Abstract
We have recently shown that alteration of the gut commensal microbiota with antibiotics can modify the susceptibility to autoimmune demyelinating processes of the central nervous system. Treatment of mice with a broad spectrum of antibiotics not only induced significant changes in the regulatory T cell populations of the gut associated lymphoid tissues (GALT) and peripheral lymphoid organs but reduced the susceptibility to EAE, the most widely used animal model for human multiple sclerosis. Here, we show further that oral antibiotic treatment of EAE mice induced a CD5+B cell subpopulation that conferred protection against the disease. Protection was associated with an enhanced frequency of CD5+B cells in distal lymphoid sites such as cervical LN. In vitro stimulation with LPS increased the production of IL-10 by splenic CD5+B cells. Adoptive transfer of CD5+B cells from antibiotic treated mice reduced significantly the severity of EAE by shifting the immune responses from Th1/Th17 towards anti-inflammatory Th2-type responses. Our results demonstrate that this specific B cell population appears to be involved in the immune regulation of autoimmunity, in particular this experimental demyelinating disease of the central nervous system by gut commensal microflora.
Key words: B cells, commensal bacteria, autoimmune regulation, IL-10, EAE/MS
Introduction
Multiple sclerosis (MS) is a chronic human demyelinating disease associated with inflammation and neurodegeneration of the central nervous system (CNS).1 In experimental allergic encephalomyelitis (EAE), encephalitogenic CD4+ T cells react with self-antigens of the neuronal myelin sheet causing cell infiltration into the CNS, demyelination and eventually neuronal damage.2 Th1 and Th17 antigen-specific cells are believed to be involved in the disease progression,3 whereas FoxP3+Treg cells control natural recovery4 and protection.5
The mammalian mucosal gastrointestinal (GI) tract is a complex ecological environment that contains a heterogeneous population of >1014 microorganisms that belong to ∼1,000 species.6–8 Studies in germ-free animals, born and raised in sterile conditions, have demonstrated that early exposure to both pathogens and non-pathogenic microbes is necessary for the complete development of the gut-associated lymphoid tissues (GALT) and balanced immune development.9 We have recently demonstrated that modification of the bacterial populations of the gut alters the clinical outcome of EAE in mice.10 Oral treatment of mice with antibiotics reduced the clinical severity of disease that was associated with both diminished pro-inflammatory responses and conversely enhanced FoxP3+Treg cells that accumulated in mesenteric and cervical lymph nodes (LN). Adoptive transfer of IL-10-producing Treg cells conferred protection against EAE.
The role of B cells in EAE and MS is of increasing interest. Distinct B cell subtypes have been recently associated with EAE disease progression and regulation.11 B cells with a plasmacytoid type phenotype can control EAE in mice by the acquisition of regulatory features.12 Recent Phase I and Phase II clinical trials in relapsing and progressive MS focused on B cell depletion13–15 have demonstrated a potentially important role for B cells in disease progression and regulation. In this addendum, we show that oral treatment with a combination of broad spectrum antibiotics induced changes in the B cell subsets of GALT and peripheral C57BL/6 mice. Treatment with antibiotics enhanced a regulatory-type phenotype in B cells. CD5+ B cells obtained from mice with altered gut microbiota demonstrated enhanced IL-10 levels and conferred EAE protection after adoptive transfer.
