Abstract
We report the case of a 40-year-old man diagnosed with a scleroderma-like disease. Clinical similarities with graft versus host disease prompted initial testing for chimerism employing fluorescence in situ hybridization (FISH). Female cells were observed within peripheral blood mononuclear cells from the patient.
Because maternal cells have been detected in healthy immunologically competent adults and patients with autoimmune conditions, we hypothesized that these cells were of maternal origin. Contrary to our expectations, HLA-specific quantitative PCR (QPCR) ruled out maternal microchimerism. However, HLA-specific QPCR testing was positive for the paternal HLA haplotype that the patient did not inherit. We reasoned that the most likely origin of chimerism with non-inherited paternal HLA alleles was from an unrecognized “vanished” twin. The patient had never received a blood transfusion.
This report suggests that cells from a vanished twin are a possible source of chimerism. The frequency of chimerism from this source is not yet known and whether the scleroderma-like disease observed in the patient is anecdotal or implies a potential association with autoimmune disease remains to be elucidated.
Key words: chimerism, twin, scleroderma, gender, HLA-specific PCR
Introduction
Systemic Sclerosis (SSc) or scleroderma, from the Greek skleros (hard or indurated) and derma (skin), is an autoimmune disease characterized by skin induration and thickening accompanied by various degrees of tissue fibrosis and chronic inflammation involving numerous organs, functional and structural abnormalities of the vascular system and cellular immune alterations.
Female sex is one of the strongest risk factors for development of autoimmune disease. SSc does not escape this rule with a female:male ratio between 4:1 and 15:1.1
We and others have previously shown that microchimerism (Mc) arising from pregnancy may contribute to the pathogenesis of SSc in women.2–4 Indeed naturally acquired fetal Mc enters the maternal circulation and can be found decades later, with higher frequencies and quantities observed in parous women with SSc compared to healthy matched controls.
Scleroderma also affects men, although to a lesser extent. Alternative sources of Mc that could affect males include cells from a blood transfusion, a twin or their mother.5
Maternal Mc has been demonstrated in cord blood samples wherein female cells were detected in male cord blood by fluorescence in situ hybridization and PCR methods.6,7 Long-term persistence of maternal cells was first described in peripheral blood from infants with severe combined immunodeficiency,8 and subsequently detected in immuno-competent individuals5,9 and implicated in women with SSc. Microchimeric cells can also be transferred in utero from a twin and potentially from an unrecognized (vanished) twin. A vanished twin is relatively common in healthy pregnancies.10 We present here the case of a 40-year-old male with a scleroderma-like disease who was studied for maternal Mc and for Mc from a vanished twin.
Patient History and Results
Patient history.
The patient was a 40-year-old Belgian male with a history of professional exposure to hydrocarbons at the age of 20, who developed skin and lung inflammation within months after the toxic exposure. Rapidly progressive cutaneous inflammation associated with lung fibrosis led to the presumed diagnosis of environmental scleroderma, although the patient did not have Raynaud's phenomenon or antinuclear antibodies. Over the following years this condition evolved into a chronic inflammatory disease, reminiscent of graft-versus-host disease with lymphocytic infiltrates and involvement at other locations including small bowel, uvea and liver. His disease has been partially controlled by chronic immunosuppressive therapy with low-dose corticosteroids and azathioprine, and at the time of enrollment in the current studies his physical findings included digital vitiligo, disseminated patchy erythematous lesions and nail involvement (20 of 20 nails).
The patient did not fulfill the American College of Rheumatology classification criteria for SSc,11 i.e., major criterion (symmetrical thickening, tightening and induration of the skin of the fingers and the skin proximal to the metacarpophalangeal or metatarsophalangeal joints), or presence of two minor criteria (sclerodactyly, digital pitting scars/loss of substance of the finger pad and basilar pulmonary fibrosis). Therefore his disease is referred to as “scleroderma-like”.
We investigated this man, who never received a blood transfusion, initially for Mc from his mother and subsequently for Mc that could have originated from a vanished twin and persisted 40 years after his birth.
Results
Studies for maternal Mc.
