Abstract
Mucins are a family of heavily glycosylated proteins that are the major organic components of the mucus layer, the protective layer covering the epithelial cells in many human and animal organs, including the entire gastro-intestinal tract. Microbes that can associate with mucins benefit from this interaction since they can get available nutrients, experience physico-chemical protection and adhere, resulting in increased residence time. Mucin-degrading microorganisms, which often are found in consortia, have not been extensively characterized as mucins are high molecular weight glycoproteins that are hard to study because of their size, complexity and heterogeneity. The purpose of this review is to discuss how advances in mucus and mucin research, and insight in the microbial ecology promoted our understanding of mucin degradation. Recent insight is presented in mucin structure and organization, the microorganisms known to use mucin as growth substrate, with a specific attention on Akkermansia muciniphila, and the molecular basis of microbial mucin degradation owing to availability of genome sequences.
Key words: mucus, mucins, gastrointestinal tract, mucin degradation, intestinal microbiota, host-microbe interactions, mucin-degrading enzymes
Introduction
Mucus is present at the interface between many epithelial surfaces and their extracorporeal environments, including the oculo-rhino-otolaryngeal tracts, the respiratory tract, the gastrointestinal (GI) tract and the reproductive tract. Mucus is a complex viscous secretion, which often forms a continuous layer. The mucus is a highly hydrated gel (∼95% water) formed by large glycoproteins, called mucins, which further contains salts, lipids1 and proteins that are involved in defense such as defensins, growth factors, immunoglobulins, lysozym and trefoil factors and many other intestinal proteins.2 Humans secrete large volumes of mucus that amounts to approximately 10 liters of mucus per day.3 Mucus has many roles and can act as: (1) a lubricant, as in the airways and when facilitating the passage of food in the intestine; (2) a selective barrier by allowing passage of low molecular weight components, such as nutrients to the epithelial cells; and (3) a defense system that protects the underlying epithelial cells from mechanical damage or entrance of harmful substances of either chemical nature, such as drugs, toxins or heavy metals or biological nature, such as luminal pepsin, organic acids, pathogenic bacteria, viruses or parasites.4–7 Another important function of mucus, which has come to light more recently, is to serve as a substrate for the growth, adhesion and protection of the trillions of microbial cells that are present in the lumen in the GI tract. This review addresses specifically this microbial dimension and summarizes recent insight in mucus structure and organization, the microbes known to use mucin as growth substrate, and the molecular basis of microbial mucin degradation.
Mucus
Mucus is constantly produced, secreted, and shed, a process that can take from a few minutes to several hours depending on the organ and the situation, such as invasion by pathogens that requires a fast response of the epithelium.8 The thickness and dynamics of the mucus layer differ highly among organs. The oral cavity is covered by a salivary film consisting of water, mucins, and other proteins. Assuming that the saliva is distributed evenly over the tissues the calculated thickness of the salivary film is 70 to 100 µm.9 The stomach presents a thicker mucus layer (approximately 300 µm), which protects the underlying gastric epithelium against the hostile acidic conditions.10 The small intestine is mainly covered by a loosely attached mucus layer (150–400 µm), which is thinnest in the jejunum, where the major nutrient uptake takes place.10 In the colon, the mucus layer thickness follows a gradient from the caecum to the rectum from thin to a thick mucus layer, respectively, reaching a thickness of 800–900 µm in the distal colon.
Histochemical analysis of human biopsies revealed two different mucus sub-layers, as described in the colon.11 The outer layer designated as a mobile or non-adherent layer, is largely soluble and constantly removed, and acts as a lubricant by expulsing potentially dangerous agents (like microorganisms and viruses) trapped in this layer.10 The underlying inner layer, also termed adherent layer, is firmly adherent to the epithelial surfaces and not soluble in water. This latter sub-layer acts as a selective barrier, and allows only passage of smaller molecules.4 After removal by suction of the loosely attached outer mucus layer from the firm inner layer, the loosely attached layer is replenished quickly as was studied in tissue of live animals.2,10 The relative thickness of the outer and inner mucus layer depends on the organs, e.g., in stomach and colon the inner layer is thick (100–150 µm), whereas the firm inner layer is hardly existent in the jejunum.10 Recently, the inner mucus layer that adheres closely to the epithelium and the more loosely attached outer mucus layer were described in detail in mice.2 It appears that in the colon the firm inner layer contains hardly any bacteria, but has a similar composition than the outer ‘sloppy’ layer, which contains a high concentration of bacteria. In both sub-layers, the mucin Muc2 was invariably the main structural constituent, yet the transition from the one to the other layer is abrupt. It is not yet known what defines both sub-layers.2
Disturbance of the structure and function of the mucus layer is characteristic for the pathology of many diseases of the respiratory tract, such as asthma, chronic obstructive pulmonary diseases or cystic fibrosis, which are all characterized by overproduction of mucus.12 A disturbed mucus layer integrity is also invariably part of the pathology of disorders of the GI tract like Crohn disease and ulcerative colitis. In case of ulcerative colitis, the mucus layer was found to be thinner in the inflamed part of the tissue, whereas in Crohn disease, the mucus thickness was normal or even higher than usual.13 The protecting role of the colonic mucus layer has been recently demonstrated in genetically engineered mice deficient in Muc2, which is the dominant structural mucin of intestinal mucus in human and mouse.14,15 These mice spontaneously develop colitis in the absence of the Muc2 glycoprotein, and also Muc2+/− heterozygous mice have a significantly increased sensitivity towards the colitis-inducing agent dextran sulfate sodium.16 Thus, the integrity of the mucus layer is crucial to ensure the protection of the underlying epithelium, at least in the mouse colon.
Mucins
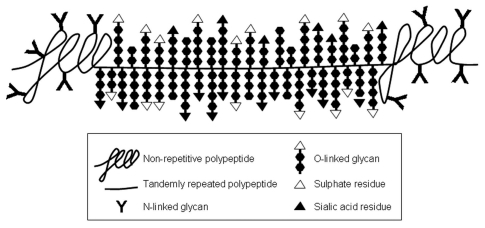
Mucus layers consist mainly of gel-forming mucin-type glycoproteins, which are heavily O-glycosylated molecules. These mucins are usually produced in specialized mucous cells of glandular tissues and in goblet cells of the GI tract.17–21 Biophysically, mucins are responsible for the visco-elastic properties of the mucus-gel, and are characterized by 50–90% O-linked glycans (by weight of the mucin molecules), which are attached to the protein backbone (apomucin). This resuls in very high molecular weight complexes of many millions of Daltons, which are to date impossible to measure accurately. To date 17 human mucin genes have been assigned to the MUC gene family, according to the Human Genome Organization (HUGO) Gene Nomenclature Committee (HGNC; www.gene.ucl.ac.uk/nomenclature/index.html) (Table 1). Based on sequence homology, several mucin families can be distinguished: such as mucus-forming mucins at human chromosome locus 11p15, which have probably evolved through gene duplication of one ancestral gene,22 and a number of membrane-bound mucins at locus 7q22, which might also share a common ancestral gene.23 Genes encoding mucin-type glycoproteins have appeared at different moments and different locations in our ancestral genome, as excellently (reviewed in ref. 24) Lang et al. Mucin polypeptides have distinct domains: a central domain on which O-glycosylation is concentrated; the so-called ‘PTS region’ (i.e., region rich in the amino acid residues, proline, threonine and serine, Fig. 1). Within the PTS region there is usually a repetitive array of relatively short amino acid sequences, in tandem repeats, which are often variable in number among individuals. The central part of the mucin peptide core is bordered at both ends with amino acid sequences referred as ‘unique regions’, which sequences differ from the central or PTS region, and have a more normally distributed amino acid composition (Fig. 1).
Table 1.
Classification of human mucins: Length of the tandemly repeated amino acid sequence (TR), location of protein expression and chromosomal localization
| MUC protein | Amino acids in TR | Main location of expression | Chromosome locus | References |
| Secreted: | ||||
| MUC2 | 23/16 | small intestine, colon, tracheobronchial tissue | 11p15.5 | 128–131 |
| MUC5AC | 8/5 | stomach, respiratory tissue, Brunner's glands | 11p15.5 | 128, 132–135 |
| MUC5B | 29 | salivary glands, tracheobronchial tissue, Brunner's glands, endocervix, gall bladder, pancreas | 11p15.5 | 21, 70, 128, 136, 137 |
| MUC6 | 169 | stomach, gall bladder, Brunner's glands | 11p15.5 | 70, 128, 138, 139 |
| MUC7 | 23 | salivary glands | 4q13.3 | 140–142 |
| MUC8 | 13/41 | tracheobronchial tissue | 12q24.3 | 143, 144 |
| MUC19 (predicted protein) | 7/7/15/16/9/8/5 | salivary glands, tracheobronchial tissue | 12q12 | 145 |
| Membrane-bound: | ||||
| MUC1 | 20 | all epithelia | 1q22 | 146–148 |
| MUC3A | 17/375 | small intestine, colon, gall bladder | 7q22 | 129, 149–151 |
| MUC3B | 17/375 | small intestine, colon | 7q22.1 | 149–151 |
| MUC4 | 16 | virtually all epithelia | 3q29 | 152, 153 |
| MUC12 | 28 | colon | 7q22.1 | 154 |
| MUC13 | 15 | trachea, small intestine, colon | 3q21.2 | 155 |
| MUC15 | none | spleen, small intestine, colon, prostate, lung | 11p14.2 | 156 |
| MUC16 | 156 | ovarian tissue, ocular tissue | 19p13.2 | 157–159 |
| MUC17 | 59 | pancreas, small intestine, colon | 7q22.1 | 20, 35, 160 |
| MUC20 | 19 | renal tissue | 3q29 | 161 |
Figure 1.
Schematic representation of a mucin molecule. The part of the polypeptide where the O-linked glycans are concentrated is not drawn to scale. Based on electron micrographs of isolated mucin molecules, this glycopeptide comprises at least 70% of the length of the mucin molecule.40
To the tandemly repeated amino acids sequences of the polypeptide a very large number of O-linked carbohydrate chains are added in the Golgi apparatus during biosynthesis. These are linked to the apoprotein via N-acetylgalactosamine (GalNac) that is coupled to the hydroxyl group of either a serine or threonine residue via an O-glycosidic linkage (Fig. S1). Then galactose and/or N-acetylglucosamine (GlcNAc) residues are added to this initial GalNAc to form the various core structures, of which there are eight different forms, and 4 of these are the most common on mucins (Fig. S1). The backbone region of these glycans consists of successive additions of galactose and either GalNac or GlcNac residues, which may come in three types: type-1 chain (Galβ1-3GlcNAc), type-2 chain (Galβ1-4GlcNAc) or type-3 chain (Galβ1-4GalNAc). The peripheral sugars of the oligosaccharide chains often have an arrangement identical to those found in the ABH histo-blood group antigens present on red blood cells (Fig. S1).25 Other terminal groups may constitute, e.g., the Lewis histo-blood group structures (by addition of fucose residues), and other more rare blood group structures. For example, human salivary MUC5B glycosylation reflects the ABH and Lewis histo-blood group antigen status of individual.26,27 Further modification of the glycan structure of mucins often occurs via the addition of sialic acid and/or sulfate residues that give the mucins their overall negative charge and which contributes to the specific functions of mucin. The O-glycosylation that can be found on a single MUC-type mucin from one source is usually very heterogeneous. For example human colonic MUC2 contains more than 100 different O-linked glycans, which range in size from to 2 to 12 monosaccharides, most of which are based on the core 3 structure.28 Strikingly, the spectrum of these O-glycans was very uniform among human individuals.28 In the oral cavity, it was demonstrated that the glycosylation of the salivary mucins MUC5B and MUC7 is heterogeneous and can differ between individuals even with the same blood group. These different mucin glycoforms are secreted in the oral cavity by physically separated salivary glands.29–32 The production and presence of these various glycoforms of the mucins could well play a role in the adhesion of the various oral bacteria that also show a preferred localization at the various sites in the oral cavity (see below).
