Abstract
The different protein isoforms generated from the vascular endothelial growth factor-A (VEGF-A) gene, an essential regulator of blood vessel formation, differ in biochemical property and functional activity. Despite the relevance of VEGF-A for both normal and pathologic angiogenesis, our understanding of the molecular mechanisms governing alternative splicing of its pre-mRNA is still in its infancy.
Key words: alternative splicing, angiogenesis, cancer, Neuropilin-1, transcription, vascular endothelial growth factor
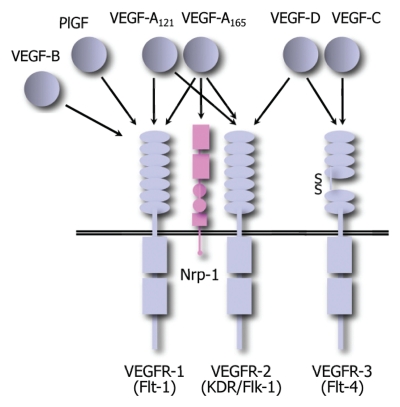
Members of the vascular endothelial growth factor (VEGF) family of secreted proteins play an essential role both during embryonic development and in the adult life. In mammals, the family consists of five members, each one encoded by a different gene (VEGF-A, -B, -C, -D and the placental growth factor PlGF). The proteins encoded by these genes act as homo- or hetero-dimers through their interaction with three structurally related tyrosine kinase receptors (VEGFR-1/Flt1, VEGFR-2/Flk-1/KDR and VEGFR-3/Flt-2) expressed on the surface of endothelial cells as well on that of several other cell types. In particular, VEGF-B and PlGF specifically interact with VEGFR-1, VEGF-C and VEGF-D with VEGFR-3, while VEGF-A interacts with both VEGFR-1 and VEGFR-2. In addition, the various VEGF family members also exert their variegated functions by interacting with the co-receptors Neuropilin-1 and -2 (Nrp-1 and -2), and by the modulation of VEGFR activity through their interaction with other cell surface molecules such as integrins and heparan sulfate proteoglycans (HSPGs), (Fig. 1; reviewed in refs. 1 and 2).
Figure 1.
Schematic representation of the interactions between the VEGF family of ligands and receptors.
A crucial angiogenic factor is VEGF-A, which is pivotal in driving vascular development in the embryo as well as regulating formation of new blood vessel and vessel homeostasis in the adult. In addition, this factor is the major angiogenic agent driving pathological blood vessel formation during tumorigenesis and vascular retinopathies, thus becoming an appealing target of various antiangiogenic therapies.
The need for timely and precisely regulated VEGF-A gene expression is highlighted by the remarkable observation that deletion of a single VEGF-A gene allele3,4 or modest gene overexpression5 both result in embryonic lethality. Not surprisingly, therefore, regulation of VEGF-A production occurs at multiple levels, including transcription, splicing, mRNA stability, translation and subcellular localization of the various factor isoforms.
Protein Isoforms Generated by Alternative Splicing of the VEGF-A pre-mRNA
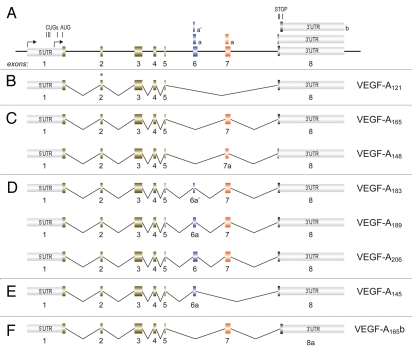
A schematic representation of the VEGF-A gene is shown in Figure 2A. The gene consists of eight exons and encompasses approximately 14 kilobases. Both the exons and introns are highly conserved between human, rodent, dog and chicken.
Figure 2.
