Abstract
Type-III or type-IV secretion systems of many Gram-negative bacterial pathogens inject effector proteins into host cells that modulate cellular functions in their favour. A preferred target of these effectors is the actin-cytoskeleton as shown by studies using the gastric pathogens Helicobacter pylori (H. pylori) and enteropathogenic Escherichia coli (EPEC). We recently developed a co-infection approach to study effector protein function and molecular mechanisms by which they highjack cellular signalling cascades. This is exemplified by our observation that EPEC profoundly blocks H. pylori-induced epithelial cell scattering and elongation, a disease-related event requiring the activity of small Rho GTPase Rac1. While this suppressive effect is dependent on the effector protein Tir and the outer-membrane protein Intimin, it unexpectedly revealed evidence for Tir-signalling independent of phosphorylation of Tir at tyrosine residues 454 and 474. Instead, our studies revealed a previously unidentified function for protein kinase A (PKA)-mediated phosphorylation of Tir at serine residues 434 and 463. We demonstrated that EPEC infection activates PKA for Tir phosphorylation. Activated PKA then phosphorylates Rac1 at its serine residue 71 associated with reduced GTP-load and inhibited cell elongation. Phosphorylation of Rho GTPases such as Rac1 might be an interesting novel strategy in microbial pathogenesis.
Key words: molecular pathogenesis, pathogenicity island, type III and type IV secretion, virulence
Many signal transduction pathways in both eukaryotes and prokaryotes involve post-translational modification of proteins by phosphorylation.1–3 Protein phosphorylation was originally described in the mid-1950s and is now considered a major regulatory mechanism which profoundly changes the biochemical characteristics of a given protein having a significant role in numerous cellular processes. Using ATP as substrate, specific protein kinases can covalently attach a phosphoryl group to the side chains of histidine, serine, threonine and tyrosine residues, while protein phosphatases catalyse the removal of the phosphoryl group in a reversible reaction. When we look back at the discovery of tyrosine phosphorylation, however, a direct link to infection biology becomes apparent. One of the first described tyrosine-phosphorylated proteins was the middle T-antigen of polyoma virus.4 Since these early days most of the published work focused on the role of tyrosine phosphorylation in mammalian cells, but tyrosine kinases are also present in distinct bacteria.3 Interestingly, it was also discovered that a growing number of effector proteins of so-called type-IV secretion systems (T4SS) and type-III secretion systems (T3SS) in bacterial pathogens including EPEC, Helicobacter, Bartonella, Chlamydia and others have adapted a mechanism for their tyrosine phosphorylation by eukaryotic kinases.2,5 Previous evidence indicated that one of the best-characterized effector proteins in EPEC and EHEC, the Translocated Intimin receptor (Tir), may also use serine phosphorylation by a host cell kinase as a strategy to communicate with host target cells, but its significance for the infection process was widely unclear.6–9 Novel findings using a co-infection approach with H. pylori indicated that there is a downstream consequence of serine-phosphorylated EPEC Tir which led to the discovery of a novel pathway regulating the small Rho GTPase Rac1.10 Rho GTPases are key regulators of many fundamental biological functions including actin-cytoskeletal dynamics and cycle between their active and inactive state by binding GTP and by hydrolysis of GTP to GDP.11 In this addendum we discuss these new findings and their possible impact on host cell signal transduction cascades.