EAE Protection by Alteration of the Gut Microbiota is Associated to Augmented CD5+ B cell Proportions
Recent findings have revealed the critical importance of B cells not only as antibody producers but also as a population of cells that exhibit regulatory function.11,16–19 Deficiency of B cells in C57BL/6 mice exacerbates EAE disease severity18,20,21 and adoptive transfer of IL-10 producing B cells can protect IL-10 deficient mice against the disease.21 The role of distinct B cell subsets in the development or control of EAE was recently analyzed by Matsushita et al.11 EAE regulation was associated with a small proportion (1–2%) of splenic B cells with a CD1dhighCD5+ phenotype that produced IL-10. TLR2/4 activated B cells through MyD88 signaling pathway were able to protect against EAE implicating a potential role for bacterial antigens in the regulation of disease progression through B cells.22 In vitro stimulation of splenic CD1dhighCD5+ B cells with bacterial LPS enhanced the secretion of IL-10.19
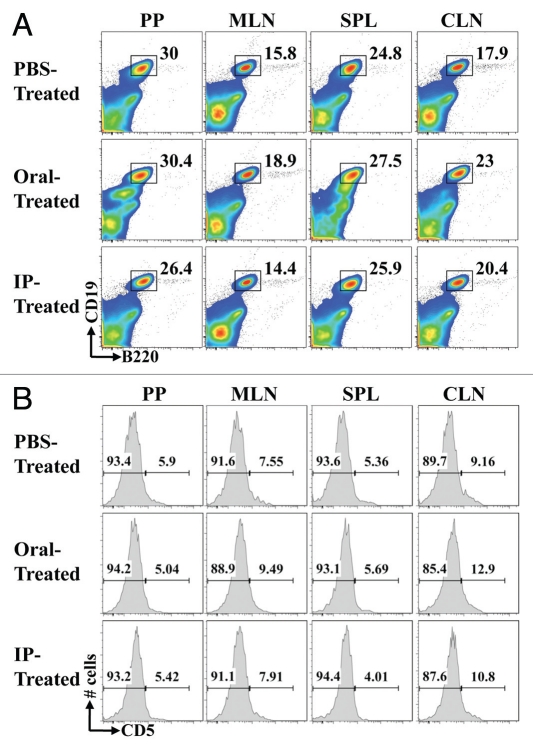
Our reported studies demonstrate that the oral treatment of mice with antibiotics against the gut microbiota reduced the severity of EAE. To assess whether B cells are associated with disease severity and progression in this antibiotic treated model, C57BL/6 mice were treated with the same antibiotic cocktail with ampicilin (1 g/L), metronidazole (1 g/L), vancomycin (1 g/L), and neomycin sulfate (1 g/L) by either oral or intraperitoneal (IP) route. Oral administration was via dissolution of antibiotics in drinking water whereas IP treatment consisted on 200 µl daily injections. EAE was induced by subcutaneous injection of 250 µg of MOG35–55 and two IP doses of 400 ng of pertussis toxin (days 0 and +2). One week of oral treatment with antibiotics but not IP, protected mice against EAE, as previously described.10 Interestingly, the percentages of B cells analyzed by flow cytometry (CD19+B220+) were enhanced in mesenteric LN, spleens and cervical LN of protected mice (Oral-Treatment) when compared to IP- and PBS-Treated mice (Fig. 1A). Moreover, the CD19+CD5+ B cell percentages were enhanced in mesenteric and cervical LN of EAE protected mice when compared to PBS-Treated mice used as EAE control group. No significant differences were observed in IP-Treated mice when compare to PBS-Treated mice (Fig. 1B).
Figure 1.
Oral treatment with antibiotics modifies the CD5+ B cell percentages in EAE protected mice. One week of oral treatment with antibiotics protected C57BL/6 mice against EAE, as previously published.10 Peyer's patches, Mesenteric and cervical LN, as well as spleens were harvested and total B cell populations (CD19+B220+) (A) and CD5+/− B cells (B) compared by flow cytometry. A representative analysis of two combined experiments for 10 mice/group is shown.
Oral Treatment with Antibiotics Enhances the Frequency of IL-10 Producing CD1dhighCD5+ B Cells
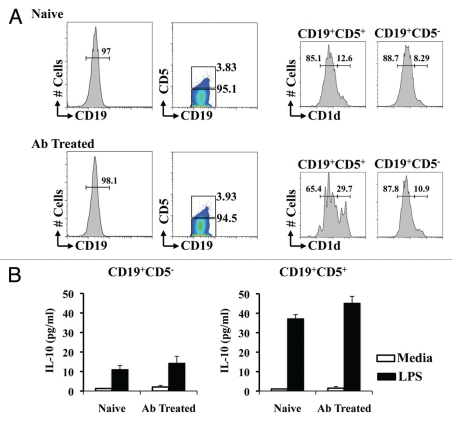
Flow cytometry analysis showed a significant (p < 0.05) increase of CD1dhigh expression in CD5+ B cells when compared to CD5− B cells (8.1 ± 1.1 vs. 12.2 ± 1.3% in naïve, and 10.2 ± 2.3 vs. 28.6 ± 3.2% in Ab Treated) (Fig. 2A). More interestingly, the frequency of CD1dhigh in CD5+CD19+ B cells of mice treated with antibiotics was enhanced significantly when compared with CD5+CD19+ B cells of naïve C57BL/6 mice (12.2 ± 1.3% in naïve vs. 28.6 ± 3.2% in Ab Treated mice; p < 0.05).
Figure 2.
