Abstract
The serine protease autotransporters of Enterobacteriaceae (SPATEs) represent a large class of proteases with contributions to virulence. They are synthesized with a C-terminal domain that forms a β-barrel pore in the outer membrane implicated in translocation of the N-terminal ‘passenger’ domain across the outer membrane. The most recent model for autotransporter secretion comprises entry to the periplasm via the Sec apparatus, followed by the insertion of the C-terminus into the outer membrane as a β-barrel protein and accompanied by translocation of the passenger domain to the bacterial cell surface, all of this with the assistance of the Bam complex insertase/foldase and periplasmic chaperone proteins. We have recently observed direct involvement of periplasmic chaperones in the biogenesis of EspP, a prototypical autotransporter protein produced by Escherichia coli O157:H7. Using molecular and biophysical approaches we demonstrated for the first time, direct protein-protein interactions between the periplasmic SurA and DegP chaperones and either the EspP-β or EspP passenger domains. Such chaperone interactions took place on conserved aromatic residues on the SPATE family. In this report, we now demonstrate direct binding of the periplasmic chaperone FkpA to the EspP passanger domain in Surface Plasmon Resonance experiments with relatively high affinity. We also provide evidence of interaction between the SurA and Skp chaperones with the Bam. These findings in conjunction with newly published data support the role of chaperones in preventing misfolding of AT passenger domains before translocation throughout the Bam complex.
Key words: EspP, autotranspoter, FkpA, surA, DegP, Skp, chaperone, YaeT
SPATE Proteins Secreted by the “Autotransporter” Mechanism
At least 8 protein secretion pathways have been described in Gram-negative bacteria, which have been proposed to be designated types I to VIII.1 Classified in the type V secretion system, the serine protease autotransporters of the Enterobacteriaceae (SPATEs) constitute a group of proteases secreted by pathogenic enteric bacteria.2 SPATEs comprise a large group of trypsin-like serine proteases, which are secreted by Shigella spp., Uropathogenic E. coli, and all of the diarrheagenic E. coli pathotypes.2 The toxins are translocated across the outer membrane by the autotransporter (AT) mechanism, in which translocation requires a dedicated C-terminal β-barrel domain. The N-terminal, mature SPATE toxins harbors a typical serine protease catalytic domain, followed by a C-terminal highly conserved β-helix structure, which is present in nearly all autotransporters. Based on sequence similarities and biological functions, we have classified the SPATE family into class-I and class-II categories.3 Members of the Class I SPATEs, include EspP from enterohemorrhagic E. coli (EHEC),4 Pet from enteroaggregative E. coli (EAEC),5 SigA from Shigella flexneri,6 EspC from enteropathogenic E. coli (EPEC),7 and Sat from uropathogenic and diffusely adhering E. coli (DAEC).8,9 Pet, Sat, SigA and EspC have shown to be cytotoxic to epithelial cells.2,10 The class II SPATEs are diverse, many with unidentified functions, but they seem to target extracellular host proteins such as mucin, coagulation Factor V and complement proteins.2 Some members of the class II SPATE include Pic and SepA from Shigella flexneri,11,12 PicU from Uropathogenic E. coli (UPEC),13 Hbp from human septicemic E. coli14 and Tsh from avian pathogenic E. coli.15
Recently, several periplasmic and an outer membrane protein have been implicated in the targeting and assembly of extracytoplasmic proteins, principally outer membrane proteins (OMPs). The OMP protein assembly machinery designated the Bam complex, was first identified in Neisseria meningitidis, where a highly conserved OMP, denominated Omp85, was essential for viability and required for the folding and assembly of all OMPs examined.16 On the other hand, the periplasmic proteins implicated in the targeting and assembly of extracytoplasmic proteins have shown three biological functions: molecular chaperones such as DegP, SurA, Skp and FkpA,17–20 which stabilize non-native conformations of target proteins and facilitate their folding; peptidy prolyl cis-trans isomerases, such as SurA, PpiD and FkpA,21–23 which catalyze the rate-limiting steps of isomerization during folding; and proteases, such as DegP and DegQ,24 which degrade unproductive or misfolded proteins.
