Abstract
We previously showed that fetal and maternal exposure to non-inherited maternal antigens (NIMA) during gestation and nursing resulted in lifelong tolerance to NIMA in some offspring. This NIMA-specific tolerance was mediated by regulatory T cells (Tregs) and was correlated with the level of multi-lineage maternal microchimerism (Mc) indicating a causative link between Mc and Treg development. To determine if transfer of fetal cells into mothers resulted in a similar tolerance to fetal cells, we used qPCR to detect rare fetal derived cells and a delayed type hypersensitivity (DTH) assay to detect fetal alloantigen-specific effector and regulatory T cells in mothers. We found that 5/8 B6 mothers of H2b/d offspring were sensitized to the alloantigens H2d and HY, indicating a dominance of alloantigen-specific effector T cells. Though these sensitized mothers did not have detectable fetal Mc (FMc) in any of the organs tested, they had very high levels of fetus-derived c-kit+ stem cells in their bone marrow. The remaining 3/8 B6 mothers that were not sensitized to the fetal antigens had detectable FMc found mostly in heart, lungs and liver, and in 2/3, we could detect alloantigen-specific regulatory T cells. This data indicates that, as in NIMA-specific tolerance, tolerance in multiparous females to inherited paternal antigens (IPA) expressed by the fetus is associated with the presence of fetal Mc in differentiated cell subsets. Surprisingly, robust lin−c-kit+ bone marrow cell fetal Mc can occur in sensitized mothers. This suggests a continuous source of allospecific priming, coupled with active elimination of mature IPA-expressing lin+ cells by effector T cells of the maternal host.
Key words: Fetal microchimerism, stem cells, sensitization
Introduction
Although fetal and maternal circulations are completely separated, fetal tissue is bathed with maternal blood in animals having a hemochorial placenta (mouse and human).1,2 Exchange of cells at the fetal-maternal interface during pregnancy is bidirectional3,4 and includes both stem cells5 and multilineage mature populations.6 Chen et al.7 showed that injected human mesenchymal stem cells crossed the placenta of pregnant rats and differentiated into different human mesenchymal cells in the organs of the offspring. In a separate study, we detected maternally derived stem cells including mesenchymal stem cells, cardiac stem cells and hematopoietic stem cells in adult murine offspring.5 These maternally derived stem cells differentiate into mature lineages throughout the lifespan of an offspring. In addition to in-utero exposure, the offspring may be exposed to maternal cells and antigens during nursing, which may also contribute to maternal microchimerism (MMc).8–10 The role of microchimerism in tolerance has been suggested by different studies11–13 and MMc has most recently been linked to the development of NIMA-specific Treg cells in mice.8
Fetal cells may also cross the placenta and give rise to fetal microchimerism (FMc) in mothers.14–19 There is evidence that stem cells of fetal origin are present in mothers.7,18,20 Khosrotehrani et al.21 crossed Rag-deficient female mice with wild type male mice and recovered functional T and B cells of fetal origin from the spleen of the female mice during pregnancy indicating transfer of fetal progenitor cells in the female mice. Bianchi et al.8 using a nested PCR, detected CD34+ male progenitor cells in the peripheral blood of one out of eight women who were previously pregnant with male offspring at least >27 years before the blood sampling.
FMc may have deleterious effects. Involvement of FMc in systemic sclerosis has been implicated by different studies.22–24 Nelson et al.24 initially reported that women with systemic sclerosis who had given birth to sons had higher levels of male DNA in their blood than healthy women who had sons. Some women with systemic sclerosis who gave birth decades previously had male DNA at levels as high as that which is found in healthy women who are currently pregnant with a male fetus. Other studies identified male DNA in skin biopsies22 with higher levels in systemic sclerosis patients compared to controls.23 MMc has also been implicated in systemic sclerosis, reported by Lambert et al.25 The frequency of MMc in peripheral blood mononuclear cells was greater in women suffering from systemic sclerosis than healthy controls. Other studies have implicated FMc in other autoimmune diseases, e.g., Hashimoto disease26 and Graves disease.27 How FMc contributes to autoimmune diseases is not clear.