Major mRNAs generated by alternative splicing of the VEGF-A pre-mRNA. (A) Schematic representation of the VEGF-A gene intron and exon organization. Arrows indicate transcription start site. The positions of the translation initiation and termination codons are shown. (B) mRNA with exon 5/8 junction. (C) mRNAs including exon 7. (D) mRNAs including exons 6 and 7. (E) mRNA including exon 6. (F) mRNA containing alternative exon 8b.
Canonic transcription of the VEGF-A gene is driven by a TATA-less promoter, which is exquisitely sensitive to hypoxia and to stimulation by various cytokines and growth factors; a second internal promoter instead directs basal levels of transcription in a more constitutive manner. The pre-mRNA that is generated then undergoes complex alternative processing, leading to the production of at least seven different protein isoforms of 206, 189, 183, 165, 148, 145 and 121 amino acids after removal of the signal peptide (all isoforms are one amino acid shorter in mice). The three most abundant isoforms detected in vivo are VEGF-A121, VEGF-A165 and VEGF-A189.
All the mRNAs coding for the different protein isoforms contain exons 1 to 5 in their 5′ portion and are characterized by various combinations of exons 6, 7 and 8, or part of these, in their 3′ regions (Fig. 2B–F). In the mRNA coding for the shorter isoform (VEGF-A121), exon 5 is directly spliced to the 6 aa-encoding exon 8 (Fig. 2B). Two mRNAs also contain exon 7 (coding for 44 aa) or its 5′ portion (coding for 32 aa) only. In particular, in the mRNA coding for VEGF-A165, the whole exon 7 is included between exon 5 and exon 8, while in that coding for VEGF-A148 the 5′ part of exon 7 (7a) is spliced to exon 8, generating a different open reading frame that terminates protein translation in exon 8 by the inclusion of an additional single amino acid (Fig. 2C). Three mRNA isoforms contain complete exon 6 (coding for 41 aa) or parts of exon 6 shorter at their 3′ ends, in addition to complete exon 7. These are the mRNAs coding for the longest VEGF-A isoform (VEGF-A206, containing whole exon 6) and the mRNAs coding for VEGF-A189 and VEGF-A183, which are generated using alternative 5′ donor sites that shorten the coding region to 24 aa (exon 6a) and 18 aa (exon 6a′), respectively (Fig. 2D). Finally, only one isoform (VEGF-A145) selectively contains exon 6a inserted between exons 5 and 7 (Fig. 2E).
Additional variability is generated when a 3′ splice site distal to that of exon 8 is used. This defines a shorter exon (exon 8b), the 5′ of which codes for 6 different amino acids from those encoded by classic exon 8. This distal splice site choice can occur in conjunction with exon 6 or 7 inclusion or exclusion, thus leading to the production of a family of polypeptides differing from those described above by the last six amino acids. The most prominent of these polypeptides is VEGF-A165b (Fig. 2F). Interest for the protein isoforms encoded by these exon 8b-containing mRNAs has recently risen, due to their potentially inhibitory, rather than stimulatory, effect on the VEGF receptors (reviewed in ref. 6).
Non-Redundant Functions of VEGF-A Isoforms
What is the biological significance of such a complex pattern of alternative splicing regulation? The N-terminal portion of VEGF-A, encoded by exons 1–5, is sufficient to bind and activate the canonical VEGF receptors. As a consequence, all the VEGF-A splicing isoforms can interact with VEGFR-2 and, with an affinity that is 10 times higher, with VEGFR-1. Exons 6 and 7 at the C-terminus of the protein, however, are essential in determining its bioavailability and biodistribution. In particular, the 24 aa and 44 aa stretches encoded by exons 6a and 7, respectively, are highly enriched in clusters of basic amino acids, defined by their capacity to bind negatively charged heparin (heparin binding domains, HBDs). These HBDs confer upon the protein isoforms containing them the capacity to bind cell surface and extracellular matrix heparan sulfate proteoglycans (HSPGs) and thus essentially modulate their localization in the extracellular space. VEGF-A121, the shortest isoform, lacks any HBD and is weakly acidic; thus, it is freely diffusible upon secretion. In contrast, the longer splice forms VEGF-A189 and VEGF-A206 have two HBDs, and are almost completely sequestered in the extracellular matrix and on the cell surface. VEGF-A165, the prototypic and most abundant VEGF-A isoform, differs from VEGF-A121 only by the inclusion of exon 7; its moderate affinity for heparin enables this isoform to act as both a soluble and a cell-bound factor (reviewed in ref. 7 and 8).