EPEC is a leading cause of infantile diarrhea linked to the formation of attaching and effacing (A/E) lesions, which are characterised by intimate bacterial binding to intestinal epithelial cells prior to triggering the loss (effacement) of absorptive microvilli and formation of actin-rich pedestal-like structures beneath the attached bacteria.12–17 The formation of A/E lesions depends on the locus of enterocyte effacement (LEE) pathogenicity island in the EPEC chromosome that encodes genes for the surface protein Intimin, the T3SS as well as the translocated effector proteins EspB, EspF, EspG, EspH, EspZ, Map and Tir. EPEC also employs its T3SS machinery to secrete and/or deliver additional non-LEE encoded proteins into the host cells, and the recent completion of the EPEC genome sequence suggests that its effector protein repertoire consists of at least 21 factors.18,19 However, Tir is the only effector shown to be essential for disease development.20,21 Early work has demonstrated that EPEC injects Tir into target cells where Tir molecules insert into the host cell membrane and bind Intimin, thereby acting as a receptor for the bacteria.22 Tir exhibits a hairpin-like conformation with two predicted transmembrane domains (residues 234–259 and 353–382), exposing a large extracellular loop (residues 260–352). This loop contains the Intimin-binding domain (IBD) that serves as a binding site for Intimin and thus intimate bacterial adherence.23 The IBD is flanked by amino-terminal (residues 1–233) and carboxy-terminal (residues 383–550) regions that are located in the host cell cytoplasm allowing interactions with host proteins. Importantly, IBD-Intimin interaction apparently unleashes Tir signalling leading for the production of actin-rich pedestals.23 The latter proceeds in a manner dependent on phosphorylation of tyrosine residue 474 (Y-474) in Tir by redundant host tyrosine kinases, namely the Src family member Fyn and Tec/Abl family kinases.24–27 Phosphorylated Y-474 serves as a binding site for the SH2 domain of the adaptor protein Nck to enable the N-WASP-Arp2/3 complex to polymerise actin beneath attached bacteria.28,29 Interestingly, the latter signalling events do not require the activity of small Rho GTPases Rac1, Cdc42 or RhoA.30 However, Tir also nucleates actin by Nck-independent mechanisms in an inefficient manner linked to a second tyrosine phosphorylation site at Y-454.31 In addition, in vitro phosphorylation assays identified two serine residues in Tir (at position S-434 and S-463) as putative PKA substrate sites.6 However, at this time it remained unknown whether PKA is activated by EPEC and can phosphorylate Tir in vivo. The entire scenario becomes even more complicated considering the findings that a large number of additional host cytoskeletal proteins are also recruited into these actin-rich pedestals including vinculin, cortactin, talin and α-actinin as well as phosphoinositide 3-kinase (PI3K), tyrosine-phosphatase Shp-2, GTPase activating protein Ras-GAP, ubiquitin ligase Cbl and others (Fig. 1A and B) which were all reported to directly interact with Tir.5,32–37 This demonstrates that Tir signalling is highly complex and still not fully understood.
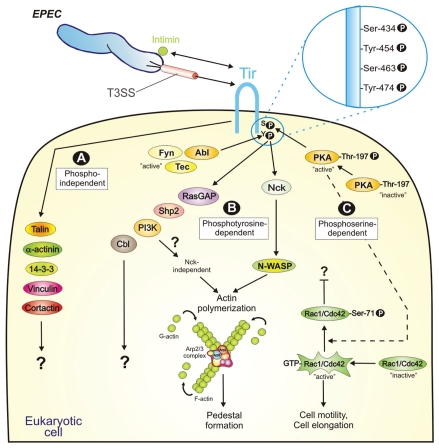
Figure 1.
Model for host-cell signalling induced by EPEC Tir. Tir is an effector protein of EPEC, which is injected into host cells by a type-III secretion system (T3SS). Translocated Tir is rapidly inserted into the host cell membrane where interaction with the bacterial surface protein Intimin triggers signalling leading to disease-related events. Tir membrane insertion results in a hairpin-like structure of the protein and correlates with multiple phosphorylation events at indicated serine/tyrosine residues. While the tyrosine residues Y-454 and Y-474 can be phosphorylated by Fyn, Tec and/or Abl kinases, the serine residues S-434 and S-463 are phosphorylated by PKA. Injected Tir has at least 10 interacting host cell proteins as indicated. The induced downstream signal cascades can be classified in three categories: (A) phosphorylation-independent, (B) dependent on Tir tyrosine phosphorylation, and (C) dependent on Tir serine phosphorylation. For more details see text.