In addition to deleterious effects of FMc, beneficial effects of FMc have also been suggested. Fetal stem cells may have tissue regeneration capability by differentiating into organ-specific cells in some patients with thyroid and liver damage.28,29 Involvement of FMc in protecting against breast cancer has also been suggested.30
In this study, we investigated whether exposure to fetal antigens via pregnancy can induce maternal Tregs specific for inherited paternal antigens of the offspring, and if the Treg induction is correlated with establishment of multilineage FMc in the mother.
Results
Fetal antigen specific effector and regulatory T cells.
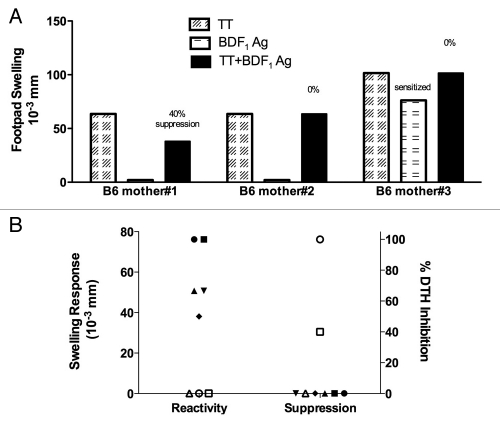
After giving birth to 4–5 litters, female B6 breeders mated with male BDF1 mice were immunized with tetanus toxoid (TT) and sacrificed two weeks later. Splenocytes were tested using a DTH transfer assay to detect antigen-specific effector and/or regulatory T cells of the indirect allorecognition pathway. Using this assay, three phenotypic responses can be found: regulator (no response to donor antigen and bystander suppression), non-regulator (no donor antigen-specific response and no bystander suppression) and sensitized (positive response to donor antigen, no bystander suppression). Representative data for each of these phenotypes are shown in Figure 1A. Splenocytes from mothers #1 and 2 did not respond to the fetal antigens (non-sensitized to fetal antigens). Furthermore, splenocytes from mother #1 were able to suppress a recall tetanus (TT)-induced DTH swelling by 40% in the presence of BDF1 antigens, indicating predominance of Tregs over T effector cells responsive to inherited paternal antigens of the offspring. Splenocytes from mother #3 produced a positive swelling response to fetal (BDF1) antigens, which indicated dominance of fetal antigen-specific effector T cells. Of the eight mothers tested, we found five mothers were sensitized to BDF1 antigens at the DTH level (Fig. 1B). Of these five mice, two were tested with B6 male antigens and both were found to be sensitized also to the fetal H-Y antigens (data not shown). Of the three B6 mothers that were not sensitized to fetal antigens, two were regulators (amount of DTH suppression= 40% and 100%) and one was a non-regulator (Fig. 1B).
Figure 1.
Trans-vivo DTH reveals maternal effector and regulatory T cells specific for fetal alloantigens. (A) Example of regulator, non-regulator and sensitized phenotypes in tvDTH assay. Splenocytes (SC) collected from multiparous female mice immunized with tetanus toxoid (TT) were injected along with different antigens (TT, BDF1 cell sonicate or TT + BDF1) or PBS into footpads of naïve B6 mice. Net footpad swellings (over PBS + SC control) were measured after 24 hours. (B) Dot plot summarizing responses to BDF1 Ag (left) and % suppression of TT-specific DTH induced by co-injection of BDF1 Ag (right) of individual mice are shown. Open symbols = regulator or non-sensitized; closed symbols = sensitized mice.
Fetal microchimerism (FMc) in B6 mothers.