Based on these observations, it was originally proposed that binding to the insoluble extracellular matrix allowed the HBD-containing VEGF-A isoforms to form a concentration gradient that radiates from the ischemic areas, and that this gradient was essential for proper angiogenic stimulation. Consistent with this notion, various studies have shown that soluble VEGF-A121 is less effective than the heparin-binding isoforms in supporting the neovascularization required for tumor growth.9,10
Essential evidence of the non-redundant function of the different VEGF-A proteins was further provided by a series of genetic studies in isoform-specific knock-in mice. Whereas animals expressing only VEGF-A164 develop and grow normally, animals expressing exclusively VEGF-A120 die perinatally. These animals also show impaired coronary vascularization and ischemic cardiomyopathy, with the formation of irregular, tortuous and dilated capillaries, indicative of incomplete vessel remodeling.11 Incomplete pericyte coverage of vessels was also detected in other organs such as kidney and retina.12,13 Conversely, the insoluble isoform VEGF-A188 alone was also incapable of driving normal vessel development, resulting in abnormalities in arterial formation and vessel branching.12 Thus, these animal studies seem to indicate that a balance between diffusibility and gradient formation through HSPG binding, such as that achieved by VEGF-A164, is required for proper vascular formation.
Might the profound developmental defects observed in mice lacking VEGF-A164 and the effects on tumor vascularization only derive from the differential capacity of the various VEGF-A isoforms to bind HSPGs? An additional, crucial level of complexity to our understanding of the significance of the VEGF-A isoforms, possibly providing an answer to this question, was provided by the discovery that the 44 aa encoded by VEGF-A exon 7 are also essential to bind the co-receptor Nrp-1.14 This non-tyrosine kinase receptor was originally discovered as the main receptor for class 3 semaphorins, which provide repulsive guiding cues to axonal growth cones in the developing nervous system. On endothelial cells, Nrp-1 significantly increases the affinity of VEGF-A165, but not of VEGF-A121 lacking exon 7, to VEGFR-2, enhancing receptor activity and signaling.15–17
We have recently observed that the prolonged expression of VEGF-A165 in both normoperfused and ischemic skeletal muscle or hearts upon AAV-mediated gene transfer exerts a profound angiogenic effect, inducing the formation of a large network of capillary vessels and arterioles covered by NG2+, α-SMA+ pericytes.18–20 In sharp contrast, however, we also observed that gene transfer and prolonged expression of VEGF-A121 markedly increases capillary sprouting, but is ineffective in determining maturation of the newly formed vasculature.18 These differential effects of the two VEGF-A isoforms were correlated with their differential capacity to recruit, to the sites of neoangiogenesis, a novel population of CD11b+, Gr-1− circulating cells, deriving from the bone marrow and expressing Nrp-1. More recently, we have observed that NEMs act in a paracrine fashion by secreting PDGF-B, TGFβ and various chemokines that promote vessel maturation, thus determining a higher degree of α-SMA+/NG2+ mural cell coverage and decreased vascular leakiness (Carrer A et al., manuscript in preparation). Taken together, these observations strongly suggest that VEGF-A121 is insufficient for normal vasculogenesis in the embryo and angiogenesis in the adult, due not only to its incapacity to determine a concentration gradient through HSPG binding, but also because it fails to induce peri-endothelial cell coverage and vessel maturation through NEM recruitment by exon 7-mediated Nrp1 binding.