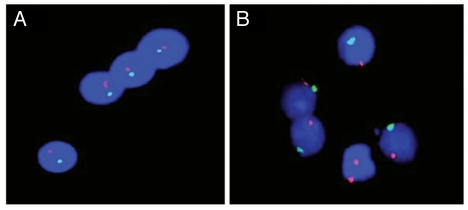
We first investigated peripheral blood mononuclear cells (PBMC) from this male patient for female cells employing fluorescence in situ hybridization with X- and Y-chromosome specific probes (Fig. 1). Among 40,600 PBMC examined seven cells with two distinct red signals were found, corresponding to two X chromosomes within a clearly defined nucleus.
Figure 1.
Fluorescence in situ hybridization of Y (green) and X (red) chromosomes in PBMCs from males. (A) Absence of female cells in a healthy male. (B) Presence of female cells in a male patient with “scleroderma-like” disease.
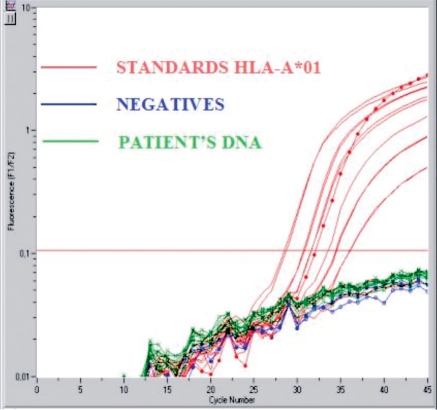
We next conducted HLA-specific quantitative PCR (QPCR) testing for a maternal HLA allele that was not transmitted to the patient. This is referred to as targeting the non-inherited maternal HLA allele (NIMA) (Fig. 2). For this purpose, we developed a new QPCR assay that was specific for HLA-A*01 (NIMA). However, we found no evidence of Mc from the mother as DNA from the patient's blood did not amplify, despite testing a total of 642,935 DNA cell equivalents in two different experiments. Figure 3 illustrates one of the two experiments.
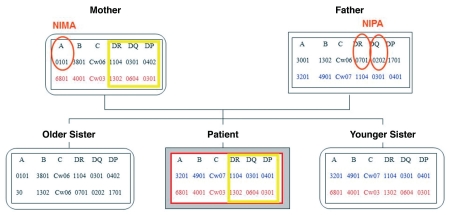
Figure 2.
Familial HLA genotyping results. NIMA: non-inherited maternal HLA alleles of the patient; NIPA: non-inherited paternal HLA alleles of the patient. To the left of the patient's HLA-typing results are results for the patient's older sister and to the right a younger sister.
Figure 3.
QPCR targeting HLA-A*01, representing an HLA allele that was not inherited by the patient, demonstrating the absence of maternal Mc.
Studies for twin Mc.
Female cells observed by FISH could not have originated from a blood transfusion as the patient had never been transfused. We then questioned whether the female cells could have originated from a female twin, although the patient was born as a singleton child.
Should this hypothesis be correct, the chance of detecting DNA from a vanished twin was 50% because if the twin had inherited the same paternal HLA haplotype as the patient, then Mc would not be distinguishable. Conversely, if the twin had inherited the other paternal haplotype, QPCR testing for non-inherited paternal HLA alleles (NIPA: DRB1*07-DQB1*02 haplotype) would allow detection of Mc by NIPA-specific QPCR.
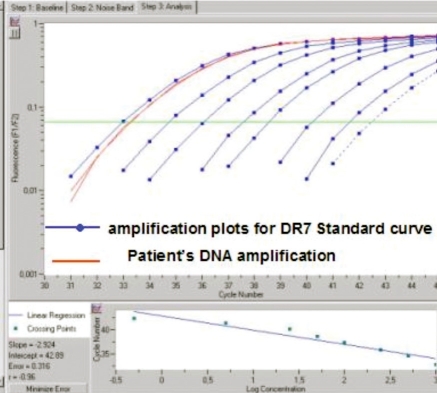
We had previously developed QPCR assays for HLA-DRB1*07 and DQB1*02,9,12 and employed each of these assays to test DNA extracted from the patient's PBMC. The patient's DNA amplified for both assays, confirming the NIPA haplotype and supporting the interpretation that the patient acquired cells from a vanished twin (Fig. 4). Both assays gave identical results with, respectively for DRB1*07 and DQB1*02 QPCR assays, 844 and 845 genome equivalent of cells (gEq) for a total of 160,000 gEq patient cells tested. These results suggest high levels of chimerism, reaching 5%. Testing by FISH identified lower levels of 0.02%.
Figure 4.