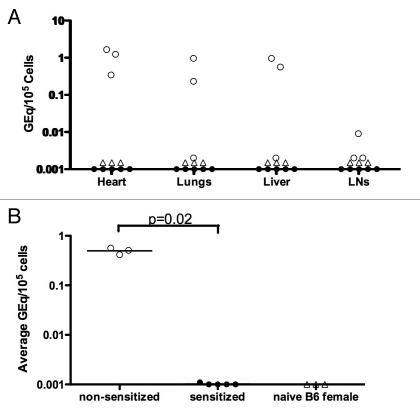
To determine the level and location of FMc, we collected various organs from the B6 mothers of H2b/d offspring and performed qPCR for H2Dd genomic DNA. We found that only the non-sensitized B6 mothers (i.e., the two regulators and one non-regulator) had detectable FMc, whereas all sensitized mothers were negative for FMc in the organs tested (Fig. 2A). When present, FMc was prevalent in the heart, lungs and liver (Fig. 2A), similar to the distribution pattern for MMc in murine offspring.8 Nulliparous B6 female mice had no detectable levels of H2Dd genomic DNA and served as a control. When we averaged the total FMc found in all organs, the non-sensitized mothers had significantly higher levels of FMc than the sensitized mothers (p = 0.02) (Fig. 2B).
Figure 2.
Fetal microchimerism (FMc) in NIPA D mothers. (A) DNA was extracted from different organs of mothers and naïve (nulliparous) B6 females. Fetal-derived H2Dd DNA was quantified with a qPCR assay. FMc is expressed as gene equivalents in 105 maternal cells (GEq/105 cells). (B) FMc in different organs was averaged and the means in individual mice plotted. p value by Wilcoxon rank sums test, where mean values below detection level were assigned a value of 0.001. Open circles = regulator or non-sensitized mothers, closed circles = sensitized mothers, and open triangles = naive B6 females.
IPA-sensitized B6mothers have high levels of offspring-derived c-kit+ stem cells.
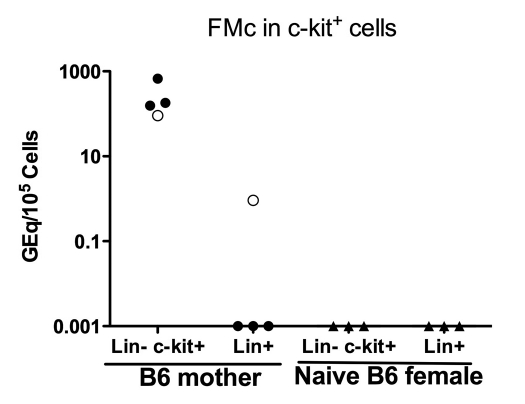
In a previous study, we found that maternally derived H2d+ stem cells were present in 85% of offspring, even though only 40–50% had detectable differentiated maternal cells in the various organs.5 This raises the possibility that lin−c-kit+ stem cells may persist in mice that fail to develop regulatory T cell responses specific for the semi-allogeneic fetal Mc. To investigate whether B6 mothers had offspring-derived stem cells, we sorted bone marrow cells into two subsets: lineage− c-kit+ stem cells and lineage+ c-kit−. Out of four B6 mothers of H2b/d offspring tested, three were sensitized by DTH assay and found to be negative for FMc in various organs,; nonetheless each of these three contained a very high quantity (>100 GEq/105 cells) of FMc in the stem cell, with no signal in the lineage+ population. The one regulator that tested positive for FMc in heart, lung and liver had FMc in both progenitor and differentiated cell populations (Fig. 3).
Figure 3.