Formerly, an AT translocation model was proposed in which the AT proteins are targeted to the periplasmic space via the Sec apparatus, followed by formation of an outer membrane (OM) β-barrel, which was believed to mediate passage of unfolded or partially folded N-terminal passenger domain to the extracellular milieu.25 A recent model of AT translocation has now been proposed based on experimental evidence, where the Bam, in conjunction with the periplasmic chaperones, assists the translocation of ATs throughout the OM.26–28 The term “autotransporter” was initially proposed on the assumption that all necessary information for movement to the extracellular space was encoded in the polypeptide itself. However, it is now evident that translocation of ATs requires accessory proteins; our group and others have reported that several periplasmic proteins are involved in the targeting and assembly of SPATEs.26–29
Recently, we demonstrated direct interaction of DegP and SurA with the EspP passenger and β-domain species.26 Likewise, interactions of SurA and Skp, as well as the BamA(YaeT) protein, with AT species have been demonstrated in vivo by using stalled AT-translocation intermediates.27,28 Moreover, we demonstrated that in the protease-deficient DegP mutant, SurA as well as FkpA were capable of restoring EspP secretion in MC4100degP strain to 70–90% of wild-type levels.26
We have now extended these studies to show that FkpA, a periplasmic chaperone belonging to the peptidyl-prolyl cis-trans isomerase/chaperone family, is also able to bind unfolded EspP passenger domain with high affinity. In addition, we also provide evidence that periplasmic chaperones SurA and Skp do not just interact with the EspP passenger species, but also with YaeT. These findings are in agreement with the recent model for AT translocation, where the periplasmic chaperone proteins assist the large 100–120 kDa passenger domain species to maintain a translocation competent state before BamA mediates translocation to the exterior.27,28 The precise events required for AT translocation across the OM are being illuminated. Nevertheless, more efforts are needed to completely understand the AT secretion mechanism.
Interaction of Chaperone Proteins with the EspP Passenger Domain and with the YaeT Protein
Interaction of FkpA to EspP passenger domain.
We recently demonstrated in yeast two-hybrid assays (Y2H) and Surface Plasmon Resonance (SPR) experiments that DegP and SurA interacted with aromatic amino acids within the N-terminal passenger domain of EspP. Similarly, Skp was shown to bind the EspP and Hbp autotransporters in a stalled AT-translocation model. FkpA has also shown involvement in the autotransporter IgA protease secretion.30 FkpA is a heat-shock periplasmic peptidyl-prolyl cis/trans isomerase (PPIase) with chaperone activity20,31 and its expression is activated via the σE pathway.32 Overexpression of FkpA was shown to reduce the σE-dependent response induced by misfolded proteins in the periplasm,33 suppress the formation of inclusion bodies from a defective maltose-binding protein and promote the reactivation of denatured citrate synthase.20 Our previous work showed that secretion of EspP was dramatically reduced in a DegP mutant strain; however, overexpression of the SurA or FkpA proteins significantly improved the EspP secretion in this mutant.
In order to assess the possibility of direct binding of FkpA to the EspP passenger species, we performed Y2H experiments to probe protein-protein interactions and SPR to confirm and measure the affinity of these interactions. Accordingly to previous findings in Y2H experiments, we observed interaction between the EspP-β domain and the SurA, Skp and YaeT proteins (Fig. 1A, top), evidenced by the growth of strains harboring the YTH constructs on L/T/H/A minimal medium and by quantifying the expression of the lacZ reporter gene (Fig. 1B, top). In contrast to the other chaperones, SurA was the only protein interacting with both the EspP passenger and EspP-β species (Fig. 1A and B).
Figure 1.
Y2H analyses of the interactions of EspPα and EspPβ domains with periplasmic chaperones. (A) Yeast strains expressing the Gal4 DNA BD-EspPβ protein (top) or Gal4 DNA BD-EspPα (bottom) were transformed with each Gal4 AD-chaperone construct (SurA, Skp, FkpA, PpiA, PpiD and DegP) and with the Gal4 AD-yaeT construct. The strains harboring plasmid combinations were selected in SD L/T minimal medium and the protein-protein interactions were evidenced by the ability to grow in SD L/T/H/A minimal medium. The vector pGADT7 (empty), which lacks an insert and the P53 + T interaction are the negative and positive controls of the system, respectively. (B) Interactions between the putative chaperones and either EspPβ (top) or EspPα (bottom) were quantified by assaying β-galactosidase production from the colonies screened in L/T minimal medium.
