Abstract
While our current knowledge of probiotic interaction in the developing gut remains poorly understood, emerging science is providing greater biological insight into their mechanism of action and therapeutic potential for human disease. Given their beneficial effects, probiotics remain promising agents in neonatal gastrointestinal disorders. Probiotics may restore or supply essential bacterial strains needed for gut maturation and homeostasis, particularly in hosts where this process has been disrupted. Here we highlight the unique characteristics of developing intestinal epithelia with a focus on gut development and colonization as well as the inflammatory propensity of immature epithelia. Additionally, we review potential mechanisms of beneficial probiotic interaction with immature intestinal epithelia including immunomodulation, upregulation of cytoprotective genes, prevention and regulation of apoptosis and maintenance of barrier function. Improved knowledge of gut-probiotic interaction in developing epithelia will allow for a better understanding of how probiotics exert their beneficial effects and help guide their therapeutic use.
Key words: probiotics, commensal bacteria, ontogeny, intestinal epithelia, gut inflammation, necrotizing enterocolitis
Introduction
Defined as ‘live microorganisms, which when administered in adequate amounts confer a health benefit to the host,’1 probiotics are promising agents in the treatment and prevention of gastrointestinal diseases. Several studies have demonstrated therapeutic potential for probiotics in the prevention of neonatal necrotizing enterocolitis (NEC), a disease unique in its developmental susceptibility where the majority of those afflicted are born prematurely and suffer considerable morbidity and mortality.2–4 Since administering live probiotic bacteria to immunocompromised neonatal hosts with developmentally immature intestinal epithelia has the potential for harm, understanding the underlying mechanisms of probiotic action is of particular importance. While great interest and study has been focused on the mechanisms by which probiotics exert their beneficial effects, little is known about their effects in the immature gut. Here we aim to review our current understanding of the mechanisms by which commensal microbes and probiotics exert their beneficial effects on developing intestinal epithelia.
Gut Microbial Cross-Talk
Establishing an intestinal microbiome is a necessary event for intestinal development and defense. Recent advances in non-culture based methods of evaluating intestinal microbiota are improving our knowledge of the ontogeny of gut colonization. The sterile fetal gut undergoes rapid maturation after birth, and gut colonization is an essential effector of these changes. Understanding microbial-epithelial cross-talk in immature epithelia is fundamental in appreciating how the developing intestine achieves tolerance to bacteria and how dysregulation of this process may predispose the gut to inflammation and disease.
Acquisition of commensal bacteria.
Soon after birth, the sterile gut begins to acquire an enormously diverse complement of commensal flora. These commensal bacteria, over time, grow to outnumber their host by a factor of ten to one.5–7 The symbiotic relationship between host and microbe provides a hospitable, temperature-stable, nutrient-rich environment for bacteria while receiving, in return, a number of contributions including facilitation of digestion, nutrient absorption and storage,8,9 homeostasis and protection against injury,7,10 and development of epithelial immune responses and function.11,12
In adults, the density of bacteria is relatively low in the proximal small intestine (102–3 cells/g)and increases in the distal intestine (107–8 cells/g).13 The greatest number of bacteria is located in the colon (1011 cells/g) where there is a large diversity of species of anaerobes such as Bacteroides spp. and Bifidobacterium spp. and aerobes such as Escherichia coli, Lactobacillus spp. and Enterobacter spp.14,15 In contrast, the fetal and newborn gut is typically sterile and rapidly begins to acquire commensal flora soon after birth. The initial acquisition of commensal bacteria is dependent on mode of delivery, and in vaginally delivered infants, usually includes maternal vaginal and colonic flora such as Enterobacteriae, Enterococci and Staphylococci.16 As feeding is introduced, particularly human milk, and then with weaning from milk, the flora is further altered and expanded with complete adult colonization by two years of age.16 Emerging science aided by modern ribosomal RNA (rRNA) techniques that allow for better characterization of bacterial diversity have aided our understanding of the diversity of the intestinal microbiome in infants. Some studies demonstrate differences in bacterial populations related to mode of delivery where infants delivered by cesarean have decreased Lactobacillus and Bifidobacterium species.17 Neonatal diet, particularly human or bovine milk feeding and environmental factors are also likely to play an important role.18,19 However, there is a significant degree of inter-individual variation, and individual environmental encounters with each particular opportunistic organism have a large influence on commensal colonization patterns.20 A recent longitudinal study of commensal diversity in infants demonstrated no specific pattern of acquisition, suggesting that intestinal colonization may be as much a host-specific as an environmentally driven process.20
Bacterial colonization and necrotizing enterocolitis.
In contrast to term infants, preterm infants tend to be colonized with fewer organisms with a shift towards the Enterobacteriaceae family21 and have delayed colonization of commensal bacteria, particularly Bifidobacterium.22 A number of factors likely contribute to abnormal colonization in premature neonates. Antenatal and postnatal antibiotic therapy, both of which have been associated with an increased risk of NEC,23,24 decrease colonization of beneficial organisms such as Bifidobacterium, Lactobacillus and Bacteroides.25,26 Delayed initiation of enteral feeding and an abnormal hospital environment are also likely to play a role. Recent studies evaluating stool flora in neonates with and without NEC have yielded differing results, likely influenced by timing of sample acquisition. Wang et al. demonstrated decreased stool microbial diversity using 16s rRNA assays in neonates with NEC.27 These infants were already being treated for NEC. Therefore, the decreased microbial diversity that was seen could be explained by lack of enteral feeds and treatment with broad spectrum antibiotics. In contrast, Mshvildadze et al. obtained stool samples prospectively, and evaluated those collected approximately one week prior to the onset of NEC. These authors noted increased Citrobacter- and Enterococcus-like sequences in infants who went on to develop NEC, although they demonstrated no significant differences in microbiota profiles in infants with NEC compared to matched controls. In addition, factors noted to have an effect on stool microbial diversity included intention to breast feed, chorioamnionitis and prematurity (<30 weeks of gestation). Ongoing studies evaluating the microbiome in premature neonates will allow for a better understanding of microbial diversity and its influence on diseases affecting the developing human intestine.28–30 Since abnormal bacterial colonization likely plays a role in the pathogenesis of NEC, probiotic bacteria may exert their beneficial effects by restoring or supplying the essential commensal strains necessary for protection against inflammation and disease.
Probiotics in necrotizing enterocolitis.
Since the preterm gut demonstrates delayed commensal colonization and low bacterial diversity, it may be particularly amenable to therapeutic manipulation by probiotic administration. In keeping with this idea, several clinical studies have demonstrated the benefit of probiotic administration in reducing the incidence and severity of NEC.31,32 Most clinical trials of probiotic therapy in neonates have used sub-species of Lactobacillus or Bifidobacterium although dosing and duration of therapy vary significantly. Several animal studies support the rationale for clinical use of probiotics and have identified potential mechanisms of action.33 Caplan et al. demonstrated that supplementation of Bifidobacterium infantis led to rapid intestinal colonization and reduction in the incidence of NEC in a rat model.34 Additionally, the authors demonstrated that expression of plasma endotoxin and intestinal phospholipase A2 were lower in probiotic treated rats, suggesting that control of the inflammatory response seen in NEC may be an important target for probiotics. Dvorak et al. administered Bifidobacterium bifidum in their rat model of NEC and demonstrated similar benefit with decreases in the pro-inflammatory cytokine, IL-6.35 It is important to note that while current rodent NEC models may cause NEC-like histologic intestinal injury, they may not accurately recapitulate the pathophysiology of human preterm NEC. Nevertheless, both human and animal studies have provided encouraging results regarding the benefit of probiotic therapy in reducing the incidence and severity of NEC in the immature neonate. However, widespread use of probiotics is limited by the lack of knowledge regarding the optimal species of probiotics, dosing and duration of therapy and potential side effects.
