgCl2 also generated a unique liver-specific antibody response not found in mice exposed to a single toxicant. This finding stresses the importance of including mixtures in assessments of chemical immunotoxicity.
Keywords: mercury, trichloroethylene, MRL+/+ mice, Co-exposure, liver, autoimmune hepatitis, CD4+ T cells cytokines, autoimmunity
There is increasing evidence that chronic exposure to certain toxicants at levels too low to be overtly toxic can harm human health by altering immune function. Although many of the environmental chemicals that affect the immune system in humans are suppressive, others such as trichloroethylene (TCE) can be proinflammatory. TCE is a solvent and degreasing agent whose improper disposal has led to it becoming a widespread environmental pollutant. Although exposure to TCE is relatively common, it rarely occurs at levels thought to be directly toxic. However, a number of epidemiological reports and case studies have linked chronic low-level TCE exposure to a variety of autoimmune diseases in humans including lupus, scleroderma, bullous pemphigoid, diabetes, and immune-mediated hepatitis (Byers et al., 1988; Dubrow and Gute, 1987; Flindt-Hansen and Isager, 1987; Gist and Burg, 1995; Hansen and Isager, 1988; Lockey et al., 1997; Saihan et al., 1978; Yanez Diaz et al., 1992). Even if overt disease is not diagnosed, signs of immune activation including increased numbers of T cells and elevated serum levels of autoantibodies (antinuclear antibodies [ANA]) have been associated with chronic exposure to a domestic water supply contaminated with TCE (Byers et al., 1988; Clark et al., 1994; Kilburn and Washaw, 1992; Nietert et al., 1998). Similarly, occupational exposure to TCE resulted in significantly increased serum levels of the T cell–derived proinflammatory cytokine interferon-gamma (IFN-γ) (Iavicoli et al., 2005). Aside from humans, mice chronically exposed (26–48 weeks) to low-level TCE have demonstrated an increased incidence of autoimmune hepatitis (AIH) (Griffin et al., 2000b) and increased production of lupus-related autoantibodies (Cai et al., 2008; Keil et al., 2009).
Delineating the role of TCE in the development of autoimmune disease would be easier if it were understood why only some people seemingly exposed to similar amounts of the toxicant develop autoimmune disease. This discrepancy may be due in part to genetic differences in susceptibility factors. However, equally important is the fact that no individual is exposed to a single toxicant but rather a unique but ill-defined set of environmental toxicants with potentially interactive effects. Multiple chemicals can have discrete mechanisms of action with the cumulative effect on the immune system difficult to predict. Little is known about the possible modulating effects of other chemicals on TCE-induced immunotoxicity. Most coexposure studies with TCE have used the liver or kidney as endpoints. Only a few studies have examined the effects of TCE-containing mixtures on the immune system, and because these examined a mixture of at least four chemicals, it was impossible to determine the contribution of TCE (Vodela et al., 1997; Yang, 1993). The weight-of-evidence approach to examining mixtures dictates that a mixture is broken down into binary mixtures in order to examine the interaction potential of each pair. Consequently, this study examined TCE immunotoxicity in the context of a TCE-containing binary mixture.
The second chemical that was selected for examination here was mercury. Human exposure to mercury is widespread because of its existence as a natural element in the environment and its anthropogenic release from industrial use. A recent analysis of women in the National Health and Nutrition Examination Survey 1999–2006 data sets showed that inorganic mercury detection increased dramatically from 2% in 1999–2000 to 30% in 2005–2006 with compelling evidence of age-dependent mercury deposition (Laks, 2009). Mercury has been implicated as a cofactor in systemic human autoimmunity, where studies showed that mercury exposure increased levels of antinucleolar antibodies (ANoA) (Cooper et al., 2004; Gardner et al., 2010). The ability of mercury to promote autoimmunity has been especially well documented in mice where it promotes autoantibodies specific for fibrillarin and other nuclear antigens, such as chromatin, and induces immune complex-mediated lupus nephritis (Hultman et al., 1996; Pollard et al., 1997).
The 2007 Comprehensive Environmental Response, Compensation and Liability Act Priority List of Hazardous Substances, compiled by the Agency for Toxic Substances and Disease Registry after considering toxicity, frequency, and potential for human exposure, listed mercury and TCE as 3rd and 16th, respectively, out of 275 environmental toxicants. They are both considered Superfund chemicals and are often found together. According to the National Priorities List, approximately 44% of the active Superfund sites contaminated with TCE also list mercury as a contaminant. Because exposure to both TCE and mercury is not unlikely, the question of how exposure to a binary mixture of these toxicants affects the immune system is a relevant one. The two toxicants appear to have effects that are overlapping. Both TCE and mercury increase CD4+ T-cell production of IFN-γ and inhibit activation-induced cell death in CD4+ T cells (Gilbert et al., 2006; Laiosa et al., 2007). In view of their similar effects, it is possible that coexposure to mercury and TCE could induce autoimmunity under conditions when single toxicant exposures would not. This possibility was tested here.
MATERIALS AND METHODS
Mice.
Eight-week-old female MRL+/+ mice (Jackson Laboratories, Bar Harbor, ME) were housed in polycarbonate ventilated cages and provided with laboratory drinking water ad libitum. TCE (purity 99+%; Aldrich Chemical Co. Inc., Milwaukee, WI) was suspended in drinking water with 1% emulsifier Alkamuls EL-620 from Rhone-Poulenc (Cranbury, NJ). The mice (six mice per group) received 0, 0.1, or 2.0 mg/ml TCE in their drinking water for 8 weeks. Freshly made TCE-containing drinking water was provided every 2–3 days. Subsets of mice were injected sc twice per week for 8 weeks with 100 μl PBS alone or containing 40 μg mercuric chloride (HgCl2). The mice were weighed weekly. In addition to prebleeds, retro-orbital blood samples were obtained at 4, 6, and 8 weeks of treatment. All studies were approved by the Animal Care and Use Committee at the University of Arkansas for Medical Sciences.
Histopathology.
At the time of sacrifice, dorsal skin, the right kidneys, and the caudal lobes of the livers were fixed in 10% neutral buffered formalin. Tissues were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. Liver, skin, and kidney sections were examined microscopically and scored in a blinded manner by a veterinary pathologist for the severity of mononuclear cell infiltration and other tissue-specific changes in morphology based on a four-point scale. The location and cellular composition of all inflammatory infiltrates were recorded. A chi-square test was conducted to compare cumulative tissue pathology scores in toxicant-treated versus control mice.
Western blotting to detect tissue-specific antibody production.
Equal amounts (30 μg) of microsomal or cytosolic liver protein (prepared as described; Halmes et al., 1996) or brain (cerebellum) protein obtained from the control or treated MRL+/+ mice were separated on 12% SDS-polyacrylamide gel electrophoresis (PAGE), electrotransferred onto nitrocellulose, and subsequently immunoblotted with pooled sera (1:1000) from control or treated MRL+/+ mice collected at 4- or 8-week time points followed by HRP-conjugated goat anti-mouse immunoglobulin G (IgG) (1:10,000). Immunodetection was performed by Super Signal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL). In one set of experiments, recombinant heat shock proteins (HSP)-90α protein (StressMarq Biosciences, Canada) (4 micrograms per lane) was run as the target protein on the SDS-PAGE and then immunoblotted with the pooled 8-week sera as described above. In some cases, 200 μg of pooled microsomal liver or brain protein were incubated with Dynabeads M-280 Streptavidin (Invitrogen Life Sciences, Carlsbad, CA), which had been preconjugated with biotinylated donkey anti-mouse IgG (Jackson ImmunoResearch). The beads were removed using a Dynal MPC magnet, and the beads and remaining protein lysates were separated by SDS-PAGE and then immunoblotted with the goat-anti-mouse IgG.