Interestingly, in Y2H experiments we did not see interaction of DegP or FkpA with the passenger domain, though DegP did interact with the EspP passenger domain in SPR experiments.26 We then hypothesized that protein-protein interaction in the Y2H system was limited to monomeric proteins. The DegP and FkpA chaperones naturally multimerize forming hexamers (up to 24-mers) and homodimers, respectively. Fusion of chaperone sequences to the activation domain and DNA binding domain of Gal4 protein in the Y2H system may have interfered with oligomerization of these proteins and, as a consequence, with the ability to interact with their substrates.
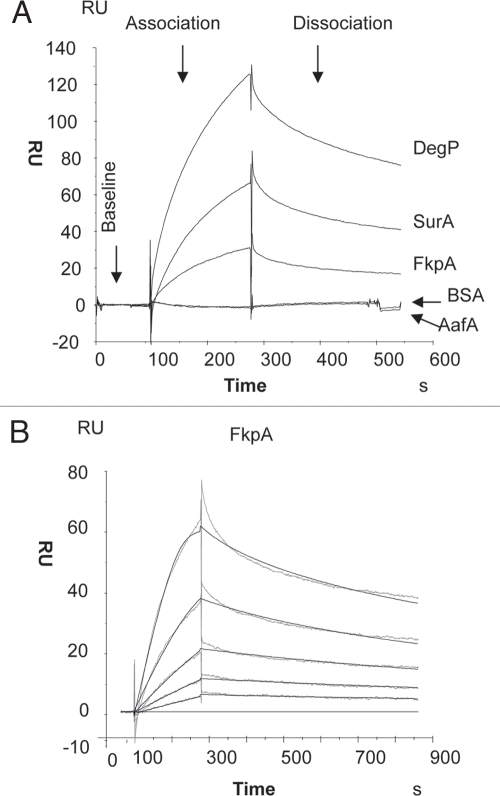
The advantage of the SPR approach to study protein-protein interactions lies in the fact that it employs low concentrations of purified native proteins (without tags) and binding interactions can be studied quantitatively under controlled conditions. The EspP passenger was immobilized onto the sensor surface and chaperone proteins were then injected in aqueous solution through the flow cell under continuous flow. In this system, like DegP and SurA, FkpA interacted with the unfolded EspP passenger domain (Fig. 2A), but was unable to bind folded EspP species (data not shown). The binding of these chaperone proteins to the EspP passenger domain was specific, given that equimolar concentrations of the negative control proteins (BSA and AafA) did not bind to the EspP protein (Fig. 2A). The relative binding affinity of purified FkpA to the unfolded EspP passenger species was determined by using a range of analyte concentrations (Fig. 2B). The equilibrium dissociation constant (KD) for binding of FkpA to EspP was 64.2 nM, which is at least 19 times lower than the measured affinity of SurA for EspP passenger. This relative lower binding affinity of FkpA to EspP when compared to SurA, led us to hypothesize that SurA has the primary role in chaperoning the EspP protein; however, in the event of chaperone shortage, FkpA may play a supplementary chaperone role in AT translocation in vivo.
Figure 2.
FkpA chaperone binds to unfolded but not native EspP protein in Biacore SPR. (A) BIAcore sensorgram for interactions of DegP, SurA, FkpA, BSA and AafA (ligands) with purified denature EspP passenger domain (analyte). (B) SPR measurement of the relative affinities of FkpA to denature EspP was determined by using a range of analyte concentrations (0 to 100 nM). Values for KD (affinity) are: ka (1/Ms),1.36 × 104; kd (1/s), 8.27 × 10−4; KD, 64.2 nM; StDev KD, 20.2 nM. The time course of change in the refractive index (RU) is illustrated in real time. Data were analyzed with BIAevaluation 3.2 software using a 1:1 Langmuir binding model, which describes a simple reversible interaction between two molecules in a 1:1 complex. Each panel illustrates a representative experiment of three performed.