Production of IL-10 by CD5+ B cells is enhanced in mice treated with antibiotics. Splenic and lymph node cells from naïve C57BL/6 and mice treated orally with antibiotics for one week were sorted into CD19+B220+ B cells and subsequently into CD5+ and CD5− by flow cytometry, and CD1d expression was compared (A). CD19+B220+CD5+ and CD19+B220+CD5− cells were cultured for 12 h in RPMI complete media with 100 ng/ml of LPS. IL-10 secreted to the culture media was compared by cytokine ELISA (B). A representative analysis of two combined experiments for six mice/group is shown.
The role of a splenic CD1dhighCD5+ B cell phenotype that produced IL-10 in the regulation of EAE has been recently described.11 We next compared the IL-10 production by CD5+ and CD5− B cells from naïve and mice treated with antibiotics (Fig. 2B). CD19+B220+ B cells were FACS sorted into CD5+ and CD5− subpopulations. Cells were cultured for 72 h in media or 100 µg/ml of LPS (Sigma-Aldrich, St. Louis, MO). Similar to the observations with the CD1dhigh frequency among CD5− and CD5+ CD19+B cells, the level of IL-10 production B cells was polarized significantly towards the CD5+ subpopulation. CD5−CD19+ B cells from naïve and mice treated with antibiotics restimulated with LPS released less than significant levels of IL-10 (10.5 ± 2.1 and 13.2 ± 2.6 pg/ml). When CD5+CD19+ B cells were restimulated with LPS significant increased levels of IL-10 were detected in the media when compared to CD5−CD19+ B cells (36.5 ± 2.4 in naïve and 45.2 ± 4.4; p < 0.05). Although the IL-10 levels produced by CD5+CD19+ B cells sorted from mice treated with antibiotics were enhanced when compared with naïve CD5+CD19+ B cells, the differences observed were not statistically significant (Fig. 2B).
Adoptively Transferred CD5+ B Cells Reduce the Severity of EAE in Conventional Mice
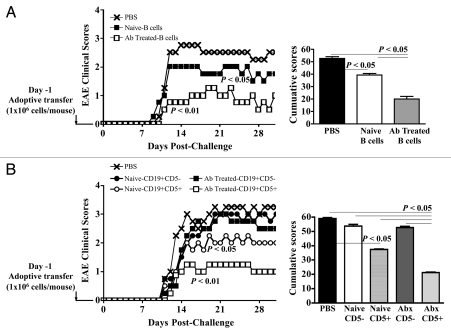
To examine whether CD5+ B cells would play a role in the control of EAE associated with alteration of the gut microbiota following oral antibiotic treatment, we compared the protective effect of total CD19+ as well as sorted CD5−CD19+ B cells and CD5+CD19+ B cells from naïve and mice treated with antibiotics (Fig. 3). Naïve C57BL/6 recipient mice were subjected to the adoptive transfer of one million CD19+ B cells obtained from spleens of donor naïve, and from mice treated with antibiotics (Fig. 3A) and one million of CD5+CD19+ or CD5−CD19+ B cells from spleens of donor naïve, and from mice treated with antibiotics (Fig. 3B). One day following the adoptive transfers, EAE was induced.
Figure 3.
CD5+ B cells from mice with altered microbiota protect against EAE. Splenic and lymph node cells from naïve C57BL/6 and mice treated orally with antibiotics for one week were sorted into CD19+B220+ B cells and subsequently into CD5+ and CD5− by flow cytometry. 1 × 106 CD19+ B cells (A) and cells CD5+ or CD5− B cells (B) from naïve and C57BL/6 mice treated with antibiotics were adoptively transferred into naïve recipient C57BL/6 mice. EAE was induced one day after the adoptive transfers. The graphs show the averages of combined EAE clinical scores and cumulative scores from two independent experiments for eight mice/group. p < 0.05 and p < 0.01 (Mann-Whitney U-test).
The adoptive transfer of total CD19+ B cells from mice treated with antibiotics reduced significantly the severity of EAE (p value: 0.02). Interestingly, naïve CD19+ B cells transferred into recipient mice conferred partial protection against the disease when compared to PBS-Treated mice (p value: 0.009) (Fig. 3A). The reduction in the EAE severity and the cumulative clinical scores was significantly more relevant (p value: 0.001) after the adoptive transfer of cells obtained from mice treated with antibiotics than from naïve mice.
