Abstract
Involvement of intestinal microbes in the pathogenesis of chronic inflammatory bowel diseases (IBD, including Crohn disease and ulcerative colitis) is well established. However, the mechanisms by which bacteria lead to intestinal injury in IBD remain unclear and are the focus of current research. Using adherent-invasive Escherichia coli (AIEC) strain LF82, which is linked to Crohn disease, we recently demonstrated the ability of these intestinal microbes to disrupt the integrity of epithelial cells in an in vitro cell model. This disruption provides the bacteria a capacity to penetrate into and beyond the epithelial monolayer, replicate in cells, disseminate within the host, and induce a chronic immune response. These findings provide a link between microbes related to IBD, disruption of the intestinal epithelial cell barrier, and disease pathogenesis.
In this addendum, we provide a synopsis on current data concerning the role of AIEC in the pathogenesis of intestinal inflammation, summarise our recent findings, and highlight the central role of the epithelium in mucosal defence. We also discuss, in more detail, the potential implications of our findings and present ideas for future studies and targets for intervention.
Key words: inflammatory bowel diseases, adherent-invasive Escherichia coli, intestinal barrier, microbial pathogenesis, host-microbial interactions
Introduction
Microbes are involved in the pathogenesis of inflammatory bowel diseases (IBD).
Crohn disease and ulcerative colitis, together known as IBD, are chronic intestinal diseases that develop due to an aberrant immune response to intestinal microbes in genetically susceptible hosts.1 The involvement of luminal bacteria in the pathogenesis of IBD is supported by a plethora of research findings, including both human data and murine models. For example, diversion of the fecal flow away from the bowel results in an alleviation of mucosal inflammation.2 Moreover, inflammation can be induced by introducing fecal material, including its microbial content, into non-inflamed bowel loops in patients with IBD.3 Many of the human genes recently shown to be associated with susceptibility to IBD, including those involving innate immune responses and autophagy encode proteins involved in host-microbial interactions.4 The finding that colitis develops in most gene knock-out models of IBD only when animals are grown in the presence of bacteria—and not when they are reared in germ-free conditions—further supports the involvement of microbes in the pathogenesis of IBD.5,6
Several specific organisms are proposed as a cause of IBD; however, there is still no compelling evidence that any one microbe is the etiologic agent. Using metagenomic approaches, Frank et al. identified a reduction in the phyla Firmicutes and Bacteroidetes in stool and biopsy samples from IBD patients, relative to healthy controls.7 Some investigators have suggested that Mycobacterium avium subspecies paratuberculosis is involved in inducing IBD,8 although this remains an area of ongoing controversy.9 Alternatively, a protective role for commensal organisms is suggested by the recent finding that the absence of Faecalibacterium prausnitzii from the ileum of patients with Crohn disease undergoing surgical resection is associated with recurrence of gut inflammation.10
Adherent-invasive E. coli and IBD.
Escherichia coli strains are commonly found in the lumen of the gut and are mostly considered to be non-harmful. Nevertheless, some E. coli strains, for example enterohemorrhagic E. coli serotype O157:H7, are identified as enteric pathogens with well defined virulence factors, such as the outer membrane protein adhesion intimin and the elaboration of phage encoded Shiga-like toxins. Studies in IBD patients have identified an increased presence of E. coli strains that do not contain any of the previously described virulence genes that have a capacity to adhere to and invade epithelial cells in vitro.11 It is this phenotype of adherence to intestinal epithelial cells, invasion into the cytoplasm of the infected eukaryotic cell, and intracellular replication in epithelial cells and macrophages, in the absence of previously known virulence factors that led to the proposition that adherent-invasive E. coli strains (also termed AIEC) should be considered a separate pathogenic category of E. coli causing intestinal diseases in humans. Subsequent studies suggested that AIEC strains may be involved in the pathogenesis of IBD.12 In fact, AIEC isolates are isolated in 36% of ileal lesions in Crohn disease patients after surgical resection, compared to just 6% of healthy controls,13 and there is an increased prevalence and diversity of AIEC strains in patients with Crohn disease.14 E. coli, strain LF82 (serotype O83:H1) was originally isolated from an ileal lesion in a patient from France with Crohn disease, and this isolate has been generously shared for extensive use by multiple investigators as a prototype AIEC strain.15 Testing of other E. coli isolates from patients with Crohn disease with similar properties suggests that these observations are generalizable.16 However, not all of these phenotypically-defined strains (that is, based on invasive capacity) may share all of the same proposed virulence genes.14,17–19
Although some of the mechanisms by which these bacteria lead to colonization and intestinal injury, such as induction of carcinoembryonic antigen-related cell-adhesion molecule (CEACAM)-6 receptor expression by TNFα,20,21 and expression of a unique type of type 1 binding pilus22 have been described, it is highly likely that other virulence traits still remain to be discovered.