The folding-assistance function of FkpA was previously suggested to be in early folding periplasmic intermediates for preventing their aggregation and, in reactivation of inactive proteins, possibly by binding to partially unfolded species.34 Here, we indeed provide evidence of direct binding of FkpA to unfolded EspP passenger species. The involvement of FkpA, particularly in AT secretion, was previously anticipated in a report, which described FkpA increased the efficiency of displaying an immunoglobulin variable domain fused to the Neisseria IgA protease AT.30 Likewise, enhanced yellow fluorescence protein display on the surface of E. coli by EstA autotransporter was observed when coexpressed with FkpA.35
Accordingly to the proposed genetic model for chaperone function, the periplasm of E. coli contains two parallel pathways for chaperone activity; Skp and DegP are components of the same pathway and that SurA is a component of a separate pathway. This model was based on the fact that only certain pairwise combinations of skp, degP and surA null mutations cause synthetic lethality. Thus, losing one component of either pathway alone is tolerated because the other, parallel pathway can still function. But, losing one component of each pathway simultaneously (e.g., surA and degP) results in a lethal phenotype because both pathways for chaperone activity were compromised.36 We assume that FkpA would fit in the SurA pathway since both have similar functions, beside their chaperoning role, SurA and FkpA have also shown PPIase activity.20,37
On the other hand, YaeT, a component of the Bam complex, was also found to be involved in the secretion of ATs. An initial study in Neisseria meningitidis showed that depletion of Omp85 protein, the E. coli YaeT homolog, dramatically reduced OM proteins, including the IgA protease AT.16 Further studies in a YaeT-depleted Shigella strain demonstrated clear involvement of YaeT in the secretion of two more AT proteins, IcsA and SepA.38 Most recently, the depletion of YaeT in E. coli severely affected the secretion of Hbp through the bacterial envelope.28 In addition, we and others have found that two SPATE proteins, EspP and Hbp interacted with YaeT in vitro and in vivo.26,28 Together, these results suggest that SPATEs proteins are transported through the OM via a mechanism depending on the YaeT protein.
Since periplasmic chaperones deliver proteins destined to be assembled in the bacterial surface to the Bam complex, we hypothesized that chaperones, which interact with EspP, may also interact with the YaeT protein to accomplish with their delivery fuction. To test this hypothesis, we used the YTH system to examine whether SurA and Skp interact with YaeT protein. We found that both, Skp and SurA were able to bind the YaeT molecule expressed in yeast strains, as determined for the growth phenotype in L/T/H/A minimal medium (Fig. 3A) and their ability to induce β-galactosidase activity (Fig. 3B). In agreement with our data, pull-down experiments in a stalled AT translocation model have shown the copurification of both SurA and Skp periplasmic chaperones along with subunits of the Bam complex.27,28 Likewise, in vivo cross-linking strategy to covalently attach chaperone proteins to a component of the Bam complex identified interacting chaperones following copurification using affinity chromatography.39 These results suggest that periplasmic chaperones bind to AT species to prevent nonproductive aggregation and premature folding, but also may suggest direct delivery of AT proteins to the Bam complex. Nevertheless, since SurA and Skp have predilection for OMPs, we do not rule out the possibility of chaperone binding to the transmembrane β barrels of YaeT. All members of the BamA family contain one or more polypeptide transport-associated (POTRA) domains N-terminal to their transmembrane β barrels.40,41 Their function is unclear, but recent studies have shown that the POTRA domain binds periplasmic substrates destined to the OM.41 One would imply that binding of SurA and Skp to the POTRA domain may be related to their chaperone/delivery function on ATs, but binding to the YaeT-β barrel may suggest the requirement of chaperones for the assembly of YaeT itself into the OM.
Figure 3.
Yeast two-hybrid analysis of SurA and Skp interaction with the YaeT protein. (A) Growth phenotype of the yeast AH109 strain transformed with the two hybrid system constructs. The yeast strain expressing the Gal4 DNA-BD-YaeT protein was transformed with Gal4 AD-Skp or Gal4 AD-SurA chaperone constructs and selected on L/T minimal medium. Protein-protein interactions were evaluated in L/T/H/A minimal medium from 4 individual colonies and (B) quantified by β-galactosidase assays carried out in triplicate. Minimal medium L/T, medium without Leucine and Tryptophan. L/T/H/A, medium without Leucine, Tryptophan, Histidine and Adenine. Empty = Y2H plasmid without YaeT. Lam + T Ag and P53 + T Ag are the negative and positive control of the Y2H system, respectively.