Sepsis has been reported in probiotic therapy given to immunocompromised children36,37 and the potential for harm exists in similarly compromised immature neonatal hosts.38 In a multicenter trial administering the probiotics Bifidobacterium bifidum and Lactobacillus acidophilus for the prevention of NEC in very low birth weight infants, there was a trend towards increasing sepsis, particularly with gram-positive organisms, in the smallest neonates with birth weights of 500–750 grams.4 In addition, two recently published reports highlight this concern. Guenther et al. described severe sepsis in a premature neonatal host after administration of the probiotic Escherichia coli NISSLE for treatment of viral gastroenteritis39 and Bifidobacterium breve septicemia was reported by Ohisi et al. in a neonate with omphalocele.40 Understanding the mechanism of probiotic action may allow for therapies targeting the beneficial effector pathways while avoiding the potential side effects. These include the emerging therapeutic use of non-viable bacteria or purified bacterial components which may replicate the beneficial effects of live probiotic bacteria without the associated risk of sepsis.
Recognition of bacteria by pattern recognition receptors.
As the gut is colonized, bacteria located within the intestinal lumen undergo constant surveillance by Toll-like Receptors (TLRs), a key constituent of the innate immune system. These pattern recognition receptors (PRRs) interact with and identify microbial associated molecular patterns (MAMPs) on both commensal and pathogenic bacteria. The various MAMPs, including lipopolysaccharide, peptidoglycan and flagellin, are TLR-specific ligands (Table 1). TLR signaling, while once thought to exclusively yield pro-inflammatory activation by pathogenic bacteria, is now known to be differentially activated by commensal bacteria to induce pathways involved in gut homeostasis, cytoprotection, epithelial cell proliferation, regulation of tight junctions and antimicrobial peptide secretion.10,12,41
Table 1.
Toll-like receptors and their ligands
| Toll-like receptor | Ligand |
| TLR1 | Lipopeptides |
| TLR2 | Lipoprotein, lipoteichoic acid, others |
| TLR3 | Double-stranded RNA |
| TLR4 | Lipopolysaccharide (LPS) |
| TLR5 | Flagellin |
| TLR6 | Lipoprotein, lipoteichoic acid, others |
| TLR7 | Viral RNA |
| TLR8 | Viral RNA |
| TLR9 | Unmethylated CpG-containing DNA |
| TLR10 | Unknown |
| TLR11 | Profillin |
There are currently over ten known Toll-like receptors that bind microbial associated molecular patterns (MAMPs) from bacteria, fungi and viruses.
Rakoff-Nahoum et al. demonstrated that mice deficient of MyD88, a necessary downstream effector of most TLR signaling, were more prone to intestinal injury and mortality in a model where mice developed colitis following DSS administration.10 In the same study, mice with intact MyD88 who were depleted of commensal flora by broad spectrum antibiotics demonstrated similar intestinal injury and mortality, suggesting that commensal interaction through TLRs are vital in maintaining the intestinal barrier.
However, in immature epithelia, the TLR response to a newly colonizing gut may exhibit aberrant responses. Studies on fetal enterocyte lines (H4) and fetal small intestinal samples suggest that TLR-2 and TLR-4 are expressed in human epithelia as early as 18 to 21 weeks gestation.42 In the developing murine small intestine, Lotz et al. demonstrated that the LPS receptor complex, TLR-4/MD-2, was present in intestinal epithelial cells (IEC) isolated from fetal (one day prior to scheduled birth), pre-weaned neonatal (six days postnatal age) and post-weaned (28 days postnatal age) mice.43 Importantly, the response to LPS was developmentally regulated. While fetal IEC demonstrated a robust inflammatory response to LPS, more mature cells had attenuated inflammatory responses. The authors suggested that the postnatal loss of LPS responsiveness was associated with a posttranscriptional downregulation of the interleukin 1 receptor-associated kinase 1, an essential component of TLR-4 signaling. This suggests that tolerance to MAMPs such as LPS may require early exposure to bacterial ligands for desensitization to the ongoing acquisition and inhabitation of commensal flora.
Interestingly, these authors also looked at mode of delivery and found that immediate postnatal epithelial induction of MIP-2, an inflammatory chemokine, was detected in vaginally born mice but completely absent in neonatal mice delivered by cesarean. When these cesarean-delivered mice were given exogenous LPS, they developed MIP-2 induction similar to vaginally-delivered counterparts. This suggests that while colonization of the gut occurs over several weeks in the postnatal murine intestine,44 activation by microbial ligands acquired from vaginal flora in immature epithelia have the potential to occur very soon after birth and this activation may, as previously discussed, allow for desensitization and tolerance of later LPS exposure. Additionally, under-expressed negative regulators of the TLR pathway in fetal intestine may contribute to the exaggerated inflammatory signaling that has been described in the immature gut.45 In addition to having exaggerated responses to LPS induced TLR-4 activation, immature epithelia also demonstrate abnormal upregulation of TLR-4 expression during periods of stress and injury in a rodent NEC model.46 Increased TLR-4 expression may potentiate an already robust inflammatory signaling response to LPS in immature epithelia and help explain why neonatal C3H/HeJ mutant mice, which have dysfunctional TLR-4 receptors, are protected from the development of intestinal injury in a rodent NEC model.47 Recent studies suggest that probiotics may modulate these aberrant TLR-4 responses and protect against excessive inflammatory signaling in immature epithelia. LPS-mediated TLR-4 activation and IL-8 production is inhibited by Lactobacillus bulgaricus treatment in adult gastric cells48 and LGG decreases LPS-induced systemic inflammatory response in neonatal rats.49
While TLRs remain the key PRRs in intestinal epithelia, other PRRs such as cytoplasmic NOD1, NOD2 and FPR are likely to play a role in epithelial-microbial cross-talk in the developing intestine. NOD1 and NOD2 have similar structure and activity as TLRs and recognize LPS and peptidoglycan with downstream activation of the NF.B pathway. While NOD2 has been implicated in the pathogenesis of intestinal inflammatory disorders such as IBD,50 limited studies suggest it is not involved in NEC pathogenesis. Le Mandat Schultz et al. demonstrated that NOD2 expression, unlike TLR-2 and TLR-4 expression, was unaltered in a rat model of NEC.51 Additionally, genetic alterations in NOD2/CARD15, shown to confer susceptibility to inflammatory bowel disease (IBD), were not associated with predisposition to NEC in small clinical studies.52,53 Further studies are needed to better understand the role of non-TLR PRRs in the developing intestine.