FMc in lin− c-kit+ cells. Hematopoietic progenitor c-kit+ cells were sorted from lineage− bone marrow cells of multiparous (back crossed to BDF1 male) female B6 and naïve B6 females. Levels of H-2Dd Mc in DNA from lin− c-kit+ cells and lin+ cells are shown. Open circles = regulator or non-sensitized mothers, closed circles = sensitized mothers, and open triangles = naive B6 females
Discussion
In previous studies, we found that the level of systemic MMc in F1 backcross offspring was directly correlated with the level of Treg cells specific for non-inherited maternal antigens as measured by several different methods.8 While FMc could potentially induce a similar immune-regulatory response to fetal antigens in mothers, we considered this unlikely due to the maturity of mother's immune system at the time of Ag exposure. To investigate this, we tested eight B6 mothers exposed to fetal H2d antigens during multiple (minimum of four) pregnancies with 10–12 offspring in each pregnancy. About half of them were male offspring. We found that 5/8 had a positive response and only 2/8 suppressed a recall TT response in presence of fetal (H-2d and HY) antigens. This result indicates a predominance of effector T cells specific for IPA antigens expressed by the fetus in most parous females exposed to H2d via pregnancy. These T effectors may then proceed to delete multi-lineage FMc in sensitized mothers5 since antigen-specific T effector cells may participate in active deletion of microchimeric cells expressing the fetal antigens. Why FMc mostly induced an effector response while MMc induced a regulatory response is an interesting question. Offspring are exposed to maternal antigens/cells in fetal and neonatal life when their immune systems are immature. Exposure to an alloantigen in fetal life often results in lifelong tolerance to allograft containing the alloantigen.31 However, mothers are exposed to fetal antigens/cells when their immune systems are mature, resulting mainly in sensitization to fetal antigens, which was supported by a study done more than fifty years ago.32 Most Rh− women born of a Rh+ mother failed to produce antibody (Ab) against the Rh antigen when they gave birth to Rh+ children indicating that exposure to the maternal Rh antigen in these Rh− women resulted in tolerance to the Rh antigen. In contrast, Rh− women birthed by Rh− mothers did produce anti-Rh Ab when exposed to fetal Rh antigen indicating that in absence of regulation to maternal Rh antigen the women were sensitized to fetal Rh antigen.
Another possible explanation for sensitization to fetal antigens is absence of oral exposure to the antigens. We previously found that, in absence of nursing by biological mothers, offspring became sensitized to maternal antigens and deleted MMc.8 Since oral tolerance is known to generate TGFβ-producing Tregs,33,34 oral exposure to maternal MHC antigens present in breast milk9 may generate maternal antigen-specific Tregs, which may prevent deletion of maternal cells by maternal antigen-specific effector T cells resulting in high level of MMc. In the current study most (5/8) of the B6 mothers were sensitized to their fetal antigens. However, it is clear that 2/8 B6 mothers developed a regulatory response to the H2d antigens of the fetus, a response that was correlated with multi-lineage Mc. Since the number of parities in all of the B6 mothers tested was similar (4–5), we can rule out the difference in Ag exposure to explain why some mothers could develop tolerance instead of becoming sensitized. A similar heterogeneity in response to HY minor histocompatibility antigens in mothers of male children was recently reported.35 In this regard, the finding that BDF1 and B6 male Ag elicited a positive DTH response in sensitized mothers indicates that minor HY-specific responses parallel those to the major histocompatibility complex antigens of the fetus. However, we did not test HY reactivity in B6 regulator mothers, which would be necessary to confirm HY-specific regulation in the latter mice.
We previously found that while 50% of NIMA-exposed offspring had detectable MMc in differentiated lineage+ cells, the majority (85%) had maternally derived lineage− c-kit+ stem cells in bone marrow.5 When we analyzed FMc in the B6 mothers, all had high levels (100 GEq/105 cells) of fetus-derived stem cells in their bone marrow, even though the sensitized mothers did not have detectable FMc in any of the organs tested. This remarkable finding may be due to inability of fetal antigen-specific maternal effector T cells to recognize fetal stem cells since stem cells are immune privileged. Embryonic stem cells have been shown to express low levels of MHC class I and no MHC class II and thus, can avoid adaptive immune-mediated rejection.36 However, they can be recognized by innate immune system, e.g., NK cells.37,38 Presence of fetal mesenchymal stem cells in women decades after pregnancies with male children has been reported.20 Mesenchymal stem cells are considered immunosuppressive since they produce low levels of Th1 cytokines, e.g., IFNγ and high levels of immunosuppressive cytokines, e.g., TGFβ and IL-10 favoring regulatory T cell induction39,40 and thus, can evade maternal immune system. However, when self-renewing stem cells differentiate into mature cell lineages, they express MHC class I and in some lineages, class II molecules and thereby can easily be detected by maternal effector T cells. Unilineage Mc as FMc only in c-kit+ cells in the sensitized B6 mothers may result in sensitization to the antigens, which is known as ‘split tolerance’.41 However, multilineage FMc in different organs of non-sensitized B6 mothers may induce regulatory response to the antigen (a robust tolerance).