Quantitative PCR.
Fluorescence-based quantitative PCR (qPCR) was conducted using RNA isolated (using RNEasy, Qiagen, Germantown, MD) from frozen (−80°C) liver and cerebella of individual mice. For each sample, complementary DNA (cDNA) was synthesized with random hexamers and 0.5 μg of total RNA using an iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Inc., Hercules, CA). PCR primer sequences were designed using the National Center for Biotechnology Information gene databases and synthesized by IDT, Inc. (Coralville, IA) using PrimerQuest. All qPCR reactions were carried out using iQ SYBR Green Supermix (Bio-Rad Laboratories) and the IQ5 iCycler (Bio-Rad Laboratories, Inc.) according to manufacturer’s protocols. No-template controls were included to detect primer-dimer artifacts. DNA melting curve analysis was used as another control against primer-dimer formation and to ensure the lack of contaminating DNA in the RNA preparations. Cq values > 36 were not reported because of implied low efficiency. The relative gene expression was calculated using the delta-delta Cq method based on normalization to the reference gene eukaryotic translation elongation factor 2. The Cq value of this reference gene did not vary more than 5% among the treatment groups and was found to be superior to β-actin, 18S ribosomal RNA, and glyceraldehyde-3-phosphate dehydrogenase in terms of copy number and treatment-associated invariance in the tissues tested. Primer optimization was conducted, and the PCR amplification efficiency for the reference gene and the genes of interest was > 90% but < 110%, with the R2 value > 0.9. The results were reported as fold change in liver or brain tissue from treated compared with expression in tissue from control mice.
Phenotypic analysis of spleen cells.
The flow cytometric analysis of spleen cells from six individual mice per group was conducted as previously described (Blossom et al., 2007). The phenotypic analysis of 30,000 events per sample was carried out using a CyFlow ML (Partec GmbH, Munster, Germany), and the data were presented as mean ± SE of the different cell subsets. Nonviable cells, based on low forward scatter and side scatter, were excluded in each sample. Fluorescence Minus One controls and isotype Ig controls were included.
Cytokine analysis.
CD4+ T cells isolated from the spleens of individual mice using Dynabeads Mouse CD4 (Invitrogen Corp.) were stimulated with immobilized anti-CD3 antibody (10 μg/ml) and anti-CD28 antibody (1 μg/ml) for 24 h. The 99+% culture supernatants were examined using ELISA kits for IFN-γ (OptEIA Set Mouse IFN-γ, BD Biosciences, San Diego, CA), interleukin (IL)-17 (RayBiotech, Inc., Norcross, GA), or osteopontin (Assay Designs, Detroit, MI).
Detection of ANA, ANoA, and total IgG.
ANA and ANoA were examined in the sera obtained from each animal after 4, 6, or 8 weeks of toxicant exposure. This was done using indirect immunofluorescence and commercially available fixed HEp-2 cell substrates (Antibodies, Inc., Davis, CA). The sera from individual mice were tested at 1:50, 1:100, and 1:200 dilutions. Detecting antibodies specific for mouse IgG1 and IgG2a were used to examine isotype-specific ANA and ANoA. Each ANA/ANoA result was interpreted under blinded conditions, and the results were represented as the mean reciprocal titer that provided a positive score. Total levels of IgG1 and IgG2a in the blood from individual mice were determined in triplicate wells using an ELISA as described (Blossom et al., 2007). A standard curve was used to determine concentrations of total IgG2a, although consistent problems with the myeloma used to generate the IgG1 standard curve resulted in total IgG1 levels being reported in terms of relative optical density.
Statistics.
The data are presented as means and SDs. Most assays, unless stated in the figure legends, were conducted using samples from six individual mice per treatment group. Among-group comparisons of mean values were made using a one-way ANOVA and post hoc analysis. Chi-square distribution was used to determine the association between the categorical variables for tissue pathology and treatment groups using SAS v 9.2 (SAS Institute, Inc., Cary, NC). The threshold for statistical significance was set at α = 0.05.
RESULTS
Coexposure Promoted AIH
Based on water intake, body weight, and measured TCE degradation in the water bottles, the mice given water with TCE present at 0.1 or 2.0 mg/ml were exposed to an average of 9.9 or 186.9 mg/kg/day TCE, respectively. The current 8-h permissible exposure limit (PEL) established by the Occupational Safety and Health Administration for TCE is 100 ppm or approximately 76 mg/kg/day. Thus, the concentrations of TCE used in the current study were approximately 13 or 246% of the PEL. The mice that were exposed to HgCl2 received approximately 260 μg/kg/day of this second toxicant. To put this in perspective, the Environmental Protection Agency has designated the Lowest-Observed-Adverse-Effect-Level for autoimmune effects following chronic oral exposure to HgCl2 as 317 μg/kg/day.
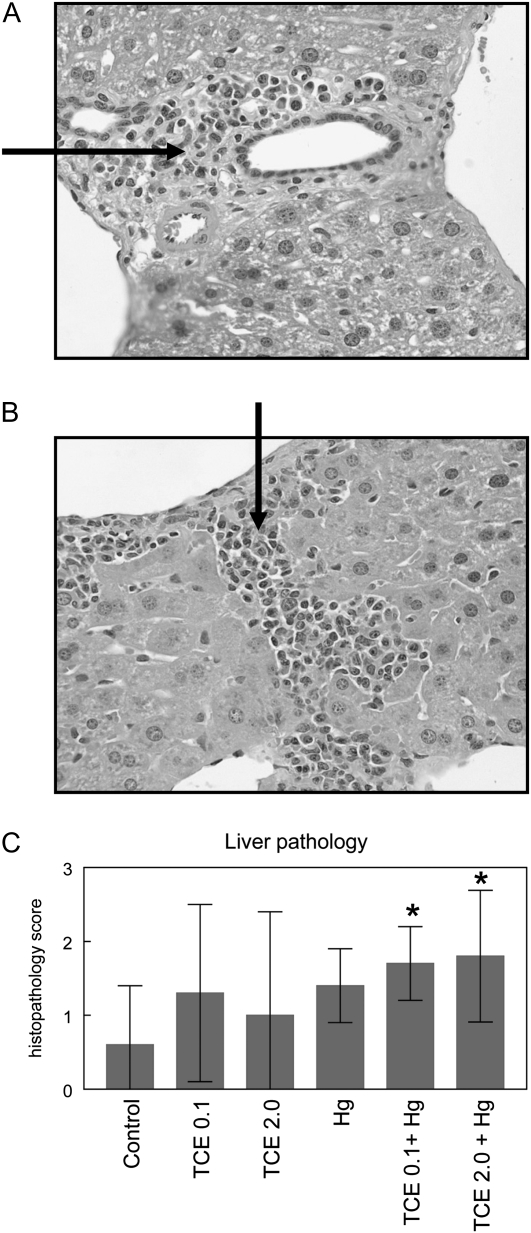
Histological analysis of liver, kidney, and skin from each mouse was conducted to examine possible TCE- and/or HgCl2-induced tissue pathology. Based on cumulative scores concerning mononuclear cell infiltration, fibrosis, necrosis, and hepatocellular enlargement, liver pathology from mice exposed to HgCl2 and either 0.1 or 2.0 mg/ml TCE, 0.1 was significantly different from that of control mice ( and , respectively). Representative liver sections from an HgCl2- and TCE-treated mouse are presented in Figure 1. The livers of mice exposed to HgCl2 or TCE alone for 8 weeks exhibited no significant pathology. None of the mice exhibited significant kidney or skin pathology regardless of treatment. Taken together, the results indicate that although an 8-week exposure to TCE alone at the concentrations used here was not sufficient to promote pathology, coexposure to both TCE and HgCl2 did induce significant early liver pathology. Thus, coexposure to HgCl2 appeared to enhance the autoimmune-promoting effects of TCE.