Conclusion
Our data suggest participation of periplasmic pathways of chaperone activity in AT biogenesis. We previously found that FkpA, most likely a member of the SurA chaperone pathway, was able to rescue secretion of EspP in the degP mutant strain. Moreover, here we show that FkpA interacts with the EspP passenger species with relatively high affinity in vitro, but to a lesser extent when compared with SurA or DegP chaperones, which suggests an alternative role of FkpA in the secretion of EspP. Furthermore, we have also shown direct interaction of periplasmic chaperones to the YaeT protein.
Our data support the overlapping role of chaperones in preventing nonproductive aggregation and premature folding of passenger domains before translocation through the Bam complex.
Proteins secreted by pathogenic bacteria play essential roles in colonization, infection and virulence. Vast amounts of newly identified AT protein are continuously reported in the scientific literature. However, the mechanism of secretion and the factors that govern their biogenesis are still under investigation.
Acknowledgements
This work was supported by Public Health Service grants AI33096 and AR43615 to J.P.N. and BBSRC/E021174/1 and MRC/G0700151 to I.R.H.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/13436
References
- 1.Desvaux M, Hebraud M, Talon R, Henderson IR. Secretion and subcellular localizations of bacterial proteins: a semantic awareness issue. Trends Microbiol. 2009;17:139–145. doi: 10.1016/j.tim.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Yen YT, Kostakioti M, Henderson IR, Stathopoulos C. Common themes and variations in serine protease autotransporters. Trends Microbiol. 2008;16:370–379. doi: 10.1016/j.tim.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Dutta PR, Cappello R, Navarro-Garcia F, Nataro JP. Functional comparison of serine protease autotransporters of enterobacteriaceae. Infect Immun. 2002;70:7105–7113. doi: 10.1128/IAI.70.12.7105-7113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunder W, Schmidt H, Karch H. EspP a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol Microbiol. 1997;24:767–778. doi: 10.1046/j.1365-2958.1997.3871751.x. [DOI] [PubMed] [Google Scholar]
- 5.Navarro-Garcia F, Sears C, Eslava C, Cravioto A, Nataro JP. Cytoskeletal effects induced by pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect Immun. 1999;67:2184–2192. doi: 10.1128/iai.67.5.2184-2192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Hasani K, Henderson IR, Sakellaris H, Rajakumar K, Grant T, Nataro JP, et al. The sigA gene which is borne on the she pathogenicity island of Shigella flexneri 2a encodes an exported cytopathic protease involved in intestinal fluid accumulation. Infect Immun. 2000;68:2457–2463. doi: 10.1128/iai.68.5.2457-2463.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellies JL, Navarro-Garcia F, Okeke I, Frederickson J, Nataro JP, Kaper JB. espC pathogenicity island of enteropathogenic Escherichia coli encodes an enterotoxin. Infect Immun. 2001;69:315–324. doi: 10.1128/IAI.69.1.315-324.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guyer DM, Radulovic S, Jones FE, Mobley HL. Sat, the secreted autotransporter toxin of uropathogenic Escherichia coli, is a vacuolating cytotoxin for bladder and kidney epithelial cells. Infect Immun. 2002;70:4539–4546. doi: 10.1128/IAI.70.8.4539-4546.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boisen N, Ruiz-Perez F, Scheutz F, Krogfelt KA, Nataro JP. Short report: high prevalence of serine protease autotransporter cytotoxins among strains of enteroaggregative Escherichia coli. Am J Trop Med Hyg. 2009;80:294–301. [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Hasani K, Navarro-Garcia F, Huerta J, Sakellaris H, Adler B. The immunogenic SigA enterotoxin of Shigella flexneri 2a binds to HEp-2 cells and induces fodrin redistribution in intoxicated epithelial cells. PLoS One. 2009;4:8223. doi: 10.1371/journal.pone.0008223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson IR, Czeczulin J, Eslava C, Noriega F, Nataro JP. Characterization of pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect Immun. 1999;67:5587–5596. doi: 10.1128/iai.67.11.5587-5596.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benjelloun-Touimi Z, Sansonetti PJ, Parsot C. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol Microbiol. 1995;17:123–135. doi: 10.1111/j.1365-2958.1995.mmi_17010123.x. [DOI] [PubMed] [Google Scholar]