QPCR targeting HLA-DRB1*07, representing an HLA allele that was not inherited by the patient, demonstrating the presence of Mc.
Follow-up over time.
We investigated twin Mc in a follow-up blood sample drawn 15 months later. HLA-DRB1*07 and HLA-DQB1*02 PCR were both negative. However, the two samples also had an important difference in that the first (positive for twin Mc) was obtained by cytapheresis [without Granlocyte Macrophage Colony Stimulating Factor (GM-CSF) mobilization], whereas the second (negative for Mc) was a standard blood sample drawn into heparinized vacutainer tubes. There was no difference in the patient's clinical status or treatment between the two dates.
Studies of the mother for fetal Mc from prior pregnancy with a vanished twin.
A peripheral blood sample was obtained from the mother who was 60 years-old at the time of the blood draw. The same HLA-DRB1*07 and DQB1*02 QPCR assays were used to test DNA extracted from the mother's PBMC for evidence of fetal Mc from a prior pregnancy with a vanished twin. Results were negative for both assays (results not shown).
Discussion
We report here the case of a man presenting with marked lymphocytic inflammation involving the skin, lungs and digestive tract, who had a previous exposure to hydrocarbons. Although initially diagnosed as environmental scleroderma, diagnosis was revised in the absence of sufficient criteria for this disease to that of a scleroderma-like condition reminiscent of graft-vs-host-disease. These characteristics prompted initial studies employing FISH to assess for chimerism with female cells. The presence of cells with two X chromosomes among male PBMC suggested maternal Mc.
However, contrary to our expectations, female cells were not maternal in origin as the patient's DNA did not amplify by HLA-specific QPCR when tested for a non inherited maternal HLA allele (NIMA).
We then hypothesized that the female cells were from an unrecognized vanished twin, as this phenomenon is relatively common in healthy pregnancies.10 The patient tested positively for non inherited paternal HLA alleles (NIPA) with amplification for two different HLA class II alleles by QPCR, supporting this hypothesis. An alternative explanation could be Mc from an older sister passed via maternal blood to the patient during his fetal life or from a miscarriage (recognized or unrecognized) of the mother prior to the patient's birth. However, the patient tested negatively for HLA-A*01 and his older sister had inherited the maternal haplotype containing HLA-A*01. Additionally, the patient's mother had no history of miscarriage. Thus the most likely interpretation of our results is that this study represents the first report of persistent Mc from an unrecognized twin 40 years after birth.
We were unable to extend experiments to include testing for HLA class I NIPA (A*30 and B*13), because the remaining DNA was insufficient for Mc testing.
Quantitative assessment of Mc is difficult to evaluate in this patient as FISH results indicate that Mc represented 0.02% of total host cells, whereas QPCR showed levels as high as 5%. Two separate HLA-specific QPCR assays, DRB1*07 and HLA-DQB1*02, were concordant for Mc levels of 5%. Previous Mc studies have reported variations in Mc quantities depending on the method used, with FISH being reputedly less sensitive than PCR. The manufacturer's instructions (Abbott laboratories, Illinois, USA) report that the probe kit used for FISH offers a limit of detection of 1% through a combination of CEP X and CEP Y fluorescently labeled DNA probes for specific regions of chromosome X and chromosome Y, respectively. In parallel HLA-specific QPCR offers a limit of detection of 0.005%, which could in part explain a difference in results. QPCR for additional HLA-specificities, such as HLA-A*30 or B*13, would have been useful but were not feasible in this case.
We also observed a difference in Mc in two different time points. While fluctuation of Mc levels has been described, including in transplant recipients, the difference in the two time points in our study could be due to the method of blood sampling, as the first time point (positive for Mc) was from a cytapheresis and the second time point (negative for Mc) from a standard blood test. Alternatively, changes in levels could reflect “physiological” fluctuations of cells depending on recruitment to tissues.
It has recently been shown in women undergoing surgery for suspected lung cancer that male stem cells, presumably of fetal origin, are present at sites of tissue injury.14 These cells could be recruited from a natural niche, such as bone marrow or proliferate locally and either way could result in dissimilar amounts in peripheral blood at different time points. In our patient, clinical status and treatment were very similar between the two blood draws, arguing against variations in Mc related to inflammation and recruitment. Thus it seems most likely that technical aspects related to cytapheresis resulted in higher levels of Mc compared to a standard blood draw.