Development of Intestinal Barrier Function
Eukaryotic organisms rely on an effective intestinal barrier to protect against pathogenic prokaryotes while appropriately housing beneficial commensal symbiotes. In the developing immature host, an ineffective barrier can predispose to aberrant inflammatory and/or apoptotic responses to bacteria.54,55 When considering administration of probiotic therapy to premature infants who are likely developmentally immunodeficient, immature intestinal barrier function is particularly relevant because it potentially allows these beneficial bacteria access to the sensitive submucosa where it may exert pathologic effects. As discussed previously, probiotic associated sepsis has been reported in premature neonates and remains a significant concern mitigating its widespread clinical use.56
To understand the unique susceptibility of the developing immature intestine to potentially harm from probiotic therapy, it is important to review the maturation of intestinal barrier function, both in utero and postnatally. The primitive structural barrier of the human intestinal epithelia is formed during the first three months of gestation.57 Enterocytes appear at eight weeks of gestation with intercellular tight junctions detected at ten weeks.58 Thus, within the first trimester of human gestation, the early structural barrier of the gut is formed. The development of the functional immune barrier follows, with production of antimicrobial defensins and protective mucin gel layers. Paneth cells appear by 12 weeks and produce antimicrobial defensins and lysozyme soon after59 although they are developmentally deficient in number in the premature 24-week gestation neonate.60 Mucins are expressed as early at 6.5 weeks gestation and undergo maturation throughout gestation.61 While development of barrier function occurs in utero, there is ongoing maturation postnatally. Extracellular signals such as growth factors, hormones, nutrients and microbes all aid in postnatal gut maturation. Given the benefit of a mature epithelial barrier in preventing injury and inflammation, encouraging growth of the appropriate complement of commensal bacteria may be of particular benefit in premature neonates who are deprived of the benefits of an in utero environment.62 Potential mechanisms whereby probiotics can promote maturation in developing epithelia will be discussed later in this review.
Gut Inflammation—A Key Target for Probiotic Action
Tolerance of commensal bacteria.
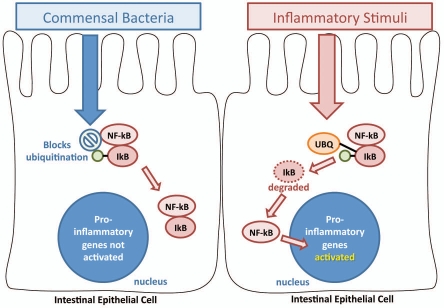
Despite a large number of Toll-like receptors on the intestinal surface which constantly sample luminal microbes, eukaryotes have a unique ability to tolerate commensal bacteria. Bacteria reduce their ability to induce inflammation in the host by preventing their recognition through masking or modifying MAMPs or by suppressing downstream pathways, particularly the pro-inflammatory NFκB pathway.63 A key mechanism by which intestinal epithelia tolerate commensal bacteria without inflammatory detriment was demonstrated by Neish et al.64 Using a non-pathogenic strain of Salmonella, the authors demonstrated that inflammatory activation of the NFκB pathway was prevented by blocking degradation of IκB, an inhibitor of the pathway (Fig. 1). Probiotics demonstrate similar activity in their ability to attenuate inflammatory responses by regulating NFκB activation through IκB.65,66
Figure 1.
Bacterial inhibition of NFκB activation through IκB ubiquitination. Commensal bacteria are able to inhibit innate pro-inflammatory signaling pathways by blockade of IκB ubiquitination (UBQ). Activation of NFκB occurs through stimulation of effector pathways, including the interaction of Pattern Recognition Receptors (PRRs) with bacterial products such as peptidoglycan and lipopolysaccharides. Commensal bacteria, as demonstrated by Neish et al.64 are able to block ubiquitination of IκB, an inhibitor that keeps NFκB sequestered in the cytoplasm. When IκB undergoes ubiquitination, NFκB is tranlocated into the nucleus where it activates the transcription of pro-inflammatory genes such as TNFα and IL-8.
Lessons from gnotobiotic animals.
Studies of germ-free (gnotobiotic) mice have highlighted important effects of commensal microbes, particularly in the development of the host immune system. Gnotobiotic mice demonstrate several immunological defects including fewer and smaller Peyer's patches and mesenteric lymph nodes, as well as defective development of gut associated lymphoid tissues.11,67,68 These mice have an abnormal composition of CD4+ T cells with an unconventional population of T cells as well as altered IgA producing B cells.69,70 And IgA-mediated immunity may be necessary for host tolerance to bacterial symbiotes.71 This suggests that some immune activation is necessary for normal immune development and function. Gnotobiotic mice also have abnormal intestinal architecture, demonstrated by altered patterns of microvilli formation and decreased rates of cell turnover.72 In addition, certain deficiencies in commensal bacteria-mediated regulation of intestinal development, such as fucosylation, are normalized once these germ-free mice are exposed to commensal strains.73 These findings support the critical importance of commensal colonization in gut development and immunity and provide a rationale for promoting the growth of helpful commensal bacteria either through judicious use of antibiotics or the administration of prebiotic or probiotic agents in conditions, such as prematurity, where this essential process is disrupted.
Inflammatory propensity in the developing gut.
Inflam-mation protects against harmful pathogens through active recruitment of effectors of immune defense. However, uncontrolled inflammation can damage tissues. The host may respond to this damage with additional inflammatory responses setting up a potential vicious cycle resulting in an acute severe sepsis syndrome or chronic inflammation. This is of particular relevance in the immature gut, which may be predisposed to exaggerated inflammatory responses.74 Implications of this exaggerated inflammation are seen in several intestinal inflammatory syndromes in newborns and children.75–77 These include NEC2,3 and IBD.78
Several studies have suggested that immature intestinal epithelia are prone to exuberant inflammatory responses. Nathankumar et al. reported increased inflammatory signaling in immature intestinal epithelia in in vitro and ex vivo studies.77 In their in vitro studies, the authors compared secretion of the pro-inflammatory cytokine, IL-8, in response to both LPS and IL-1β in human fetal intestinal cells (H4) and mature human enterocytes (Caco-2) and found that H4 cells had 8-fold and 20-fold increases in IL-8 secretion to LPS and IL-1β, respectively. The authors then validated these findings ex vivo by comparing IL-8 secretion in response to LPS and IL-1β in small intestine organ cultures from fetuses and older children. IL-8 secretion was increased 2.5 fold in response to LPS and 200-fold in response to IL-1β. Claud et al. comparing T84 and H4 cell lines and neonatal (ten days) and post-weaned (five weeks) rat enterocytes, found similar propensity for increased inflammatory signaling.75 IL-8 secretion in response to pathogenic Salmonella SL3201 and commensal E. coli were significantly higher in H4 cells. IL-6 secretion was also significantly higher in neonatal rat enterocytes. Developmental changes in expression of IκB, an inhibitor of the NFκB pathway, may be one explanatory mechanism for these findings as IκB expression was increased in mature epithelia. The authors suggested that low levels of IκB expression in immature epithelia may be partially responsible for the heightened inflammatory responses of immature intestinal epithelia as transfection of immature H4 cells with an IκB expression construct attenuated IL-8 secretion.