- 13.Parham NJ, Srinivasan U, Desvaux M, Foxman B, Marrs CF, Henderson IR. PicU, a second serine protease autotransporter of uropathogenic Escherichia coli. FEMS Microbiol Lett. 2004;230:73–83. doi: 10.1016/S0378-1097(03)00862-0. [DOI] [PubMed] [Google Scholar]
- 14.Otto BR, van Dooren SJ, Nuijens JH, Luirink J, Oudega B. Characterization of a hemoglobin protease secreted by the pathogenic Escherichia coli strain EB1. J Exp Med. 1998;188:1091–1103. doi: 10.1084/jem.188.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dozois CM, Dho-Moulin M, Bree A, Fairbrother JM, Desautels C, Curtiss R., 3rd Relationship between the Tsh autotransporter and pathogenicity of avian Escherichia coli and localization and analysis of the Tsh genetic region. Infect Immun. 2000;68:4145–4154. doi: 10.1128/iai.68.7.4145-4154.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science. 2003;299:262–265. doi: 10.1126/science.1078973. [DOI] [PubMed] [Google Scholar]
- 17.Krojer T, Garrido-Franco M, Huber R, Ehrmann M, Clausen T. Crystal structure of DegP (HtrA) reveals a new protease-chaperone machine. Nature. 2002;416:455–459. doi: 10.1038/416455a. [erratum appears in Nature 2002; 417:102] [DOI] [PubMed] [Google Scholar]
- 18.Chen R, Henning U. A periplasmic protein (Skp) of Escherichia coli selectively binds a class of outer membrane proteins. Mol Microbiol. 1996;19:1287–1294. doi: 10.1111/j.1365-2958.1996.tb02473.x. [DOI] [PubMed] [Google Scholar]
- 19.Bitto E, McKay DB. Crystallographic structure of SurA, a molecular chaperone that facilitates folding of outer membrane porins. Structure. 2002;10:1489–1498. doi: 10.1016/s0969-2126(02)00877-8. [DOI] [PubMed] [Google Scholar]
- 20.Arie JP, Sassoon N, Betton JM. Chaperone function of FkpA, a heat shock prolyl isomerase, in the periplasm of Escherichia coli. Mol Microbiol. 2001;39:199–210. doi: 10.1046/j.1365-2958.2001.02250.x. [DOI] [PubMed] [Google Scholar]
- 21.Rouviere PE, Gross CA. SurA, a periplasmic protein with peptidyl-prolyl isomerase activity, participates in the assembly of outer membrane porins. Genes Dev. 1996;10:3170–3182. doi: 10.1101/gad.10.24.3170. [DOI] [PubMed] [Google Scholar]
- 22.Dartigalongue C, Raina S. A new heat-shock gene, ppiD, encodes a peptidyl-prolyl isomerase required for folding of outer membrane proteins in Escherichia coli. EMBO J. 1998;17:3968–3980. doi: 10.1093/emboj/17.14.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramm K, Pluckthun A. The periplasmic Escherichia coli peptidylprolyl cis, trans-isomerase FkpA II. Isomerase-independent chaperone activity in vitro. J Biol Chem. 2000;275:17106–17113. doi: 10.1074/jbc.M910234199. [DOI] [PubMed] [Google Scholar]
- 24.Kolmar H, Waller PR, Sauer RT. The DegP and DegQ periplasmic endoproteases of Escherichia coli: specificity for cleavage sites and substrate conformation. J Bacteriol. 1996;178:5925–5929. doi: 10.1128/jb.178.20.5925-5929.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacob-Dubuisson F, Fernandez R, Coutte L. Protein secretion through autotransporter and two-partner pathways. Biochim Biophys Acta. 2004;1694:235–257. doi: 10.1016/j.bbamcr.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz-Perez F, Henderson IR, Leyton DL, Rossiter AE, Zhang Y, Nataro JP. Roles of periplasmic chaperone proteins in the biogenesis of serine protease autotransporters of Enterobacteriaceae. J Bacteriol. 