In contrast to EPEC which uses a T3SS to locally target the host actin cytoskeleton, H. pylori utilizes a T4SS to induce global actin-cytoskeletal rearrangements involved in cell scattering and elongation.38,39 This T4SS, which injects the CagA protein into host target cells in a beta1 integrin-dependent manner, is encoded by the cag pathogenicity island.40 CagA is the only described H. pylori T4SS effector protein and several lines of evidence have recently emerged that provide insights into the mechanisms by which CagA modulates the actin cytoskeleton.38,39 First, translocated CagA is phosphorylated by Src and Abl kinases on tyrosine residues in the so-called EPIYA sequence repeat motifs.41 Secondly, transfected and/or injected CagA can interact with at least 16 host signalling molecules [Shp-2, C-terminal Src kinase (Csk), the adapter proteins Grb2 and Crk, the kinases Src, Abl, Par1b, TAK1, PI3K and others] in a manner dependent or independent of CagA phosphorylation.5,38,39 Binding of CagA to Src inactivates the kinase leading to the dephosphorylation of the actin-binding proteins cortactin, ezrin and vinculin.39 Interestingly, the cell scattering phenotype induced by H. pylori also involves the activity of Rho GTPase family member Rac1 which is required for the induction of cell motility.42 As mentioned above, in a recent study we carried out co-infection experiments of H. pylori with EPEC.10 Here we found that EPEC inhibits H. pylori-induced AGS cell scattering and elongation, and identified a consequence for PKA-mediated phosphorylation in Tir function. These findings provide new insights on how the involved signalling intersects with CagA-mediated responses as discussed below.
We originally developed the co-infection approach to dissect novel signalling pathways of CagA-induced rearrangements in the actin-cytoskeleton using EPEC with its well-known effector repertoire as a tool. We indeed found that the presence of EPEC during infection efficiently inhibited the CagA-induced effects on actin-cytoskeleton, even at low multiplicity of infection of about 20 bacteria per cell.10 First, we excluded the possibility that this inhibitory activity was due to reduced cell binding of H. pylori or EPEC affecting the viability of H. pylori or changes in CagA injection levels. Second, we were able to demonstrate that the EPEC-blocking activity on the elongation phenotype was dependent on host cell contact of EPEC (using a transwell system separating EPEC from the AGS epithelial cells) and a functional T3SS (using EPEC mutants deficient in the expression of the T3SS). Third, we could exclude the involvement of the T3SS effector proteins EspF, EspH or Map, while mutants of Tir and Intimin abolished this effect and were shown to be crucial. Next, we applied several EPEC strains expressing deletion variants of Tir. In this way, we could show that removal of one or both transmembrane-spanning domains in Tir significantly reduced Tir's inhibitory function. Clearly, because mutation of Intimin also abolished the blocking effect, our results strongly suggested that binding of Tir to Intimin is important for the Tir-dependent blocking activity on cell elongation. We then confirmed in the AGS cell model that EPEC can induce all necessary signalling events that are required for pedestal formation, indicating that Intimin/Tir induces downstream signalling which may interfere with that of injected CagA.
As described above, several host cell proteins including tyrosine kinases and Nck are recruited to the EPEC attachment site leading to N-WASP-dependent actin polymerization by the Arp2/3-complex.28,35,43,44 Therefore, it was tempting to postulate that Tir may directly compete with CagA for their tyrosine kinases, the actin-nucleating activity of N-WASP, Arp2/3 or other proteins. However, tyrosine phosphorylation of CagA and Tir were not significantly affected during the co-infections, thereby ruling out the possibility that these effector proteins compete for tyrosine kinases of the Abl and Src family. Moreover, expression of Tir carrying substitutions of the two known tyrosine-phosphorylation sites (Y454F, Y474F and/or Y454/474F), still efficiently blocked cell elongation. In line with these observations, we found that expression of EHEC-Tir, which lacks the Y-474 phosphorylation site and is not tyrosine-phosphorylated,45 also blocked the H. pylori-induced phenotypes. Importantly, we detected essential roles for either of two serine residues in Tir, S-434 and S-463, which are the sites for PKA phosphorylation in vitro.6 Expression of Tir S434A, S463A or S434/463A substitution mutants entirely abrogated the ability of Tir to block H. pylori-induced cell scattering and elongation. Therefore, we provided the first example and clear evidence that both serine residues exhibit function(s) in cell signalling, and are crucial in enabling Tir to block the CagA-mediated phenotypes.