Initially we were puzzled by the finding that B6 mothers maintain sensitization to the fetal antigens in absence of detectable FMc in differentiated cells. Finding FMc in the lin− c-kit+ cells of bone marrow, but nowhere else in sensitized mothers, suggests that the cells which differentiated from the stem cells of fetal origin were being produced, but were continuously being eliminated by maternal effector T and B cells, while boosting the immunity to fetal (inherited paternal) antigens. If correct, this hypothesis has important implications in solid organ and hematopoietic cell transplantation since mothers are commonly used as transplant donors and memory T cells may be an important risk factor in determining outcome. There may be important implications for autoimmunity here also, since the tolerant mothers, in whom fetal stem cells give rise to immunocompetent T and B cell progeny, may be at risk for low-level chronic graft versus host disease, which may activate autoimmune T cells.42,43
Materials and Methods
Source of mice and breeding.
C57 BL/6 (B6; H-2b/b), DBA/2 (H-2d/d) and B6D2F1 (BDF1; H-2b/d) were purchased from Harlan Sprague Dawley (Indianapolis, IN). B6 female mice were crossed with BDF1 male mice resulting in approximately 50% of the offspring inheriting H2b/d. During pregnancy, B6 mothers might receive H2b/d+ cells from the fetuses, which would result in FMc. We tested eight B6 females who were mated with BDF1 males. All the experimental B6 mothers experienced at least four or five pregnancies. Each B6 female had 10–12 offspring on average in each pregnancy. About half of the offspring were males. Naïve B6 females served as controls. B6 male mice were used as a source of HY antigens to test for reactivity to inherited paternal minor H antigen (Ag) of male offspring in multiparous female responders.
DNA extraction and quantitative polymerase chain reaction (qPCR) and analysis.
The detailed technique of DNA extraction, qPCR and the sequences of primers and probe specific for H2Dd sequence were described in detail previously in reference 8. Briefly, DNA was extracted from different tissues (heart, lungs, liver, brain, blood, spleen, lymph nodes, bone marrow and thymus) and sorted cells using QIAamp DNA extraction kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. The extracted DNA was quantified using a nanodrop (NanoDrop products, Wilmington, DE). DNA (1 µg) was amplified by qPCR using an iCycler thermal cycler (Bio-Rad Laboratories, Hercules, CA). BDF1 DNA was diluted into B6 DNA in different ratios (1:10–1:106) and run with the samples to obtain a standard curve. Fetal H2Dd DNA in the maternal samples was quantified using the standard curve. The reaction was performed in 50 µl of total reaction volume with 20 pm of each primer, 7.5 pm of probe and 25 µl of Taqman Universal PCR Mastermix (Applied Biosystem, Carlsbad, CA). The qPCR program used was 50°C for 2 minutes, 95°C for 10 minutes, followed by fifty cycles of 95°C for 15 seconds and 60°C for 1 minute. A qPCR was performed at least twice for each sample.
Cell sorting.
Bone marrow cells were collected from femur, tibia and radius of B6 mothers. Single cell suspension from bone marrow was prepared and the cells were stained with magnetic bead-conjugated lineage cocktail antibodies [CD5, CD45R (B220), CD11b, Gr-1 (Ly-6G/C), 7-4 and Ter-119] (Miltenyi Biotech, Auburn, CA). The lin+ and lin− cells were sorted using a MACS sorter (Miltenyi Biotech, Auburn, CA) according to the manufacturer's protocol. Lin− cells were stained with magnetic bead-conjugated anti-c-kit antibody and the stained cells were sorted using the MACS sorter.
Adoptive transfer delayed type hypersensitivity (DTH) assay.