Based on their structure, mucins are usually subdivided into two classes: secretory and membrane-bound mucins. Secretory mucins are apically secreted by specialized cells, such as the goblet cells in the intestines and airways, and characterized by their high molecular weight, and their ability to form a viscous gel. Membrane-bound mucins, in contrast, are synthesized by epithelial cells, such as MUC1 and MUC3 by enterocytes in the intestine, and integrated into their apical plasma membranes. Secretory mucins contain cysteine-rich sequences, located in the N- and C-terminal regions, which allow the formation of disulfide bridges to form either filamentous multimers,33,34 or more complex network-like covalent structures as demonstrated for human MUC2.35 Membrane-bound mucins, such as MUC1 or MUC3, do not form covalent multimers and are not gel-forming, but do contain a C-terminal trans-membrane anchor.36 Membrane-bound mucins have been thought to play a role as cell surface receptors and sensors, which translate information about external conditions into cellular responses including proliferation, differentiation, apoptosis or secretion of specialized cellular products.37
Depending on the sugar composition, mucins can be differentiated bio- and histochemically as neutral or acidic mucins. Acidic mucins contain substantial amounts of sialic acid (‘sialomucins’) and/or sulfate residues (‘sulfomucins’) giving a strong negative charge to the mucin molecule. This distinction between sialomucins/sulfomucins and acidic/neutral mucins is hardly ever absolute. Most mucins carry at least some of these negatively charged groups and at thus not neutral in the strict sense. Also many mucins might carry both sulfate—and sialic acid residues, and the name sialo/sulfomucins in fact only indicates the predominance of one or the other negatively charged group. The sulfation has been proposed to provide extra protection of the underlying epithelium against degradation by the high density of bacteria, because sulfation confers relative resistance to most bacterial mucin-degrading enzymes.38,39 There is usually also a substantial amount of N-glycosylation found on mucins (e.g., 40 chains per MUC2 molecule), but relative to the O-glycosylation this makes a relatively small contribution to the molecular size of the mucin molecules.33 The extensive glycosylation of mucins strongly affects their physical properties, giving the molecules, e.g., a very high buoyant density of around 1.4 g/ml, and extending the monomeric mucin molecules into very long filaments.34,40 The glycosylation can mediate specific binding of immune cells, and pathogenic and commensal microbes, and plays a role in inflammation and cancer metastasis.37,41–43 Several studies indicate that changes in mucin-structure change their protective properties. When in mice the O-glycosylation is altered, by knocking out either a specific core 3 type glycosyltransferase or a set of three core 2 type glycosyltransferase, the mice are rendered more susceptible to development of colitis.44,45 A similar effect is caused by knocking out the intestinal sulfate transporter, which leads to under-sulfated intestinal mucin, which also increases the sensitivity to small intestinal infections and the development of experimental colitis in these mice.46
In diverse intestinal infection models in the mouse it was clearly demonstrated that mucin production as well as mucin structure was influenced by the presence of pathogens. One example is the intestinal infection of mice by the intestinal nematode Nippostrongylus brasiliensis, which remains in the gut lumen for several weeks. These studies show that the predominant secretory mucin of the small intestine Muc2 is specifically upregulated, but also that the structure of the mucins change in a specific pattern over time during the infection.47,48 For the duration of the infection by these nematodes, increasing numbers of mucin-producing goblet cells contain sulfomucins, and also transient modifications in the terminal sugars were observed,49 indicating the adaptability of the epithelium as well as the increased necessity to produce mucins in order to expel the nematodes. Another example is intestinal infection in mouse by rotavirus; a virus that replicates within intestinal epithelial cells, thereby destroying the enterocytes in a process that is self-limiting in a few days. Upon rotavirus infection there is an increase in Muc2 synthesis shortly after virus infection. During the following phase of villus atrophy, the Muc2 synthesis is largely maintained, despite severe pathology.50 Also in these studies of rotavirus infection it appears that the Muc2 structure changes, since the mucins produced contain much less sulfation than in under non-infected situations.50
Mucus and Mucin-associated Microbes
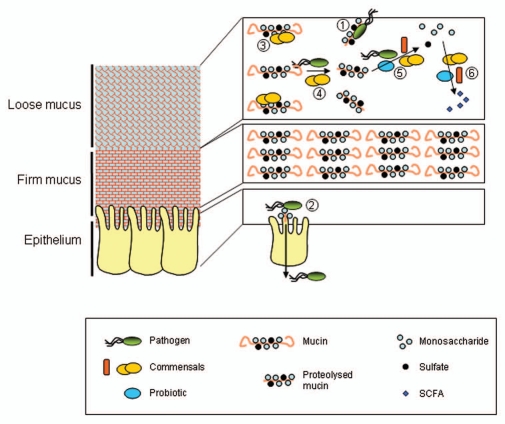
The mucus layer is considered the first line of defense between the bacteria in the lumen and the host cells, and also serves as the initiation surface for host-microbe interactions. The GI-tract contains a complex ecosystem that is composed of trillions of microbial cells. Bacteria associated with mucus probably gain an advantage over the luminal or planktonic bacteria. Indeed, bacteria colonizing the mucus gel are less susceptible to elimination by the passage of luminal contents, and have increased access to carbon sources provided by the mucus layer, compared to luminal bacteria.51 The mucus has a dual role in relation to microbiota; it protects the underlying mucosa from undesired interactions with microbes such as pathogens; besides it provides an initial adhesion site, nutrient source, and matrix on (and/or in) which bacteria can proliferate and thrive (Fig. 2). This dualistic role of mucins, i.e., keeping the bacteria at bay and at the same time provide attachment sites, has been noted earlier by Van Klinken et al.19
Figure 2.
Schematic representation of the different associations of gel forming mucins with intestinal bacteria. Pathogens can be bound by proteolytically degraded mucin in the lumen (1), as mucins contain similar carbohydrate structures as found on the epithelium and thus be expelled. Similarly pathogens might be bound by the mucins in the mucus layer and therefore not reach the epithelium. When pathogens reach the epithelium they can bind to receptors (often carbohydrate structures) and might translocate across the epithelium (2). Normally, this will primarily occur when the mucus layer is gone as in severe pathology or concern microbes that can achieve mobility through the mucus layer, such as H. pylori in gastric mucus. Commensal bacteria can be present as planktonic bacteria in the lumen, live bound to the mucins in the mucus layer or live protected within the mucus without direct interactions (3). Commensal bacteria might compete with pathogens for binding sites on the mucins, thereby denying access to the pathogens (1 vs. 3). Some commensal as well as some pathogenic bacteria are able to proteolytically degrade mucins (4). Commensals, pathogens and probiotics are able to use mucins as energy source by degrading the O-glycans (5); the latter could happen within the mucus layer as well as in the lumen. The released monosaccharides are amongst others converted by commensal and probiotic bacteria into secondary metabolites (6), such as short chain fatty acids (SCFA), which are, e.g., essential for the colonic epithelium as an energy source. Please notice the scale of the components of the drawing. Mucin molecules (polymers) are several micrometers long, and are thus in the same range of size as the bacteria. In contrast, the epithelial cells are about 20 µm tall, whereas the mucus layer in, e.g., the colon can reach up to 800 µm in thickness.
In general, commensal GI bacteria benefit from the mucus-layer by (1) attaching to mucus (retention at mucosal surface), (2) ‘hiding’ in the outer mucus-layer (which needs some form of mobility of the individual bacterium by the diverse mechanism as recently reviewed by Jarrell and McBride,52 or a form of ‘mixing’ of the bacteria with the luminal contents) and (3) using the mucin as nutrient source (which needs specific enzymes, and requires cooperation among consortia). From the point of view of our body, the mucus-layer thus has two main purposes. First, it serves as a protective secretion; a physical layer. Second, mucins are provided to the luminal GI bacteria as a kind of ‘prebiotic’ to (at least partly) manage the growth of intestinal luminal microbiota. Mucins have no known or recorded anti-bacterial activity. If mucins were purely a defensive layer, as postulated in early mucus research, these molecules would probably have acquired such a function during evolution. Thus, their partial consumption by bacteria seems at least a part of their physiological functions. As mucins vary widely in their extent and form of glycosylation, this could well constitute a means of the body to stimulate bacterial growth in an organ-specific manner. Interestingly, the ‘protective’ and ‘prebiotic’ functions of the mucins collide, since both are affected by changes in glycan structure, which will both affect physical properties (as in gel-formation), but also the abilities for degradation by bacterial enzymes. However, these two roles can be balanced, i.e., separately regulated, by the type of mucin (different MUC genes in different organs, and regulation of the levels of their respective productions), by physico-chemical composition of the mucin molecules that is determined by type of glycosylation and extent of oligomerization of the mucins, and by cellular localization: i.e., secretory vs. membrane-bound mucins.
It was shown in a model reactor with an artificial mucus layer that the microbial community living inside the mucus matrix differed phylogenetically and metabolically from the luminal community.53 Luminal mucosa-associated bacteria varied inter-individually, and showed intra-individual similarity on different sites of the colon.54–56 The mucosa-associated bacteria are in close contact with/proximity to the epithelial cells, and therefore can have a major impact on these cells. However, a study based on fluorescent in situ hybridization (FISH) with a combination of a general 16S rRNA oligonucleotide probe targeting all bacteria, and specific probes targeting particular groups of bacteria, showed that there was no specific population present in the mucus layer, relative to commensal bacteria as located in the lumen.57 In contrast, human and animal studies using FISH, light and scanning electron microscopy, showed bacteria embedded in the mucus layer.7,58 Johansson et al. investigated the presence of bacteria in the inner and outer mucus layer of colonic tissues. Semi-quantitative PCR and FISH using a general 16S rRNA probe showed that very low numbers of bacteria were present in the inner mucus layer, while large numbers of bacteria were detected in the outer mucus layer, suggesting that the inner mucus layer acts as a major physical barrier against penetration of bacteria into the epithelium.2
Mucin Binding by Bacteria
Carbohydrate structures present on mucins are extremely diverse (Figs. 1 and S1). As a result, mucins offer an ‘oasis’ of binding sites for microbial (pathogens and commensals) adhesion. By offering binding sites similar to those of epithelial cells, mucin can prevent pathogen adhesion to the underlying epithelial cells, and further translocation into the mucosa. Human saliva, e.g., contains two mucin-type glycoproteins: the high molecular weight, gel-forming mucin MUC5B, and the low molecular weight, soluble mucin MUC7. Secretions of the human submandibular, sublingual and minor salivary glands contain MUC5B and MUC7.59 Several investigations have demonstrated that MUC5B and MUC7 alone or together, interact with oral microorganisms in order to protect the underlying epithelium (summarized in Table 1S). Another example of this ‘scavenging’ function of mucins can be found for MUC1 from human breast milk.60 MUC1 adheres to pathogenic microorganisms, such as rotavirus, Campylobacter, Escherichia coli (ETEC), which interferes with their colonization in the infant GI tract.61–63 The ability to adhere to mucins has been suggested to be one of the criteria for the selection of lactic acid bacteria with probiotic activity.64,65 However, as comparative studies on the efficiency of probiotic strains are scarce, the validation of this attribute has to be confirmed. For this type of study, the proper procedure would be to isolate the genuine mucin(s) from the organ of interest. As indicated above, even pure mucins (e.g., human colonic MUC228) are very heterogeneous in nature due to their extensive and variable O-glycosylation, and the glycosylation varies from organ to organ even for one specific MUC-type mucin. In other words, only mucins from the organ of interest will do as appropriate targets for bacterial adhesion and/or degradation studies. Thus, identification of relevant bacterial-mucin interactions needs to be performed ‘pair-wise,’ i.e., by using the proper mucin from the organ of interest in combination with (a community of) the relevant bacteria known to reside in that organ. These essential studies have as yet not been done, largely hampered by lack of proper (human) mucin-sources, which would allow for isolation of sufficient amounts of native mucins to enable these experiments.
The molecular mechanism by which binding to mucins may occur is receiving increasing attention. Several genomes of probiotic lactic acid bacteria contain genes with predicted mucin-binding activity.66 One of them, the protein GroEL of Lactobacillus johnsonni was shown to bind to mucins and aggregate Helicobacter pylori, suggesting that the protein could facilitate clearance of this pathogen during mucus flushing.67 The carbohydrate structures on mucins can also promote the invasion of specialized pathogens by providing them a first attachment site that facilitates further access to epithelial cells. The recent study of Celli et al. showed that the pathogenic bacterium H. pylori, responsible of gastric ulcers, achieves motility by modifying the rheological properties of the mucus layer.68 Previously, it was demonstrated in the antrum of H. pylori-infected individuals, that H. pylori co-localizes in situ with the extracellular mucin MUC5AC (the predominant gel-forming mucin in the human stomach) as well as with the apical domain of MUC5AC-producing cells of the superficial gastric epithelium.69 We showed that the histo-blood group antigen Lewis b, present on gastric mucin MUC5AC, is the primary adhesion site for H. pylori in the stomach.70 This is in line with the earlier work of Boren et al. who showed that Lewis b structures mediated the attachment of H. pylori to human gastric epithelium, when assayed on tissue sections.71 The H. pylori adhesin, which interacts with Lewis b, has been cloned and was designated as blood group antigen-binding adhesin (BabA).72 Recently, it was discovered that one of the most widely consumed probiotic lactic acid bacteria, L. rhamnosus GG, contains cell-envelope bound filaments, known as pili. These pili contain the SpaC pilus protein, which is able to mediate strong binding to host mucins.73 This finding might explain the ability of the probiotic strain to persist in the GI tract as well as to stimulate the host immune system.
Mucin Degradation by Bacteria
Besides providing attachment sites to bacteria, mucins can be an important factor for bacterial colonization by providing an energy source. Mucin is an important carbon source for bacteria, mainly in the distal colon where the availability of carbohydrates is limited.74 The carbohydrate structures account for about 80% of mass the mucin molecules and hence constitute a significant endogenous carbon and energy source for intestinal microbes able to cleave the relevant glycosidic linkages.