Previous work has demonstrated that purified EPEC Tir and its carboxy-terminal domain (residues 385–550 including the PKA substrate sites) exist in an equilibrium of monomers, dimers, and in the case of full-length Tir, higher oligomers, which depends on the serine residues at S-434 and S-463.7 It has been proposed that these serine sites may induce changes in the three-dimensional structure of Tir that either aid additional kinase-dependent modification and/or to promote insertion of Tir into the host plasma membrane.6–8,46 In fact, serine-phosphorylated Tir in vitro has been linked to shifts in apparent molecular mass of the protein on western blots and the serine sites are required for efficient pedestal formation.6,22,23 However, if EPEC can induce the kinase activity of PKA during infection and the functional importance of Tir phosphorylation on serine residues in vivo were unclear at this time. We could demonstrate that serine phosphorylated Tir is indeed associated with elevated PKA kinase activity as quantitated on the basis of induced phosphorylation at this kinase on threonine residue T-197.10 Interestingly, activation of PKA required the Tir serine residues at S-434 and/or S-463 (Fig. 1C). Our PKA inhibitor and siRNA studies also confirmed that injected Tir and CagA do not compete for PKA itself, because PKA is not activated by H. pylori and inhibition of PKA by the cell-permeable drugs PKI and H-89 did not block the elongation phenotype but partially inhibited Tir activities. However, the PKA-dependent assembly of Tir in the host cell membrane during infection is still not fully elucidated. A possible scenario is that multimerization of Tir is promoted by interactions in addition to those of its carboxy-terminal domain.7 This idea is consistent with the observed dimerization of the extracellular IBD bound to Intimin.47 We postulate that PKA-mediated phosphorylation of Tir during infection in vivo has a function in the correct insertion and/or interaction of Tir with the cell membrane for Intimin binding to further enhance the activity of PKA which inhibits the H. pylori-induced elongation phenotype. In line with this hypothesis, we observed that cell elongation was restored in the presence of the PKA inhibitors and is therefore a reversible process.10
Given the observation that EPEC stimulates cellular PKA during infection,10 we proposed that activated PKA may phosphorylate and therefore alter the activity of downstream factors in the target cell, which then may interfere with the CagA-induced cell elongation phenotype. Interestingly, we found that infection of AGS cells with EPEC leads to inhibition of cell migration and scattering as indicated by signs of detachment of cells from the substratum and cell rounding. Such phenotype typically indicates that the activity of small Rho GTPases maybe downregulated. A major driver in controlling cell motility is the Rho GTPase member Rac1,11,42,48,49 and Rac1 has been shown to be crucial for the H. pylori-induced elongation phenotype.42,49 Thus, we monitored the activity of Rac1 during the course of infection. Indeed, we found that injection of Tir lead to a rapid decrease of Rac1-GTP levels in an S-434/S-463-dependent manner, implying that EPEC inhibits the latter response by controlling the activity of Rac1 at the level of GTP-binding (Fig. 1C). Using phospho-specific antibodies, we found that the GTP-load inversely correlated with increasing phosphorylation of Rac1 at serine residue S-71.10 In fact, Tir induced the phosphorylation of Rac1 at S-71 by PKA which was confirmed by siRNA knockdown and pharmacological inhibition of PKA. These data indicate that PKA is a novel kinase of Rac1,10 and probably also Cdc42 which is also recognized by the phospho-antibody. Our data also showed that the latter response widely correlates with PKA-mediated activation and the production of serine-phosphorylated Tir. Interestingly, phosphorylation of Rac1 at S-71 by another cellular kinase, Akt, was shown to negatively regulate its GTP- binding in vitro.50 This indicates that PKA may directly control the activity of Rac1 in infected AGS cells by phosphorylating S-71 followed by inactivation of Rac1. However, sole treatment of cells with PKA-activating compounds such as forskolin or cAMP did not induce Rac1 phosphorylation at S-71 (our unpublished data), indicating that Tir may use a highly specific mechanism to stimulate the PKA→Rac1 pathway and/or parallel signal cascade to trigger Rac1 inactivation. Importantly, the inhibitory response on Rac1 could be widely rescued either by expression of constitutive-active Rac1 or treatment of cells with cytotoxic necrotizing factor-1 (CNF-1), which has the ability to permanently activate Rac1 by glutamine-deamidation.51 Taken together, the latter findings highlight the fact that we have discovered a novel PKA-dependent signalling pathway and the first identified functional role of serine-phosphorylated Tir (Fig. 1C). Thus, our co-infection strategy aided in the definition of pathways subverted by two different pathogens, EPEC and H. pylori, and helped defining signalling cascades that control the CagA-mediated cell elongation. Both EPEC and H. pylori colonize very different niches in the human gastrointestinal tract and therefore may not directly interfere with each other during possible co-infections in vivo. However, our studies illustrate the value of co-infection studies in vitro for identifying novel functions for effector proteins and also provided a means to define a signalling role for PKA-mediated phosphorylation in Tir function. We propose that phosphorylation of Rac1 by PKA during infection with EPEC10 (and probably also EHEC) could contribute to the formation of A/E lesions and subsequently disruption of epithelial cell-cell interactions in the gastrointestinal tract. Our findings will undoubtedly rekindle interest in Tir serine phosphorylation in relation to the functioning of this critical virulence factor, the role for the PKA-Rac1 pathway in disease-related cellular changes and the identification of new serine phosphorylation-dependent cellular responses.
