Abstract
We have demonstrated that direct antigen sampling of bacteria by intestinal dendritic cells (DCs) is accompanied by a rapid migration of CD11c+CX3CR1+MHCII+CD8α-CD11b− DCs into the intestinal lumen upon exposure to non-invasive ΔSPI1-Salmonella. Importantly, intraluminal DCs internalized Salmonella but were not able to cross the epithelium to return into tissue, thus showing that these DCs do not function as antigen-presenting cells and participate in the conventional regulation of immune responses to intestinal pathogens. Here we show that the presence of the chemokine receptor CX3CR1, that plays a vital role in DC-mediated antigen sampling and clearance in the gut, is also instrumental for the transepithelial migration of DCs. The latter observation, along with the notion that CX3CR1-deficient mice displayed higher susceptibility to Salmonella infection compared to wild-type mice raises the possibility that Salmonella-induced migration of “bacteria-capturing” DCs into the lumen may be an important mechanism of mucosal defence and clearance.
Key words: dendritic cell, mucosal immunity, Salmonella, antigen sampling, cell migration, immune exclusion, mucosal clearance
Salmonella Induces Flagellin- and MyD88-Dependent Migration of Bacteria-Capturing Dendritic Cells into the Gut Lumen
Intestinal DCs play a critical role in the orchestration of mucosal immune responses,1 however in the gut-DCs appeared to be also directly involved in sampling luminal antigens by extending cellular processes between epithelial cells and shuttle them across the epithelial barrier.2–4 This event is facilitated by the expression of tight junction protein that enables sampling without altering the integrity of the intestinal barrier. It has been hypothesized that direct DC sampling is followed by migration of bacteria-loaded DCs to the mesenteric lymph node (MLN) where they present antigens to T cells.5 We have recently described that DC direct sampling is not the sole event taking place at mucosal interface in the gut during Salmonella infection.6 Indeed, using both isolated intestinal loops and oral delivery of Salmonella we observed that a significant number of CD11c+CX3CR1+MHCII+CD8α−CD11b−DCs traversed the epithelial barrier and moved into the lumen prior or following internalization of GFP-labelled Salmonella. Post Salmonella-challenge analysis of the epithelial barrier, that involved detailed study of tight junctions and intestinal permeability showed that DCs migration was not due to Salmonella-associated damages of the epithelium. The numbers of DCs within the gut lumen at 1.5 h after the introduction of Salmonella were 50-fold greater than after challenge with E. coli or PBS and after 3 h, the number of luminal DCs increased another ∼3-fold in the lumen of Salmonella-treated mice.
Our work also determined that flagellin is a key signal, although not the only one, for DC migration. This was assessed by the use of Salmonella variant (ΔfliC ΔfljB) that lack flagellin and transgenic mice that lack the adaptor molecule MyD88, that is required for the activation signals following flagellin/TLR5 engagement.7 The role of flagellin was also assessed by generating a double SPI1-SPI2 deficient Salmonella double mutant (ΔSPI1 ΔssrA) and a flagellated variant of E. coli K12. In either case the bacterial challenge did not induce DC migration suggesting an important role for the SPI2-mediated vescicular intracellular transport of flagellin,8 and the requirement for additional pathogen-specific signal that were absent in the flagellated, but not pathogenic variant of E. coli used. Parallel experiments also showed that soluble flagellin did not induced DCs migration. It is also interesting to highlight that the lack of migration upon challenge with different variants of E. coli showed that LPS is not involved in this event. Also, by challenging different areas of the gut by using the isolated loops technique we determined that DC migration took place exclusively in the small intestine, including jejunum and ileum, but not in the colon. We also demonstrated that intraluminal DCs internalized Salmonellae prior or following migration into the gut lumen and that DC traffic was unidirectional; DCs were not able to cross the epithelial barrier and return into the intestinal tissue. The latter observations ruled out the possibility that these DCs play a role in antigen-presentation. Taken together, these data demonstrated that the introduction of non-invasive Salmonella in the small intestine triggers at least two different events; DCs can either sample Salmonella and shuttle these bacteria back across the epithelial barrier or they can migrate into the intestinal lumen prior to or following internalization of Salmonella. These data also show that transepithelial DC protrusions do not always represent antigen-sampling devises; instead it appeared that the majority of these structures identify DCs in their migration into the intestinal lumen.