Splenocytes were collected from TT/DT vaccinated B6 female breeders, two weeks after the vaccination. Ten million splenocytes were injected into footpads of naïve B6 recipients with coinjection of PBS, BDF1 Ag (20 µg) or 0.25 lf of TT/DT. In some cases, B6 male Ag (splenocyte sonicate from a B6 male mouse) was tested to see if there was male-specific Ag sensitization. To measure bystander suppression, splenocytes were co-injected with BDF1 Ag and 0.25 lf of TT/DT. Changes in footpad swelling were measured after 24 hours post-injection using a dial-thickness gauge to measure DTH reaction.
Acknowledgements
This work was supported by 5R01AI066219-04 from the NIH (to William J. Burlingham).
Abbreviations
- FMc
fetal microchimerism
- IPA
inherited paternal antigens
- MMc
maternal microchimerism
- NIMA
non-inherited maternal antigen
- NIPA
non-inherited paternal antigen
- TR
T regulatory cells
- TE
T effector cells
- Lin
hematopoietic lineage+ cells
Footnotes
Previously published online: www.landesbioscience.com/journals/chimerism/article/14295
References
- 1.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 2.Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 3.Lo YM, Lo ES, Watson N, Noakes L, Sargent IL, Thilaganathan B, Wainscoat JS. Two-way cell traffic between mother and fetus: biologic and clinical implications. Blood. 1996;88:4390–4395. [PubMed] [Google Scholar]
- 4.Rinkevich B. Human natural chimerism: an acquired character or a vestige of evolution? Hum Immunol. 2001;62:651–657. doi: 10.1016/s0198-8859(01)00249-x. [DOI] [PubMed] [Google Scholar]
- 5.Dutta P, Burlingham WJ. Stem cell microchimerism and tolerance to non-inherited maternal antigens. Chimerism. 2010;1:1–9. doi: 10.4161/chim.1.1.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutta P, Burlingham WJ. Tolerance to noninherited maternal antigens in mice and humans. Curr Opin Organ Transplant. 2009;14:439–447. doi: 10.1097/MOT.0b013e32832d6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CP, Lee MY, Huang JP, Aplin JD, Wu YH, Hu CS, et al. Trafficking of multipotent mesenchymal stromal cells from maternal circulation through the placenta involves vascular endothelial growth factor receptor-1 and integrins. Stem Cells. 2008;26:550–561. doi: 10.1634/stemcells.2007-0406. [DOI] [PubMed] [Google Scholar]
- 8.Dutta P, Molitor-Dart M, Bobadilla JL, Roenneburg DA, Yan Z, Torrealba JR, Burlingham WJ. Microchimerism is strongly correlated with tolerance to noninherited maternal antigens in mice. Blood. 2009;114:3578–3587. doi: 10.1182/blood-2009-03-213561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molitor ML, Haynes LD, Jankowska-Gan E, Mulder A, Burlingham WJ. HLA class I noninherited maternal antigens in cord blood and breast milk. Hum Immunol. 2004;65:231–239. doi: 10.1016/j.humimm.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L, Yoshimura Y, Huang Y, Suzuki R, Yokoyama M, Okabe M, Shimamura M. Two independent pathways of maternal cell transmission to offspring: through placenta during pregnancy and by breast-feeding after birth. Immunology. 2000;101:570–580. doi: 10.1046/j.1365-2567.2000.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bemelman F, Honey K, Adams E, Cobbold S, Waldmann H. Bone marrow transplantation induces either clonal deletion or infectious tolerance depending on the dose. J Immunol. 1998;160:2645–2648. [PubMed] [Google Scholar]