FIG. 1.
Coexposure to TCE and HgCl2 promoted AIH. Shown are representative liver sections (×400) demonstrating portal inflammation with fibrosis (A) and centrilobular inflammation (B) in liver sections from mice exposed to both TCE (2 mg/ml) and HgCl2. The arrows indicate areas of mononuclear infiltration. (C) Cumulative histopathology scores for liver sections. *Statistically different from the results obtained using liver sections from control mice.
Physical Characteristics
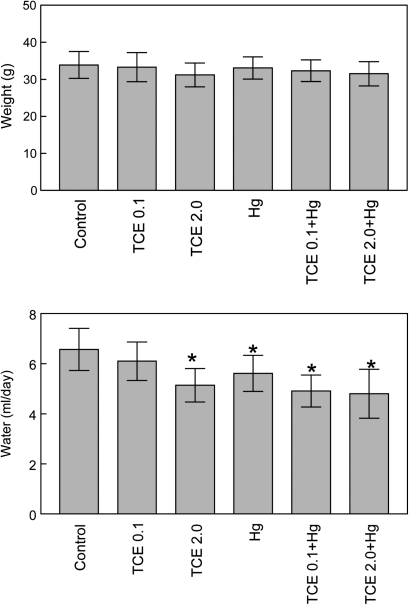
Body weight was not affected by exposure to TCE and/or HgCl2 (Fig. 2). A decrease in water consumption was seen in several of the groups exposed to TCE and/or HgCl2. However, because the toxicant-treated mice exhibited normal weight gain and showed no signs of dehydration (e.g., piloerection, lethargy, or recessed eyes), it was not clear that their decreased water consumption was functionally significant.
FIG. 2.
Effects of TCE and HgCl2 on weight gain and water consumption. Female MRL+/+ mice were given water alone (controls), water with TCE, and/or HgCl2 for 8 weeks. Body weight was monitored over time and is presented as mean ± SD. Water consumption was monitored over time and is presented as mean ± SD per mouse per day for the entire 8-week time period. *Statistically different from the results obtained from control mice.
Coexposure Did Not Alter Lymphoid Organ Cellularity
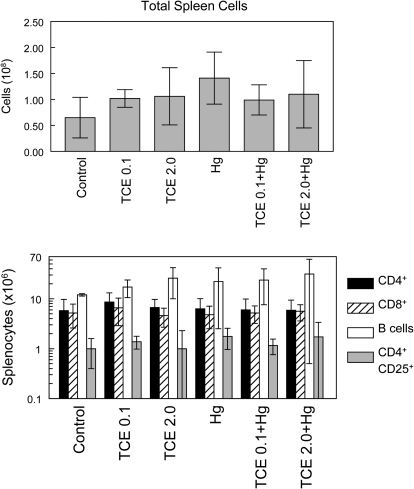
Cellularity of the spleen and lymph nodes from individual mice was examined as a potential measurement of toxicant-induced immunotoxicity. As shown in Figure 3, no differences in total spleen cell numbers were observed in any of the groups. Subsets of splenocytes were also examined. None of the toxicant combinations altered numbers of total splenic CD4+ T cells, CD8+ T cells, or B cells. The number of splenic CD4+ CD25+ T cells (a phenotype often used to designate TReg cells but also found on some activated non-TReg CD4+ T cells) was also unaltered by toxicant exposure. Similarly, none of the treatment regimens altered the percentages of CD4+ T cells or CD8+ T cells in lymph nodes (data not shown). Thus, the tissue pathology observed in the coexposed mice could not be attributed to an alteration in the percentage of the measured lymphoid subsets.
FIG. 3.
Effects of TCE and HgCl2 on splenic cellularity. Spleen cells from six individual mice per group treated with water alone (controls), water with TCE, and/or HgCl2 for 8 weeks were evaluated for total cell number. The spleen cells were also evaluated for total numbers of CD4+ T cells, CD8+ T cells, B cells, and CD4+ CD25+ cells. The data are presented as the mean ± SD.
Effects of Coexposure on Cytokine Production
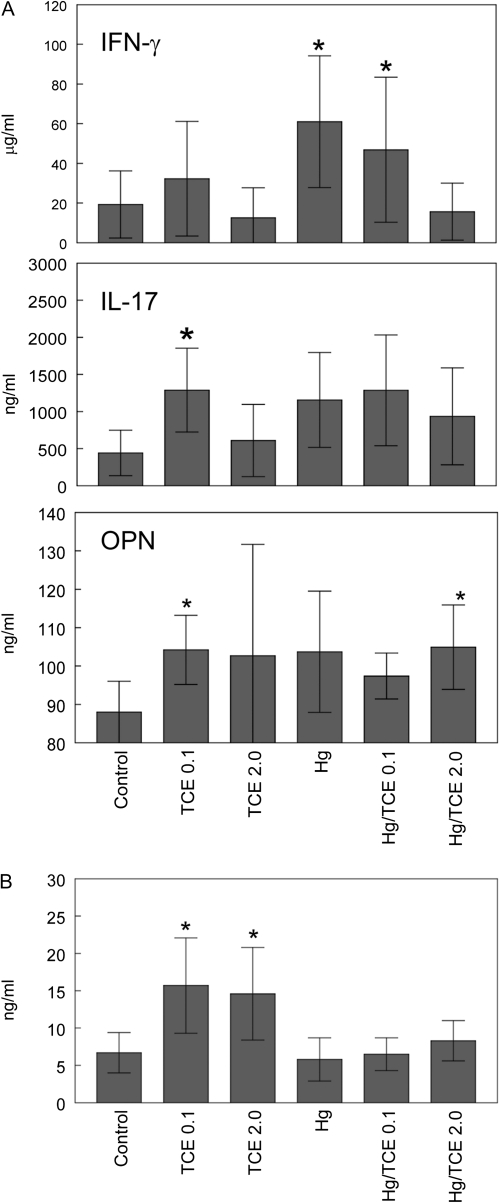
CD4+ T-cell production of cytokines associated with autoimmune inflammation, including IFN-γ, IL-17, and osteopontin, was examined using culture supernatants of isolated CD4+ T cells stimulated with anti-CD3 and anti-CD28 antibodies. As shown in Figure 4A, intragroup variation was large resulting in only a few groups demonstrating statistical difference from controls. Exposure to 0.1 mg/ml TCE alone increased production of both IL-17 and osteopontin. Osteopontin was also produced in higher amounts by CD4+ T cells from the mice exposed to TCE (2.0 mg/ml) and HgCl2. Because blood levels of osteopontin, unlike many other cytokines, can change during autoimmune disease development, we also examined this cytokine in the mouse sera. Interestingly, mice exposed to either concentration of TCE alone demonstrated increased levels of osteopontin in the blood (Fig. 4B). Thus, levels of IFN-γ, IL-17, and osteopontin did get upregulated in response to TCE and/or HgCl2, even if these effects were not directly correlated to pathology.