Mucin degradation.
Mucin degradation by bacteria is often regarded as an initial stage in pathogenesis, since it would disturb the protection of the host mucosal surfaces. The fact of mucin degradation also offers ecological advantages to certain bacteria that scavenge released products from mucin degradation such as oligosaccharides or sulfate. One example is the relation between sulfate reducing-bacteria (SRB) and mucin degraders. Sulfomucins offer a potential source of sulfate for subsequent reduction into hydrogen sulfide, which has been shown to be highly toxic for the intestinal epithelium, promoting cell proliferation.75 When fecal microbiota was inoculated into a three-stage continuous culture system supplemented with mucin, stimulation of the growth of SRB, especially Desulfovibrio sp. and subsequent production of sulfide were observed.76 Interestingly, a co-culture of B. fragilis and D. desulfuricans enhanced sulfide production.77 Hence, mucin degradation is sometimes viewed as negative effect, although this might hold only for the activities of specific bacteria. Mucin degradation has been recognized as a normal process of mucus turn-over in the GI tract, starting a few months after birth.78 Midtvedt et al. studied the establishment of the mucin-degrading microbiota from 30 Swedish children from birth to the age of two years old, by analyzing their fecal samples. They found that the establishment of mucin-degrading bacteria starts during the first months of life, and is completed when the children are around two years old. Interestingly, a relation with diet was also observed: breast-fed babies showed a delay in the mucin degradation profile as compared with babies fed with formula milk. An explanation might be that mucins and the other mucin-like glycoproteins present in the breast milk compete with endogenous GI mucins as microbial substrates.79 A specific transcriptional response to mucin-type oligosaccharides was recently observed in Bifidobacterium spp. in breast fed babies, confirming the reaction of intestinal microbiota to mucin.80
Mucin degradation has also been found to affect the host. The epithelium of germ-free mice differs morphologically from that of conventionally raised animals: non-degraded mucin is found in feces, their goblet cells are smaller and less abundant, the colonic mucus layer is approximately two times thicker and the weight of the cecum can reach up to eight times that of conventional animals.81–84 The swelling of the cecum in germ-free animals is due to the accumulation of mucus, and the resulting retention of water, due to the absence of mucin-degrading bacteria.85 In addition, when fecal suspensions or pure cultures of Clostridium, Bacteroides or Peptococcus were introduced into germ-free rodents, the cecum showed a striking reduction to its normal weight,86,87 suggesting the beneficial workings of mucin-degrading bacteria. In the study of Schwerbrock et al. on the conventionalization of germ-free mice, the intestinal production of the major intestinal mucin Muc2 was carefully characterized and quantified as a function of time after colonization.82 In healthy control mice the level of Muc2 production was steady after the conventionalization, however over time there was a marked increase in the level of sulfation of the Muc2 molecules. These results were compared to an IL10 knockout mouse, which develops spontaneous colitis when colonized by bacteria. The IL10 knockout mice have lower level of Muc2 synthesis in germ-free conditions and react to the conventionalization by a quick upsurge in Muc2 production, which however quickly falls to very low levels. At the same time the level of sulfation of the Muc2 molecules produced in the colon of the IL10 knockout mouse decrease to barely detectable levels. The results indicate that bacteria in normal and pathological conditions can have quite opposite effects on mucin production and on mucin structure, as exemplified by the level of sulfation.82
Isolation of mucin-degrading microbes.
Enrichment of dental plaque microbiota on saliva-containing culture media demonstrated that cell-bound microbial enzymes almost completely degrade salivary proteins, including mucins.88 Further studies showed that oral Streptococcus species were capable to grow in defined medium containing pig gastric mucin and used this as a nutritional substrate.89
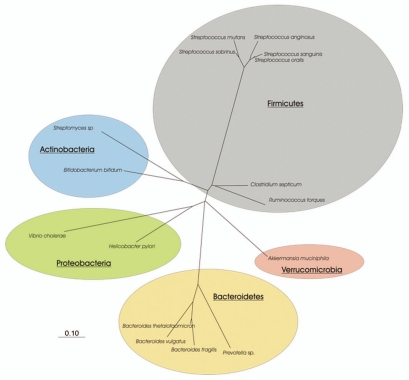
In a series of pioneering studies, Hoskins and co-workers studied the adult fecal microbiota, which was able to degrade pig gastric mucin in vitro. They first quantified the microbiota using most probable number analysis using mucin-based medium after fecal inoculation.90 This study revealed that 1% of the total fecal microbiota was able to use mucin as carbon source, including the genera Ruminococcus and Bifidobacterium, although complete degradation of mucin required the action of a specific consortium.77 Bacteroides species were also shown to ferment mucins.91 Very recently, the use of a basal medium supplemented with mucin as single carbon source as an alternative to the use of complex and rich media for the isolation of new intestinal strains has resulted in the isolation of novel bacteria, i.e., Akkermansia muciniphila from human fecal samples and Enterorhabdus mucosicola from ileal samples from mouse.92 This suggests that there are many more bacteria that can use mucin as primary carbon-source, which wait to be discovered. A phylogenetic overview of cultivated mucin-degrading bacteria present in the human digestive tract is depicted in Figure 3.
Figure 3.
Phylogenetic tree derived from the 16S rRNA sequence data of isolated and cultured mucin-degrading bacteria in the human digestive tract. Bar represents 10% divergence.
Isolation of Akkermansia muciniphila and its abundance in humans and animals.
Recently, the use of a targeted cultivation approach allowed the isolation of a novel microorganism, Akkermansia muciniphila, capable of growth on mucin as sole carbon and nitrogen source.93 This is the first intestinal member belonging to the recently discovered Verrucomicrobia phylum. Members of this bacterial phylum have been detected using molecular techniques in a variety of ecosystems, including soil, water and GI tract and increasing numbers of isolates.94 Using periodic acid-Schiff staining (that stains mucin molecules) on A. muciniphila cultures growing on mucin, it was estimated that 80% of the mucin was degraded and metabolized in a growing culture, indicating the efficient breakdown of the mucin molecule (Derrien M et al., unpublished results). A variety of enzymes targeting a wide range of mucin carbohydrates have been identified in grown cultures of A. muciniphila (Table 2). The available genome sequence is being now analyzed and will provide new information about the genetic capacity of A. muciniphila to degrading mucin (Table 3). In addition to genetic information, 16S rRNA-based surveys, including clone libraries, showed that A. muciniphila was detected as a dominant bacterium in the GI-tract. The study of Wang et al. reported that the 16S rRNA gene sequence of A. muciniphila was detected in the distal ileum, ascending colon and rectum with levels of 5, 6 and 9% of the clone libraries from a healthy 54-year-old female, respectively.95 In a larger scale study from Eckburg et al. in which 13,355 prokaryotic ribosomal RNA gene sequences were examined from various colonic mucosal sites and feces of three healthy subjects, A. muciniphila was detected in every site from one subject, from the ascending colon, descending colon and stool of another subject, but was not detected in appreciable numbers in the third subject.58 Sequences corresponding to A. muciniphila and related species have been detected in 16S rRNA clone libraries, originating from human biopsies, feces derived from healthy adults,58,96,97 and inflammatory bowel disease patients.98 A recent molecular inventory revealed that Akkermansia species are widely distributed amongst wild and zoo mammals, with a strong predominance in herbivores.99 Other studies also reported the presence of A. muciniphila in mice,100 herbivores101 and hamsters.102
Table 2.
Bacterial mucin-degrading enzymes identified in the human digestive tract
| Organisms | Enzymes | References |
| Oral cavity | ||
| Streptococcus anginosus | β-N-acetyl-D-glucosaminidase, α- and β-D-galactosidase | 89 |
| Streptococcus mitis | β-N-acetyl-D-galactosaminidase, β-N-acetyl-D-glucosaminidase, α- and β-D-galactosidase, α-L-fucosidase, neuraminidase | 89 |
| Streptococcus mutants | β-N-acetyl-D-glucosaminidase, α- and β-D-galactosidase | 89 |
| Streptococcus oralis | β-N-acetyl-D-galactosaminidase, β-N-acetyl-D-glucosaminidase, α- and β-D-galactosidase, α-L-fucosidase, neuraminidase, protease | 88, 89 |
| Streptococcus sanguinis | β-N-acetyl-D-galactosaminidase, β-N-acetyl-D-glucosaminidase, α- and β-D-galactosidase, α-L-fucosidase, protease | 88, 89 |
| Streptococcus sobrinus | β-N-acetyl-D-glucosaminidase, β-D-galactosidase | 89 |
| Gastrointestinal tract | ||
| Akkermansia muciniphila | α- and β-D-galactosidase, α-L fucosidase, α- and β-N-acetylgalactosaminidase, β-N-acetylglucosaminidase, neuraminidase, sulfatase. | 162 |
| Bacteroides fragilis | protease, α-N-acetylgalactosaminidase, β-galactosidase, β-N-acetyl-D-glucosaminidase, α-L-fucosidase, neuraminidase, sulfatase | 163–165 |
| Bacteroides thetaiotaomicron | α-fucosidase, β-galactosidase, α-N-acetylgalactosaminidase, β-N-acetylglucosaminidase, neuraminidase, sulfatase | 166, 167 |
| Bacteroides vulgatus | α- and β-galactosidase, α-fucosidase, β-N-acetyl-D-glucosaminidase, α- and β-N-acetylgalactosaminidase, neuraminidase | 168 |
| Bifidobacterium sp., Bifidobacterium bifidum | α-L-fucosidase, α-N-acetylgalactosaminidase, galactosyl-N-acetylhexosamine phosphorylase | 169 |
| Clostridium cocleatum | β-galactosidase, β-N-acetylglucosaminidase, α-N-acetylgalactosaminidase, neuraminidase | 170 |
| Clostridium septicum | β-Galactosidase, β-N-acetyl-D-glucosaminidase, glycosulfatase, neuraminidase | 171 |
| Helicobacter pylori | Glycosulfatase | 172 |
| Prevotella sp. RS2 | Sulfoglycosidase, glycosulfatase | 173, 174 |
| Ruminococcus torques | α-N-acetylgalactosaminidase | 175 |
| Streptomyces sp. | α-L-fucosidase | 176 |
| Vibrio cholerae | Neuraminidase, β-N-acetylhexosaminidase, proteinase. | 177 |
Table 3.
Mining for candidate mucin-degrading enzymes in seven sequenced genomes
| Candidate mucin-degrading enzymes4 | ||||||||||
| Phylum | Species name1 | Size (Mbp) | # CDS | # Signal P2 (%) | # UF3 (%) | GH | P | Su | Si | Total |
| Verrucomicrobia | A. muciniphila | 2.7 | 2176 | 576 (26) | 270 (47) | 35 | 13 | 11 | 2 | 61 |
| Bacteroidetes | B. vulgatus | 5.2 | 4076 | 1200 (29) | 510 (43) | 145 | 42 | 4 | 7 | 198 |
| B. thetaiotaomicron | 6.3 | 4816 | 1574 (33) | 777 (49) | 171 | 30 | 11 | 9 | 221 | |
| P. gingivalis | 2.4 | 2090 | 451 (22) | 248 (55) | 9 | 28 | 0 | 1 | 38 | |
| Proteobacteria | H. pylori | 1.6 | 1494 | 311 (21) | 141 (45) | 0 | 6 | 0 | 0 | 6 |
| Firmicutes | S. mutans | 2.0 | 1960 | 331 (17) | 140 (42) | 4 | 10 | 0 | 0 | 14 |
| Actinobacteria | Bif. longum subsp. infantis | 2.8 | 2486 | 462 (19) | 181 (39) | 6 | 7 | 1 | 0 | 15 |
The accession numbers for the different species are NC_010655 (A. muciniphila), NC_009614 (B. vulgatus), NC_004663 (B. thetaiotaomicron), NC_010729 (P. gingivalis), NC_000921 (H. pylori), NC_004350 (S. mutans), NC_011593 (B. longum subsp. infantis).
These numbers (percentages) represent the protein coding genes that encode a putative signal peptide.
These numbers (percentages) represent the protein coding genes that encode a putative signal peptide and lack a predicted function.
The candidate mucin-degrading enzymes are categorized into four broad categories; glycosyl hydrolases (GH), proteases/peptidases (P), sulfatases (Su) and sialidases/neuraminidases (Si). For details of the list, see Table S2.