The intestinal epithelial barrier: gatekeeper of the gut.
The main physical portion of the intestinal barrier consists of a simple columnar epithelial monolayer that is in a state of constant renewal. Intercellular junctional complexes, which maintain barrier integrity, are composed of the apical junctional complex (AJC), which includes tight junctions and adherens junctions. Defects in the structure and function of AJCs are implicated in both patients with IBD and in animal models of IBD.23,24 Barrier dysfunction and increased intestinal permeability are observed in non-inflamed ileum in Crohn disease patients and in unaffected first-degree relatives,25 as well as preceding the relapse of Crohn disease in asymptomatic patients.26
Abnormal distribution patterns of tight junction proteins, which correlate with increased gut permeability, are found in IBD patients.27,28 However, changes in barrier permeability can also be directly induced in vitro by pro-inflammatory cytokines (e.g., IFNγ and TNFα),29 which raises the question whether barrier defects are the primary mediator of disease pathogenesis, or secondary to cytokine-induced inflammatory processes.
Microbes target the epithelial barrier.
Components of both tight junctions and adherens junctions are common targets for bacterial virulence. For example, Clostridium perfringens enterotoxin (CPE) is a 35-kDa toxin that forms complexes at the host membrane, capable of binding and internalizing occludin and claudin-4. This leads to depletion of these essential proteins from AJCs and compromise of epithelial barrier integrity.30 Another example is Salmonella enterica serovar Typhimurium, which through pathogenicity island (SPI-1)-dependent type 3 secretion of several effectors (SopB, SopE, SopE2 and SipA) leads to structural and functional alterations in intercellular tight junctions.31 We have recently shown that another enteric pathogen linked to IBD,32 Campylobacter jejuni, disrupts AJCs through invasion into epithelial cells33 and that this can be prevented by probiotics.34
In this context, adverse effects of microbes on intercellular junctions offer potential bridges connecting bacteria to the pathogenesis of IBD. For these reasons, the aim of the study reviewed by this addendum was to define the ability of AIEC strain LF82, to disrupt model epithelial cell polarized monolayers as a potential mechanism for tissue damage and immune stimulation in patients with IBD.
AIEC and the Epithelial Barrier
AIEC infection increases permeability of epithelial cell monolayers.
In order to characterize the capacity of AIEC to disrupt the epithelial barrier, we used an in vitro cell model with T84 and Madin-Darby Canine Kidney (MDCK)-I cells. When polarized, these epithelial cells form mature AJCs, resulting in high electrical resistance, and are widely used for studying the effects of bacteria on permeability.35,36 We found that after 16 h of infection with AIEC strain LF82 there was a 50–60% reduction in transepithelial electrical resistance (TER) in both cell lines.37 This effect was comparable to that of enterohemorrhagic E. coli (EHEC) serotype O157:H7, which was used as a positive control. Interestingly, when the AIEC strain was introduced into the basolateral aspect of monolayers there was an 81% reduction in TER, relative to sham control monolayers, compared to a 50% reduction with EHEC infection. Other enteric pathogens, such as C. jejuni, invade epithelial cells from the basolateral membrane,38 which may explain the relevance of the preferred basolateral effect of AIEC in this setting. Therefore, it is possible that these bacteria more effectively invade polarized cells from the basolateral side, which results in a more profound effect on monolayer integrity. Dextran flux was used to measure paracellular macromolecular permeability.39 Transcytosis of a 10-kDa dextran probe across monolayers supported the TER results, since infection with AIEC also resulted in increased macromolecular permeability in MDCK-I cells.