An important question which remained open is whether activated PKA is the sole factor involved in the observed suppression of Rac-GTP load during infection with EPEC. In fact, very recent data showed that Rac1 phosphorylated at S-71 by Akt kinase remained in its GTP-bound conformation as proven by pulldown assays using the PAK-CRIB domain.52 In the latter study, phosphorylation at S-71 did not only fail to inactivate Rac1 but interfered with recognition of Rac1 by glucosylating bacterial toxins such as TcdA from Clostridium difficile. Both aspects resulted in a protective effect against disturbance of colonic barrier functions induced by clostridial glucosylating toxins. Moreover, the role of phosphorylated Rac1 at S-71 was investigated by expression of a phosphomimetic Rac1-S71E mutant which induced a specific phenotype and was able to reduce toxin-induced morphological changes of target cells.52 As long as it is not fully clear whether the active and/or inactive form of Rac1 can be phosphorylated, and how phosphorylation modulates specific effector protein coupling, more detailed studies are necessary to investigate the observed phenomenon. However, these reports clearly indicate that phosphorylation of Rho GTPases is not restricted to EPEC and therefore might be an interesting novel mechanism for modulation of other microbial infections, especially when Rac1 is involved. Interestingly, the S-71 site is conserved in another member of this Rho family GTPases, Cdc42, which can also undergo phosphorylation.52 Since both of these GTPases are targets for a variety of microbial virulence factors and crucial for both invasive and non-invasive bacteria, phosphorylation of Rac1/Cdc42 at S-71 could therefore be of general importance in bacterial-host interactions. Future studies should also examine pathogen-induced alteration on the three groups of regulatory proteins in the Rho GTPase cycle: the guanine dissociation inhibitors (GDIs), the guanine nucleotide exchange factors (GEFs), and GTPase-activating proteins (GAPs). It will be also interesting to investigate whether EHEC Tir, which uses serine residues S-436 and S-437 as PKA phosphorylation sites in vitro,9 also affects GTPase phosphorylation during infection. Such studies will undoubtedly contribute to our knowledge on both GTPase signalling in general and in microbial pathogenesis.
Abbreviations
- cagA
cytotoxin-associated gene A
- PKA
protein kinase A
- T3SS
type-III secretion system
- T4SS
type-IV secretion system
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/11437
References
- 1.Pawson T. Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Cell. 2004;116:191–203. doi: 10.1016/s0092-8674(03)01077-8. [DOI] [PubMed] [Google Scholar]
- 2.Backert S, Selbach M. Tyrosine-phosphorylated bacterial effector proteins: the enemies within. Trends Microbiol. 2005;13:476–484. doi: 10.1016/j.tim.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Grangeasse C, Cozzone AJ, Deutscher J, Mijakovic I. Tyrosine phosphorylation: an emerging regulatory device of bacterial physiology. Trends Biochem Sci. 2007;322:86–94. doi: 10.1016/j.tibs.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Eckhart W, Hutchinson MA, Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979;18:925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- 5.Selbach M, Paul FE, Brandt S, Guye P, Daumke O, Backert S, et al. Host cell interactome of tyrosine-phosphorylated bacterial proteins. Cell Host Microbe. 2009;5:397–403. doi: 10.1016/j.chom.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Warawa J, Kenny B. Phosphoserine modification of the enteropathogenic Escherichia coli Tir molecule is required to trigger conformational changes in Tir and efficient pedestal elongation. Mol Microbiol. 2001;42:1269–1280. doi: 10.1046/j.1365-2958.2001.02693.x. [DOI] [PubMed] [Google Scholar]