Specific 16S rRNA primers and probes have brought new information about this microorganism. Its abundance over life time has been measured using qPCR on fecal samples from babies, children, adults and elderly people. FISH revealed that A. muciniphila is a common member of the human intestinal tract with a high prevalence and variable abundance, and that its colonization starts in early life and develops within a year to a level close to that observed in adults (108 cells/g feces), but decreases in the elderly. Interestingly we also found that the 16S rRNA sequence of A. muciniphila contains two mismatches with the eubacterial probe (EUB-338, commonly used as positive control) and that A. muciniphila cells showed only a weak signal after EUB-338 hybridization. With the increase of availability of 16S rRNA sequences, it was shown that EUB-338 does not cover all bacterial phyla, such as the Planctomycetales and Verrucomicrobia. Therefore, two additional probes, EUB-338-II and EUB-338-III, targeting these two groups respectively were designed. When combined, these three probes allow for detection of all the phyla of the bacteria domain.103 The EUB-338-III is 100% complementary only to members of the divisions 1 to 4 of the Verrucomicrobia phylum.104 Nevertheless, in intestinal studies, this specific probe has not been often employed, suggesting that A. muciniphila has been missed in most analyses so far and thus its presence was underestimated. The widespread occurrence of A. muciniphila in the intestine of diverse hosts supports its importance among the members of intestinal consortia. An obvious advantage of mucin-degraded bacteria over food-dependent bacteria is their ability to survive in more extreme GI conditions, such as lack of food or severe diarrhea. A recent study on cecal microbiota of fasted Syrian hamsters reported that A. muciniphila significantly increased, supporting its ability to survive over species belonging to Firmicutes and Bacteroidetes. This ability also offered advantage to bacteria belonging to Proteobacteria due to released products from mucins degradation and most likely the release of sulfate from the mucins.102 Many authors hypothesized that mucin-degraders are related to pathogenicity in the intestine as they might undermine the protective nature of the mucus layer. However, A. muciniphila has so far not been correlated to any disease or sign of pathogenicity. Its high prevalence in diverse gut ecosystems also supports its benign nature and probably also its importance for the microbial ecosystem. Moreover, colonization of germ-free mice with A. muciniphila did not reveal any sign of discomfort or pathology (Derrien et al. manuscript submitted). Moreover, a recent study from Swidsinski reported that it was negatively correlated with acute appendicitis, together with Faecalibacterium prausnitzii, Eubacterium rectale and Bacteroides spp.105
Molecular analysis of mucin-degrading bacteria.
The last decade, the advent of molecular approaches based on the 16rRNA has allowed insight into the unseen microbiota and a more precise description of the intestinal microbiota. In an in vitro fermentation system mimicking the human intestine, Macfarlane et al. reported that a heterogeneous bacterial community was colonizing mucin.53 These authors used a combination of cultivation and visualization of the bacterial community by FISH, based on specific and general 16S rRNA probes. They observed that this community consisted of members of the Bacteroides fragilis group, bifidobacteria, enterobacteria and clostridia. Further studies based on construction of 16S rRNA genes of enriched fecal mucin-degrading bacteria revealed the diversity of mucolytic consortia.93 Mucolytic bacteria were enriched from fecal samples inoculated on a mucin-limited medium and monitored by denaturing gel gradient electrophoresis of 16S rRNA gene amplicons. This molecular fingerprint approach showed that the profiles from different subjects were highly diverse, with a similarity index of 57.4 ± 9.2%, indicating that the mucin-degrading bacterial community is roughly similar within the whole population, but that there is still substantial individual variation. In addition, cloning and sequencing of 16S rRNA genes revealed that the majority (69%) of these genes derived from not-yet cultivated species. The 16S rRNA gene sequences that showed similarities higher than 98% to known database entries were related to the Gram-positive genera Clostridium and Ruminococcus (Clostridium clusters IV, XIVa and XVIII), whereas the remaining ones were related to species belonging to Verrucomicrobia and Proteobacteria.93 In addition, recently Leitch et al. studied the colonization of insoluble substrates including pig gastric mucin by fecal bacteria in an anaerobic in vitro continuous flow system. They showed by a cloning—and sequencing strategy and FISH based on 16S rRNA gene, that Bifidobacteria were the principal species (Bif. bifidum and Bif. breve), followed by bacteria belonging to Clostridium cluster XIVa (41% of the mucin-associated sequences) including Ruminococcus lactaris and a closely related group of uncultured bacteria. Bacteria belonging to Bacteroidetes such as B. vulgatus were also detected on mucin.106 These molecular based studies provided evidence for the fact that fecal samples contain a diverse population of mucin-associated bacteria, the composition of which differs from one person to the other.
Mucin degrading enzymes.
Mucin degradation is achieved by a combination of mainly saccharolytic enzymes from the bacteria and proteolytic enzymes from the host and bacteria. As discussed above, the composition of O-linked glycans on the mucins, their size, linkages and terminal sugar-residues differs along the GI tract, being more neutral in the upper part, while more acidic in the lower part. Mucin-degrading bacteria can adapt to the host mucins by producing specific enzymes, which are able to degrade the histo-blood group antigens (oligosaccharides).107–109 Due to their high complexity and diversity, mucins can only be completely degraded by a panel of diverse enzymes, including proteases, glycosidases, sialidases and sulfatases, together designated as ‘mucinases’.109,110 Mucin degradation in vivo starts probably with cleavage of the non-glycosylated regions of the polypeptide backbone performed by proteolytic enzymes (Fig. 2). Subsequently, the oligosaccharide chains are degraded by a panel of diverse glycosidases and finally followed by proteolytic degradation of the exposed protein core. Proteases are secreted by both host and bacteria, whereas glycosidases capable of degrading mucin-type O-linked glycans are only secreted by bacteria and not by the host tissues. The bacterial glycosidases include mainly β-N-acetyl-D-galactosaminidase, β-N-acetyl-D-glucosaminidase, α- and β-D-galactosidase and α-D-mannosidase; whereas the latter plays only a minor role in degradation of mucins that are relatively poor in N-linked glycans (mannose is only present in N-linked glycans). Sialidases (neuraminidases), sulfatases and α-fucosidases act on the terminal ends of the oligosaccharide chains and will often act as initiators of mucin oligosaccharide degradation, as sulfate, sialic acids and α-fucose (the latter in particular in the diverse blood group structures) form the usual terminal structures on the mucin-type O-glycans (examples in Fig. S1). Obviously, mucin degradation requires the subsequent actions of several microbial enzymes, mainly glycosidases, each having the specificity to degrade a specific glycoside linkage. Only a few bacteria produce all the enzymes necessary for complete mucin degradation. Several bacteria belonging to the genera Streptococcus, Helicobacter, Akkermansia, Bacteroides, Bifidobacterium, Clostridium, Prevotella, Ruminococcus, Streptomyces produce one, several or all mucin-degrading enzymes (Table 2). The ratio of production and degradation of mucin is usually stable in healthy individuals, leading to a dynamic mucus-layer with over time a stabile thickness, composition and consistency. However, this status quo can be affected in pathogenesis as seen in tracheobronchial diseases, e.g., chronic obstructive pulmonary diseases and cystic fibrosis, in which the mucus layer is abnormally thick and the mucus has a very pronounced viscosity, disabling the proper clearance of mucus and bacteria from the airways. Also in intestinal pathology the mucin production and mucus layer is affected, mucin mRNA expression has been shown to be disturbed for several MUC genes in ileal and colonic mucosal biopsies from inflammatory bowel diseases patients such as ulcerative colitis and Crohn disease.111 Changes in the properties of mucins and mucus thickness have been noted in ulcerative colitis and Crohn disease, such as decrease of the length of the O-glycans, a decrease in the degree of sulfation and increase of sialylation of the oligosaccharide chains.112 Moreover, the thickness of the mucus layer is altered in these patients, leading to a weakness in the protective barrier and thus contributing to the pathology by increasing the vulnerability of the underlying epithelium.13,41,113 In ulcerative colitis, the mucus layer is thinner, the epithelium harbors a reduced proportion of goblet cells and there are increased levels of fecal mucin-degrading enzymes, including sulfatases, as compared to healthy individuals, contributing to a general dysfunction of the mucosal barrier. The rate of mucin turn-over can be modified by bacterial colonization,114,115 short chain fatty acids,116 starvation,117 and intake of fibers and other oligosaccharides.116,118–123 Colonic fermentation (including that of mucins) has a major impact on our human metabolism,99,124 thus the selective retention of and the presence of metabolic substrates for, bacteria are important mechanism to maintain body homeostasis. Also changed microbiota has been associated with changed health status such as obesity,125 indicating that specific bacterial substrates such as endogenous mucins, are probably very important to regulate the microbial balance in the gut.
Genome mining for candidate genes involved in mucin degradation.
Besides the purification and study of specific mucin-degrading enzymes, the increasing number of genome sequencing projects is providing a glimpse into the genetic basis of mucin breakdown. However, the complex nature of mucins frustrates the straightforward identification of the range of genes responsible for its degradation. Identifying candidate mucinases from the genetic repertoires of sequenced bacterial genomes relies partially on certain prediction tools, since for many species experimental data for these activities are lacking. Due to the large molecular size of mucins, mucin-degrading enzymes are expected to be either secreted proteins or proteins associated with the outside of the cell wall and using dedicated in silico secretion predictions tools, candidate mucin-degrading enzymes can be listed for a range of mucin-degrading bacteria (Table 3).
Genome-wide predictions of signal sequences, indicative of secretion via the classical pathway,126 reveal general characteristics of a genome's capacity for mucin degradation. As an example, a well-studied intestinal bacterium that can thrive on mucins is B. thetaiotaomicron, a common inhabitant of the human and mouse GI tract. The pioneering work of Salyers et al. in the 1970s has brought about information on the capacity of this organism to use host glycans and mucins in vitro.91 Later studies from Gordon and coworkers revealed that B. thetaiotaomicron is a very well adapted bacterium to the intestinal environment, with a flexible ability to switch to mucins when polysaccharides are depleted from the diet.127 Of the analyzed genomes, B. thetaiotaomicron shows the largest number of genes that encode putative signal sequences (1,574, 32.7% of the protein coding repertoire). It also contains the largest number of putatively secreted genes with glycosidase, sialidase, protease or sulfatase activities. Comparable numbers of these genes are encountered in Bacteroides vulgatus. Other known mucin-degrading GI bacteria, such as Helicobacter pylori, Streptococcus mutans and Bifidobacterium longum subsp. infantis show lower counts of these genes, and each is lacking at least one category of the functionalities for complete mucin degradation. The recently identified and characterized Verrucomicrobium Akkermansia muciniphila, which was isolated by dilution-to-extinction on mucin as a single carbon and nitrogen source, has predicted secreted genes for glycosidase, sialidase, protease and sulfatase activities. With this repertoire of functionalities in its relatively small genome, A. muciniphila seems therefore to be specialized in mucin degradation; more so than the Bacteroides spp., which have much larger genomes. On the other hand, the large numbers of unknown functions in all seven of these predicted secretomes may hold a plethora of activities towards mucin degradation.
Conclusion and Perspectives
Mucus overlying the epithelium in the oral cavity and the GI tract and the bacteria residing in these organs maintain intimates relationships. The combined information in this review has brought new insight in the specific bacterial community present in the digestive tract. Many members of this community are able to associate with mucins and an increasing number of bacteria are found to be able to degrade mucins that offer alternative nutrients during changes of diet, and is an integral part of the bacterial survival strategy in this complex ecology. The increasing availability of sequenced genomes of mucin-degraders helps us to understand the mucin degradation and its relevance for microbial ecology. The high degree of diversity of the mucin oligosaccharide chains, with their many potential binding sites and metabolic substrates for bacteria, is likely an important determinant in the site-specific colonization of bacteria along the digestive tract. The mucus layer and more specifically the constituting mucins are thus a major determinant of bacterial colonization. This notion is an important extension of the views advocated in the early years of mucin research that state that the mucus layer has only a barrier function. Now that it is becoming more and more clear that important commensal and probiotic bacteria can degrade mucins and use these as substrates, it seems that bacterial fermentation of mucins is an important feature to selectively favor the growth of certain classes of bacteria. Thus, aside from their well-described role in host mucosal defense, the mucins of the digestive tract could be designated as endogenous ‘prebiotics’. The lumen/mucus interface and the mucus-layer itself harbors many ecological niches, which are sought for by beneficial commensal bacteria as well as by pathogenic organisms. We are still far from being able to understand these many and complex interactions between microbes and the mucus and mucins. Yet, the examples in this review indicate that there is a battle fought over the mucins by the oral and intestinal microbiota on a daily basis in GI tract, and that the outcome is an important factor in human health.