Morphological alterations of cell monolayers infected with AIEC.
Zonula occludens-1 (ZO-1) is an intracellular adaptor protein that connects transmembrane tight junction proteins (e.g., claudins and occludin) to the cell cytoskeleton. Infection of MDCK-I monolayers with AIEC strain LF82 led to profound disruption of ZO-1 with large gaps between cells, as shown by confocal microscopy of monolayers. This finding reflects the ability of AIEC to disrupt tight junctions, similar to other enteric pathogens.40 This effect was also demonstrated by transmission electron microscopy, showing changes in infected monolayers as early as 4 h after infection with AIEC, including disruption of intercellular spaces, loss of cellular polarity, and invasion of multiple bacteria into cells.
AIEC bacteria replicate in epithelial cells and are found within late endosomes.
Invasive AIEC were present in membrane-bound compartments 4 h after infection and appeared to replicate within vacuoles, since multiple organisms were seen within a single compartment on transmission electron microscopy. Bacteria co-localized with the late endosomal marker LAMP1 (lysosomal-associated membrane protein 1) at the same time point, suggesting that bacteria were directed to the endosomal pathway in epithelial cells, similar to the case in infection of macrophages.41 The vacuole membrane appeared to be partially missing, which suggests that bacteria may have been escaping this compartment.37
Unresolved issues.
Our recent findings describe the ability of AIEC to subvert and penetrate the first line of host innate defences, the polarized epithelial cell barrier. Disruption of the epithelial monolayer enables luminal antigens and microbes to penetrate the mucosa, which could then stimulate pro-inflammatory responses, leading to chronic intestinal and systemic diseases, including IBD.1,42 The importance of barrier maintenance in IBD is further highlighted by the development of colitis in mice expressing constitutively active myosin light chain kinase, which is involved in regulating integrity of the epithelial barrier.43 Epithelial barriers are common targets of bacterial virulence, as displayed by multiple infection models disrupting epithelial barrier function.44
Most studies on AIEC have focused on bacterial adhesion, invasion and replication in both epithelial cells and macrophages, as well as the accompanying inflammatory response.45 However the ability of these microbes to disrupt the integrity of the epithelial barrier has not been extensively studied to date. Only a single previously published study describes AIEC-induced barrier disruption with reduced TER of Caco-2 monolayers and displacement of both ZO-1 and E-cadherin from AJCs.17 Eaves-Pyles et al.18 also used polarized epithelial monolayers to demonstrate chemokine secretion by AIEC-infected Caco-2 and T84 monolayers leading to transmigration of innate immune cells.