FIG. 4.
Effects of TCE and HgCl2 on cytokine production. (A) CD4+ T cells isolated from the spleens of individual mice were stimulated with anti-CD3 and anti-CD28 antibodies. The resulting culture supernatants were tested for levels of IFN-γ, IL-17, and osteopontin. (B) Sera were tested for levels of osteopontin. The data are presented as the mean ± SD, with asterisks designating results statistically different from controls.
Alterations in Liver and Brain Gene Expression
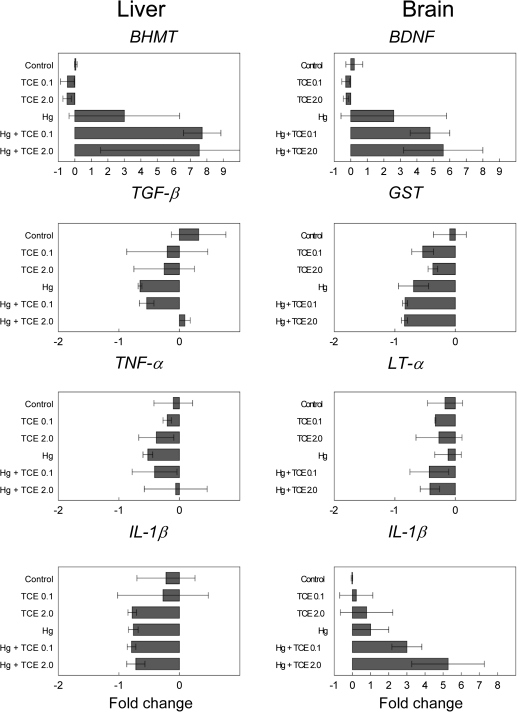
Hepatic expression of genes that might promote liver inflammation was examined for a potential role in the generation of the liver-reactive antibodies. No increase was observed in the expression of proinflammatory cytokines IL-1β or tumor necrosis factor-α (TNF-α) (Fig. 5). Expression of IFN-γ was undetectable in the liver. Depletion of the immunosuppressive cytokine transforming growth factor-β (TGF-β) in the liver has been shown to promote T cell–mediated inflammatory liver disease (Gorham et al., 2001). Although TGF-β expression was decreased in some of the treatment groups in this study, the decrease did not correlate with liver pathology. Neither CYP2E1 nor CYP1A2, genes that encode for TCE-metabolizing enzymes, was increased in the liver tissue from any treatment groups (data not shown). Only betaine homocystein methytransferase (BHMT) was increased in the livers from the coexposed mice. Because BHMT upregulation is usually thought of as a compensatory protective mechanism designed to maintain levels of S-adenosylmethionine, the likelihood that this alteration promoted autoimmunity seems low. However, this effect did underscore the additive effect of coexposure on liver gene expression.
FIG. 5.
Effects of coexposure to TCE and HgCl2 on gene expression in liver and cerebellum. qPCR was performed using liver or cerebellum from three individual mice per treatment group collected after 8 week exposure to TCE and/or HgCl2.
The HgCl2 did not induce measurable nephritis. However, the central nervous system is even more sensitive to the effects of mercury than the kidney. Consequently, toxicant-induced alterations in the expression of genes associated with inflammation and repair were examined in the cerebellum, a brain region likely to demonstrate inflammatory and oxidative stress–related events. As shown in Figure 5, TCE at the concentrations used here did not alter expression of the genes selected. However, when used in combination with HgCl2, TCE increased expression of brain-derived neurotrophic factor (BDNF), an active member of the neurotrophin family that is often altered in brain-associated disease states. Unlike in the liver, coexposure also increased expression of the proinflammatory cytokine IL-1β in the cerebellum. Neither TCE nor HgCl2 alone altered expression of these two genes. Exposure to HgCl2 and/or TCE did not increase glutathione S-transferase, Lymphotoxin-α (Fig. 5), or other inflammation-related genes such as inducible nitric oxide synthase, TNF-α, or IL-6 (data not shown). Even though the functional consequences of the coexposure-induced increased expression of BDNF and IL-1β in the cerebellum are not yet known, this finding once again demonstrated that mixtures can be more powerful than single chemicals.
Coexposure Increased Serum Parameters of Autoimmunity
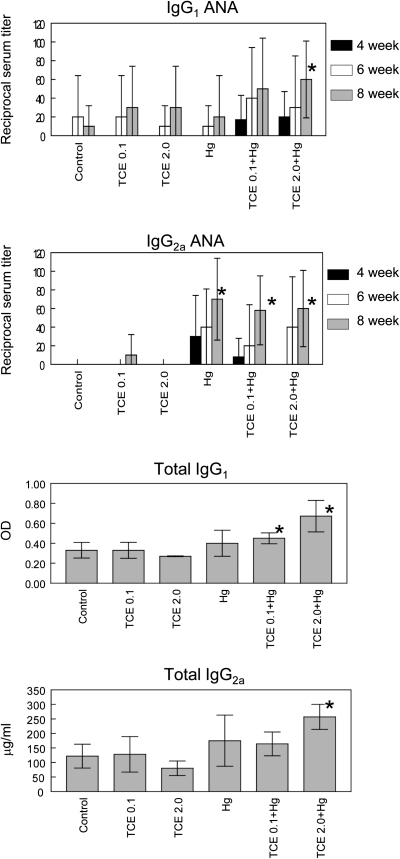
At certain concentrations, TCE has been shown to transiently increase the production of ANA in MRL+/+ mice (Cai et al., 2008). HgCl2, on the other hand, is most noted for its ability to induce ANoA that target fibrillarin (Pollard et al., 1997). Although the role of ANA and ANoA in tissue pathology is not clear, they can be used for diagnostic purposes. Consequently, both ANA and ANoA were examined in the serum of individual mice exposed to TCE and/or HgCl2. As shown in Figure 6, IgG1 ANA levels were increased at the final time point in the mice exposed to both Hg and 2.0 mg/ml TCE, whereas IgG2a ANA was increased at that time point in all the groups exposed to HgCl2. Levels of ANoA were low and not altered in any of the groups (data not shown). Autoimmunity can also be associated with an increase in total Ig levels. As shown in Figure 5, coexposure to TCE and HgCl2 increased levels of total IgG1 and IgG2a. This effect was also evident at the 6-week time point (data not shown). Taken together, although none of the mice developed detectable nephritis, the immune pathology most often associated with autoantibody production, coexposure to HgCl2 and TCE did increase levels of ANA and total IgG1 and IgG2a. Once again, coexposure promoted serum parameters of autoimmunity, whereas single exposures were ineffective at the time points and concentrations used here.
FIG. 6.