Lin et al. further support the finding that immature intestinal epithelial inflammatory responses appear to be a developmentally regulated phenomenon.79 In mice, TNFα secretion appears to peak at 2 weeks postnatal age in ex vivo small intestinal organ culture treated with Salmonella typhimurium. This developmental increase in pathogen-induced inflammatory signaling at two weeks of postnatal age in the murine intestine has particular relevance in neonatal NEC as exaggerated inflammation is thought to play a key role in the pathogenesis of NEC74 and there appears to be a developmental window of susceptibility to NEC.80,81 Since commensal bacteria are known to regulate inflammatory signaling,13,63,64,82,83 it is not surprising that recent studies have demonstrated a link between prolonged empiric antibiotic therapy in neonates and an increased incidence of NEC and death.23
Mechanisms of probiotic action in developing epithelia.
Much of our understanding of the mechanisms of specific probiotic action is derived from studies involving mature intestinal epithelial cell lines or adult rodent models. While our knowledge of the effect of probiotics in the immature gut remains limited, probiotics have been shown to protect the developing epithelia through control of inflammation; upregulation of cytoprotective genes; prevention and regulation of apoptosis; and maintenance of barrier function (Fig. 2). Lactobacillus, Bifidobacterium and Saccharomyces are the most commonly used probiotic strains although numerous other strains have been studied; and it is important to note that their effects are likely dependent on factors involving both the host and specific probiotic strain.
Figure 2.
Mechanisms of probiotic action in developing epithelia. Probiotics have several potential mechanisms of action. These include: maintenance of barrier function and attenuation of changes in intestinal permeability through effects on tight junctions, regulation and prevention of apoptosis, induction of cytoprotective genes and immunomodulatory effects that decrease abnormal inflammatory signaling including signaling through generation of reactive oxygen species (ROS).
Control of inflammation.
Since uncontrolled inflammatory responses play a key role in the pathogenesis of disorders of the immature gut such as NEC, probiotics may prove invaluable agents in specific clinical settings. Probiotics may control inflammation by restoring balance to an intestine exerting intolerant responses to a “dysbiotic” microbiome. Additionally, probiotics can restore commensal microbes in situations where they are abnormally depleted such as through the use of broad spectrum antibiotics. While several studies have documented anti-inflammatory effects in adult cell lines66,84,85 and rodent colitis models,86,88 there are few studies of similar probiotic effects in models of immature gut. Li et al. using a 7-day-old infant rat model, demonstrated that enteral supplementation of live and heat-killed Lactobacillus rhamnosus GG (LGG) reduces LPS-induced inflammation by reducing pro-inflammatory mediators and increasing anti-inflammatory mediators.89 In premature neonates, Bifidobacterium breve supplementation may exert anti-inflammatory effects by enhancing TGFβ1 signaling.90 As discussed previously, Dvorak et al. demonstrated that Bifidobacterium bifidum was protective in their rat model of NEC with associated decreases in expression of the pro-inflammatory cytokine, IL-6.35
While probiotics are commonly thought to reduce inflammation in the intestine, low-level stimulation of inflammatory signaling by microbes through TLRs and their downstream pathways, including NFκB, may also be cytoprotective. In contrast, high-level NFκB stimulation may lead to too much inflammation which can cause secondary tissue damage. This is highlighted by a recent study demonstrating that probiotics exert their beneficial influence through stimulation of innate immunity. The authors reported that VSL#3, a probiotic mixture of eight bacterial strains, administered to SAMP mice resulted in activation of the NFκB pathway with increases in TNFα that were associated with decreases in intestinal permeability and ileitis.91 NFκB activation is also known to be cytoprotective by inducing increased expression of anti-apoptotic proteins.92 Thus, some probiotics may exert their beneficial effects through activation of NFκB-induced cytoprotection without overt inflammation. Further studies examining probiotic influence on inflammation, particularly in the developing gut, are necessary to better understand their effects in both physiologic and pathologic states.
Upregulation of cytoprotective genes.
Hooper et al. demonstrated that commensal bacteria modulate expression of genes involved in several essential intestinal functions, including maintenance of barrier function, nutrient absorption, angiogenesis and postnatal intestinal maturation.7 Although research into the mechanisms by which commensal bacteria exhibit these influences are ongoing, these findings suggest that their effects are widespread in many key facets of intestinal homeostasis.
Nasr et al. demonstrated similar effects in the developing murine intestine.93 They administered LGG to 2-week-old immature mice and demonstrated upregulation of genes known to control proliferation and migration as well as mitogen-activated protein kinase (MAPK) pathways important in growth, differentiation and cytoprotection. These effects were demonstrated without the generation of inflammatory responses which were in contrast to pathogenic bacteria such as Salmonella typhimurium, where these genes are upregulated through NFκB mediated pro-inflammatory activation.92
Prevention of apoptosis.
Aberrant apoptosis had been implicated in developmental gut disorders such as NEC. Apoptosis is a necessary mechanism for cells to undergo programmed death from injury and prevent damage from release of cellular contents as seen in necrosis.92 Normal apoptotic processes during periods of health maintain a balance between proliferation and death, while aberrant and uncontrolled apoptosis may worsen tissue injury.94 Attenuation of excessive apoptosis is one mechanism by which probiotics promote intestinal health.
In immature epithelia, Nasr et al. demonstrated that the probiotic LGG reduces chemically induced intestinal epithelial apoptosis both in vitro and ex vivo.93 This may be accomplished by upregulation of known anti-apoptotic genes, including Ku70, Sox4, PHIP, API5 and Jak2,95–99 and candidate genes such as Dusp3 which regulate MAP-kinase pathways.100 This supports findings by Yan et al. in mature murine and human colonic epithelia. They demonstrated that LGG prevented cytokine-induced apoptosis by activating anti-apoptotic genes (Akt/protein kinase B) and inhibiting activation of pro-apoptotic genes (p38/MAP-kinase).101 The same group later demonstrated that soluble proteins, p75 and p40, from LGG mediate the cytoprotective effects, highlighting the potential benefit of bacterial products rather than bacteria itself.102
Generation of ROS.