- 12.Bonilla WV, Geuking MB, Aichele P, Ludewig B, Hengartner H, Zinkernagel RM. Microchimerism maintains deletion of the donor cell-specific CD8+ T cell repertoire. J Clin Invest. 2006;116:156–162. doi: 10.1172/JCI26565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burlingham WJ, Grailer AP, Fechner JH, Jr, Kusaka S, Trucco M, Kocova M, et al. Microchimerism linked to cytotoxic T lymphocyte functional unresponsiveness (clonal anergy) in a tolerant renal transplant recipient. Transplantation. 1995;59:1147–1155. [PubMed] [Google Scholar]
- 14.Fujiki Y, Johnson KL, Peter I, Tighiouart H, Bianchi DW. Fetal cells in the pregnant mouse are diverse and express a variety of progenitor and differentiated cell markers. Biol Reprod. 2009;81:26–32. doi: 10.1095/biolreprod.108.074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson KL, Tao K, Stroh H, Kallenbach L, Peter I, Richey L, et al. Increased fetal cell trafficking in murine lung following complete pregnancy loss from exposure to lipopolysaccharide. Fertil Steril. 2010;93:1718–1721. doi: 10.1016/j.fertnstert.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans PC, Lambert N, Maloney S, Furst DE, Moore JM, Nelson JL. Long-term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma. Blood. 1999;93:2033–2037. [PubMed] [Google Scholar]
- 17.Aractingi S, Berkane N, Bertheau P, Le Goue C, Dausset J, Uzan S, Carosella ED. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898–1901. doi: 10.1016/S0140-6736(98)05121-6. [DOI] [PubMed] [Google Scholar]
- 18.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA. 1996;93:705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo ES, Lo YM, Hjelm NM, Thilaganathan B. Transfer of nucleated maternal cells into fetal circulation during the second trimester of pregnancy. Br J Haematol. 1998;100:605–606. doi: 10.1046/j.1365-2141.1998.0636a.x. [DOI] [PubMed] [Google Scholar]
- 20.O'Donoghue K, Chan J, de la Fuente J, Kennea N, Sandison A, Anderson JR, et al. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet. 2004;364:179–182. doi: 10.1016/S0140-6736(04)16631-2. [DOI] [PubMed] [Google Scholar]
- 21.Khosrotehrani K, Leduc M, Bachy V, Nguyen Huu S, Oster M, Abbas A, et al. Pregnancy allows the transfer and differentiation of fetal lymphoid progenitors into functional T and B cells in mothers. J Immunol. 2008;180:889–897. doi: 10.4049/jimmunol.180.2.889. [DOI] [PubMed] [Google Scholar]
- 22.Artlett CM, Smith JB, Jimenez SA. Identification of fetal DNA and cells in skin lesions from women with systemic sclerosis. N Engl J Med. 1998;338:1186–1191. doi: 10.1056/NEJM199804233381704. [DOI] [PubMed] [Google Scholar]
- 23.Ohtsuka T, Miyamoto Y, Yamakage A, Yamazaki S. Quantitative analysis of microchimerism in systemic sclerosis skin tissue. Arch Dermatol Res. 2001;293:387–391. doi: 10.1007/s004030100245. [DOI] [PubMed] [Google Scholar]
- 24.Nelson JL, Furst DE, Maloney S, Gooley T, Evans PC, Smith A, et al. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet. 1998;351:559–562. doi: 10.1016/S0140-6736(97)08357-8. [DOI] [PubMed] [Google Scholar]
- 25.Lambert NC, Erickson TD, Yan Z, Pang JM, Guthrie KA, Furst DE, Nelson JL. Quantification of maternal microchimerism by HLA-specific real-time polymerase chain reaction: studies of healthy women and women with scleroderma. Arthritis Rheum. 2004;50:906–914. doi: 10.1002/art.20200. [DOI] [PubMed] [Google Scholar]
- 26.Klintschar M, Schwaiger P, Mannweiler S, Regauer S, Kleiber M. Evidence of fetal microchimerism in Hashimoto's thyroiditis. J Clin Endocrinol Metab. 2001;86:2494–2498. doi: 10.1210/jcem.86.6.7540. [DOI] [PubMed] [Google Scholar]
- 27.Ando T, Imaizumi M, Graves PN, Unger P, Davies TF. Intrathyroidal fetal microchimerism in Graves' disease. J Clin Endocrinol Metab. 2002;87:3315–3320. doi: 10.1210/jcem.87.7.8656. [DOI] [PubMed] [Google Scholar]