Salmonella-Induced DC Migration, but Not Bacterial Translocation Is Impaired in CX3CR1-Deficient Mice
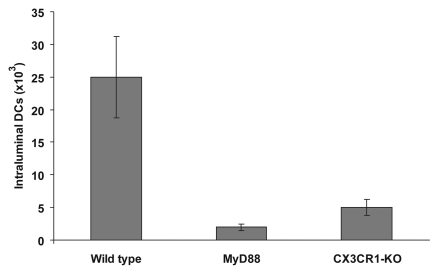
Conflicting results have been reported on the role of the chemokine receptor CX3CR1 on the ability of DCs to form transepithelial dendrites and sample directly luminal bacteria. CX3CR1+ DCs are the target for the CX3CL1/fraktalkine, a transmembrane chemokine expressed at the surface and basolateral compartment of intestinal epithelial cells (IEC) predominantly, but not exclusively in the terminal ileum.3,4,9 One study reporting that CX3CR1-deficient mice were unable to sample luminal Salmonella,4 was followed by another one suggesting that CX3CR1 was not directly involved in the formation of DC protrusions across the intestinal epithelium.3 Important, both studies were done in mice of identical genetic make up (C57BL/6), thus showing that this was not the origin of this discrepancy. Most important, in addressing the role of CX3CR1+ DC it was reported that CX3CR1-deficient mice showed increased susceptibility to Salmonella infection, thus suggesting that these antigen-sampling DCs are a major component of defence against pathogenic microorganisms.4 We then extended our investigation of Salmonella-induced DC migration in CX3CR1 knock-out (KO) mice and we observed that following short-term (3 h) challenge, carried out using isolated loops DC migration is drastically reduced (Fig. 1). Numbers of DCs into the lumen were significantly reduced compared to wild-type mice and comparable to levels observed in MyD88 mice. In addition, early translocation (5 h) of non-invasive Salmonella in the jejunum and ileum was not significantly affected in CX3CR1-KO mice (Table 1) confirming the finding that the absence of transepithelial dendrites in the terminal ileum of CX3CR1-KO did not impair bacteria entry to the lamina propria and suggesting the existence of multiple entry pathways for pathogens and an overall minor role for DC-mediated sampling.10
Figure 1.
Isolated loops were carried out in small intestine of wild-type C57BL/6 and transgenic MyD88 and CX3CR1-KO mice of same genetic background. The small intestine was isolated by ligatures at the level of terminal duodenum and terminal ileum. Saline suspensions containing bacteria (4 × 108/ml) was injected in the loop and the intestine was returned to the abdominal cavity for 3 h. Luminal contents were then carefully recovered by gently flushing the intestine with PBS and the numbers of DCs assessed by flow cytometry. DC migration was completely abolished in CX3CR1-KO mice and was not different from levels observed in MyD88 where the numbers of DCs did not differ from PBS-treated mice.6
Table 1.
Transepithelial transport of non-invasive salmonella after oral challenge CFU/gr. of tissue
| Jejunum | Ileum | Colon | |
| Wild-type | 680 ± 230 | 895 ± 380 | 257 ± 98 |
| CX3CR1-KO | 590 ± 332 | 1012 ± 426 | 390 ± 57 |
CX3CR1-KO (C57BL/6 background) and wild-type mice and (n = 6/group) were orally administrated with 5 × 108–109 non-invasive ΔSPI1-Salmonella. Tissues were removed after 5 h, washed in antibiotics and subsequently homogenized in HBSS buffer. Serial dilutions of the homogenates were plated on LB agar plates overnight at 37°C and then colonies were counted.
Protection against Bacteria Infection in the Gut: DC-Sampling vs DC-Migration?
Is it possible that both DC-sampling and migration are required for protection against bacterial infections? Also, do these events complement each other by playing a role at different stages of the immune response to pathogens? In regard to DC sampling, its biological relevance and role in the generation of immune responses remains to be determined. To this end it is interesting to notice that following oral challenge a very small number of E. coli was translocated by the lamina propria (LP)-DCs while that vast majority (<100-fold difference) crossed the epithelial barrier via M cells of Peyer's patch.4 Interestingly, this result was obtained by comparing levels of CFU within the MLNs and PPs as parameters to evaluate DC and M cell transport, respectively. However, this way of assessing DC-mediated sampling has to been interpreted cautiously. Indeed, determining levels of CFU within the MLN does not take into consideration both the contribution of villous-M cells,11,12 and the fact that bacteria transported by PPs also end up in MLN.13,14 In regard to the latter observation, the finding that no bacteria were recovered in the MLN of CX3CR1-KO mice despite an intact M cell-mediated transport is particularly intriguing.4 This would mean that pathogens that invade the host via PP-associated M cells would not reach the MLN and in so doing they would escape, critical immunological check points associated to the intestinal immune system. However, this is not the case. Indeed, it has been demonstrated that also pathogens invading the host exclusively via the PPs are subsequently transported to the MLN;15–17 thus showing that both PP and LP drain to the MLN. This notion is also supported by a detailed anatomical investigation of the mouse intestine;18 this study confirmed that the entire small intestine, including PPs drained into the MLN and did not detect any alternative pathway for PP-derived lymph and its cellular contents.