Acknowledgements
The authors gratefully acknowledge Dr. Erwin G. Zoetendal for his critical reading and suggestions for the manuscript. M.W.Jv.P. is funded by the Netherlands Organization for Scientific Research (NWO) via a VENI grant.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/12778
Supplementary Material
References
- 1.Allen A. The structure and function of gastrointestinal mucus. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Vol. 1. New York Raven Press; 1981. pp. 617–639. [Google Scholar]
- 2.Johansson MEV, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. PNAS. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore E. Physiology of intestinal and electrolyte absorption. American Gastroenterological Society. Baltimore: Milner-Fenwick; 1976. [Google Scholar]
- 4.Allen A, Flemstrom G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol. 2005;288:1–19. doi: 10.1152/ajpcell.00102.2004. [DOI] [PubMed] [Google Scholar]
- 5.Allen A. Mucus—a protective secretion of complexity. Trends Biochem Sci. 1983;8:169–173. [Google Scholar]
- 6.Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr. 2001;73:1131–1141. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- 7.Forstner JF, Forstner GG. Gastrointestinal mucus. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York Raven Press; 1994. pp. 1255–1283. [Google Scholar]
- 8.Bell A, Sellers L, Allen A, Cunliffe W, Morris E, Ross-Murphy S. Properties of gastric and duodenal mucus: effect of proteolysis, disulfide reduction, bile, acid, ethanol and hypertonicity on mucus gel structure. Gastroenterology. 1985;88:269–280. doi: 10.1016/s0016-5085(85)80180-3. [DOI] [PubMed] [Google Scholar]
- 9.Collins LM, Dawes C. The surface area of the adult human mouth and thickness of the salivary film covering the teeth and oral mucosa. J Dent Res. 1987;66:1300–1302. doi: 10.1177/00220345870660080201. [DOI] [PubMed] [Google Scholar]
- 10.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol. 2001;280:922–929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo K, Ota H, Akamatsu T, Sugiyama A, Katsuyama T. Histochemistry of the surface mucous gel layer of the human colon. Gut. 1997;40:782–789. doi: 10.1136/gut.40.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards R, Rodriguez-Brito B, Wegley L, Haynes M, Breitbart M, Peterson D. Using pyrosequencing to shed light on deep mine microbial ecology. BMC Genomics. 2006;7:57. doi: 10.1186/1471-2164-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pullan RD, Thomas GA, Rhodes M, Newcombe RG, Williams GT, Allen A, et al. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994;35:353–359. doi: 10.1136/gut.35.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Klinken BJ-W, Einerhand AWC, Duits LA, Makkink MK, Tytgat KMAJ, Renes IB, et al. Gastrointestinal expression and partial cDNA cloning of murine Muc2. Am J Physiol Gastrointest Liver Physiol. 1999;276:115–124. doi: 10.1152/ajpgi.1999.276.1.G115. [DOI] [PubMed] [Google Scholar]
- 15.Tytgat KMAJ, Buller HA, Opdam F, Kim Y, Einerhand AWC, Dekker J. Biosynthesis of human colonic mucin: Muc2 is the prominent secretory mucin. Gastroenterology. 1994;107:1352–1363. doi: 10.1016/0016-5085(94)90537-1. [DOI] [PubMed] [Google Scholar]
- 16.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol. 1995;57:607–634. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- 18.Strous GJ, Dekker J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol. 1992;27:57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- 19.Van Klinken BJ, Dekker J, Buller HA, Einerhand AW. Mucin gene structure and expression: protection vs. adhesion. Am J Physiol. 1995;269:613–627. doi: 10.1152/ajpgi.1995.269.5.G613. [DOI] [PubMed] [Google Scholar]
- 20.Van Klinken BJ, Dekker J, Buller HA, de Bolos C, Einerhand AW. Biosynthesis of mucins (MUC2-6) along the longitudinal axis of the human gastrointestinal tract. Am J Physiol. 1997;273:296–302. doi: 10.1152/ajpgi.1997.273.2.G296. [DOI] [PubMed] [Google Scholar]
- 21.Van Klinken BJ, Dekker J, van Gool SA, van Marle J, Buller HA, Einerhand AW. MUC5B is the prominent mucin in human gallbladder and is also expressed in a subset of colonic goblet cells. Am J Physiol. 1998;274:871–878. doi: 10.1152/ajpgi.1998.274.5.G871. [DOI] [PubMed] [Google Scholar]
- 22.Desseyn JL, Aubert JP, Porchet N, Laine A. Evolution of the large secreted gel-forming mucins. Mol Biol Evol. 2000;17:1175–1184. doi: 10.1093/oxfordjournals.molbev.a026400. [DOI] [PubMed] [Google Scholar]
- 23.Dekker J, Rossen JW, Buller HA, Einerhand AW. The MUC family: an obituary. Trends Biochem Sci. 2002;27:126–131. doi: 10.1016/s0968-0004(01)02052-7. [DOI] [PubMed] [Google Scholar]
- 24.Lang T, Hansson GC, Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. PNAS. 2007;104:16209–16214. doi: 10.1073/pnas.0705984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carraway KL, Hull SR. Cell surface mucin-type glycoproteins and mucin-like domains. Glycobiology. 1991;1:131–138. doi: 10.1093/glycob/1.2.131. [DOI] [PubMed] [Google Scholar]
- 26.Thomsson KA, Schulz BL, Packer NH, Karlsson NG. MUC5B glycosylation in human saliva reflects blood group and secretor status. Glycobiology. 2005;15:791. doi: 10.1093/glycob/cwi059. [DOI] [PubMed] [Google Scholar]
- 27.Prakobphol A, Leffler H, Fisher SJ. The high-molecular-weight human mucin is the primary salivary carrier of ABH, Le(a) and Le(b) blood group antigens. Crit Rev Oral Biol Med. 1993;4:325–333. doi: 10.1177/10454411930040031001. [DOI] [PubMed] [Google Scholar]
- 28.Holmen Larsson JM, Karlsson H, Sjovall H, Hansson GC. A complex, but uniform O-glycosylation of the human MUC2 mucin from colonic biopsies analyzed by nanoLC/MSn. Glycoconj J. 2009;19:756–766. doi: 10.1093/glycob/cwp048. [DOI] [PubMed] [Google Scholar]
- 29.Thornton DJ, Khan N, Mehrotra R, Howard M, Veerman E, Packer NH, et al. Salivary mucin MG1 is comprised almost entirely of different glycosylated forms of the MUC5B gene product. Glycobiology. 1999;9:293. doi: 10.1093/glycob/9.3.293. [DOI] [PubMed] [Google Scholar]
- 30.Wickström C, Davies JR, Eriksen GV, Veerman ECI, Carlstedt I. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: Identification of glycoforms and C-terminal cleavage. Biochem J. 1998;334:685. doi: 10.1042/bj3340685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ligtenberg AJ, Walgreen-Weterings E, Veerman EC, de Soet JJ, de Graaff J, Amerongen AV. Influence of saliva on aggregation and adherence of Streptococcus gordonii HG 222. Infect Immun. 1992;60:3878–3884. doi: 10.1128/iai.60.9.3878-3884.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramasubbu N, Reddy MS, Bergey EJ, Haraszthy GG, Soni SD, Levine MJ. Large-scale purification and characterization of the major phosphoproteins and mucins of human submandibular-sublingual saliva. Biochem J. 1991;280:341–352. doi: 10.1042/bj2800341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dekker J, Strous GJ. Covalent oligomerization of rat gastric mucin occurs in the rough endoplasmic reticulum, is N-glycosylation-dependent and precedes initial O-glycosylation. Glycoconj J. 1990;265:18116–18122. [PubMed] [Google Scholar]
- 34.Dekker J, van der Ende A, Aelmans P, Strous G. Rat gastric mucin is synthesized and secreted exclusively as filamentous oligomers. Biochem J. 1991;279:251–256. doi: 10.1042/bj2790251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Godl K, Johansson MEV, Lidell ME, MÃrgelin M, Karlsson H, Olson FJ, et al. The N terminus of the MUC2 mucin forms trimers that are held together within a trypsin-resistant core fragment. J Biol Chem. 2002;277:47248–47256. doi: 10.1074/jbc.M208483200. [DOI] [PubMed] [Google Scholar]
- 36.Corfield A, Shukla A. Mucins: Vital components of the mucosal defensive barrier. Am Gen Prot Technol. 2003:20–23. [Google Scholar]
- 37.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 38.Roberton AM, McKenzie CG, Sharfe N, Stubbs LB. A glycosulphatase that removes sulphate from mucus glycoprotein. Biochem J. 1993;293:683–689. doi: 10.1042/bj2930683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brockhausen I. Sulphotransferases acting on mucin-type oligosaccharides. Biochem Soc Trans. 2003;31:318–325. doi: 10.1042/bst0310318. [DOI] [PubMed] [Google Scholar]
- 40.Dekker J, Van Beurden-Lamers W, Oprins A, Strous G. Isolation and structural analysis of rat gastric mucus glycoprotein suggests a homogeneous protein backbone. Biochem J. 1989;260:717–723. doi: 10.1042/bj2600717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhodes JM. Colonic mucus and ulcerative colitis. Gut. 1997;40:807–808. doi: 10.1136/gut.40.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brockhausen I. Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 2006;7:599–604. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robbe C, Calliope C, Maes E, Rousset M, Zweibaum A, Zanetta JP, et al. Evidence of regio-specific glycosylation in human intestinal mucins. J Biol Chem. 2003;278:46337–46348. doi: 10.1074/jbc.M302529200. [DOI] [PubMed] [Google Scholar]
- 44.An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3 derived O-glycans. J Exp Med. 2007;204:1417–1429. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stone EL, Ismail MN, Lee SH, Luu Y, Ramirez K, Haslam SM, et al. Glycosyltransferase Function in Core 2-Type Protein O Glycosylation. Mol Cell Biol. 2009;29:3770–3782. doi: 10.1128/MCB.00204-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dawson P, Huxley S, Gardiner B, Tran T, McAuley J, Grimmond S, et al. Reduced mucin sulfonation and impaired intestinal barrier function in the hyposulfataemic NaS1 null mouse. Gut. 2009;58:910–919. doi: 10.1136/gut.2007.147595. [DOI] [PubMed] [Google Scholar]
- 47.Soga K, Yamauchi J, Kawai Y, Yamada M, Uchikawa R, Tegoshi T, et al. Alteration of the expression profiles of acidic mucin, sialytransferase and sulfotransferases in the intestinal epithelium of rats infected with the nematode Nippostrongylus brasiliensis. Parasitol Res. 2008;103:1427–1434. doi: 10.1007/s00436-008-1152-8. [DOI] [PubMed] [Google Scholar]
- 48.Junko Y, Yuichi K, Minoru Y, Ryuichi U, Tatsuya T, Naoki A. Altered expression of goblet cell- and mucin glycosylation-related genes in the intestinal epithelium during infection with the nematode Nippostrongylus brasiliensis in rat. APMIS. 2006;114:270–278. doi: 10.1111/j.1600-0463.2006.apm_353.x. [DOI] [PubMed] [Google Scholar]
- 49.Holmén J, Olson F, Karlsson H, Hansson G. Two glycosylation alterations of mouse intestinal mucins due to infection caused by the parasite Nippostrongylus brasiliensis. Glycoconj J. 2002;19:67–75. doi: 10.1023/a:1022589015687. [DOI] [PubMed] [Google Scholar]
- 50.Boshuizen J, JH R, Korteland-van Male A, van Ham V, Bouma J, Gerwig G, et al. Homeostasis and function of goblet cells during rotavirus infection in mice. Virology. 2005;337:210–221. doi: 10.1016/j.virol.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 51.Probert H, Gibson G. Bacterial biofilms in the human gastrointestinal tract. Curr Issues Intest Microbiol. 2002;3:23–27. [PubMed] [Google Scholar]
- 52.Jarrell KF, McBride MJ. The surprisingly diverse ways that prokaryotes move. Nat Rev Micro. 2008;6:466–476. doi: 10.1038/nrmicro1900. [DOI] [PubMed] [Google Scholar]