Emerging evidence indicates that physiologic ROS signaling regulates many necessary homeostatic processes.103 While oxidative stress has been implicated in several developmental diseases including retinopathy of prematurity and chronic lung disease, physiological levels of ROS have been shown to regulate homeostatic cellular processes by transient oxidative inactivation of catalytic cysteine residues on key regulatory enzymes. Through this activity, ROS has the ability to regulate apoptotic, proliferative and inflammatory signaling.104 Specifically in the gut, commensal and probiotic bacteria have been shown to regulate these pathways by inducing local epithelial ROS generation.82
In intestinal epithelia, ROS reduces inflammatory signaling through oxidative inactivation of Ubc12, a key enzyme regulating NFκB activation. Ubc12 is responsible for activation of the specific ubiquitin ligase complex SCF-βTRCP through neddylation of its cullin-1 (Cul1) subunit.82 When Cul1 remains de-neddylated, SCF-βTRCP fails to ubiquitinate the inhibitor of NFκB (IκBα), a modification that normally targets IκBα for proteasomal degradation.64 NFκB thus remains trapped in the cytosol by IκBα, unable to translocate to the nucleus to activate transcription of inflammatory mediators. In human intestinal cells and human colonic mucosa, Kumar et al. demonstrated that probiotic generation of ROS are modulated by the bacterial fermentation product butyrate.105 Given normal commensal bacteria produce butyrate and other short chain fatty acids, ROS generation by these products may be an important pathway in which commensal and probiotic bacteria regulate inflammation in the immature intestine. While several commensal species including Bacteroides thetaiotaomicron and Escherichia coli demonstrate the ability to induce intestinal epithelial ROS generation, the effect was greatest with LGG.82 This suggests that probiotic therapy with LGG may be unique in its potent induction of epithelial ROS generation and, potentially, increased anti-inflammatory potential.
ROS signaling regulates developmental processes in the fetus and premature neonate via tightly regulated changes in cellular localization and concentration.106 Lin et al. demonstrated that the probiotic LGG can block activation of the classic pro-inflammatory transcription factor NFκB in the distal small intestines of immature mice by inducing epithelial ROS generation and preventing Cul1 neddylation required for activation of the ubiquitin ligase complex.79 In their study, human fetal intestinal cells and small intestine from neonatal mice treated with LGG demonstrated epithelial generation of ROS. Differential responsiveness to LGG in distal versus proximal immature intestinal epithelia may be due to underlying differences in local concentrations of commensal bacteria or inherent differences in epithelial responsiveness to bacteria.107 How ROS signaling may influence intestinal epithelial proliferation and differentiation critical to maturation of the premature intestine remains to be studied.
Maturation of barrier function and regulation of intestinal permeability.
Increased intestinal permeability predisposes the gut to invasion of toxins and bacteria located within the gut lumen resulting in both inflammation and injury.108 An important characteristic of the immature gut in humans and other mammals is a leaky epithelial barrier. Over time, the intestinal barrier tightens and becomes more selective in paracellular permeability to both large and small molecules.109–111 Commensal colonization may be an important driving influence by which the developing intestine reduces intestinal permeability and probiotic bacteria may replicate these effects.
In T84 monolayers, the probiotic compound VSL#3 results in enhanced epithelial barrier function as measured by transepithelial resistance.80 In animal models, probiotics have been demonstrated to restore compromised barrier function following infectious insult. Lactobacillus rhamnosus protects against intestinal permeability alterations induced by Rotaviral infections in immature sucking rats. Additionally, treatment with Lactobacillus in a sepsis model with rats undergoing cecal ligation and perforation results in decreased bacterial translocation and maintenance of epithelial tight junction expression.112 Probiotics also promote intestinal integrity in injury models of immature intestine. Khalilova et al., in a rat model of NEC, demonstrated that Bifidobacterium bifidum ameliorated disruption of tight junction expression and localization.35 Similar to the effect of VSL#3 described above, Bifidobacterium bifidum attenuated increases in gene expression of the pro-inflammatory cytokine IL-6 that were seen after intestinal injury.113,114 In mature rats, similar benefits in barrier function have been demonstrated. Adult rats administered Lactobacillus rhamnosus demonstrated decreased intestinal permeability following hemorrhagic shock.115
In a study of preterm infants (27 to 36 weeks gestation) administered Bifidobacterium lactis supplemented formula, intestinal permeability measured by sugar absorption tests decreased significantly at both 1 week and 1 month.116 Although this study was small, there was also a trend towards decreasing sepsis and NEC in infants given formula supplemented with Bifidobacterium lactis which corroborates other studies demonstrating the benefit of probiotics in premature infants.31,117 While probiotics clearly attenuate inflammatory responses in the intestine and reduction in inflammation may be an important component of reducing barrier dysfunction, other yet uncovered non-immune pathways are likely to play a role. Future studies aimed at understanding how the developing gut transitions from a leaky to more selective epithelial barrier, with a focus on the role of tight junctions, may yield important insights into therapies aimed at regulating and reducing intestinal permeability.
Summary
While the developmental biology of gut-probiotic interaction remains poorly understood, emerging studies are elucidating important mechanisms relevant to beneficial epithelial-microbial symbiosis. Given the unique characteristics of immature epithelia, including compromised barrier function; propensity for inflammation; and need to transition from a sterile to microbial environment, the extrapolation of gut-probiotic interaction in mature epithelia to the developing, immature intestine should be made with caution. A better understanding of gut-probiotic interaction may potentially allow for development of novel therapies for intestinal diseases such as NEC. Given the myriad of probiotic species, further studies are needed to distinguish their unique benefits and guide their therapeutic use in both human health and disease.
Acknowledgements
This review was supported by NIH R01 HD059122 (Patricia W. Lin).
Abbreviations
- cul1
cullin-1
- IBD
inflammatory bowel disease
- LGG
Lactobacillus rhamnosus GG
- NEC
necrotizing enterocolitis
- PRR
pattern-recognition receptor
- ROS
reactive oxygen species
- TLR
toll-like receptor
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/12484
References
- 1.FAO/WHO, author. Food and Argricultural Organization / World Health Organization Report—Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria. Cordoba, Argentina: 2001. [Google Scholar]