Furthermore, another study reported that orally delivered fluorescence-labelled Enterobacter cloacae were recovered only within the PPs but not within the LP suggesting that, at least in this case DCs did not sample luminal bacteria.19 These results taken together showed that PP-M cells are the major site of antigen up-take, a critical step for the induction of mucosal and systemic immune responses and that DC-sampling, as a whole represents a small scale event, thus making it difficult to envisage its biological relevance. In addition, other relevant features of DC sampling are the subject of debate. For example, in one case high numbers of DC extensions were detected in the jejunum and a small number in the terminal ileum, where their numbers increased following challenge with non-invasive Salmonella;3 in contrast others have reported that transepithelial dendrites were observed only in the terminal ileum and not in the remaining areas of the small intestine.4 Also the underlying molecular mechanism appeared to vary according to the different areas of the gut with DC-sampling being TLR-dependent in the small intestine,3 but TLR-independent in the colon.20 Interestingly, the formation of DC extensions is also strain-specific.10 These were observed in C57BL/6 but were absent in BALB/c mice and this led to suggest that DC-mediated antigen sampling is not a universal phenomenon.13 To this end it would be interesting to dissect in detail immune responses and determine the rate of mortality in these two mouse strains after challenge with invasive Salmonella.
At present much less is known on the potential role of DCs that migrate rapidly into the intestinal lumen. Indeed, this event poses several key questions. Firstly, do sampling and migration involve different DC subpopulations? The possibility that different subpopulations of DCs may actively sample luminal antigens is strongly suggested by the observation that DC protrusions in CX3CR1-GFP and MHCII-GFP mice only partially overlapped.21 Also, it would be interesting to determine the expression of CD103 on DCs involved in both antigen-sampling and migration into the intestinal lumen.22,23 Secondly, and most compelling, what is the function, if any, of these cells into the gut lumen? The observation that CX3CR1-KO mice are not able to send DCs into the intestinal lumen upon Salmonella infection may enable us to formulate some hypothesis on their role. Indeed, as discussed above, it was reported that CX3CR1 deficient mice showed higher rate of mortality following Salmonella infection and albeit direct evidence was lacking this was attributed to their inability to form intraepithelial DC protrusions and sample luminal antigens.4 Although a quantitative analysis of Salmonella-induced recruitment of DCs in the LP of CX3CR1-KO mice is currently lacking, in light of our previous paper,6 and the present work on the role of CX3CR1 receptor in DC migration and bacterial transport to the LP we would like to propose that the increased susceptibility of these CX3CR1-deficient mice to Salmonella infection could also reflect the absence of “Salmonella-capturing” DCs into the lumen at the early stage of infection. Indeed, the lack of DC migration, along with other defects in monocyte (GR1+) recruitment may contribute to an overall diminished capability to cope with mucosal infection.24 As we have previously discussed,6 at present we can not rule out the possibility that DC migration is simply a physical consequence of the mechanical pressure of cells that migrate within the LP towards the intestinal lumen to perform antigen-sampling following certain bacterial stimuli. However, we feel it is unlikely that DC migration into the lumen would represent an accidental event as it would inevitably lead to the loss of important regulatory cells. Alternatively, these cells may be part of a defence mechanism of cell (phagocyte)-mediated immune exclusion that limits the number of pathogens crossing the epithelial barrier that would complement and potentiate the mucous and IgA antibody secretion-mediated system. A similar hypothesis was first proposed by Bellamy and Nielsen.25 The authors observed that a large number of phagocytes moved into the lumen following exposure to enteric antigen and suggested that antigen “escaping” the immune exclusion barrier provided by both specific IgA and mucous and penetrating the epithelial barrier triggered a rapid migration of cells with the task of removing and eliminating the offending pathogen. Furthermore, the observation that DC migration is restricted to the small bowel also would lend support to this hypothesis; indeed, in the past it was observed that small intestine juices upregulated phagocytosis;26 this feature along with the known opsonising properties of IgA would make the small intestine the ideal environment for the implementation of such a defence strategy.
Concluding Remarks
This work demonstrates that CX3CR1 plays a critical role in Salmonella-induced migration into the intestinal lumen but it does not significantly affect early transport of bacteria to the LP. This would suggest that intraluminal DC have an important role in mucosal clearance during the early phase of bacterial infection in the gut; the lack of this defence mechanism, as seen in CX3CR1-KO mice may determine higher susceptibility to Salmonella infection. Finally, it is worth to highlight that the co-existence of DC-sampling and migration in response to the same antigenic stimulus, in this case non-invasive Salmonella, illustrates the highly dynamic nature of both gut epithelium and immune system in response to bacterial infections and the complexity of the signalling network that governs host-pathogen interaction at mucosal interface. The challenge now is to identify the host molecular mechanisms and the bacterial signals triggering these two events and to determine their biological relevance in regard to immunity and mucosal clearance of intestinal pathogens.