Coexposure to TCE and HgCl2 increased autoantibody production. Sera obtained at 4, 6, or 8 weeks from individual mice treated with water alone (controls), water with TCE, and/or HgCl2 were evaluated for IgG1 and IgG2a ANA. The ANA results are presented as the inverse of the highest dilution that provided a positive score. These results duplicate those found in an earlier analysis conducted with fewer sera but a wider dilution range. Levels of total IgG1 and IgG2a in the sera collected at the 8-week time point are also shown. *Statistically different from results in control mice.
Toxicant-Induced Tissue-Reactive Antibodies Minimal at 4 Weeks
TCE has been shown to stimulate the time- and dose-dependent generation of antibodies that react with liver proteins (Gilbert et al., 2009). The autoantibodies induced by HgCl2 are best known as targeting nucleolar proteins; their specificity for tissue proteins such as those found in the liver has not been extensively examined. Because autoantibodies can be used to help identify the autoantigens targeted by an autoimmune response, we tested the sera from the mice exposed here to TCE and/or HgCl2 for their immunoreactivity with different target tissues including microsomal liver and cerebellum.
The tissue-specific reactivity was tested using sera collected at 4 and 8 weeks. The anti-liver staining pattern produced by sera collected at the 4-week time point showed little differences among the groups exposed to TCE alone (Fig. 7A). However, liver proteins between 30 and 37 kDa were detected by antibodies in the sera mice exposed to HgCl2 ± TCE. Another exception was the appearance of an antibody in the mice treated with TCE (2.0 mg/ml) and HgCl2 that recognized a protein between 75 and 100 kDa in liver tissue. Interestingly, a brain protein of similar molecular weight was stained by sera from the same treatment group.
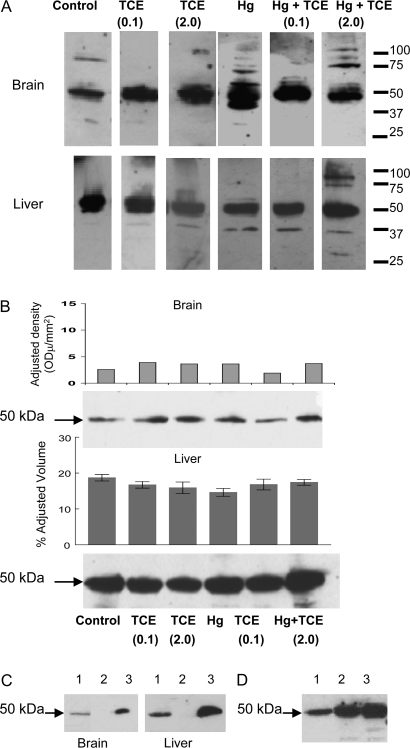
FIG. 7.
Detecting TCE- and HgCl2-induced antibodies after 4 week exposure. (A) Pooled microsomal liver or brain protein collected from control MRL+/+ mice (treated with water alone for 8 weeks) was separated by SDS-PAGE and immunoblotted using pooled sera from pooled control or treated MRL+/+ mice collected at the 4-week time point, followed by a goat anti-mouse IgG antibody. (B) Brain or microsomal liver proteins from control MRL+/+ mice or MRL+/+ mice exposed to either TCE and/or HgCl2 for 8 weeks were separated by SDS-PAGE and immunoblotted with goat anti-mouse IgG antibody alone. Densitometric analysis of the 50-kDa protein was conducted. This experiment was repeated with brain tissue, and mean densitometric values (± SD) from three experiments are presented. (C) Pooled brain or microsomal liver protein from control MRL+/+ mice was separated by SDS-PAGE and immunoblotted with goat anti-mouse IgG antibody (lane 1). Alternatively, IgG was immunoprecipitated from the protein lysates using beads conjugated with donkey anti-mouse IgG; the remaining lysates (lanes 2) or resulting beads (lanes 3) were separated by SDS-PAGE and immunoblotted with goat anti-mouse IgG. (D) Microsomal liver protein from an untreated MRL+/+ mouse (lane 1), C57BL/6 mouse (lane 2), or C3H/HeJ mouse (lane 3) was separated by SDS-PAGE and immunoblotted with goat anti-mouse IgG antibody.
All the Western blots obtained using 4-week sera, regardless of treatment group, revealed a protein of approximately 50 kDa in both brain and liver. A subsequent analysis in which brain and liver tissue from all the groups were immunoblotted with only the secondary goat anti-mouse IgG detected bands at a similar molecular weight (Fig. 7B). Densitometric analysis showed no differences among the groups in terms of the amount of the 50 kDa detected in liver or brain. Immunoprecipitation with anti-mouse IgG removed the 50-kDa protein from the protein (Fig. 7C), further suggesting that both brain and liver from the MRL+/+ mice, regardless of treatment, contained mouse IgG, the heavy chain of which is approximately 50 kDa. The same 50-kDa protein was found in microsomal liver protein from non-autoimmune C57Bl/6 and C3H/HeJ mice (Fig. 7D). The significance of IgG in the microsomal fraction of liver protein is not known; however, the detection of the 50-kDa protein in the liver and brain lysates provided an internal reference point for the individually stained Western blots.
Coexposure for 8 Weeks Promoted Unique Patterns of Tissue-Reactive Antibodies
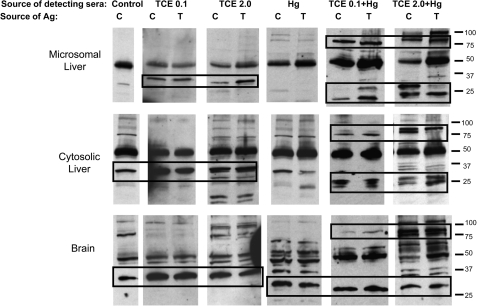
Unlike the 4-week sera, sera from mice exposed to TCE and/or HgCl2 for 8 weeks revealed several interesting differences in immunoreactivity among the groups. In case reactivity depended upon toxicant-induced adduct formation, protein from both control and treated mice was used as a target for immunoblotting. For example, a preparation of pooled sera from mice treated with TCE at 0.1 mg/ml in the water was tested for its ability to stain control brain protein as well as brain protein from the mice treated with TCE at 0.1 mg/ml. Using this approach, we found that sera from mice exposed to either 0.1 or 2.0 mg/ml TCE in drinking water contained antibodies that recognized liver microsomal proteins around 30–37 kDa (Fig. 8). Interestingly, this reactivity was observed regardless of whether the source of the liver tissue was control mice or the same mice as the source of sera. Unlike exposure to TCE alone, exposure to TCE plus HgCl2 or HgCl2 alone generated little or no antibodies specific for 30- to 33-kDa liver proteins. Indeed, the liver-specific immunoblotting detected using sera from mice exposed to HgCl2 alone was similar to that of control mice. However, sera from mice exposed to both HgCl2 and TCE produced a unique staining pattern for liver microsomal proteins of approximately 90 kDa as well as proteins of approximately 20–30 kDa. Neither of these sets of proteins was detected by sera from mice exposed to TCE or HgCl2 alone. Thus, coexposure to TCE and HgCl2 generated liver-specific antibodies not found in the blood of mice exposed to either toxicant alone. Antibodies generated by exposure to TCE and HgCl2 appeared to recognize liver microsomal proteins from either control or toxicant-treated mice. In other words, the toxicant-induced liver-specific antibodies were not restricted to haptenated proteins. Very similar results were obtained if cytosolic rather than microsomal liver protein was used as the target of the immunoblotting.
FIG. 8.