- 2.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–1283. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 3.Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol. 2008;32:70–82. doi: 10.1053/j.semperi.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics. 2008;122:693–700. doi: 10.1542/peds.2007-3007. [DOI] [PubMed] [Google Scholar]
- 5.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 6.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 7.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 8.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 10.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 13.Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Claud EC, Walker AW. The Intestinal Microbiota and the Microbiome. New York: Saunders; 2008. [Google Scholar]
- 17.Biasucci G, Benenati B, Morelli L, Bessi E, Boehm G. Cesarean delivery may affect the early biodiversity of intestinal bacteria. J Nutr. 2008;138:1796–1800. doi: 10.1093/jn/138.9.1796S. [DOI] [PubMed] [Google Scholar]
- 18.Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30:61–67. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91:48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 20.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosloske AM. Epidemiology of necrotizing enterocolitis. Acta Paediatr Suppl. 1994;396:2–7. doi: 10.1111/j.1651-2227.1994.tb13232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gewolb IH, Schwalbe RS, Taciak VL, Harrison TS, Panigrahi P. Stool microflora in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 1999;80:167–173. doi: 10.1136/fn.80.3.f167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123:58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenyon S, Boulvain M, Neilson J. Antibiotics for preterm premature rupture of membranes. Cochrane Database Syst Rev. 2001:001058. doi: 10.1002/14651858.CD001058. [DOI] [PubMed] [Google Scholar]
- 25.Sudo N, Yu XN, Aiba Y, Oyama N, Sonoda J, Koga Y, et al. An oral introduction of intestinal bacteria prevents the development of a long-term Th2-skewed immunological memory induced by neonatal antibiotic treatment in mice. Clin Exp Allergy. 2002;32:1112–1116. doi: 10.1046/j.1365-2222.2002.01430.x. [DOI] [PubMed] [Google Scholar]
- 26.Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 2004;12:562–568. doi: 10.1016/j.tim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3:944–954. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mshvildadze M, Neu J, Shuster J, Theriaque D, Li N, Mai V. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J Pediatr. 2010;156:20–25. doi: 10.1016/j.jpeds.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mshvildadze M, Neu J. The infant intestinal microbiome: Friend or foe? Early Hum Dev. 12010 doi: 10.1016/j.earlhumdev.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morowitz MJ, Poroyko V, Caplan M, Alverdy J, Liu DC. Redefining the role of intestinal microbes in the pathogenesis of necrotizing enterocolitis. Pediatrics. 2010;125:777–785. doi: 10.1542/peds.2009-3149. [DOI] [PubMed] [Google Scholar]
- 31.Alfaleh K, Bassler D. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2008:005496. doi: 10.1002/14651858.CD005496.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Deshpande G, Rao S, Patole S. Probiotics for prevention of necrotising enterocolitis in preterm neonates with very low birthweight: a systematic review of randomised controlled trials. Lancet. 2007;369:1614–1620. doi: 10.1016/S0140-6736(07)60748-X. [DOI] [PubMed] [Google Scholar]
- 33.Sodhi C, Richardson W, Gribar S, Hackam DJ. The development of animal models for the study of necrotizing enterocolitis. Dis Model Mech. 2008;1:94–98. doi: 10.1242/dmm.000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caplan MS, Miller-Catchpole R, Kaup S, Russell T, Lickerman M, Amer M, et al. Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology. 1999;117:577–583. doi: 10.1016/s0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 35.Khailova L, Dvorak K, Arganbright KM, Halpern MD, Kinouchi T, Yajima M, et al. Bifidobacterium bifidum Improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2009 doi: 10.1152/ajpgi.00141.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunz AN, Fairchok MP, Noel JM. Lactobacillus sepsis associated with probiotic therapy. Pediatrics. 2005;116:517–518. doi: 10.1542/peds.2005-0475. [DOI] [PubMed] [Google Scholar]
- 37.Land MH, Rouster-Stevens K, Woods CR, Cannon ML, Cnota J, Shetty AK. Lactobacillus sepsis associated with probiotic therapy. Pediatrics. 2005;115:178–181. doi: 10.1542/peds.2004-2137. [DOI] [PubMed] [Google Scholar]
- 38.Bell EF. Preventing necrotizing enterocolitis: what works and how safe? Pediatrics. 2005;115:173–174. doi: 10.1542/peds.2004-2360. [DOI] [PubMed] [Google Scholar]
- 39.Guenther K, Straube E, Pfister W, Guenther A, Huebler A. Sever sepsis after probiotic treatment with Escherichia coli NISSLE 1917. Pediatr Infect Dis J. 2010;29:188–189. doi: 10.1097/INF.0b013e3181c36eb9. [DOI] [PubMed] [Google Scholar]
- 40.Ohishi A, Takahashi S, Ito Y, Ohishi Y, Tsukamoto K, Nanba Y, et al. Bifidobacterium septicemia associated with postoperative probiotic therapy in a neonate with omphalocele. J Pediatr. 2010;156:679–681. doi: 10.1016/j.jpeds.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 41.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 42.Fusunyan RD, Nanthakumar NN, Baldeon ME, Walker WA. Evidence for an innate immune response in the immature human intestine: toll-like receptors on fetal enterocytes. Pediatr Res. 2001;49:589–593. doi: 10.1203/00006450-200104000-00023. [DOI] [PubMed] [Google Scholar]
- 43.Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203:973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaedler RW, Dubos R, Costello R. The Development of the Bacterial Flora in the Gastrointestinal Tract of Mice. J Exp Med. 1965;122:59–66. doi: 10.1084/jem.122.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanderson IR, Walker WA. TLRs in the Gut I. The role of TLRs/Nods in intestinal development and homeostasis. Am J Physiol Gastrointest Liver Physiol. 2007;292:6–10. doi: 10.1152/ajpgi.00275.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caplan MS, Simon D, Jilling T. The role of PAF TLR and the inflammatory response in neonatal necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:145–151. doi: 10.1053/j.sempedsurg.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. 2006;177:3273–3282. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou C, Ma FZ, Deng XJ, Yuan H, Ma HS. Lactobacilli inhibit interleukin-8 production induced by Helicobacter pylori lipopolysaccharide-activated Toll-like receptor 4. World J Gastroenterol. 2008;14:5090–5095. doi: 10.3748/wjg.14.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L, Li N, des Robert C, Fang M, Liboni K, McMahon R, et al. Lactobacillus rhamnosus GG decreases lipopolysaccharide-induced systemic inflammation in a gastrostomy-fed infant rat model. J Pediatr Gastroenterol Nutr. 2006;42:545–552. doi: 10.1097/01.mpg.0000221905.68781.4a. [DOI] [PubMed] [Google Scholar]