- 7.Hawrani A, Dempsey CE, Banfield MJ, Scott DJ, Clarke AR, Kenny B. Effect of protein kinase A-mediated phosphorylation on the structure and association properties of the enteropathogenic Escherichia coli Tir virulence protein. J Biol Chem. 2003;278:25839–25846. doi: 10.1074/jbc.M212409200. [DOI] [PubMed] [Google Scholar]
- 8.Race PR, Solovyova AS, Banfield MJ. Conformation of the EPEC Tir protein in solution: investigating the impact of serine phosphorylation at positions 434/463. Biophys J. 2007;93:586–596. doi: 10.1529/biophysj.106.101766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen-Vercoe E, Waddell B, Toh MC, DeVinney R. Amino acid residues within enterohemorrhagic Escherichia coli O157:H7 Tir involved in phosphorylation, alpha-actinin recruitment, and Nck-independent pedestal formation. Infect Immun. 2006;74:6196–6205. doi: 10.1128/IAI.00753-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandt S, Kenny B, Rohde M, Martinez-Quiles N, Backert S. Dual infection system identifies a crucial role for PKA-mediated serine phosphorylation of the EPEC-Tir-injected effector protein in regulating Rac1 function. Cell Microbiol. 2009;11:1254–1271. doi: 10.1111/j.1462-5822.2009.01330.x. [DOI] [PubMed] [Google Scholar]
- 11.Nobes C, Hall A. Regulation and function of the Rho subfamily of small GTPases. Curr Opin Genet Dev. 1994;4:77–81. doi: 10.1016/0959-437x(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 12.Moon HW, Whipp SC, Argenzio RA, Levine MM, Giannella RA. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke SC, Haigh RD, Freestone PP, Williams PH. Virulence of enteropathogenic Escherichia coli, a global pathogen. Clin Microbiol Rev. 2003;16:365–378. doi: 10.1128/CMR.16.3.365-378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 15.Dean P, Maresca M, Kenny B. EPEC's weapons of mass subversion. Curr Opin Microbiol. 2005;8:28–34. doi: 10.1016/j.mib.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Frankel G, Phillips AD. Attaching effacing Escherichia coli and paradigms of Tir-triggered actin polymerization: getting off the pedestal. Cell Microbiol. 2008;10:549–556. doi: 10.1111/j.1462-5822.2007.01103.x. [DOI] [PubMed] [Google Scholar]
- 17.Weflen AW, Alto NM, Hecht GA. Tight junctions and enteropathogenic E. coli. Ann N Y Acad Sci. 2009;1165:169–174. doi: 10.1111/j.1749-6632.2009.04060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iguchi A, Thomason NR, Ugura Y, Saunders D, Ooka T, Henderson IR, et al. The complete genome sequence and comparative genome analysis of enteropathogenic E. coli O122:H6 strain E2348/69. J Bacteriol. 2009;191:347–354. doi: 10.1128/JB.01238-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dean P, Kenny B. The effector repertoire of enteropathogenic E. coli: ganging up on the host cell. Current Opinion in Microbiology. 2009;12:101–109. doi: 10.1016/j.mib.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marches O, Nougayrede JP, Boullier S, Mainil J, Charlier G, Raymond I, et al. Role of tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2. Infect Immun. 2000;68:2171–2182. doi: 10.1128/iai.68.4.2171-2182.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng W, Vallance BA, Li Y, Puente JL, Finlay BB. Citrobacter rodentium translocated intimin receptor (Tir) is an essential virulence factor needed for actin condensation, intestinal colonization and colonic hyperplasia in mice. Mol Microbiol. 2003;48:95–115. doi: 10.1046/j.1365-2958.2003.03429.x. [DOI] [PubMed] [Google Scholar]
- 22.Kenny B, DeVinney R, Stein M, Reinscheid DJ, Frey EA, Finlay BB. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 23.Kenny B. Phosphorylation of tyrosine 474 of the enteropathogenic Escherichia coli (EPEC) Tir receptor molecule is essential for actin nucleating activity and is preceded by additional host modifications. Mol Microbiol. 1999;31:1229–1241. doi: 10.1046/j.1365-2958.1999.01265.x. [DOI] [PubMed] [Google Scholar]