Interestingly, our experiments did not show evidence of fetal Mc from prior pregnancy with a “vanished fetus” in PBMC from the patient's mother. One could suggest, as previously described by our group, that the host's genotype might influence persistence of Mc, but in this particular case the patient and his mother had very similar HLA typing, at least for HLA class II, where they were HLA-identical. Besides genetic factors, chimerism in this case may be dependent on other factors such as inflammation, environmental exposure (e.g., hydrocarbons), timing or the method used to obtain and isolate PBMC. A decade ago, in a mouse model Christner et al.15 showed marked proliferation of pre-existing microchimeric cells of fetal origin following intraperitoneal injections of vinyl chloride, associated with the development of fibrosis and a heavy mononuclear infiltration in the dermis. We suggest that a similar expansion and recruitment of microchimeric cells may have occurred in this scleroderma-like patient after hydrocarbon exposure, in contrast to his mother, who had no such exposure.
Parous women are potential hosts to at least two sources of Mc, maternal and fetal. We and others, proposed that this particular condition might contribute to female preponderance in auto-immune diseases.16 This hypothesis has been most extensively tested for SSc, a condition resembling graft-versus-host-disease, the latter a condition of chimerism that clearly leads to inflammation. Higher levels of Mc in women with SSc compared to matched healthy women have reinforced this hypothesis.
Our current study suggests that a vanished twin represents an additional potential source of Mc that can occur in men as well as women. Further studies are needed to determine the frequency of vanished twin Mc and to elucidate whether the autoimmune-like disease in the current report is anecdotal or whether vanished twin Mc might predispose to autoimmunity.
Methods
Peripheral blood samples.
PBMCs were obtained on two occasions for the current study: the first sample derived from cytapheresis without stem cell mobilization (March 2005) and the second sample was a standard heparinized blood sample (June 2006). At both time points, the patient was taking immunosuppressive medications including low-dose corticosteroids and azathioprine. The patient gave informed consent according to the Declaration of Helsinki.13
Familial HLA typing.
HLA-A, B, DR, DQ and DP typing was done in the Laboratory of Immunology-Hematology-Transfusion of Erasme Hospital, in Brussels, Belgium.
Fluorescence in situ hybridization (FISH).
To test for chimerism with female cells FISH was employed and the patient's PBMCs were probed, after Ficoll density separation, with CEP X/Y DNA Probe Kit according to manufacturer's instruction (Abbot laboratories, Illinois, USA). The CEP X/Y probe is a mixture of a Spectrum Orange labeled CEP X DNA probe and a Spectrum Green labeled CEP Y DNA probe specific for the alpha satellite centromeric region of chromosome X and the satellite III (Yq12) region of chromosome Y. The limit of detection sensitivity, according to the manufacturer's instructions, is 1%. A healthy male donor was tested in parallel as a control (Fig. 1).
HLA-specific quantitative PCR (QPCR).
In order to identify and quantify maternal Mc, we first examined HLA typing results for the patient and his mother. The patient and his mother were HLA-DR and DQ identical (Fig. 2) so we could not employ any of the HLA class II specific QPCR assays that had previously been developed. We therefore designed a new QPCR assay that was specific for a maternal HLA class I allele that the patient did not inherit (NIMA), HLA-A* 01; the assay was validated with a sensitivity of 0.005%, as the DNA-equivalent of one HLA-A*01 cell was detectable in a background of the DNA-equivalent of 20,000 non HLA-A*01 cells (Fig. 3).
To investigate Mc from a vanished twin the patient samples were tested with QPCR assays that were specific to the patient's non-inherited paternal HLA alleles (NIPA). These experiments were performed employing previously developed QPCR assays for HLA-DRB1*07 and DQB1*02.9,12
Acknowledgements
We are grateful to Olivier Michel, MD, PhD, Clinique d'Immuno-Allergologie, Centre Hospitalier Universitaire Brugmann, Brussels, Belgium, who referred his patient for the investigations reported herein, and to Marc Andrien from the Laboratory of Immunology, Erasme Hospital, who performed the detailed HLA typing of the patient and his parents and siblings. This work was supported by the Fonds Erasme, Brussels.