- 50.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le Mandat Schultz A, Bonnard A, Barreau F, Aigrain Y, Pierre-Louis C, Berrebi D, et al. Expression of TLR-2, TLR-4, NOD2 and pNFkB in a neonatal rat model of necrotizing enterocolitis. PLoS One. 2007;2:1102. doi: 10.1371/journal.pone.0001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szebeni B, Szekeres R, Rusai K, Vannay A, Veres G, Treszl A, et al. Genetic polymorphisms of CD14, toll-like receptor 4 and caspase-recruitment domain 15 are not associated with necrotizing enterocolitis in very low birth weight infants. J Pediatr Gastroenterol Nutr. 2006;42:27–31. doi: 10.1097/01.mpg.0000192246.47959.b2. [DOI] [PubMed] [Google Scholar]
- 53.Zouali H, Bonnard A, De Lagausie DL, Farnoux C, Aigrain Y, Cezard JP, et al. CARD15/NOD2 is not a predisposing factor for necrotizing enterocolitis. Dig Dis Sci. 2005;50:1684–1687. doi: 10.1007/s10620-005-2915-z. [DOI] [PubMed] [Google Scholar]
- 54.Louis NA, Lin PW. The Intestinal Immune Barrier. NeoReviews. 2009;10:180–190. [Google Scholar]
- 55.Lin PW, Neish AS. Innate immunity and epithelial biology; special considerations in the neonatal gut. New York: Saunders; 2008. [Google Scholar]
- 56.Caplan MS. Probiotic and prebiotic supplementation for the prevention of neonatal necrotizing enterocolitis. J Perinatol. 2009;29:2–6. doi: 10.1038/jp.2009.21. [DOI] [PubMed] [Google Scholar]
- 57.Rumbo M, Schiffrin EJ. Ontogeny of intestinal epithelium immune functions: developmental and environmental regulation. Cell Mol Life Sci. 2005;62:1288–1296. doi: 10.1007/s00018-005-5033-3. [DOI] [PubMed] [Google Scholar]
- 58.Polak-Charcon S, Shoham J, Ben-Shaul Y. Tight junctions in epithelial cells of human fetal hindgut, normal colon and colon adenocarcinoma. J Natl Cancer Inst. 1980;65:53–62. [PubMed] [Google Scholar]
- 59.Mallow EB, Harris A, Salzman N, Russell JP, DeBerardinis RJ, Ruchelli E, et al. Human enteric defensins. Gene structure and developmental expression. J Biol Chem. 1996;271:4038–4045. doi: 10.1074/jbc.271.8.4038. [DOI] [PubMed] [Google Scholar]
- 60.Salzman NH, Polin RA, Harris MC, Ruchelli E, Hebra A, Zirin-Butler S, et al. Enteric defensin expression in necrotizing enterocolitis. Pediatr Res. 1998;44:20–26. doi: 10.1203/00006450-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Buisine MP, Devisme L, Savidge TC, Gespach C, Gosselin B, Porchet N, et al. Mucin gene expression in human embryonic and fetal intestine. Gut. 1998;43:519–524. doi: 10.1136/gut.43.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lebenthal A, Lebenthal E. The ontogeny of the small intestinal epithelium. JPEN J Parenter Enteral Nutr. 1999;23:3–6. doi: 10.1177/014860719902300502. [DOI] [PubMed] [Google Scholar]
- 63.Neish AS. Molecular aspects of intestinal epithelial cell-bacterial interactions that determine the development of intestinal inflammation. Inflamm Bowel Dis. 2004;10:159–168. doi: 10.1097/00054725-200403000-00015. [DOI] [PubMed] [Google Scholar]
- 64.Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V, et al. Prokaryotic regulation of epithelial responses by inhibition of IκB-α ubiquitination. Science. 2000;289:1560–1563. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 65.Iyer C, Kosters A, Sethi G, Kunnumakkara AB, Aggarwal BB, Versalovic J. Probiotic Lactobacillus reuteri promotes TNF-induced apoptosis in human myeloid leukemia-derived cells by modulation of NFκB and MAPK signalling. Cell Microbiol. 2008;10:1442–1452. doi: 10.1111/j.1462-5822.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- 66.Tien MT, Girardin SE, Regnault B, Le Bourhis L, Dillies MA, Coppee JY, et al. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J Immunol. 2006;176:1228–1237. doi: 10.4049/jimmunol.176.2.1228. [DOI] [PubMed] [Google Scholar]
- 67.Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev. 1998;62:1157–1170. doi: 10.1128/mmbr.62.4.1157-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 69.Helgeland L, Vaage JT, Rolstad B, Midtvedt T, Brandtzaeg P. Microbial colonization influences composition and T-cell receptor V β repertoire of intraepithelial lymphocytes in rat intestine. Immunology. 1996;89:494–501. doi: 10.1046/j.1365-2567.1996.d01-783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Umesaki Y, Setoyama H, Matsumoto S, Okada Y. Expansion of αβT-cell receptor-bearing intestinal intraepithelial lymphocytes after microbial colonization in germ-free mice and its independence from thymus. Immunology. 1993;79:32–37. [PMC free article] [PubMed] [Google Scholar]
- 71.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 72.Abrams GD, Bauer H, Sprinz H. Influence of the normal flora on mucosal morphology and cellular renewal in the ileum. A comparison of germ-free and conventional mice. Lab Invest. 1963;12:355–364. [PubMed] [Google Scholar]
- 73.Bry L, Falk PG, Midtvedt T, Gordon JI. A model of host-microbial interactions in an open mammalian ecosystem. Science. 1996;273:1380–1383. doi: 10.1126/science.273.5280.1380. [DOI] [PubMed] [Google Scholar]
- 74.Grave GD, Nelson SA, Walker WA, Moss RL, Dvorak B, Hamilton FA, et al. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res. 2007;62:510–514. doi: 10.1203/PDR.0b013e318142580a. [DOI] [PubMed] [Google Scholar]
- 75.Claud EC, Lu L, Anton PM, Savidge T, Walker WA, Cherayil BJ. Developmentally regulated IκB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. Proc Natl Acad Sci USA. 2004;101:7404–7408. doi: 10.1073/pnas.0401710101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liboni KC, Li N, Scumpia PO, Neu J. Glutamine modulates LPS-induced IL-8 production through IκB/NFκB in human fetal and adult intestinal epithelium. J Nutr. 2005;135:245–251. doi: 10.1093/jn/135.2.245. [DOI] [PubMed] [Google Scholar]
- 77.Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci USA. 2000;97:6043–6048. doi: 10.1073/pnas.97.11.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 79.Lin PW, Myers LE, Ray L, Song SC, Nasr TR, Berardinelli AJ, et al. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Radic Biol Med. 2009;47:1205–1211. doi: 10.1016/j.freeradbiomed.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 81.Neu J. The ‘myth’ of asphyxia and hypoxia-ischemia as primary causes of necrotizing enterocolitis. Biol Neonate. 2005;87:97–98. doi: 10.1159/000081898. [DOI] [PubMed] [Google Scholar]
- 82.Kumar A, Wu H, Collier-Hyams LS, Hansen JM, Li T, Yamoah K, et al. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J. 2007;26:4457–4466. doi: 10.1038/sj.emboj.7601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Collier-Hyams LS, Sloane V, Batten BC, Neish AS. Cutting edge: bacterial modulation of epithelial signaling via changes in neddylation of cullin-1. J Immunol. 2005;175:4194–4198. doi: 10.4049/jimmunol.175.7.4194. [DOI] [PubMed] [Google Scholar]
- 84.Zhang L, Li N, Caicedo R, Neu J. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-a-induced interleukin-8 production in Caco-2 cells. J Nutr. 2005;135:1752–1756. doi: 10.1093/jn/135.7.1752. [DOI] [PubMed] [Google Scholar]