- 28.Srivatsa B, Srivatsa S, Johnson KL, Samura O, Lee SL, Bianchi DW. Microchimerism of presumed fetal origin in thyroid specimens from women: a case-control study. Lancet. 2001;358:2034–2038. doi: 10.1016/S0140-6736(01)07099-4. [DOI] [PubMed] [Google Scholar]
- 29.Stevens AM, McDonnell WM, Mullarkey ME, Pang JM, Leisenring W, Nelson JL. Liver biopsies from human females contain male hepatocytes in the absence of transplantation. Lab Invest. 2004;84:1603–1609. doi: 10.1038/labinvest.3700193. [DOI] [PubMed] [Google Scholar]
- 30.Gadi VK, Nelson JL. Fetal microchimerism in women with breast cancer. Cancer Res. 2007;67:9035–9038. doi: 10.1158/0008-5472.CAN-06-4209. [DOI] [PubMed] [Google Scholar]
- 31.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 32.Owen RD, Wood HR, Foord AG, Sturgeon P, Baldwin LG. Evidence for Actively Acquired Tolerance to Rh Antigens. Proc Natl Acad Sci USA. 1954;40:420–424. doi: 10.1073/pnas.40.6.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonnella PA, Kodali D, Weiner HL. Induction of low dose oral tolerance in monocyte chemoattractant protein-1- and CCR2-deficient mice. J Immunol. 2003;170:2316–2322. doi: 10.4049/jimmunol.170.5.2316. [DOI] [PubMed] [Google Scholar]
- 34.Marth T, Strober W, Kelsall BL. High dose oral tolerance in ovalbumin TCR-transgenic mice: systemic neutralization of IL-12 augments TGFbeta secretion and T cell apoptosis. J Immunol. 1996;157:2348–2357. [PubMed] [Google Scholar]
- 35.van Halteren AG, Jankowska-Gan E, Joosten A, Blokland E, Pool J, Brand A, et al. Naturally acquired tolerance and sensitization to minor histocompatibility antigens in healthy family members. Blood. 2009;114:2263–2272. doi: 10.1182/blood-2009-01-200410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Baroja ML, Majumdar A, Chadwick K, Rouleau A, Gallacher L, et al. Human embryonic stem cells possess immune-privileged properties. Stem Cells. 2004;22:448–456. doi: 10.1634/stemcells.22-4-448. [DOI] [PubMed] [Google Scholar]
- 37.Durkin ET, Jones KA, Rajesh D, Shaaban AF. Early chimerism threshold predicts sustained engraftment and NK-cell tolerance in prenatal allogeneic chimeras. Blood. 2008;112:5245–5253. doi: 10.1182/blood-2007-12-128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tabayoyong WB, Salas JG, Bonde S, Zavazava N. HOXB4-transduced embryonic stem cell-derived Lin-c-kit+ and Lin-Sca-1+ hematopoietic progenitors express H60 and are targeted by NK cells. J Immunol. 2009;183:5449–5457. doi: 10.4049/jimmunol.0901807. [DOI] [PubMed] [Google Scholar]
- 39.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 40.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 41.Chan WF, Razavy H, Luo B, Shapiro AM, Anderson CC. Development of either split tolerance or robust tolerance along with humoral tolerance to donor and third-party alloantigens in nonmyeloablative mixed chimeras. J Immunol. 2008;180:5177–5186. doi: 10.4049/jimmunol.180.8.5177. [DOI] [PubMed] [Google Scholar]
- 42.Scaletti C, Vultaggio A, Bonifacio S, Emmi L, Torricelli F, Maggi E, et al. Th2-oriented profile of male offspring T cells present in women with systemic sclerosis and reactive with maternal major histocompatibility complex antigens. Arthritis Rheum. 2002;46:445–450. doi: 10.1002/art.10049. [DOI] [PubMed] [Google Scholar]
- 43.Lambert N, Nelson JL. Microchimerism in autoimmune disease: more questions than answers? Autoimmun Rev. 2003;2:133–139. doi: 10.1016/s1568-9972(02)00149-0. [DOI] [PubMed] [Google Scholar]