2009;191:6571–6583. doi: 10.1128/JB.00754-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ieva R, Bernstein HD. Interaction of an autotransporter passenger domain with BamA during its translocation across the bacterial outer membrane. Proc Natl Acad Sci USA. 2009;106:19120–19125. doi: 10.1073/pnas.0907912106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauri A, Soprova Z, Wickstrom D, de Gier JW, Van der Schors RC, Smit AB, et al. The Bam (Omp85) complex is involved in secretion of the autotransporter haemoglobin protease. Microbiol. 2009;155:3982–3991. doi: 10.1099/mic.0.034991-0. [DOI] [PubMed] [Google Scholar]
- 29.Purdy GE, Fisher CR, Payne SM. IcsA surface presentation in Shigella flexneri requires the periplasmic chaperones DegP, Skp and SurA. J Bacteriol. 2007;189:5566–5573. doi: 10.1128/JB.00483-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veiga E, de Lorenzo V, Fernandez LA. Structural tolerance of bacterial autotransporters for folded passenger protein domains. Mol Microbiol. 2004;52:1069–1080. doi: 10.1111/j.1365-2958.2004.04014.x. [DOI] [PubMed] [Google Scholar]
- 31.Saul FA, Arie JP, Vulliez-le Normand B, Kahn R, Betton JM, Bentley GA. Structural and functional studies of FkpA from Escherichia coli, a cis/trans peptidyl-prolyl isomerase with chaperone activity. J Mol Biol. 2004;335:595–608. doi: 10.1016/j.jmb.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 32.Raivio TL, Silhavy TJ. The sigmaE and Cpx regulatory pathways: overlapping but distinct envelope stress responses. Curr Opin Microbiol. 1999;2:159–165. doi: 10.1016/S1369-5274(99)80028-9. [DOI] [PubMed] [Google Scholar]
- 33.Missiakas D, Betton JM, Raina S. New components of protein folding in extracytoplasmic compartments of Escherichia coli SurA, FkpA and Skp/OmpH. Mol Microbiol. 1996;21:871–884. doi: 10.1046/j.1365-2958.1996.561412.x. [DOI] [PubMed] [Google Scholar]
- 34.Ramm K, Pluckthun A. The periplasmic Escherichia coli peptidylprolyl cis, trans-isomerase FkpA II. Isomerase-independent chaperone activity in vitro. J Biol Chem. 2000;275:17106–17113. doi: 10.1074/jbc.M910234199. [DOI] [PubMed] [Google Scholar]
- 35.CP NNaC. Periplasmic chaperone FkpA reduces extracytoplasmic stress response and improves cell-surface display on Escherichia coli. Enzyme and Microbiol Technol. 2008;42:506–513. [Google Scholar]
- 36.Rizzitello AE, Harper JR, Silhavy TJ. Genetic evidence for parallel pathways of chaperone activity in the periplasm of Escherichia coli. J Bacteriol. 2001;183:6794–6800. doi: 10.1128/JB.183.23.6794-6800.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rouviere PE, Gross CA. SurA, a periplasmic protein with peptidyl-prolyl isomerase activity, participates in the assembly of outer membrane porins. Genes Dev. 1996;10:3170–3182. doi: 10.1101/gad.10.24.3170. [DOI] [PubMed] [Google Scholar]
- 38.Jain S, Goldberg MB. Requirement for YaeT in the outer membrane assembly of autotransporter proteins. J Bacteriol. 2007;189:5393–5398. doi: 10.1128/JB.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sklar JG, Wu T, Kahne D, Silhavy TJ. Defining the roles of the periplasmic chaperones SurA, Skp and DegP in Escherichia coli. Genes Dev. 2007;21:2473–2484. doi: 10.1101/gad.1581007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez-Pulido L, Devos D, Genevrois S, Vicente M, Valencia A. POTRA: a conserved domain in the FtsQ family and a class of beta-barrel outer membrane proteins. Trends Biochem Sci. 2003;28:523–526. doi: 10.1016/j.tibs.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Knowles TJ, Jeeves M, Bobat S, Dancea F, McClelland D, Palmer T, et al. Fold and function of polypeptide transport-associated domains responsible for delivering unfolded proteins to membranes. Mol Microbiol. 2008;68:1216–1227. doi: 10.1111/j.1365-2958.2008.06225.x. [DOI] [PubMed] [Google Scholar]