- 85.Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-g and RelA. Nat Immunol. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 86.Grabig A, Paclik D, Guzy C, Dankof A, Baumgart DC, Erckenbrecht J, et al. Escherichia coli strain Nissle 1917 ameliorates experimental colitis via toll-like receptor 2- and toll-like receptor 4-dependent pathways. Infect Immun. 2006;74:4075–4082. doi: 10.1128/IAI.01449-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O'Mahony C, Scully P, O'Mahony D, Murphy S, O'Brien F, Lyons A, et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NFκB activation. PLoS Pathog. 2008;4:1000112. doi: 10.1371/journal.ppat.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peran L, Camuesco D, Comalada M, Bailon E, Henriksson A, Xaus J, et al. A comparative study of the preventative effects exerted by three probiotics, Bifidobacterium lactis, Lactobacillus casei and Lactobacillus acidophilus, in the TNBS model of rat colitis. J Appl Microbiol. 2007;103:836–44. doi: 10.1111/j.1365-2672.2007.03302.x. [DOI] [PubMed] [Google Scholar]
- 89.Li N, Russell WM, Douglas-escobar M, Hauser N, Lopez M, Neu J. Live and heat-killed Lactobacillus rhamnosus GG: effects on proinflammatory and anti-inflammatory cytokines/chemokines in gastrostomy-fed infant rats. Pediatr Res. 2009;66:203–207. doi: 10.1203/PDR.0b013e3181aabd4f. [DOI] [PubMed] [Google Scholar]
- 90.Fujii T, Ohtsuka Y, Lee T, Kudo T, Shoji H, Sato H, et al. Bifidobacterium breve enhances transforming growth factor β1 signaling by regulating Smad7 expression in preterm infants. J Pediatr Gastroenterol Nutr. 2006;43:83–88. doi: 10.1097/01.mpg.0000228100.04702.f8. [DOI] [PubMed] [Google Scholar]
- 91.Pagnini C, Saeed R, Bamias G, Arseneau KO, Pizarro TT, Cominelli F. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci USA. 2010;107:454–459. doi: 10.1073/pnas.0910307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zeng H, Wu H, Sloane V, Jones R, Yu Y, Lin P, et al. Flagellin/TLR5 responses in epithelia reveal intertwined activation of inflammatory and apoptotic pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:96–108. doi: 10.1152/ajpgi.00273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin PW, Nasr TR, Berardinelli AJ, Kumar A, Neish AS. The probiotic Lactobacillus GG may augment intestinal host defense by regulating apoptosis and promoting cytoprotective responses in the developing murine gut. Pediatr Res. 2008;64:511–516. doi: 10.1203/PDR.0b013e3181827c0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ramachandran A, Madesh M, Balasubramanian KA. Apoptosis in the intestinal epithelium: its relevance in normal and pathophysiological conditions. J Gastroenterol Hepatol. 2000;15:109–120. doi: 10.1046/j.1440-1746.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- 95.Mazumder S, Plesca D, Kinter M, Almasan A. Interaction of a cyclin E fragment with Ku70 regulates Bax-mediated apoptosis. Mol Cell Biol. 2007;27:3511–3520. doi: 10.1128/MCB.01448-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Podcheko A, Northcott P, Bikopoulos G, Lee A, Bommareddi SR, Kushner JA, et al. Identification of a WD40 repeat-containing isoform of PHIP as a novel regulator of β-cell growth and survival. Mol Cell Biol. 2007;27:6484–6496. doi: 10.1128/MCB.02409-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pramoonjago P, Baras AS, Moskaluk CA. Knockdown of Sox4 expression by RNAi induces apoptosis in ACC3 cells. Oncogene. 2006;25:5626–5639. doi: 10.1038/sj.onc.1209566. [DOI] [PubMed] [Google Scholar]
- 98.Ruchatz H, Coluccia AM, Stano P, Marchesi E, Gambacorti-Passerini C. Constitutive activation of Jak2 contributes to proliferation and resistance to apoptosis in NPM/ALK-transformed cells. Exp Hematol. 2003;31:309–315. doi: 10.1016/s0301-472x(03)00007-9. [DOI] [PubMed] [Google Scholar]
- 99.Tewari M, Yu M, Ross B, Dean C, Giordano A, Rubin R. AAC-11, a novel cDNA that inhibits apoptosis after growth factor withdrawal. Cancer Res. 1997;57:4063–4069. [PubMed] [Google Scholar]
- 100.Cerignoli F, Rahmouni S, Ronai Z, Mustelin T. Regulation of MAP kinases by the VHR dual-specific phosphatase: implications for cell growth and differentiation. Cell Cycle. 2006;5:2210–2215. doi: 10.4161/cc.5.19.3267. [DOI] [PubMed] [Google Scholar]
- 101.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959–50965. doi: 10.1074/jbc.M207050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–575. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 104.Salmeen A, Barford D. Functions and mechanisms of redox regulation of cysteine-based phosphatases. Antioxid Redox Signal. 2005;7:560–577. doi: 10.1089/ars.2005.7.560. [DOI] [PubMed] [Google Scholar]
- 105.Kumar A, Wu H, Collier-Hyams LS, Kwon YM, Hanson JM, Neish AS. The bacterial fermentation product butyrate influences epithelial signaling via reactive oxygen species-mediated changes in cullin-1 neddylation. J Immunol. 2009;182:538–546. doi: 10.4049/jimmunol.182.1.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Covarrubias L, Hernandez-Garcia D, Schnabel D, Salas-Vidal E, Castro-Obregon S. Function of reactive oxygen species during animal development: passive or active? Dev Biol. 2008;320:1–11. doi: 10.1016/j.ydbio.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 107.O'Hara AM, Shanahan F. Gut microbiota: mining for therapeutic potential. Clin Gastroenterol Hepatol. 2007;5:274–284. doi: 10.1016/j.cgh.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 108.Bjarnason I. Intestinal permeability. Gut. 1994;35:18–22. doi: 10.1136/gut.35.1_suppl.s18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Urao M, Okuyama H, Drongowski RA, Teitelbaum DH, Coran AG. Intestinal permeability to small- and large-molecular-weight substances in the newborn rabbit. J Pediatr Surg. 1997;32:1424–1428. doi: 10.1016/s0022-3468(97)90553-4. [DOI] [PubMed] [Google Scholar]
- 110.van Elburg RM, Fetter WP, Bunkers CM, Heymans HS. Intestinal permeability in relation to birth weight and gestational and postnatal age. Arch Dis Child Fetal Neonatal Ed. 2003;88:52–55. doi: 10.1136/fn.88.1.F52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weaver LT, Laker MF, Nelson R. Intestinal permeability in the newborn. Arch Dis Child. 1984;59:236–241. doi: 10.1136/adc.59.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qin HL, Shen TY, Gao ZG, Fan XB, Hang XM, Jiang YQ, et al. Effect of Lactobacillus on the gut microflora and barrier function of the rats with abdominal infection. World J Gastroenterol. 2005;11:2591–2596. doi: 10.3748/wjg.v11.i17.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Isolauri E, Kaila M, Arvola T, Majamaa H, Rantala I, Virtanen E, et al. Diet during rotavirus enteritis affects jejunal permeability to macromolecules in suckling rats. Pediatr Res. 1993;33:548–553. doi: 10.1203/00006450-199306000-00002. [DOI] [PubMed] [Google Scholar]
- 114.Isolauri E, Majamaa H, Arvola T, Rantala I, Virtanen E, Arvilommi H. Lactobacillus casei strain GG reverses increased intestinal permeability induced by cow milk in suckling rats. Gastroenterology. 1993;105:1643–1650. doi: 10.1016/0016-5085(93)91059-q. [DOI] [PubMed] [Google Scholar]
- 115.Luyer MD, Buurman WA, Hadfoune M, Speelmans G, Knol J, Jacobs JA, et al. Strain-specific effects of probiotics on gut barrier integrity following hemorrhagic shock. Infect Immun. 2005;73:3686–3692. doi: 10.1128/IAI.73.6.3686-3692.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stratiki Z, Costalos C, Sevastiadou S, Kastanidou O, Skouroliakou M, Giakoumatou A, et al. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev. 2007;83:575–579. doi: 10.1016/j.earlhumdev.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 117.Martin CR, Walker WA. Probiotics: role in pathophysiology and prevention in necrotizing enterocolitis. Semin Perinatol. 2008;32:127–137. doi: 10.1053/j.semperi.2008.01.006. [DOI] [PubMed] [Google Scholar]