Our results confirm these findings in additional polarized epithelial cell lines and also reveal an increase in macromolecular permeability of infected monolayers, as well as morphological defects in the structure of AJCs in infected polarized epithelial cell monolayers. Since AIEC strains are associated with IBD, host cell invasion and barrier disruption are mechanisms that could contribute to intestinal injury and immune stimulation in affected patients. We also found that AIEC can replicate in membrane-bound vesicles, which positively stain with the late endosomal marker LAMP1. The ability of AIEC to survive and replicate within the cytoplasm of epithelial cells is of relevance in IBD, since defects in the handling of intracellular microbes are considered to contribute to disease pathogenesis.6 For example, transgenic mice that lack the gene encoding the Nod-like receptor NOD2 are more susceptible to infection with intracellular pathogens, such as Mycobacterium tuberculosis.46 Furthermore, mice lacking the autophagy protein Atg16L1 have more severe chemically-induced colitis than wild-type animals.47 Therefore, it is plausible that impaired handling of invasive AIEC strains in genetically susceptible individuals with defects in microbial processing contributes to intestinal injury. This is also demonstrated by increased in vitro response of monocytes derived from Crohn disease patients with NOD2 mutations to AIEC infection.48
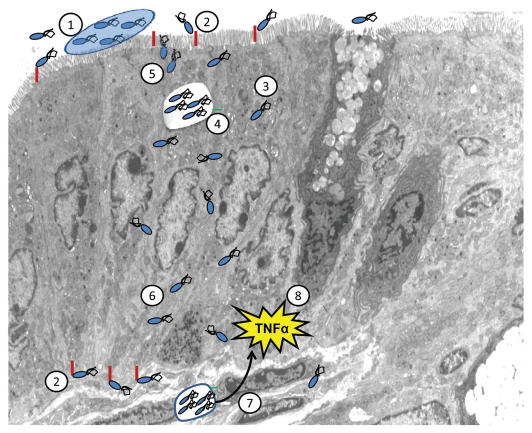
AIEC strains are now linked to the pathogenesis of IBD by a sequence of events, as summarized in Figure 1. It remains unclear whether these strains are present in all individuals, initially below standard detection rates, and then become more prominent due to genetic and environmental factors that lead to disease development, or whether disease is directly induced when AIEC strains colonize the bowel. Nevertheless, factors that favor their colonization have been identified, including CEACAM receptor overexpression in the gut20,21 and the capacity of the pathogen to form biofilms.49 After establishing colonization, bacteria invade and replicate in epithelial cells, modify host signaling cascades, escape the endosomal pathway, and disrupt the integrity of the epithelial layer. AIEC strains can then translocate across polarized epithelia either through a paracellular route (passing through AJCs and between cells), via transcellular migration (through epithelial cells), or both. After penetrating the epithelial barrier, these strains are then taken up by intestinal immune cells where they further replicate and modify eukaryotic cell responses. This could then lead to chronic immune stimulation and the tissue inflammation and damage typically seen in patients with IBD.
Figure 1.
AIEC disruption of epithelial integrity—a model for bacterial involvement in IBD pathogenesis. AIEC strains create a niche in the intestinal mucus layer, possibly by forming biofilms (1). Bacteria then adhere to specific receptors on epithelial cells (e.g., CEACAM6) (2) and invade epithelial cells (3). Within these cells AIEC strains replicate, likely within the late endosomal compartment (4). Parallel to this, there is an increase in permeability to macromolecules of the polarized epithelial monolayer, as reflected by changes in apical junctional complexes (5) and translocation of bacteria (6). As a result, these microbes gain access to submucosal immune cells (7) and induce pro-inflammatory chemokine and cytokine responses (8), typically seen in patients with IBD.
Unresolved Issues, Future Directions and Potential Applications
Recent studies on the role of microbes in IBD, and specifically AIEC strains, have introduced potentially novel insights into disease pathogenesis and provide substantial support for microbial involvement in the disease. Nevertheless, many issues remain to be clarified and validated before this knowledge can be fully applied to humans with IBD. For example, there is a need to identify additional virulence factors common to the various AIEC isolates identified in patients with IBD and define just how they contribute to disease pathogenesis.
Current data are limited by the lack of in vivo models confirming a role for AIEC in experimental colitis, with the exception of a single study where CEABAC10 mice (overexpressing human CEACAMs) developed colitis after DSS treatment and AIEC infection.21 Therefore, expanding experimental data to include additional animal models is required to better define the importance of the in vitro findings.
Our recent findings focus attention to the epithelial barrier as a key regulator of intestinal homeostasis. The findings described here now need to be confirmed both in appropriate, relevant animal models and in human subjects. Furthermore, there is also a need to clarify whether increases in permeability are a direct adverse effect of bacterial infection or whether they are a consequence of tissue inflammation.50
Defining virulence mechanisms of AIEC strains presents opportunities for novel approaches to reduce the effects of mucosal infection and control inflammatory disease. For example, limiting bacterial invasion by probiotics, likely through competitive exclusion,51 could reduce tissue damage. Enhancing the epithelial barrier, by use of novel therapies, such as zonulin peptide inhibitors, shown to protect interleukin 10 gene knockout mice from colitis,52 could enhance mucosal protection. Further research into specific interactions between AIEC strains and epithelial cells will help identify additional targets for interrupting the infectious process. Addressing these knowledge gaps is now within our reach, thanks to advances in cell and molecular biology and metagenomic approaches, and the focus on translation of findings to disease states.