- 24.Kenny B. The enterohaemorrhagic Escherichia coli (serotype O157:H7) Tir molecule is not functionally interchangeable for its enteropathogenic E. coli (serotype O127:H6) homologue. Cell Microbiol. 2001;3:499–510. doi: 10.1046/j.1462-5822.2001.00133.x. [DOI] [PubMed] [Google Scholar]
- 25.Phillips N, Hayward RD, Koronakis V. Phosphorylation of the enteropathogenic E. coli receptor by the Src-family kinase c-Fyn triggers actin pedestal formation. Nat Cell Biol. 2004;6:618–625. doi: 10.1038/ncb1148. [DOI] [PubMed] [Google Scholar]
- 26.Swimm A, Bommarius B, Li Y, Cheng D, Reeves P, Sherman M, et al. Enteropathogenic Escherichia coli use redundant tyrosine kinases to form actin pedestals. Mol Biol Cell. 2004;15:3520–3529. doi: 10.1091/mbc.E04-02-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bommarius B, Maxwell D, Swimm A, Leung S, Corbett A, Bornmann W, et al. Enteropathogenic Escherichia coli Tir is an SH2/3 ligand that recruits and activates tyrosine kinases required for pedestal formation. Mol Microbiol. 2007;63:1748–1768. doi: 10.1111/j.1365-2958.2007.05626.x. [DOI] [PubMed] [Google Scholar]
- 28.Gruenheid S, DeVinney R, Bladt F, Goosney D, Gelkop S, Gish GD, et al. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat Cell Biol. 2001;3:856–859. doi: 10.1038/ncb0901-856. [DOI] [PubMed] [Google Scholar]
- 29.Campellone KG, Giese A, Tipper DJ, Leong JM. A tyrosine-phosphorylated 12-amino-acid sequence of enteropathogenic Escherichia coli Tir binds the host adaptor protein Nck and is required for Nck localization to actin pedestals. Mol Microbiol. 2002;43:1227–1241. doi: 10.1046/j.1365-2958.2002.02817.x. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Ami G, Ozeri V, Hanski E, Hofmann F, Aktories K, Hahn KM, et al. Agents that inhibit Rho, Rac and Cdc42 do not block formation of actin pedestals in HeLa cells infected with enteropathogenic Escherichia coli. Infect Immun. 1998;66:1755–1758. doi: 10.1128/iai.66.4.1755-1758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campellone KG, Leong JM. Nck-independent actin assembly is mediated by two phosphorylated tyrosines within enteropathogenic Escherichia coli Tir. Mol Microbiol. 2005;56:416–432. doi: 10.1111/j.1365-2958.2005.04558.x. [DOI] [PubMed] [Google Scholar]
- 32.Freeman NL, Zurawski DV, Chowrashi P, Ayoob JC, Huang L, Mittal B, et al. Interaction of the enteropathogenic Escherichia coli protein, translocated intimin receptor (Tir), with focal adhesion proteins. Cell Motil Cytoskeleton. 2000;47:307–318. doi: 10.1002/1097-0169(200012)47:4<307::AID-CM5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 33.Cantarelli VV, Takahashi A, Akeda Y, Nagayama K, Honda T. Interaction of enteropathogenic or enterohemorrhagic Escherichia coli with HeLa cells results in translocation of cortactin to the bacterial adherence site. Infect Immun. 2000;68:382–386. doi: 10.1128/iai.68.1.382-386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantarelli VV, Takahashi A, Yanagihara I, Akeda Y, Imura K, Kodama T, et al. Talin, a host cell protein, interacts directly with the translocated intimin receptor, Tir, of enteropathogenic Escherichia coli, and is essential for pedestal formation. Cell Microbiol. 2001;3:745–751. doi: 10.1046/j.1462-5822.2001.00156.x. [DOI] [PubMed] [Google Scholar]
- 35.Goosney DL, DeVinney R, Finlay BB. Recruitment of cytoskeletal and signaling proteins to enteropathogenic and enterohemorrhagic Escherichia coli pedestals. Infect Immun. 2001;69:3315–3322. doi: 10.1128/IAI.69.5.3315-3322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang L, Mittal B, Sanger JW, Sanger JM. Host focal adhesion protein domains that bind to the translocated intimin receptor (Tir) of enteropathogenic Escherichia coli (EPEC) Cell Motil Cytoskeleton. 2002;52:255–265. doi: 10.1002/cm.10050. [DOI] [PubMed] [Google Scholar]