Coexposure to TCE and HgCl2 for 8 weeks generated unique pattern of anti-liver and anti-brain antibodies. Pooled microsomal liver, cytosolic liver, or brain protein collected at the 8-week time point from control (C) mice (treated with water alone) or from mice treated (T) with TCE and/or HgCl2 was separated by SDS-PAGE. This protein was then immunoblotted using pooled sera from control or treated mice collected at the 8-week time point, followed by a goat anti-mouse IgG antibody. In all cases, the mice that provided the protein in the “T” lane were the same mice that provided the detecting sera.
When antibodies reactive with brain antigens were examined, it appeared that sera from mice exposed to TCE alone differed very little when compared with sera from control mice (Fig. 8). In contrast to control sera, sera from mice exposed to HgCl2 alone stained an array of brain proteins. Most striking was the fact that HgCl2 ± TCE stimulated production of an antibody specific for a brain protein of approximately 30–33 kDa. Interestingly, mice exposed to both HgCl2 and TCE, but not those exposed to HgCl2 alone, generated antibodies that recognized a brain protein of approximately 90 kDa. Thus, several of the brain proteins recognized by the sera from the toxicant-treated mice were of similar molecular weight to liver microsomal proteins recognized by the same sera. Also similar was the fact that coexposure to both TCE and HgCl2 generated some brain-specific and liver-specific antibodies not found in sera from mice exposed to either toxicant alone.
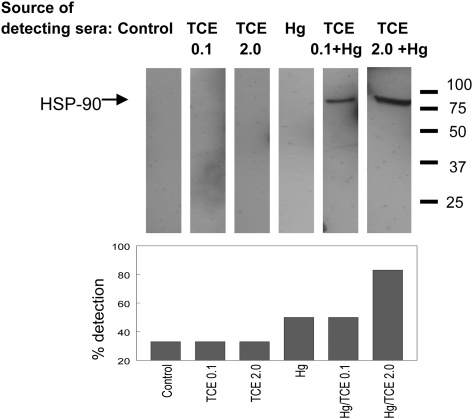
A recent proteomic study using sera from humans with idiopathic AIH demonstrated the presence of an autoantibody specific for HSP-90, a 90-kDa protein in the liver plasma membrane (Tahiri et al., 2008). Using recombinant HSP-90 as the target antigen in Western blotting, we found that sera from mice coexposed to TCE and HgCl2 were much more likely than sera from mice exposed to TCE or HgCl2 alone to contain anti-HSP antibodies (Fig. 9).
FIG. 9.
Detecting anti-HSP-90 antibodies after 8 week exposure to TCE and HgCl2. SDS-PAGE was performed using recombinant HSP-90α as the source of protein. The protein was then immunoblotted using pooled detecting sera collected from the different treatment groups at 8 weeks, followed by a goat anti-mouse IgG antibody. This approach was repeated using individual or pairs of pooled sera (n = 3 or 4 per group), and the bar graph describes the percentage of these Western blots in which anti-HSP-90 was detected.
DISCUSSION
Previous studies have shown that MRL+/+ mice given drinking water with 0.5 or 2.5 mg/ml TCE for 26–32 weeks developed liver inflammation commensurate with AIH (Gilbert et al., 2009; Griffin et al., 2000a). Liver inflammation was not seen in mice exposed to those concentrations of TCE for a shorter (4 weeks) time period. Similarly, in the study here, liver inflammation was not found in MRL+/+ mice treated here with TCE alone (0.1 or 2.0 mg/ml) in drinking water for 8 weeks. However, modest but significant liver inflammation was observed if the TCE-treated mice were coexposed to HgCl2. Exposure to HgCl2 is not known to promote liver inflammation. It is more likely that HgCl2 augmented the liver inflammation induced by TCE. HgCl2 has similarly been shown in a mouse model of arthritis to enhance rather than initiate disease development (Hansson et al., 2005).
The assessment of autoantibodies and total serum Ig indicated that coexposure to TCE and HgCl2 generated the most robust increase. Exposure to approximately 1.5 mg/kg/week HgCl2 for 4–8 weeks has been shown to alter several serological parameters in mice including the generation of ANoA and an increase in total IgG1 and IgG2a. Mouse strains vary in their susceptibility to these effects of HgCl2 with H-2s mice such as B10.S and A.SW being particularly susceptible. However, MRL+/+ mice are also considered susceptible to the effects of HgCl2 (Pollard et al., 1999). In the current study, the mice exposed to HgCl2 alone did demonstrate increased levels of IgG2a ANA. Conversely, other serum parameters of autoimmunity were only found in mice exposed to both TCE and HgCl2. Because TCE exposure alone was not sufficient to promote the increase in ANA or total IgG1 or IgG2a, it must be inferred that HgCl2 contributes to these increases.
TCE and HgCl2 also appeared to work together to generate a unique pattern of liver-associated antibodies. Hepatic seropositivity is an important component of a definitive diagnosis of AIH in humans. In the current study, sera from mice coexposed to TCE and HgCl2 contained antibodies that stained liver proteins of approximately 90 kDa as well as proteins in the 25- to 35-kDa range. Unlike coexposure, exposure to just TCE or HgCl2 alone did not appear to stimulate antibodies for these liver proteins.
Sera from the mice were also tested for brain-reactive antibodies. Neither TCE nor HgCl2 as individual chemicals is known for their ability to generate anti-brain antibodies. In this study, sera from mice treated with TCE alone immunoblotted brain protein in a manner indistinguishable from control sera. Immmunoblotting using sera from mice exposed to HgCl2, alone or together with TCE, demonstrated reactivity with a brain protein between 25 and 37 kDa. However, coexposure to HgCl2 and TCE generated unique brain-reactive antibodies not found in mice exposed to either toxicant alone. Most notable was the immunoblotting of a brain protein of approximately 90 kDa similar to that seen using liver as the target antigen. Thus, similar to the findings obtained used liver as the target tissue, coexposure appeared to generate a unique pattern of brain-specific autoantibodies.
HSP-90 was considered as a candidate for the high molecular weight liver and brain protein targeted by autoantibodies in the coexposed mice. HSP-90 is an abundant and widely distributed protein found in both liver and brain. Using recombinant HSP-90 as an antigen, Western blotting showed that this protein was recognized by antibodies in sera from mice coexposed to TCE and HgCl2. HSP-90 is a constitutively expressed protein that can be upregulated by various environmental stresses including high temperature, infections, and chemical exposure. HSPs associate with a broad arrays of proteins in the cell, and there is extensive evidence that HSP-protein complexes generated in the cell and released after cell death are very immunogenic, generating immune responses to both protein and HSP (Nemoto et al., 1997). How exactly HSPs act as adjuvants is not known but HSPs complexed to peptides have been shown to enhance peptide processing and Major Histocompatability complex class II presentation to T cells (Tobian et al., 2004).
Blood and/or tissue levels of HSP-90, as well as anti-HSP-90 autoantibodies, have been shown to be increased in several autoimmune diseases including rheumatoid arthritis, Systemic Lupus Erythematosus, and AIH (Hayem et al., 1999; Ripley et al., 2001; Tahiri et al., 2008). Anti-HSP-90 antibodies have been shown to develop in MRL+/+ mice as they age, becoming detectable when they develop lupus around 40 weeks of age (Faulds et al., 1995). Because the MRL+/+ mice in the current study were approximately 20 weeks of age at the end of the experiment, it appears that coexposure to TCE and mercury accelerated the generation of anti-HSP-90 antibodies. It is not clear whether these antibodies are pathogenic, protective, or merely serve as markers of inflammation. AIH is after all thought to be primarily T-cell mediated. The generation of anti-HSP-90 antibodies may involve activation of HSP-90–specific CD4+ T cells. Alternatively, these antibodies may be generated via linked recognition by CD4+ T cells specific for some other self-antigen complexed to HSP-90. In either case, the pathogenicity of the CD4+ T cells involved in anti-HSP-90 antibody production remains to be determined.