Acknowledgements
Supported by operating grants from the Crohn's and Colitis Foundation of Canada (Fay Shapiro Cutler Grant in Aid of Research) and the Canadian Institutes of Health Research (CIHR). E.W. was supported by a fellowship award from the Canadian Association of Gastroenterology/CIHR/Astra Zeneca and by the Canadian Child Health Clinician Scientist Program. P.M.S. is the recipient of a Canada Research Chair in Gastrointestinal Disease.
Abbreviations
- AIEC
adherent-invasive Escherichia coli
- AJCs
apical junctional complexes
- CEACAM
carcinoembryonic antigen-related cell-adhesion molecules
- EHEC
enterohemorrhagic Escherichia coli
- IBD
inflammatory bowel diseases
- LAMP
lysosomal-associated membrane protein
- MDCK
madin-darby canine kidney
- TER
transepithelial electrical resistance
- ZO-1
zonula occludens-1
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/11142
References
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Shanahan F. Probiotics and inflammatory bowel disease: is there a scientific rationale? Inflamm Bowel Dis. 2000;6:107–115. doi: 10.1097/00054725-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 3.D'Haens GR, Geboes K, Peeters M, Baert F, Penninckx F, Rutgeerts P. Early lesions of recurrent Crohn's disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262–267. doi: 10.1016/s0016-5085(98)70476-7. [DOI] [PubMed] [Google Scholar]
- 4.Marx J. Biomedicine. Puzzling out the pains in the gut. Science. 2007;315:33–35. doi: 10.1126/science.315.5808.33. [DOI] [PubMed] [Google Scholar]
- 5.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 6.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 7.Frank DN, St. Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naser SA, Ghobrial G, Romero C, Valentine JF. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease. Lancet. 2004;364:1039–1044. doi: 10.1016/S0140-6736(04)17058-X. [DOI] [PubMed] [Google Scholar]
- 9.Abubakar I, Myhill D, Aliyu SH, Hunter PR. Detection of Mycobacterium avium subspecies paratuberculosis from patients with Crohn's disease using nucleic acid-based techniques: a systematic review and meta-analysis. Inflamm Bowel Dis. 2008;14:401–410. doi: 10.1002/ibd.20276. [DOI] [PubMed] [Google Scholar]
- 10.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boudeau J, Glasser AL, Masseret E, Joly B, Darfeuille-Michaud A. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect Immun. 1999;67:4499–4509. doi: 10.1128/iai.67.9.4499-4509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnich N, Darfeuille-Michaud A. Role of bacteria in the etiopathogenesis of inflammatory bowel disease. World J Gastroenterol. 2007;13:5571–5576. doi: 10.3748/wjg.v13.i42.5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127:412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Medina M, Aldeguer X, Lopez-Siles M, Gonzalez-Huix F, Lopez-Oliu C, Dahbi G, et al. Molecular diversity of Escherichia coli in the human gut: new ecological evidence supporting the role of adherent-invasive E. coli (AIEC) in Crohn's disease. Inflamm Bowel Dis. 2009;15:872–882. doi: 10.1002/ibd.20860. [DOI] [PubMed] [Google Scholar]
- 15.Darfeuille-Michaud A, Neut C, Barnich N, Lederman E, Di Martino P, Desreumaux P, et al. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology. 1998;115:1405–1413. doi: 10.1016/s0016-5085(98)70019-8. [DOI] [PubMed] [Google Scholar]
- 16.Sepehri S, Kotlowski R, Bernstein CN, Krause DO. Phylogenetic analysis of inflammatory bowel disease associated Escherichia coli and the fimH virulence determinant. Inflamm Bowel Dis. 2009;15:1737–1745. doi: 10.1002/ibd.20966. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki M, Sitaraman SV, Babbin BA, Gerner-Smidt P, Ribot EM, Garrett N, et al. Invasive Escherichia coli are a feature of Crohn's disease. Lab Invest. 2007;87:1042–1054. doi: 10.1038/labinvest.3700661. [DOI] [PubMed] [Google Scholar]