Acknowledgements
The authors wish to thank L. Zuidmeer for helpful discussions and suggestions.
This work was funded by the Biotechnology and Biological Sciences Research Council, UK (to Claudio Nicoletti) and University of Siena, Italy (to Eugenio Bertelli).
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/11711
References
- 1.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nature Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 3.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:248–254. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 5.Niedergang F, Didierlaurent A, Kraehenbuhl JP, Sirard JC. Dendritic cells: the host Achille's heel for mucosal pathogens? Trends Microbiol. 2004;12:79–88. doi: 10.1016/j.tim.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Arques JL, Hautefort I, Ivory K, Bertelli E, Regoli M, Claire S, et al. Salmonella induces flagellin- and MyD88-dependent migration of bacteria-capturing dendritic cells into the gut lumen. Gastroenterology. 2009;137:579–587. doi: 10.1053/j.gastro.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 8.Lyons S, Wang L, Casanova JE, Sitaraman SV, Merlin D, Gewirtz AT. Salmonella typhimurium transcytoses flagellin via an SPI2-mediated vesicular transport pathway. J Cell Sci. 2004;117:5771–5780. doi: 10.1242/jcs.01500. [DOI] [PubMed] [Google Scholar]
- 9.Muehlhoefer A, Saubermann LJ, Gu X, Heckenkamp KL, Xavier R, Blumberg RS, et al. Fraktalkine is an epithelial and endothelial cell-derived chemoattractan for intraepithelial lymphocytes in the small intestinal mucosa. J Immunol. 2000;164:3368–3376. doi: 10.4049/jimmunol.164.6.3368. [DOI] [PubMed] [Google Scholar]
- 10.Vallon-Eberhard A, Landsman L, Yogev N, Verrier B, Jung S. Transepithelial pathogen uptake into the small intestinal lamina propria. J Immunol. 2006;176:2465–2469. doi: 10.4049/jimmunol.176.4.2465. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Terahara K, Sasakawa C, Suzuki T, Nochi T, Yokota Y, et al. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci USA. 2004;101:6110–6115. doi: 10.1073/pnas.0400969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knoop KA, Kumar N, Butler BR, Sakthivel SK, Taylor RT, Nochi T, et al. RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J Immunol. 2009;183:5738–5747. doi: 10.4049/jimmunol.0901563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 14.Johansson-Lindbom B, Agace WW. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol Rev. 2007;215:226–242. doi: 10.1111/j.1600-065X.2006.00482.x. [DOI] [PubMed] [Google Scholar]
- 15.McDonald TT, Carter PB. Cell-mediated immunity to intestinal infection. Infect Immun. 1980;28:516–532. doi: 10.1128/iai.28.2.516-523.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marco AJ, Prats N, Ramos JA, Briones V, Blanco M, Dominguez L, et al. A microbiological, histopathological and immunohistological study of the intragastric inoculation of Listeria monocytogenes in mice. J Comp Pathol. 1992;107:1–9. doi: 10.1016/0021-9975(92)90090-h. [DOI] [PubMed] [Google Scholar]
- 17.Marco AJ, Altimira J, Prats N, Lòpez S, Dominguez L, Domingo M, et al. Penetration of Listeria monocytogenes in mice infected by the oral route. Microb Pathog. 1997;23:255–263. doi: 10.1006/mpat.1997.0144. [DOI] [PubMed] [Google Scholar]
- 18.Carter PB, Collins FM. The route of enteric infection in normal mice. J Exp Med. 1974;134:1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 20.Hapfelmeier S, Müller AJ, Stecher B, Kaiser P, Barthel M, Endt K, et al. Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in DeltainvG S. typhimurium colitis. J Exp Med. 2008;205:437–450. doi: 10.1084/jem.20070633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rescigno M. Before they were gut dendritic cells. Immunity. 2009;31:454–456. doi: 10.1016/j.immuni.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung S, Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 24.Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, et al. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206:595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellamy JEC, Nielsen NO. Immune-mediated emigration of neutrophils into the lumen of the small intestine. Infect Immun. 1974;9:615–619. doi: 10.1128/iai.9.4.615-619.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girard JP, de Kalbermatten A. Antibody activity in human duodenal fluid. Eur J Clin Invest. 1970;1:188–195. doi: 10.1111/j.1365-2362.1970.tb00616.x. [DOI] [PubMed] [Google Scholar]