Toxicant-induced liver inflammation in the current study may depend on toxicant-induced protein alterations. TCE exposure has been shown to modify liver proteins in MRL+/+ mice in a manner that increases their immunogenicity (Halmes et al., 1996). It has been proposed that metabolites of TCE form adducts with self-proteins in the liver and stimulate an immune response against the modified (haptenated) proteins. Haptenated proteins have been shown to have unusually immunostimulatory potential demonstrated at the level of antibody production and at the level of T-cell proliferation (Willis et al., 2003). However, there is some disagreement over whether haptenated proteins promote an immune response to the haptenated protein, the carrier protein, or both. If TCE-induced haptenation does promote liver-specific autoimmunity in the present study, it must be concluded that coexposure to mercury somehow enhances this effect because autoimmunity was not observed in mice exposed for 8 weeks to TCE alone. Second, if haptenation is involved in initiating liver-specific autoimmunity, the response it generates must encompass both modified and unmodified liver proteins; the sera from the coexposed mice reacted similarly to tissue from untreated and treated mice.
Delineating the mechanisms of immunotoxicity in humans is complicated by the need to consider the cumulative effect of multiple toxicants. The majority of current toxicological research with animal models focuses on a single substance. However, it is possible that exposure to mixtures of immunotoxicants with additive or synergistic effects can promote autoimmunity at concentrations that would not generate measurable responses for single toxicants. This might explain why autoimmunity can be linked to a chemical such as TCE even though it is rarely found at concentrations thought to be directly toxic. On the other hand, simultaneous exposure to an immunostimulatory chemical and one with immunosuppressive capacity could result in a null response. The cumulative effect of multiple toxicants with competing or additive mechanisms presumably determines the final immunological response.
FUNDING
National Institutes of Health (1R01ES017286 to K.M.G.); Organic Compounds Property Contamination class action settlement (CV 1992-002603) and the Arkansas Biosciences Institute to K.M.G.
Acknowledgments
We would like to gratefully acknowledge the excellent technical assistance of Ashley Nelson, Trey Fleet, Meagan Kreps, and Chase Lambert.
References
- Blossom SJ, Doss JC, Gilbert KM. Chronic exposure to a trichloroethylene metabolite in autoimmune-prone MRL+/+ mice promotes immune modulation and alopecia. Toxicol. Sci. 2007;95:401–411. doi: 10.1093/toxsci/kfl149. [DOI] [PubMed] [Google Scholar]
- Byers VS, Levin AS, Ozonoff DM, Baldwin RW. Association between clinical symptoms and lymphocyte abnormalities in a population with chronic domestic exposure to industrial solvent-contaminated domestic water supply and a high incidence of leukemia. Cancer Immunol. Immunother. 1988;27:77–82. doi: 10.1007/BF00205762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai P, Konig R, Boor PJ, Kondraganti S, Kaphalia BS, Khan MF, Ansari GA. Chronic exposure to trichloroethene causes early onset of SLE-like disease in female MRL +/+ mice. Toxicol. Appl. Pharmacol. 2008;228:68–75. doi: 10.1016/j.taap.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LC, Giulano A, Walsh B, Guernsey de Zaplen J, Alberts DS, Meister J, Manson TS. The Santa Cruz County Community Health Survey. Phoenix, AZ: Arizona Department of Health Services; 1994. [Google Scholar]
- Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkeson GS, Dooley MA. Occupational risk factors for the development of systemic lupus erythematosus. J. Rheumatol. 2004;31:1928–1933. [PubMed] [Google Scholar]
- Dubrow R, Gute DM. Cause-specific mortality among Rhode Island jewelry workers. Am. J. Ind. Med. 1987;12:579–593. doi: 10.1002/ajim.4700120511. [DOI] [PubMed] [Google Scholar]
- Faulds G, Conroy S, Madaio M, Isenberg D, Latchman D. Increased levels of antibodies to heat shock proteins with increasing age in Mrl/Mp-lpr/lpr mice. Br. J. Rheumatol. 1995;34:610–615. doi: 10.1093/rheumatology/34.7.610. [DOI] [PubMed] [Google Scholar]
- Flindt-Hansen H, Isager H. Scleroderma after occupational exposure to trichloroethylene and trichlorethane. Toxicol. Lett. 1987;95:173–181. [PubMed] [Google Scholar]
- Gardner RM, Nyland JF, Silva IA, Ventura AM, de Souza JM, Silbergeld EK. Mercury exposure, serum antinuclear/antinucleolar antibodies, and serum cytokine levels in mining populations in Amazonian Brazil: a cross-sectional study. Environ. Res. 2010;110:345–354. doi: 10.1016/j.envres.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert KM, Przybyla B, Pumford NR, Han T, Fuscoe J, Schnackenberg LK, Holland RD, Doss JC, MacMillan-Crow LA, Blossom SJ. Delineating liver events in trichloroethylene-induced autoimmune hepatitis. Chem. Res. Toxicol. 2009;22:626–632. doi: 10.1021/tx800409r. [DOI] [PubMed] [Google Scholar]
- Gilbert KM, Pumford NR, Blossom SJ. Environmental contaminant trichloroethylene promotes autoimmune disease and inhibits T-cell apoptosis in MRL+/+ mice. J. Immunotoxicol. 2006;3:263–267. doi: 10.1080/15476910601023578. [DOI] [PubMed] [Google Scholar]
- Gist GL, Burg JR. Trichloroethylene—a review of the literature from a health effects perspective. Toxicol. Ind. Health. 1995;11:253–307. doi: 10.1177/074823379501100301. [DOI] [PubMed] [Google Scholar]
- Gorham JD, Lin JT, Sung JL, Rudner LA, French MA. Genetic regulation of autoimmune disease: BALB/c background TGF-beta 1-deficient mice develop necroinflammatory IFN-gamma-dependent hepatitis. J. Immunol. 2001;166:6413–6422. doi: 10.4049/jimmunol.166.10.6413. [DOI] [PubMed] [Google Scholar]
- Griffin JM, Blossom SJ, Jackson SK, Gilbert KM, Pumford NR. Trichloroethylene accelerates an autoimmune response in association with Th1 T cell activation in MRL+/+ mice. Immunopharmacology. 2000a;46:123–137. doi: 10.1016/s0162-3109(99)00164-2. [DOI] [PubMed] [Google Scholar]
- Griffin JM, Gilbert KM, Lamps LW, Pumford NR. CD4+ T cell activation and induction of autoimmune hepatitis following trichloroethylene treatment in MRL+/+ mice. Toxicol. Sci. 2000b;57:345–352. doi: 10.1093/toxsci/57.2.345. [DOI] [PubMed] [Google Scholar]