- 18.Eaves-Pyles T, Allen CA, Taormina J, Swidsinski A, Tutt CB, Jezek GE, et al. Escherichia coli isolated from a Crohn's disease patient adheres, invades and induces inflammatory responses in polarized intestinal epithelial cells. Int J Med Microbiol. 2008;298:397–409. doi: 10.1016/j.ijmm.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Medina M, Mora A, Blanco M, Lopez C, Alonso MP, Bonacorsi S, et al. Similarity and divergence among adherent-invasive Escherichia coli and extraintestinal pathogenic E. coli strains. J Clin Microbiol. 2009;47:3968–3979. doi: 10.1128/JCM.01484-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnich N, Carvalho FA, Glasser AL, Darcha C, Jantscheff P, Allez M, et al. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest. 2007;117:1566–1574. doi: 10.1172/JCI30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carvalho FA, Barnich N, Sivignon A, Darcha C, Chan CH, Stanners CP, Darfeuille-Michaud A. Crohn's disease adherent-invasive Escherichia coli colonize and induce strong gut inflammation in transgenic mice expressing human CEACAM. J Exp Med. 2009;206:2179–2189. doi: 10.1084/jem.20090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnich N, Boudeau J, Claret L, Darfeuille-Michaud A. Regulatory and functional co-operation of flagella and type 1 pili in adhesive and invasive abilities of AIEC strain LF82 isolated from a patient with Crohn's disease. Mol Microbiol. 2003;48:781–794. doi: 10.1046/j.1365-2958.2003.03468.x. [DOI] [PubMed] [Google Scholar]
- 23.Bruewer M, Samarin S, Nusrat A. Inflammatory bowel disease and the apical junctional complex. Ann N Y Acad Sci. 2006;1072:242–252. doi: 10.1196/annals.1326.017. [DOI] [PubMed] [Google Scholar]
- 24.Weber CR, Turner JR. Inflammatory bowel disease: is it really just another break in the wall? Gut. 2007;56:6–8. doi: 10.1136/gut.2006.104182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Inca R, Annese V, di Leo V, Latiano A, Quaino V, Abazia C, et al. Increased intestinal permeability and NOD2 variants in familial and sporadic Crohn's disease. Aliment Pharmacol Ther. 2006;23:1455–1461. doi: 10.1111/j.1365-2036.2006.02916.x. [DOI] [PubMed] [Google Scholar]
- 26.Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
- 27.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85:1139–1162. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 28.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by upregulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClane BA, Chakrabarti G. New insights into the cytotoxic mechanisms of Clostridium perfringens enterotoxin. Anaerobe. 2004;10:107–114. doi: 10.1016/j.anaerobe.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Grassl GA, Finlay BB. Pathogenesis of enteric Salmonella infections. Curr Opin Gastroenterol. 2008;24:22–26. doi: 10.1097/MOG.0b013e3282f21388. [DOI] [PubMed] [Google Scholar]
- 32.Gradel KO, Nielsen HL, Schonheyder HC, Ejlertsen T, Kristensen B, Nielsen H. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology. 2009;137:495–501. doi: 10.1053/j.gastro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Wine E, Chan VL, Sherman PM. Campylobacter jejuni mediated disruption of polarized epithelial monolayers is cell-type specific, time dependent and correlates with bacterial invasion. Pediatr Res. 2008;64:599–604. doi: 10.1203/PDR.0b013e31818702b9. [DOI] [PubMed] [Google Scholar]
- 34.Wine E, Gareau MG, Johnson-Henry K, Sherman PM. Strain-specific probiotic (Lactobacillus helveticus) inhibition of Campylobacter jejuni invasion of human intestinal epithelial cells. FEMS Microbiol Lett. 2009;300:146–152. doi: 10.1111/j.1574-6968.2009.01781.x. [DOI] [PubMed] [Google Scholar]