- 53.Macfarlane S, Woodmansey EJ, Macfarlane GT. Colonization of mucin by human intestinal bacteria and establishment of biofilm communities in a two-stage continuous culture system. Appl Environ Microbiol. 2005;71:7483–7492. doi: 10.1128/AEM.71.11.7483-7492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zoetendal EG, von Wright A, Vilpponen-Salmela T, Ben-Amor K, Akkermans ADL, de Vos WM. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl Environ Microbiol. 2002;68:3401–3407. doi: 10.1128/AEM.68.7.3401-3407.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nielsen DS, Moller PL, Rosenfeldt V, Paerregaard A, Michaelsen KF, Jakobsen M. Case study of the distribution of mucosa-associated Bifidobacterium species, Lactobacillus species and other lactic acid bacteria in the human colon. Appl Environ Microbiol. 2003;69:7545–7548. doi: 10.1128/AEM.69.12.7545-7548.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lepage P, Seksik P, Sutren M, de la Cochetiere MF, Jian R, Marteau P, et al. Biodiversity of the mucosa-associated microbiota is stable along the distal digestive tract in healthy individuals and patients with IBD. Inflamm Bowel Dis. 2005;1:473–480. doi: 10.1097/01.mib.0000159662.62651.06. [DOI] [PubMed] [Google Scholar]
- 57.van der Waaij L, Harmsen H, Madjipour M, Kroese F, Zwiers M, van Dullemen H, et al. Bacterial population analysis of human colon and terminal ileum biopsies with 16S rRNA-based fluorescent probes: commensal bacteria live in suspension and have no direct contact with epithelial cells. Inflamm Bowel Dis. 2005;11:865–871. doi: 10.1097/01.mib.0000179212.80778.d3. [DOI] [PubMed] [Google Scholar]
- 58.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tabak LA. In defense of the oral cavity: structure, biosynthesis and function of salivary mucins. Annu Rev Physiol. 1995;57:547–564. doi: 10.1146/annurev.ph.57.030195.002555. [DOI] [PubMed] [Google Scholar]
- 60.Shimizu M, Yamauchi K. Isolation and characterization of mucin-like glycoprotein in human milk fat globule membrane. J Biochem (Tokyo) 1982;91:515–524. doi: 10.1093/oxfordjournals.jbchem.a133724. [DOI] [PubMed] [Google Scholar]
- 61.Martin-Sosa S, Martin MJ, Hueso P. The sialylated fraction of milk oligosaccharides is partially responsible for binding to enterotoxigenic and uropathogenic Escherichia coli human strains. J Nutr. 2002;132:3067–3072. doi: 10.1093/jn/131.10.3067. [DOI] [PubMed] [Google Scholar]
- 62.Yolken RH, Peterson JA, Vonderfecht SL, Fouts ET, Midthun K, Newburg DS. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J Clin Invest. 1992;90:1984–1991. doi: 10.1172/JCI116078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha1, 2Gal beta1, 4GlcNAc) and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem. 2003;278:14112–14120. doi: 10.1074/jbc.M207744200. [DOI] [PubMed] [Google Scholar]
- 64.Kirjavainen PV, Ouwehand AC, Isolauri E, Salminen SJ. The ability of probiotic bacteria to bind to human intestinal mucus. FEMS Microbiol Lett. 1998;167:185–189. doi: 10.1111/j.1574-6968.1998.tb13226.x. [DOI] [PubMed] [Google Scholar]
- 65.Salminen S, Laine M, von Wright A, Vuopio-Varkila J, Korhonen T, Mattila-Sandholm T. Development of selection criteria for probiotic strains to access their potential in functional food. A nordic and European approach. Biosci Microflora. 1996;2:23–28. [Google Scholar]
- 66.Boekhorst J, Helmer Q, Kleerebezem M, Siezen RJ. Comparative analysis of proteins with a mucus-binding domain found exclusively in lactic acid bacteria. Microbiology. 2006;152:273–280. doi: 10.1099/mic.0.28415-0. [DOI] [PubMed] [Google Scholar]
- 67.Bergonzelli GE, Granato D, Pridmore RD, Marvin-Guy LF, Donnicola D, Corthesy-Theulaz IE. GroEL of Lactobacillus johnsonii La1 (NCC 533) is cell surfaceassociated: potential role in interactions with the host and the gastric pathogen Helicobacter pylori. Infect Immun. 2006;74:425–434. doi: 10.1128/IAI.74.1.425-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Celli JP, Turner BS, Afdhal NH, Keates S, Ghiran I, Kelly CP, et al. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. PNAS. 2009;106:14321–14326. doi: 10.1073/pnas.0903438106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van den Brink GR, Tytgat KM, Van Der Hulst RW, Van Der Loos CM, Einerhand AW, Buller HA, et al. H pylori colocalises with MUC5AC in the human stomach. Gut. 2000;46:601–607. doi: 10.1136/gut.46.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van de Bovenkamp JHB, Korteland-Van Male AM, Büller HA, Einerhand AWC, Dekker J. Metaplasia of the duodenum shows a Helicobacter pylori-correlated differentiation into gastric-type protein expression. Hum Pathol. 2003;34:156–165. doi: 10.1053/hupa.2003.15. [DOI] [PubMed] [Google Scholar]
- 71.Boren T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 72.Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, Incecik ET, et al. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 73.Kankainen M, Paulin L, Tynkkynen S, von Ossowski I, Reunanen J, Partanen P, et al. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. PNAS. 2009;106:17193–17198. doi: 10.1073/pnas.0908876106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rabiu BA, Gibson GR. Carbohydrates: a limit on bacterial diversity within the colon. Biol Rev Camb Philos Soc. 2002;77:443–453. doi: 10.1017/s1464793102005961. [DOI] [PubMed] [Google Scholar]
- 75.Attene-Ramos MS, Nava GM, Muellner MG, Wagner ED, Plewa MJ, Gaskins HR. DNA damage and toxicogenomic analyses of hydrogen sulfide in human intestinal epithelial FHs 74 Int cells. Environ Mol Mutagen. 2010;51:304–314. doi: 10.1002/em.20546. [DOI] [PubMed] [Google Scholar]
- 76.Gibson G, Cummings J, MacFarlane G. Use of a three-stage continuous culture system to study the effect of mucin on dissimilatory sulfate reduction and methanogenesis by mixed populations of human gut bacteria. Appl Environ Microbiol. 1988;54:2750–2755. doi: 10.1128/aem.54.11.2750-2755.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Willis C, Cummings JH, Neale G, Gibson GR. In vitro effects of mucin fermentation on the growth of human colonic sulphate-reducing bacteria. Anaerobe. 1996;2:117–122. [Google Scholar]
- 78.Norin K, Gustafsson BE, Lindblad B, Midtvedt T. The establishment of some microflora associated biochemical characteristics in feces from children during the first years of life. Acta Paediatr Scand. 1985;74:207–212. doi: 10.1111/j.1651-2227.1985.tb10951.x. [DOI] [PubMed] [Google Scholar]
- 79.Midtvedt A, Carlstedt-Duke B, Midtvedt T. Establishment of a mucin-degrading intestinal microflora during the first two years of human life. J Pediatr Gastroenterol Nutr. 1994;18:321–326. doi: 10.1097/00005176-199404000-00012. [DOI] [PubMed] [Google Scholar]
- 80.Klaassens ES, Boesten RJ, Haarman M, Knol J, Schuren FH, Vaughan EE, et al. Mixed-species genomic microarray analysis of fecal samples reveals differential transcriptional responses of bifidobacteria in breast- and formula-fed infants. Appl Environ Microbiol. 2009;75:2668–2676. doi: 10.1128/AEM.02492-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kandori H, Hirayama K, Takeda M, Doi K. Histochemical, lectin-histochemical and morphometrical characteristics of intestinal goblet cells of germfree and conventional mice. Exp Anim. 1996;45:155–160. doi: 10.1538/expanim.45.155. [DOI] [PubMed] [Google Scholar]
- 82.Schwerbrock NM, Makkink MK, van der Sluis M, Buller HA, Einerhand AW, Sartor RB, et al. Interleukin 10-deficient mice exhibit defective colonic Muc2 synthesis before and after induction of colitis by commensal bacteria. Inflamm Bowel Dis. 2004;10:811–823. doi: 10.1097/00054725-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 83.Szentkuti L, Riedesel H, Enss ML, Gaertner K, Von Engelhardt W. Pre-epithelial mucus layer in the colon of conventional and germ-free rats. Histochem J. 1990;22:491–497. doi: 10.1007/BF01007234. [DOI] [PubMed] [Google Scholar]
- 84.McCracken VJ, Lorenz RG. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell Microbiol. 2001;3:1–11. doi: 10.1046/j.1462-5822.2001.00090.x. [DOI] [PubMed] [Google Scholar]
- 85.Lindstedt G, Lindstedt S, Gustafsson BE. Mucus in intestinal contents of germfree rats. J Exp Med. 1965;121:201–213. doi: 10.1084/jem.121.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Loesche WJ. Effect of bacterial contamination on cecal size and cecal contents of gnotobiotic rodents. J Bacteriol. 1969;99:520–526. doi: 10.1128/jb.99.2.520-526.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Skelly BJ, Trexler PC, Tanami J. Effect of a Clostridium species upon cecal size of gnotobiotic mice. Proc Soc Exp Biol Med. 1962;10:455–458. doi: 10.3181/00379727-110-27548. [DOI] [PubMed] [Google Scholar]
- 88.De Jong MH, Van der Hoeven JS. The growth of oral bacteria on saliva. J Dent Res. 1987;66:498–505. doi: 10.1177/00220345870660021901. [DOI] [PubMed] [Google Scholar]
- 89.Van der Hoeven JS, Van den Kieboom CW, Camp PJ. Utilization of mucin by oral Streptococcus species. Antonie Van Leeuwenhoek. 1990;57:165–172. doi: 10.1007/BF00403951. [DOI] [PubMed] [Google Scholar]
- 90.Miller R, Hoskins L. Mucin degradation in human colon ecosystems. Fecal population densities of mucin-degrading bacteria estimated by a “most probable number” method. Gastroenterology. 1981;81:759–765. [PubMed] [Google Scholar]
- 91.Salyers AA, Vercellotti JR, West SE, Wilkins TD. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977;33:319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clavel T, Charrier C, Braune A, Wenning M, Blaut M, Haller D. Isolation of bacteria from the ileal mucosa of TNFdeltaARE mice and description of Enterorhabdus mucosicola gen. nov., sp. nov. Int J Syst Evol Microbiol. 2009;59:1805–1812. doi: 10.1099/ijs.0.003087-0. [DOI] [PubMed] [Google Scholar]
- 93.Derrien M, Adawi D, Ahrne S, Jeppsson B, Molin G, Osman N, et al. The intestinal mucosa as a habitat of the gut microbiota and a rational targert for probiotic functionality and safety. Microb Ecol Health Dis. 2004;16:137–144. [Google Scholar]
- 94.Hugenholtz P, Goebel BM, Pace NR. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J Bacteriol. 1998;180:4765–4774. doi: 10.1128/jb.180.18.4765-4774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang M, Ahrné S, Jeppsson B, Molin G. Comparison of bacterial diversity along the human intestinal tract by direct cloning and sequencing of 16S rRNA genes. FEMS Microbiol Ecol. 2005;54:219–231. doi: 10.1016/j.femsec.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 96.Hayashi H, Sakamoto M, Benno Y. Fecal microbial diversity in a strict vegetarian as determined by molecular analysis and cultivation. Microbiol Immunol. 2002;46:819–831. doi: 10.1111/j.1348-0421.2002.tb02769.x. [DOI] [PubMed] [Google Scholar]
- 97.Hold GL, Pryde SE, Russell VJ, Furrie E, Flint HJ. Assessment of microbial diversity in human colonic samples by 16S rDNA sequence analysis. FEMS Microbiol Lett. 2002;39:33–39. doi: 10.1111/j.1574-6941.2002.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 98.Mangin I, Bonnet R, Seksik P, Rigottier-Gois L, Sutren M, Bouhnik Y, et al. Molecular inventory of faecal microflora in patients with Crohn's disease. FEMS Microbiol Ecol. 2004;50:25–36. doi: 10.1016/j.femsec.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 99.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 100.Salzman NH, de Jong H, Paterson Y, Harmsen HJM, Welling GW, Bos NA. Analysis of 16S libraries of mouse gastrointestinal microflora reveals a large new group of mouse intestinal bacteria. Microbiology. 2002;148:3651–3660. doi: 10.1099/00221287-148-11-3651. [DOI] [PubMed] [Google Scholar]
- 101.Nelson KE, Zinder SH, Hance I, Burr P, Odongo D, Wasawo D, et al. Phylogenetic analysis of the microbial populations in the wild herbivore gastrointestinal tract: insights into an unexplored niche. Environ Microbiol. 2003;5:1212–1220. doi: 10.1046/j.1462-2920.2003.00526.x. [DOI] [PubMed] [Google Scholar]
- 102.Sonoyama K, Fujiwara R, Takemura N, Ogasawara T, Watanabe J, Ito H, et al. Response of gut microbiota to fasting and hibernation in syrian hamsters. Appl Environ Microbiol. 2009;75:6451–6456. doi: 10.1128/AEM.00692-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Daims H, Brühl A, Amann R, Schleifer K, Wagner M. The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol. 1999;22:434–444. doi: 10.1016/S0723-2020(99)80053-8. [DOI] [PubMed] [Google Scholar]