We next compared the protective effect of CD5+CD19+ or CD5−CD19+ B cells from spleens of donor naïve, and from mice treated with antibiotics (Fig. 3B). Only CD5+CD19+ B cells reduced significantly the EAE severity and cumulative clinical scores of recipient mice when compared to PBS-untreated mice (Naïve CD5+CD19+ B cells: p value: 0.015; Ab-Treated CD5+CD19+ B cells; p value: 0.009). As observed for total CD19+ B cells, the adoptive transfer of naïve CD5+CD19+ B cells diminished significantly the severity of EAE, yet the protection conferred by CD5+CD19+ B cells harvested from mice subjected to the treatment with antibiotics was more pronounced (p value: 0.019) (Fig. 3B).
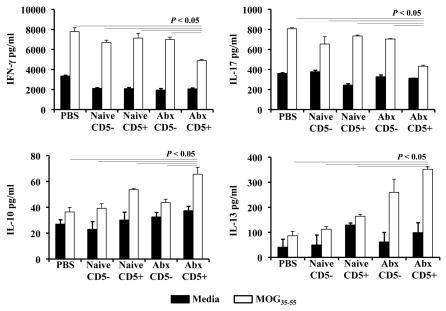
In order to compare the MOG-specific responses in mice subjected to adoptive transfer of CD19+ B cell subsets, splenic cells of EAE mice were stimulated for 72 h with MOG35–55 or media (Fig. 4). Cells obtained from PBS-Treated mice (EAE control) at day 20 post-disease induction produced significant levels of IFNγ and IL-17 upon stimulation with MOG35–55 when compared with cells cultured in the absence of self-antigen. IFNγ and IL-17 production was reduced significantly in splenic cells of recipient mice of CD5+CD19+ B cells from mice treated with antibiotics. No significant differences in the IFNγ and IL-17 levels were observed between splenocytes of mice subjected to adoptive transfer with naïve CD5−CD19+ B cells, naïve CD5+CD19+ B cells and Ab-Treated CD5−CD19+ B cells when compared to PBS-untreated mice. By contrast, we detected a significant increase in IL-10 produced by splenic cells of mice treated with CD5+CD19+ B cells of mice with altered microbiota when compared to the EAE control group and mice treated with naïve CD5+CD19+ B cells, naïve CD5− CD19+ B cells and Ab-Treated CD5−CD19+ B cells. Similarly, the level of IL-13 from Ab-Treated CD5+CD19+ B cells was augmented significantly when compared to mice treated with PBS-, naïve CD5−CD19+ B cells, naïve CD5+CD19+ B cells, and Ab-Treated CD5−CD19+ B cells. These results demonstrate that the adoptive transfer of CD5+CD19+ B cells of mice with altered microbiota induced a significant shift of the immune responses of recipient mice following EAE induction from Th1/Th17 towards Th2-type immune responses.
Figure 4.
Adoptive transferred CD5+ B cells from mice with altered microbiota shift the cytokine patterns towards a Th2-type in EAE mice. Splenic cells from EAE mice and mice subjected to adoptive transfers of CD5+ and CD5− CD19+ B cells were cultured in the presence of 100 µg/ml MOG35–55 or media for 72 h. Specific ELISA were used to detect cytokines from culture supernatants. Results represent the combination of four mice/group.
Finally, we compared the frequencies of regulatory T and B cell phenotypes in spleens and cervical lymph nodes of the recipient mice subjected to adoptive transfers. No significant differences in FoxP3+Treg and CD5+CD1dhigh B cells were observed (not shown).
Conclusions
We recently published that treatment of mice with a broad spectrum of antibiotics induces the accumulation of regulatory T cells that mediate a reduced susceptibility to EAE, and are protective after adoptive transfer.10 The role of NKT cells in the protection against EAE by oral treatment with antibiotics has been described as well.23 We questioned whether the alteration of the gut microbiota would modify the different subpopulations of B cells that have been associated with the control of EAE and perhaps human multiple sclerosis.11,16–19 Our results suggest that alterations of the gut microbiota could induce strong immune shift that modifies the regulation of inflammatory processes of the CNS by regulatory T10 and B cell subpopulations. Both cell subpopulation appear to function as modulators of inflammation by the production of IL-10, however, IL-13 might be playing a significant role in the protection. Adoptive transfer of IL-10 producing CD5+CD19+ B cells of mice treated with oral antibiotics into naïve recipient mice significantly reduced the severity of EAE, and that this protection was associated with a shift of the cytokine patterns favoring Th2-type polarization versus pro-inflammatory Th1/Th17.