- 35.Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–1434. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson-Henry KC, Donato KA, Shen-Tu G, Gordanpour M, Sherman PM. Lactobacillus rhamnosus strain GG prevents enterohemorrhagic Escherichia coli O157:H7-induced changes in epithelial barrier function. Infect Immun. 2008;76:1340–1348. doi: 10.1128/IAI.00778-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wine E, Ossa JC, Gray-Owen SD, Sherman PM. Adherent-invasive Escherichia coli, strain LF82 disrupts apical junctional complexes in polarized epithelia. BMC Microbiol. 2009;9:180. doi: 10.1186/1471-2180-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monteville MR, Konkel ME. Fibronectin-facilitated invasion of T84 eukaryotic cells by Campylobacter jejuni occurs preferentially at the basolateral cell surface. Infect Immun. 2002;70:6665–6671. doi: 10.1128/IAI.70.12.6665-6671.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raimondi F, Santoro P, Barone MV, Pappacoda S, Barretta ML, Nanayakkara M, et al. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am J Physiol Gastrointest Liver Physiol. 2008;294:906–913. doi: 10.1152/ajpgi.00043.2007. [DOI] [PubMed] [Google Scholar]
- 40.Saadat I, Higashi H, Obuse C, Umeda M, Murata-Kamiya N, Saito Y, et al. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature. 2007;447:330–333. doi: 10.1038/nature05765. [DOI] [PubMed] [Google Scholar]
- 41.Bringer MA, Glasser AL, Tung CH, Meresse S, Darfeuille-Michaud A. The Crohn's disease-associated adherent-invasive Escherichia coli strain LF82 replicates in mature phagolysosomes within J774 macrophages. Cell Microbiol. 2006;8:471–484. doi: 10.1111/j.1462-5822.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 42.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 43.Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136:551–563. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laukoetter MG, Bruewer M, Nusrat A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr Opin Gastroenterol. 2006;22:85–89. doi: 10.1097/01.mog.0000203864.48255.4f. [DOI] [PubMed] [Google Scholar]
- 45.Barnich N, Darfeuille-Michaud A. Adherent-invasive Escherichia coli and Crohn's disease. Curr Opin Gastroenterol. 2007;23:16–20. doi: 10.1097/MOG.0b013e3280105a38. [DOI] [PubMed] [Google Scholar]
- 46.Divangahi M, Mostowy S, Coulombe F, Kozak R, Guillot L, Veyrier F, et al. NOD2-deficient mice have impaired resistance to Mycobacterium tuberculosis infection through defective innate and adaptive immunity. J Immunol. 2008;181:7157–7165. doi: 10.4049/jimmunol.181.10.7157. [DOI] [PubMed] [Google Scholar]
- 47.Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 48.Peeters H, Bogaert S, Laukens D, Rottiers P, De Keyser F, Darfeuille-Michaud A, et al. CARD15 variants determine a disturbed early response of monocytes to adherent-invasive Escherichia coli strain LF82 in Crohn's disease. Int J Immunogenet. 2007;34:181–191. doi: 10.1111/j.1744-313X.2007.00670.x. [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Medina M, Naves P, Blanco J, Aldeguer X, Blanco JE, Blanco M, et al. Biofilm formation as a novel phenotypic feature of adherent-invasive Escherichia coli (AIEC) BMC Microbiol. 2009;9:202. doi: 10.1186/1471-2180-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Ingrassia I, Leplingard A, Darfeuille-Michaud A. Lactobacillus casei DN-114 001 inhibits the ability of adherent-invasive Escherichia coli isolated from Crohn's disease patients to adhere to and to invade intestinal epithelial cells. Appl Environ Microbiol. 2005;71:2880–2887. doi: 10.1128/AEM.71.6.2880-2887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arrieta MC, Madsen K, Doyle J, Meddings J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut. 2009;58:41–48. doi: 10.1136/gut.2008.150888. [DOI] [PMC free article] [PubMed] [Google Scholar]