- 104.Vandekerckhove TTM, Coomans A, Cornelis K, Baert P, Gillis M. Use of the Verrucomicrobia-specific probe EUB338-III and fluorescent in situ hybridization for detection of “Candidatus xiphinematobacter” cells in nematode hosts. Appl Environ Microbiol. 2002;68:3121–3125. doi: 10.1128/AEM.68.6.3121-3125.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Swidsinski A, Dorfeel Y, Loening-Baucke V, Theissig F, Ruckert J, Ismail M, et al. Acute appendicitis is characterized by local invasion with Fusobacterium nucleatum/necrophorum. Gut. 2010 doi: 10.1136/gut.2009.191320. In press. [DOI] [PubMed] [Google Scholar]
- 106.Leitch EC, Walker AW, Duncan SH, Holtrop G, Flint HJ. Selective colonization of insoluble substrates by human faecal bacteria. Environ Microbiol. 2007;9:667–679. doi: 10.1111/j.1462-2920.2006.01186.x. [DOI] [PubMed] [Google Scholar]
- 107.Hoskins L, Agustines M, McKee W, Boulding E, Kriaris M, Niedermeyer G. Mucin degradation in human colon ecosystems. Isolation and properties of fecal strains that degrade ABH blood group antigens and oligosaccharides from mucin glycoproteins. J Clin Invest. 1985;75:944–953. doi: 10.1172/JCI111795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hoskins L, Boulding E. Degradation of blood group antigens in human colon ecosystems. I. In vitro production of ABH blood group-degrading enzymes by enteric bacteria. J Clin Invest. 1976;57:63–73. doi: 10.1172/JCI108270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hoskins LC, Boulding ET. Mucin degradation in human colon ecosystems. evidence for the existence and role of bacterial subpopulations producing glycosidases as extracellular enzymes. J Clin Invest. 1981;67:163–172. doi: 10.1172/JCI110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Corfield AP, Wagner SA, Clamp JR, Kriaris MS, Hoskins LC. Mucin degradation in the human colon: production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase and glycosulfatase activities by strains of fecal bacteria. Infect Immun. 1992;60:3971–3978. doi: 10.1128/iai.60.10.3971-3978.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Christoph M, Nikolaus A, Thomas L, Charalampos A, Alexander K, Olga KM, et al. Aberrant intestinal expression and allelic variants of mucin genes associated with inflammatory bowel disease. J Mol Med. 2006;84:1055–1066. doi: 10.1007/s00109-006-0100-2. [DOI] [PubMed] [Google Scholar]
- 112.Shirazi T, Longman RJ, Corfield AP, Probert CSJ. Mucins and inflammatory bowel disease. Postgrad Med J. 2000;76:473–478. doi: 10.1136/pmj.76.898.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schultsz C, Van Den Berg FM, Ten Kate FW, Tytgat GN, Dankert J. The intestinal mucus layer from patients with inflammatory bowel disease harbors high numbers of bacteria compared with controls. Gastroenterology. 1999;117:1089–1097. doi: 10.1016/s0016-5085(99)70393-8. [DOI] [PubMed] [Google Scholar]
- 114.Lievin-Le Moal V, Servin AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides and microbiota. Clin Microbiol Rev. 2006;19:315–337. doi: 10.1128/CMR.19.2.315-337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 2003;52:827–833. doi: 10.1136/gut.52.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barcelo A, Claustre J, Moro F, Chayvialle JA, Cuber JC, Plaisancie P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut. 2000;46:218–224. doi: 10.1136/gut.46.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Smirnov A, Sklan D, Uni Z. Mucin dynamics in the chick small intestine are altered by starvation. J Nutr. 2004;134:736–742. doi: 10.1093/jn/134.4.736. [DOI] [PubMed] [Google Scholar]
- 118.Vahouny G, Le T, Ifrim I, Satchithanandam S, Cassidy M. Stimulation of intestinal cytokinetics and mucin turnover in rats fed wheat bran or cellulose. Am J Clin Nutr. 1985;41:895–900. doi: 10.1093/ajcn/41.5.895. [DOI] [PubMed] [Google Scholar]
- 119.Kleessen B, Blaut M. Modulation of gut mucosal biofilms. Br J Nutr. 2005:35–40. doi: 10.1079/bjn20041346. [DOI] [PubMed] [Google Scholar]
- 120.Ten Bruggencate SJM, Bovee-Oudenhoven IMJ, Lettink-Wissink MLG, Van der Meer R. Dietary fructooligosaccharides increase intestinal permeability in rats. J Nutr. 2005;135:837–842. doi: 10.1093/jn/135.4.837. [DOI] [PubMed] [Google Scholar]
- 121.Satchithanandam S, Vargofcak-Apker M, Calvert R, Leeds A, Cassidy M. Alteration of gastrointestinal mucin by fiber feeding in rats. J Nutr. 1990;120:1179–1184. doi: 10.1093/jn/120.10.1179. [DOI] [PubMed] [Google Scholar]
- 122.Cabotaje L, Shinnick F, Lopez-Guisa J, Marlett J. Mucin secretion in germfree rats fed fiber-free and psyllium diets and bacterial mass and carbohydrate fermentation after colonization. Appl Environ Microbiol. 1994;60:1302–1307. doi: 10.1128/aem.60.4.1302-1307.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schmidt-Wittig U, Enss M, Coenen M, Gartner K, Hedrich H. Response of rat colonic mucosa to a high fiber diet. Ann Nutr Metab. 1996;40:343–350. doi: 10.1159/000177936. [DOI] [PubMed] [Google Scholar]
- 124.Nicholson JK, Holmes E, Wilson ID. Gut microorganisms, mammalian metabolism and personalized health care. Nat Rev Micro. 2005;3:431–438. doi: 10.1038/nrmicro1152. [DOI] [PubMed] [Google Scholar]
- 125.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. 2009;587:4153–4158. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 127.Sonnenburg JL, Xu J, Leip DD, Chen C-H, Westover BP, Weatherford J, et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 128.Pigny P, Guyonnet-Duperat V, Hill AS, Pratt WS, Galiegue-Zouitina S, d'Hooge MC, et al. Human mucin genes assigned to 11p15.5: identification and organization of a cluster of genes. Genomics. 1996;38:340–352. doi: 10.1006/geno.1996.0637. [DOI] [PubMed] [Google Scholar]
- 129.Griffiths B, Matthews DJ, West L, Attwood J, Povey S, Swallow DM, et al. Assignment of the polymorphic intestinal mucin gene (MUC2) to chromosome 11p15. Ann Hum Genet. 1990;54:277–285. doi: 10.1111/j.1469-1809.1990.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 130.Gum JR, Byrd JC, Hicks JW, Toribara NW, Lamport DT, Kim YS. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989;264:6480–6487. [PubMed] [Google Scholar]
- 131.Toribara NW, Gum JR, Jr, Culhane PJ, Lagace RE, Hicks JW, Petersen GM, et al. MUC-2 human small intestinal mucin gene structure. Repeated arrays and polymorphism. J Clin Invest. 1991;88:1005–1013. doi: 10.1172/JCI115360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Escande F, Aubert JP, Porchet N, Buisine MP. Human mucin gene MUC5AC: organization of its 5′-region and central repetitive region. Biochem J. 2001;358:763–772. doi: 10.1042/0264-6021:3580763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Van de Bovenkamp JHB, Hau CM, Strous GJ, Büller HA, Dekker J, Einerhand AWC. Molecular cloning of human gastric mucin MUC5AC reveals conserved cysteine-rich D-domains and a putative leucine zipper motif. Biochem Biophys Res Commun. 1998;245:853–859. doi: 10.1006/bbrc.1998.8535. [DOI] [PubMed] [Google Scholar]
- 134.Meerzaman D, Charles P, Daskal E, Polymeropoulos MH, Martin BM, Rose MC. Cloning and analysis of cDNA encoding a major airway glycoprotein, human tracheobronchial mucin (MUC5) J Biol Chem. 1994;269:12932–12939. [PubMed] [Google Scholar]
- 135.Nguyen VC, Aubert JP, Gross MS, Porchet N, Degand P, Frezal J. Assignment of human tracheobronchial mucin gene(s) to 11p15 and a tracheobronchial mucin-related sequence to chromosome 13. Hum Genet. 1990;86:167–172. doi: 10.1007/BF00197699. [DOI] [PubMed] [Google Scholar]
- 136.Desseyn JL, Guyonnet-Duperat V, Porchet N, Aubert JP, Laine A. Human mucin gene MUC5B, the 10.7-kb large central exon encodes various alternate subdomains resulting in a super-repeat. Structural evidence for a 11p15.5 gene family. J Biol Chem. 1997;272:3168–3178. doi: 10.1074/jbc.272.6.3168. [DOI] [PubMed] [Google Scholar]
- 137.Dufosse J, Porchet N, Audie JP, Guyonnet Duperat V, Laine A, Van-Seuningen I, et al. Degenerate 87-base-pair tandem repeats create hydrophilic/hydrophobic alternating domains in human mucin peptides mapped to 11p15. Biochem J. 1993;293:329–337. doi: 10.1042/bj2930329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Toribara NW, Roberton AM, Ho SB, Kuo WL, Gum E, Hicks JW, et al. Human gastric mucin. Identification of a unique species by expression cloning. J Biol Chem. 1993;268:5879–5885. [PubMed] [Google Scholar]
- 139.Vinall LE, Hill AS, Pigny P, Pratt WS, Toribara N, Gum JR, et al. Variable number tandem repeat polymorphism of the mucin genes located in the complex on 11p15.5. Hum Genet. 1998;102:357–366. doi: 10.1007/s004390050705. [DOI] [PubMed] [Google Scholar]
- 140.Bobek LA, Tsai H, Biesbrock AR, Levine MJ. Molecular cloning, sequence and specificity of expression of the gene encoding the low molecular weight human salivary mucin (MUC7) J Biol Chem. 1993;268:20563–20569. [PubMed] [Google Scholar]
- 141.Biesbrock AR, Bobek LA, Levine MJ. MUC7 gene expression and genetic polymorphism. Glycoconj J. 1997;14:415–422. doi: 10.1023/a:1018587031814. [DOI] [PubMed] [Google Scholar]
- 142.Bolscher JG, Groenink J, van der Kwaak JS, van den Keijbus PA, van 't Hof W, Veerman EC, et al. Detection and quantification of MUC7 in submandibular, sublingual, palatine and labial saliva by anti-peptide antiserum. J Dent Res. 1999;78:1362–1369. doi: 10.1177/00220345990780071101. [DOI] [PubMed] [Google Scholar]
- 143.Shankar V, Gilmore MS, Elkins RC, Sachdev GP. A novel human airway mucin cDNA encodes a protein with unique tandem-repeat organization. Biochem J. 1994;300:295–298. doi: 10.1042/bj3000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shankar V, Pichan P, Eddy RL, Jr, Tonk V, Nowak N, Sait SN, et al. Chromosomal localization of a human mucin gene (MUC8) and cloning of the cDNA corresponding to the carboxy terminus. Am J Respir Cell Mol Biol. 1997;16:232–241. doi: 10.1165/ajrcmb.16.3.9070607. [DOI] [PubMed] [Google Scholar]
- 145.Chen Y, Zhao YH, Kalaslavadi TB, Hamati E, Nehrke K, Le AD, et al. Genome-wide search and identification of a novel gel-forming mucin MUC19/Muc19 in glandular tissues. Am J Respir Cell Mol Biol. 2004;30:155–165. doi: 10.1165/rcmb.2003-0103OC. [DOI] [PubMed] [Google Scholar]
- 146.Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J, et al. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990;265:15286–15293. [PubMed] [Google Scholar]
- 147.Gendler S, Taylor-Papadimitriou J, Duhig T, Rothbard J, Burchell J. A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J Biol Chem. 1988;263:12820–12823. [PubMed] [Google Scholar]
- 148.Swallow DM, Gendler S, Griffiths B, Kearney A, Povey S, Sheer D, et al. The hypervariable gene locus PUM, which codes for the tumour associated epithelial mucins, is located on chromosome 1, within the region 1q21-4. Ann Hum Genet. 1987;51:289–294. doi: 10.1111/j.1469-1809.1987.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 149.Gum JR, Jr, Ho JJ, Pratt WS, Hicks JW, Hill AS, Vinall LE, et al. MUC3 human intestinal mucin. Analysis of gene structure, the carboxyl terminus and a novel upstream repetitive region. J Biol Chem. 1997;272:26678–26686. doi: 10.1074/jbc.272.42.26678. [DOI] [PubMed] [Google Scholar]
- 150.Pratt WS, Crawley S, Hicks J, Ho J, Nash M, Kim YS, et al. Multiple transcripts of MUC3: evidence for two genes, MUC3A and MUC3B. Biochem Biophys Res Commun. 2000;275:916–923. doi: 10.1006/bbrc.2000.3406. [DOI] [PubMed] [Google Scholar]