Finally, VEGF-A165b differs from VEGF-A165 only in the C-terminal six amino acids. The factor still binds VEGF receptors with equal affinity as the other VEGF-A ligands, but is a poor agonist of VEGFR-2, determining a distinct pattern of tyrosine phosphorylation and, most notably, fails to bind HSPGs and interact with Nrp-1, similar to VEGF-A121.21,22 Thus, VEGF-A165b might act competitively with the other VEGF-A isoforms and quench their angiogenic activity.
Regulation of VEGF-A Gene Splicing
Despite the important differences in the biological activity of the different VEGF-A isoforms, information on the molecular mechanisms involved in alternative splicing of the VEGF-A gene in physiologic and pathologic conditions is still very scant. It is very likely that some of the molecular mechanisms leading to increased transcription from the inducible VEGF-A promoter might also determine activation of various SR proteins involved in splicing regulation. Consistent with this possibility, in endometrial carcinoma cells, increased production of VEGF-A in response to hypoxia and acidosis was paralleled by a shift of isoform ratio towards the production of VEGF-A121 concomitant with p38 MAPK activation and consequent increase in different SR proteins (SF2/ASF, SRp20 and SRp40) activity.23 In breast cancer cells, siRNA-mediated silencing of one member (CAPERα, also known as hCC1.3) of the U2AF65 family of splicing regulators, which couple splicing to transcriptional activation in the hormone receptor-responsive promoters, was also found to selectively increase VEGF-A121 production compared to the longer isoforms, suggesting that this protein might conversely favor proximal splice site selection.24
Very little information is as yet available on the specific molecular mechanisms leading to exon 7 inclusion or exclusion. Cohen and coworkers25 reported that this exon might contain a purine-rich exonic splicing enhancer potentially binding the alternative splicing regulatory protein SLM-2. Consistent with this conclusion, expression of this protein in podocytes was found to correlate with relatively higher production of VEGF-A165 compared to other isoforms.
The difference between VEGF-A189, having high affinity for HSPGs, and the more soluble VEGF-A165 is the presence of the alternatively spliced exon 6A, which is present in the longer isoform and missing in the shorter. By use of a reporter minigene, Wang and coworkers identified the presence of an exonic splicing silencer (ESS) within exon 6a; the identity of the proteins interacting with this ESS are still uncharacterized.26
As far as exon 8b is concerned, this is likely to be included in the mRNA in place of canonical exon 8 as a consequence of distal splice site selection. Bioinformatics analysis indicates that there is a cluster of ASF2/ASF (which favor proximal splice site selection) adjacent to the proximal splice site and a cluster of SRp55-binding sites just distal to the distal splice sites.27 In a consistent manner, treatment of retinal pigment epithelial cells and podocytes with TGFβ1 was found to determine accumulation of poorly angiogenic exon 8b-containing VEGF-As by increasing the levels of SRp55, possibly indirectly through the activation of the MAPK p38.27 In contrast, cell treatment with IGF-1 also increased the amount of total VEGF-A; however, it decreased the levels of the isoforms containing 8b through a cascade of signals involving first activation of protein kinase C (PKC) and later that of the splicing factor kinase SRPK1, the end results being activation of nuclear ASF/SF2 and consequent proximal splice site selection.28
The Importance of Understanding VEGF-A Gene Alternative Splicing
Despite the vast body of information already available on the different functions of the various VEGF-A isoforms, it can be safely concluded that our understanding of the molecular mechanisms regulating alternative splicing of the VEGF-A pre-mRNA and, most notably, their connection with physiological and pathologic conditions is still in its infancy. However, this appears to be an important topic for investigation for a number of reasons. First, the non-equivalent function of the different VEGF-A isoforms indicates that their precise regulation is critical for blood vessel formation. In the adult, the modulation of VEGF-A pre-mRNA splicing might thus lead to unanticipated effects on tumor vascularization or impact on tumor vessel stabilization, with potentially important consequences on tumor growth, invasion and propensity to metastasize. Second, the induction of therapeutic angiogenesis by gene therapy holds great promise for the revascularization of otherwise ischemic tissues. A correct choice of the VEGF-A isoform cDNA to be delivered thus appears essential. In this respect, the apparent lack of satisfactory results so for obtained might be, at least in part, also attributed to improper choice of the delivered VEGF-A cDNA. Third, over the last several years, it has become evident that expression of the VEGF receptors is not restricted to endothelial cells and that their ligands exert a variety of fundamental functions in other cell types, including cardiomyocytes, muscle satellite cells, neurons, hepatocytes, osteoblasts, monocytes and hematopoietic stem cells (reviewed in ref. 18, 29 and 30). Very little is so far known, however, on the differential role of the various VEGF-A isoforms on all these non-angiogenic activities of the factor.