- Halmes NC, McMillan DC, Oatis JE, Pumford NR. Immunochemical detection of protein adducts in mice treated with trichloroethylene. Chem. Res. Toxicol. 1996;9:451–458. doi: 10.1021/tx950171v. [DOI] [PubMed] [Google Scholar]
- Hansen BL, Isager H. A scleroderma-resembling disease-exposure to trichloroethylene and trichloroethane, is there a causal connection? Ugeskr. Laeger. 1988;150:805–808. [PubMed] [Google Scholar]
- Hansson M, Djerbi M, Rabbani H, Mellstedt H, Gharibdoost F, Hassan M, Depierre JW, Abedi-Valugerdi M. Exposure to mercuric chloride during the induction phase and after the onset of collagen-induced arthritis enhances immune/autoimmune responses and exacerbates the disease in DBA/1 mice. Immunology. 2005;114:428–437. doi: 10.1111/j.1365-2567.2005.02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayem G, De BM, Palazzo E, Roux S, Combe B, Eliaou JF, Sany J, Kahn MF, Meyer O. Anti-heat shock protein 70 kDa and 90 kDa antibodies in serum of patients with rheumatoid arthritis. Ann. Rheum. Dis. 1999;58:291–296. doi: 10.1136/ard.58.5.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman P, Turley SJ, Enestrom S, Lindh U, Pollard KM. Murine genotype influences the specificity, magnitude and persistence of murine mercury-induced autoimmunity. J. Autoimmun. 1996;9:139–149. doi: 10.1006/jaut.1996.0017. [DOI] [PubMed] [Google Scholar]
- Iavicoli I, Marinaccio A, Carelli G. Effects of occupational trichloroethylene exposure on cytokine levels in workers. J. Occup. Environ. Med. 2005;47:453–457. doi: 10.1097/01.jom.0000161728.23285.66. [DOI] [PubMed] [Google Scholar]
- Keil DE, Peden-Adams MM, Wallace S, Ruiz P, Gilkeson GS. Assessment of trichloroethylene (TCE) exposure in murine strains genetically-prone and non-prone to develop autoimmune disease. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2009;44:443–453. doi: 10.1080/10934520902719738. [DOI] [PubMed] [Google Scholar]
- Kilburn KH, Washaw RW. Prevalence of symptoms of systemic lupus erythematosus (SLE) and of fluorescent antinuclear antibodies associated with chronic exposure to trichloroethylene and other chemicals in well water. Environ. Res. 1992;57:1–9. doi: 10.1016/s0013-9351(05)80014-3. [DOI] [PubMed] [Google Scholar]
- Laiosa MD, Eckles KG, Langdon M, Rosenspire AJ, McCabe MJ., Jr Exposure to inorganic mercury in vivo attenuates extrinsic apoptotic signaling in Staphylococcal aureus enterotoxin B stimulated T-cells. Toxicol. Appl. Pharmacol. 2007;225:238–250. doi: 10.1016/j.taap.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laks DR. Assessment of chronic mercury exposure within the U.S. population, National Health and Nutrition Examination Survey, 1999-2006. Biometals. 2009;22:1103–1114. doi: 10.1007/s10534-009-9261-0. [DOI] [PubMed] [Google Scholar]
- Lockey JE, Kelly CR, Cannon GW, Colby TV, Aldrich V, Livingston GK. Progressive systemic sclerosis associated with exposure to trichloroethylene. J. Occup. Med. 1997;29:493–496. [PubMed] [Google Scholar]
- Nemoto T, Sato N, Iwanari H, Yamashita H, Takagi T. Domain structures and immunogenic regions of the 90-kDa heat-shock protein (HSP90). Probing with a library of anti-HSP90 monoclonal antibodies and limited proteolysis. J. Biol. Chem. 1997;272:26179–26187. doi: 10.1074/jbc.272.42.26179. [DOI] [PubMed] [Google Scholar]
- Nietert PJ, Sutherland SE, Silver RM, Pandey JP, Knapp RG, Hoel DG, Dosemeci M. Is occupational organic solvent exposure a risk factor for scleroderma? Arthritis Rheum. 1998;41:1111–1119. doi: 10.1002/1529-0131(199806)41:6<1111::AID-ART19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Pollard KM, Lee DK, Casiano CA, Bluthner M, Johnston MM, Tan EM. The autoimmunity-inducing xenobiotic mercury interacts with the autoantigen fibrillarin and modifies its molecular and antigenic properties. J. Immunol. 1997;158:3521–3528. [PubMed] [Google Scholar]
- Pollard KM, Pearson DL, Hultman P, Hildebrandt B, Kono DH. Lupus-prone mice as models to study xenobiotic-induced acceleration of systemic autoimmunity. Environ. Health Perspect. 1999;107(Suppl. 5):729–735. doi: 10.1289/ehp.99107s5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley BJ, Isenberg DA, Latchman DS. Elevated levels of the 90 kDa heat shock protein (hsp90) in SLE correlate with levels of IL-6 and autoantibodies to hsp90. J. Autoimmun. 2001;17:341–346. doi: 10.1006/jaut.2001.0549. [DOI] [PubMed] [Google Scholar]
- Saihan EM, Burton JL, Heaton KW. A new syndrome with pigmentation, scleroderma, gynaecomastia, Raynaud's phenomenon and peripheral neuropathy. Br. J. Dermatol. 1978;99:437–440. doi: 10.1111/j.1365-2133.1978.tb06184.x. [DOI] [PubMed] [Google Scholar]
- Tahiri F, Le NF, Huguet S, Lai-Kuen R, Samuel D, Johanet C, Saubamea B, Tricottet V, Duclos-Vallee JC, Ballot E. Identification of plasma membrane autoantigens in autoimmune hepatitis type 1 using a proteomics tool. Hepatology. 2008;47:937–948. doi: 10.1002/hep.22149. [DOI] [PubMed] [Google Scholar]
- Tobian AA, Canaday DH, Harding CV. Bacterial heat shock proteins enhance class II MHC antigen processing and presentation of chaperoned peptides to CD4+ T cells. J. Immunol. 2004;173:5130–5137. doi: 10.4049/jimmunol.173.8.5130. [DOI] [PubMed] [Google Scholar]
- Vodela JK, Renden JA, Lenz SD, McElhenney WH, Kemppainen BW. Drinking water contaminants (arsenic, cadmium, lead, benzene, and trichloroethylene). 1. Interaction of contaminants with nutritional status on general performance and immune function in broiler chickens. Poult. Sci. 1997;76:1474–1492. doi: 10.1093/ps/76.11.1474. [DOI] [PubMed] [Google Scholar]
- Willis MS, Thiele GM, Tuma DJ, Klassen LW. T cell proliferative responses to malondialdehyde-acetaldehyde haptenated protein are scavenger receptor mediated. Int. Immunopharmacol. 2003;3:1381–1399. doi: 10.1016/S1567-5769(03)00136-X. [DOI] [PubMed] [Google Scholar]
- Yanez Diaz S, Moran M, Unamuno P, Armijo M. Silica and trichloroethylene-induced progressive systemic sclerosis. Dermatology. 1992;184:98–102. doi: 10.1159/000247513. [DOI] [PubMed] [Google Scholar]
- Yang R. NTP technical report on the toxicity studies of a Chemical Mixture of 25 Groundwater Contaminants Administered in Drinking Water to F344/N Rats and B6C3F(1) Mice. Toxic. Rep. Ser. 1993;35:1–I12. [PubMed] [Google Scholar]