- 37.Patel A, Cummings N, Batchelor M, Hill PJ, Dubois T, Mellits KH, et al. Host protein interactions with enteropathogenic Escherichia coli (EPEC): 14-3-3tau binds Tir and has a role in EPEC-induced actin polymerization. Cell Microbiol. 2006;8:55–71. doi: 10.1111/j.1462-5822.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 38.Hatakeyama M. SagA of CagA in Helicobacter pylori pathogenesis. Curr Opin Microbiol. 2008;11:30–37. doi: 10.1016/j.mib.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Backert S, Selbach M. Role of type IV secretion in the pathogenesis of Helicobacter pylori. Cell Microbiol. 2008;10:1573–1581. doi: 10.1111/j.1462-5822.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 40.Kwok T, Zabler D, Urman S, Rohde M, Hartig R, Wessler S, et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007;449:862–866. doi: 10.1038/nature06187. [DOI] [PubMed] [Google Scholar]
- 41.Wessler S, Backert S. Molecular mechanisms of epithelial-barrier disruption by Helicobacter pylori. Trends Microbiol. 2008;16:181–189. doi: 10.1016/j.tim.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Brandt S, Shafikhani S, Balachandran P, Jin S, Hartig R, König W, et al. Use of a novel co-infection system reveals a role for Rac, Ras and CrkII phosphorylation in Helicobacter pylori-induced host cell actin cytoskeletal rearrangements. FEMS Immunol Med Microbiol. 2007;50:190–205. doi: 10.1111/j.1574-695X.2007.00234.x. [DOI] [PubMed] [Google Scholar]
- 43.Kalman D, Weiner OD, Goosney DL, Sedat JW, Finlay BB, Abo A, et al. Enteropathogenic E. coli acts through WASP and Arp2/3 complex to form actin pedestals. Nat Cell Biol. 1999;1:389–391. doi: 10.1038/14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lommel S, Benesch S, Rohde M, Wehland J, Rottner K. Enterohaemorrhagic and enteropathogenic Escherichia coli use different mechanisms for actin pedestal formation that converge on N-WASP. Cell Microbiol. 2004;6:243–254. doi: 10.1111/j.1462-5822.2004.00364.x. [DOI] [PubMed] [Google Scholar]
- 45.DeVinney R, Stein M, Reinscheid D, Abe A, Ruschkowski S, Finlay BB. Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated. Infect Immun. 1999;67:2389–2398. doi: 10.1128/iai.67.5.2389-2398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kenny B. Enteropathogenic Escherichia coli (EPEC)—a crafty subversive little bug. Microbiology. 2002;148:1967–1978. doi: 10.1099/00221287-148-7-1967. [DOI] [PubMed] [Google Scholar]
- 47.Luo Y, Frey EA, Pfuetzner RA, Creagh AL, Knoechel DG, Haynes CA, et al. Crystal structure of enteropathogenic Escherichia coli intimin-receptor complex. Nature. 2000;405:1073–1077. doi: 10.1038/35016618. [DOI] [PubMed] [Google Scholar]
- 48.Churin Y, Kardalinou E, Meyer TF, Naumann M. Pathogenicity island-dependent activation of Rho GTPases Rac1 and Cdc42 in Helicobacter pylori infection. Mol Microbiol. 2001;40:814–823. doi: 10.1046/j.1365-2958.2001.02443.x. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki M, Mimuro H, Suzuki T, Park M, Yamamoto T, Sasakawa C. Interaction of CagA with Crk plays an important role in Helicobacter pylori-induced loss of gastric epithelial cell adhesion. J Exp Med. 2005;202:1235–1247. doi: 10.1084/jem.20051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon T, Kwon DY, Chun J, Kim JH, Kang SS. Akt protein kinase inhibits Rac1-GTP binding through phosphorylation at serine 71 of Rac1. J Biol Chem. 2000;275:423–428. doi: 10.1074/jbc.275.1.423. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- 52.Schoentaube J, Olling A, Tatge H, Just I, Gerhard R. Serine-71 phosphorylation of Rac1/Cdc42 diminishes the pathogenic effect of Clostridium difficile toxin A. Cell Microbiol. 2009;11:1816–1826. doi: 10.1111/j.1462-5822.2009.01373.x. [DOI] [PubMed] [Google Scholar]