- 151.Fox MF, Lahbib F, Pratt W, Attwood J, Gum J, Kim Y, et al. Regional localization of the intestinal mucin gene MUC3 to chromosome 7q22. Ann Hum Genet. 1992;56:281–287. doi: 10.1111/j.1469-1809.1992.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 152.Porchet N, Nguyen VC, Dufosse J, Audie JP, Guyonnet-Duperat V, Gross MS, et al. Molecular cloning and chromosomal localization of a novel human tracheo-bronchial mucin cDNA containing tandemly repeated sequences of 48 base pairs. Biochem Biophys Res Commun. 1991;175:414–422. doi: 10.1016/0006-291x(91)91580-6. [DOI] [PubMed] [Google Scholar]
- 153.Nollet S, Moniaux N, Maury J, Petitprez D, Degand P, Laine A, et al. Human mucin gene MUC4: organization of its 5′-region and polymorphism of its central tandem repeat array. Biochem J. 1998;332:739–748. doi: 10.1042/bj3320739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Williams SJ, McGuckin MA, Gotley DC, Eyre HJ, Sutherland GR, Antalis TM. Two novel mucin genes downregulated in colorectal cancer identified by differential display. Cancer Res. 1999;59:4083–4089. [PubMed] [Google Scholar]
- 155.Williams SJ, Wreschner DH, Tran M, Eyre HJ, Sutherland GR, McGuckin MA. Muc13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J Biol Chem. 2001;276:18327–18336. doi: 10.1074/jbc.M008850200. [DOI] [PubMed] [Google Scholar]
- 156.Pallesen LT, Berglund L, Rasmussen LK, Petersen TE, Rasmussen JT. Isolation and characterization of MUC15, a novel cell membrane-associated mucin. Eur J Biochem. 2002;269:2755–2763. doi: 10.1046/j.1432-1033.2002.02949.x. [DOI] [PubMed] [Google Scholar]
- 157.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 158.O'Brien TJ, Beard JB, Underwood LJ, Dennis RA, Santin AD, York L. The CA 125 gene: an extracellular superstructure dominated by repeat sequences. Tumour Biol. 2001;22:348–366. doi: 10.1159/000050638. [DOI] [PubMed] [Google Scholar]
- 159.O'Brien TJ, Beard JB, Underwood LJ, Shigemasa K. The CA 125 gene: a newly discovered extension of the glycosylated N-terminal domain doubles the size of this extracellular superstructure. Tumour Biol. 2002;23:154–169. doi: 10.1159/000064032. [DOI] [PubMed] [Google Scholar]
- 160.Moniaux N, Junker WM, Singh AP, Jones AM, Batra SK. Characterization of human mucin MUC17: Complete coding sequence and organization. J Biol Chem. 2006;281:23676–23685. doi: 10.1074/jbc.M600302200. [DOI] [PubMed] [Google Scholar]
- 161.Higuchi T, Orita T, Nakanishi S, Katsuya K, Watanabe H, Yamasaki Y, et al. Molecular cloning, genomic structure and expression analysis of MUC20, a novel mucin protein, upregulated in injured kidney. J Biol Chem. 2004;279:1968–1979. doi: 10.1074/jbc.M304558200. [DOI] [PubMed] [Google Scholar]
- 162.Derrien M. Mucin utilisation and host interactions of the novel intestinal microbe Akkermansia muciniphila. Wageningen, The Netherlands: Wageningen University; 2007. Ph.D. Thesis. [Google Scholar]
- 163.Macfarlane G, Gibson G. Formation of glycoprotein degrading enzymes by Bacteroides fragilis. FEMS Microbiol Lett. 1991;61:289–293. doi: 10.1016/0378-1097(91)90567-t. [DOI] [PubMed] [Google Scholar]
- 164.Berg J, Lindqvist L, Andersson G, Nord C. Neuraminidase in Bacteroides fragilis. Appl Environ Microbiol. 1983;46:75–80. doi: 10.1128/aem.46.1.75-80.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Macfarlane G, Macfarlane S, Gibson G. Synthesis and release of proteases by Bacteroides fragilis. Curr Microbiol. 1992;24:55–59. [Google Scholar]
- 166.Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, et al. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003;299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 167.Tsai H, Sunderland D, Gibson G, Hart C, Rhodes J. A novel mucin sulphatase from human faeces: its identification, purification and characterization. Clin Sci. 1992;82:447–454. doi: 10.1042/cs0820447. [DOI] [PubMed] [Google Scholar]
- 168.Ruseler-van Embden JGH, van der Helm R, vanLieshout LMC. Degradation of intestinal glycoproteins by Bacteroides vulgatus. FEMS Microbiol Lett. 1989;58:37–42. doi: 10.1016/0378-1097(89)90338-8. [DOI] [PubMed] [Google Scholar]
- 169.Katayama T, Fujita K, Yamamoto K. Novel bifidobacterial glycosidases acting on sugar chains of mucin glycoproteins. J Biosci Bioeng. 2005;99:457–465. doi: 10.1263/jbb.99.457. [DOI] [PubMed] [Google Scholar]
- 170.Boureau H, Decre D, Carlier JP, Guichet C, Bourlioux P. Identification of a Clostridium cocleatum strain involved in an anti-Clostridium difficile barrier effect and determination of its mucin-degrading enzymes. Res Microbiol. 1993;144:405. doi: 10.1016/0923-2508(93)90198-b. [DOI] [PubMed] [Google Scholar]
- 171.Macfarlane S, Hopkins M, Macfarlane GT. Toxin synthesis and mucin breakdown are related to swarming phenomenon in Clostridium septicum. Infect Immun. 2001;69:1120–1126. doi: 10.1128/IAI.69.2.1120-1126.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Slomiany BL, Murty VL, Piotrowski J, Liau YH, Sundaram P, Slomiany A. Glycosulfatase activity of Helicobacter pylori toward gastric mucin. Biochem Biophys Res Commun. 1992;183:506–513. doi: 10.1016/0006-291x(92)90511-i. [DOI] [PubMed] [Google Scholar]
- 173.Wright D, Rosendale D, Robertson A. Prevotella enzymes involved in mucin oligosaccharide degradation and evidence for a small operon of genes expressed during growth on mucin. FEMS Microbiol Lett. 2000;190:73–79. doi: 10.1111/j.1574-6968.2000.tb09265.x. [DOI] [PubMed] [Google Scholar]
- 174.Rho JH, Wright DP, Christie DL, Clinch K, Furneaux RH, Roberton AM. A novel mechanism for desulfation of mucin: identification and cloning of a mucin-desulfating glycosidase (sulfoglycosidase) from Prevotella strain RS2. J Bacteriol. 2005;187:1543–1551. doi: 10.1128/JB.187.5.1543-1551.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hoskins LC, Boulding ET, Larson G. Purification and characterization of blood group A-degrading isoforms of alpha -N-acetylgalactosaminidase from Ruminococcus torques strain IX-70. J Biol Chem. 1997;272:7932–7939. doi: 10.1074/jbc.272.12.7932. [DOI] [PubMed] [Google Scholar]
- 176.Goso Y, Ishihara K, Sugawara S, Hotta K. Purification and characterization of alpha-L-fucosidases from Streptomyces sp. OH11242. Comp Biochem Physiol B Biochem Mol Biol. 2001;130:375–383. doi: 10.1016/s1096-4959(01)00442-0. [DOI] [PubMed] [Google Scholar]
- 177.Stewart-Tull D, Ollar R, Scobie T. Studies on the Vibrio cholerae mucinase complex. I. Enzymic activities associated with the complex. J Med Microbiol. 1986;22:325–333. doi: 10.1099/00222615-22-4-325. [DOI] [PubMed] [Google Scholar]
- 178.Groenink J, Ligtenberg AJ, Veerman EC, Bolscher JG, Nieuw Amerongen AV. Interaction of the salivary low-molecular-weight mucin (MG2) with Actinobacillus actinomycetemcomitans. Antonie Van Leeuwenhoek. 1996;70:79–87. doi: 10.1007/BF00393572. [DOI] [PubMed] [Google Scholar]
- 179.Prakobphol A, Tangemann K, Rosen SD, Hoover CI, Leffler H, Fisher SJ. Separate oligosaccharide determinants mediate interactions of the low- molecular-weight salivary mucin with neutrophils and bacteria. Biochemistry. 1999;38:6817–6825. doi: 10.1021/bi990145m. [DOI] [PubMed] [Google Scholar]
- 180.Biesbrock AR, Reddy MS, Levine MJ. Interaction of a salivary mucin-secretory immunoglobulin A complex with mucosal pathogens. Infect Immun. 1991;59:3492–3497. doi: 10.1128/iai.59.10.3492-3497.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Moshier A, Reddy MS, Scannapieco FA. Role of type 1 fimbriae in the adhesion of Escherichia coli to salivary mucin and secretory immunoglobulin A. Curr Microbiol. 1996;33:200. doi: 10.1007/s002849900100. [DOI] [PubMed] [Google Scholar]
- 182.Murray PA, Prakobphol A, Lee T, Hoover CI, Fisher SJ. Adherence of oral streptococci to salivary glycoproteins. Infect Immun. 1992;60:31–38. doi: 10.1128/iai.60.1.31-38.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Takamatsu D, Bensing BA, Prakobphol A, Fisher SJ, Sullam PM. Binding of the streptococcal surface glycoproteins GspB and Hsa to human salivary proteins. Infect Immun. 2006;74:1933. doi: 10.1128/IAI.74.3.1933-1940.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu B, Rayment SA, Gyurko C, Oppenheim FG, Offner GD, Troxler RF. The recombinant N-terminal region of human salivary mucin MG2 (MUC7) contains a binding domain for oral Streptococci and exhibits candidacidal activity. Biochem J. 2000;345:557–564. [PMC free article] [PubMed] [Google Scholar]
- 185.Liu B, Rayment S, Oppenheim FG, Troxler RF. Isolation of human salivary mucin MG2 by a novel method and characterization of its interactions with oral bacteria. Arch Biochem Bioph. 1999;364:286. doi: 10.1006/abbi.1999.1141. [DOI] [PubMed] [Google Scholar]
- 186.Schenkels LC, Ligtenberg AJ, Veerman EC, Van Nieuw Amerongen A. Interaction of the salivary glycoprotein EP-GP with the bacterium Streptococcus salivarius HB. J Dent Res. 1993;72:1559–1565. doi: 10.1177/00220345930720120501. [DOI] [PubMed] [Google Scholar]
- 187.Plummer C, Douglas CW. Relationship between the ability of oral streptococci to interact with platelet glycoprotein Ibalpha and with the salivary low-molecular-weight mucin, MG2. FEMS Immunol Med Microbiol. 2006;48:390–399. doi: 10.1111/j.1574-695X.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- 188.Veerman EC, Bank CM, Namavar F, Appelmelk BJ, Bolscher JG, Nieuw Amerongen AV. Sulfated glycans on oral mucin as receptors for Helicobacter pylori. Glycobiology. 1997;7:737–743. doi: 10.1093/glycob/7.6.737. [DOI] [PubMed] [Google Scholar]
- 189.Prakobphol A, Boren T, Ma W, Zhixiang P, Fisher SJ. Highly glycosylated human salivary molecules present oligosaccharides that mediate adhesion of leukocytes and Helicobacter pylori. Biochemistry. 2005;44:2216–2224. doi: 10.1021/bi0480180. [DOI] [PubMed] [Google Scholar]
- 190.Namavar F, Sparrius M, Veerman EC, Appelmelk BJ, Vandenbroucke-Grauls CM. Neutrophil-activating protein mediates adhesion of Helicobacter pylori to sulfated carbohydrates on high-molecular-weight salivary mucin. Infect Immun. 1998;66:444–447. doi: 10.1128/iai.66.2.444-447.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Koop HM, Valentijn-Benz M, Nieuw Amerongen AV, Roukema PA, de Graaff J. Involvement of human mucous saliva and salivary mucins in the aggregation of the oral bacteria Streptococcus sanguis, Streptococcus oralis and Streptococcus rattus. Antonie Van Leeuwenhoek. 1990;57:245–252. doi: 10.1007/BF00400156. [DOI] [PubMed] [Google Scholar]
- 192.Veerman EC, Ligtenberg AJ, Schenkels LC, Walgreen-Weterings E, Nieuw Amerongen AV. Binding of human high-molecular-weight salivary mucins (MG1) to Hemophilus parainfluenzae. J Dent Res. 1995;74:351–357. doi: 10.1177/00220345950740011101. [DOI] [PubMed] [Google Scholar]
- 193.Gururaja TL, Levine JH, Tran DT, Naganagowda GA, Ramalingam K, Ramasubbu N, et al. Candidacidal activity prompted by N-terminus histatin-like domain of human salivary mucin (MUC7) Biochim Biophys Acta. 1999;1431:107. doi: 10.1016/s0167-4838(99)00034-5. [DOI] [PubMed] [Google Scholar]
- 194.Habte HH, Mall AS, de Beer C, Lotz ZE, Kahn D. The role of crude human saliva and purified salivary MUC5B and MUC7 mucins in the inhibition of Human Immunodeficiency Virus type 1 in an inhibition assay. Virol J. 2006;3:99. doi: 10.1186/1743-422X-3-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hoffman MP, Haidaris CG. Analysis of Candida albicans adhesion to salivary mucin. Infect Immun. 1993;61:1940–1949. doi: 10.1128/iai.61.5.1940-1949.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Satyanarayana J, Situ H, Narasimhamurthy S, Bhayani N, Bobek LA, Levine MJ. Divergent solid-phase synthesis and candidacidal activity of MUC7 D1, a 51-residue histidine-rich N-terminal domain of human salivary mucin MUC7. J Pept Res. 2000;56:275–282. doi: 10.1034/j.1399-3011.2000.00765.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.