Given all of the above mentioned unknown elements and the central importance of VEGF-A and its receptors in so many different biological and clinical settings, further investigation on the molecular mechanisms regulating splicing of its pre-mRNA are definitely warranted.
Acknowledgements
Financial support from Advanced Grant 20090506 from the European Research Council (ERC) is acknowledged. The author wishes to thank Suzanne Kerbavcic for excellent editorial assistance.
Footnotes
Previously published online: www.landesbioscience.com/journals/transcription/article/13229
References
- 1.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 2.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 5.Miquerol L, Langille BL, Nagy A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development. 2000;127:3941–3946. doi: 10.1242/dev.127.18.3941. [DOI] [PubMed] [Google Scholar]
- 6.Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8:880–887. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrara N. Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Mol Biol Cell. 2010;21:687–690. doi: 10.1091/mbc.E09-07-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krilleke D, Ng YSE, Shima DT. The heparin-binding domain confers diverse functions of VEGF-A in development and disease: a structure-function study. Biochem Soc Trans. 2009;37:1201–1206. doi: 10.1042/BST0371201. [DOI] [PubMed] [Google Scholar]
- 9.Guo P, Xu L, Pan S, Brekken RA, Yang ST, Whitaker GB, et al. Vascular endothelial growth factor isoforms display distinct activities in promoting tumor angiogenesis at different anatomic sites. Cancer Res. 2001;61:8569–8577. [PubMed] [Google Scholar]
- 10.Grunstein J, Masbad JJ, Hickey R, Giordano F, Johnson RS. Isoforms of vascular endothelial growth factor act in a coordinate fashion to recruit and expand tumor vasculature. Mol Cell Biol. 2000;20:7282–7291. doi: 10.1128/mcb.20.19.7282-7291.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmeliet P, Ng YS, Nuyens D, Theilmeier G, Brusselmans K, Cornelissen I, et al. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med. 1999;5:495–502. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- 12.Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002;109:327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattot V, Moons L, Lupu F, Chernavvsky D, Gomez RA, Collen D, et al. Loss of the VEGF(164) and VEGF(188) isoforms impairs postnatal glomerular angiogenesis and renal arteriogenesis in mice. J Am Soc Nephrol. 2002;13:1548–1560. doi: 10.1097/01.asn.0000013925.19218.7b. [DOI] [PubMed] [Google Scholar]
- 14.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform- specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 15.Whitaker GB, Limberg BJ, Rosenbaum JS. Vascular endothelial growth factor receptor-2 and neuropilin-1 form a receptor complex that is responsible for the differential signaling potency of VEGF(165) and VEGF(121) J Biol Chem. 2001;276:25520–25531. doi: 10.1074/jbc.M102315200. [DOI] [PubMed] [Google Scholar]
- 16.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia H, Bagherzadeh A, Hartzoulakis B, Jarvis A, Lohr M, Shaikh S, et al. Characterization of a bicyclic peptide neuropilin-1 (NP-1) antagonist (EG3287) reveals importance of vascular endothelial growth factor exon 8 for NP-1 binding and role of NP-1 in KDR signaling. J Biol Chem. 2006;281:13493–13502. doi: 10.1074/jbc.M512121200. [DOI] [PubMed] [Google Scholar]