Footnotes
Previously published online: www.landesbioscience.com/journals/chimerism/article/14294
References
- 1.Morrow J, Nelson JL, Watts R, Isenberg D. Scleroderma. In: Maddison P, Isenberg D, Glass D, Woo P, editors. Autoimmune Rheumatic Disease. Oxford University Press; 1999. pp. 194–215. [Google Scholar]
- 2.Nelson JL, Furst DE, Maloney S, Gooley T, Evans PC, Smith A, et al. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet. 1998;351:559–562. doi: 10.1016/S0140-6736(97)08357-8. [DOI] [PubMed] [Google Scholar]
- 3.Artlett CM, Smith JB, Jimenez SA. Identification of fetal DNA and cells in skin lesions from women with systemic sclerosis. N Engl J Med. 1998;338:1186–1191. doi: 10.1056/NEJM199804233381704. [DOI] [PubMed] [Google Scholar]
- 4.Rak JM, Pagni PP, Tiev K, Allanore Y, Farge D, Harle JR, et al. Male microchimerism and HLA compatibility in French women with sclerodema: a different profile in limited and diffuse subset. Rheumatology. 2009;48:363–366. doi: 10.1093/rheumatology/ken505. [DOI] [PubMed] [Google Scholar]
- 5.Maloney S, Smith A, Furst DE, Myerson D, Rupert K, Evans PC, Nelson JL. Microchimerism of maternal origin persists into adult life. J Clin Invest. 1999;104:41–47. doi: 10.1172/JCI6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall JM, Lingenfelter P, Adams SL, Lasser D, Hansen JA, Bean MA. Detection of maternal cells in human umbilical cord blood using fluorescence in situ hybridization. Blood. 1995;86:2829–2832. [PubMed] [Google Scholar]
- 7.Bauer M, Orescovic I, Schoell WM, Bianchi DW, Pertl B. Detection of maternal deoxyribonucleic acid in umbilical cord plasma by using fluorescent polymerase chain reaction amplification of short tandem repeat sequences. Am J Obstet Gynecol. 2002;186:117–120. doi: 10.1067/mob.2002.118306. [DOI] [PubMed] [Google Scholar]
- 8.Pollack MS, Kapoor N, Sorell M, Kim SJ, Christiansen FT, Silver DM, et al. DR-positive maternal engrafted T cells in a severe combined immunodeficiency patient without graft-versus-host disease. Transplantation. 1980;30:331–334. doi: 10.1097/00007890-198011000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Lambert NC, Erickson TD, Yan Z, Pang JM, Guthrie KA, Furst DE, Nelson JL. Quantification of maternal microchimerism by HLA-specific real-time polymerase chain reaction—Studies of healthy women and women with scleroderma. Arthritis Rheum. 2004;50:906–914. doi: 10.1002/art.20200. [DOI] [PubMed] [Google Scholar]
- 10.Robinson HP, Caines JS. Sonar evidence of early pregnancy failure in patients with twin conceptions. Br J Obstet Gynaecol. 1977;84:22–25. doi: 10.1111/j.1471-0528.1977.tb12460.x. [DOI] [PubMed] [Google Scholar]
- 11.LeRoy EC, Medsger TA., Jr Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001;28:1573–1576. [PubMed] [Google Scholar]
- 12.Rak JM, Maestroni L, Balandraud N, Guis S, Boudinet H, Guzian MC, et al. Transfer of the shared epitope through microchimerism in women with rheumatoid arthritis. Arthritis Rheum. 2009;60:73–80. doi: 10.1002/art.24224. [DOI] [PubMed] [Google Scholar]
- 13.Vollmann J, Winau R. Informed consent in human experimentation before the Nuremberg code. Bmj. 1996;313:1445–1449. doi: 10.1136/bmj.313.7070.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Donoghue K, Chan J, de la Fuente J, Kennea N, Sandison A, Anderson JR, et al. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet. 2004;364:179–182. doi: 10.1016/S0140-6736(04)16631-2. [DOI] [PubMed] [Google Scholar]
- 15.Christner PJ, Artlett CM, Conway RF, Jimenez SA. Increased numbers of microchimeric cells of fetal origin are associated with dermal fibrosis in mice following injection of vinyl chloride. Arthritis Rheum. 2000;43:2598–2605. doi: 10.1002/1529-0131(200011)43:11<2598::AID-ANR30>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Nelson JL. Microchimerism: incidental byproduct of pregnancy or active participant in human health? Trends Mol Med. 2002;8:109–113. doi: 10.1016/s1471-4914(01)02269-9. [DOI] [PubMed] [Google Scholar]