We are currently investigating the specific bacterial population(s) involved in the immune regulation observed. Our preliminary data suggest that the treatment with antibiotics not only significantly reduced the total numbers of bacteria present in the gut, but also altered significantly the heterogeneity of their populations. Recent work has shown that the homeostasis and control of inflammatory Th17 and regulatory Treg responses depend on the composition of the gut microbiota.24,25 These reports and our results demonstrating that changes in the gut flora modifies the outcome of autoimmune processes such as EAE suggest that the selective manipulation of microbial communities or their antigenic products could have potential benefits in the treatment of human autoimmune diseases such as multiple sclerosis.
Acknowledgements
This work was supported by the Dartmouth-Hitchcock Foundation (Javier Ochoa-Repáraz; Tiffany Blake Fellowship # 205-702B) and training grants from TEVA Neuroscience (Lloyd H. Kasper; grant # 50-2033-TEVA Neuroscience Murray B. Bornstein Fund) and the National Multiple Sclerosis Society (Lloyd H. Kasper; grant # CA1027A1/3). The authors wish to thank Dr. Azizul Haque, Dr. Jacqueline Y. Channon Smith, Mark Christy, John DeLong, Kathleen Smith and Alan J. Bergeron for technical support and critical review of the manuscript.
Abbreviations
- CNS
central nervous system
- EAE
experimental allergic or autoimmune encephalomyelitis
- GALT
gut associated lymphoid tissues
- LN
lymph nodes
- PP
peyer's patches
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/11515
References
- 1.Bhat R, Steinman L. Innate and adaptive autoimmunity directed to the central nervous system. Neuron. 2009;64:123–132. doi: 10.1016/j.neuron.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Mix E, Meyer-Rienecker H, Zettl UK. Animal models of multiple sclerosis for the development and validation of novel therapies—potential and limitations. J Neurol. 2008;255:7–14. doi: 10.1007/s00415-008-6003-0. [DOI] [PubMed] [Google Scholar]
- 3.Awasthi A, Kuchroo VK. Th17 cells: from precursors to players in inflammation and infection. Int Immunol. 2009;21:489–498. doi: 10.1093/intimm/dxp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 5.O'Connor RA, Anderton SM. Foxp3+ regulatory T cells in the control of experimental CNS autoimmune disease. J Neuroimmunol. 2008;193:1–11. doi: 10.1016/j.jneuroim.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Zoetendal EG, Rajilic-Stojanovic M, de Vos WM. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut. 2008;57:1605–1615. doi: 10.1136/gut.2007.133603. [DOI] [PubMed] [Google Scholar]
- 7.Booijink CC, Zoetendal EG, Kleerebezem M, de Vos WM. Microbial communities in the human small intestine: coupling diversity to metagenomics. Future Microbiol. 2007;2:285–295. doi: 10.2217/17460913.2.3.285. [DOI] [PubMed] [Google Scholar]
- 8.Zoetendal EG, Vaughan EE, de Vos WM. A microbial world within us. Mol Microbiol. 2006;59:1639–1650. doi: 10.1111/j.1365-2958.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- 9.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183:6041–6050. doi: 10.4049/jimmunol.0900747. [DOI] [PubMed] [Google Scholar]
- 11.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rafei M, Hsieh J, Zehntner S, Li M, Forner K, Birman E, et al. A granulocyte-macrophage colony-stimulating factor and interleukin-15 fusokine induces a regulatory B cell population with immune suppressive properties. Nat Med. 2009;15:1038–1045. doi: 10.1038/nm.2003. [DOI] [PubMed] [Google Scholar]
- 13.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 14.Bar-Or A, Calabresi PA, Arnold D, Markowitz C, Shafer S, Kasper LH, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63:395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- 15.Hawker K, O'Connor P, Freedman MS, Calabresi PA, Antel J, Simon J, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66:460–471. doi: 10.1002/ana.21867. [DOI] [PubMed] [Google Scholar]
- 16.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 17.Bouaziz JD, Yanaba K, Venturi GM, Wang Y, Tisch RM, Poe JC, et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci USA. 2007;104:20878–20883. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushita T, Fujimoto M, Hasegawa M, Komura K, Takehara K, Tedder TF, et al. Inhibitory role of CD19 in the progression of experimental autoimmune encephalomyelitis by regulating cytokine response. Am J Pathol. 2006;168:812–821. doi: 10.2353/ajpath.2006.050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 22.Lampropoulou V, Hoehlig K, Roch T, Neves P, Calderon Gomez E, Sweenie CH, et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol. 2008;180:4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 23.Yokote H, Miyake S, Croxford JL, Oki S, Mizusawa H, Yamamura T. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am J Pathol. 2008;173:1714–1723. doi: 10.2353/ajpath.2008.080622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]