- 18.Zacchigna S, Pattarini L, Zentilin L, Moimas S, Carrer A, Sinigaglia M, et al. Bone marrow cells recruited through the Neuropilin-1 receptor promote arterial formation at the sites of adult neoangiogenesis. J Clin Invest. 2008;118:2062–2075. doi: 10.1172/JCI32832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zentilin L, Tafuro S, Zacchigna S, Arsic N, Pattarini L, Sinigaglia M, et al. Bone marrow mononuclear cells are recruited to the sites of VEGF-induced neovascularization but are not incorporated into the newly formed vessels. Blood. 2006;107:3546–3554. doi: 10.1182/blood-2005-08-3215. [DOI] [PubMed] [Google Scholar]
- 20.Tafuro S, Ayuso E, Zacchigna S, Zentilin L, Moimas S, Dore F, et al. Inducible adeno-associated virus vectors promote functional angiogenesis in adult organisms via regulated vascular endothelial growth factor expression. Cardiovasc Res. 2009;83:663–671. doi: 10.1093/cvr/cvp152. [DOI] [PubMed] [Google Scholar]
- 21.Cebe Suarez S, Pieren M, Cariolato L, Arn S, Hoffmann U, Bogucki A, et al. A VEGF-A splice variant defective for heparan sulfate and neuropilin- 1 binding shows attenuated signaling through VEGFR-2. Cell Mol Life Sci. 2006;63:2067–2077. doi: 10.1007/s00018-006-6254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamura H, Li X, Harper SJ, Bates DO, Claesson-Welsh L. Vascular endothelial growth factor (VEGF)-A165b is a weak in vitro agonist for VEGF receptor-2 due to lack of coreceptor binding and deficient regulation of kinase activity. Cancer Res. 2008;68:4683–4692. doi: 10.1158/0008-5472.CAN-07-6577. [DOI] [PubMed] [Google Scholar]
- 23.Elias AP, Dias S. Microenvironment changes (in pH) affect VEGF alternative splicing. Cancer Microenviron. 2008;1:131–139. doi: 10.1007/s12307-008-0013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowhan DH, Hong EP, Auboeuf D, Dennis AP, Wilson MM, Berget SM, et al. Steroid hormone receptor coactivation and alternative RNA splicing by U2AF65-related proteins CAPERalpha and CAPERbeta. Mol Cell. 2005;17:429–439. doi: 10.1016/j.molcel.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Cohen CD, Doran PP, Blattner SM, Merkle M, Wang GQ, Schmid H, et al. Sam68-like mammalian protein 2, identified by digital differential display as expressed by podocytes, is induced in proteinuria and involved in splice site selection of vascular endothelial growth factor. J Am Soc Nephrol. 2005;16:1958–1965. doi: 10.1681/ASN.2005020204. [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Crystal RG, Hackett NR. Identification of an exonic splicing silencer in exon 6A of the human VEGF gene. BMC Mol Biol. 2009;10:103. doi: 10.1186/1471-2199-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nowak DG, Woolard J, Amin EM, Konopatskaya O, Saleem MA, Churchill AJ, et al. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci. 2008;121:3487–3495. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowak DG, Amin EM, Rennel ES, Hoareau-Aveilla C, Gammons M, Damodoran G, et al. Regulation of vascular endothelial growth factor (VEGF) splicing from pro-angiogenic to anti-angiogenic isoforms: a novel therapeutic strategy for angiogenesis. J Biol Chem. 2010;285:5532–5540. doi: 10.1074/jbc.M109.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, et al. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther. 2004;10:844–854. doi: 10.1016/j.ymthe.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Zentilin L, Puligadda U, Lionetti V, Zacchigna S, Collesi C, Pattarini L, et al. Cardiomyocyte VEGFR-1 activation by VEGF-B induces compensatory hypertrophy and preserves cardiac function after myocardial infarction. FASEB J. 2009;24:1467–1478. doi: 10.1096/fj.09-143180. [DOI] [PubMed] [Google Scholar]